Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.


of drinks and consumption data difficult, so assess-
ment and comparison of data from many countries
would be greatly facilitated by the uniform expres-
sion of alcohol intakes and by the adoption of
a uniform international definition of a standard
drink.
Alcohol Control Policies
0032 In order to reduce the prevalence of alcohol-related
problems, many countries apply control measures
that affect the availability and/or the price of alco-
holic drinks. In addition, governments may use
public health campaigns and alcohol advertising as
instruments to diminish the prevalence of alcohol-
related problems. Alcohol control policies may be
directed at reducing the average alcohol consumption
among the total population (public health approach)
or at reducing the number of heavy users or drinking
in high-risk situations (high-risk approach). Alcohol
control measures are more effective as public support
is higher, and so education of the public and possibly
self-regulation by the alcohol industry may be
important contributory measures.
Price
0033 Taxation of alcoholic beverages is essentially for
the purpose of raising revenue. It is not generally
regarded as a primary tool for controlling consump-
tion, although it is used as such in some countries. In
most countries, alcoholic drinks are perceived to be
luxury items and, as such, candidates for taxation.
Alcoholic drinks are price-elastic, which means that
the demand is sensitive to price changes. This is the
main reason for the argument that taxation should be
used to reduce consumption. However, it has been
found that elasticity values relating to different
regions, different periods, and different types of
alcohol vary considerably.
0034More important is the effect of price increases
on different types of consumers. Some claim that
heavy drinkers are at least as responsive to price
increases than light-to-moderate consumers. Others
claim that heavy drinkers reduce their consumption
much more than other drinkers after excise duties are
increased.
Advertising and Alcohol Availability
0035A commonly held assumption is that alcohol adver-
tising increases alcohol consumption. The main
objective of advertising is to persuade customers to
continue to enjoy, or to switch their preference to, the
brand being advertised. Several studies have dem-
onstrated that advertising does not increase con-
sumption. Also, restrictions on advertising do not
automatically result in a decline in total alcohol
consumption.
0036It has been suggested that there is an association
between hours of sale and outlet density, and the
consumption of alcohol, but some studies show no
tbl0004 Table 4 Alcohol content of a ‘standard drink’ as defined in various countries, and some daily consumption guidelines recommended
by governments or learned bodies
Country Size of standard drink
(grams of absolute alcohol)
Daily consumption guidelines
(grams of absolute alcohol)
Men Women
Australia 10 40 20
Austria 10 30 20
Canada 13.5 13.5 13.5
Denmark 12 36 27
Finland 11 41 27
France 12 60 36
Hungary 17
Iceland 9.5
Ireland 8
Italy 10
Japan 19.75 39.5
Netherlands 9.9 2–3 units a few times a week 2–3 units a few times a week
New Zealand 10 30 20
Portugal 14
Spain 10 30 20
UK 8 24–32 16–24
USA 14 28 14
Data adapted from ICAP (1998b) Safe Alcohol Consumption: A Comparison of Nutrition and Your Health: Dietary Guidelines for Americans and Sensible Drinking.
ICAP Report No. 1. Washington, DC: International Center for Alcohol Policies; ICAP (1998c) What is a ‘Standard Drink’? ICAP Report No. 5. Washington, DC:
International Center for Alcohol Policies.
124 ALCOHOL/Alcohol Consumption

such association. Prohibition of alcohol has been
shown to be an ineffective and even counterproduc-
tive measure. Although the alcoholic cirrhosis mor-
tality and other alcohol problems may decrease, it
might not produce positive results in public health
terms as, for example, organized crime increased in
the United States after complete alcohol ban.
Drinking Age Limits
0037 Drinking age laws cover a broad spectrum of behav-
iors concerned with where, when, and under what
circumstances alcoholic beverages can be purchased
and consumed. Some countries focus their legislation
on both a minimum legal drinking age and a
minimum age at which beverage alcohol can be
purchased, and these ages may be different. Other
countries do not address a minimum age for con-
sumption. Table 5 shows some minimum drinking
and purchasing age laws. Both consumption and
purchasing laws in most countries are set at 18. The
national laws generally apply to drinking age limits
for venues outside the home, such as taverns, bars,
restaurants, nightclubs, and similar establishments.
The UK is the only country that legislates a minimum
consumption age in the home (from age 5 with
parental consent).
Effectiveness of Alcohol Control Policies
0038 Price policy may be an effective means of reducing
alcohol consumption and alcohol-related problems as
long as increases in price keep up with or outstrip
rises in income. However, the effectiveness may
depend very much on the population group affected
and on socio-economic and demographic factors.
Also, stable political and economic conditions as
well as appropriate law enforcement and administra-
tive systems are prerequisites for success.
See also: Alcohol: Properties and Determination;
Metabolism, Beneficial Effects, and Toxicology; Body
Composition; Dietary Surveys: Measurement of Food
Intake; Surveys of National Food Intake; Surveys of Food
Intakes in Groups and Individuals
Further Reading
Boffetta P and Garfinkel L (1990) Alcohol drinking and
mortality among men enrolled in an American Cancer
Society prospective study. Epidemiology 1: 342–348.
Grant M and Litva J (eds) (1998) Drinking Patterns &
Their Consequences. London: Taylor & Francis.
ICAP (1998a) Drinking Age Limits. ICAP Report No. 4.
Washington, DC: International Center for Alcohol
Policies.
ICAP (1998b) Safe Alcohol Consumption: A Comparison
of Nutrition and Your Health: Dietary Guidelines for
Americans and Sensible Drinking. ICAP Report No. 1.
