Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.


objectives of agitation are to keep solids in suspension
and to disperse them uniformly so that any draw-off
would have an identical solid concentration. Impel-
ler-agitated devices, described earlier, are generally
useful for these purposes; propellers, in particular,
are known to be efficient for suspending solids in
low-viscosity liquids. For a given impeller and solid–
liquid system, there is a minimum rotational speed
below which the impeller does not produce sufficient
‘lift force’ to suspend the solids. The threshold rota-
tional speed, in general, depends on the size, shape
and density of the solid particles, the solid concen-
tration, the density and viscosity of the liquid, and the
geometry of the mixing vessel. In food systems, the
density difference between the particles to be sus-
pended and the liquid phase is generally not high. It
follows that the minimum rotational speed for solid
suspension is not high. Nevertheless, it is quite prob-
lematic to ensure homogeneity after the particles have
been suspended, especially when the particles are sen-
sitive to collision and high shear. While information
on threshold suspension speeds is readily available,
the number of studies on the homogeneity of such
dispersions in mixing vessels is very limited.
Air–Liquid Agitation
0033 During the agitation of food products, it is generally
desirable to exclude air, since entrained air can cause
spoilage during storage. However, there are certain
situations where aeration is desirable: aerobic fer-
mentations generally demand a continuous supply
of air; processes such as cream whipping and ice-
cream preparation involve air inclusion. Both
aeration and deaeration are therefore important to
food processing.
0034 Aeration, whether desirable or not, can occur from
the surface of an impeller-agitated vessel. The action
of the impeller induces circulation and turbulence in
the liquid. When strong eddies are generated at the
surface, air is entrained from the head-space to form
bubbles, which are then dragged into the bulk by
circulation currents. These bubbles are stabilized
by surface-active agents that are invariably present in
food systems, and the two phases coexist. It has been
noted that surface aeration occurs above a minimum
impeller speed, and it is possible to estimate its value
for a variety of systems. If aeration is detrimental to
product storage, then either the impeller speed should
be less than the minimum speed for surface aeration,
or agitation should be followed by de-aeration.
0035 Aerobic fermenters invariably need a continuous
supply of air to sustain microbial growth. The main
effect of sparging air into an impeller-agitated tank is
to lower its power consumption. The reduction in
power is a consequence of the formation of stable
air cavities behind the impeller blades; the extent of
the reduction depends on the size and shape of the
cavities. At a given impeller speed, an increase in air
flow rate results in an increase in cavity size. How-
ever, the cavity size cannot increase indefinitely: once
the cavities have grown to their maximum size at a
given flow rate, further increases in air flow rate cause
some of the excess air to bypass the cavities and hence
the impeller blades. The cavities, in certain cases, may
also coalesce. As a consequence, the impeller virtually
stops pumping, and the phenomenon is described as
‘impeller flooding.’ An impeller-agitated fermenter
operates well away from the flooding point. In prac-
tise, the disc turbine of Figure 2a is the preferred
impeller used in aerobic fermentation, mainly owing
to dispersion ability. This impeller, however, suffers
from certain drawbacks: it is a high-shear impeller,
and therefore causes shear damage to certain sub-
stances, its power consumption is very high, and,
more importantly, a large fraction of the supplied
power is dissipated in a relatively low volume fraction
of the vessel. These disadvantages have been found to
be critical to several processes, and there is an attempt
to use alternative devices, such as the hydrofoil
impeller of Figure 2d.
Agitation of Particulate Material
0036Particle mixing is an extensive food processing oper-
ation used for mixing materials that include flour,
sugar, dried milk, salt, flavoring materials, cereal
flakes, etc. Wide differences among properties such
as particle size, shape, density, and surface character-
istics (e.g., frictional and electrostatic), make particle
mixing a difficult and a complex operation. The pro-
cess can be further complicated in food systems by a
high moisture content, friability, complex flow prop-
erties, and agglomeration or segregation. The desired
end point of solid-phase mixing is the attainment of
a truly random distribution.
0037The following differences between mixing in
particulate systems and fluid systems have been
recognized:
1.
0038There is no particulate motion equivalent to
molecular diffusive transport in liquids and
gases. Thus, when two miscible liquids or gases
are in contact with each other, complete mixing
eventually occurs. However, blending of particu-
late materials cannot occur without some input
from external energy.
2.
0039Unlike fluids, mixing of particles is reversible, i.e.,
mixed solids, on storage, tend to segregate, pri-
marily because of size differences; even marginal
differences of 15–20% can cause ‘unmixing.’ Dif-
ferences in other properties, such as density and
94 AGITATION

shape, can only accelerate the process of unmix-
ing. It has been recognized that, in general, heav-
ier, smaller, or smoother and rounder particles
tend to percolate and sink through lighter, larger
or jagged particles.
3.
0040 Mixing in liquids and gases is far more intimate
than in solids. The ultimate mixing elements of a
particulate mixture have a coarser texture, and are
of a poorer quality.
Particulate materials may be broadly classified into
two groups: (1) free-flowing or cohesionless powders
and (2) cohesive powders. Cohesionless powders are
mixed by convective transport, surface mixing and/or
interparticle percolation; mixing and segregation
occur simultaneously. Particles in cohesive powders
move in clusters.
0041 Mixers used for solids are normally batch type.
Solid mixers can be broadly classified as: (1) tumbler
mixers, (2) convective mixers, and (3) hopper mixers.
A V-shaped tumbler mixer is shown in Figure 6. The
vessel is typically filled to about half its volumetric
capacity and is rotated on an axis between bearings,
thus causing the particles to tumble and roll over each
other. In the case of convective mixers, the mixing
vessels are stationary, and the solids are agitated by
impeller blades, usually of a ribbon or screw type.
Cohesionless powders can also be mixed by discharg-
ing a mixture of the components through hoppers.
