Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.


(1) antioxidant nutrient components; (2) absorption
and bioavailability; (3) food processing and storage;
(4) food additives and nutritional supplements;
(5) chiral form and other chemical characteristics of
nutrients. A dietary antioxidant is a substance that
significantly decreases the adverse effects of reactive
oxygen species, reactive nitrogen species, or both on
normal physiological functions in humans. The diet
contains antioxidants recognized as essential nutri-
ents or nonnutrients, and such nutrient antioxidants
include: vitamin E (tocopherols and tocotrienols),
vitamin A, ascorbic acid, and nutrients essential for
the normal function of endogenous enzymatic anti-
oxidant systems, such as Cu, Mn, Zn (cofactors of
superoxide dismutase), Se (cofactor of glutathione
peroxidase) and Fe (cofactor of catalase and peroxid-
ases). The diet also contains nonnutrient antioxi-
dants, including a large number of phytochemicals.
Dietary Antioxidants
Mechanisms of Antioxidant Action and Relation
with CHD Pathophysiology
0004 Vitamin E (tocopherols and tocotrienols) Vitamin E
is the generic term used to describe at least eight
naturally occurring compounds that exhibit the bio-
logical activity of a-tocopherol. It includes a-, b-, g-,
and d-tocopherol and a-, b-, g-, and d-tocotrienol.
The naturally occurring RRR stereoisomer and the
synthetic all-rac-a-tocopherol are equally well absorb-
ed, yet levels of a-tocopherol in the blood and tissues
increase significantly more than all-rac. In the liver,
the a-tocopherol transfer protein preferentially
selects RRR-a-tocopherol over g- and other toco-
pherols for incorporating into very-low-density lipo-
protein (VLDL), which then enters into systemic
circulation. Tocotrienols appear in the blood and
tissues at significantly lower levels than tocopherols,
even when ingested at equivalent or higher amounts.
0005 The antioxidant activity of the tocopherols and
tocotrienols is mainly due to their ability to donate
their phenolic hydrogens to lipid free radicals.
a-Tocopherol acts as a chain-breaking antioxidant
by donating its phenolic hydrogen to the chain-
propagating lipid peroxyl radical (LOO
) in mem-
branes and lipoproteins and replacing the latter with
the less reactive a-tocopheroxyl radical (a-TO
). Al-
ternatively, a-tocopherol may react directly with the
initiating radical to prevent LOO
formation. LOO
may also be eliminated via a radical–radical reaction
with a-TO
. Ascorbate, ubiquinone 10 and other co-
antioxidants, donates electrons to the a-TO
reducing
it back to a-tocopherol. Both, a- and g-tocopherol,
the principal forms of vitamin E in the diets of
Europe and the USA, respectively, protect against
peroxynitrite-induced lipid peroxidation. This pro-
cess is inhibited more effectively by g-tocopherol
than a-tocopherol. These results suggest that
g-tocopherol can act as a trap for membrane-soluble
electrophilic nitrogen oxides, forming stable carbon-
centered adducts through its nucleophilic 5-position,
which is blocked in a-tocopherol. Besides the reac-
tions with free radicals, tocopherols also react with
singlet oxygen either by physical quenching or by
chemical reactions. In physical quenching, excited
state singlet oxygen (O
2
1
D
g
) is deactivated to
ground-state triplet oxygen (O
2
3
S
g
) through a
charge-transfer mechanism. The tocopherols also
react chemically with singlet oxygen and become des-
troyed after the reaction.
0006LDL particles may contain approximately
12 mol of vitamin E per mol of LDL. The oxidative
resistance of LDL is increased in vitamin E-supple-
mented individuals, especially at intakes 269–
336 mg of a-tocopherol equivalents, and there are
strong correlations between oxidative resistance and
LDL-a-tocopherol concentrations in these subjects.
In vitamin E-supplemented subjects, the rate of oxi-
dation was significantly decreased at 269 and 537 mg
of a-tocopherol equivalents per day, leading to the
conclusion that vitamin E was the most important
variable that determined oxidative resistance of
LDL. However, this relationship becomes weaker in
unsupplemented subjects. In contrast, some studies
on LDL oxidation have suggested that a-tocopherol
accelerates the peroxidation of LDL lipids under mild
free-radical generation and in the absence of co-
antioxidants, such as ascorbate and coenzyme Q
10
.
0007The bioactivity of
NO is particularly sensitive to
oxidative stress. The superoxide radical combines
readily with
NO in a diffusion-limited reaction to
form peroxynitrite. In atherosclerosis, hypercholes-
terolemia, hypertension, and diabetes, the superoxide
flux in the arterial wall is increased, leading to
reduced
NO bioavailability/activity. a-Tocopherol
localizes mainly in lipoproteins and membranes,
where it serves to scavenge lipid peroxyl radicals.
Despite this action, atherosclerosis is characterized
by lipid peroxidation within the arterial wall, even
in the presence of a-tocopherol. Experimental studies
have shown that arteries deficient in a-tocopherol
showed a dose-dependent impairment of
NO-
mediated arterial relaxation upon exposure to
oxLDL. In contrast, vessels containing a high amount
of a-tocopherol were resistant to this effect of oxLDL.
This effect may be accounted for by inhibition of
protein kinase C-a phosphorylation by a-tocopherol,
which will prevent protein kinase C stimulation by
oxLDL. One hallmark of atherosclerotic plaque is
1656 CORONARY HEART DISEASE/Antioxidant Status

proliferarion of vascular smooth muscle. Consider-
able in-vitro data indicate that a-tocopherol inhibits
the proliferation of smooth muscle cells. This effect
was also associated with the inhibitory effect of a-
tocopherol on protein kinase C. Thrombus formation
within the vessel lumen, an important event in patho-
physiology of cardiovascular disease, is often precipi-
tated by the adhesion and aggregation of platelets to a
ruptured atherosclerotic plaque. It has been reported
that platelet a-tocopherol content is an important
determinant in platelet responsiveness toward protein
kinase C-dependent stimuli leading to inhibition of
platelet aggregation. Moreover, platelet a-tocopherol
status appears to be important in regulating
NO and
superoxide radical production, which could also con-
tribute to inhibit platelet aggregation. Recent data
indicating that patients treated with vitamin E
showed a high risk for hemorrhagic stroke could be
related to its modulation on platelet function.
