Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.


Others additionally transform the alkaloids into
pheromones or utilize them as morphogens. Well-
studied examples have been published for pyrroli-
zidine and QAs. Vertebrate herbivores (humans
included) have effective liver enzymes that can detox-
ify xenobiotics. Often, substances become hydroxy-
lated, conjugated, and then excreted via the feces or
the kidney and urine.
See also: Alkaloids: Toxicology; Chromatography: Thin-
layer Chromatography; High-performance Liquid
Chromatography; Gas Chromatography;
Immunoassays: Radioimmunoassay and Enzyme
Immunoassay
Further Reading
Bell EA and Charlwood BV (1980) Secondary plant
products. In: Encyclopedia of Plant Physiology, vol. 8.
Berlin: Springer.
Blum MS (1981) Chemical Defenses of Arthropods. New
York: Academic Press.
Conn EE (1981) Secondary plant products. In: Stumpf PK
and Conn EE (eds) The Biochemistry of Plants, vol. 7.
New York: Academic Press.
Harborne JB (1988) Introduction to Ecological Biochemis-
try, 3rd edn. London: Academic Press.
Hegnauer R (1962–1990) Chemotaxonomie der Pflanzen,
vols 1–10. Basel: Birkha
¨
user.
Luckner M (1990) Secondary Metabolism in Microorgan-
isms, Plants, and Animals, 3rd edn. Berlin: Springer.
Mothes K, Schu
¨
tte HR and Luckner M (1985) Biochemistry
of Alkaloids. Weinheim: Verlag Chemie.
Roberts MF and Wink M (1998) Alkaloids: Biochemistry,
Ecology and Medicinal Applications. New York:
Plenum.
Robinson T (1981) The Biochemistry of Alkaloids, 2nd
edn. Heidelberg: Springer.
Rosenthal GA and Berenbaum MR (1991) The chemical
participants. In: Herbivores – Their Interactions with
Secondary Plant Metabolites, vols 1 and 2. London:
Academic Press.
Rosenthal GA and Janzen DH (1979) Herbivores: Their
Interactions with Secondary Plant Metabolites. London:
Academic Press.
Rosenthal GA (1982) Plant Nonprotein Amino Acids and
Imino Acids. London: Academic Press.
Schultes RE and Hofmann A (1980) The Botany and Chem-
istry of Hallucinogens. Springfield, IL: Charles Thomas.
Southon IW and Buckingham J (1989) Dictionary of
Alkaloids. London: Chapman & Hall.
Wink M (1999) Biochemistry of plant secondary metabol-
ism. In: Annual Plant Reviews, vol. 2. Sheffield, UK:
Sheffield Academic Press and CRC Press.
Wink M (1999) Function of plant secondary metabolites
and their exploitation in biotechnology. In: Annual Plant
Reviews, vol. 3, Sheffield, UK: Sheffield Academic Press
and CRC Press.
Toxicology
M Wink, University of Heidelberg, Heidelberg,
Germany
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Background
0001Apparently, most alkaloids play an important role in
the ecology of plants or animals. They serve as defense
chemicals against herbivores and predators. To a lesser
degree they protect against bacteria, fungi, and viruses
or provide a means for plant–plant interactions. To be
effective defense chemicals, alkaloids must closely
interact with specific targets in herbivores, predators,
microorganisms, or competing plants, i.e., they must
either inhibit or otherwise deregulate important
processes that are vital for these organisms. For this
purpose the molecular shape of alkaloids has appar-
ently been optimized during a million years of evolu-
tion in a process which could be termed ‘evolutionary
molecular modeling.’
0002While the structures of more than 12 000 individ-
ual alkaloids have been reported, rather limited
knowledge is available for most of them in terms of
biological activities and functions. In this chapter the
modes of action of the better known alkaloids, espe-
cially those found in food plants, are summarized and
discussed, considering interactions with organs or
complete organisms first and then molecular targets.
These interactions are the base for understanding
the toxic or antinutritional effects that are observed
if humans or animals have ingested alkaloids with
their diet.
Toxic and Pharmacological Effects at the
Organ Level
0003Many alkaloids are known for their toxic or adverse
effects on animals (Table 1). In many cases, only the
toxicity of an alkaloid has been reported evidencing
substantial interactions, but the exact mode of action
has not yet been elucidated or is rather complex,
involving several molecular targets and organs.
0004In medicine, alkaloids are employed as local anes-
thetics, as narcotics, analgesics, as cardiac, uterine
and respiratory stimulants, or to raise blood pressure,
dilate pupils, and to relax skeletal muscles (Table 2).
The use of alkaloids as narcotics and hallucinogens
causes major social problems.
0005Ultimately, the toxic and pharmacological effects
(Tables 1 and 2) observed must be the result of inter-
actions of alkaloids with molecular targets present in
or on cells.
134 ALKALOIDS/Toxicology

Central Nervous System and Neuromuscular
Junctions
0006A remarkable number of alkaloids interfere with the
metabolism and activity of neurotransmitters in the
brain and nerve cells. A disturbance of metabolism or
binding of neurotransmitters and related signal path-
ways impairs learning and memory, sensory faculties
(smell, vision, or hearing), and coordination of bodily
functions, or produces euphoric or hallucinogenic
effects.
0007Muscle activity (skeletal, heart, etc.) is controlled
by acetylcholine (Ach) and norepinephrine (nor-
adrenaline). Any inhibition or overstimulation of
neurotransmitter-regulated ion channels will severely
influence muscular activity and thus the mobility or
organ function (such as heart, lungs, gut). When there
is inhibition, muscles will relax; when there is over-
stimulation, they will be tense or in tetanus, leading to
a general paralysis and/or respiratory failure (which
is the effect of many of the more toxic alkaloids).
Alkaloids which activate (so-called parasympathomi-
metics) or inhibit (parasympatholytics) neuromuscu-
lar action are tabulated in Table 3. These compounds
are usually considered to be strong poisons (Table 1).
