Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.


0023 Although most cases of dental caries originate with
dissolution of the enamel, the structure of the teeth is
such that should the gingival tissues or gums recede,
the neck of the tooth may be exposed. If this occurs,
dentine is quickly exposed, leading to sensitivity of
the tooth and, subsequently, dentinal or root caries.
As receding gingiva occurs with age, so root caries is
associated with the aging dentition and is seen mostly
in patients over 50 years of age.
Cementum
0024 The dentine of the root of the tooth is covered by a
thin layer of cementum. This structure, also of
mesenchymal origin, is again composed of inorganic
and organic material but is closer to bone in the
proportion of the two components. (See Bone.)
0025 Cementum provides a mean of bonding the teeth
into the bony sockets of the jaws by means of strands
of collagen, known as periodontal fibers. The whole
root is surrounded by many of these fibers, attaching
the tooth to the bone, and providing a system of
‘elastic bands,’ or a hydraulic system, so that the
tooth can be pressed deeper into its socket under biting
forces. This mechanism takes up heavy forces on the
tooth without them being transferred directly to the
bone, which would be very uncomfortable on biting.
0026 The cementum may break down as part of the dental
caries process or during gum disease, or be worn away
by excessively hard tooth brushing. Because of its
lower inorganic content, its resistance to decay or
wear is far less than either enamel or dentine, and if
exposed, it can wear away quite quickly.
See also: Bone; Calcium: Physiology
Further Reading
Nikiforuk G (1985) Understanding Dental Caries: 1.
Etiology and Mechanisms. Basic and Clinical Aspects.
Basel: Karger.
Thylstrup A and Fejerskov O (1986) Textbook of Cariol-
ogy, 1st edn. Copenhagen: Munksgaard.
Etiology of Dental Caries
M S Duggal and M E J Curson, University of Leeds,
Leeds, UK
This article is reproduced from Encyclopaedia of Food Science,
Food Technology and Nutrition, Copyright 1993, Academic Press.
Introduction
0001 Dental caries or tooth decay is a pathological process
of localized destruction of tooth tissues. It is a form of
progressive loss of enamel, dentine, and cementum
initiated by microbial activity at the tooth surface.
Loss of tooth substances is preceded by a partial
dissolution of mineral ahead of the total destruction
of the tissue. It is because of this characteristic that
dental caries can be differentiated from other destruc-
tive processes of the teeth, such as abrasion through
mechanical wear and erosion by acid fluids.
Current Concepts of Caries Etiology
0002There is overwhelming evidence that the initial phase
of dental caries involves demineralization of the tooth
enamel by localized high concentrations of organic
acids. These acids are produced by the fermentation
of common dietary carbohydrates by bacteria that
accumulate in dental plaque on the teeth. This con-
cept of dental caries etiology is based essentially on
the interplay of four principal factors: a susceptible
host (the tooth), microflora with an acidogenic
potential, a suitable substrate available locally to the
pathodontic bacteria (carbohydrate), and a fourth
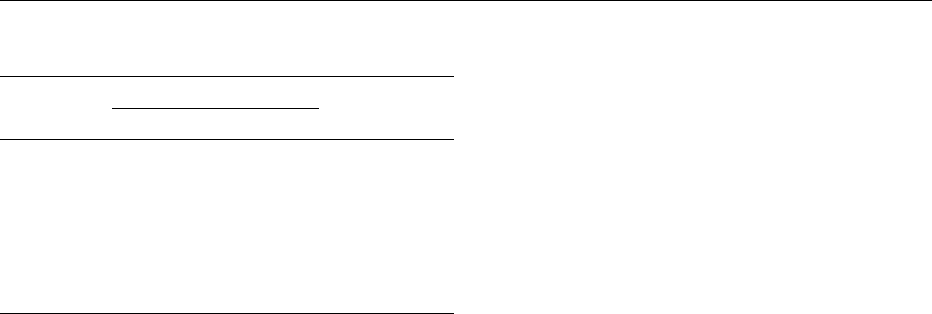
important dimension, time (Figure 1). Because of the
multiplicity of factors which influence the initiation
and progression of dental caries it is often referred to
B
u
f
f
e
r
i
n
g
c
a
p
a
c
i
t
y
p
H
c
o
m
p
o
s
i
t
i
o
n
F
l
o
w
r
a
t
e
p
H
Tooth
Tooth
Time
Saliva
Saliva
Saliva
Saliva
Age
Fluorides
Morphology
Nutrition
Trace elements
Carbonate level
Substrate
Substrate
Micro-
organisms
Oral clearance
Oral hygiene
Detergency of food
Frequency of eating
Carbohydrate (type,
concentration)
Microorganisms
(Substrate)
Oral hygiene
Fluoride in plaque
Streptococcus mutans
Caries
fig0001Figure 1 Diagrammatic representation of the parameters in-
volved in the carious process. All factors must be acting concur-
rently for caries to develop. Dental Disease/Aetiology of Dental
Caries, Reproduced from Encyclopaedia of Food Science, Food
Technology and Nutrition, Macrae R, Robinson RK and Sadler MJ
(eds), 1993, Academic Press.
1746 DENTAL DISEASE/Etiology of Dental Caries

as a disease of a ‘multifactorial etiology’, an old but
appropriate description. In this article the role of
microflora and the host susceptibility are discussed.
The role of carbohydrates in the diet is discussed
later. (See Carbohydrates: Digestion, Absorption, and
Metabolism; Requirements and Dietary Importance.)
The Role of Microorganisms
0003 Considerable difference of opinion exists within the
dental research community as to which microorgan-
isms cause dental caries and whether indeed a specific
strain of bacteria is responsible in its causation. How-
ever, there is evidence that microorganisms, which
accumulate in dental masses (dental plaque) on the
teeth, cause fermentation of dietary carbohydrates,
producing organic acids which cause demineraliza-
tion of the tooth enamel. This concept is a combin-
ation of Miller’s acidogenic theory of 1889 and that
of dental plaque introduced by Williams in 1897.
Subsequent research has supported this original view
and the evidence implicating oral microorganisms in
the etiology of dental caries is summarized below:
1.
0004 Germfree animals do not develop dental caries.
2.
0005 Addition of penicillin to the diet of rats prevents
caries.
3.
