Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.

was positively correlated to the percentage protein
in the flour, whereas lipid and cell wall material was
negatively correlated. Starch and protein separating
efficiencies generally increase with increasing pro-
tein content. An unpublished rule of thumb has
been applied to evaluating the potential of legume
seed for air classification. If the flour is finely milled
and a 33% fines fraction yield is sought, the protein
content of that fraction is apt to be 2–2.5 times the
initial protein value of the flour. To insure uniform
products by air classification technology, it is sug-
gested that, in any particular legume being processed,
the seed falls within a narrow range of protein
content.
0012 Shifting of other components also occurs as a result
of air classification. In terms of fiber material present
in the flour, the hull fiber tends to segregate into the
coarse fraction and the cell wall fiber into the fine
fraction. Dehulling may enhance the efficiency of
the process somewhat and may be a prerequisite if
colored hulls are present. If the coarse (starch) frac-
tion is of lesser importance, dehulling need not be
practiced as it only has a small effect on the crude
fiber content of the protein fraction.
0013 Legume flours containing a-galactosides, especially
raffinose, manninotriose, stachyose, and verbascose,
generally show the protein fraction to be 40–90%
higher in these components than the initial flour,
while the starch fractions become depleted. Legumes
with trypsin inhibitors, hemagglutinating factors,
saponins, phytic acid, vicine, and convicine will
exhibit elevated concentrations of these components
in the protein fractions.
0014 The protein fraction from legumes also tends to be
enriched in ash and lipid, the latter having undesir-
able ramifications in storage of the concentrate. The
lipids likely occur as a result of the presence of a lipid-
rich coating on the protein bodies.
0015Protein concentrates prepared by air classification
may be used as emulsifiers or supplements in meat
products and patties, beverages, bread, noodles, and
spaghetti. Cowpea flour fractionated into enriched
protein and starch concentrates has been applied to
produce emulsifiers and starch jellies. There may be a
requirement to inactivate some of the antinutritional
factors. The starch fractions are increasingly being
utilized in the paper industry, especially carbonless
paper. Some use of pea starch was made in ore refin-
ing, but this has been sporadic. Nevertheless, peas
have shown the greatest potential for commercial
application of air-classification technology. There
are few major nutritional or technological problems
except for the ‘pea flavor’ and, in some cases, a darker
green color in some end products. The main obstacles
to success in classification stem back to basic agro-
nomics, the guarantee of a year-round supply at a
competitive price and of high quality, and establish-
ment of markets for the products.
Application to Cereal Grains
Wheat Flour
0016The earliest work in application of air classification
techniques to food products began in several United
States labs (Peoria, Illinois and Manhattan, Kansas)
with protein-shifting in wheat flours. Classification
has generally been less efficient than in legume flours
because of the high proportion of small starch gran-
ules that cannot be readily separated from the fine
protein particles. Approximately 81% of the granules
fall below 7.5 mm, the quantity by weight represented
tbl0001 Table 1 Yield, compositional data
a
, and separation efficiency of fine and coarse fractions from air classification of milled legume
flours
b
Legume species Starting flour
(% protein)
Fraction yield Protein content Starch content Proteinrecovery
in fines
Starchrecovery
in coarse
Fine Coarse Fine Coarse Fine Coarse
Field pea 20.4–25.3 2.3–53.0 77.7–47.0 62.5–42.6 14.5–6.5 1.4–24.7 60.0–86.2 58.1–88.8 99.8–75.3
Fababean 29.8–33.0 21.1–45.8 78.9–54.2 71.8–60.6 19.6–4.2 0–9.5 57.5–91.1 61.3–92.6 100–95.0
Navy bean 24.5–30.4 19.4–44.6 80.6–55.4 63.8–46.2 21.9–5.7 0.1–6.9 58.0–84.9 49.1–87.1 100–96.7
Lima bean 22.7–22.8 17.7–32.0 82.3–68.0 49.6–39.3 18.7–14.1 0–5.9 56.9–69.6 32.0–55.4 99.1–96.1
Great Northern
bean
22.6–24.0 22.5–42.2 77.5–57.8 53.5–45.6 15.6–6.1 0–4.2 51.5–92.3 50.0–87.0 100–96.6
Lentil 22.6–27.1 21.9–44.6 78.1–55.4 64.6–46.5 14.5–3.3 0.2–19.3 65.8–89.7 63.0–91.9 99.2–84.3
Cowpea 23.6–24.4 24.1–33.5 75.9–66.5 51.6–41.8 17.2–14.2 3.2–14.6 67.8–73.7 48.7–60.0 97.0–89.2
Mung bean 25.8–26.5 27.1–29.0 72.9–71.0 61.4–60.4 12.3–11.7 2.0–6.1 67.7–74.0 66.1 96.4–96.3
Chickpea 17.7–19.5 8.5–29.0 91.5–71.0 40.0–28.9 15.5–11.4 3.7–30.3 53.1–62.7 19.2–50.2 94.5–81.4
Soybean 52.5 72.0 28.0 54.2 50.5 74.1
a
On a percentage dry-weight basis.
b
Data are shown as a range of reported values from assorted classifier types and settings.
104 AIR CLASSIFICATION/Uses in the Food Industry

in those fractions being equal to 4, 3, and 93%,
respectively. In some studies, protein fractions having
up to 40–50% protein (but yields of only 1.5%) were
generated accompanied by a 20–30% protein frac-
tion (< 15 mm) at a yield of 11–25%. Although this
fraction could be used to enhance the protein content
of foods, commercial use has not occurred. The starch
fraction can be further processed by water extraction
to remove the protein generating purified starch for
food (e.g., puddings and thickeners) and industrial
applications.
