Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.

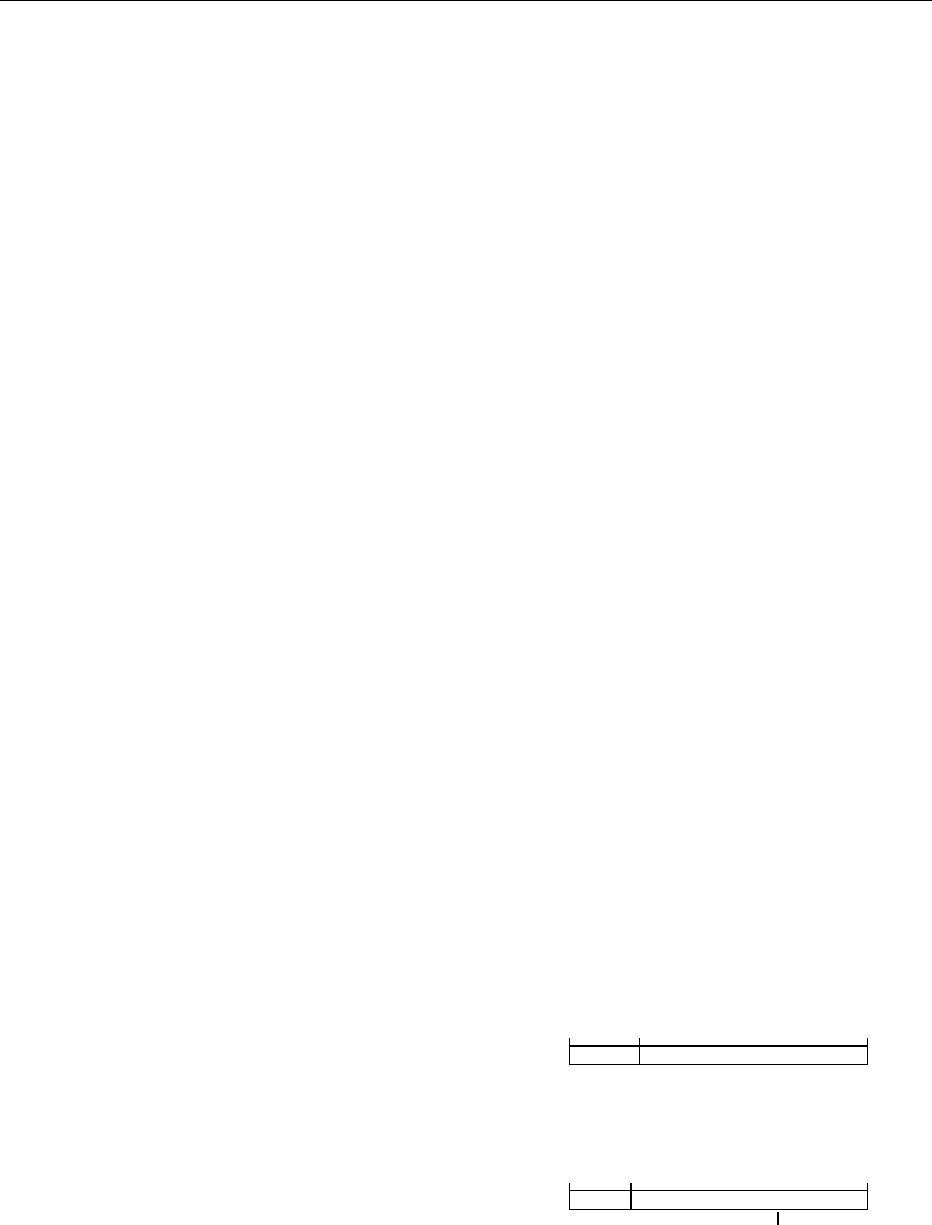
release of immune mediators, such as histamine,
which actually cause the symptoms associated with
allergic reactions. (See Food Intolerance: Food Aller-
gies for more information.)
0003 The sites on a protein that are recognized by an
antibody are known as epitopes and can be either
linear or conformational. In the former, only the pri-
mary sequence of a polypeptide is involved in anti-
body recognition, but in conformational epitopes, the
three-dimensional structure of a protein is important.
Thus, a number of segments of the polypeptide chain
that may be quite distant in the amino acid sequence
of a protein are brought together spatially as a conse-
quence of its tertiary and quaternary structure, to
form an epitope. Most epitopes are thought to be
conformational in nature and are particularly diffi-
cult to define in relation to food allergens where
processing can have such a disruptive effect on native
protein structure (see below).
Allergen Nomenclature
0004 The World Health Organization and the Inter-
national Union of Immunological Societies produce
an official list of allergens, which are designated by
the Allergen Nomenclature subcommittee. Allergens
included in this listing must induce IgE-mediated
(atopic) allergy in humans with a prevalence of IgE
reactivity above 5%. An allergen is termed major if it
is recognized by IgE from at least 50% of a cohort of
allergic individuals but does not carry any connota-
tion of allergenic potency; allergens are otherwise
termed ‘minor.’ The allergen designation is then
based on the Latin name of the species from which
it originates and is composed of the first three letters
of the genus, followed by the first letter of the species
finishing with an arabic number, e.g., Ara h 1 relates
to an allergen from Arachis hypogea (peanuts).
Types of Food Allergens
0005 There appear to be around eight foods, including
those of both plant and animal origin, that are re-
sponsible for causing the majority of food allergies,
namely peanuts and tree nuts, wheat, soya, cows’
milk, egg, shellfish, and fish. The geographical distri-
bution of these allergies reflects patterns of food con-
sumption with, for example, buckwheat allergy being
much more common in the Far East, where they are
staple foods, than is the case in Western countries.
The distribution of allergies also differs across
Europe, with reactions to fish and shellfish being
higher in Mediterranean countries, where fish con-
sumption is higher.
0006 In addition to foods that sensitize individuals dir-
ectly (probably via the gastrointestinal tract), there is
a group of fruit and vegetable allergies that arise
from the presence of cross-reactive epitopes in the
allergens. Thus, individuals who become sensitized
to pollen are more likely to develop IgE, which recog-
nizes homologous proteins present in fresh fruits and
vegetables. Around 70% of individuals who develop
birch pollen allergy with IgE towards the pollen pro-
tein Bet v 1, develop allergies to apples and other
fruits of the Rosacea because the anti-Bet v 1 IgE
reacts with fruit homologs such as the apple protein
Mal d 1. Similarly, many of those who develop allergy
to latex have IgE, which recognizes the chitin-binding
domain of the hevein polypeptides that make up the
rubber latex network. An almost identical domain
is found in a group of enzymes, the class I chitinases,
found in a number of fruits including avocado, chest-
nut, and banana, which the anti-latex IgE recognizes.
As a consequence of this cross-reactivity, individuals
with latex allergy frequently suffer from allergic
reactions on consuming these fruits (Figure 1.)