Washington, DC: International Center for Alcohol
Policies.
ICAP (1998c) What is a ‘Standard Drink’? ICAP Report
No. 5. Washington, DC: International Center for
Alcohol Policies.
MacDonald I (ed.) (1999) Health Issues Related to Alcohol
Consumption. Brussels: ILSI Europe.
tbl0005 Table 5 Minimum drinking and purchasing age laws in various countries
Country Minimum drinking age (MDA) Minimum purchasing age (MPA)
Australia 18 18
Austria 16 16
18 (spirits)
Canada In Alberta and Quebec: 19 All provinces have their own legal MPA
In all other provinces: 18
Denmark 18
Finland 18 18 (for all beverages up to 21% alcohol by volume)
20
France 16 16
Hungary 18
Iceland 20
Ireland 18 18
Italy 16 16
Japan 20 20
Netherlands 16 (beer and wine)
18 (spirits, 16 if accompanied by an adult)
New Zealand 20 20
Portugal No MDA No MPA
Spain 16 16
UK 18 18
USA 21 21
Data adapted from ICAP (1998) Drinking Age Limits. ICAP Report No. 4. Washington, DC: International Center for Alcohol Policies.
ALCOHOL/Alcohol Consumption 125

Pikaar NA, Wedel M and Hermus RJJ (1988) Influence of
several factors on blood alcohol concentrations after
drinking alcohol. Alcohol & Alcoholism 23: 289–297.
Van Tol A and Hendriks HFJ (2001) Moderate alcohol
consumption: Effects on lipids and cardiovascular dis-
ease risk. Current Opinion in Lipidology 12: 19–23.
World Drink Trends 2000 International Beverage Con-
sumption and Production Trends. Henley-on-Thames,
UK: NTC Publications, Commodity Board for the Dis-
tilled Spirits Industry.
Ales See Beers: History and Types; Wort Production; Raw Materials; Chemistry of Brewing; Biochemistry of
Fermentation; Microbreweries
Algae See Single-cell Protein: Algae; Yeasts and Bacteria; Marine Foods: Production and Uses of Marine
Algae; Edible Animals Found in the Sea; Marine Mammals as Meat Sources
ALKALOIDS
Contents
Properties and Determination
Toxicology
Properties and Determination
M Wink, University of Heidelberg, Heidelberg,
Germany
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Background
0001 Plants produce a wide variety of secondary metabol-
ites, many of which function as allelochemicals or
signal compounds to attract pollinating insects and
seed- or fruit-dispersing animals. Their main function
is chemical defense against herbivores, microrganisms,
or competing plants. Over 13 000 nitrogen-containing
secondary metabolites have been described so far in
plants. Alkaloids contribute over 12 000 compounds,
followed by amines, nonprotein amino acids, cyano-
genic glycosides, and glucosinolates.
Definition
0002 The definition of alkaloids has changed throughout
the years. Formerly, this class of secondary compounds
was restricted to plant bases with a heterocyclic
nitrogen atom. Exocyclic nitrogen bases were termed
‘pseudoalkaloids.’ Other definitions demanded that
the skeleton of alkaloids should derive from amino
acids or that these bases have explicit pharmaco-
logical activities. At present, alkaloids are defined in
a more pragmatic way; they include all nitrogen-
containing natural products that are not otherwise
classified as peptides, nonprotein amino acids,
amines, cyanogenic glycosides, glucosinolates, cofac-
tors, phytohormones, or primary metabolites (such as
purine and pyrimidine bases). Even a number of anti-
biotics produced by bacteria or fungi are therefore
included in the group of alkaloids.
Occurrence
0003Alkaloids have been detected in about 15% of plants,
bacteria, fungi, and even in animals. Within the plant
kingdom, they occur in primitive groups such as
Lycopodium or Equisetum, in gymnosperms and
angiosperms. In higher plants (angiosperms) some
families contain more alkaloid-containing taxa than
126 ALKALOIDS/Properties and Determination

others. Such alkaloid-rich’ taxa include Papaveraceae,
Berberidaceae, Fabaceae, Boraginaceae, Apocyna-
ceae, Asclepiadaceae, Asteraceae, Liliaceae, Gne-
taceae, Ranunculaceae, Rubiaceae, Solanaceae, and
Rutaceae. Also, several food plants and food items
may contain alkaloids (Table 1).
0004 It has been speculated that alkaloids evolved early
in evolution and were already present at the time
(c. 200 million years ago) when the angiosperms
began to radiate. In general, specific alkaloid types
are restricted to particular systematic units and are
therefore of some importance for the systematics,
taxonomy, and phylogeny of plants. For example,
benzylisoquinoline alkaloids are typical for Papaver-
aceae, Berberidaceae, and Ranunculaceae, which
seem to be phylogenetically related. Other alkaloids
occur in phylogenetically unrelated plant families.
Ergot alkaloids occur in fungi (Claviceps) but also in
members of the Convolvulaceae; quinolizidine alka-
loids are typical for some Fabaceae, but have also
been detected in Berberidaceae (Caulophyllum, Leon-
tice). It has been speculated that the genes encoding
enzymes leading to the main quinolizidine alkaloid
skeleton are distributed much more widely in the
plant kingdom, but are normally switched off. Alter-
natively, convergent evolution and horizontal gene
transfer have been suggested.