0008 The antioxidant efficacy of tocotrienols in mem-
branes is higher than that of tocopherols, in spite of
their uptake and biodistribution after oral ingestion
being lower than that of a-tocopherol. Cell-culture
studies indicate that tocotrienols inhibit cholesterol
synthesis by directly regulating the expression of
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(HMGR), through a posttranscriptional process in-
volving accelerated degradation of the reductase
protein. The molecular mechanism for this suppres-
sion by tocotrienols was ascribed to their side-
chain’s unique ability to increase cellular farnesol,
a mevalonate-derived product, which signals the
proteolytic degradation of HMGR. Some studies
in humans have shown that tocotrienol supplements
decreased LDL-cholesterol, apolipoprotein B (apoB),
lipoprotein (a) (Lp(a)), thromboxane B
2
, and platelet
factor 4, suggesting hypolipidemic and antithrombo-
tic effects. However, supplementation studies with a-
tocotrienyl acetate, which is hydrolyzed, absorbed,
and detectable in human plasma, did not lower chol-
esterol in hypercholesterolemic subjects, although it
was potent in decreasing LDL oxidizability. In-vitro
studies with HepG2 cells suggest that a-tocotrienol is
effective at levels of 10 mmol l
1
. Interestingly, g- and
d-tocotrienol, which lack the 5-methyl substituents
present in a-tocotrienol, show a higher HMGR sup-
pression. This structure–activity relationship indicates
that in addition to the requirements of the prenyl side-
chain for HMGR supression, changes in the methyl
substitution on the chromanol ring may also lead to a
divergent effect on HMGR activity. The conflicting
results obtained in human studies might also be related
to differences in the effective concentrations in cells
necessary to inhibit HMGR. Moreover, it has recently
been found that humans do not respond uniformily to
the cholesterol-lowering action of tocotrienols, par-
ticularly when cholesterol and alcohol intakes are
not controlled. Tocotrienols, as well as other HMGR
inhibitors, lower apoB levels partly by upregulating
LDL receptors in the liver. This facilitates the liver
uptake of circulating apoB-LDL. In addition, tocotrie-
nol has been shown to increase the intracellular pro-
teolytic degradation of apoB and alter the assembly
process of VLDL. Thus, it seems that the ability of
tocotrienol to reduce apoB levels in blood plasma
depends on both the clearance rate of LDL and the
production of VLDL. A novel tocotrienol fraction
from specially processed rice bran oil enriched with
didesmethyl-tocotrienol (with no methyl group on the
chromanol ring) has been shown to decrease plasma
Lp(a). Additionally, long-term prevention studies in
humans are needed to validate this effect, which may
have important implications in the prevention of ath-
erosclerosis and thromboembolism.
0009Carotenoids Carotenoids are pigments found only
in plants and microorganisms. Most carotenoids in
the diet are provided by deeply pigmented vegetables
and fruits. Nearly 600 of these compounds have been
identified in nature. Less than 10% of the carotenoids
can be metabolized to retinol and act as vitamin A
precursors. The predominant carotenoids found in
blood plasma (i.e., about 90%) are b-carotene, lyco-
pene, lutein, b-cryptoxanthin and a-carotene. The
structure of carotenoids is a key determinant of their
physical properties, chemical reactivity, and bio-
logical functions. The unique chemical features of
each carotenoid, such as size, shape, hydrophobicity,
and polarity, determine its ability to be incorporated
into the molecular microenvironment and its bio-
logical function. Thus, when carotenoids are con-
sumed by humans from the diet, these structural
properties influence the absorption, bioavailability,
and biodistribution, and may affect their actions
at the subcellular level and on biochemical pathways.
Carotenoids exist in different geometric forms (cis-
and trans-isomers), which can be interconverted
by light, thermal energy, or chemical reactions. After
passive absorption by the enterocyte, unmetabo-
lized carotenoids are incorporated into chylo-
micra followed by the uptake of remnants by liver.
Cis-b-carotene appears in blood and tissues at signifi-
cantly lower concentrations than the corresponding
trans form, even when ingested at equivalent or
higher amounts. After absorption, the cis form of b-
carotene is converted to the trans form. This conver-
sion seems to increase the bioavailability of the trans
form at the expense of the cis form. The more non-
polar carotenoids (e.g., b-carotene, a-carotene, lyco-
pene) are predominatly within LDL. The more polar
CORONARY HEART DISEASE/Antioxidant Status 1657

carotenoids (e.g., lutein) are associated with high-
density lipoprotein (HDL). Approximately four ca-
rotenoid molecules are associated with each VLDL
and one with each LDL particle, whereas for every
1000 HDL particles, only one contains carotenoids.
An inverse relation between body mass and plasma
concentrations of carotenoids, irrespective of dietary
carotenoid intake, has been found in both epidemi-
ological and clinical studies. High concentrations of
carotenoids are found in tissues that are rich in LDL
receptors, such as the corpus luteum, adrenal gland,
and testes.
0010 Carotenoids are efficient quenchers of singlet
oxygen and directly scavenge free radicals. In com-
parison, vitamin A is a poor antioxidant. Lycopene
exhibits a better antioxidant capability than b-
carotene and lutein. The in-vivo antioxidant actions
of carotenoids is mainly due to inhibition of lipid
peroxidation. Moreover, cell-culture studies and
very small clinical trials suggest that lycopene may
inhibit macrophage cholesterol synthesis, secondary
to the inhibition of HMGR, and increases macro-
phage LDL receptors. It is worth noting that lipid-
lowering pharmacological agents can reduce serum
concentrations of lycopene, via a reduction in lipo-
protein particle size and competition between the
drug and carotenoids for incorporation into VLDL.