0006 Plaque bacteria can demineralize enamel in vitro
when incubated with dietary carbohydrates.
4.
0007 Microorganisms can be found invading carious
enamel and dentine.
5.
0008 Animals in which diet or dietary carbohydrates are
delivered directly into the stomach through a tube
do not develop caries.
Role of Dental Plaque
0009 Dental plaque is the name given to the aggregations of
bacteria and their products which accumulate on the
tooth surface. Plaque collects rapidly in the mouth,
although the actual rate of formation varies from one
individual to another. When plaque accumulates on the
crowns of teeth the natural, smooth, shiny appearance
of the enamel is lost and a dull, matt effect is produced.
As it builds up, masses of plaque become more readily
visible to the naked eye. In direct smears, the early
plaque is dominated by cocci and rods, most of which
are Gram-positive. In the mature plaque (after about 7
days) the percentage of cocci in the plaque decreases
rapidly and filaments and rods constitute about 50% of
organisms in plaque. The ecological term for this shift
from a predominantly coccal type in the beginning to a
predominantly mixed, filamentous flora a few days
later is bacterial succession.
0010 There is no doubt that the presence of plaque is
required for caries development in humans. The best
available evidence suggests that a carious lesion results
from the action of bacterial metabolic end products
localized to the enamel surface by dental plaque. It is
therefore the acidity generated by the microorganisms
in the plaque after exposure to carbohydrates that is
directly responsible for causing demineralization. This
is under the influence of many factors.
Regulators of Plaque Acidity
0011Various parameters have been studied for their effect
on plaque acidity, and hence their effect on caries;
these are listed in Table 1. The role of saliva will be
discussed later, and carbohydrates as regulators of
plaque pH are discussed elsewhere.
0012Bacterial mass in relation to caries Plaque is re-
quired for caries initiation, although the relationship
between the plaque quantity and dental caries activity
is unclear. Some earlier studies, using a crude method-
ology, showed a positive correlation between caries
increment and oral hygiene, but more controlled
investigations failed to establish this finding. Many
individuals remain caries-free in spite of an unfavor-
able oral hygiene. This lack of a clear-cut relationship
between amount of plaque and dental caries suggests
that plaques vary in their microbial composition and,
although present in equal amounts, might vary in
their cariogenicity. Impressive evidence indicates
that the qualitative nature of the plaque flora deter-
mines the metabolism and the potential for caries
production. This view has been referred to in the
literature as the specific plaque hypothesis.
0013Bacterial composition of plaque and caries Most
published data on gnotobiotic animals have concen-
trated on the cariogenic potential of streptococci,
especially Streptococcus mutans.However,several
organisms have been found capable of inducing
carious lesions when used as monocontaminants in
gnotobiotic rats. These include S. mutans, S. salivarius,
S. sanguis, S. milleri, Lactobacillus acidophilus,
Peptostreptococcus intermedius, Actinomyces visco-
sus,andA. naeslundii. However, not all organisms are
equally cariogenic. Table 2 shows the acidogenicity and
tbl0001Table 1 Factors influencing pH of dental plaque
Carbohydrates Saliva Plaque
Quantity of intake Flow rate Bacterial mass
Frequency of intake Buffer capacity Bacterial composition
Retentiveness Other factors,
e.g., urea,
ammonia
Acid production
Modifying effects
of other ingredients
of foodstuffs
Acid tolerance
Acid retention
DENTAL DISEASE/Etiology of Dental Caries 1747
acid tolerance of plaque bacteria. In evaluating data
shown in Table 2 it should be noted that the final pH
represents the pH observed after incubation of pure
bacterial cultures in broth with excess carbohydrates.
Table 2 also indicates the tolerance of bacterial systems
of plaque to acid. Though these data do not reveal the
rate at which acid is produced or the pH at which
growth ceases, they give some insight into the relative
acidogenic, i.e. cariogenic, potential of plaque bacteria.
Bacterial Specificity and Dental Caries
0014 Investigations into the relationship between various
plaque organisms and dental caries have mainly
focused on streptococci, lactobacilli, and filamentous
bacteria.
Streptococci
0015 Studies of S. mutans strongly suggest its active in-
volvement in the initiation and progression of dental
caries. Members of this species are nonmotile, cata-
lase-negative, Gram-positive cocci. On Mitis salivar-
ius agar they grow as highly convex colonies. When
cultured with sucrose they form polysaccharides
which are insoluble. This property of forming insol-
uble polysaccharides from sucrose is regarded as an
important characteristic contributing to the caries-
inducing properties of the species. S. mutans can
invariably be isolated from incipient lesions as well
as lesions that have cavitated on pits and fissures,
approximal and smooth surfaces. It can also be isol-
ated with highest frequency from plaque over carious
lesions, and the isolation frequency decreases when
the samples are taken further away from the lesions.
0016 S. sanguis is also one of the predominant groups
of streptococci which colonize the tooth surface.
Evidence suggests that caries from this strain is
significantly less extensive than that from S. mutans
and occurs primarily in pits and fissures, whereas
S. mutans causes smooth surface caries as well.
0017It would therefore appear that S. mutans is the
most cariogenic of the family of streptococci. Its car-
iogenicity is attributed to several important proper-
ties:
1.
0018It synthesizes insoluble polysaccharides from
sucrose.
2.
0019It colonizes all tooth surfaces.
3.
0020It is a homofermentative producer of lactic acid.
4.
0021It is more acidogenic than other streptococci.
Lactobacilli
0022Lactobacilli have also been implicated in the etiology
of dental caries for many decades. Although a few
studies have suggested their involvement in the initi-
ation of caries, they are most often found in large
numbers in carious cavities. This and the fact that
lactobacilli have a relatively low affinity for the
tooth surface suggest that they might have a more
important role to play in the progression rather than
initiation of dental caries.
Filamentous Bacteria
0023Filamentous bacteria, especially actinomycetes, have
been found to be associated with root surface caries.
Actinomyces viscosus, an acidogenic bacterium, is
almost always isolated from plaques overlying root
lesions. The role of A. viscosus in the initiation of root
lesions is difficult to assess because they are often
predominant on the sound root surfaces in subjects
experiencing and resisting root caries. More definitive
studies are needed to determine the association of
A. viscosus and root caries.
0024It must be remembered that most of the micro-
organisms implicated in dental caries etiology are
indigenous organisms and exist in a dynamic relation-
ship with the host. Disease is not a necessary outcome
of this association but it may ensue when the balance
is greatly disturbed. With S. mutans this may occur,
for example, when its numbers on teeth are increased
owing to a higher or more frequent consumption of
carbohydrates.