Radial and axial mixing is achieved by returning the
discharged material to the top of the hopper and
repeating the process until the solids are completely
homogeneous.
Mixing of Doughs and Batters
0042 Dough and batter, used in the making of bakery
products, are formed by a series of agitation-induced
interactions of such diverse components as water,
flour, lipids, enzymes, salt, sweeteners, yeast, air,
and oxidizing and reducing agents. When classified
on the basis of the desired agitating action, most
bakery products fall into two broad categories, exten-
sible doughs, such as those used to make bread or puff
pastry, and flowable or friable mixtures, used in the
preparation of cake batters, icings, etc. (See Bread:
Dough Mixing and Testing Operations.)
0043 Mixing of doughs is more complex and energy-
intensive. Although continuous processes are now
being developed, mixing of doughs is invariably
carried out batchwise in one or many stages. Single-
stage mixing is mainly adopted by smaller bakeries,
and it aims to complete dough development within its
duration. However, in a two-stage process, the initial
stage has a shorter duration, and it accomplishes
blending and flour hydration (or hydration of
the gluten proteins). Dough development and
strengthening of the gluten network occurs more
gradually during the second stage, promoted by
repeated folding and stretching action caused by the
mixer. An undesirable effect of the intense shearing
action, especially in high-speed mixers, is the rapid
increase in dough temperature. Provision for simul-
taneous cooling therefore exists in most mixers. Care-
ful control of the process is necessary to obtain a
dough structure optimum for baking purposes: at
the right end point, the dough has maximum consist-
ency or minimum mobility; it appears smooth with a
dry surface, and its elastic character is optimized. The
end point is reached when the dough starts to pull
away from the mixer walls. Overmixed dough, how-
ever, is sticky and difficult to handle, and its surface
possesses a characteristic sheen.
0044The agitator in a dough mixer is mounted either on
a horizontal shaft or on a vertical shaft. Vertical shafts
generally rotate about a fixed axis; in some cases,
planetary motion is also superimposed. In either case,
there is a close clearance between the agitating blades
and the mixing vessel, in order to eliminate stagnant
regions and build-up of sticky material on the wall.
See also: Aerated Foods; Bread: Dough Mixing and
Testing Operations; Emulsifiers: Organic Emulsifiers;
Plant Design: Designing for Hygienic Operation
Further Reading
Harnby N, Edwards MF and Nienow AW (eds) (1992)
Mixing in the Process Industries, 2nd edn. Oxford:
Butterworth-Heinemann.
Jones RL (1985) Mixing equipment for powders and pastes.
The Chemical Engineer 419: 41, 43.
Lindlay JA (1991a) Mixing for agricultural and food
materials: 1. Fundamentals of mixing. Journal of
Agricultural and Engineering Research 48: 153–170.
Lindlay JA (1991b) Mixing for agricultural and food ma-
terials: 2. Highly viscous liquids and cohesive materials.
Journal of Agricultural and Engineering Research 48:
229–247.
Lindlay JA (1991c) Mixing for agricultural and food mater-
ials: 3. Powders and particulates. Journal of Agricultural
and Engineering Research 49: 1–19.
Niranjan K, Smith DLO, Rielly CD, Lindlay JA and Phillips
VR (1994) Mixing for agricultural and food materials:
5. Review of mixer types. Journal of Agricultural and
Engineering Research 59: 145–161.
Niranjan K (1995) An apprisal of the characteristics of food
mixing. In: Singh RK (ed.) Food Process Design and
Evaluation, pp. 47–67. Lancaster, PA: Technomic.
Rielly CD, Smith DLO, Lindlay JA, Niranjan K and Phillips
VR (1994) Mixing for agricultural and food materials:
4. Assessment and monitoring of mixing systems. Jour-
nal of Agricultural and Engineering Research 59: 1–18.
AGITATION 95

AIDS See HIV Disease and Nutrition
AIR CLASSIFICATION
Contents
Principles of Operation
Uses in the Food Industry
Principles of Operation
P Fedec, POS Pilot Plant Corporation, Saskatoon,
Saskatchewan, Canada
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Background
0001 In simplistic terms, air classification is a means of
using air to effect a dry separation of objects having
certain characteristics. These characteristics include
physical properties such as size, shape, density, and
physicochemical nature. Air classification is an oper-
ation that applies the technology to fractionation of
nonhomogenous particles, suspended in an air stream,
into classes of fairly uniform size based on a common
criterion of density or mass. Whereas some segrega-
tion of light and heavy particles can be achieved by
aspiration where airflow patterns are generated by
only a vacuum, true air classification makes use of
airflow induced through centrifugal motion with
the assistance of a vacuum. Generally, air classifiers
will segregate a heterogeneous particulate into two
subclasses, one primarily below a targeted particle
size (fine fraction) and the remainder above this
size (coarse fraction). Air classification becomes a
possible alternative when ordinary sieving fails to
effect separation below 40 mm. Applications of air
classification in the food industry separate par-
ticulates in the range of 2–60 mm, seldom exceeding
100 mm.
Factors Affecting Classification
0002 In the ideal case, classification can be best achieved if
the particles are uniform and homogeneous, spherical
and smooth, and dry and easily dispersible. Practic-
ally, very few finely granulated mixtures exhibit these
ideal properties. Other features such as particle geom-
etry can affect the tragectory of particles in an air
stream. Classification is also affected if some of the
particles are porous, allowing fine particles to hide
within the pores of the coarser particles.
0003Dispersibility is a key issue during classification.
Materials containing moisture or oil, or exhibiting
hygroscopic tendencies, are likely to agglomerate
and resist classification. To minimize these effects,
pre-classification techniques need to be considered,
which may include additional grinding, vacuum
drying, extraction by solvent, blending, dispersion,
or deagglomeration.