The biological link between high levels of carotenoids
and a reduced risk of cardiovascular diseases has been
suggested to be the antioxidant effect of carotenoids
in LDL. Moreover, carotenoids have been detected in
lipid-rich atherosclerotic plaques, although concen-
trations are very low. The serum concentrations of
b-carotene achieved with supplementation are much
higher than those achieved with the consumption of a
carotenoid-rich diet. This fact may have contributed
to the adverse effects observed in some intervention
trials (ATBC, Table 1). Thus, a diet intervention strat-
egy is advantageous considering that: (1) it is risk-free
because, until now, no adverse effects have been
associated with consuming more carotenoids from
food; and (2) benefits may be gained, even if other
biologically active compounds, other than carote-
noids, are the truly active agents or act in synergism
with carotenoids.
0011 Ascorbic acid Ascorbic acid (vitamin C) is an essen-
tial micronutrient required for normal metabolic func-
tions in the organism. Like other primates, humans
lost the ability to synthesize ascorbic acid, as a result of
a mutation in the gene coding for l-gulonolactone
oxidase, required for the biosynthesis of ascorbic
acid via the glucuronic acid pathway. A lack of ascor-
bic acid in the diet causes the disease scurvy, which can
be prevented with 10 mg per day, an amount easily
obtained by consumption of fruit and vegetables.
Tissue saturation in healthy men occurs at ascorbic
acid intakes of approximately 0.1 g per day. Thus,
further increases over this intake may have minimal
or no additional effect on tissue ascorbic acid concen-
tration, and hence disease risk. Ascorbic acid is a
cofactor for several enzymes involved in the biosyn-
thesis of neurotransmitters, collagen, and carnitine.
Ascorbic acid has also been implicated in the catabol-
ism of cholesterol to bile acids by the enzyme choles-
terol 7a-monooxygenase and in the steroid
metabolism in the adrenals. The role of ascorbic acid
in these metabolic pathways is basically to reduce the
central metal ion of the mono- and dioxygenases,
acting as a cosubstrate in these reactions. Ascorbic
acid is an important water-soluble antioxidant in bio-
logical fluids. Ascorbic acid scavenges reactive oxygen
and nitrogen species, such as superoxide, hydroxyl,
nitroxide and aqueous peroxyl radicals, singlet
oxygen, ozone, peroxynitrite, nitrogen dioxide, and
hypochlorous acid. Two major properties of ascorbic
acid make it a strong antioxidant: (1) the low one-
electron reduction potentials of both ascorbate and its
one-electron oxidation product, the ascorbyl radical,
which allows both forms to react with and reduce
basically all physiologically relevant oxidants; (2) sta-
bility and low reactivity of the ascorbyl radical. The
latter readily dismutates to form ascorbate and dehy-
droascorbic acid, or is reduced back to ascorbate by an
NADH-dependent semidehydroascorbate reductase.
Ascorbic acid can also act as a co-antioxidant by
regenerating a-tocopherol from a-TO
, although
in vivo, this interaction is not clear.
0012The effect of ascorbic acid on hypercholesterolemia
has been investigated in numerous studies, although
results are still controversial. In one supplementation
study, consumption of 1.0 g of ascorbic acid per day
for 4 weeks resulted in a reduction in total choles-
terol, whereas in another study, supplementation
with (0.060–6.0) g per day for 2 weeks had no effect.
The positive effect of ascorbate may be related to its
role as a cofactor for cholesterol 7a-monooxygenase,
or, its modulating effect on HMGR. Several observa-
tional studies have found a significant association
between elevated plasma ascorbic acid and increased
concentrations of HDL-cholesterol and reduced con-
centrations of LDL-cholesterol. In relation to throm-
bosis, two studies found an inverse association
between serum ascorbate concentrations and coagu-
lation factors, as well as a positive association be-
tween low serum ascorbate and elevated coagulation
activation markers. However, these effects were not
confirmed by further studies. In-vitro studies have
shown that physiologic concentrations of ascorbic
acid increase PGE
1
(protaglandin E
1
) and PGI
1
1658 CORONARY HEART DISEASE/Antioxidant Status

(prostacyclin) production, reducing platelet aggrega-
tion and thrombus formation. Low concentrations of
ascorbate have also been associated with increased
concentration of plasminogen activator inhibitor 1,
a protein that inhibits fibrinolysis. High doses of
ascorbate, administered either orally or by intra-
arterial infusion, have shown beneficial effects on
vasodilation. Four studies investigated vasodilation
in patients with CVD and found increases of
45–220% in vasodilation after administration of
ascorbate (1.0–2.0 g oral or 0.025 g min
1
infusion).
A 100% reversal of epicardial artery vasoconstriction
was observed in coronary spastic angina patients in-
fused with 0.010 g of ascorbate per minute. In the
studies reporting an inverse association between
plasma ascorbate and angina pectoris and CHD, the
association was reduced after adjusting for smoking,
suggesting that smokers may need additional ascorbic
acid intakes. The possible mechanisms to explain
the positive effect of ascorbate on vasodilation are
related to its antioxidant activity and are suggested
as the following: (1) ascorbate may spare
NO by
scavenging superoxide radicals or preventing the for-
mation of oxLDL; (2) maintenance of intracellular
concentrations of glutathione by a sparing effect or
regeneration of thiols from thyil radicals, which may
enhance the bioavailability of
NO or increase
the stabilization of
NO through the formation of
S-nitrosothiols.
0013Phytochemicals (polyphenols) The secondary me-
tabolism of plants (e.g., shikimate and acetate path-
ways) generates products that are grouped under the
term phytochemicals or polyphenols. The term poly-
phenols includes several classes of compounds that
share a common structure; among them, flavonoids
constitute the most important group, including more
than 5000 compounds already identified. Subclasses
of flavonoids are flavonols, flavones, catechins, fla-
vanones, anthocyanidins, and isoflavonoids. Pheno-
lics are derivatives of benzene with one or more
hydroxyl groups associated with the aromatic rings.