The Host Factors
Saliva
0025Saliva significantly influences the carious process.
For example, removal of the salivary glands of
hamsters greatly enhanced the caries activity when
they were fed a high-sucrose diet. Human studies
have demonstrated the same results and have shown
that if the exposure of teeth to saliva is restricted by
blocking the opening of the salivary glands the pH
values of plaque, after exposure to carbohydrates, are
lower.
tbl0002 Table 2 Acidogenicity and acid tolerance of some plaque
bacteria
pH Initiationof growth
< 4.0 4.1^4.5 4.6^5.0 > 5.0
Streptococcus
mutans
þþ 5.2
S. sanguis þþ 5.2–5.6
S. salivarius þþ 5.2
S. mitis þþ 5.2–5.6
Lactobacillus þþ <5.0
Actinomyces þþ?
Enterococci þþþ?
Adapted from Van Houte J (1980) Bacterial specificity in the etiology of
dental caries. International Dental Journal 30: 305–325.
1748 DENTAL DISEASE/Etiology of Dental Caries

0026 Salivary flow and caries rate Humans suffering
from xerostomia or decreased secretion of saliva as
a consequence of a pathological condition such as
sarcoidosis, Sjo
¨
gren’s syndrome, etc., often experi-
ence a higher rate of caries. The tooth destruction as
a result of desalivation is typically quite rapid. Ram-
pant caries has been demonstrated in patients who
have been treated by radiotherapy to the head and
neck as irradiation of the salivary glands leads to
decreased salivary flow and usually leads to extensive
caries in the cervical region of the teeth. These obser-
vations have led to the conclusion that saliva is in
some way important to maintain the integrity of the
tooth substance.
0027 Salivary buffering and dental caries Saliva contains
bicarbonate-carbonic acid and phosphate buffer
systems. The buffering capacity acquired by saliva
by virtue of these ion systems tends to correct pH
changes caused by concentration changes of acidic
ions produced by fermentation of carbohydrates. A
typical reaction of plaque to carbohydrate challenge
is an immediate drop in plaque pH followed by a
gradual rise to the resting value. However, if plaque
and teeth are isolated from the influence of saliva the
pH of plaque drops further and remains low for
prolonged periods. Thus the buffering capacity of
saliva is an important factor in reducing the time the
tooth enamel is exposed to the acidogenic challenge.
0028 Other protective factors in saliva Saliva contains a
number of factors of glandular origin, including
lysozymes and a peroxidase system. Interest has also
been focused on the immunological aspect of caries,
and specific immunoglobulin A (IgA) antibodies to
S. mutans have been detected in saliva by immune
assays. The concentration of secretory IgA in whole
saliva has been reported to be significantly less in
subjects with a high caries rate as compared with
those with a lower caries experience. Although sub-
stantial evidence suggests that the aforementioned
factors form an antibacterial system in saliva, the
extent to which they contribute to caries resistance
of the host is not yet clear.
Tooth
0029 Tooth morphology and dental caries A susceptible
host is one of the factors which is required for caries
to occur. The morphology of the tooth has long been
known to be one of the major determinants of its
susceptibility. Clinical observations have suggested
that pits and fissures of posterior teeth are highly
susceptible to caries. This is thought to result from
impaction of food and microorganisms in the fissures
which are also difficult to reach with routine oral
hygiene aids. An interesting observation is that there
is a positive correlation between the depth of the
fissures and caries susceptibility.
0030It has also been observed that certain surfaces of a
tooth are more prone to caries than other surfaces. For
example, the occlusal surface and the buccal surface of
the lower first permanent molar are more likely to
develop caries as compared with the lingual, mesial,
or distal surfaces, probably because of the presence of
the fissures on the occlusal surfaces and the buccal pit
on the buccal surface. Similarly, an intraoral variation
in susceptibility to caries exists between different
teeth. The caries susceptibility by tooth type, in des-
cending order, is as follows: first permanent molar,
second permanent molar, second premolar, upper in-
cisor, first premolar, with the lower incisors and
canines least likely to develop caries.
0031Arch morphology and caries Irregularities in the
arch form and imbrication of the teeth also favor
the development of caries. It has been shown experi-
mentally that enamel in stagnation areas is more
likely to develop demineralization, which is mani-
fested as white spot lesions.
0032Tooth composition and dental caries It is well
known that the surface enamel is more resistant to
caries attack than the subsurface. This is attributed
to a higher concentration of fluoride and other
trace metals, such as zinc and lead, which are thought
to protect the surface enamel from demineraliza-
tion. It is because of this that dental caries is often
said to be subsurface in origin. Although there is
plenty of evidence to support the direct relationship
between the fluoride content of the surface layer of
enamel and its resistance to caries attack, the relation-
ship of other trace elements is unclear and still being
investigated.
See also: Carbohydrates: Digestion, Absorption, and
Metabolism; Requirements and Dietary Importance;
Immunology of Food
Further Reading
Newbrun E (1979) Cariology, 2nd edn. Baltimore: Williams
and Wilkins.
Silverstone LM, Johnson NW, Hardie JM and Williams
RAD (1981) Dental Caries – Aetiology, Pathology and
Prevention. London: Macmillan.
Thylstrup A and Fejerskov O (1986) Textbook of Cariol-
ogy, 1st edn. Copenhagen: Munksgaard.
Van Houte J (1980) Bacterial specificity in the etiology of
dental caries. International Dental Journal 30: 305–325.
DENTAL DISEASE/Etiology of Dental Caries 1749
Role of Diet
M S Duggal and M E J Curson, University of Leeds,
Leeds, UK
This article is reproduced from Encyclopaedia of Food Science,
Food Technology and Nutrition, Copyright 1993, Academic Press.