0017 To obtain a protein-rich flour, a separation cut
point of 15 mm is usually sufficient, especially in soft
wheat. Two separations may be required, however, to
obtain a low-protein starch fraction. As an example,
10.8% protein content soft wheat flour can be frac-
tionated into three cuts: < 15, 15–35, and 35 mm, with
yields of 12, 43, and 45% having 19.5, 7.1, and
12.0% protein content, respectively. In a single oper-
ation, a 20% yield of 21–22% protein fine fraction
and an 80% yield of a coarse fraction having 7.5–8%
protein can be achieved from soft wheat flour of
around 10.5% initial protein.
0018 Using soft wheat flour, air-classification methods
have generated a very fine subfraction that is light,
fluffy, and high in protein and total dietary fiber.
Applications requiring such high-gluten flour are
found in the bagel industry.
0019 The protein-rich fractions generated from wheat
can be used as the basis for preparation of protein
isolates, or the high-protein fraction can be added to
low-protein base flours to improve the dough and
baking characteristics. This enhancement is due to
the nature of the protein components present and
the alteration of starch and ash components during
air classification. As in legumes, the soluble sugars are
also concentrated into the high protein fraction.
0020 To improve the efficiency of fine grinding and sub-
sequent air classification, it has been suggested that a
treatment of 0.1–1.0% sulfurous acid be applied for
several hours as an aid in loosening the starch protein
complex. Sorghum subjected to such treatment has
shown increased yields of protein-rich and starch-rich
fractions.
Miscellaneous Cereal Flours
0021 Barley, and especially dehulled malted barley, lends
itself to successful separation by air classification.
Dehulled malted seed with 11.5% protein can be
impact-milled and classified into a high-protein,
low-fiber fraction with 26–27% protein content and
a starch portion of about 9.5% protein. The split
ratio is in the range of 16/84. Regular dehulled barley
with 15% protein has up to 40% protein in the fine
fraction with a 17.5% yield. The benefits of such
application may be in a defibered starchy malt or
barley flour or a high-starch malt flour that may be
used in rapidly brewed malt drinks. Applications for
generating starch have shown that up to three passes
have been used in some programs, thereby yielding
fractions rich in large granule starch (77–78%
starch), which, upon subjecting to wet extraction,
produce an almost pure large-granule starch with a
higher extraction efficiency than conventional extrac-
tion processes.
0022Similarly, high-fiber byproduct streams from the
milling of rye have been impact-milled and reclassi-
fied to yield fractions that have enhanced soluble and
dietary fiber levels.
b-Glucan-enriched Flours
0023Nonconventional use of the air-classification tech-
nique has been applied to oats for the purpose of
generating a b-glucan rich bran fraction. More re-
cently, focus has also turned to barley flour. Instead
of classifying finely ground flour, oat groats have been
coarsely milled and then subjected to air classification
in order to produce a high-bran coarse fraction and a
fine flour fraction. The bran fraction can serve as an
enriched fiber source for cereal applications or as a
starting material for the isolation of b-glucan concen-
trates. Other researchers have defatted the oat flour
prior to air classification and noted that the coarse
fraction (> 30 mm) had a b-glucan content of about
17% for a 30% yield. Inactivation of enzymes would
be a prerequisite for the preparation of b-glucan
fractions by wet extraction. Preparation of barley
fractions (2–4% yield) has shown b-glucan values of
15–18%. Numerous researchers have confirmed
these results with various waxy and nonwaxy barleys.
Other Minor Uses
0024Cottonseed proteins are rarely used in edible foods
because of pigment glands containing gossypol. Air
classification has been shown to produce an edible
protein fraction if there is minimal pigment gland
damage during the milling of extracted flakes. Yields
of 35–40% protein-rich fractions with up to 65%
protein and meeting food and drug directorate stand-
ards are possible.
0025Finely ground soybean hulls have been air-classified
into fine and coarse fractions with enriched fiber.
Application of this fraction in frying batters reduces
oil absorption. Substitution of wheat flour by 20% in
cakes increases volume and acceptability. The protein
fraction from the soybean hulls may yield increased
quantities of trypsin inhibitor with anticarcinogenic
properties.
AIR CLASSIFICATION/Uses in the Food Industry 105

0026 Utilization of rapeseed and canola meals could be
enhanced if crude fiber levels could be reduced by on-
half or less. Small protein shifts of 11–17% have been
achieved in roughly 50/50 splits, but in spite of the
fiber being shifted into the coarse fraction, the two
end products are not sufficiently improved to warrant
commercialization.
0027 Although rice protein has a high nutritional value,
attempts at recovery of this protein by air classifica-
tion have failed to demonstrate any significant pro-
tein shifting. Air classification of defatted finely
ground rice bran has provided a high-protein bran
fraction of a 50% yield and 23% protein content
from an initial 19.4% protein level in the flour. Un-
fortunately, the ash content of the protein-enriched
fraction is also increased, rendering the product
unsuitable for direct food use.
0028 Potato granules produced by spray drying from a
wet milling process have been successfully classified.
Granules with about 10% protein have been classi-
fied into a 15/85 fine:coarse ratio having 38 and
3.1% protein, respectively. Assuming that the eco-
nomics of spray drying is favorable, the protein frac-
tion can provide a source of nondenatured protein
concentrate for food applications.
0029 Other miscellaneous applications of air-classifica-
tion principles to food products include the produc-
tion of fine confectioners sugar (< 5 mm) and fine
cocoa powder (< 75 mm), the latter being used to
make top-quality, smooth chocolate products. Recent
investigations have shown a possible means of gener-
ating flax lignan involving the use of hull material
removed from flaxseed by pearling then subjected to
classification yielding a fraction rich in lignan and
water-soluble fiber. The lignan can be extracted
sequentially and used in the nutraceutical industry.
0030 The combined air-classifier mills have recently
found some applications in the food industry, includ-
ing milk powder, sugar, alginates, gelatine, wheat
gluten and flour, cocoa powder, casein, and starches.