Protein Properties and Allergenicity
0007Two factors that are thought to contribute to the
allergenic potential of food proteins are their resist-
ance to the effects of thermal processing used in food
preparation (discussed below) and breakdown by
digestive enzymes in the gastrointestinal tract. The
stability of a number of allergenic proteins to pepsin,
including those from peanuts, soya, and cows’ milk,
has been compared with that of nonallergenic
proteins. It was found that all the allergens either
remained undigested or gave stable fragments that
persisted for 8–60 min (depending upon the allergen),
whereas the nonallergens were completely digested
after less than 15 s. As peptides are required to have
a molecular weight of greater than 3000 Da in order
to stimulate an immune response, large stable
The major latex allergen prohevein (Hev b 6.01)
Latex-fruit syndrome allergen class I Chitinases
1 43 187
Hevein Carboxy-terminal domain
1 43 328
Hevein
Catalytic domain
Hev b 6.02, chitin-binding domain
Chitin-binding domain
NPT
N-glycosylation site
fig0001Figure 1 Presence of conserved hevein domains in latex and
the class I chitinases of fruits responsible for many latex–fruit
cross-reactive allergies.
144 ALLERGENS

fragments, as well as intact proteins, have the poten-
tial to act as sensitizers. Similarly, such large frag-
ments may be able to cross-link mast cell IgE,
leading to histamine release. Whilst the bulk of food
proteins are broken down into immunologically in-
active fragments, very small proportions (milligram
amounts) of material, which are still immunologically
important, may escape digestion. Circumstantial evi-
dence that food proteins can resist digestion and cross
the gut barrier is offered by the presence of antibodies
to a wide range of food proteins in the circulation of
normal individuals. The presence of proteins, such
as ovalbumin in the blood of individuals after con-
sumption of egg, also supports the premise that intact
proteins, albeit in small quantities, can enter the body.
However, although the nature of the transported pro-
teins has not been clearly defined, a large proportion
of cow’s milk allergic patients have specific IgE, which
recognizes proteolyzed b-Lg, some of which reacts
better with the hydrolyzed protein than the intact
form. Thus, the resistance of allergens to the effects
of the digestive process plays an important role in
determining the allergenic potency of food proteins.
Allergens of Plant Origin
0008 Many plant food allergens are pathogen-related (PR)
proteins, which are produced by the plant as a defense
against pathogenic microorganisms, insects, and
invertebrate pests. They have been classified into a
number of families on the basis of sequence similar-
ities or enzymic properties and are receiving attention
from plant breeders, as a tool for GM crops, because
of their protective activities against agricultural pests.
However, they are cross-reactive allergens in fruits,
vegetables, and pollen. Allergens, other than PR
proteins, are inhibitors of proteases and a-amylases,
profilins (actin-binding proteins), seed-storage pro-
teins (albumins, globulins, and prolamins), proteases
and lectins (agglutinins). These are summarized in
Table 1.
Fruit and Vegetables
0009 Fruit allergy is increasing and is often associated with
birch- and grass pollinosis, as well as latex–fruit syn-
drome. Oral allergy syndrome is a manifestation of
food allergy, where certain foods cause itching or
hives when they touch the lips and mouth. Fruit and
vegetables are often responsible for this reaction,
owing to pollen cross-reactivity, and many fruit
allergens are PR proteins.
0010 Allergens homologous to the major birch pollen
allergen Bet v 1 (PR group 10), are found in apple
Mal d 1, cherry Pru av 1, apricot Pru ar 1, pear Pyr
c1, carrot Dau c 1, and celery Api g 1, but may trigger
responses to many other fruits and vegetables. By
contrast, in areas free of birch, the major allergens
in patients sensitized to Rosaceae fruits are lipid
transfer proteins (LTP, PR group 14), 9-kDa proteins
that often have antifungal and antibacterial activities.
LTP allergens characterized include apple Mal d 3 and
peach Pru p 3, but homologous proteins are found in
a range of plant-derived foods (vegetables, nuts, and
seeds), and as a consequence, LTPs have been de-
scribed as panallergens.
0011Class-I chitinases (PR group 3) are a group of car-
bohydrases active against the exoskeletons of insects
and the cell walls of fungi. They are widely distrib-
uted in plants and are also termed panallergens, with
characterized allergens including those from avocado
(Prs a 1) and banana (Mus p 1.2). Wound-induced
chitinases (Win) have been extracted from virus-
infected leaves (PR group 4) and have been reported
in turnip, tomato, and potato. Another group of
carbohydrases, the glucanases (PR group 2), are
active against fungal cell walls, have been isolated
from latex, and are recognized by IgE from food-
allergic patients with hypersensitivity to banana,
potato, and tomato. Lastly, thaumatin-like proteins
(PR group 5) are another group of allergens that have
antifungal and/or antibacterial activity, allergens
having been characterized in apple (Mal d 2)and
cherry (Pru a 2).
0012NonPR protein allergens include the profilins, ori-
ginally identified from birch pollen, Bet v2, which are
now recognized as cross-reactive plant allergens in a
wide range of fruits, vegetables, nuts, and seeds. Thiol
proteases (proteolytic enzymes) have also been iden-
tified as allergens in kiwi Act c 1, papaya, fig, pine-
apple, and soybean. Patatin, a storage protein from
potato, has also been shown to be an allergen in raw
potato and cross-react with the latex allergen, Hev v 7.
Tree Nut and Peanuts
0013Nut allergy is the most common form of food allergy
and the most common cause of death by anaphylaxis.