Introduction
0001 The driving force for the development of preventive
dentistry and its effective use in patient management
has been the expanding understanding of the disease
of dental caries itself. As already discussed, dental
caries is now recognized as a disease of altered ecology
in which the host, oral microflora, and diet interact to
present a challenge too strong for the normal defense
mechanisms. It is wrong, however, to regard caries as a
simple, continuing acid demineralization of the tooth
enamel. Although teeth may be frequently exposed to
acid environment, caries does not always arise as it is a
result of a dynamic interaction of demineralization
and remineralization. These are under the influence
of a whole range of both cariogenic and protective
factors (Figure 1). It is clear from this model that diet
forms a part of a large spectrum of etiological factors
involved in the caries process. Sugar and other fer-
mentable carbohydrates in the diet constitute an es-
sential aspect of cariogenic potential of food, with
other factors, such as the frequency of intake, reten-
tiveness, and buffering potential of foods, also playing
an important part. These are in addition to the tooth
structure, saliva flow and composition, and the pres-
ence of oral bacteria. This article is concerned with the
discussion of the dietary considerations related to the
etiology of dental caries.
The Role of Diet in the Etiology of Dental
Caries
0002 Epidemiological studies have been used to try to
establish the relationship of various types of diets
and dietary components to caries incidence. Circum-
stantial evidence linking sucrose consumption and
prevalence of dental caries can be readily found in
several epidemiological surveys. For example, the
prevalence of caries among the native population of
Tristan da Cunha, and among Eskimos and Austra-
lian Aborigines, was low before western-type food
was introduced into their diets. Controlled human
studies, mostly on institutionalized subjects, also in-
dicated that sucrose-containing diets were cariogenic.
0003 It is well known that when eating foods containing
fermentable carbohydrates the pH of plaque drops,
implying localized generation of acid. The drop in pH
is the result of fermentation carbohydrates by the
plaque bacteria, producing lactic and other organic
acids, and is followed by a gradual return to the
resting or near-resting pH. This recovery depends on
a number of factors, including the buffering potential
of plaque and the food, retention, or the rapidity of
clearance from the mouth. The whole cycle of pH
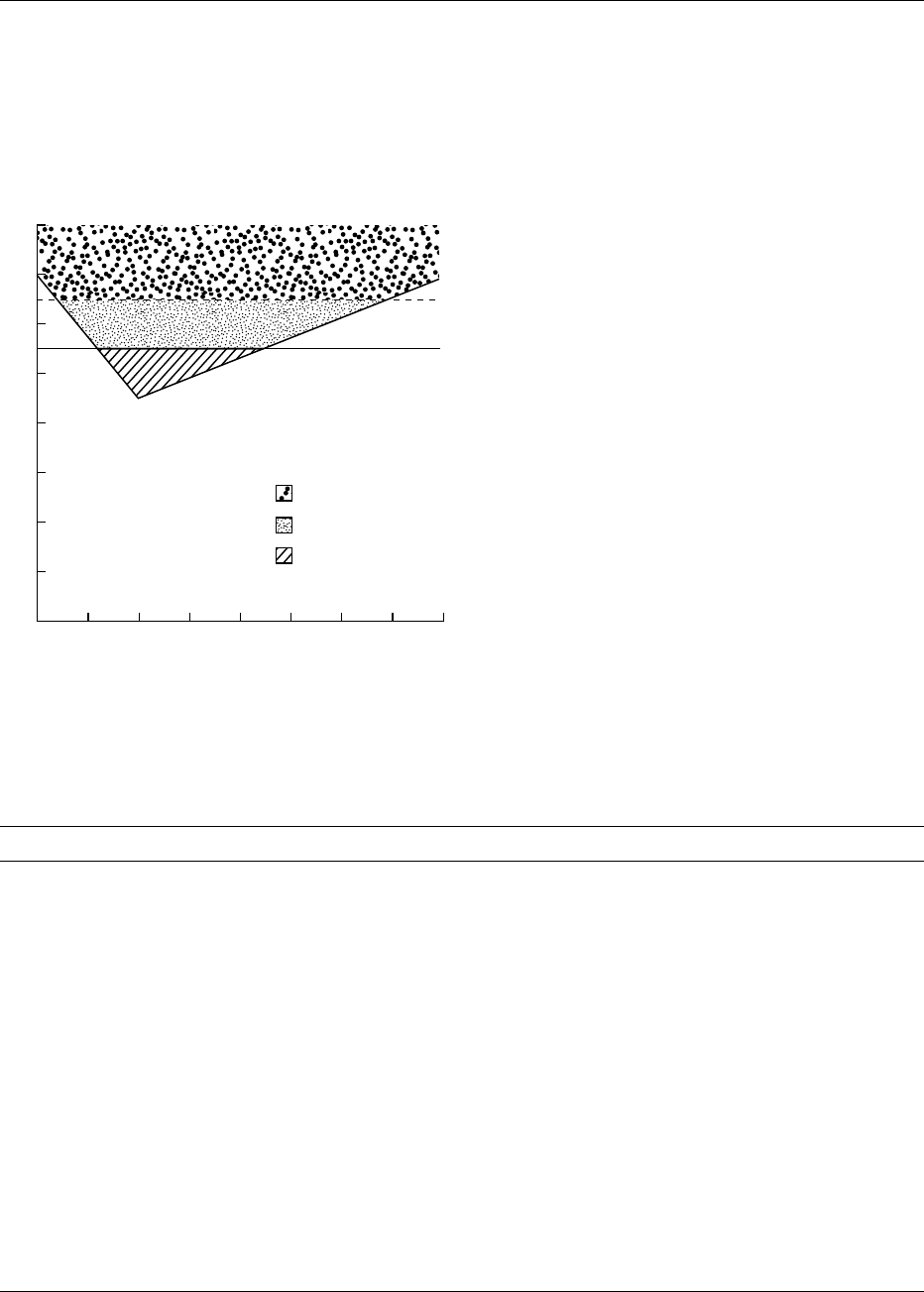
drop and recovery was first described by Stephan in
1940 and is commonly known as the Stephan curve
(Figure 2). (See Carbohydrates: Digestion, Absorp-
tion, and Metabolism; Requirements and Dietary
Importance.)
0004It is agreed that foods which produce a pH drop
below pH 5.5, known as critical pH, are considered
detrimental to teeth. The pH value 5.5 is considered
critical because it is thought that enamel demineraliza-
tion occurs below this point. Some authors regard
values below 5.7 as being cariogenic. Foods giving a
pH drop between 5.5 and 6.0 are also dubious. This
form of ranking is referred to as relative potential car-
iogenicity. Of greater importance is the relative cario-
genic potential of foods compared with known foods of
high cariogenicity (sucrose) and low cariogenicity
(sorbitol), as agreed at the international conference on
cariogenicity of foods at San Antonio in 1985.