0031 Air-classification techniques applied to finely
ground flours have produced a wide range of pro-
tein/starch-rich fractions and with variable degrees
of success. The process is advantageous in that drying
and effluent disposal costs for wet plants are reduced
or excluded, a significant factor from an environmen-
tal viewpoint. There is also some flexibility for the
processor to vary the resulting flours to suit the end-
user. Increases in capital and operating costs for
the operation must be considered in light of these
benefits.
See also: Air Classification: Principles of Operation;
Barley; Cocoa: Chemistry of Processing; Production,
Products, and Use; Flour: Roller Milling Operations;
Analysis of Wheat Flours; Dietary Importance; Rice
Further Reading
Andersson AAM, Andersson R and Aman P (2000) Air
classification of barley flours. Cereal Chemistry 77:
463–467.
Gueguen J (1983) Legume seed protein extraction, process-
ing and end product characteristics. Qualitas Plan-
tarum, Plant Foods and Human Nutrition 32: 267–303.
Kadan RS, Freeman DW, Ziegler GM Jr. and Spadaro JJ
(1979) Air classification of defatted, glanded cottonseed
flours to produce edible protein product. Journal of
Food Science 44: 1522–1524.
King RD and Dietz HM (1987) Air classification of rape-
seed meal. Cereal Chemistry 64: 411–413.
Kohnhorst AL, Uebersax MA and Zabik ME (1990) Pro-
duction and functional characteristics of protein concen-
trates. Journal of American Oil Chemists’ Society 67:
285–292.
Mok C, Park D-J and Ku K-H (1995) Air classification of
barley flour. Foods and Biotechnology 4(1): 21–24.
Reichert RD (1982) Air classification of peas (Pisum sati-
vum) varying widely in protein content. Journal of Food
Science 47: 1263–1271.
Sosulski FW, Walker AF, Fedec P and Tyler RT (1987)
Comparison of air classifiers for separation of protein
and starch in pin-milled legume flours. Lebensmittel-
Wissenschaft & Technologie 20: 221–225.
Sosulski F and Youngs CG (1979) Yield and functional
properties of air-classified protein and starch fractions
from eight legume flours. Journal of American Oil
Chemists’ Society 56: 292–295.
Thomas ND (1999) The MikoPul air classifier mill. Inter-
national Sugar Journal 101: 480–481.
Tyler RT, Youngs CG and Sosulski FW (1981) Air classifi-
cation of legumes. I. Separation efficiency, yield and
composition of the starch and protein fractions. Cereal
Chemistry 58: 144–148.
Vasanthan T and Bhatty RS (1995) Starch purification after
pin milling and air classification of waxy, normal and
high amylose barleys. Cereal Chemistry 72(4): 379–384.
Vose JR, Basterrechea MJ, Gorin PAJ, Finlayson AJ and
Youngs CG (1976) Air classification of field peas and
horsebean flours: chemical studies of starch and protein
fractions. Cereal Chemistry 53: 928–936.
Wu YV and Stringfellow AC (1992) Air classification of
flours from wheats with varying hardness: protein shifts.
Cereal Chemistry 69(2): 188–191.
Wu YV and Stringfellow AC (1995) Enriched protein and
b-glucan fractions from high-protein oats by air classifi-
cation. Cereal Chemistry 72(1): 132–134.
106 AIR CLASSIFICATION/Uses in the Food Industry

Air Drying See Drying: Theory of Air-drying; Drying Using Natural Radiation; Fluidized-bed Drying; Spray
Drying; Dielectric and Osmotic Drying; Physical and Structural Changes; Chemical Changes; Hygiene; Equipment
Used in Drying Foods
ALCOHOL
Contents
Properties and Determination
Metabolism, Beneficial Effects, and Toxicology
Alcohol Consumption
Properties and Determination
A T Bakalinsky and M H Penner, Oregon State
University, Corvallis, OR, USA
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Introduction
0001 The earliest written records indicate that alcohol
(ethanol or ethyl alcohol) has been enjoyed in the
human diet for thousands of years. In spite of past
and present prohibitions among some groups, an im-
pressive number and variety of alcoholic beverages
have been developed, refined, and extolled in an
extensive and rich literature. While the discovery of
alcohol was undoubtedly accidental, its microbial
origin was only established some 120 years ago
through the work of Louis Pasteur. Microbial physi-
ologists have since shown that alcohol formed
through different fermentative pathways in various
microbial species always serves the same purpose:
regeneration of an oxidized cofactor, usually the
oxidized form of nicotinamide adenine dinucleotide
(NAD
þ
).
0002 Major classes of alcoholic beverages include pri-
mary products of alcoholic fermentations (beer and
wine), products of mixed alcoholic–lactic acid fer-
mentations, and since the discovery of distillation –
known to the Chinese at least 3000 years ago and
to the ancient Egyptians even earlier – distilled and
fortified beverages. Because alcohol is an excellent
extractant, exhibits low toxicity, and possesses anti-
microbial activity, it also enjoys wide use as a solvent
for food ingredients such as spices and flavors. (See
Lactic Acid Bacteria.)
Physical and Sensory Properties
0003Ethanol is a clear, colorless, flammable liquid mis-
cible with water and many organic solvents in all
ratios. It is hygroscopic and relatively nontoxic, ex-
hibiting an oral LD
50
in rats of 13.7 g kg
1
(grams of
ethanol per kilogram of body weight required to kill
50% of the animals). When ethanol is added to water,
a rise in temperature due to the heat of solvation
occurs with a consequent increase in volume. When
the mixture cools to the original temperature, the
volume decreases, becoming slightly less than the
sum of the initial water and ethanol volumes. Max-
imum contraction occurs at a molar ratio of eight
parts of water to one part of ethanol. At a pressure
of 101.3 kPa, a mixture of 95.6% ethanol and 4.4%
water on a mass basis forms a constant-boiling-point
mixture called an azeotrope. In practice, this means
that the concentration of ethanol cannot be increased
beyond 95.6%, by simple distillation. Some import-
ant physical properties of ethanol are listed in Table 1.