Inhibition of the Digestive Process
0008Food uptake can be reduced by pungent or bitter taste
in the first instance. The next step can be the induc-
tion of vomiting, which is a common reaction to
the ingestion of a number of alkaloids; the alkaloid
emetine already implies this activity in its name!
Causing diarrhea, or the opposite, constipation,
would be another activity which negatively influences
the digestive system. Many intoxications with
alkaloid-containing plants have diarrhea as one of
the symptoms. Another way to interfere would be
the inhibition of digestive enzymes or of transport
proteins for amino acids, sugars, or lipids.
Modulation of Liver and Kidney Function
0009Nutrients and xenobiotics (such as secondary meta-
bolites) are transported to the liver after resorption in
tbl0001 Table 1 LD
50
values of some alkaloids
Alkaloid Test system LD
50
(mgkg
1
)
Alkaloids derived from tryptophan
Brucine Rat p.o. 1
Cinchonidine Rat i.p. 206
Cinchonine Rat i.p. 152
Ellipticine Mouse i.v. 1.2
Ergocryptine
a
Rabbit i.v. 1.1
Ergometrine
a
Mouse i.v. 0.15
Ergotamine
a
Mouse i.v. 62
Harman Mouse i.p. 50
Harmine Mouse i.v. 38
Physostigmine Mouse p.o. 4.5
Psilocybin Mouse i.v. 285
Quinidine Rat i.v. 30; p.o. 263
Quinine
a
Agelaius p.o. 100
Reserpine Agelaius p.o. 100
Strychnine Agelaius p.o. 6
Rat i.v. 0.9
Vinblastine Mouse i.v. 9.5
Vincamine Mouse i.v. 75
Vincristine Mouse i.p. 5.2
Alkaloids derived from phenylalanine/tyrosine
Aristolochic acid Mouse i.v. 38–70; p.o. 56–106
Berberine Mouse i.p. 23
Bulbocapnine Mouse p.o. 413
Canadine Mouse p.o. 940
Chelerythrine Mouse s.c. 95
Chelidonine Mouse i.v. 35
Codeine Mouse s.c. 300
Colchicine Mouse i.v. 4.1
Humans p.o. 0.1–0.3
Emetine Mouse s.c. 32
Galanthamine Mouse i.v. 8; p.o. 18.7
Morphine Mouse i.v. 226–318
Papaverine Mouse i.v. 27.5; s.c. 150
Protopine Mouse i.p. 36–102
Sanguinarine Mouse s.c. 102; i.v. 16
Thebaine Mouse i.p. 20
Tubocurarine Mouse p.o. 33.2
Steroid alkaloids
Jervine Mouse i.v. 9.3
Protoveratrine Rabbit i.p. <0.1
Samandarine Mouse i.p. <3.4
Solanine
a
Mouse i.p. 42
Veratridine Mouse i.p. 1.4
Tropane alkaloids
Atropine Rat p.o. 750
Cocaine Rat i.v. 17.5
Pyrrolizidine alkaloids
Echimidine
a
Rat i.p. 200
Heliotrine Rat i.p. 300
Jacobine Rat i.p. 138
Monocrotaline Rat i.p. 175, p.o. 71
Senecionine Rat i.p. 85
Seneciphylline Rat i.p. 77
Quinolizidine alkaloids
Cytisine Mouse i.v. 1.7
13-Hydroxylupanine
a
Mouse i.p. 172
Lupanine
a
Mouse i.p. 80
N-Methylcytisine Mouse i.v. 21; i.p. 51
Sparteine
a
Mouse i.p. 55–67; p.o. 350–510
Miscellaneous alkaloids
Aconitine Mouse i.v. 0.17; p.o. 1
a-Amanitin Mouse i.p. 0.1
Arecoline
a
Mouse s.c. 100
Caffeine
a
Mouse p.o. 127–137
Coniine Agelaius p.o. 56
Delphinine Rabbit i.p. 1.5–3.0
Maytansine Rat s.c. 0.48
Muscimol Rat p.o. 45
Nicotine
a
Agelaius p.o. 17.8
Mouse i.v. 0.3; p.o. 230
Tetrodotoxin
a
Mouse i.p. 0.01; s.c. 0.008
a
Encountered in food plants or food items.
i.p., intraperitoneal; i.v., intravenous; p.o., oral; s.c., subcutaneous.
ALKALOIDS/Toxicology 135

the intestine. In the liver the metabolism of carbo-
hydrates, amino acids, and lipids and the subsequent
synthesis of proteins and glycogen takes place. The
liver is also the main site for the detoxification of
xenobiotics. Lipophilic compounds, which are easily
resorbed from the diet, are often hydroxylated and
then conjugated with a polar, hydrophilic molecule,
such as glucuronic acid, sulfate, or an amino acid.
These conjugates are exported via the blood to the
kidney for elimination via the urine. Both organ
systems are affected by a variety of secondary metab-
olites: pyrrolizidine alkaloids are activated during the
detoxification process and are converted into potent
carcinogens, causing liver cancer. Many other meta-
bolic inhibitors, discussed below, are also liver toxins.
Many alkaloids are known for their diuretic activity.
Increased diuresis would also mean an increased elim-
ination of water and essential ions. Since Na
þ
ions are
already limited in plant food, long-term exposure
to diuresis-inducing compounds would reduce the
fitness of a herbivore substantially.