0004The cell structure of food materials is one of the
most important factors for the mechanical separation
or dry processing. Application has primarily been in
protein and starch separations of legume and cereal
flours. Within the cell, the protein forms a matrix in
which the starch granules are embedded. During
comminution, the breakdown of this matrix into
wedge protein or granular protein (and its size or
homogeneity) is important and will affect the effi-
ciency of classification (Figure 1). The cell matrix
must shatter easily, and in addition, the protein
matrix must disintegrate readily. If compound starch
granules are present, they must remain intact. A sig-
nificant number of particles (e.g., starch granules)
must be above the selected cut point. Typically,
impact milling is the comminution of choice.
0005Three particle-related factors of primary import-
ance in achieving separation include: particle density,
shape, and size. The difference between density of
starch (1500 kg m
3
) and protein (1300 kg m
3
)is
too small for density to have a significant role.
Shape plays some role; in particular, starch granules
flattened by impact milling exhibit increased air drag
and unfortunately may concentrate in the fine frac-
tion. Experience has shown that particle size is
the predominating influence on the quality of air
classification.
0006The particle size of the starting material is an
important factor in that, generally, the particle must
96 AIR CLASSIFICATION/Principles of Operation
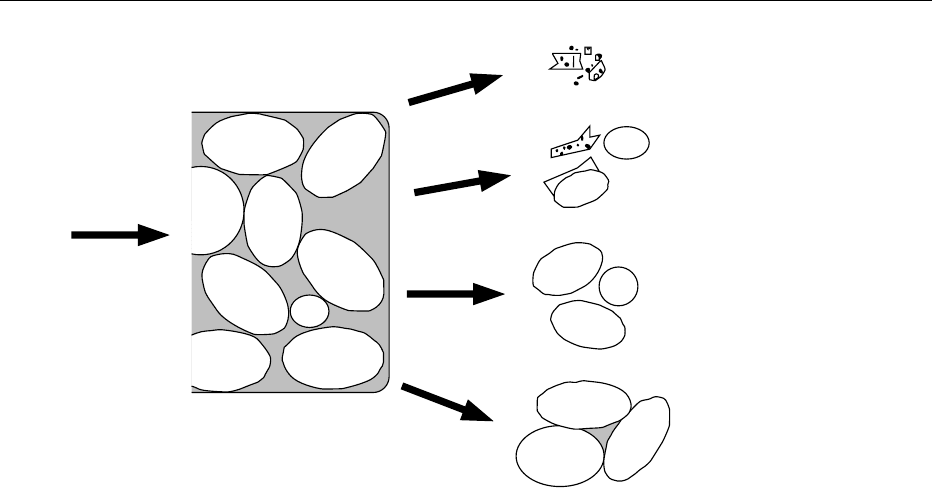
be less than 100 mm and preferably lower, with a
significant number being below the selected cut size.
For example, if the granular and wedge protein can be
pulverized into aggregates of 2–10 mm in size, while
the starch particles remain intact and are in the range
of 15–45 mm, separation is possible (Figure 1). Some
wedge protein of larger size will spill over into the
coarse particle cut, and some small granules will spill
into the protein or fine fraction. Products that do
not have a significant difference in the sizes of
components are not suitable candidates for air classi-
fication.
0007 The moisture content of grains and legumes affects
the ability to disintegrate the cell contents and dis-
lodge the protein from the starch. Practice suggests
that the moisture content for satisfactory comminu-
tion preceding air classification should be around
8%. Moisture contents above this value do not lead
to an ideal size reduction, but a moisture below 6%
can lead to starch granule fragmentation and subse-
quent spill-over into the fine fraction.
0008 No well-documented research has been carried out
on the influence of fat content of the starting material
on air classification, but in general, fat contents have
to be kept low in order to achieve dispersion in the
centrifugal zone and minimize bridging in and around
rotors, vanes, and passage-ways. Generally, a 1–2%
fat content is the limit, although some results have
indicated that flours having up to 7% fat are separ-
able by some classifier models, but prolonged operat-
ing times with sustained quality of separation have
not been established.
0009Sharpness of classification is affected by the feed
rate, and generally, as rates are elevated above opti-
mal, the sharpness decreases.
Air-classifier Operations
0010Air-classifier operation is influenced by air-flow dy-
namics on suspended/dispersed particles of typically
less than 100 mm. The term ‘cut size’ is used in con-
junction with discussing the segregation of the two
fractions.
0011If a predetermined particle size were selected (e.g.,
typically 15 mm in flour applications), a theoretical
ideal classification would permit a subdivision of a
mixed particle feed stock into two fractions: a fine
fraction below the predetermined size and a coarse
fraction above it. Figure 2a depicts such a segregation
of fine (shaded) and coarse (clear) fractions.
0012Under practical conditions, such a sharp separation
cannot be achieved, and the separation is more realis-
tically observed as shown by the curves in Figure 2b.
A single ‘cut size’ is not achieved; rather, there is a
range of particle sizes present in both the fines and
coarse fractions, and these overlap in the region of a
nominal cut size. The less the overlap, the better is the
separation efficiency.
0013The two major forces affecting air classification are
the drag or frictional force exerted on the particle by
the air flow and an inertial force exerted by the accel-
erated motion of the particles. The latter is generally
achieved by a centrifugal field or rotational air flow
through use of a rotor. A typical example of forces
Impact
milling
Endosperm cell
Free protein
(incl. wedge protein)
= 0.5 − 5 mm
Wedge protein, clusters
and detached small
granules
= 2 −15 mm
Detached large
starch granules
= 15 − 35 mm
Starch and protein
clusters
>35mm
fig0001 Figure 1 Conceptualized presentation of endosperm cells subjected to milling and subsequent fragmentation.