Isolation and Detection
0005Alkaloids form free bases in alkaline solutions. The
free base is usually lipophilic and mostly insoluble
in water but soluble in organic solvents (ethanol,
methanol, diethylether, methylene chloride). At
higher hydrogen ion concentrations (i.e., pH < 7),
alkaloids occur in a protonated state and are usually
tbl0001 Table 1 Alkaloids present in food plants, beverages, stimulants, or hallucinogens
Substance Occurrence Activity
Alkaloids in food
Solanine and other steroid alkaloids Solanum (potato, tomato) Membrane disruption, Neuroreceptor
interaction, mutagenicity
Pyrrolizidine alkaloids Symphytum (comfrey); honey
(if bees have visited PA plants)
DNA and protein alkylation; mutagenicity,
cancer
Cycasin Cycas, other cycads Mutagenicity
Ergotalkaloids Claviceps purpurea Neuroreceptor interaction;
vasoconstriction; uterus contraction
neurotoxin (Na
þ
channel)
Saxitoxin Algae; ends up in food chain (molluscs;
fish)
Lupanine and other quinolizidine alkaloids Lupinus; other genistoids Interaction with AChR
a
,Na
þ
,K
þ
channels
Pelletierine Punica granatum Interaction with AChR
Alkaloids in beverages
Caffeine, theophylline, theobromine Coffea,Thea, Paullinia, Ilex paraguarensis Stimulant
Quinine Cinchona succirubra Bitter tonic; neuroreceptor and ion
channel interaction; central stimulant
Ephedrine and related compounds Ephedra; Catha edulis
Alkaloids as stimulants and hallucinogens
Morphine Papaver somniferum, opium Hallucinogen
N-Methyltryptamine Fungi, plants Central stimulant
N, N-dimethyltryptamine, 5-methoxy-N,
N-dimethyltryptamine
Mimosaceae: Anadenanthera
(syn. Piptadenia), Mimosa hostilis;
Myristicaceae: Virola; Malpighiaceae:
Banisteriopsis, Poaceae: Phalaris
Central stimulant, hallucinogen
Serotonin Fungi (Amanita); stinging hairs of Urtica,
Laportea, Jatropha urens, Mucuna
pruriens; seeds and fruits
Bufotenine Fungi (Amanita); animal poisons (Cnidaria,
spider, scorpions, wasps, toads)
Psilocybin, psilocin Fungi (Psilocybe, Stropharia, Conocybe,
Panaeolus, Gymnophilus, Psathyrella,
etc.)
Mescalin Lophophora williamsii; other cacti Hallucinogen
Harmalin and other b-carboline alkaloids Peganum harmala, Banisteriopsis caapi Hallucinogen
Cocaine Hallucinogen
Arecoline Nuts of Areca palm Stimulant
a
AChR, acetylcholine receptor.
ALKALOIDS/Properties and Determination 127

soluble in water but insoluble in apolar organic solv-
ents. The different solubilities are useful to isolate and
purify alkaloids. In the laboratory, alkaloids are often
solubilized from plant material by dissolving them in
acidic solutions (e.g., 0.5 M HCl). Treatment of this
solution with organic solvents will remove nonalka-
loidal substances. The solutions are brought to pH
> 12 in the next step and extracted with CH
2
Cl
2
,
ethylacetate, or ethylether to yield free alkaloids.
0006 Plant extracts are often analyzed by thin-layer
chromatography (TLC) as a first screening to find
out whether alkaloids are present or not. A number
of reagents give typical color reactions, such as Dra-
gendorff’s, Mayer’s reagent, etc. Because alkaloids are
typically present in complex mixtures consisting of
two to five main and up to 20–50 minor alkaloids,
TLC does not have sufficient separation capacities.
Better methods are high-performance liquid chroma-
tography (HPLC) and capillary gas–liquid chro-
matography (GLC). The latter method is extremely
useful because it has a strong separation power and is
very sensitive and selective if a nitrogen-specific de-
tector is used. Furthermore, GLC can be directly
coupled with a mass spectrometer, allowing mass
spectra to be obtained, even from very minor com-
ponents. Since many alkaloids have been analyzed by
mass spectrometry, already a large collection of mass
spectra is available, making it possible to identify
many of the known alkaloids unambiguously. Usu-
ally, mass spectra are recorded in the electron impact
(EI) mode, which promotes fragmentation. If infor-
mation on the molecular ions is needed (which can be
elusive in EI-MS), other MS techniques, such as
chemical ionization (CI), field desorption (FD), or
fast-atom bombardment (FAB) are the methods of
choice.
0007 HPLC tends to be less sensitive and of lower separ-
ation capacity than capillary GLC. However, modern
photodiode array detectors are very helpful to iden-
tify known metabolites by UV-VIS spectroscopy.
Today, also, HPLC and capillary electrophoresis can
be coupled to a mass spectrometer, thus widening the
use of MS for the analysis of natural products.
0008 In addition, HPLC has a major advantage in that it
is possible to isolate a compound in milligram quan-
tities that allow structural elucidation by nuclear
magnetic resonance (
1
H,
13
C). Nuclear magnetic res-
onance is the method of choice if unknown structures
are to be elucidated, whereas mass spectrometry is
extremely useful for identifying previously described
substances or substances that are slightly different to
known compounds.
0009 If small quantities (femtogram or nanogram
amounts) of known alkaloids need to be detected
routinely, immunological procedures such as
radioimmunoassays (RIA) or enzyme immunoassays
(EIA, ELISA) should be appropriate. In order to
obtain specific antibodies, the alkaloid in question
has to be coupled chemically to a large protein, such
as albumin, usually by some sort of spacers (e.g.,
succinic acid).