Disturbance of Reproduction
0010Quite a number of allelochemicals are known to
influence the reproductive system of animals, which
will ultimately reduce their numbers (and fitness as a
species). Antihormonal effects could be achieved by
mimicking the structure of sexual hormones, such as
coumarins which dimerize to dicoumarols, or isofla-
vones. The next target is the gestation process itself.
tbl0002 Table 2 Pharmacological and medicinal properties of alkaloids
Type Alkaloid Activity
Poison Pyrrolizidine alkaloids (from Senecio,
Heliotropium, Crotalaria)
Conversion to DNA and protein alkylating
agent in liver
Causing liver cirrhosis, mutations, cancer
Ergot alkaloids (from Claviceps purpureus) Cause vasoconstrictions, hallucinogenic
effects, gangrenic limps; disease named
ergotism
Aconitine (Aconitum) Local anesthetic, general paralytic effect
Analgesics Morphine (Papaver somniferum) Very effective painkiller (used since ancient
times); addictive properties
Codeine (Papaver) Pain and cough depression
Cocaine (Erythroxylon coca) Local anesthetic
Cardiac stimulants Quinidine (Cinchona spp.) Antiarrhythmic properties at heart auricle
Sparteine (Cytisus scoparius) Antiarrhythmic properties
Ajmaline (Rauwolfia serpentina) Antiarrhythmic properties at ventricle
Respiratory stimulant Nicotine (Nicotina), cytisine (Laburnum) Stimulation of respiration is followed by
respiratory depression, asphyxia or even
respiratory failure
Lobeline (Lobelia spp.) Stimulant; used in bronchial asthma
Coniine (Conium maculatum) Used as a potent poison in antiquity
(Socrates)
Constriction of blood vessels Ergot alkaloids (Claviceps purpurea) Used in obstetrics
Ephedrine (Ephedra spp.) Employed in the treatment of bronchial
asthma,
cold, sinusitis
Scopolamine (Hyoscyamus, Atropa, Datura) Dilatator of vessels
Muscle relaxant Tubocurarine (Chondodendron tomentosum) Block nAChR*; used in surgery
Hyoscyamine (atropine, Hyoscyamus, Atropa, Datura) Antispasmodic at smooth muscles
(gastrointestine, bladder)
Papaverine (Papaver somniferum) Smooth-muscle relaxant
Antiparasitic and antimicrobial
activity
Berberine (Berberis, Mahonia) Intercalates DNA and inhibits parasites and
microorganisms
Emetine (Cephaelis acuminata) Intestinal amoebiasis, emetic drug
Boldine (Peumus boldo) Anthelmintic activity
Quinine (Cinchona succirubra) Antimalarial
Antiinflammatory activity Colchicine (Colchicum autumnale) Treatment of acute gout, recurrent gout
Eye treatments Physostigmine (Physostigma venenosum) Reduces intraocular pressure (glaucoma)
Pilocarpine (Pilocarpus jaborandi) Miotic used in the treatment of open-angle
glaucoma
Cytostatic treatment Taxol (Ta x u s br e vif olia ) Treatment of breast and ovary carcinoma;
other malignancies
Vinblastine, vincristine (Catharanthus roseus) Treatment of lymphomas and other tumors
*nAChR, nicotinic acetylcholine receptor.
136 ALKALOIDS/Toxicology

As outlined below, a number of alkaloids are muta-
genic and lead to malformation of the offspring or
directly to the death of the embryo. The last step
would be premature abortion of the embryo. This
dramatic activity has been reported for a number of
allelochemicals, including many mono- and sesquiter-
penes and alkaloids. Some alkaloids achieve this by
the induction of uterine contraction, as do the ergot
and lupin alkaloids.
Molecular Targets of Alkaloids
0011In the following a number of important cellular
molecular targets (Figure 1) have been addressed
tbl0003 Table 3 Examples of alkaloids which bind to neurotransmitter receptors and neurotransmitter-degrading enzymes
Target Ligand Alkaloid Occurrence
Acetylcholine receptors
Nicotinic receptor Acetylcholine Nicotine Nicotiana, Duboisia
C-toxiferine Strychnos
Tubocurarine Chondodendron
Coniine Conium
Cytisine and other QA Several legumes
Lobeline Lobelia
Anabasine Anabasis, Nicotiana
Muscarinic receptor Acetylcholine Hyoscyamine (atropine) Atropa, Hyoscyamus,
Datura, Mandragora
Scopolamine Several Solanaceae
Arecoline Areca
Pilocarpine Pilocarpus
Muscarine Amanita, Inocybe, Clitocybe,
other fungi
Sparteine and other QA Several legumes
Adrenergic receptors Norepinephrine(noradrenaline)/
(adrenaline) epinephrine
Ergot alkaloids Claviceps
Yohimbine Pausinystalia, Aspidosperma
Rauwolscine Rauwolfia
Corynanthine Rauwolfia
Norlaudanosoline Papaveraceae
Ephedrine, norephedrine Ephedra
Serotonin receptor Serotonin Ergot alkaloids Claviceps
Psilocin, psilocybine Psilocybe, other fungi
N,N-dimethyltryptamine Several plants and toads
Bufotenine Virola, Anadenanthera
b-carboline alkaloids Banisteriopsis, Peganum
Mescaline Lophophora, other cacti
Dopamine receptor Dopamine Ergot alkaloids Claviceps
Bulbocapnine Corydalis
GABA receptor GABA Bicuculline Dicentra cucullaria and other
Corydalis species
Muscimol Amanita
b-carboline alkaloids Peganum, Banisteriopsis
Adenosine receptor Adenosine Caffeine Coffea, Camellia, Ilex, Paullinia
Theophylline, theobromine Theobroma
Glycine receptor Glycine Brucine Strychnos
Strychnine Strychnos
Opioid receptor Endorphins Morphine Papaver somniferum
Acetylcholine esterase Acetylcholine Physostigmine (eserine) Physostigma venenosum
Berberine Several Papaveraceae
Coptisine Several Papaveraceae
Galanthamine Several Amaryllidaceae
Solanine and other
steroid alkaloids Solanum
Huperzine A Huperzia serrata
Monoamine oxidase (MAO) Norepinephrine, dopamine,
serotonin, histamine
Harmaline, harmine Peganum
Salsolinol Chenopodiaceae
Ephedrine Ephedra
Catechol-O-methyltransferase Norepinephrine, epinephrine,
dopamine
Tetrahydroisoquinoline Papaveraceae
QA, quinolizidine analogs; GABA, g-aminobutyric acid.