0004Ethanol has a slightly sweet taste and a character-
istic aroma. The aroma threshold determined in one
study ranged from 4 to 5 mg of ethanol per 100 ml of
tbl0001Table 1 Physical properties of ethanol
Formula CH
3
CH
2
OH
Molecular weight (Da) 46.07
Boiling point (
C) 78.32
Freezing point (
C) 114.1
Density, d
4
20
(g ml
1
) 0.7893
Refractive index, n
D
20
1.361
Viscosity at 20
C (cP) 1.17
Dielectric constant at 20
C 25.7
Heat of fusion (J g
1
) 104.6
Heat of vaporization at 78.32
C(Jg
1
) 839.31
ALCOHOL/Properties and Determination 107

total aqueous solution. At high concentrations, it
causes a burning sensation in the mouth. It moderates
the taste of acids, as shown by the greater tartness of
dealcoholized wines relative to the same untreated
wines. It is said to impart ‘body,’ which may be due
to the fact that at room temperature it is more viscous
than water. Addition of sugar to an ethanol–water
solution increases the threshold for ethanol, indicat-
ing that sugar masks its taste and/or aroma.
Sources of Alcohol
0005 The natural source of alcohol is fermentation which
has been defined as the oxidation of organic com-
pounds (generally carbohydrates) in the absence of
external electron acceptors. Pasteur called it ‘life
without air.’ Microorganisms ferment in order to
obtain the energy necessary for growth and reproduc-
tion and alcohol is one of several possible fermenta-
tion products. In most countries, use of alcohol in
beverages is restricted to that made by fermentation.
The vast amount of alcohol used industrially as a
solvent and substrate in chemical syntheses is made
synthetically from ethylene. The amount derived
from the fermentation of agricultural products is
largely dependent on economic considerations,
e.g., alcohol produced by fermentation is used as a
substitute for petrol in Brazil.
Alcoholic Beverages
0006 All alcoholic beverages are derived either directly or
indirectly from fermented products. As indicated in
Table 2, the major groups are fermented beverages,
products of mixed alcoholic–lactic acid fermenta-
tions, beverages made by distillation and by fortifica-
tion. Fruits containing high sugar concentrations at
maturity and nutrients at levels sufficient to support
growth of fermenting yeasts, primarily Saccharo-
myces cerevisiae, have traditionally been the raw
materials from which wines are made. Grapes are
unusual in that they contain sufficiently high levels
of sugars, nutrients, and acids to produce wines that
are microbiologically stable.
0007Beer and sake are produced from barley (other
grains are possible sources) and rice, respectively.
Unlike wines, these beverages are derived from carbo-
hydrates that are not initially fermentable. Conversion
of carbohydrates into fermentable sugars requires the
action of amylases produced by barley in the case of
beer, and by the fungus Aspergillus oryzae during
sake production. Kefir and koumiss are examples of
beverages produced by the fermentation of cows’ and
mares’ milk, respectively, by a mixture of lactic acid
bacteria and lactose-fermenting yeasts. Although
almost unknown in some western countries, kefir
and koumiss continue to enjoy great popularity in
eastern Europe. (See Beers: History and Types; Fer-
mented Milks: Other Relevant Products.)
0008Distilled beverages are derived from fermented
grains and potatoes (whiskies and vodka), sugar
cane byproducts (rum), fruits (brandies), and other
plants such as mezcal (tequila). Liqueurs are distilled
beverages that have been flavored and sweetened.
Fortified beverages require the addition of alcohol
during production, generally in the form of brandy.
Special fruit preserves are fortified to assure preserva-
tion. The intact fruits, usually cherries, are steeped in
brandy for a period of months prior to consumption.
(See Brandy and Cognac: Armagnac, Brandy, and
Cognac and their Manufacture; Rum; Whisky, Whis-
key, and Bourbon: Products and Manufacture.)
Use as a Preservative
0009Ethanol is not particularly toxic. As a sole agent of
preservation in beverages, minimum concentrations
ranging from 18 to 21% by volume are required to
assure microbiological stability. Table wines which
contain significantly less alcohol are stable because
of additional factors: their natural high acidity, low
pH, high content of phenolic compounds, and lack of
sugar. The toxicity of ethanol towards microorgan-
isms is due to a number of effects. At very high
concentrations, as in flavor extracts, ethanol acts as
a desiccant and protein denaturant. At lower concen-
trations, 10–20% by volume, the toxicity is believed
to result primarily from interactions with cell mem-
branes. In the yeast S. cerevisiae, ethanol has been
shown to inhibit several solute transport systems. In
the presence of sugar, the toxicity of ethanol is en-
hanced. This explains the stability of a class of sweet
tbl0002 Table 2 Major classes of alcoholic beverages
Fermented
Beer
Wine
Sake
Mixed alcoholic^lactic acid fermentation
Kefir
Koumiss
Distilled
Whiskies, vodka
Rum
Brandy
Tequila
Liqueurs
Fortified
Port
Sherry
Vermouth
108 ALCOHOL/Properties and Determination

dessert wines which contain several percent sugar and
only 7–10% ethanol.
Analysis
0010 Several methods are available for quantitative meas-
urement of ethanol. Some are based on ethanol-
specific chemical reactions, such as dichromate and
enzyme-catalyzed oxidation. Others are based on
selected physical properties of the product or sample
which are functions of its ethanol content, such as
boiling point or relative density. Often, separation of
the ethanol from other compounds by chromatog-
raphy, distillation, or other means is required prior
to quantification. The choice of method depends on
the need for accuracy and precision, analysis time,
potential for interfering substances, and expense.
(See Chromatography: Principles.)
0011 With respect to interfering substances, it is worth
noting that methanol is a minor but potentially toxic
constituent of distilled beverages derived from fruits.