The major allergens are 2S albumins and the 7S and
11/12S globulins. In peanut, three of the major
allergens are major storage proteins: Ara h 1 is the
7S globulin, conarachin, a vicilin-like glycoprotein;
Ara h 2 is a 2S albumin; and Ara h 3, is a subunit of
the legumin-like 11S globulin, arachin. The IgE bind-
ing epitopes of Ara h 1 and 2 have been determined
and mutagenized to alter their activity. Brazil nut
contains a 2S storage protein allergen, Ber e 1;a
major allergen is a 2S albumin with a high methionine
content. Related 2S albumins are found in walnut,
Jug r 1, which also contains a 7S globulin allergen,
Jug r 2. In addition to storage protein allergens, chest-
nut contains an endochitinase allergen Cas s 1,and
ALLERGENS 145

tbl0001 Table 1 Major food allergens of plant origin
Food Allergen Allergen designation Molecular weight Functionand stability
Fruit
Apple Hom: Bet v 1 Mal d 1 17 700 Possible sterol binding protein (PR-10)
Hom: thaumatin Mal d 2 31 000 Antifungal activity (PR-5)
LTP Mal d 3 9 000 Antifungal and antimicrobial activity, heat-stable
(PR-14)
Apricot Hom: Bet v 1 Pru ar 1 17 700 Possible sterol binding protein (PR-10)
LTP Pru ar 3 9 000 Antifungal and antimicrobial activity, heat-stable
(PR-14)
Avocado Endochitinase Prs a 1 32 000 Degrade chitin (PR-3)
Banana Chitinase Mus p1.1 32 000 Degrade chitin (PR-3)
Chitinase Mus p1.2 34 000 Degrade chitin (PR-3)
Cherry Hom: Bet v 1 Pru av 1 17 700 Possible sterol binding protein (PR-10)
Hom: thaumatin Pru av 2 23 300 Antifungal activity (PR-5)
Profilin Pru av 4 15 000 Actin (cytoskeleton)-binding protein
Kiwi Cysteine protease Act c 1 30 000 Proteolytic enzyme
Peach LTP Pru p 3 9 000 Antifungal and antimicrobial activity, heat-stable
(PR-14)
Pear Hom: Bet v 1 Pyr c 1 18 000 Possible sterol binding protein (PR-10)
Profilin Pyr c 4 14 000 Actin (cytoskeleton) binding protein
Isoflavone reductase Pyr c 5 33 500 Enzyme involved in secondary metabolism
Plum LTP Pru d 3 9 000 Antifungal and antibacterial activity, heat-stable
(PR-14)
Vegetables
Carrot Hom: Bet v 1 Dau c 1 16 000 Possible sterol binding protein (PR-10)
Celery Hom: Bet v 1 Api g 1 16 000 Possible sterol binding protein (PR-10)
Profilin Api g 4 14 300 Actin (cytoskeleton)-binding protein
Unknown Api g 5 55/58 000 Unknown
Potato Patatin Sola t 1 43 000 Storage protein
Turnip Hom: prohevein Bra r 2 25 000 Defense protein
Nuts
Brazil nut 2S albumin Ber e 1 9 000 Storage protein
Cashew nut Anacardien Ana o 50,0000 Storage protein
Chestnut Chitinase Cas s 1 30 000 Degrade chitin (PR-3)
Hazelnut Hom: Bet v 1 Cor a 1.0401 17 000 Possible sterol binding protein (PR-10)
Peanut Cor Arachin Ara h 1 63 500 Storage protein
Conglutin Ara h 2 17 000 Storage protein
Arachin Ara h 3 60 000 Storage protein
Arachin Ara h 4 37 000 Storage protein
Profilin Ara h 5 15 000 Actin (cytoskeleton)-binding protein
2S albumin Ara h 6 15 000 Storage protein
2S albumin Ara h 7 15 000 Storage protein
Walnut 2S albumin Jug r 1 15 000 Storage protein
Vicilin Jug r 2 44 000 Storage protein
Seeds
Barley Inhibitor Hor v 1 15 000 Glycosylated a-amylase inhibitor
Inhibitor CMb 16 000 Glycosylated subunit of barley tetrameric
a-amylase inhibitor
Castor bean 2S albumin Ric c 1 10 000 Storage protein
Lentil Vicilin Len c 1 45-50,0000 Storage protein
Maize LTP Zea m 14 9 000 Antifungal and antibacterial activity (PR-14)
Mustard 2S albumin Bra j 1 14 000 Storage protein
2S albumin Sin a 1 14 000 Storage protein
Rice RAP 14 800 Rice allergenic protein
Rye Inhibitor Sec c 1 14 000 a-Amylase/trypsin inhibitor
Sesame 2S albumin Ses i 1 10 000 Storage protein
Vicilin Ses i 3 60,0000 Storage protein
Soybean Cys protease
(inactive)
Gly m 1 7 000 Function
Gly m 2 8 000 Unknown
Profilin Gly m 3 14 000 Actin (cytoskeleton)-binding protein
Wheat Inhibitor CM16* 16 000 Glycosylated subunit of wheat tetrameric
a-amylase inhibitor
Prolamin g-gliadin 30-50,0000 Storage protein of glutenin
146 ALLERGENS

peanuts a profilin, Ara h 5. Other nuts, such as
cashew, hazelnut, pecan, and pine nuts are known to
be allergenic, and contain storage globulin, 2S albu-
min and LTP allergens.
Seeds
0014 Seed allergens are predominantly nonPR-type pro-
teins, comprising storage proteins, proteases and a-
amylase inhibitors. Wheat and related cereals, barley
and rye, contain a range of allergens including the
prolamins (alcohol soluble storage proteins), which
are responsible for food-dependent exercise-induced
anaphylaxis and atopic dermatitis; the same proteins
are active in celiac disease and dermatitis herpetifor-
mis. Inhibitors of proteases and a-amylases protect
the seed from microorganisms and insect pests, char-
acterized allergens including the 12–15-kDa proteins
and inhibit a-amylases or trypsin and are both
inhalant allergens (e.g., baker’s asthma) and food
allergens. Those with the highest activity are glycosy-
lated subunits of tetrameric a-amylase inhibitors
from wheat (CM16) and its homologs from barley
(CMb) and BMAI-1 and rye Sec c 1, similar proteins
being found in rice. The major allergens in rice are a-
amylase and trypsin inhibitors, and in buckwheat, the
major allergen is another trypsin inhibitor. Peroxid-
ases in wheat and barley (PR group 9) of 36 kDa have
been identified as allergens in baker’s asthma.
0015 The major allergens in yellow mustard Sin a 1 and
oriental mustard Bra j 1, castor bean, cottonseed,
chickpea, oilseed rape BnIII, and sunflower SFA8
are 2S albumins (apart from sunflower, which con-
sists of a single polypeptide chain), and they consist
of two polypeptide chains linked via disulfide bonds.
In soybean, the two major storage globulins,
b-conglycinin and glycinin, are major allergens;
other allergens are profilin Gly m 3 and a papain-
related thiol protease Gly m Bd28.
Allergens of Animal Origin
Egg
0016 The allergens primarily originate from egg white, the
major allergens being ovomucoid (Gal d 1) and oval-
bumin (Gal d 2), which constitute 10 and 50% of
white proteins, respectively. Both proteins are heavily
glycosylated with 25% of the mass of ovomucoid
comprising carbohydrate. However, in ovomucoid,
the IgE epitopes are clustered in seven regions of the
protein sequence and do not encompass glycosylation
sites. Whilst IgE epitopes of ovomucoid appear to be
conformational in newly acquired sensitivities to egg,
linear epitopes are more important in long-standing
egg allergy. The IgE epitopes of ovalbumin are also
resistant to enzymic digestion and denaturation, and
seven IgE-binding regions have been identified, which
tend to cluster at the N- and C-terminal regions of
the protein. Ovalbumin has also been found in an
immunologically active form in the blood of human
subjects following consumption of raw egg. Two
minor allergens, ovotransferrin (Gal d 3) and lyso-
zyme (Gal d 4), have also been identified in egg white.
In general, cooking has been found to reduce the
allergenic activity of egg.