ALKALOIDS/Toxicology 137
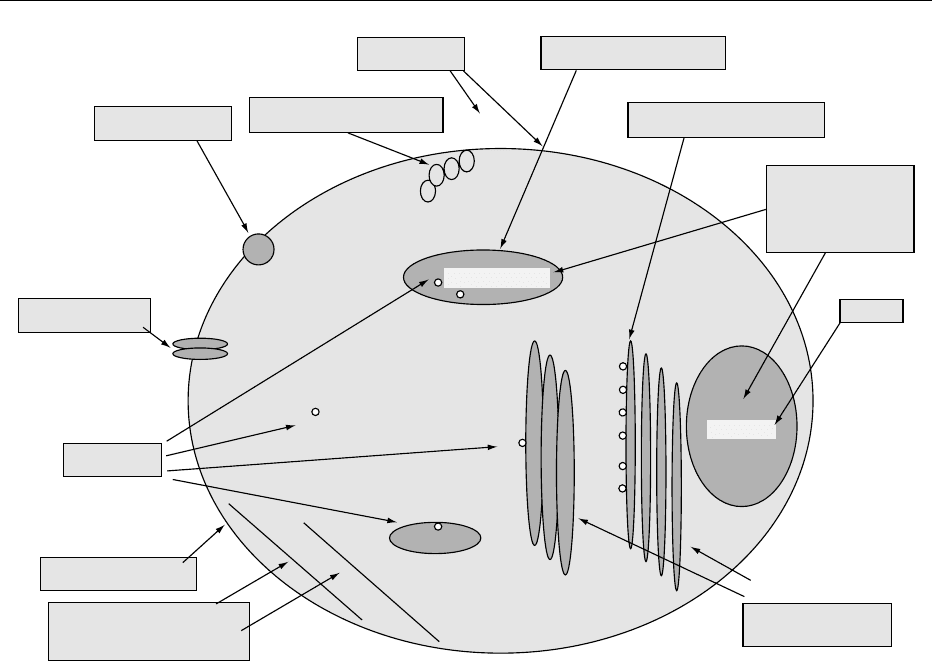
which are often affected by alkaloids and other plant
toxins.
Biomembranes, membrane transport, and neuronal
signal transduction
0012 Cells can only operate effectively if their biomem-
branes (cytoplasmic membrane, internal membranes)
are intact. Biomembranes are almost impermeable for
ions and polar molecules. As an exchange of these
molecules must take place between cells and organs,
specific membrane proteins, which can be ion chan-
nels, pores, or carrier proteins, mediate the controlled
flux of these compounds across biomembranes. The
biomembranes and the complex transport systems are
targets of many natural products.
0013 Steroidal alkaloids, such as solanine and tomatine,
which are present in many members of the Solanaceae
(including potatoes and tomatoes), can form com-
plexes with the cholesterol present in biomembranes.
While the steroidal moiety ‘dives’ into the lipophilic
interior of the membrane and interacts with the struc-
turally similar cholesterol, the hydrophilic side chain
remains outside and binds to external sugar receptors.
Since phospholipids are in a continuous motion, a
tension easily builds up which leads to membrane
disruption; transient ‘holes’ occur in the biomem-
brane, rendering the cell leaky. A similar mechanism
has been postulated for saponins, a widely distributed
group of natural products, to which the steroidal
alkaloids may be assigned. Steroidal alkaloids can
also interact with other targets, such as neurorecep-
tors or even with DNA; malformations have been
observed in animal embryos after having been
exposed to Solanum alkaloids.
0014Communication between cells is especially import-
ant for nerve cells. Signal transduction in the central
nervous system and in neuromuscular junctions is
mediated by receptor proteins residing in the mem-
brane which are directly or indirectly coupled with
ion channels. The neurotransmitters involved in-
clude, among others, norepinephrine (noradrenaline),
epinephrine (adrenaline), serotonin, dopamine, hista-
mine, glycine, g-aminobutyric acid (GABA), glutam-
ate, and acetylcholine (ACh).
0015Neuroreceptors can be ligand-gated channels, i.e.,
a receptor which is part of an ion-channel complex.
When the neurotransmitter binds, a conformational
change induces the opening of a Na
þ
/K
þ
channel for
Receptors
Transporters
Signal transduction
Electron transport
Protein biosynthesis
DNA replication
DNA transcription
DNA repair
RNA
Posttranslational
protein modification
Mitochondrion
Ion channels
Enzymes
Cell membrane
Cytoskeleton
− actin, microtubules
Lysosome
Nucleus
Y
Y
Y
fig0001 Figure 1 Molecular targets of animal cells that are affected by alkaloids.