0005In one study, using the plaque-harvesting method, a
representative sample of plaque was removed from
human mouths before and after consumption of
foods, and the pH response of that plaque was
Total cariogenic load
Frequency
Cariostatic factors
Buffering
Sound
enamel
Dental
caries
Demineralization
Remineralization
Carbohydrates
Retention/clearance
Total protective factors
Fluorides
Fissure sealants
Oral hygiene
Salivary components
Host susceptibility
fig0001Figure 1 A dynamic model of caries. Reproduced from Dental
Disease/Role of Diet, Encyclopaedia of Food Science, Food Tech-
nology and Nutrition, Macrae R, Robinson RK and Sadler MJ
(eds), 1993, Academic Press.
1750 DENTAL DISEASE/Role of Diet
assessed. The response followed a typical Stephan
curve and the potential acidogenicity of the foods
was compared by measuring the minimum pH
recorded and the area enclosed by the curve under
the resting pH value. Acidogenicity in a range of
snack foods is assessed in Table 1. Interestingly,
some foods which are perceived to be ‘better for
teeth’ actually fare quite badly in such a ranking,
compared with foods traditionally thought of as
‘bad for teeth,’ such as chocolate.
0006This study bore out a previous study demonstrating
that ingestion of chocolate or even apples resulted in a
similar pH response. Later work, using the same test
procedure, also showed that a wide range of foods
containing either sugar or starches, or combinations
thereof, are potentially acidogenic and thus possibly
cariogenic.
0007It is important to note that the concentration of
fermentable carbohydrate in a food does not affect
the pH drop in the mouth, although the period of
time taken to return to normal pH levels may be related
to concentration. This return to resting pH is as much
related to the buffering capacity of the saliva or plaque,
buffering capacity of the foods, and the physical prop-
erties of the food itself. The retentiveness, and hence
clearance rate, of a food is therefore an important
determinant of the cariogenic potential of that food.
The concentration of a single ingredient of a food has
little relation to the cariogenicity; it is the ability of the
whole food to promote caries that is important.
Tests for Cariogenicity of Foods
0008Animal experiments have been used to rank different
foods in the order of their cariogenicity. In one such
study a selection of human snack foods was ranked
by their cariogenic potential index (CPI) by feeding
5
0
1
2
3
4
5
6
7
8
10 15 20
Time
Critical pH
Safe
pH
Doubtful
Demineralization
25 30 35 40
fig0002 Figure 2 A typical Stephan’s curve. Reproduced from Dental
Disease/Role of Diet, Encyclopaedia of Food Science, Food Tech-
nology and Nutrition, Macrae R, Robinson RK and Sadler MJ
(eds), 1993, Academic Press.
tbl0001 Table 1 Acidogenic potential of carious snack foods (US) grouped by category and acidogenicity
Group Beverages Fruit, etc. Baked goods Sweets
Least acidogenic Milk Peanuts
Chocolate milk Potato chips (crisps) Bread and butter
Apple Graham crackers Caramels
Sugared gum
Chocolate
Licorice
Sugarless gum
Carbonated beverages Banana Cream-filled cakes
Sandwich cookies Orange jellies
Apple juice Dates Doughnut
Raisins Bread and jam
Orange juice Sweetened cereal Whole-wheat bread
Plain sweet biscuits/cakes
Apple pie Rock candy
Chocolate Clear mints
Graham
Angel food cake
Most acidogenic Sourballs
Fruit gums
Fruit
Lollipops
After Edgar WM (1981) Plaque pH assessments related to food cariogenicity. In: Hefferen JJ (ed.) Foods, Nutrition and Dental Health, vol. 1, pp. 137–150.
Illinois: Pathotox.
DENTAL DISEASE/Role of Diet 1751

laboratory rats via a gastric tube, thus bypassing the
mouth. Sucrose was used as a reference food and
given a CPI of 1.0. Foods with a score of less than
1.0 were considered less cariogenic than sucrose,
while those with a score above 1.0 were thought to
be more cariogenic. Interestingly, the concentration
of sucrose in a breakfast cereal made little or no
difference to the CPI. The results, given in Table 2,
show that potato chips (crisps) actually score higher
than chocolate bars. It is clear from most of these
experiments that any foodstuff containing carbohy-
drate has the potential to cause significant amounts of
acid to be produced at certain sites in the dentition,
which can be followed by demineralization of the
enamel and subsequent caries. However, it must
be remembered that not all occasions of a drop in
plaque pH are accompanied by demineralization of
the enamel. This has prompted many investigators
to question the significance of acid production as a
measure of the cariogenicity of the foods. It has also
been shown that the total amount of titratable acid
produced by the foods does not necessarily parallel
the amount of enamel it will dissolve. It is now well
accepted that the cariogenic potential of a food is
influenced by a number of other factors, including
the ability of the foods to remain in the oral cavity
and, in some cases, the sequence of food intake. Thus,
studies on the relationship between food and dental
caries should consider not only foods in themselves
but also their relationship to other items of diet with
regard to their nature, timing, and order of usage.
Cariostatic Factors in Food
0009 Some components of foods may be cariostatic. Pro-
teins may assist remineralization of enamel or reduce
the rate of crystal dissolution. Some fatty acids have
been shown to reduce caries in rat studies while phos-
phates have been shown to have a marked protective
effect. Inorganic phosphates have been demonstrated
to have a protective influence when added to a
cariogenic diet. Organic phosphates such as phytates
and glycerophosphates also have a cariostatic action
and are thought to reduce the cariogenicity of diets.
Although the exact mechanism of action is unknown,
studies have indicated that it might be a local modify-
ing influence in the oral cavity, rather than a systemic
effect through ingestion. The local effects of phos-
phates can be attributed to various properties:
1.
0010Phosphates are good buffers; thus they can buffer
organic acids produced by plaque flora.
2.
0011Phosphates are known to reduce the rate of dissol-
ution of hydroxyapatite.
3.