Cows’ Milk
0017Allergens are found in both the whey and casein
fractions, the most common allergens being b-lacto-
globulin and a-casein, although other IgE-reactive
proteins have been identified (Table 2). b-Lactoglo-
bulin is highly resistant to proteolysis and is taken up
in an intact form by the gut in experimental systems,
with the major IgE epitopes having been in four main
regions located on the more mobile surface loops
of the protein. The caseins also appear to contain
thermostable epitopes, with IgE binding being located
in around seven different regions of the protein.
Fish
0018The major allergen in fish is the muscle protein parv-
albumin (Gad c 1), which belongs to a group of such
proteins unique to fish and amphibians. Whilst it has
only been characterized in depth for cod and salmon,
parvalbumin is conserved across fish species, a factor
responsible for the cross-reactive nature of allergens
in cod, salmon, mackerel, herring, and plaice, amongst
many other fish species. It acts as a calcium buffer
protein in fast muscle, binding the Ca
2þ
ions in a
calcium-binding motif known as an EF-hand. Gad
c1has been shown to bind IgE in five regions evenly
distributed along the length of the protein, one of
which encompassed one of the Ca
2þ
-binding sites.
Like other calcium-binding proteins, Gad c 1 is
heat-stable, with the holo-form being both more
IgE-reactive and more heat-stable than the apo
form.
Shellfish and Seafood
0019Tropomyosin, a heat-stable muscle protein, is the
major allergen in shellfish and seafood, with highly
homologous proteins being found in the commonly
edible crustaceans. These homologies are responsible
for the cross-reactive allergies observed between vari-
ous types of seafood including shrimps, lobsters, crab,
squib, and abalone, and inhalant insect allergens,
such as those from cockroaches. Two main linear
IgE-binding sites have been identified in the shrimp
allergen, Pen i 1, one in the N-terminus, which
ALLERGENS 147

showed no homology with vertebrate tropomyosin,
and another in the C-terminal region. The first two
residues of the C-terminal epitope appear to be cru-
cial for IgE binding and are not found in vertebrate
tropomyosin. As a consequence of the lack of hom-
ology in the IgE epitopes, there is no cross-reactivity
between IgE from shell-fish allergic individuals and
animal muscle tropomyosins. In addition to being
found in cooked meat, the allergen also leaches into
cooking water.
Meat
0020 Meat allergy is rare, the major allergens having been
identified as serum albumins and IgG, both of which
are also minor milk allergens. However, unlike shell-
fish, muscle proteins such as tropomyosin do not
appear to be involved. Processing has been found to
reduce the allergenic activity of meat allergens, with
more severe processes, such as sterilization, causing
the greatest reduction.
Factors Altering the IgE reactivity of Food
Allergens
0021 Postharvest treatments and processing can potentially
alter the IgE reactivity of allergens by altering the
levels of allergens present in raw materials and
altering IgE-epitope presentation.
Effects of Raw Material and Postharvest Handling
0022A number of plant protein allergens belong to multi-
gene families, where a number of isoforms differing in
only a few amino acid residues may be present. In
addition, the levels of different isoforms may vary
between cultivars. These changes have been demon-
strated to make a difference particularly with regard
to the apple allergen Mal d 1, where the IgE reactivity
of certain cultivars is demonstrably higher than
others. Furthermore, one variant, Mal d 1a, has a
higher IgE-binding capacity than another variant
Mal d 1b, which differs by only 15 amino acid
residues. Allergen levels appear to increase in apples
following harvest and during storage at 4
C over 130
days, probably because of their association with the
ripening process, and the fact that they are ‘PR’ pro-
teins and hence synthesized in response to environ-
mental stress. Storage under modified atmospheres
can reduce these increases in allergen levels, as
might be anticipated from its ability to delay fruit
ripening.
Effects of Processing
0023There are several food proteins that do not adopt a
compact globular three-dimensional structure, but
are highly mobile, adopting an ensemble of conform-
ations. These include the g-gliadin from wheat and
caseins from milk. The IgE epitopes on such proteins
tbl0002 Table 2 Major food allergens of animal origin
Food Allergen Allergen designation Molecular weight Functionand stability
Hen’s egg Ovomucoid Gal d 1 28 000 Protein source with protease inhibitor
Ovalbumin Gal d 2 42 000 Serpin family member
Ovotransferrin Gal d 3 70 000 Iron-binding protein and bacteriocide
Lysozyme Gal d 4 14 300 Carbohydrase and bacteriocide
Cows’ milk b-Lactoglobulin Bos d 5 14 000 (monomer) Dimeric protein belonging to the lipocalin
superfamily that binds a number of small
lipophiles such as retinol
a-Lactalbumin Bos d 4 14 200 Calcium-binding protein
Serum albumin Bos d 6 66 000 Fatty-acid-binding protein
IgG Bos d 7 150 000 Immunoglobulin
a-Casein Bos d 8 32 400 Phosphoprotein able to chelate calcium
b-Casein Bos 26 600 Phosphoprotein able to chelate calcium
Fish Parvalbumin Gad c 1 (cod) 12 500 Calcium-binding muscle protein that is
pH- and heat-stableSal s 1 (salmon)
Shellfish Tropomyosin Pen a 1 (northern brown shrimp) 36 000 Contractile muscle protein that is heat-stable
Pan i 1 (Indian white shrimp) 38 000
Met e 1 (greasy black shrimp) 34 000
Pan s 1 (spiny lobster)
Hom a 1 (American lobster) 34 000
Cha f 1 (crab) 34 000
To d p 1 (squid) 34 000
Hal m1 (abalone) 38 000
49 000
Meat Serum albumin Bos d 6 (beef) 68 000 Fatty-acid-binding protein
Bos d 7 (beef) 150 000 Immunoglobulin
148 ALLERGENS
are likely to be linear and hence less affected by
thermal treatments than those on globular proteins.
In contrast, globular proteins undergo a marked de-
naturation at around 50–60
C, which is accompan-
ied by a loss of tertiary and secondary structure and is
usually followed by aggregation.
0024 Cross-reactive allergens, such as those involved in
the pollen–fruit and latex–fruit allergy syndromes,
are generally destroyed by cooking, and hence the
allergic reactions are confined to raw produce. In
addition, heating has been found to reduce the
allergenicity of beef and purified bovine allergens.