AIR CLASSIFICATION/Principles of Operation 97
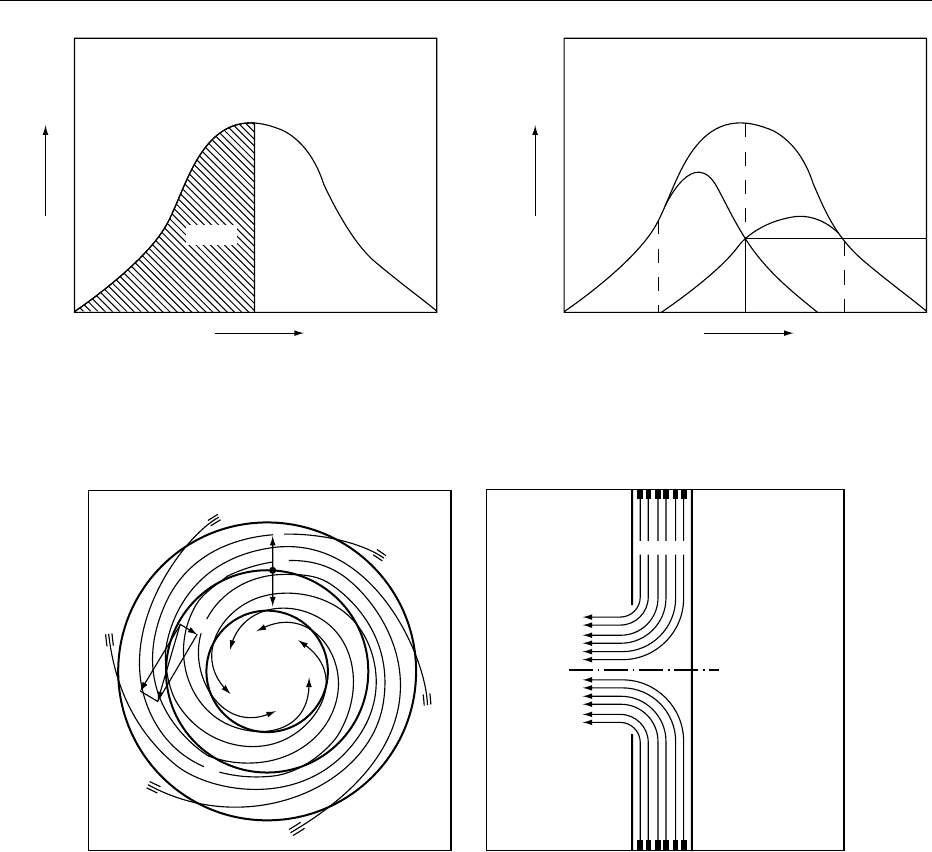
acting within a classifier is shown in Figure 3.A
particle (‘G’) of a certain diameter and density is
exposed to two opposing forces, the centrifugal force
(F) produced by a rotating assembly and frictional
force (R) produced by an air flow. Additional and
more complex mathematical models can be found in
articles by Vose and de Silva. As the air flows inwards
in a spiral path, particles entrained in the flow are
subjected to the opposing forces F and R. Larger
particles are dominated by the mass-dependent cen-
trifugal force, whereas small particles are influenced
by the frictional force. When the forces are equal, a
cut point or particle of a definite size can be estab-
lished. The cut size can be adjusted by manipulating
these forces within the classifier.
Design Styles of Air Classifiers
0014Many air-classifier variations are on the market. They
can be subdivided into types based on their principles
of operation. Some of the common types are detailed
below.
x min
x
q(x)
x max
Fines
Coarse
x min
x
q(x)
x max
Fines Coarse
(a) (b)
fig0002 Figure 2 Classification phenomena: (a) an ideal classification; (b) a real classification. Adapted from de Silva SR (1983) Develop-
ments in air classifier theory and practice. In: Institution of Chemical Engineers (Gr. Br.) Symposium Series, vol. 69, pp. 387–410, with
permission.
Cu
Cr
C
SS SSS
S
K
R
G
F
SSSSSS
= Centrifugal forceF
= Frictional forceR
= Cut size particleG
= Circular path
= Spiral flow line
= Air velocity
= Peripheral component
= Radial componentK
S
C
Cu
Cr
fig0003 Figure 3 Forces interacting in the spiral flow of an air classifier. From Hosokawa Micron International Inc. Catalogue 31/6e. Summit,
NJ with permission.
98 AIR CLASSIFICATION/Principles of Operation
Gravity Principle
0015 This is the simplest design, has no moving parts, and
consists of a zigzag-shaped classifying channel with
internal baffling. It is generally used for gross separ-
ations, often for dissimilar materials that may be
difficult to separate by simple separation techniques
such as screening.