0012Phosphates can desorb proteins from the enamel
surface; thus they can possibly have a modifying
influence on acquired pellicle.
The protection afforded by fluoride is well docu-
mented and has led some researchers to refer to dental
caries as a fluoride-deficiency disease. These mater-
ials are all components of various foods. Further-
more, some cariostatic agents have been isolated
from cereals and cocoa and these factors may all
influence the level of caries caused. Accordingly the
level of fermentable carbohydrate in a food will not
be directly related to the degree of caries caused.
Food Retention
0013Tests have illustrated that, contrary to popular opin-
ion, foods that are perceived to be ‘sticky,’ such as
caramel, tend to clear from the oral cavity faster than
many other foods considered cariogenic. As Table 3
shows, after 15 min, white bread was retained in
higher quantities in the oral cavity than cake,
chocolate, or hard mint. After 30 min, there was
more residue from raisins than from caramel. Raisins
have been consistently shown to be highly cariogenic.
0014Beverages, which are perceived to clear quickly
from the mouth, actually sustain a low pH level for
the same period as a ‘sticky’ confectionery. In a recent
study a range of fruit drinks which were advertised as
‘sugar-free’ or as ‘no added sugar’ were assessed on
the basis of their ability to reduce the pH of plaque. It
was found that the fact that most of these drinks had
little natural sugar (sucrose) did not affect their ability
to reduce the pH of plaque; when compared with a
standard 10% sucrose rinse they were equally acido-
genic. This was attributed to the presence of natural
sugars – fructose and glucose – in these drinks. These
so-called natural sugars are also fermentable by oral
tbl0002 Table 2 Selected rankings of cariogenic potential index (CPI)
in rats using human food as snacks
Food tested CPI
Sucrose 1.0
Filled chocolate cookie 1.4
Cereal (14% sucrose) 1.1
Cereal (8% sucrose) 1.0
Cereal (60% sucrose) 0.9
Coated chocolate candy 0.9
Potato chips 0.8
Caramel 0.7
Chocolate bar 0.7
Cereal (2% sucrose) 0.5
Starch 0.5
Sucrose plus 5% Dical 0.4
No meals by mouth 0.0
After Bowen WH, Amsbaugh SM, Monnel-Torrens S et al. (1980) A method
to assess cariogenic potential of foodstuffs. Journal of the American Dental
Association 100: 677–681.
1752 DENTAL DISEASE/Role of Diet
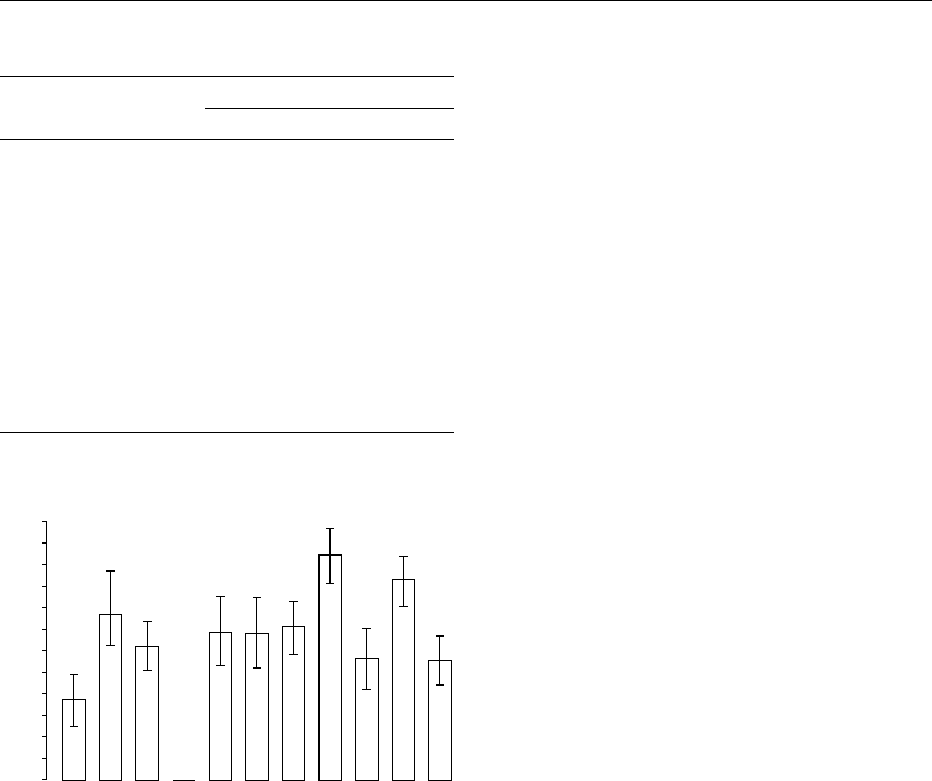
bacteria and their presence instead of sucrose does
not render the drinks any safer for teeth. In fact,
when the fruit drinks were compared with 10%
sucrose it was obvious that the pH recovery was
slower after consumption of fruit drinks and the pH
remained below 5.5 for a longer time (Figure 3). This
is because of an inherent buffering potential of fruit
drinks which gives them an ability to resist any
attempts by saliva to buffer the acid. These drinks
can therefore be deemed to be more cariogenic
than a pure 10% sucrose solution. This research
highlighted the fact that the concentration of sugars
alone is not the sole determinant of the acidogenicity,
and hence cariogenicity, and other factors discussed
above are equally important. (See Carbohydrates:
Metabolism of Sugars.)
Eating Pattern and Frequency
0015On a population level, the average amount of sucrose
consumed per capita relates to the average level of
caries in the population. However, more detailed
studies show the relationship to be less consistent
and of low statistical significance. In the classical
study often referred to as the Vipeholm study, inmates
of a Swedish Medical Institute were fed increased
sucrose or other foods in different patterns and caries
experience was monitored. Groups of patients receiv-
ing high levels of sucrose (up to 330 g day
1
) with
their meals experienced minimal increase in caries.
But if smaller quantities of sucrose were consumed
between meals, very high levels of caries ensued. The
relationship was not therefore between quantity of
sucrose and caries but rather frequency of intake
and caries experience. This relationship, which has
been confirmed in human and animal research, sheds
light on why population studies do not demonstrate
a clear and consistent relationship between sugar
consumption and caries. For example, long-term
diet–caries studies carried out in the UK showed
that statistical correlation of sugar concentration to
caries was 0.16 and explained only 4% of the caries
variance.