It is formed from hydrolysis of methoxylated fruit
pectin and does not interfere with enzymatic or chro-
matographic methods of ethanol analysis.
Boiling Point Determinations (Ebulliometry)
0012 Boiling point depression is probably the most com-
monly used measure of ethanol in liquid systems. The
method is based on Raoult’s law of partial pressures.
Under ideal conditions, the law states that the total
pressure above a mixture of two miscible liquids is
equal to the sum of their partial pressures, and that
the partial pressure of each component is directly
proportional to its mole fraction in the mixture.
Stated mathematically:
P
T
¼ P
A
þ P
B
¼ P
0
A
X
A
þ P
0
B
X
B
ð1Þ
where P
T
is the total pressure above a solution of
liquids A and B, P
A
is the partial pressure of compon-
ent A, P
B
is the partial pressure of component B, P
0
A
is
the vapor pressure of component A above pure liquid
A, X
A
is the mole fraction of component A in the
mixture, P
0
B
is the vapor pressure of component B
above pure liquid B, and X
B
is the mole fraction of
component B in the mixture. Ethanol–water mixtures
have boiling points ranging from slightly less than
78.32
C (100% ethanol) to 100
C (0% ethanol) at
101.3 kPa pressure. The ethanol–water azeotropic
mixture (95.6% ethanol, w/w) boils at 78.2
C. Devi-
ations from Raoult’s law are exhibited by mixtures of
nonideal liquids, in which significant molecular inter-
actions occur between components. Ethanol–water
mixtures are examples of nonideal systems because
ethanol exhibits significant hydrophilic character.
Generally, the boiling point of pure water is measured
in addition to that of the sample and tabulated
ethanol content–boiling point data are adjusted ac-
cordingly, to account for variations in atmospheric
pressure. To prevent significant evaporative loss of
ethanol during boiling and measurement, ebulli-
ometers are fitted with a small condenser, and read-
ings are taken shortly after a constant temperature is
attained.
0013Boiling point measurements require relatively large
samples – up to 50 ml – which limits their utility.
Their accuracy is reduced in the presence of high
concentrations of other dissolved solutes. Since ebul-
liometers are simple devices, relatively inexpensive,
and sufficiently accurate for production purposes,
small wineries have used them extensively.
Relative Density Measurements (Hydrometry)
0014Relative density (specific gravity) is the ratio of the
density of a solution to the density of a reference
solution (usually water at 4
C). Relative densities of
aqueous solutions are dependent on the concentra-
tion of dissolved solutes. Two general approaches
are used to obtain relative densities.
0015The first is to measure the mass of known volumes
of sample and standard reference solutions. This
is accomplished by use of a pycnometer, a small,
relatively lightweight flask of known volume. Since
specific volume is temperature-dependent, the pycno-
meter is immersed in a water bath maintained at a
standard temperature. The mass of the sample and
reference solutions is then determined on an analyt-
ical balance and compared to tabulated standard
values. The relative density of aqueous ethanol solu-
tions reflects the concentration of ethanol, assuming
it is the only (or major) dissolved solute.
0016The second general approach is based on Archime-
des’ principle, which holds that an object immersed in
a liquid appears to lose an amount of mass equivalent
to the mass of liquid it displaces. The Westphal bal-
ance illustrates this principle. Its design is similar to
that of an equal-arm, two-pan balance. At one end, a
tube of known volume and weight is immersed in a
reference solution and the beam is balanced by add-
ition of weights to compensate for the buoyancy of
the liquid. The procedure is repeated by immersion in
the unknown liquid sample. In this manner, a relative
density is determined. A more commonly used device,
based on the same principle, is the hydrometer. Hy-
drometers are glass instruments with narrow, cali-
brated stems much like a thermometer at the top,
but with much expanded bottom halves that are
weighted with lead to insure that they float upright.
When the hydrometer is placed in a liquid sample, it
sinks to a depth that results in displacement of a
volume of liquid equal to its weight. The alcohol
ALCOHOL/Properties and Determination 109

content is read from the calibrated stem at the point
where it intersects the liquid surface. Hydrometers
designed for alcohol measurement are standardized
for use at a specific temperature and calibrated either
in percent alcohol (v/v), or
proof, where
proof ¼
2 % ethanol (v/v). Correction tables are available
for measurements taken at nonstandard tempera-
tures. Hydrometers are accurate over a limited range
of alcohol concentrations. Consequently, a set of
instruments may be required to cover the range of
interest.
0017 Since the relative density of a solution depends on
the concentration of all dissolved solutes, it is often
necessary to separate the ethanol from the other
solutes. To accomplish this, the ethanol contained in
a specified volume of product is separated by distilla-
tion. The distillate is diluted to a known volume with
water, and the relative density of the resulting solu-
tion is measured. In fermented beverages, the concen-
tration of other volatiles that codistill is significant
from a sensory perspective, but is very low compared
to that of ethanol and does not significantly affect the
relative density. In some beverages, such as bourbon
whiskey, the concentration of soluble solids other
than ethanol is sufficiently low to permit direct
measurement of relative density without need for
distillation.
0018 The international Association of Official Analyt-
ical Chemists (AOAC) has approved methods based
on specific gravity for determination of ethanol in
distilled beverages, beers, and wines. The methods
require relatively large samples, and are somewhat
tedious due to the need for distillation and strict
temperature control. However, the equipment is rela-
tively inexpensive and readily available, and precise
results can be expected from a skilled technician.
Enzymatic Determination
001 9 The enzymatic analysis of ethanol is generally based
on the reaction catalyzed by alcohol dehydrogenase.
As shown in eqn (2), the oxidation of ethanol by
NAD
þ
yields acetaldehyde and NADH.
CH
3
CH
2
OH
Ethanol
þ NAD
þ
!