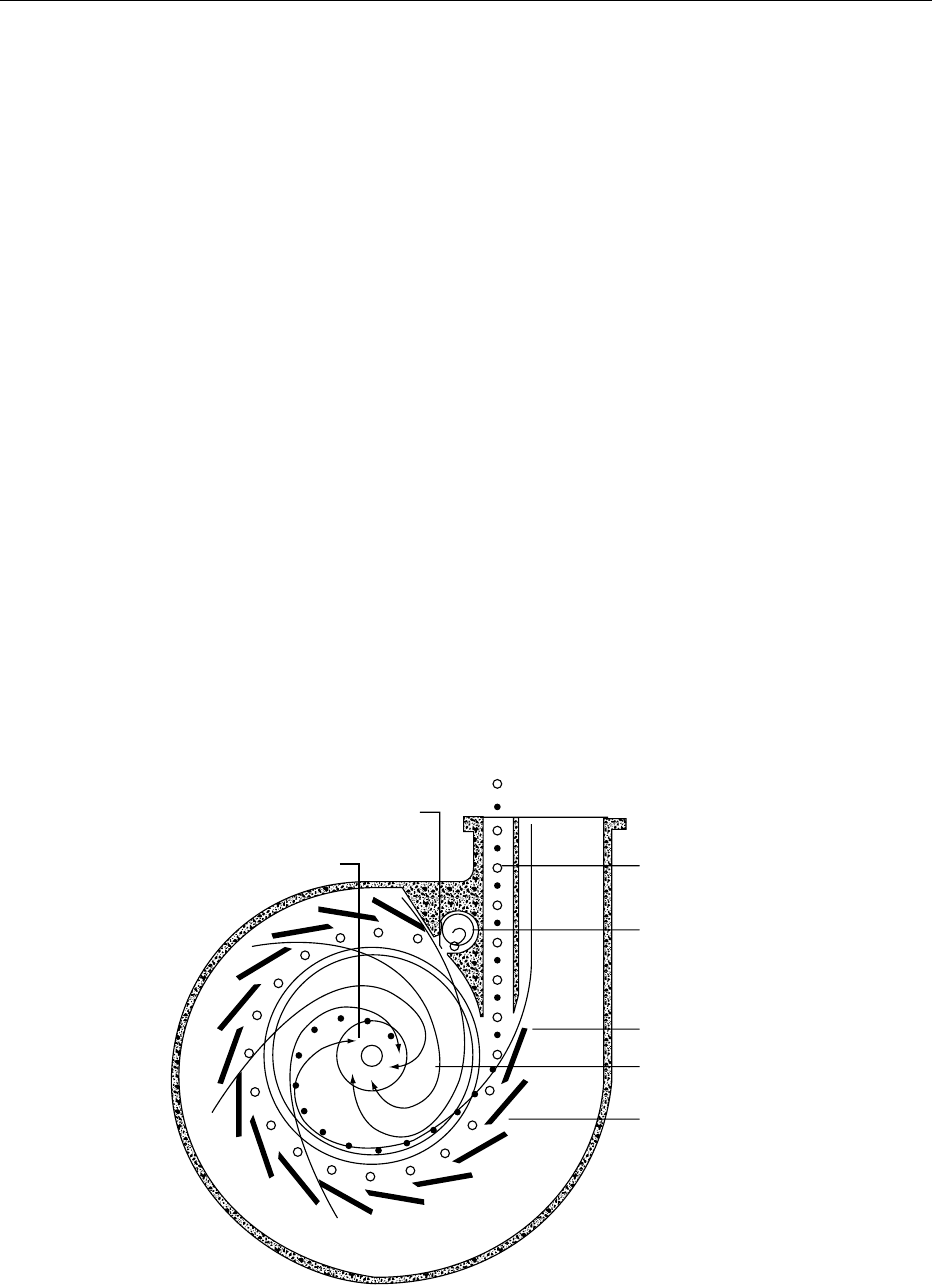
Free Vortex Principle
0016 Air classification is achieved by adjusting a set of
louvers located at the periphery of the classifying
zone, which allows the superimposed radial air flow
to enter the zone at an angle. Typical of this group are
designs such as Pallmann Galaxy and Alpine Mikro-
plex. The latter (Figure 4) has been used in a large
number of research studies, and significant data have
been published. This design incorporates a rotor on a
horizontal axis, resulting in a vertical plane of centri-
fugal force, whereas most others use a horizontal
plane that favors greater throughput but with a re-
duced precision in cut size. The Mikroplex MPS
model has been discontinued, but many are still in
industrial operation. Feed rates vary from 50 to
5000 kg h
1
, depending on the model size. Classifiers
manufactured by Larox operate on the same
principle, but the peripheral vanes are not adjustable.
The cut size is adjusted by manipulating the air flow
produced by a blower.
Forced Vortex Principle
0017The force is provided by means of a turbine or rotor
that disperses the particles into an air stream applied
by a suction fan. Variations in equipment design then
use air streams to select fine or coarse particles. The
Bauer Centri-Sonic was typical of this design and was
used commercially for cake flour separation as well as
much research, but its precision honeycomb rotor
was costly to manufacture, and the equipment has
been discontinued. A new breed of classifier, often
referred to as the turbine classifier, is now being util-
ized for fine powder separation (3–150 mm). These
may incorporate single or multiple turbine classifier
wheels in either vertical or horizontal configuration.
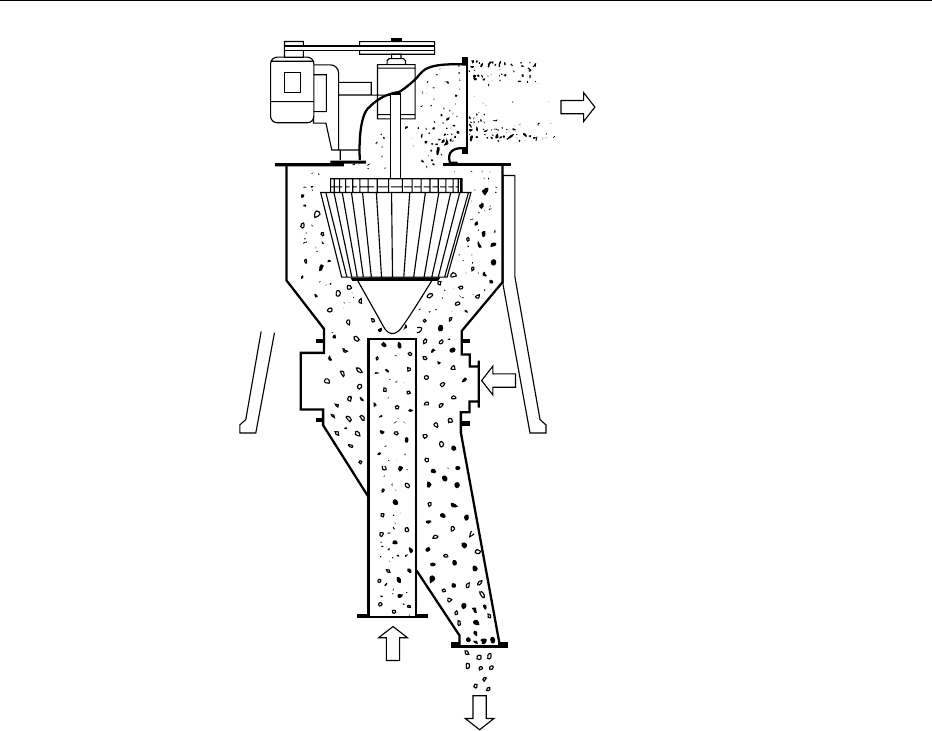
The MikroPul Micron Separator illustrates this con-
cept (Figure 5). Other manufacturers using this
principle include Hosokawa Micron, ABB Raymond,
and Alpine ATP series classifiers, as well as those
manufactured by Nisshin, Sturtevant, and Matter
and Partner.