0016Experience from primitive and developing cultures
with little access to sucrose but abundant access to
starch is often cited as evidence that sucrose and not
starch results in dental caries. This evidence purports
to be strengthened by the fact that introduction of
western diet (including sucrose) immediately results
in development of dental caries. It can be argued that
introduction of a western diet is accompanied by
increased affluence and an altered eating pattern.
Such changes also include differences in the use
of cooked starches as much as differences in use of
sucrose. Frequency of intake of any food increases
dramatically, along with the potential incidence of
dental caries. It is also interesting to note that caries
has been shown to be associated with a diet consisting
of sago starch, as in the Sepik villages in Papua New
Guinea.
Sugars and their Role in Caries
0017In western society, eating frequency has generally
increased and snacking has become an accepted
aspect of life. This change took place over a long
period of the nineteenth and early twentieth centuries
12
11
10
9
8
7
6
5
4
3
2
1
0
Apple/chR
Apple/chD
Apple/bcCG
Apple orange D
Ribena app/bcB
Ribena orange B
Sucrose
Time (min)
Gripe water W
Summer fruits CG
Pear/peach CG
Apple/bcD
fig0003 Figure 3 Bar graph showing mean time spent below pH 5.5 for
fruit drinks compared with a 10% sucrose rinse. Reproduced
from Dental Disease/Role of Diet, Encyclopaedia of Food Science,
Food Technology and Nutrition, Macrae R, Robinson RK and Sadler
MJ (eds), 1993, Academic Press.
tbl0003 Table 3 Representative figures (mg) for food retention in
mouth after eating
Food Food retention (mg) after
5 min 15 min 30 min
Peanuts 4.9 3.3 2.6
Dentyne gum 5.0 3.9 3.1
7-Up 6.3 2.4 2.1
Chocolate milk 7.4 3.8 1.9
Potato chip 12.3 4.9 2.5
White bread 16.1 10.0 3.6
Raisins 16.8 5.7 3.0
Sponge cake 18.8 6.0 4.2
Caramel 19.0 4.2 2.5
Milk chocolate 19.0 6.8 3.0
Cracker (oil-sprayed) 23.8 8.5 3.7
Hard mint 31.9 9.4 2.5
Cracker (plain) 33.6 10.4 3.3
Sandwich cookie 35.0 8.4 4.9
After Bibby BG (1981) Foods and dental caries. In: Hefferren J (ed.) Foods,
Nutrition and Dental Health, vol. 1, pp. 257–278. Illinois: Pathotox.
DENTAL DISEASE/Role of Diet 1753

when the incidence of dental caries increased. How-
ever, in the past 20 years, while sucrose usage has not
changed, dental caries has dramatically decreased. In
the case of 5-year-olds, the percentage with tooth
decay fell from 73% in 1973 to 48% in 1983.
0018 Reducing the frequency of eating just one of these
foods, or reducing the concentration of sugars in a
food, is unlikely to have a significant effect on the
incidence of caries as nearly all foods contain some
fermentable carbohydrate. It has been assumed tacitly
both by practicing dentists and by too many dental
investigators that the cariogenicity of an individual
food is directly proportional to its content of sucrose
or other fermentable carbohydrates. There are no
quantitative data to support this belief. The effects
of high sucrose concentrations in increasing the rate
of food clearance of some foods from the mouth and
in inhibiting the fermentation process make it seem
improbable that high sugar content of itself would be
particularly damaging to teeth. The Vipeholm study
has been mentioned as evidence for the cariogenicity
of sucrose, although investigators have questioned
the reliability of a single clinical study from a mental
institute. There are a number of contradictory studies
that have not been widely recognized. For example,
one group of investigators, studying English children,
found that they could substantially increase sugar as
sucrose in the children’s diet without increasing the
incidence of caries.
0019 It is the frequency of consumption rather than the
amount consumed which is associated positively with
dental caries incidence. Considering the already
substantial decline in the incidence of dental caries
in the west, where frequency of eating has generally
increased, and given the fact that dietary manipula-
tion is difficult to achieve, it is reasonable to assume
that alternative preventive measures such as the use of
fluoride would be unfortunate if public hopes were
raised to believe that dietary control alone would
solve the problem of dental caries.
See also: Carbohydrates: Metabolism of Sugars;
Digestion, Absorption, and Metabolism; Requirements
and Dietary Importance
Further Reading
Bibby BG (1981) Foods and dental caries. In: Hefferren JJ
(ed.) Foods, Nutrition and Dental Health, vol. 1,
pp. 257–278. Illinois: Pathotox.
Bowen WH, Amsbaugh SM, Monnel-Torrens S et al. (1980)
A method to assess cariogenic potential of foodstuffs. Jour-
nal of the American Dental Association 100: 677–681.
Duggal MS and Curzon MEJ (1989) An evaluation of the
cariogenic potential of baby and infant fruit drinks.
British Dental Journal 160: 327–330.
Edgar WM (1981) Plaque pH assessments related to food
cariogenicity. In: Hefferren JJ (ed.) Foods, Nutrition and
Dental Health, vol. 1, pp. 137–150. Illinois: Pathotox.
Edgar WM, Bibby BG, Mundorff S and Rowley J (1975)
Acid production in plaques after eating snacks: modify-
ing factors in foods. Journal of the American Dental
Association 90: 418–425.
Gustafsson BE, Quensel CE, Lanke LS et al. (1954) The
effect of different levels of carbohydrate intake on caries
activity in 436 individuals observed for five years. The
Vipeholm Dental Caries Study. Acta Odontologica
Scandinavica 11: 232–364.
King JD, Mellanby M, Stones HH and Green HN (1955)
The effect of sugar supplement on dental caries in chil-
dren. MRC Special Report Series, no. 288. London: Her
Majesty’s Stationery Office.
Navia JM et al. (1983) Nutrition in oral health and disease.
In: Stallard RE (ed.) A Textbook of Preventive Dentistry,
pp. 90–146. Philadelphia: WB Saunders.
Rugg-Gunn AJ, EdgarWM and Jenkins GN (1978) The effect
of eating some British snacks upon the pH of human
dental plaque. British Dental Journal 145: 95–100.