CH
3
CHO
Acetaldehyde
þ NADH þ H
þ
ð2Þ
The reaction is monitored photometrically at 340 nm
since NAD has no significant absorbance at this
wavelength in the oxidized form (NAD
þ
), but has a
molar extinction coefficient of 6.23 10
3
lmol
1
cm
1
when reduced (NADH). Primary alcohols other
than ethanol can interfere, but this is rarely a problem
in foods. Determination of ethanol concentration re-
quires that conditions be adjusted such that the equi-
librium is shifted toward acetaldehyde formation.
One approach is to trap the aldehyde as it forms by
reaction with semicarbazide. Another is to carry out
the reaction at alkaline pH. A third is to couple the
reaction with a second enzyme, aldehyde dehydro-
genase, which converts the acetaldehyde to acetic
acid. In the latter case, 2mol of NADH are formed
per mole of ethanol. (See Enzymes: Uses in Analysis.)
0020Enzyme assays have high sensitivity relative to
other methods and good accuracy due to the specifi-
city of the reaction. Often, samples require treatment
such as dilution, pH adjustment, or decolorization
prior to analysis. Material needs for this type of
assay are simple: volumetric glassware, a photometer,
and reasonable temperature control. A recurring
expense is the enzyme itself, which must be stored
and handled appropriately.
Dichromate Oxidation
0021Chemical oxidation may be used to quantify the
amount of ethanol in a sample. In the dichromate
procedure, the quantity of dichromate required to
oxidize ethanol to acetic acid is measured. In complex
beverages, the ethanol is usually separated from
potentially interfering compounds by distillation.
The ethanol in the ethanol–water distillate is then
oxidized with a known excess of dichromate in the
presence of sulfuric acid. Residual dichromate is then
reduced by ferrous ammonium sulfate in a standard
oxidation–reduction titration. A useful end-point
indicator is 1,10-o-phenanthroline-ferrous sulfate,
which turns brownish purple from blue-green. Potas-
sium dichromate is available in sufficient purity to be
used as a primary standard. Ferrous ammonium sul-
fate may be used as an approximate standard, but
solutions that are not fresh must be standardized
against dichromate.
0022The dichromate oxidation method for the measure-
ment of alcohol in wines has been approved by the
AOAC. The method is appropriate for routine
analyses only when the reagents are standardized
regularly.
Refractive Index
0023The refractive index (RI) of a medium is dependent on
its chemical composition, since under controlled con-
ditions composition dictates its electrical and mag-
netic properties. The RI of a sample is defined as the
ratio of the speed of light in a vacuum to its speed
in the sample medium. Consequently, RI values are
always greater than one. The RI of aqueous ethanol
solutions can be measured directly to indicate ethanol
concentration. When interfering compounds are pre-
sent, the RI of a distillate can be measured. Since the
RI of a liquid is temperature-sensitive, measurements
110 ALCOHOL/Properties and Determination

taken at nonstandard temperatures must be corrected
by use of conversion tables.
0024 The AOAC has approved RI-based methods for
determining ethanol in beers and wines. For wines,
the measurement is made on the distillate. Measured
RI values for beer are used in conjunction with
specific gravity measurements to calculate ethanol
content.
Chromatography
0025 Chromatography is based on the separation of
sample components due to their differing affinities
within a stationary–mobile-phase system. A success-
ful chromatographic run results in separation of the
analyte of interest from all sample components that
would otherwise interfere with its analysis. The sep-
arated analyte may then be quantified by one of sev-
eral detectors. The ethanol in most samples can be
readily separated by chromatography. Since ethanol
is volatile, direct measurement by gas chromatog-
raphy (GC) is possible. Several commercial vendors
have columns specifically designed for determining
the ethanol content of beverages. Simple, 2-m packed
columns are commonly used. Depending on the
sample, it is often beneficial to carry out a simple
pretreatment to extend the life of the column by
removing nonvolatile components. Ethanol exiting a
GC column is commonly quantified with a flame
ionization detector or by thermal conductivity. Etha-
nol separations using high-pressure liquid chroma-
tography (HPLC) are also possible. An advantage of
HPLC over GC is the possibility of simultaneous
determination of certain nonvolatile sample compon-
ents. Commercial HPLC columns for ethanol analysis
are available. Chromatographic methods, which are
expensive, are widely used in analytical laboratories
because of their specificity, increasingly simple oper-
ation, and potential for automation. (See Chromatog-
raphy: High-performance Liquid Chromatography;
Gas Chromatography.)
See also: Beers: History and Types; Brandy and Cognac:
Armagnac, Brandy, and Cognac and their Manufacture;
Chromatography: Principles; High-performance Liquid
Chromatography; Gas Chromatography; Cirrhosis and
Disorders of High Alcohol Consumption; Enzymes:
Uses in Analysis; Fermented Milks: Other Relevant
Products; Lactic Acid Bacteria; Rum; Whisky, Whiskey,
and Bourbon: Products and Manufacture
Further Reading
Amerine MA and Ough CS (1980) Methods for Analysis of
Musts and Wines. New York: Wiley.
Amerine MA and Roessler EB (1983) Wines. Their Sensory
Evaluation. New York: W.H. Freeman.
Association of Official Analytical Chemists (1990) Official
Methods of Analysis, 15th edn. Arlington, Virginia:
Association of Official Analytical Chemists.
Boulton RB, Singleton VL, Bisson LF and Kunkee RE
(1996) Principles and Practice of Winemaking. New
York: Chapman Hall.
Heath HB and Reineccius G (1986) Flavor Chemistry and
Technology. New York: Van Nostrand Reinhold.
Pederson CS (1979) Microbiology of Food Fermentations,
2nd edn. Westport, CT: AVI Publishing.
Sherman PD and Kavasmaneck PR (1980) Ethanol.
Encyclopedia of Chemical Technology, 3rd edn, vol. 9.
New York.