However, many other food allergens are remarkably
heat-stable, particularly those originating from plant
seeds, which are involved in sensitization via the
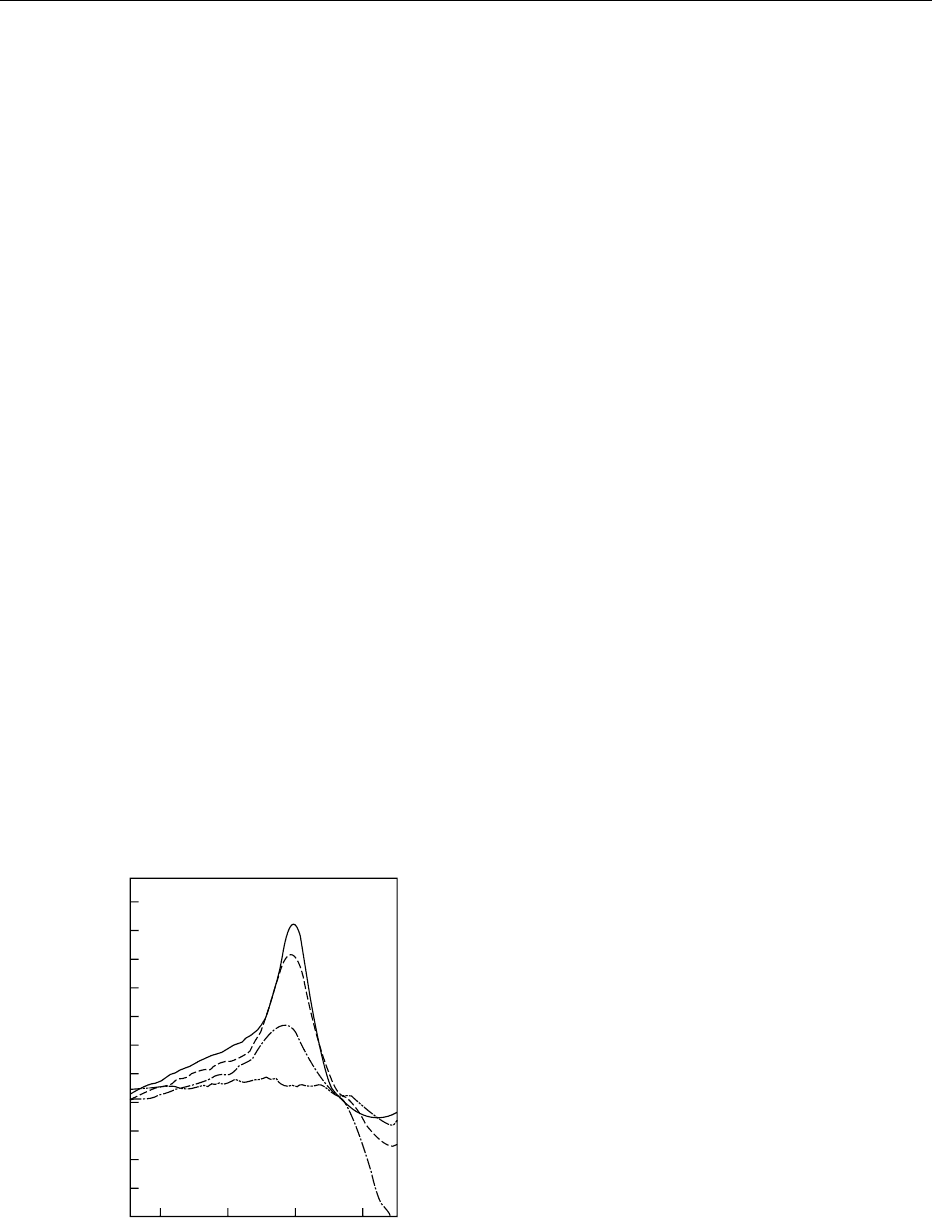
gastrointestinal tract. This is illustrated by Figure 2,
which shows the differential scanning calorimetry
(DSC) thermogram for glycinin, indicating that the
main thermal transition for this protein is around
90
C. There is also evidence that, whilst many of
highly disulfide bonded plant proteins unfold on
heating, they are able to refold on cooling to an
almost native structure. Hence, many allergenic
proteins may be able to retain both linear and con-
formational epitopes following processing, allowing
an IgE-mediated reaction towards the native and pro-
cessed protein. However, the unfolding and subse-
quent aggregation processes that occur in many food
proteins offer the possibility of introducing new epi-
tope sites. This applies to many allergens, including
those from soya, egg, and shellfish meat, which form
aggregates on heating that become incorporated into
heat-set gel networks. Thus, allergic IgE binding to
roasted nuts has been shown to be 90 times greater
than that observed towards raw peanuts, indicating
that processing does introduce additional IgE binding
sites. The technical difficulties of working with large
aggregated protein systems has meant that the char-
acterization of such processing-induced epitopes has
been difficult.
0025Carbohydrate residues on glycoproteins have also
been implicated as thermostable IgE epitopes, al-
though some workers have shown that their removal
does not affect IgE reactivity. Furthermore, because of
their sparse nature in some allergens, they do not
offer the polyvalency necessary IgE cross-linking on
mast cells and subsequent histamine release. Whilst
they have not been well characterized, there is now
evidence that Maillard browning adducts formed
following severe heat treatments contribute to the
allergenic activity of roasted nuts such as peanuts
and pecan nuts. It appears that Maillard modified
peanut allergens Ara h 1 and Ara h 2 become cross-
linked to form high-molecular-weight aggregates that
bind IgE more effectively than unmodified allergens
and are also more resistant to gastric digestion. The
role that other thermally induced modification, such
as lactosylation of milk proteins, may play in altering
the allergenic activity of products, including severely
heat-treated milks, has yet to be characterized in
detail.
Problems of ‘Hidden’ Allergens
0026Those individuals who suffer from severe forms of
food allergy, such as anaphylaxis, can have reactions
triggered by very small amounts (as little as 100 mg) of
an allergen present in a food. Indeed, traces of nuts
found in processed oils and carry-over of allergens on
utensils used for serving foods can be enough to cause
an allergic reaction. Proteins are widely used as func-
tional ingredients in foods, with soya flours being
routinely included in bread and bakery products as
an improver, whereas other soya ingredients are used
in soups and sauces as well as meat products such as
sausages. Similarly, caseinates and whey protein isol-
ates are used in a range of products such as sauces,
cakes, and confectionery.
0027Another difficulty is presented by the uncertainty
relating to accidental contamination, where process-
ing lines are shared between products utilizing differ-
ent ingredients. This is particularly problematic for
confectionery, where tree nuts and peanuts are a
favored ingredient and has led to labeling of products
with the warning ‘may contain nuts.’ Indeed, surveys
have shown that a large proportion of chocolate
confectionery is contaminated with hazelnut pro-
teins, probably as a result of cross-contamination in
−0.25
−0.20
−0.15
−0.10
−0.05
0.0
0.05
0.10
0.15
0.20
0.25
0.30
70.0 80.0 90.0 100.0
Temperature (⬚C)
Heat flow (mW)
fig0002 Figure 2 Thermostability of the soya allergen, glycinin, as
determined by DSC. Native protein (——); after treatment at
70
C, 10 min (–); 80
C, 10 min (---) and 90
C, 10 min (---).