138 ALKALOIDS/Toxicology
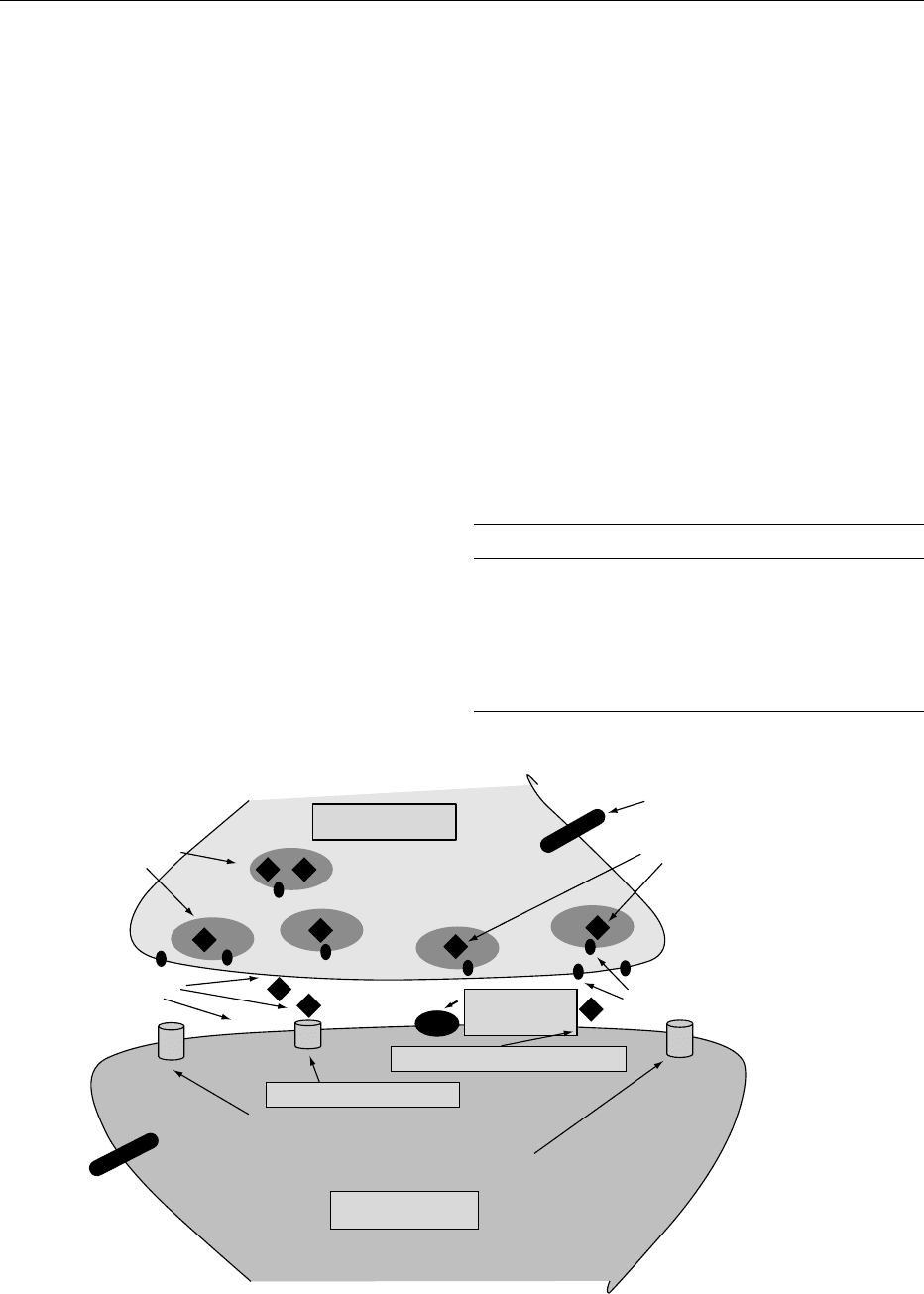
microseconds, allowing Na
þ
ions (the external con-
centration is about 145 mmol l
1
) to enter the cell
following a concentration gradient (the internal Na
þ
concentration is between 5 and 15 mmol l
1
). The
ligand quickly dissociates from the receptor and, in
the case of ACh, is hydrolyzed by ACh esterase
(Figure 2). Glutamate (N-methyl-d-aspartate,
NMDA) and GABA receptors are also ligand-gated
ion channels.
0016 More abundant are G-protein-coupled neuro-
receptors. A prominent one is the muscarinic ACh re-
ceptor; norepinephrine, serotonin, and dopamine
receptors also belong to this type. When ACh binds,
the receptor changes its conformation, inducing a
conformational change in an adjacent G-protein mol-
ecule. Its a-subunit dissociates and then activates the
enzyme adenylyl cyclase, which in turn produces
cyclic adenosine monophosphate (cAMP) from
adenosine triphosphate (ATP). The cAMP molecule,
a second messenger, activates protein kinases or Ca
2þ
channels directly.
0017 Quite a number of alkaloids are known whose
structures are more or less similar to those of
endogenous neurotransmitters. They can function
therefore as structural analogs. In addition, several
plants produce compounds which are identical to
animal neurotransmitters, such as ACh and histamine
in stinging hairs of Urtica, or serotonin and dopamine
in several species. Targets can be:
.
0018the receptor itself through inhibition or overstimu-
lation (Table 3)
.
0019the enzymes which deactivate neurotransmitters
after they have bound to a receptor (Table 3)
.
0020transport processes, which are important for the
uptake of neurotransmitters into the presynapse
or their storage in synaptic vesicles (Table 4)or
.
0021enzymes involved in the biosynthesis of a neuro-
transmitter.
The stimulation of neurotransmitter-activated ion
channels leads to a rapid influx of Na
þ
ions, which
in turn activates voltage-gated Na
þ
and K
þ
channels,
which are essential for further signal transduction.
These Na
þ
and K
þ
channels constitute another
important target for alkaloids (Table 5).