0018Additional but less popular classifier designs used
mixed vortex, countercurrent, cocurrent, or cross-
flow principles. Some are experimental, and others
have not been applied to the food industry.
Combined Mill/classifier Units
0019In the food applications area, no appreciable techno-
logical changes to classifier design have been made in
Spiral air flow
Air guide vane
Coarse fraction
discharge worm
Coarse fraction
knife edge
Fine fraction
outlet
Coarse particles
Fine particles
Inlet for material
to be classified
Classifying
chamber
fig0004 Figure 4 Frontal cross-section view of a Mikroplex MP spiral classifier.
s
, coarse particles;
.
, fine particles. From Hosokawa Micron
International Inc. Catalogue 31/6e. Summit, NJ with permission.
AIR CLASSIFICATION/Principles of Operation 99
recent years, primarily because the profit margins
are low in this industry, thus providing little incentive
for spending resources on further development and
refinement.
0020 To reduce operating costs, interest has focused on
equipment that combines the required fine grinding
with classification. One such unit is the Mikropul
ACM Air Classifier Mill. The basic operation in-
cludes a high-speed impact mill through which an
air stream is drawn containing the raw material.
The product/air mixture enters the milling chamber,
after which the product is then accelerated by the
rotor towards peripheral guide vanes into the classifi-
cation zone. Here, the particles are acted upon by
centrifugal force in a centripetal airstream, and
lower-mass fine particles are separated and conveyed
out of the mill to a collector. The coarse particles are
directed back to the grinding zone for further size
reduction. The rotor speed and air flow are adjusted
to maximize mill performance in a given application.
Plant System Designs
0021The typical installation consists of a centrifugal fan
generating a vacuum draw to a baghouse collector.
An additional scrubber for fine particulates may be
included after the baghouse filter, and some designs
use an optional cyclone ahead of the baghouse col-
lector. The fines separated by the classifier are drawn
into the collector, whereas the coarse particles are
usually rejected by one of several ways, either in an
auger system, in an air stream to a cyclone, or within
a cyclone built into the classifier. The classifier may
be fed from a silo of premilled material or may be
directly coupled to a fine grinding mill. This direct
coupling method has received criticism in that
changes in material composition or particle size,
owing to stratification, affect the classifier cut fraction
and thereby the final product composition, especially
if the latter is being packaged directly from the pro-
cess line. Ancillary equipment includes rotary air
Fine product
discharge
Secondary
air inlet
Feed and
primary air
Coarse product
discharge
fig0005 Figure 5 Typical illustration of a turbine-type fine powder classifier (MikroPul Micron Separator MS). From Hosokawa Micron
International Inc. (1988) MikroPul Micron Separators Cat. No. HMS-1 5M-4/88. Summit, NJ with permission.
100 AIR CLASSIFICATION/Principles of Operation

locks for the collectors, filter assemblies, discharge
devices, blow-out relief doors, monitoring and con-
trol devices, blowers, silencing devices, and pulse jet
filter cleaning apparatus.
0022 Some operating facilities, in particular for legumes,
have used a double milling, double classification
design sequence, where the initial mill is followed by
a classifier, the coarse fraction remilled and reclassi-
fied, thereby yielding a final coarse fraction and two
fine fractions that are generally pooled. This plan
strives to increase the quantity recovered as fines,
although their quality may be compromised, and to
improve the purity of the coarse fraction.
0023 Safety factors that should be considered in an in-
stallation include dust inhalation, noise hazard,
vibration, and dangers of explosion from fine dusts,
which are ignitable under certain conditions.
Operating Conditions
0024 In addition to the features of the feed material as given
by the composition, nature of particles, product uni-
formity, and premilling influences, the variation of cut
point, and, subsequently, the quality/content of the
separated fractions can be manipulated by classifier
variables such as the rotational speed of the rotor or
disintegrator, the amount of air flow through the clas-
sifying chamber and the velocity of the air flow (both
can be altered by either adjusting air intake or the
external booster fan), degree or angle of vane setting,
which deflects flow, and finally the rate of feed de-
livered to the classifier. These options vary in both
availability and degree of adjustment, depending on
the manufacturer of the equipment. Some classifiers
allow for easy adjustment of fineness from an external
position while the unit is operating, whereas others
can be adjusted only after partial disassembly (i.e.,
such as alteration or adjustment of numbers or sizes
of selection plates). However, with proper manipula-
tion of these parameters, the classifier can produce a
quality separation of high efficiency and yield.
0025 Supplemental options are available for special ap-
plications whereby water cooling may be applied to
the outside cone of certain separators to satisfy the
needs of the cooling product (prior to packaging) that
has developed a temperature rise during the milling
and classification sequence.
0026 When selecting systems for an application, there is
no substitute for actually trying out a system on the
materials intended for classification. Most manufac-
turers maintain laboratory and pilot plant test facil-
ities, where various types and sizes of classifiers may
be tested for separation efficiencies. Some independ-
ent contract research centers are also able to conduct
exploration and demonstration testing.
See also: Air Classification: Uses in the Food Industry;
Plant Design: Basic Principles; Separation and
Clarification
Further Reading
Alpine Aktiengesellshaft (1978) Alpine Mikroplex Spiral
Air Classifiers MP, MPS. Alpine Bulletin 31/6e. Augs-
berg, Germany.
de Silva SR (1983) Developments in air classifier theory and
practice. In: Institution of Chemical Engineers (Gr. Br.)