Sommer AE and Bu
¨
cker R (1983) Ethanol. Encyclopedia of
Chemical Processing and Design, vol. 19. New York:
Marcel Dekker.
Zoecklein BW, Fugelsang KC, Gump BH and Nury FS
(1990) Production Wine Analysis. New York: Van
Nostrand Reinhold.
Metabolism, Beneficial Effects,
and Toxicology
C H Halsted, University of California, Davis, CA, USA
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Background
0001Alcohol is both a food that provides 29.7 kJ g
1
(7.1 kcal g
1
), accounting for 5.6% of energy in
the American diet, and a drug with potential for
intoxication, addiction, and damage to several
organ systems. In the USA, alcohol is consumed to
some extent by at least two-thirds of the population,
and an estimated 5–10% consume alcohol in excess
and are at risk for addiction, liver disease, cardiomy-
opathy, pancreatic disease, and neurological defects.
The overall mortality from alcoholism in the USA is
about 100 000 deaths per year, about half due to
accidents related to intoxication, and the remainder
mainly due to chronic alcoholic liver disease and its
consequences.
Metabolism of Alcohol
0002Unlike most other drugs, alcohol is completely me-
tabolized after ingestion at a rate of 10–15 g h
1
, and
its energy content is not stored in the body. Alcohol
diffuses across all cell membrances, and after equili-
bration, its concentration is similar in all aqueous
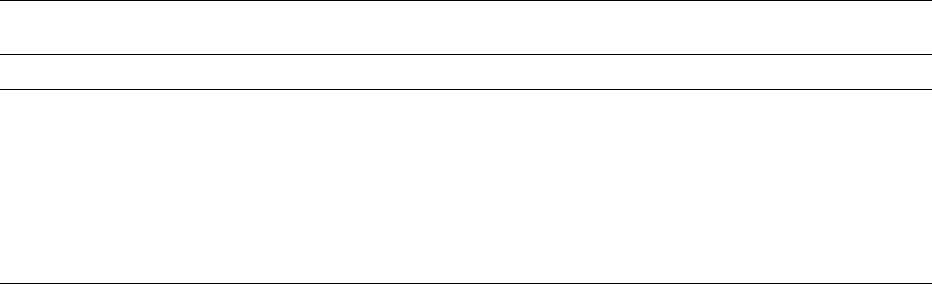
compartments. After the human ingestion of 50 g of
alcohol, its concentration in the stomach reaches
ALCOHOL/Metabolism, Beneficial Effects, and Toxicology 111
1 mol l
1
(4.2 g dl
1
), decreasing by one-half in the
upper intestine and equilibrating with the blood alco-
hol level in the distal intestine. ‘First pass’ metabolism
is achieved in the stomach by gastric alcohol dehy-
drogenase, an enzyme located in parietal cells with
K
m
500 mM that accounts for 30% of total alcohol
metabolism in men and 10% of alcohol metabol-
ism in women. This gender difference in gastric me-
tabolism partly explains the lower tolerance of
women for alcohol. Subsequently, alcohol is metabol-
ized entirely in liver cells by two enzyme systems:
cytoplasmic alcohol dehydrogenase and a specific
and inducible microsomal cytochrome P450 enzyme,
known as CYP2E1 (Table 1). Hepatic alcohol dehy-
drogenase with K
m
2 mM metabolizes alcohol at
blood levels consistent with moderate drinking.
During the oxidation of alcohol by this reaction,
NAD is reduced to NADH while producing acetalde-
hyde and subsequently net energy through the pro-
duction of ATP. However, the increased ratio of
NADH to NAD is associated with increased fatty
acid and triglyceride synthesis, reduced gluconeogen-
esis, enhanced lactate production, and reduced urin-
ary excretion of uric acid. The potential clinical
consequences of these metabolic side effects include
transient fatty liver, hypertriglyceridemia, hypogly-
cemia, acidosis, and gout. On the other hand,
CYP2E1 with a K
m
of 20 mM accounts for the me-
tabolism of higher alcohol levels as seen with chronic
excessive alcohol use. Alcohol oxidation by CYP2E1
is energy-wasteful by utilizing NADPH at a 2:1 molar
ratio while producing greater amounts of acetalde-
hyde without generation of ATP. Increased intrahepa-
tic acetaldehyde blocks mitochondrial respiration
thereby enhancing the accumulation of fat in the
liver, increases catecholamine release while promot-
ing ketoacidosis, and, by enhancing lipid peroxida-
tion and collagen synthesis, contributes to the
development of alcoholic hepatitis and cirrhosis.
The induction of CYP2E1 by frequent alcohol con-
sumption generates a free radical that can trigger
oxidative liver injury, accounts for alcohol tolerance
in habitual users, and is associated with induction
of other microsomal enzymes that accelerate the
metabolism of drugs such as acetaminophin, bar-
biturates, and coumadin, and the catabolism of vita-
min A.
Risks Versus Benefits of Alcohol
Consumption
0003The risks or benefits of alcohol consumption are based
on quantitative estimates of the numbers of drinks per
day, where one drink of 360 ml of beer, 120 ml of
wine, or 40 ml of spirits provides 15 g of alcohol.
By these standards, ‘moderate drinking’ is defined as
one to two drinks (15–30 g of alcohol) per day or
seven to 14 drinks per week on average, and excessive
drinking that increases disease risk is defined as more
than two to three drinks (30–45 g of alcohol) per day
for men and more than one or two drinks (15–30 g of
alcohol) per day for women. Excessive drinking may
occur through habituation or addiction, and in cul-
tures where wine is consumed daily as a component
of the diet (Table 2).