Biosynthesis
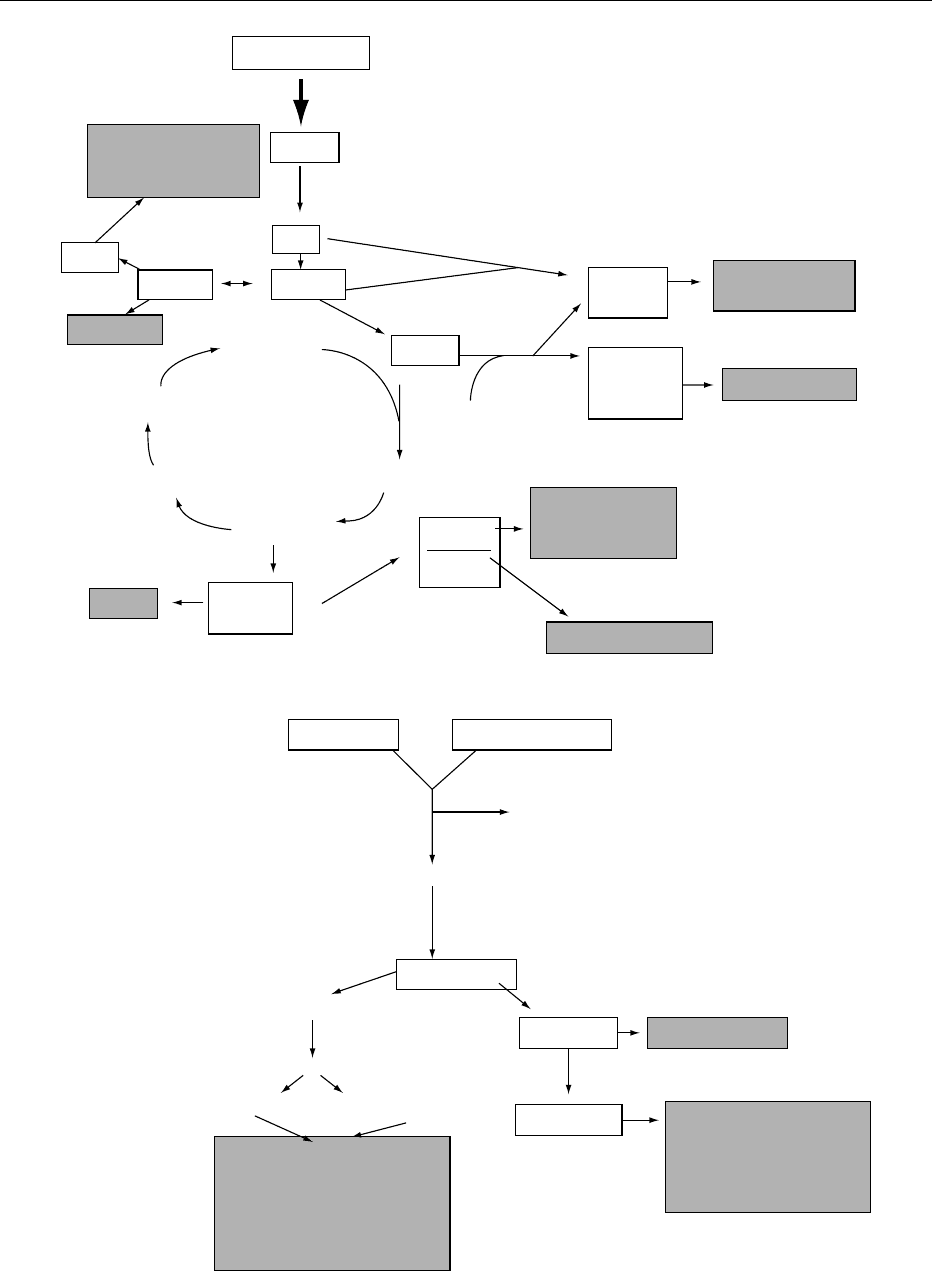
0010The skeleton of most alkaloids is derived from amino
acids (Figure 1, Table 2), although moieties from
other pathways, such as terpenoids, are often
combined. In addition, in a number of alkaloids
(e.g., steroid alkaloids), the nitrogen (deriving from
glutamine or other NH
2
sources) is added near the
end of a biosynthetic pathway, i.e., the alkaloid skel-
eton does not stem from amino acids. Biosynthetic
pathways have been worked out in detail for a few
alkaloids.
Accumulation and Storage
0011Although the exact site of alkaloid formation in a
plant cell has been elucidated for a few species only,
it is certainly correct to assume that most compounds
are synthesized in the cytoplasm. Membrane-enclosed
vesicles have been implied in the biosynthesis of ber-
berine. Not only is the chloroplast the site of photo-
synthesis and related processes, but it also harbors a
number of biosynthetic pathways, such as those for
fatty acids and amino acids. In addition, quinolizidine
alkaloids (QA) are synthesized in the chloroplast
stroma; thus, both the alkaloid and its amino acid
precursor, l-lysine, share the same compartment.
QA formation is regulated by light and displays a
diurnal rhythm. Light regulation seems to be trig-
gered by (1) lysine availability (it is also made during
the day), (2) the change in stromal hydrogen ion
concentration to pH 8 (enzymes of QA formation
have a pH optimum at pH 8), and (3) the reduction
of QA enzymes by thioredoxin (reduced thioredoxin
is generated under illumination).