ALLERGENS 149
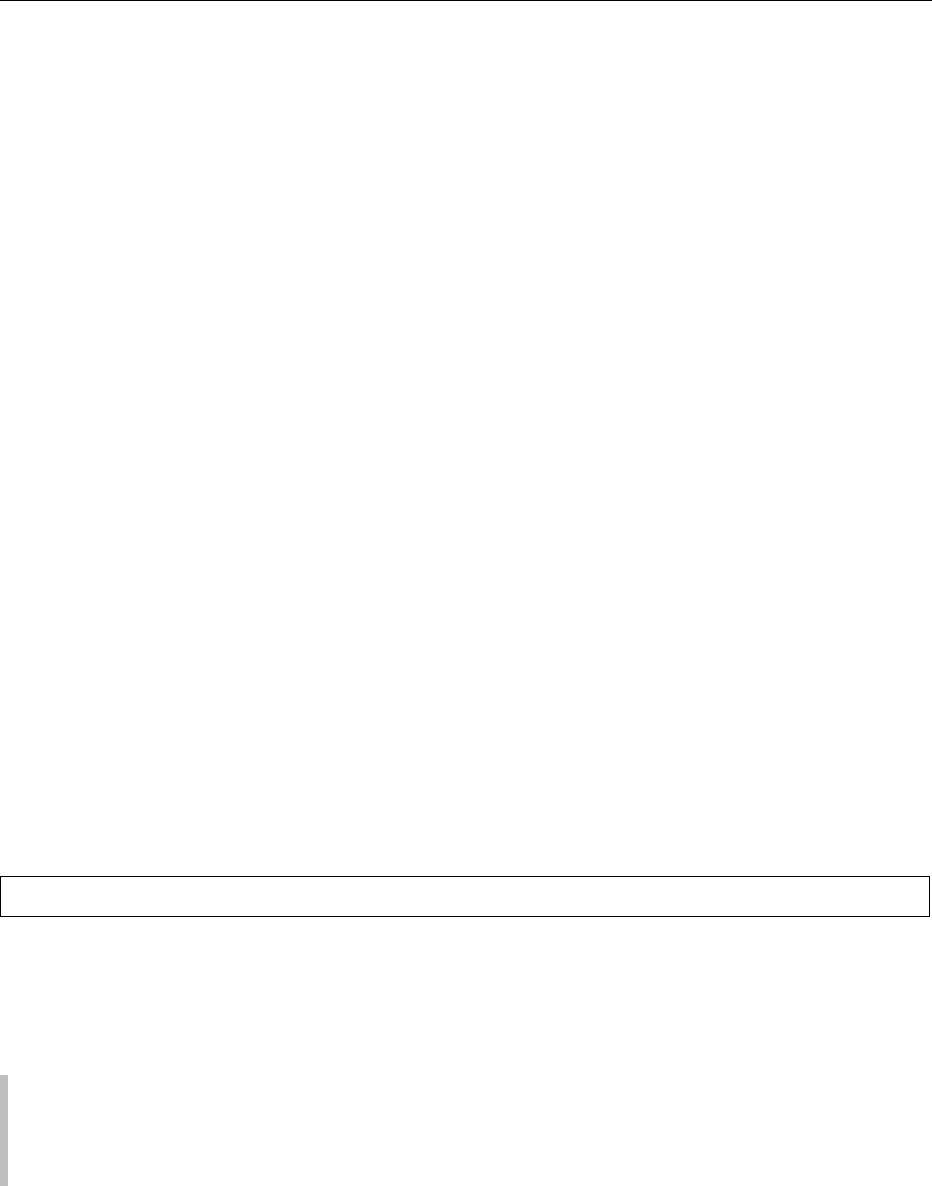
factories during manufacture. One of the conse-
quences of this for the allergic consumer is that aller-
genic proteins are hidden as ingredients in
manufactured products. Accidental exposure to aller-
gens is a frequent occurrence, and epidemiological
studies have shown that around 50% of peanut-aller-
gic individuals have a reaction at least once a year as a
consequence of accidental exposure.
0028 In order to address concerns over inadvertent
contamination of raw materials by allergenic nuts
and seeds, methodologies have been developed for
detecting allergens in raw materials and finished
products. The favored method of analysis is immuno-
assay, particularly the form known as enzyme-linked
immunosorbent assay (ELISA). In general, the
approach adopted has been to develop either a poly-
clonal or a monoclonal antibody preparation that is
specific for a major allergen present in a food stuff,
such as Ara h 1 from peanuts. Whilst it is possible to
develop sensitive calibration curves for allergens in
buffer, difficulties can be encountered when analyzing
foodstuffs, where the presence of fats and polyphenol
components found in products such as chocolate can
reduce assay sensitivity by 10-fold. Nevertheless, such
assays have been developed for a range of allergenic
proteins, including milk proteins, hazelnuts, and
peanuts, and are available for manufacturers, retail-
ers, and other analysts to detect allergen contamin-
ation. Another exciting new development is the
application of highly sensitive mass spectrometry
methods to allergen analysis, which has the potential
to offer a second orthogonal method of analysis
to immunoassay, as is desirable for purposes of
enforcing labeling regulations and hazard control
procedures.
See also: Food Intolerance: Types; Food Allergies; Milk
Allergy; Lactose Intolerance; Elimination Diets; Food
Labeling (Labelling): Applications; Immunoassays:
Principles; Radioimmunoassay and Enzyme
Immunoassay; Protein: Chemistry; Food Sources;
Determination and Characterization; Interactions and
Reactions Involved in Food Processing; Heat Treatment
for Food Proteins
Further Reading
Bousquet J, Bjorksten B, Brujijnzeel-Koomen CAFM et al.
(1998) Scientific criteria and the selection of allergenic
foods for product labelling. Allergy 53: 3–21.
Breiteneder H and Ebner C (2000) Molecular and bio-
chemical classification of plant-derived food allergens.
Journal of Allergy Clinical Immunology 106: 27–36.
Hefle SL (1999) Impact of processing on food allergens.
Advances in Experimental Medicine and Biology 459:
107–119.
Hefle SL, Nordlee JA and Taylor SL (1996) Allergenic
Foods. CRC Critical Reviews in Food Science and
Nutrition 36(S): S69–S89.
Lehrer SB, Horner WE and Reese G (1996) Why are some
proteins allergenic? Implications for biotechnology.
CRC Critical Reviews in Food Science and Nutrition
36(S): S553–S564.
Lessof M (ed.) (1997) Food Allergy Issues for the Food
Industry. Surrey: British Food Manufacturing Industries
Research Association.
Allergy See Food Intolerance: Types; Food Allergies; Milk Allergy; Lactose Intolerance; Elimination Diets
ALMONDS
D E Kester and A A Kader, University of California
Davis, Davis, CA, USA
S Cunningham, Blue Diamond Almond Growers,
Sacramento, CA, USA
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Introduction
0001 The almond is the major commercial tree nut crop
of the world. This importance has been achieved by
the very large increase in acreage and production in
California during the past 25 years. This article
reviews the areas of production, principal cultivars,
important uses, and methods of handling and storage.