Several in-vitro studies have shown that flavonoids
can modulate a variety of mammalian enzyme
systems. Some of these enzymes are involved in
important pathways that regulate cell division and
proliferation, platelet aggregation, and inflammatory
and immune responses. Soybean isoflavones, in
particular genistein and daidzein, have a weak proes-
trogenic or antiestrogenic activity, which enable them
to interact with estrogen receptors and decrease
serum cholesterol concentrations. In vitro studies
tbl0001 Table 1 Randomized trials of antioxidants in CHD
Study Participants Follow-up
(years)
Antioxidants Results
Primary
prevention trials
ATBC 29 000
male smokers
(50 – 60 years)
5 – 8 VE (50 mg per day) No effect on
coronary mortality (increased
incidence of lung cancer)b-Carotene (20 mg per day)
PHS 22 071 healthy males
(40 – 84 years)
12 b-Carotene
(50 mg every other day)
Supplementation confers
neither benefit nor harm with
respect to CHD or cancer
CARET 18 314 men and women
heavy smokers/asbestos
workers
4 b-Carotene
(30 mg per day)
Trend towards excess
cardiovascular deaths
(relative risk ¼1,26; 95%
CI ¼0.99–1.61)
Retinol (25 000 IU
per day)
Women’s
Health Study
40 000 healthy US health
professionals
(40 – 84 years)
Ongoing VE (600 mg every other day) Cancer and
cardiovascular endpointsb-Carotene (50 mg every
other day)
Su. Vi.MAX 12 735 healthy men and
women
Ongoing VC (120mg per day) Premature deaths, cancers,
cardiovascular diseases end pointsVE (30 mg per day)
b-Carotene (6 mg per day)
Selenium (100 mg per day)
Zinc (20 mg per day)
ATBC, a-tocopherol b Carotene Cancer Intervention Trial; CARET, b-carotene and Retinol Efficacy Trial; CI, confidence interval; PHS, Physicians’ Health
Study; Su. Vi.MAX, Supplementation en Vitamines et Mineraux Study; VC, vitamin C; VE, vitamin E.
CORONARY HEART DISEASE/Antioxidant Status 1659
have shown that genistein, a specific inhibitor for
tyrosine kinases, prevents the development of ather-
oma by inhibiting cell adhesion and proliferation, by
inhibiting LDL oxidation and by altering growth
factor activity. In addition, soy isoflavones improve
coronary vascular reactivity in female nonhuman pri-
mates, as well as, the endothelium-dependent flow-
mediated vasodilation and arterial compliance in
postmenopausal women. The phenolic compounds
found in tea (e.g., quercetin, kaempferol, myrecitin,
and epigallocatechin gallate) may be protective
against CHD. Some epidemiological studies have
correlated a high tea flavonoid intake with a lower
incidence of CHD (Table 2). Since the ‘French para-
dox’ was revealed, it has been demonstrated that
red wine, as well as dark grapes, contains many phen-
olic antioxidant compounds. In particular, trans-
resveratrol has been shown in vitro to act as: (1) a
strong antioxidant, (2) a vasorelaxant, (3) a phytoes-
trogen, and (4) an inhibitor of platelet aggregation,
cyclooxygenase-2, and polymorphonuclear leuko-
cytes activity. However, human studies are still
needed to confirm the effects of trans-resveratrol in
the protection against CHD. The antioxidant proper-
ties of olive oil phenolics, particularly oleuropein
and hydroxytyrosol, have also been demonstrated in
cellular and animals models. Among biological activ-
ities demonstrated by olive oil phenolics, the
following can be mentioned: inhibition of platelet
aggregation and human neutrophil respiratory burst;
reduced formation of thromboxane B
2
and leuko-
triene B
4
by human leukocytes; and increased pro-
duction of
NO by mouse macrophages. Cocoa
(Theobroma cacao L.) also contains appreciable
amounts of phenolic substances, and in-vitro and
ex-vivo studies indicate that they are endowed with
certain biological activities such as an antioxidant
capacity and immunoregulatory effects.
Epidemiological Studies on Antioxidants
and Coronary Heart Disease
0014In the last two decades, observational studies around
the world have suggested an inverse association be-
tween the consumption of fresh fruits and vegetables,
important sources of antioxidants, and the risk of
tbl0002 Table 2 Prospective studies on flavonoid intake and CHD
Population Age
(years)
Follow-
up (years)
Relative risk
a
(95% confidence
interval)
Cross-cultural study
Seven-countries Study 40–59 25 r ¼0.5; P ¼ 0.01
12 763 men with CHD
Cohort studies
Zutphen Elderly Study
(The Netherlands)
50–69 15 0.27 (0.11–0.70)
552 men
with stroke
Caerphilly Study (UK) 49–59 14 1.6 (0.9–2.9)
1 900 men with CHD
Finish Study
5 133 men þ women
with CHD
30–69 20 Men: 0.67
(0.44–1.00)
Women: 0.73
(0.41–0.32)
a
Relative risk of highest versus lowest flavonoid intake group, adjusted for
age, diet and other risk factors for CHD.
tbl0003 Table 3 Case-control studies relating antioxidants and CVD
Study Comparison groups Comments
Scotland, Finland,
and Southern
Italy men
Plasma VE, VC, b-carotene
were determined in angina
patients and healthy controls
The relative risk of angina
between the lowest and highest
quintiles of lipid-standardized
VE levels was 2.98 (95%
CI ¼1.07–6.70); after adjustment for
cigarette smoking, the relative risks for
VC and b-carotene were, respectively,
1.63 (0.76–3.49) and 1.41 (0.63–3.13); antioxidants
did not reflect regional CVD mortality rates
EURAMIC Adipose tissue levels of
a-tocopherol and b-carotene
were obtained in men from 10
European countries with first acute
MI and healthy controls
The multivariate odds ratio in the lowest
quintile b-carotene level
compared to the highest was 1.78 (95%
CI ¼1.17–2.71); the associations were
strongest among current and former
smokers. a-Tocopherol levels were
not associated with an apparent
increased risk, with an odds ratio
of MI in the lowest compared to the
highest category of 0.83 (95% CI ¼ 0.57–1.21)
MI, myocardial infarction; VC, vitamin C; VE, vitamin E.