0012Alkaloids are not formed in the extracellular space
or in the vacuole. The storage of high concentrations
of alkaloids is a prerequisite for their allelochemical
roles as defense compounds. Since these concentra-
tions would interfere with the normal metabolisms,
the allelochemicals are safely stored in the vacuole
(Table 3) of often specialized cells or tissues (such
as epidermis). The vacuole of these alkaloid-
accumulating cells has been termed the ‘toxic’ or
‘defense’ compartment. A number of plants produce
latex, which, in addition to its gluing properties
(think of insect mandibles!), often contains defense
chemicals, such as alkaloids (morphine and related
128 ALKALOIDS/Properties and Determination
Photosynthesis
Glucose
GAP
Pyruvate
Oxalacetate
Malate
Succinate
Oxoglutarate
Citrate
AcCoA
IPP
DMAPP
Fatty acids
Waxes
Polyketides
Aspartate
Lysine
Krebs Cycle
Glutamate
Glutamine
Ornithine
Arginine
Malonyl-CoA
Erythrose-4-P Phosphoenolpyruvate
Gallic acid
Shikimate
Chorismate
L-Tryptophan
Anthranilate
Prephenate
Arogenate
L-Tyrosine L-Phenylalanine
Tetrahydroisoquinoline alkaloids
Benzylisoquinoline alkaloids
Benzophenanthridine alkaloids
Morphinane alkaloids
Aporphine alkaloids
Protoberberine alkaloids
Indol alkaloids
Monoterpene indol alkaloids
β-carboline alkaloids
Ergot alkaloids
Quinoline alkaloids
Acridone alkaloids
Piperidine alkaloids
Quinolizidine alkaloids
Sedum alkaloids
Pyrimidines
Purines
Pyrrolizidine alkaloids
Tropane alkaloids
Coca alkaloids
Nicotiana alkaloids
Steroidal alkaloids
Diterpen alkaloids
Conium alkaloids
fig0001 Figure 1 Schematic overview of biosynthetic pathways leading to different groups of alkaloids.
ALKALOIDS/Properties and Determination 129
benzylisoquinoline alkaloids in Papaveraceae; proto-
berberine and benzophenanthridine alkaloids in Che-
lidonium; lobeline and other piperidine alkaloids in
Lobelia) and terpenoids (e.g., phorbole esters). The
alkaloids are selectively sequestered in small (diam-
eter < 1 mm) latex vesicles and reach local concentra-
tions of up to 1 M.
0013 Storage in the vacuole or in vesicles demands that
alkaloids pass the tonoplast and accumulate within
the vacuole against a concentration gradient. For the
passage across the tonoplast, three mechanisms are
plausible:
.
0014simple diffusion (takes place in case of lipophilic
alkaloids, e.g., nicotine, ajmalicine, vinblastine,
colchicine);
.
0015carrier-mediated transport (in case of polar and
charged alkaloids, which is the rule for most alka-
loids under physiological conditions, e.g., hyoscya-
mine, lupanine, reticuline, scoulerine, senecionine);
.
0016membrane fusion (in case of alkaloids that are
formed in vesicle-enclosed compartments, e.g.,
berberine).
Because alkaloids are sequestered against a concen-
tration gradient in the vacuole or in latex vesicles, the
driving force of uphill accumulation needs to be de-
termined. In some instances, vesicles or vacuoles con-
tain alkaloid binding or complexing compounds. For
example, latex vesicles of Chelidonium majus contain
between 500 and 1200 mM chelidonic acid, which
binds or complexes berberine and benzophenanthri-
dine alkaloids. It has been shown experimentally that
chelidonic acid acts as a trapping agent that causes
the apparent uphill transport resulting in alkaloid
concentrations in vesicles of 500–1200 mM. Protona-
tion, organic acids, and specific peptides may
constitute other trapping mechanisms. There is some
evidence that amino acids and some ions are trans-
ported into the vacuole with aid transporters that
are fueled by proton–substrate antiport mechanisms.
tbl0003 Table 3 Storage of alkaloids in the vacuole
Alkaloid Species
Leaf vacuoles
Lupanine Lupinus
Sparteine Cytisus, Lupinus
Hyoscyamine Atropa
Nicotine Nicotiana
S-Coulerine Fumaria
S-Reticuline Fumaria
Ajmalicine Catharanthus
Serpentine Catharanthus
Catharanthine Catharanthus
Betalaines Beta, Chenopodium
Senecionine-N-oxide Senecio
Capsaicin Capsicum
Latex vesicles
Sanguinarine Chelidonium
Berberine Chelidonium
Morphine and other morphinane
alkaloids
Papaver
tbl0002 Table 2 Examples for important alkaloids and their biosynthetic precursors
Amino acid Alkaloid Main occurrences Example structure
Lysine Quinolizidine alkaloids Fabaceae Lupanine, sparteine, cytisine
Lycopodium alkaloids Lycopodiaceae Lycopodine
Piperidine alkaloids Punicaceae, Crassulaceae Pelletierine, sedamine
Ornithine Tropane alkaloids Solanaceae, Erythroxylaceae Hyoscyamine, cocaine
Pyrrolizidine alkaloids Asteraceae, Boraginaceae, Fabaceae Senecionine, heliotrine
Nicotiana alkaloids Solanaceae Nicotine, anabasine
Tryptophan Monoterpene indol alkaloids Apocynaceae, Aslepiadaceae . . . Strychnine, vincamine,
Yohimbine, ajmalicine . . .