The Crop and its Importance
Global Distribution
0002The cultivated sweet almond (Prunus dulcis Miller
(D. A. Webb) syn. P. amygdalus Batsch) originated
from within the wild species known originally as
150 ALMONDS

Amygdalus communis L. which grew on the lower
slopes of mountains in central Asia. About 30 related
almond species have been described occupying spe-
cific ecological niches in the arid steppes, mountains,
and deserts of central and south-western Asia and
southern Europe.
0003 The geographical range of the cultivated almond
corresponds to the three stages of cultural evolution:
(1) Asiatic (south-west and central Asia); (2) Mediter-
ranean (countries bordering both sides of the Medi-
terranean sea); and (3) Californian (central valleys of
California, parts of Australia, central Chile, and areas
of South Africa).
0004 Almonds are adapted to Mediterranean, steppe,
and desert climates characterized by mild, rainy
winters and hot, dry summers. Although they have
traditionally been grown with other arid tree and vine
crops, such as olive, pistachio, and grape, almond
trees respond so well to supplementary irrigation,
fertilizers, good soil, disease, and insect control and
other intensive culture methods that yields can be
increased five-to 10-fold over the traditional culture
practiced for centuries.
Commercial Importance
0005 Since 1950, almonds have become the most import-
ant world tree nut crop, with present annual
production of approximately 350–400 10
6
kg
(800–900 10
6
lb). In any given year, California
produces about 70% of the world’s supply of
almonds (Figure 1), with most of the balance
coming from Mediterranean countries (Spain, Italy,
Greece, Portugal, Morocco, Tunisia, Canary Islands,
France). Almonds are also grown, often in primitive
culture, as local crops in Iran, Israel, Afghanistan,
Pakistan, south-eastern Russia, north-west India,
Syria, Turkey, and Iraq.
Cultivars
0006 Older botanical literature described two botanical
varieties as Prunus amygdalus var. dulcis, the sweet
almond, and P. amygdalus var. amara, the bitter
almond. In reality, bitter and sweet kerneled trees
exist side by side in seedling populations with the
same parents; the difference is apparently due to a
single dominant gene. Some of the more primitive
almond-growing areas (e.g., in Asia and Morocco)
still grow almond trees as seedling populations (wild
or cultivated).
0007Modern orchards grow selected cultivars, which
are clones that are vegetatively propagated by bud-
ding or grafting upon specific rootstocks. Rootstocks
include almond and peach seedlings, as well as certain
plums and peach almond hybrids. Historically,
many cultivars were apparently identified when su-
perior individual trees were selected to replace ‘bitter’
trees within seedling orchards. In recent years, con-
trolled breeding has created cultivars with special
qualities. Use of genetic engineering methods will
augment genetic improvement.
0008Although many cultivars exist in the world, a
limited number have dominated industrial and local
use within specific production areas. Cultivars are
selected for performance in the orchard. Flowers on
most current cultivars are self-incompatible, so that
two or more cultivars that bloom at nearly the same
time need to be planted together, preferably in
adjoining rows. Hives of honeybees are placed within
the orchard to effect cross-pollination. Combinations
of cultivars are also chosen to facilitate an economical
harvest. Other cultivar characteristics of importance
include yield, tree habit, disease and insect resistance,
ease of harvest and processing, and other specific
characteristics. Nuts of different cultivars vary from
very hard-shelled to soft- and paper-shelled. Cultivars
are also chosen for their marketing potential, and
specific marketing classes have evolved.
0009California Nonpareil accounts for around 44% of
the production. Kernels are uniform in shape, rela-
tively flat, smooth, attractive in appearance, easy to
blanch, and are versatile in processing and utilization.
The nut has a thin, papery shell which is easy to
remove without damaging the kernel. This shell
is subject to worm and bird damage. The cultivar is
subject to a genetic variant referred to as noninfec-
tious bud failure which is controlled through selec-
tion and management of propagation sources.
0010Mission (Texas) is an old cultivar which produces
small, round, plump nuts. Kernels have a relatively
strong almond flavor owing to higher levels of amyg-
dalin. The pellicle is difficult to remove during
blanching. The shell is hard and protective of worm
damage. Padre is a similar cultivar, the kernels of
which can be combined with Mission. Peerless is
grown to a limited amount as a pollinator of
78
0
200
400
600
800
1000
1200
1400
79 80 81 82 83 84
California
Million pounds
All other
85 86 87 88 89 90 91 92 93 94 95 96 97 98 99
fig0001 Figure 1 Almonds produced in California in comparison to
world production.
ALMONDS 151

Nonpareil. The shell is hard and the nuts are sold
primarily in the shell.
0011 Ne Plus Ultra has a large, elongated kernel, blooms
early, and cross-pollinates Nonpareil. The cultivar is
gradually becoming obsolete in California, but orch-
ards still exist and it represents 3% of the total crop.
0012 In the mid-1960s, Merced and Thompson were
planted to replace Ne Plus Ultra, Peerless, and Mis-
sion. Similarly shaped and blanchable kernels of these
and later introduced cultivars have been mixed and
marketed under the name California. Other cultivars
of this group include Price, Fritz, Monterey, and
others.
0013 Carmel became popular in the mid-1970s and is
the second most important cultivar in new plantings
(18% of almond acreage in 1996). Sonora was intro-
duced in the mid-1970s and is becoming popular as
an early-blooming pollinator for Nonpareil. Sonora
has large, elongated nuts and is sometimes marketed
with Carmel.
0014 Additional cultivars that have recently been
planted in California include Butte, Livingston,
LeGrand, Mono, and Ruby. Their nuts are grouped
either as Mission or California marketing classes.
0015 Spain Marcona and Desmayo are the most import-
ant cultivars in Spain, each accounting for about 25%
of Spanish production. Marcona has large, heart-
shaped, flat, high-quality kernels which blanch easily.
Desmayo produces large, elongated, flat kernels, the
pellicle of which is thin and can be readily removed
after roasting by rubbing between the fingers. Many
other cultivars exist in specific production areas of
Spain but their kernels are usually mixed together for
marketing.
0016 Italy Most of the older areas (Puglia, Sicily, and
Sardinia) are reducing production. Kernels of the
many cultivars are mixed together for marketing.
The main cultivars in newer orchards include Tuono,
Felipo Ceo, and Genco, which are self-fertile.
0017 France New, late-blooming, high-yielding, vigorous
cultivars derived from breeding programs are being
planted not only in France but other countries of
the Mediterranean area. These cultivars include Fer-
ragnes and Ferraduel, kernels of which are marketed
together with those from other cultivars.