1660 CORONARY HEART DISEASE/Antioxidant Status

CHD. Several case-control studies (Table 3) and
prospective cohort studies (Tables 2 and 4), compar-
ing rates of CHD with different plasma levels or
intake of antioxidants, have reported a significantly
reduced CVD incidence and mortality in subjects
or populations presenting a high intake or plasma
levels of vitamin E, vitamin C, and b-carotene, while
others have not supported these associations. How-
ever from the results of these studies, it was not
possible to establish a causal relationship and several
limitations. Some of the restrictions to the case-con-
trol studies are sample size, biases in cases and control
selection, quality of information on exposures, and
recall bias. Moreover, blood levels of antioxidants are
assessed with a single measurement some time after
the index event, thus precluding any distinctions be-
tween short- and long-term exposures. In addition,
storage of samples for long periods may decrease the
concentrations of antioxidants. The prospective
cohort studies are also limited by several factors: (1)
subjects taking vitamin supplements may also have a
healthier lifestyle than nontakers, such as practice of
physical activities, equilibrated diet, and no smoking;
(2) lack of precision and reliability of nutrient intake
tbl0004 Table 4 Prospective cohort studies of dietary intake and CVD
Study Participants Follow-up (years) Comparison groups Results
(relative risk; 95% CI)
Nurses’ Health
Survey (NHS)
121 000 female nurses
(USA) (34–59 years)
8 Upper vs. lower quintiles
of dietary and supplemental
vitamin E, vitamin C or
b-carotene intake
b-Carotene (0.78)
Vitamin E (0.66)
Vitamin C (0.80;
nonsignificant after
controlling for
vitamin E intake)
Health Professional
Follow-up Study (HPFS)
39 910 male health
professionals
(USA) (40–75 years)
4 Upper vs. lower quintiles of
dietary and supplemental
vitamin E, vitamin C or
b-carotene intake
b-Carotene (0.71)
Vitamin E (0.60)
Vitamin C (1.29)
First National Health and
Nutrition Examination Survey
(NHANES-1)
11 349 adults
(USA) (25–74 years)
10 Upper vs. lower dietary
and supplemental
vitamin C intake
Vitamin C (0.66)
Intake of other vitamin
supplements was not
taken into account
Iowa Women Health Study 34 486 postmenopausal
women (USA)
(55–69 years)
7 Upper vs. lower quintiles of
dietary and supplemental
vitamin E, vitamin A,
retinol, and
carotenoid intake
Vitamin E (0.96)
No benefit for Vitamin A,
retinol, and
carotenoids
2 748 men (Finland)
(30–69 years)
14 Upper vs. lower tertiles
of dietary and
supplemental
vitamins or
b-carotene intake
Vitamins (0.66)
b-Carotene (1.02)
2 385 women (Finland)
(30–69 years)
14 Upper vs. lower tertiles
of dietary and
supplemental
vitamins or
b-carotene intake
Vitamins (0.35)
b-Carotene (0.62)
1 899 men with
hyperlipidemia (USA)
(40–59 years)
13 Upper vs. lower
quartiles of serum
b-carotene intake
b-Carotene (0.64)
CORONARY HEART DISEASE/Antioxidant Status 1661
ascertained by food-frequency questionnaires; and (3)
confounding factors that were neither measured nor
controlled for in multivariate analysis.
0015 The randomized intervention trials (Tables 1 and 5)
have been considered as the golden standard to test
the hypothesis that antioxidant micronutrients
effectively have a causal relationship with the de-
crease of CHD morbidity/mortality. Until now, the
results of these trials have raised the possibility that
some benefits from observational epidemiology may
have been overestimated. b-Carotene and vitamin C
appear to confer no overall benefit in the primary
prevention of CVD among well-nourished subjects.
However, whether risk can be reduced among those
with disease or lower baseline levels of these micro-
nutrients remains unclear. For vitamin E, the results
are still controversial; while the results of the Cam-
bridge Heart Antioxidant Study (CHAOS) show a
tbl0005 Table 5 Randomized trials of antioxidants in CHD
Study Participants Follow-up (years) Antioxidants Results
Secondary prevention trials
CHAOS 2002 men and women with
angiographically proven CHD
510 days (3–981) VE (400 or 800 mg per day) 75% reduction in nonfatal MI;
nonsignificant increase in
cardiovascular death
HOPE 9 541 patients with CHD, stroke or pad 4.5 VE (400 mg per day) No effect on death from CVD
þ MI þ stroke
GISSI 11 324 men
and women with CHD
3.5 VE (300 mg per day) 4.7% (NS) reduction of total
mortality þ nonfatal MI
þ CVA; 35% reduction of
sudden death
WACS 8 000 women with CHD Ongoing VE (400 mg per day) MI, stroke, coronary
revascularization, and death
from CVD end points
VC (1 g per day)
b-Carotene (20 mg per day)
HPFS 20 000 patients with previous angina,
stroke, claudication, or diabetes
Ongoing VE (600 mg per day) Incidence of coronary
mortality and all-cause
mortality end points
VC (250 mg per day)
b-Carotene (20 mg per day)
CVA, cerebrovascular accidents; GISSI, Gruppo Italiano per lo Studio della Sopravvivenza nell’infarto; HOPE, Heart Outcome Prevention Evaluation;
HPFS, Heart Protection Study; MI, myocardial infarction; VC, vitamin C; VE, vitamin E: NS, nonsignificant; WACS, Women’s Antioxidant Cardiovascular
Study.