Simple indole alkaloids Fabaceae Physostigmine
Quinoline alkaloids Rubiaceae, Cornaceae Quinine, cinchonine,
camptothecine
Ergot alkaloids Claviceps, Convolvulaceae Ergotamine, lysergic acid
b-carboline alkaloids Loganiaceae, Zygophyllaceae Harman, harmaline
Phenylalanine/tyrosine Ephedra alkaloids Ephedraceae Ephedrine
Tetrahydro-isoquinoline alkaloids Rubiaceae Emetine
Benzylisoquinoline alkaloids Papaveraceae, Berberidaceae Papaverine
Benzophenanthridine alkaloids Papaveraceae Sanguinarine
Protoberberine alkaloids Berberidaceae, Papaveraceae Berberine
Morphinane alkaloids Papaveraceae Morphine, codeine
Aporphine alkaloids Monimiaceae Boldine
Phenylethyl-isoquinoline alkaloids Colchicaceae Colchicine
Aristolochia alkaloids Aristolochiaceae Aristolochic acid
Anthranilic acid Ruta alkaloids Rutaceae Skimmianine, dictamine
130 ALKALOIDS/Properties and Determination

Protons are enriched in the vacuole, which generally
has hydrogen ion concentrations of 0.001–1mM
(¼pH 6–3), by proton-translocating ATPases and
pyrophosphatases. In analogy, it has been assumed
(supported by experimental evidence) that carrier
transport of alkaloids is achieved by a proton–alkaloid
antiport mechanism or with the aid of ABC trans-
porters.
0017 Whereas a few alkaloids are formed ubiquitously in
all plant organs, organ- or even tissue-specific forma-
tion seems to be more common (Table 4). Since most
eukaryotic genes are regulated in a cell-, tissue-, and
organ-specific manner, genes of alkaloid formation
seem to be no exception. The corresponding promo-
tors are presumed to be regulated by respective regu-
latory proteins. This conclusion is important for the
interpretation of results obtained with cell suspension
cultures. Whereas alkaloid formation is active in
differentiated systems (root or shoot cultures), it is
usually reduced, or even absent, in undifferentiated
suspension cultured cells. It is likely that the corres-
ponding alkaloid genes are just ‘switched off.’ The
production of berberine and sanguinarine in cell cul-
tures seems to be more the exception than the rule.
0018 Alkaloids are stored predominantly in tissues that
are important for survival and reproduction, which
include actively growing young tissues, root and stem
bark, flowers (especially seeds), seedlings, and photo-
synthetically active tissues. Alkaloid contents in stor-
ing organs can be quite high, reaching up to 10% of
dry weight in some instances, which is important if
the alkaloids are to function as effective defense com-
pounds. In several herbaceous plants, alkaloids are
stored in epidermal and subepidermal tissues (e.g.
cocaine, colchicine, aconitine, steroidal alkaloids,
nicotine, veratrine, buxine, and coniine), which have
to ward off small enemies (insects, microorganisms)
in the first place. In lupins and broom, quinolizidine
alkaloid concentrations are up to 200 mM in epider-
mal tissue, whereas mesophyl tissue has values below
5 mM. Some plants possess typical alkaloid-storing
cells, called ‘idioblasts.’ These idioblasts have been
detected in Corydalis (for corydaline), Sanguinaria
(for sanguinarine), Ruta (for rutacridones), Cathar-
anthus (for indole alkaloids), and Macleaya (for pro-
topine). Short- and long-distance transport are often
required to reach these sites of accumulation.
0019Alkaloid patterns usually vary between the site of
synthesis and the sites of accumulation, since a
number of secondary substitutions may take place in
the latter tissues, or transport may be selective, dis-
tributing differing cocktails. In addition, alkaloid
profiles of seeds and seedlings often differ from
those of the mature plant. Both patterns and concen-
trations usually change during the development of
plants and the annual cycle. In general, alkaloid levels
are markedly reduced in senescing tissues, so that
shedded leaves are often nearly alkaloid-free. Alkal-
oid formation and storage may be influenced by
environmental stress, such as wounding or infection.
Transport and Turnover
0020A number of alkaloids are synthesized and stored in
all parts of plants, whereas others are restricted to a
particular organ. Alternatively, alkaloids are synthe-
sized but accumulated in many organs, which usually
demands long-distance transport. Theoretically, this
transport could procede sym- or apoplastically. How-
ever, utilization of established transport routes, such
as xylem or phloem, seems to be more common.
Because it is technically difficult to sample and ana-
lyze xylem and phloem sap, only a limited number
of appropriate data are available (Table 5). Besides
long-distance transport, short-distance and intra-
cellular transport need to be reviewed. In general,
alkaloids are synthesized in the cytosol or in mem-
brane-enclosed vesicles (endoplasmic reticulum,
mitochondria, chloroplasts), but are accumulated
and sequestered in the vacuole.
0021In general, alkaloids are not end products of
metabolism but can be degraded, which seems to be
tbl0004 Table 4 Examples for organ-specific biosynthesis of alkaloids
Alkaloid Organ Species
Tropane alkaloids Roots Atropa, Datura, Hyoscyamus, Mandragora
Nicotine Roots Nicotiana
Senecionine and other PA Roots Senecio
Emetine Roots Cephaelis
Sanguinarine Roots Sanguinaria
Betalaines Roots, shoots Beta
Quinine Stem bark Cinchona
Berberine Stem and root bark Berberis, Mahonia
Caffeine Green tissue Coffea
Quinolizidine alkaloids Leaves and other photosynthetic tissues Lupinus, Cytisus, Laburnum, Baptisia
Steroid alkaloids Roots, tubers, leaves Solanum
ALKALOIDS/Properties and Determination 131

plausible because nitrogen is a limited nutrient for
plants. As discussed previously, alkaloids stored in
seeds are partly degraded during germination and
seedling development, and their nitrogen is probably
used for the synthesis of amino acids. Degradative
pathways have not been worked out yet.