Symposium Series, vol. 69, pp. 387–410.
Galk J (1998) Production of fine powder with the air classi-
fier mill ACM or the newly developed multistage impact
mill MSM. Powder Handling Process 10(1): 11–13.
Hixon L (1992) Sizing up air classifiers. Chemical Engin-
eering Progress 88(7): 59–62.
Hosokawa Micron International Inc. (1988) MikroPul
Micron Separators Cat. No. HMS-1 5M-4/88. Summit,
NJ.
Jones CR (1960) New developments in milling processes.
Protein displacement. Milling 135: 494–498.
Klumpar IV, Currier FN and Ring TA (1986) Air classifiers.
Chemical Engineering 93: 77–92.
Lauer O and Prem H (1978) Protein enrichment in vege-
table products by air classification. Zeitschrift fu
¨
r
Lebensmittel - Technologie und - Verfahrenstechnik 29:
212–215.
Schubert H and Heideker H-Th (1986) Protein separation
from vegetable sources by selective commination and air
classification. In: Le Maguer M and Jelen P (eds) Food
Engineering and Process Applications II, Unit Oper-
ations, pp. 293–303. London: Elsevier.
Thomas ND (1999) The MikroPul air classifier mill. Inter-
national Sugar Journal 101: 480–481.
Vose JR (1978) Separating grain components by air classifi-
cation. Separation and Purification Methods 7: 1–29.
Uses in the Food Industry
P Fedec, POS Pilot Plant Corporation, Saskatoon,
Saskatchewan, Canada
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Background
0001Air classification is a technology that is based on
physical principles, and therefore, the use of
chemicals is precluded, and protein denaturation is
avoided, as is the formation of artifacts. This dry
treatment also avoids the production of polluting
effluents and minimizes or obviates drying costs.
Two of its negative aspects are that there may be a
retention of antinutritional or undesirable compon-
ents in one or both fractions, and neither of the
AIR CLASSIFICATION/Uses in the Food Industry 101

fractions produced is ‘pure’ when compared to
those obtained through conventional wet processing;
hence, applications for products produced often need
to be developed.
Application to Grain Legumes
0002 Considerable research work on the milling and clas-
sification of nonoleaginous, starchy legume seeds has
been carried out in Canada at the National Research
council Prairie Regional laboratory and the Univer-
sity of Saskatchewan, both in Saskatoon. Although a
wide number of species were studied for their poten-
tial to commercialization, the focus on field peas led
to the adoption of this dry separation process by
industry. Similar procedures were adopted in France
for the production of fababean protein concentrate.
Other centers that have also explored fine grinding
and air classification in legumes include the Agricul-
tural and Food Research Council Food Research
Center and the University of Reading in the UK, the
Technical University of Denmark, the Michigan State
University in the USA, and the Korean Food Research
Institute. Most researchers have employed pin mills
for fine grinding of the seed, and a majority have
focused on using the Alpine Mikroplex MPS series
classifiers, although Alpine Zig Zag and Bauer Cen-
tri-Sonic series were also used. Recently, studies have
shown that the use of jet air mills has given flour
grinds having a mean particle size of < 40 mm, which
has improved separation of flour into protein and
starch fractions.
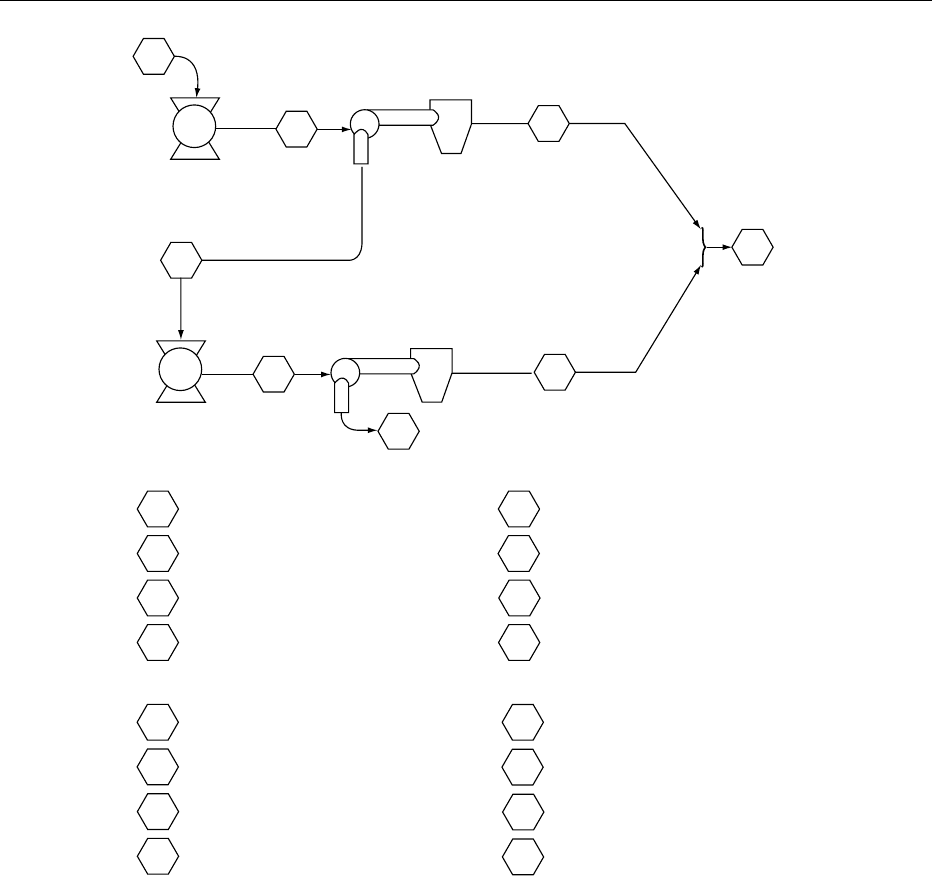
0003 A flow diagram for the process developed at the
Canadian Prairie Regional Laboratory and applied to
legumes is shown in Figure 1. Researchers have found
that the milling of the seed is of overriding import-
ance to the air-classification process. Efficient size
reduction in the initial milled flour should lead to
improved separation of the protein and starch com-
ponents. Examining Figure 1, whole or dehulled seeds
are finely ground in a pin mill and the resulting flour
classified in a spiral air stream classifier, set to a
predetermined cut point, resulting in the production
of a fine (protein) fraction (F) and a coarse (starch)
fraction (C). After the first pass, some protein bodies
still remain attached to the starch granule surface,
and some agglomerates remain, having starch gran-
ules embedded in a protein matrix. A second milling
of the starch fraction disrupts these complexes fur-
ther, and a second classification generates additional
protein (G), albeit at a reduced yield and protein cont
ent, and a final starch fraction (E) with low levels of
protein.