Lipid phase
Aqueous phase
T
TT
T
TT
ROOH
ROH
RNS
ROS
Lipids
Lipoproteins
Carotenoid
Dehydroascorbate
Carotenoid*
O
2
1
ROO /RO
Tocopherol
cycle
Asc
oxThiols
NAD(P)H
NAD(P)
+
+ H
+
TRX reductase
GSSG reductase
Lipoamida
Dehydrogenase
GSH-dependent
dehydroascorbate reductase
Thioredoxin
Thiol transferase
Thiol
cycle
redThiol
Asc
Ascorbate
cycle
Carotenoid
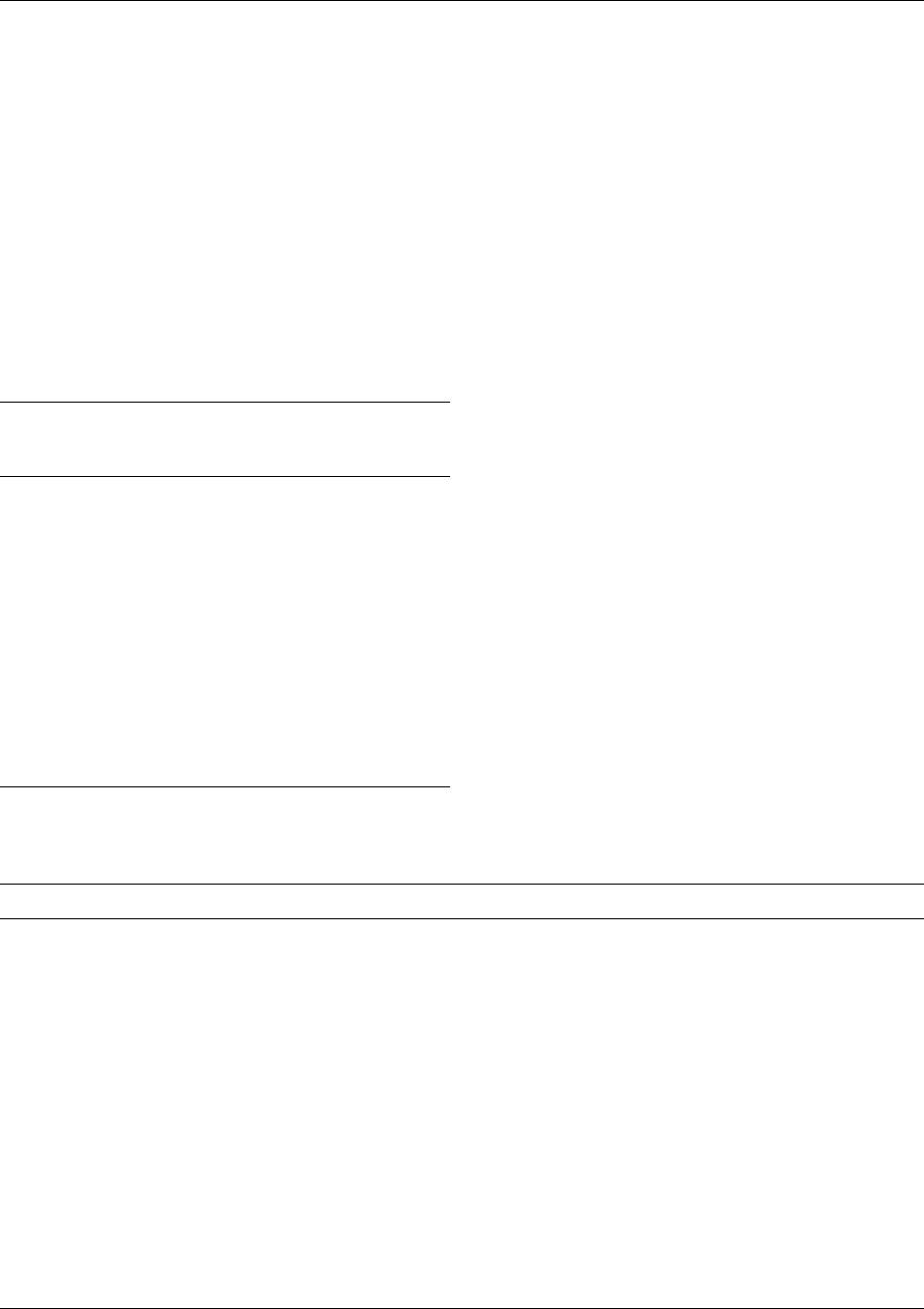
fig0002 Figure 2 Integrated action of antioxidants. Asc, ascorbate; Asc
, ascorbyl radical; GSSG, oxidized glutathione; carotenoid
, carot-
enoid-derived free radical; carotenoid
*
, excited-state carotenoids; oxThiols, oxidized thiols (glutathione, lipoic acid, thioredoxin); RO
,
alcoxyl; ROH, lipid-hydroxyl derived; ROO
, peroxyl radical; ROOH, lipid hydroperoxide; ROS, reactive oxygen species; RNS, reactive
nitrogen species; T, tocopherols; T
, tocopheroxyl radical; TT, tocotrienols; TT
, tocotrienoxyl radical; TRX, thioredoxin.
1662 CORONARY HEART DISEASE/Antioxidant Status

lower incidence of nonfatal infarction and major car-
diovascular events in supplemented subjects, HOPE
results indicate no benefit on chosen endpoints. There
are also criticisms of the randomized trials. Supple-
mentation of a diet with individual antioxidants
could not exert optimal effects because the concerted
mechanism of action of the antioxidant system
(Figure 1) could require the availability of adequate
and harmonized levels of several antioxidant com-
ponents in the diverse microenvironments in the or-
ganism. Furthermore, it may be possible that the
antioxidants chosen (vitamin C, vitamin E, and b-
carotene) could only be markers for the intake of
other, as yet unidentified, substance(s) with the true
biological effect. The rationale behind the approach
of antioxidant supplementation in order to prevent
the development and progression of CVD has not yet
been firmly established. More basic research is re-
quired to ascertain the appropriate antioxidant(s)
and doses, oxidation biomarkers, intermediate and
final end points, among other factors, before plann-
ing further large intervention trials. Thus, until the
results of these studies produce the final answers,
the consumption of fruits and vegetables and a
healthy life style would be advisable.
See also: Antioxidants: Natural Antioxidants; Synthetic
Antioxidants; Role of Antioxidant Nutrients in Defense
Systems; Ascorbic Acid: Properties and Determination;
Physiology; Carotenoids: Occurrence, Properties, and
Determination; Physiology; Soy (Soya) Beans: Dietary
Importance; Tocopherols: Properties and Determination;
Physiology
Further Reading
Antony MS (2000) Soy and cardiovascular disease: choles-
terol lowering and beyond. Journal of Nutrition 130:
662S–663S.
Carr AC and Frei B (1999) Toward a new recommended
dietary allowance for vitamin C based on antioxidant
and health effects in humans. American Journal of Clin-
ical Nutrition 69: 1006–1007.
Chisolm GM and Steinberg D (2000) The oxidative modifi-
cation hypothesis of atherogenesis: an overview. Free
Radical Biology and Medicine 28: 1815–1826.