0022 In addition to this developmentally specific recyc-
ling, there is evidence that a number of alkaloids are
turned over all the time, with half-lives of between
2 and 48 h. Examples are nicotine, QAs, and tropane
alkaloids. Alkaloid turnover is often quite substantial
in cell cultures: Lupinus callus cultures are even able
to live on the QA sparteine as a sole nitrogen source
for more than 6 months.
0023 How can we explain this phenomenon? A number
of alkaloids are allelochemicals and affect molecular
targets such as receptors of neurotransmitters (tro-
panes, nicotine, etc.). For this interaction, a correct
stereochemical configuration is required. Because
alkaloids may oxidize or give rise to racemic mixtures
spontaneously, a continuous turnover would make
sure that a sufficient concentration of active com-
pounds is always available, similar to the situation
of protein turnover.
Functions
0024 Although several alkaloids and other secondary me-
tabolites have been used by mankind for thousands
of years as dyes (e.g., indigo, shikonine), flavors
(e.g., vanillin, capsaicin, mustard oils), fragrances
(e.g., rose oil, lavender oil, and other essential oils),
stimulants (e.g., caffeine, nicotine, ephedrine), hal-
lucinogens (e.g., morphine, cocaine, mescaline,
hyoscyamine, scopolamine, tetrahydrocannabinol),
insecticides (e.g., nicotine, piperine, pyrethrin), verte-
brate and human poisons (e.g., coniine, strychnine,
aconitine) and even therapeutic agents (e.g., atropine,
quinine, codeine, cardenolides, etc.), their putative
functions have been discussed controversially.
0025Alkaloid biology is tightly connected with the basic
physiology of plants. Many of the features described
before would make no sense if these compounds
did not have a vital function for the producer. As a
common theme, it has been observed that plants that
produce seeds rich in energy supplies (carbohydrates,
lipids, proteins) concomitantly accumulate potent
chemical defense compounds, often alkaloids, non-
protein amino acids, cyanogenic glycosides, gluco-
sinolates, protease inhibitors, lectins, or other
toxalbumins. Their presence in seeds can be mutually
exclusive, i.e., legume seeds store either alkaloids
(e.g., quinolizidines, pyrrolizidines) or nonprotein
amino acids, but not both at the same time. During
germination, the breakdown of nutrient reserves
is a general procedure and usually includes the
nitrogenous defense compounds. They serve a double
purpose, i.e., that of N-storage and protection.
They are thus degradable and toxic N-storage
compounds.
0026The main function is obviously that of chemical
defense against herbivores (insects, other arthropods,
and vertebrates), which can be deduced from the fact
that many alkaloid have a high affinity for receptors
of neurotransmitters that are present only in animals.
In some instances, alkaloids play a role (additionally)
in the antimicrobial defense (against bacteria, fungi,
viruses) and even in the interaction between plants
(allelopathy).
0027Alkaloids are certainly multipurpose compounds
that, depending on the situation, may be active
in more than one environmental interaction. For
example: QAs are certainly the most important de-
fense chemical in Fabaceae against insects and other
herbivores, but they also influence bacteria, fungi,
viruses, and even the germination of other plants. In
addition, they are employed as degradable N-trans-
port and N-storage compounds.
0028Alkaloids repel or deter the feeding of many animals
(many have a bitter or pungent taste to humans and
other vertebrates) or are toxic if ingested. In micro-
organisms and competing plants, a reduction of
growth and antibiosis are usually the visible effects
of alkaloid intoxication. How are these diverse effects