PRESYNAPSE
Acetylcholine
esterase
Vesicle
Neuroreceptor
Na
+
channel
K
+
channel
POSTSYNAPSE
Ligand-gated ion channel
G-Protein-linked neuroreceptor
Neurotransmitter
transporter
Ca
2+
channel
Neurotransmitter
Y
Y
Y
Y
fig0002 Figure 2 Signal transduction in excitable synapses.
tbl0004Table 4 Alkaloids as inhibitors of neurotransmitter uptake
(transport into presynapse or into vesicles)
Transporter Alkaloid Occurrence
Norepinephrine Reserpine Rauwolfia
(noradrenaline) Ephedrine Ephedra
Biogenic amines Tetrahydro-b-carboline Peganum
Salsolinol Salsola
Tetrahydroisoquinoline Papaveraceae
Tetrahydropalmatine Berberidaceae
Dopamine Cocaine Erythroxylum
ALKALOIDS/Toxicology 139
0022 Cells carefully control ion concentrations inside
and outside of the cells with the help of specific ion
channels (e.g., Na
þ
,K
þ
,Ca
2þ
, and Cl
channels) and
of active Na
þ
,K
þ
or Ca
2þ
pumps, such as Na
þ
,
K
þ
-ATPase and Ca
2þ
-ATPase. Ion gradients and ion
fluxes mediated by these channels and pumps are
the main elements in the active transport processes,
in neuronal and neuromuscular signaling. Cardiac
glycosides are potent and well-known inhibitors of
Na
þ
,K
þ
-ATPase found in plants, some insects,
and in the skin of certain toads. A few alkaloids
such as harmaline, nitidine, sanguinarine, capsaicine,
cassaine, and solenopsine (from ants) inhibit Na
þ
,
K
þ
-ATPase.
0023 Whereas receptor/ion channel interactions repre-
sent the initial part of many signal pathways, key
enzymes which produce or inactivate second messen-
gers or amplify the signal can be important targets
further down the pathway (Table 6). These enzymes
include:
.
0024adenylyl cyclase (making cAMP),
.
0025phosphodiesterase (inactivating cAMP),
.
0026phospholipase (releasing arachidonic acid or
inositol phosphates) or
.
0027several protein kinases, such as protein kinase C
(which is activated by phorbol esters and the alkal-
oid chelerythrine) or tyrosine kinase (activating
other regulatory proteins or ion channels)
Because these targets are almost exclusively found
in animals but absent in plants, the development of
active compounds directed to these targets appears to
be advantageous for the plants producing them. They
can store these compounds without risk of being
intoxicated by their own toxins.
DNA/RNA
0028The genetic information of most organisms is mainly
encoded in DNA. Since the integrity of DNA is
important for the structure and function of rRNAs,
proteins and enzymes which are important for metab-
olism, structure and development of an organism,
DNA is a highly vulnerable target. It is not surprising
that a number of secondary metabolites became
selected during evolution which interact with DNA
or DNA-processing enzymes. Some alkaloids are
known to bind or to intercalate with DNA (Table 7).
Many of these molecules are planar, hydrophobic
molecules which fit between the planar stacks of AT
and GC base pairs. Other alkaloids act on the level of
DNA- and RNA-polymerases and DNA topoisomer-
ases, thus impairing the process of replication and
transcription.
0029The effects of DNA-binding or intercalating com-
pounds can be mutations, which may result in mal-
formations of newborn animals or in the initiation of
cancer. When anabasine, coniine, or anagyrine is ad-
ministered to pregnant cows or sheep, a large propor-
tion of the offspring develop malformations of the
tbl0006 Table 6 Alkaloids modulating enzymes involved in signal transduction
Enzyme Function Alkaloid Occurrence
Adenylyl cyclase cAMP formation Annonaine Annonaceae
b-carboline-1-propionic acid Leguminosae
Isoboldine Peumus
Tetrahydroberberine Berberidaceae
Phosphodiesterase cAMP inactivation Papaverine Papaver
Caffeine, theobromine Coffea,
Camellia,Theobroma
Theophylline Ilex paraguarensis,
Paulinia
1-ethyl-b-carboline Peganum
Protein kinases Protein phosphorylation Chelerythrine Chelidonium majus
Lyngbyatoxin A Marine seaweeds
cAMP, cyclic adenosine monophosphate.
tbl0005 Table 5 Alkaloids as modulators of Na
þ
,K
þ
, and Ca
2þ
channels
Alkaloid Occurence (genera) Action
Na
þ
and K
þ
channels
Aconitine
a
Aconitum Activation
Ajmaline
a
Rauwolfia Inhibition
Batrachotoxin
a
Frogs (Dendrobatidae) Activation
Harmalin Peganum Inhibition
Protoveratrine A, B
a
Veratrum Activation
Quinidine
a
Cinchona Inhibition
Quinine Cinchona Inhibition
Saxitoxin
a
Protogonyaulax (algae) Inhibition
Sparteine
a
Cytisus, Lupinus, Genista Inhibition
Tetrodotoxin
a
Algae/fish Inhibition
Veratridine
a
Veratrum Activation
Ca
2þ
channels
Ryanodine Ryania speciosa Inhibition
a
Na
þ
channel.
140 ALKALOIDS/Toxicology

legs – so-called ‘crooked calf disease.’ Some alkaloids
of the monocot Veratrum, such as jervine and cyclo-
pamine cause the formation of a large central eye, the
cyclopean eye, which was probably known to the
ancient Greeks and thus led to the mythical figure
of the cyclops.
0030 Other alkaloids are known as carcinogens, such as
aristolochic acid from Aristolochia and pyrrolizidine
alkaloids (PA) which are produced by approximately
3% of the higher plants, especially within the families
of Asteraceae and Boraginaceae. Aristolochic acid has
a nitro group which can be transformed into reactive
intermediates in the intestine. If resorbed, these me-
tabolites can alkylate DNA. Pyrrolizidine alkaloids
are not carcinogenic in their native form, but become
so when they are ‘detoxified’ in the liver: PA are
usually present in the plant as their N-oxides, which
are polar compounds that cannot pass biomembranes
by simple diffusion. In the intestine, PA-N-oxides are
reduced by gut bacteria. The free base is then readily
taken up by the gut cells and transported to the liver.
There, the PA are transformed into alkylating com-
pounds, which covalently bind to DNA and proteins.
As a result mutations and cancer can be initiated.
Protein Biosynthesis
0031 Protein biosynthesis is essential for all cells and
thus provides another important target. Indeed, a
number of alkaloids have been detected which
inhibit protein biosynthesis in vitro. Emetine from
Cephaelis ipecacuanha (Rubiaceae) is the most
potent plant constituent. Other alkaloids with the
same ability include harringtonine, homoharring-
tonine, cryptopleurine, tubulosine, hemanthamine,
lycorine, narciclasine, pretazettine, pseudolycorine,
tylocrepine, and tylopherine. Several alkaloids which
inhibit protein biosynthesis and are also DNA
intercalating substances can induce apoptosis in
cells.