Clinton SK (1998) Lycopene: Chemistry, biology and impli-
cations for human health and disease. Nutrition Reviews
56: 35–51.
Diplock AT, Charteux JL, Crozier-Willi G et al. (1998) Func-
tional food science and defence against reactive oxidative
species. British Journal of Nutrition 80: S77–S112.
Gaziano JM (2000) Dietary antioxidants and cardiovascu-
lar disease. Vitamins and Hormones 58: 299–320.
Hollman PCH and Katan MB (1999) Health effects and
bioavailability of dietary flavonols. Free Radical
Research 31: S75–S80.
Kamal-Eldin A and Appelqvist LA (1996) The chemistry
and antioxidant properties of tocopherols and tocotrie-
nols. Lipids 31: 671–701.
Keaney JF, Simon DI and Freedman JE (1999) Vitamin E
and vascular homeostasis: implications for atheroscler-
osis. FASEB Journal 13: 965–976.
Krinsky NI (1998) The antioxidant and biological proper-
ties of the carotenoids. Annals New York Academy of
Sciences 854: 443–447.
Marchioli R (1999) Antioxidant vitamins and prevention of
cardiovascular disease: laboratory, epidemiological and
clinical trial data. Pharmacological Research 40: 227–
238.
Packer L, Weber SU and Rimbach G (2001) Molecular
aspects of a-tocotrienol antioxidant action and cell sig-
nalling. Journal of Nutrition 131: 369S–373S.
Theriault A, Chao JT, Wang Q et al. (1999) Tocotrienol: a
review of its therapeutic properties. Clinical Biochemis-
try 32: 309–319.
Visioli F, Borsani L and Galli C (2000) Diet and prevention
of coronary heart disease: the potential role of phyto-
chemicals. Cardiovascular Research 47: 419–425.
Intervention Studies
P C Elwood, Llandough Hospital, Penarth, South
Glamorgan, UK
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Definition and Aims
0001In the section on epidemiology a number of research
strategies are discussed. These fall into two obvious
groups: observation and intervention. In general, ob-
servation studies seek to ascertain more about the
natural course of a disease process, whereas interven-
tion studies test the effect of a possible therapeutic or
preventive measure. (See Epidemiology.)
0002There is a further division of intervention studies,
and this division is of particular importance in nutri-
tion research. First, there are trials in which a food-
stuff or a nutrient is given to subjects, and the effect
upon a mechanism or a metabolic process, or the
effect on a biochemical or hematologic variant is
observed. Measuring the change in blood lipids
following changes in dietary fat intake, or measuring
the blood pressure response to coffee prepared in
different ways, illustrates this kind of study. In these,
the numbers of subjects can be relatively small, be-
cause a measurement of the outcome variable (serum
cholesterol, blood pressure, or platelet aggregation,
etc.) is obtained for every subject. Furthermore, such
trials can usually be relatively short-term, and often it
is acceptable to use a cross-over design, in which each
CORONARY HEART DISEASE/Intervention Studies 1663

patient has a period on the intervention diet or sup-
plement being evaluated, and a period on a normal
diet without the intervention, thus serving as his or
her own control. This last can be a most efficient
design, provided the order of the intervention and
control periods is random for each subject, and pro-
vided an adequate washout time is allowed between
the two periods.
0003 In the second kind of intervention trial, the effect of
a dietary change, or a nutritional supplement, on the
incidence of a disease is studied. This might be the
reduction in the mortality of patients who have had a
heart attack and who are advised to eat fatty fish, or
the effect of a magnesium supplement given to patients
judged to be at risk of a heart attack or stroke. Trials
such as these are very different in that the outcome is a
disease event, and this is likely to occur in only a very
small proportion of persons in the trial. As a conse-
quence, the numbers involved have to be very large
and/or the period of observation very prolonged.
0004 Both kinds of trials involve intervention and the
level of confidence that one can put on the results is,
in general, much higher than in studies from which the
evidence is purely observational. Most of the design
features are the same in the two kinds of intervention
trial, but the problems which arise in those which aim
to change the progress or reduce the incidence of a
disease are substantial and so most of the following
discussion will focus on the second.
Design
0005 The only design for intervention studies which is now
acceptable, fulfills the basic criteria for randomized
controlled trial (RCT). These criteria include the
following:
1.
0006 The allocation of subjects to the group which will
receive the intervention, or to the control group
which will not, must be random. Only allocation
at random is likely to insure that there are no
systematic differences or bias between the groups,
which might account for some of the effects found
and which might wrongly be attributed to the
intervention. In fact, the comparability between
the groups should later be checked by comparing
the distributions of age, social class, and perhaps
smoking, etc. in the two groups to give some as-
surance that by chance some systematic difference
or bias has not occurred.
2.
0007 The advice, handling, and monitoring of the two
subgroups of subjects – those receiving the interven-
tion and those not – must be identical in every
respect other than the intervention being tested. As
far as possible the subjects in the trial should be
‘blind’ with regard to whether or not they are receiv-
ing the intervention under test. While this last is easy
in a drug trial by the use of placebo tablets, it is
unlikely to be possible in a trial of a foodstuff.
3.
0008The outcome, whether this is a change in a bio-
chemical or other variant or a difference in disease
incidence or disease progression, must be assessed
by an observer who is blind as to whether or not
the subject has or has not been receiving the inter-
vention.
0009The setting-up and conduct of an RCT is a major
undertaking, and this is particularly true of a dietary
trial. It may be appropriate therefore to consider the
use of a factorial design. In this, two or more interven-
tions are separately randomized. Equal numbers of
subjects therefore receive each possible combination
of the interventions, with some receiving all and some
none. This design is acceptable provided that there is
no reason to expect strong interactions between the
different interventions. It is highly efficient as the main
effects are all tested simultaneously without loss of
power. For example, a trial by Burr and colleagues
evaluated the effects of three dietary changes on mor-
tality after a heart attack: a reduction in saturated fat,
an increase in fatty fish, and an increase in cereal fiber
consumption. Advice on each of these dietary changes
was given to half of the total patient group, selected for
each intervention at random.