Morphology and Anatomy
Fruit
0018 The almond fruit is known botanically as a drupe,
which is composed of three basic parts: (1) exocarp
(skin), which in almond is pubescent; (2) mesocarp,
which is fleshy and becomes the hull; (3) endocarp
(shell). This overall structure is the same as stone
fruits, such as peaches, plums, and apricots, but
differs in that the almond mesocarp does not enlarge
into fruit, but dehisces (splits) at maturity and dries.
The hull is an important feed for livestock.
Seed
0019The almond kernel is the seed which develops from
the ovules. Although two ovules are present in the
flower, only one normally develops to produce a
single kernel. The seed is edible because it lacks amyg-
dalin, the bitter compound in many seeds. The seed
consists of the embryo (also referred to as the meat),
surrounded by seed coats or testae (also referred to as
the pellicle and sometimes the skin). The embryo
consists of the hypocotyl root axis with growing
points for roots and shoots which appear upon
germination, and two massive cotyledons, which are
storage organs and contain the high-energy com-
pounds characteristic of almond.
0020Almond kernels of different cultivars have charac-
teristic sizes, shapes, appearances, thickness of pellicle
and, to some extent, flavor. Double kernels result
when two kernels develop within the same nutshell.
Twin kernels result when two or more embryos
develop within the same pellicle.
Harvesting, Handling, and Storage
Harvesting
0021Almond nuts should be harvested as soon as possible
after maturation to avoid quality losses and to min-
imize problems with fungal attack and insect infest-
ation. Indices used to determine maturity stage and
optimum harvest dates for almonds include hull
dehiscence (splitting), separation of the hull from
the shell, decrease of the fruit removal force, and
drying of hulls and kernels. Almond harvesting in
California is by machines. Almonds are shaken from
the tree to the ground by various kinds of shakers.
After about a week of drying, the nuts are raked into
rows and picked up by mechanical harvesters. They
are then transported in bulk to hulling machines
which separate the hull from the in-shell product.
Postharvest Handling
0022Dehydration of the kernel begins while the nut is still
on the tree. Further drying takes place after the nuts
are knocked to the ground. Under rainy and/or cool
weather conditions, heated-air dehydrators may be
used to reduce moisture content to 7% or less. Most
almonds sold commercially range from 4% to 5% in
152 ALMONDS

moisture. Dried almonds are transported in bulk to
processing plants where they are stored in bins, silos,
or other bulk-storage containers for a few weeks to
several months before final processing and prepar-
ation for market.
0023 In-shell almonds are fumigated with methyl brom-
ide or aluminum or magnesium phosphide, which are
lethal to all insect life stages. Residues are monitored
to insure that levels in finished products are below
legal limits. (See Fumigants.)
0024 Alternatively, almonds can be kept in a controlled
atmosphere of 0.5% oxygen and about 10% carbon
dioxide (balance nitrogen) to control insects. The
nuts can be frozen for insect control at home, or for
small-scale operations. (See Controlled-atmosphere
Storage: Applications for Bulk Storage of Foodstuffs.)
Storage
0025 Compared to other nuts, almonds and almond prod-
ucts have a long life. This is attributable in part to
their low moisture, the presence of relatively low
levels of polyunsaturated fatty acids, and high toco-
pherol levels.
0026 Raw almonds should be stored at 0–5
C, and
65–70% relative humidity (RH), to minimize deteri-
oration during storage. Prolonged exposure to direct
sunlight causes the skins to darken and reduces shelf-
life. Since almonds readily absorb odors, they should
never be exposed to pungent odors from onions, fresh
fruits, fish, cheeses, paint, chemicals, or other
products.
0027 Both the maintenance of quality and safety of
almond and the extension of storage life depend
upon the initial moisture content, the RH and tem-
perature of storage, and the exclusion of oxygen
and insect pests. (See Storage Stability: Parameters
Affecting Storage Stability.)
0028 The Food and Drug Administration (FDA) regula-
tions for tree nuts define a safe moisture level (i.e.,
moisture content which will not support fungal
growth) as a water activity level that does not exceed
0.70 at 25
C. This is equal to a moisture content
of about 7%. The optimum range for unroasted
almonds is 4–6% moisture. The equilibrium moisture
of almonds ranges from 3.8 to 5.0% at 30% RH and
from 8.1 to 10% at 75% RH at 5
C.
0029 The relationship between moisture content and
equilibrium relative humidity (ERH) is temperature-
dependent. From 20% to 80% ERH (for any given
moisture content), ERH rises approximately 3% for
every rise in temperature of 10
C. At a given RH, air
will contain more water vapor at a high temperature
than at a low temperature. Temperatures between 0
C
and 5
C are recommended for almonds; the lower
temperatures allow longer storage life (up to a year).
0030Low oxygen (0.5% or lower) atmospheres are
beneficial for keeping flavor quality, delaying rancid-
ity, and controlling stored products against insects.
Exclusion of oxygen is usually achieved by vacuum
packaging or replacement with nitrogen in storage
facilities and transport vehicles. The storage life of
raw almonds can be extended up to 2 years under
low-oxygen atmosphere at 0
C. (See Chilled Storage:
Packaging Under Vacuum.)
Quality and Safety Factors
0031Important appearance factors for almonds marketed
in the shell include shell integrity, suture opening,
and shell color. Kernel defects include damage from
insects, mold and mechanical injury, gum, callus
growth, shriveling and doubling. Kernels are graded
for size. Quality criteria used in the grade standards
include freedom from dust particles and other foreign
materials, and uniformity of shape and color.
0032Textural factors, including crispness and firmness,
are influenced by moisture content. Thus, roasted
almonds are usually more crisp than raw almonds.
0033Flavor quality depends upon sweetness, oiliness,
intensity of almond flavor, and absence of off-flavors
resulting from rancidity, staleness, or other causes. A
problem called concealed damage, which appears as
internal darkening and poor flavor after roasting, is
related to wet conditions and high temperatures
during temporary storage following harvest.
0034Safety factors relate primarily to the potential con-
tamination of almonds with mycotoxins, especially
aflatoxin produced by the fungus Aspergillus flavus.
Contamination can occur in the orchard or during
postharvest handling if recommended handling and
storage procedures are not followed. Elimination of
nuts exhibiting any symptoms of fungal contamin-
ation is an integral part of market preparation. (See
Mycotoxins: Occurrence and Determination.)
0035Residues of pesticides used to control the major
insect pest, navel orange worm (Anyelois transitella
Walk), have been reduced by 40% during the last
10 years. This reduction was made possible through
such integrated pest management procedures as orch-
ard sanitation, maintaining natural predator balance,
and prompt harvest. (See Pesticides and Herbicides:
Residue Determination.)
0036Propylene oxide, the only sterilant approved for
nuts, is generally effective against bacteria, but less
effective against yeasts and molds. The nuts are
treated in a specially designed vacuum chamber.
After treatment, a series of air washes under vacuum
removes traces of the remaining gas. Each chamber
load then enters a postconditioning staging area until
cleared for both residue level and microbial load.
ALMONDS 153