Electron Chains and Other Enzyme Activities
0032The respiratory chain and ATP synthesis in mito-
chondria or photophosphorylation in chloroplasts
demand the controlled flux of electrons. These targets
seem to be attacked by nicotine, sanguinarine, ellipti-
cine, gramine, alpinigenine, capsaicine, and a few
other alkaloids. A multitude of enzymes exist in
animal cells and several alkaloids have been reported
that interfere with at least one of them.
0033A recently discovered group of alkaloids are the
polyhydroxyalkaloids, such as swainsonine or casta-
nospermine, which inhibit hydrolytic enzymes, such
as glucosidase, galactosidase, trehalase (trehalose
is a sugar found in some beetle cocoons and fungi
which is hydrolyzed by trehalase) and mannosidase
selectively.
tbl0007 Table 7 Alkaloids interacting with DNA/RNA and related enzymes
Target Activity Alkaloid Occurrence
DNA Photoaddition Dictamnine Dictamnus
Harman Peganum
Harmine Peganum
Alkylation Pyrrolizidine alkaloids Several Asteraceae, Boraginaceae
Aristolochic acid Aristolochia
Cycasin Cycads
Intercalation Ellipticine Ochrosia
Quinine, quinidine Cinchona
Skimmianine Skimmia
Berberine Berberis, Mahonia,Thalictrum, Chelidonium
Coptisine Several Papaveraceae
Fagaronine Rutaceae
Sanguinarine Several Papaveraceae
Olivacine Aspidosperma
Ergotamine Claviceps purpurea
Harmaline, harmin Peganum harmala
Emetine Cephaelis acuminata
DNA polymerase Inhibition Fagaronine Rutaceae
Hippeastrine Hippeastrum
Lycorine Several Amaryllidaceae
DNA topoisomerase I Inhibition Camptothecin Camptotheca acuminata
Reverse transcriptase Inhibition Berberine Several Berberidaceae, Papaveraceae
Chelidonine Chelidonium
RNA polymerase Inhibition Vincristine, vinblastine Catharanthus roseus
Transcription Inhibition Colchicine Colchicum, Gloriosa
amanitin Amanita
ALKALOIDS/Toxicology 141

Cytoskeleton
0034 Microtubules, which are important for cellular move-
ments, vesicle transport in neurons, or the separation
of chromosomes during cell division, are composed
of tubulin subunits. Movements and some transport
processes are mediated through either the rapid as-
sembly or disassembly of microtubules. The assembly
of microtubules is inhibited by colchicine, and
dimeric indole alkaloids vinblastine and vincristine
(important for chemotherapy of certain cancers).
These alkaloids thus interrupt cell division. The diter-
pene alkaloid taxol (used in the treatment of ovarian
and breast cancer) affects microtubules in the oppos-
ite way; the polymerization of tubulin is enhanced by
taxol. As a consequence taxol-induced microtubules
are very stable and dividing cells are arrested in the
metaphase.
0035 Cell stability, phagocytosis, cell–cell interactions,
and cell movements are also controlled by actin fila-
ments, which are rapidly assembled or disassembled
from action monomers. Cytochalasin B and latruncu-
lin B bind to the plus end of a growing actin filament,
preventing the addition of actin monomers there.
Another alkaloid, phalloidin, produced by the fatally
poisonous toadstool Amanita phalloides, stabilizes
actin filaments and inhibits their depolymerization.
Mechanisms of Allelochemical Activities
in Antiviral, Antimicrobial, and Phytotoxic
Interactions
0036 Circumstantial evidence indicates that some alkaloids
protect the producing plant against viruses, bacteria,
fungi, and competing plants. A number of antimicro-
bial alkaloids such as sanguinarine, quinine, or ber-
berine intercalate with viral and microbial DNA or
bind to it. These compounds may thus inhibit pro-
cesses such as DNA replication and RNA transcrip-
tion which are vital for the microorganisms. Protein
biosynthesis in ribosomes is another vulnerable target,
attacked by emetine. The stability of biomembranes
can be disturbed by steroidal alkaloids and tetran-
dine. Other targets may be electron chains or just
metabolically important enzymes. Phytotoxic proper-
ties or germination inhibition, which can be observed
in plant–plant interactions, can also proceed via the
above-mentioned mechanisms. But interactions with
growth hormones and their metabolism must also
be considered.
Target specificity of alkaloids
0037 In general, the interactions of a particular alkaloid
with a molecular target (as described above) suggest a
high degree of specificity. A closer look, however,
shows that many alkaloids interfere with more than
one target. The phenomenon will be explained for
two groups of alkaloids: ergot alkaloids and quinoli-
zide alkaloids (QA).
Ergot Alkaloids
0038Ergot alkaloids, such as ergotamine, ergometrine, or
ergoclavine, are produced by fungi of the genus Cla-
viceps which lives in close contact with many grasses
(family Poaceae) such as the cereal Hordeum vulgare.
These alkaloids can modulate several receptors of
neurotransmitters, such as dopamine, serotonin, and
norepinephrine. As a consequence. the pharmaco-
logical action of ergot alkaloids is rather broad,
ranging from vasoconstriction and uterus contraction
to hallucinations. We can explain these activities
through structure similarities between the alkaloid
and the different neurotransmitters.