0010The compliance of subjects with the intervention
being tested is of great importance and should be
taken into account when drawing conclusions from
a trial. In fact, it is important to monitor both the
degree of compliance by those who have been given
advice about the dietary factor being tested and the
extent to which the control subjects may spontan-
eously change their consumption of that factor.
Ideally, compliance should be monitored in an object-
ive way; for example, the estimation of plasma eico-
sapentaenoic acid will indicate how much fatty fish
has been consumed. If an appropriate objective meas-
ure is not available, then at intervals the subjects
(both those advised and the controls) can be asked
to keep a diary of the food they consume.
0011Every reasonable effort should be made to achieve
and maintain a high level of compliance. At the same
time, whether or not a subject complies with the
intervention, and whether or not a control subject
spontaneously adopts the measure under test, all the
subjects must be retained within their originally ran-
domized group, and their results analyzed accord-
ingly. This leads to what is known as an ‘intention
to treat’ analysis. Omission of patients from one or
other group because of poor compliance or other
reason can unbalance the comparability obtained by
1664 CORONARY HEART DISEASE/Intervention Studies
the original randomization, and is highly likely to
introduce bias and invalidate any conclusions drawn
from the results of the trial.
0012 In planning a trial, careful attention should always
be given to the likely power of a study, that is, the
likelihood that the planned trial will be large enough,
and the outcome measures sensitive enough to detect
a statistically significant beneficial effect, should one
occur. A number of things affect the power of a study.
The most obvious are the number of outcome events
and the likely difference in this between the interven-
tion and the control group. Clearly, this last will not
be known, otherwise the trial would be unnecessary.
An estimate usually has to be made. If the interven-
tion involves the consumption of a foodstuff, such as
fish, or fruit and vegetables, the reproducibility of
measurement of consumption should be assessed,
and any changes in the consumption of other foods
or nutrients monitored.
0013 All these estimates should be realistic. It is all too
easy to overestimate the likely effect of advice. For
example, dietary intakes are relatively fixed, particu-
larly in older subjects, and advice to increase the
consumption of, say, fruit and vegetables, or to
reduce fat intakes, has relatively little effect.
Primary and Secondary Trials in Coronary
Heart Disease
0014 The terms ‘primary’ and ‘secondary’ are used to distin-
guish trials which test a preventive measure in patients
who have not already had a clinical episode of a disease
(primary) from those based on patients who have al-
ready had a myocardial infarct or other clinical event
(secondary trials). A distinction of this kind is most
often made in coronary heart disease (CHD).
0015 In fact, the concept of primary and secondary trials
is not helpful. The terms relate to past history, and not
to future risk. In relation to risk of a future vascular
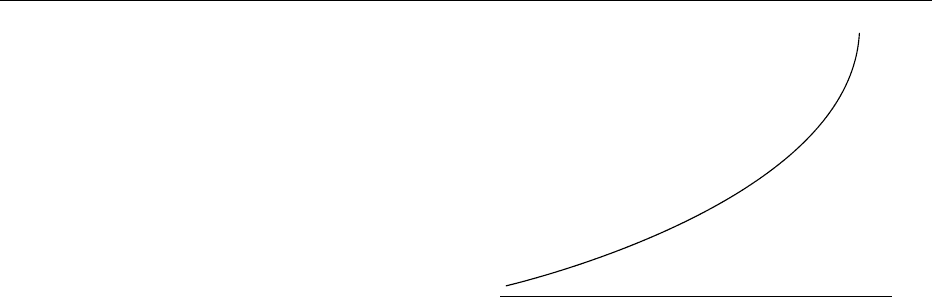
event, subjects simply form a continuum, ranging
from subjects at exceedingly low risk, such as
young, healthy subjects, to patients who have recently
had a vascular event. Ranged between these are sub-
jects who smoke, who have raised blood pressure,
raised cholesterol, etc., whose risk of a vascular
event is somewhere between those of subjects at the
two extremes. In the evaluation of the risk of any
individual, the occurrence of a past vascular event is
only one of a number of risk factors which determine
the likelihood of a future event. Figure 1 displays the
continuum of risk.
0016 Ideally, all possible preventive measures should be
tested in an RCT based upon younger healthy sub-
jects. The numbers required for a trial in such indi-
viduals are however formidable. Further difficulties
arise in persuading healthy subjects to comply with
the intervention to a sufficient degree and for a
sufficient time.
0017Intervention trials are therefore often conducted in
subjects who have evidence of an increased risk, be it
raised levels of risk factors, or some evidence of vas-
cular disease, such as angina pectoris. Trials can even
be based upon patients who have already had a CHD
event, such as a myocardial infarct. All such patients
know that they are at an increased risk of a further
event, and they are likely to be more easily persuaded
to comply with a dietary change for long periods. It
can be argued that the underlying disease of survivors
of a myocardial infarction is at an advanced stage and
may not be reversible within the time span of a trial of
a reasonable duration. There may well be some truth
in this, particularly if the aim of the intervention is to
reduce atherosclerosis.
0018Nevertheless, a myocardial or a cerebral infarct
results when a thrombus develops on top of an ather-
omatous plaque on the wall of a blood vessel. Dietary
factors are undoubtedly relevant to the risk of throm-
bosis. For example, the head of an intravascular
thrombus is usually a mass of aggregated platelets,
and alcohol reduces platelet activity. A myocardial
infarct can precipitate ventricular fibrillation and
death, and there is evidence that fish oil reduces the
electrical stability of the myocardium. Free radicals
from an ischemic lesion can damage the myocardium,
and antioxidants from fruit and vegetables may
reduce this damage to the heart muscle. There are
therefore mechanisms in vascular disease, other than
atherosclerotic disease in the vessel wall, which are
affected by dietary items and which are appropriately
tested in dietary intervention RCTs.
Young, active,
healthy subjects
Patients judged to be at
increased risk because of
age, smoking, raised blood
pressure, etc.
Low risk
High risk
Patients with a
recent vascular event
Risk of a vascular event
fig0001Figure 1 Low verus high risk of a vascular events
CORONARY HEART DISEASE/Intervention Studies 1665