Stephan RM (1940) Changes in hydrogen ion concentration
on tooth surfaces and in carious lesions. Journal of the
American Dental Association 27: 718–723.
Fluoride in the Prevention of
Dental Decay
H Whelton, University College Cork, Cork, Ireland
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Introduction
0001Dental decay or caries is a ubiquitous disease in es-
tablished market economies and in many developing
countries. Dental caries poses a threat to the dentition
throughout life. The early caries lesion is known as a
‘white spot.’ This white spot can do one of three
things. It can stay the same, progress to a cavity, or
regress to sound enamel. The fate of the white spot is
influenced by its exposure to fluoride. Fluoride can
help to prevent progression of the lesion and can aid
remineralization, in which case the early white-spot
lesion can disappear clinically.
0002Fluoride is widely used in the prevention of dental
caries. It is estimated that over one billion people use
fluoridated toothpaste worldwide. The second most
common vehicle for fluoride is water. Water fluorid-
ation is the controlled addition of fluoride to a public
water supply for the purpose of preventing dental
caries. In many parts of the world, the level of fluoride
in water supplies is adjusted to 1 part per million
(ppm). It is estimated that about 317 million people
1754 DENTAL DISEASE/Fluoride in the Prevention of Dental Decay

in 39 countries benefit from artifically fluoridated
water. A further 40 million benefit from water supplies
which are naturally fluoridated. The fluoridation of
toothpastes and public water supplies has been ac-
knowledged as a major public health success in the
prevention of dental caries. The addition of fluoride to
water supplies and to oral health products has helped
to bring about a remarkable decline in dental caries
levels since the 1960s. In this paper the occurrence of
fluoride in nature, the history of the discovery of its
effects on the dentition, its use in the prevention of
dental caries, and the risks associated with ingestion of
excess fluoride will be considered.
Fluoride in Nature
0003 Fluorine is a naturally occurring element, apparently
ubiquitous in nature. Number nine on the periodic
table of the elements, it is a halogen gas. It ranks 13th
among the elements in order of abundance in the
earth’s crust. Fluorine is the most electronegative
and reactive of all elements. It is a pale yellow, corro-
sive gas, which reacts with practically all organic and
inorganic substances. The element was isolated in
1886 by Ferdinand Frederic Henri Moisson who
used an apparatus constructed from platinum. He
won the Nobel Prize for chemistry in 1906. Fluorine
occurs chiefly in fluorspar (or fluorite, calcium
difluoride, CaF
2
), cryolite (Na
2
AlF
6
), and in many
other minerals. Ionic fluoride, also referred to as in-
organic or free fluoride, is the biologically important
form of the mineral.
0004 Fluoride is found in fresh water, sea water, and earth
and in foods. Fluoride is present in both groundwater
and surface water. The level of fluoride in groundwater
varies from less than 0.1 mg l
1
to more than 25 mg l
1
according to the geological, chemical, and physical
characteristics of the water-supplying area. The fluor-
ide concentration in fresh surface water is generally
low, ranging from 0.01 to 0.03 mg l
1
. In sea water,
fluoride is found at approximately 1.5 ppm. Marine
plants and animals are therefore constantly exposed to
large amounts of fluoride. Fluoride in soils is derived
primarily from the geologic parent material. Samples of
nonindustrially contaminated soils from Germany,
Greece, Finland, Japan, Morocco, New Zealand,
Sweden, the USA and former USSR have been reported
as containing from 30 to 500 ppm fluoride. Soils near
fluorite and other types of mineralization may show
levels up to more than 5000 ppm.
0005 The normal accumulation of soil fluoride in plants
is small. However, a few species of plants are known
to accumulate high levels of fluoride, for example tea.
The concentration of fluoride in dried tea leaves
varies widely (approximately 4–400 ppm) while
those of brewed tea range from 1 to 6 ppm depending
on the amount of dry tea used, the fluoride concen-
tration of the water, and the brewing time. High levels
of fluoride are also found in fish: early reported con-
centrations range from 0.6 to 2.7 ppm. These samples
may have contained bones. With its high affinity for
calcium, most absorbed fluoride is deposited in bones
therefore fish bones are a source of concentrated
fluoride levels. More recent studies which excluded
bone fragments from fish reported a range of fluoride
values from 0.05 to 0.17 ppm. Other dietary constitu-
ents which contain fluoride are fluoridated water and
infant formulas and beverages reconstituted with
fluoridated water. Most other foods have fluoride
concentrations well below 0.05 ppm.
History of Water Fluoridation
0006In 1901 a letter in the US Public Health Report from
JM Eager of the US Public Health Service, stationed in
Naples, Italy, reported the occurrence of ‘a dental
pecularity’ known locally as denti di chiae. Denti di
chiae were called after Prof Stefano Chiae, a cele-
brated Neapolitan who first described the appearance
of a condition which was later to become known as
dental fluorosis.
0007A similar condition, known as Colarado brown stain,
was observed in Colorado Springs, USA, in 1901 by a
dentist, Fredrick McKay. The condition manifested itself
as a stain of varying intensity from fine white patches to
a disfiguring brown mottling. Some 87% of the popula-
tion in the area were affected. In 1916, McKay, with the
help of GV Black, a prominent figure in dentistry at the
time, published a thorough description of the condition,
describing its appearance as mottled enamel. By the
1920s McKay had suspected drinking water as the
source of the problem. In Oakley, Idaho, where mottling
was severe, McKay noticed that children living on the
outskirts of town who drank from a private spring had
no mottling. He advised the town to change to this
supply, which they did in 1925. Children born in the
town subsequently had no mottling.
0008In 1931 new methods of spectrographic analysis
led to the discovery of fluoride in the water supplies
of areas where mottled enamel was endemic. The
condition then became known as dental fluorosis.
0009In 1928 McKay published the observation that, in
areas where mottled enamel was found, the preva-
lence of dental caries appeared lower than would be
expected. In 1933 Ainsworth in England showed
that dental mottling in Maldon in Essex was associ-
ated with a high level of fluoride in its water (4.5–5.5
ppm). He also noted that the prevalence of caries in
permanent teeth amongst the children in the area was
lower than that for England and Wales as a whole.
DENTAL DISEASE/Fluoride in the Prevention of Dental Decay 1755