Quinolizidine Alkaloids
0039QA, such as lupanine, sparteine, or cytisine, are pro-
duced by lupins and many members of the Legumi-
nosae. They are bitter for many animals (and plants
producing them are therefore avoided as food). If
ingested, QA exhibit a broad level of toxicity: they
interact with ACh receptors (AChR) as agonists. QA,
like many other alkaloids, occur as complex mixtures
in plants. Some QA preferentially bind to the nico-
tinic AChR, whereas others tend more to bind to the
muscarinic AChR. Some QA exhibit a prominent
cross-reactivity. Additionally, QA such as lupanine
and sparteine inhibit Na
þ
and K
þ
channels, thus
blocking the signal transduction in nerve cells at a
second critical point. A few particular QA, such as
anagyrine, cytisine, and the bipiperidine alkaloid
ammodendrine (which cooccurs with QA in many
plants), are mutagenic and lead to malformations
(see above).
0040If we accept the hypothesis that alkaloids were
developed as chemical defense compounds through
a process of ‘evolutionary molecular modeling’ the
‘cross-reactivity’ described makes sense: any com-
pound which can interfere with more than one target
or with more than one group of adverse organisms
is likely to be more effective and thus has a better
survival value in general than a more selective allelo-
chemical. In addition, herbivores will try to develop
tolerance to or resistance against the dietary toxins.
If more than one target is affected by a defense
chemical the chances of a herbivore developing
specific resistances concomitantly are much smaller
than in single-target situations. In conclusion, we
can say that Nature has obviously tried ‘to catch
as many flies with one clap as possible’ in the
selection of alkaloids during evolution. (See Trypsin
142 ALKALOIDS/Toxicology

Inhibitors; Saponins; Antibiotics and Drugs: Uses in
Food Production; Alkaloids: Properties and Deter-
mination.)
See also: Alkaloids: Properties and Determination;
Antibiotics and Drugs: Uses in Food Production;
Cereals: Dietary Importance; Coffee: Analysis of Coffee
Products; Lupin; Potatoes and Related Crops: The Root
Crop and its Uses; Plant Antinutritional Factors:
Characteristics; Saponins; Tea: Chemistry; Tomatoes;
Trypsin Inhibitors
Further Reading
Alberts B, Bray D, Lewis J, Raff M, Roberts K and Watson
JD (1993) Molecular Biology of the Cell, 3rd edn. New
York: Garland.
Harborne JB (1993) Introduction to Ecological Biochemis-
try, 4th edn. London: Academic Press.
Mann J (1992) Murder, Magic and Medicine. London:
Oxford University Press.
Roberts MF and Wink M (eds) (1998) Alkaloids: Biochem-
istry, Ecology and Medicinal Applications. New York:
Plenum.
Rosenthal GA and Berenbaum MR (1991) Herbivores:
Their Interactions with Secondary Plant Metabolites,
vol. 1. The Chemical Participants. San Diego: Academic
Press.
Rosenthal GA and Berenbaum MR (1992) Herbivores:
Their Interactions with Secondary Plant Metabolites,
vol. 2. Ecological and Evolutionary Processes. San
Diego: Academic Press.
Wink M (1998) Modes of action of alkaloids. In: Roberts
MF and Wink M (eds) Alkaloids, Biochemistry, Ecology,
and Medical Applications, pp. 301–326. New York:
Plenum.
Wink M (1999) Biochemistry of Plant Secondary Metabol-
ism. Annual Plant Reviews, vol. 2. Sheffield: Sheffield
Academic Press and CRC Press.
Wink M (1999) Function of Plant Secondary Metabolites
and their Exploitation in Biotechnology. Annual Plant
Reviews, vol. 3. Sheffield: Sheffield Academic Press and
CRC Press.
Wink M (1993) Allelochemical properties or the raison
d’e
ˆ
tre of alkaloids. In: Cordell GA (ed.) The Alkaloids.
vol. 43, pp. 1–118. San Diego: Academic Press.
Wink M (2000) Interference of alkaloids with neuro-
receptors and ion channels. In: Atta-Ur-Rahman (ed.)
Bioactive Natural Products, vol. 11, pp. 3–129. Amster-
dam: Elsevier.
Wink M and Schimmer O (1999) Modes of action of defen-
sive secondary metabolites. In: Wink M (ed.) Function
of Plant Secondary Metabolites and their Exploitation
in Biotechnology. Annual Plant Reviews, vol. 3, pp.
17–133. Sheffield: Sheffield Academic Press and CRC
Press.
ALLERGENS
E N C Mills, Institute of Food Research, Norwich, UK
A S Tatham, University of Bristol, Bristol, UK
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Introduction
0001 This encyclopedia entry describes the nature of food
allergens involved in IgE-mediated allergy, their no-
menclature, and the properties of proteins thought to
predispose them to becoming allergenic. Current
knowledge of food allergens of plant and animal
origin is summarized, and the role that cross-reacting
IgE epitopes play in the relationship between pollen
and latex allergy and allergies to certain fruits and
vegetables is discussed. The impact of postharvest
treatments and food processing on allergen activity
is described, together with the problems posed to
food allergic individuals by ‘hidden’ allergenic ingre-
dients and those that result from cross-contamination
in the factory and catering outlets.
What is an Allergen?
0002During the course of normal immune functioning, the
body produces a number of different forms, or iso-
types, of immunoglobulins such as IgA, IgG, IgM,
and IgE, which bind to ‘nonself’ molecules. These
include molecules found in microbial pathogens,
parasites, environmental agents such as pollen and
dietary proteins. However, in the allergic disease clas-
sified as a Type I hypersensitivity reaction, this anti-
body repertoire is altered, and the body synthesizes
larger quantities of IgE, an antibody type normally
produced only in response to parasitic infections. As
yet, we do not understand the mechanisms whereby
particular allergens elicit an IgE rather than the
normal IgG response in certain individuals. This IgE
is directed towards target molecules, which are usu-
ally proteinaceous in nature and are known as aller-
gens. IgE can become associated with mast cells, and
on binding multivalent allergen, it becomes ‘cross-
linked’ at the mast cell membrane, triggering the
ALLERGENS 143