being achieved? Although most compounds have not
been studied in full detail, an impressive number of
cellular and molecular targets have been identified
that are selectively inhibited or modulated by
alkaloids. As a consequence of such interactions,
organ malfunctions (heart, lung, liver, kidney, CNS
disorders) result that may impair reproduction and
fertility in animals and other organisms, or simply kill
them.
0029Because many alkaloids have been shaped during
evolution by ‘molecular modeling,’ many of them are
tbl0005 Table 5 Long-distance transport of alkaloids by phloem or
xylem
Alkaloid Xylem Phloem Occurrence
Lupanine, sparteine X Lupinus, Cytisus
Cytisine X Laburnum, Petteria,
Genista, Spartium
Matrin X Sophora
Senecionine (N-oxide) X Senecio
Aconitine ? X Aconitum
Nicotine X Nicotiana
Hyoscyamine X Atropa
Scopolamine X Datura, Hyosycamus
Swainsonine X Astragalus
132 ALKALOIDS/Properties and Determination
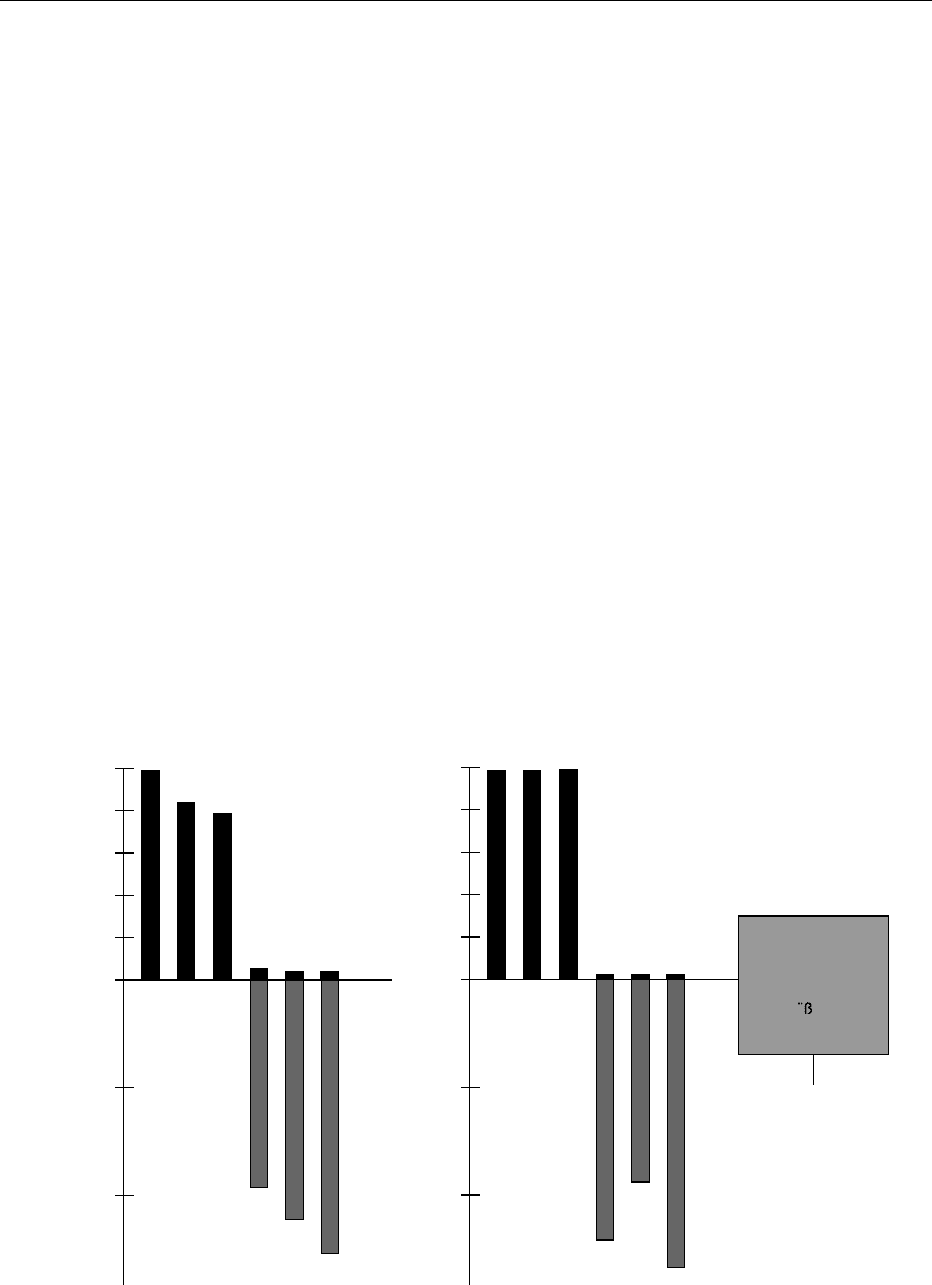
used by humans as medicinal compounds; allelo-
chemicals may have positive effects if used at non-
toxic concentrations.
Presence at the Right Concentration at
the Right Time and Place
0030 To be effective, alkaloids need to be present at the
right time, site, and concentration. Alkaloid metabol-
ism and biochemistry seem to have been optimized
and coordinated in most systems to fulfill this pre-
requisite. An interesting variation can be seen in some
plants, where alkaloid formation is enhanced by
wounding or microbial attack, i.e., in case of
emergency, the production of defense compounds is
stimulated.
0031 How effective are alkaloidal defenses? In lupins,
which normally produce high amounts of QAs,
mutants have been selected (so-called sweet lupins)
that accumulate only trace amounts of alkaloids.
When both alkaloid-rich and low-alkaloid lupins are
planted in the field without any fences and phytopro-
tectives, a dramatic effect can often be seen (Figure 2).
Whereas the low-alkaloid lupins were selectively
grazed or infested by herbivores, their alkaloid-rich
counterparts remained almost undisturbed. More
experimental data are certainly needed, but the
defensive role of alkaloids seems to be beyond doubt.
0032Similar to the situation in sweet lupins, in which
plant breeders have eliminated the alkaloid trait, also
in other crop plants, secondary metabolites, which
had evolved to serve as defense compounds, have
been bred away or strongly decreased. As a conse-
quence, many crop plants have lost their original
resistance to pests and herbivores. Man-made chem-
istry (i.e., synthetic pesticides) has to be used if these
crop plants have to be cultured.
Exceptions to the Rule – Adaptations of
Specialists
0033No defense is absolute. Whereas chemical defense
works against the majority of potential enemies, usu-
ally a few specialists have evolved during evolution
that have become specialized in the toxin-protected
ecological niche. This phenomenon can be clearly
seen in many insects, which are often highly host-
plant-specific. Some of these insects take up the
dietary alkaloids, store, and exploit them for their
own chemical defense or that of their offspring.
123456 123456
Alkaloid content (µg g
−1
FW)
Percentage completely eaten lupins
0
500
1000
20
40
60
80
100
Alkaloid content (µg g
−1
FW)
Percentage lupins with Agromyzidae
0
500
1000
20
40
60
80
100
Selective herbivory by rabbits
Selective herbivory by mining flies
1−4 = Lupinus albus
5 = L. mutabilis
6 = L. polyphyllus
1−3 = Su lupinen
Sweet lupins
fig0002 Figure 2 Selective advantage of alkaloids in lupins against herbivores (rabbits, mining flies). Lupins without alkaloids suffer heavily
from herbivory, whereas alkaloid-rich lupins are widely protected.
ALKALOIDS/Properties and Determination 133