0004 In a typical example, the two-run process on field
peas generated a combined protein fraction of 34.1%
yield and a protein content of 56.6% (Figure 1). For
fababeans, a protein fraction of 37.2% yield and a
protein content of 68.1% was produced. Starch frac-
tions contained 6–7% residual protein. Repeated pin
milling and air classification studies show that the
starch fraction can be further purified, but even
after four runs, the residual protein is still around
3% for field peas. It follows that little is to be gained
from a process based on more than two runs, and,
depending on economics, it may be sufficient to use
only a single-run procedure.
0005A comprehensive review of the published data
would be beyond this article. However, to give the
reader an overview of what may be achieved/expected
from various legume flours, a summary table (Table 1)
has been prepared. By way of caution, it should
be noted that the data are drawn from a range of
classifier types and operating parameters on starting
flours with varying initial protein levels prepared by
employing a wide assortment of grinding mills from
seed with varying moisture contents. The data are
presented as min–max ranges recorded in the litera-
ture. All data shown are based on a single impact
milling followed by a single air classification, gener-
ally using dehulled seeds.
0006Low fine fraction yields have a correspondingly
higher level of protein content as well as a higher
protein level in the coarse (starch) fraction. As the
yield of fines increases, the protein content of the
fine fraction decreases, and the quantity of the coarse
fraction will show a decrease. The protein content in
the coarse fraction will decrease, and correspond-
ingly, the starch proportion will increase. In all cases,
the recovery of protein into the fine fraction is not as
efficient as the recovery of the starch into the coarse
fraction.
0007The initial protein content of the legume flour has
an influence on the classification result. At the same
yield or split ratio, higher protein levels in the start-
ing flour will result in a higher protein content in the
fines fraction. Alternatively, if the protein content in
the fines fraction is kept constant, a higher yield of
that fraction can be expected. Finely milled, defatted
soybean meal has been added to Table 1 for compara-
tive purposes and serves to indicate that, unless there
is a high proportion of relatively large and uniform
starch granules, a shift of protein cannot be accom-
plished, despite achieving a separation into two
fractions.
0008Comparing the legumes, good separation may be
expected with faba, lima, mung, navy, Great North-
ern beans, lentils, peas, and cowpeas. Especially high
protein concentrates have been achieved from finely
ground fababean flour. Chickpeas are a poor candi-
date for air classification, presumably because of the
102 AIR CLASSIFICATION/Uses in the Food Industry
higher oil content in the flour and a high proportion
of small starch granules.
0009 The separation efficiency (i.e., the percentage of
total protein recovered in the fine fraction or the
percentage of total starch recovered in the coarse
fraction) is dependent upon moisture at the time the
legumes are milled. In peas, an increase in moisture to
14% from 10% reduces the separation efficiency by
20%. As the seed moisture decreases, there is a de-
crease in the starch yield, in the protein content of the
starch fraction and in the protein content of the pro-
tein fraction. However, there is an increase in the
yield of the protein fraction as well as the protein
separation efficiency. It has been suggested that a
moisture range of 8–10% is ideal for an impact-
milling, air-classification process operation in
legumes.
0010The effect of seed maturity on air classification
suggests that the presence of immature seeds, even
at high levels, has little effect on subsequent milling
and classification. There is concern that green and
shrunken seeds may make dehulling difficult and
translate to high fiber levels in the starch fraction.
0011The protein content of the starting flour, and ini-
tially of the seed supply prior to milling, has a signifi-
cant effect on fractionation. In a study of widely
varying protein content pea flours, it was noted that
the percentage protein in all air-classified fractions
Field peas
Dehulled seed
A
A
65.9 kg Pea starch (6.2% protein)
E
E
Air
classifier
Air
classifier
22.3 kg Protein I (59.2% protein)
F
F
11.8 kg Protein II (51.8% protein)
G
G
34.1 kg Pea protein (56.6% protein)
H
H
B
B 100 kg Pea flour (23% protein)
77.7 kg Starch I (12.4% protein)
C
C
Remilled starch I
D
D
Fababeans
Dehulled seed
A
62.8 kg Bean starch (7.2% protein)
E
21.1 kg Protein I (71.3% protein)F
16.1 kg Protein II (63.8% protein)
G
37.2 kg Bean protein (68.1% protein)
H
B 100 kg Bean flour (30.4% protein)
78.9 kg Starch (19.6% protein)
C
Remilled starch I
D
Pin
mill
Pin
mill
fig0001 Figure 1 Double-pass milling and air classification sequence for legumes. Adapted from Tyler RT, Youngs CG and Sosulski FW
(1981) Air classification of legumes. I. Separation efficiency, yield and composition of the starch and protein fractions. Cereal
Chemistry 58: 144–148, with permission.
AIR CLASSIFICATION/Uses in the Food Industry 103