0004A variety of studies from many developed countries
established a J-shaped curve for mortality risk from
alcohol consumption, wherein the lowest mortality
occurs at consumption of one or two drinks per day
compared with abstinence or to the consumption of
more than two drinks per day. Thus, the term ‘moder-
ate alcohol consumption’ is used to define consump-
tion with clinical benefit and no increased mortality
risk. Comparing all causes of mortality, the moderate
drinking level is somewhat less for women at one to
two drinks per day than in men at two to three drinks
per day. When all the causes of mortality are taken into
account, it appears that the benefit of moderate drink-
ing is confined to the prevention of coronary heart
disease and ischemic strokes, both together of which
account for the majority of deaths in the USA. Above
the level of moderation, i.e., at more than two to
three drinks per day, alcohol consumption associates
with life-threatening trauma, hemorrhagic strokes,
tbl0001 Table 1 Alcohol metabolizing enzymes
Enzyme K
m
(mM) Relatedeffects
Gastric cytosolic alcohol dehydrogenase 500 ‘First pass’; 30% in men, 10% in women
Liver cytosolic alcohol dehydrogenase (one to two drinks) 2 Increased NADH/NAD ratio promotes fatty acid synthesis
leading to fatty liver, preferential oxidation of ethanol over
lipid, and reduced gluconeogenesis, lactic acidosis, and
hyperuricemia
Liver microsomal CYP2E1 (more than two drinks) 20 Increased NADPH/NADP ratio consumes energy and
increases metabolism of many other drugs and vitamin A;
increased acetaldehyde inhibits mitochondrial functions
and stimulates lipid peroxidation and collagen synthesis
112 ALCOHOL/Metabolism, Beneficial Effects, and Toxicology

hypertension, esophageal, breast and colorectal
cancers, acute and chronic pancreatitis, malnutrition,
and alcoholic liver disease.
0005 Interest in the cardiovascular protective effects
of alcohol stemmed from the French report of
the paradoxical improved mortality among wine-
drinkers in the Mediterranean provinces compared
with residents of northeastern provinces, in spite of
the similar content of high-fat foods in the diet and
overall alcohol consumption. A subsequent in vitro
study at the University of California Davis demon-
strated that the oxidation of low-density lipoprotein
could be prevented by coincubation with phenolic
compounds extracted from red wine, suggesting
that the improved cardiovascular risk of French red
wine-drinkers was related to these nonalcoholic
compounds. The principal flavonoid of red wine is
catechin, which is also present in nonfermented grape
juice. The biological availability of catechin for
human subjects is similar in red wine to that in deal-
coholized red wine, but a greater amount of catechin
may be extracted from fermented grapes during
wine-making than from nonfermented grape juice. A
long-term prospective Danish study of residents of
Copenhagen concluded that the lowest mortality
rates occur in wine-drinkers, with the highest mortal-
ity in consumers of liquor. A study from Northern
California concluded that the improved mortality
in wine-drinkers related to their healthy dietary life-
style, and that red and white wine provided equal
protection.
0006 There are several explanations for the cardiopro-
tective effects of moderate alcohol consumption, in
particular the effects of red wine. Moderate consump-
tion of all types of alcoholic beverages has a posi-
tive effect on the circulating lipoprotein profile,
principally by elevating the levels of high-density lipo-
protein. Furthermore, moderate alcohol consumption
by women results in modest reductions in low-density
lipoprotein levels and increases Apo A-1 but de-
creases Apo B lipoproteins. Whereas serum triglycer-
ides increase in male alcohol consumers, they may
decrease in postmenopausal women drinkers. Moder-
ate alcohol consumption also benefits the clotting
process by increasing levels of tissue plasminogen
activator. In addition to the benefit of wine flavonoids
in reducing the oxidation of low-density lipoprotein,
they also improve the clotting risk by reducing
platelet adhesiveness.
Alcohol Toxicity
0007With the exception of traffic fatalities that may occur
after consumption of an intoxicating dose of alcohol
pending timing after the last drink, the deleterious
effects of alcohol consumption are limited to those
who consume excessive amounts of alcohol (on aver-
age, more than two to three drinks per day) on a
chronic basis. The clinical risks of alcohol toxicity
increase in proportion to increasing average daily
amount of alcohol and duration of excessive alcohol
consumption. In many instances, the development of
these clinical syndromes is modulated by nutrient
deficiency, and in the case of pancreatic insufficiency
and alcoholic liver disease, the clinical syndromes can
contribute to malnutrition.
Clinical Effects of Alcohol Toxicity
0008Excessive alcohol consumption contributes to
increased risks of alcoholic liver disease, cardiomyop-
athy, chronic pancreatitis and insufficiency, nutri-
tional anemia, certain cancers, abnormal energy
metabolism, and malnutrition that is more pro-
nounced after the development of alcoholic liver dis-
ease. Abnormal nutrient availability and metabolism
play a role in all of these clinical complications of
chronic alcoholism (Table 3).
0009Increased cancer risks Increased risk of cancer of
the oropharynx and esophagus is directly related to
the amount and duration of alcoholism, is tripled in
heavy smokers, and may be related in part to abnor-
mal carcinogen metabolism secondary to zinc and
vitamin A deficiency. The incidence of breast cancer
is increased in women who drink, in part due to
enhanced estrogen secretion, and particularly in
women who consume inadequate folate in their
diets. Colorectal cancer risk is increased in excessive
drinkers of both sexes and is related to both alcohol
toxicity and low folate status.
tbl0002 Table 2 Benefits and risks of alcohol consumption
Benefits (one to two drinks per
day)
Protection against ischemic
cardiovascular disease
Improved lipoprotein profile
Increased tissue plasminogen
activator
Wine flavonoids decrease LDL
oxidation, platelet
adhesiveness
Risks (more than three drinks
per day)
Hypertension
Cardiomyopathy
Hemorrhagic stroke
Pancreatitis
Anemia
Neurologic disorders
Cancer of esophagus, breast,
colon
Alcoholic liver disease
Malnutrition
ALCOHOL/Metabolism, Beneficial Effects, and Toxicology 113
