Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.

using appropriate software (for example, library
search or quantification routines).
0006 In the special case of SIM, preselected masses
are scanned in preference to the entire spectrum.
This yields much greater sensitivity and is used for
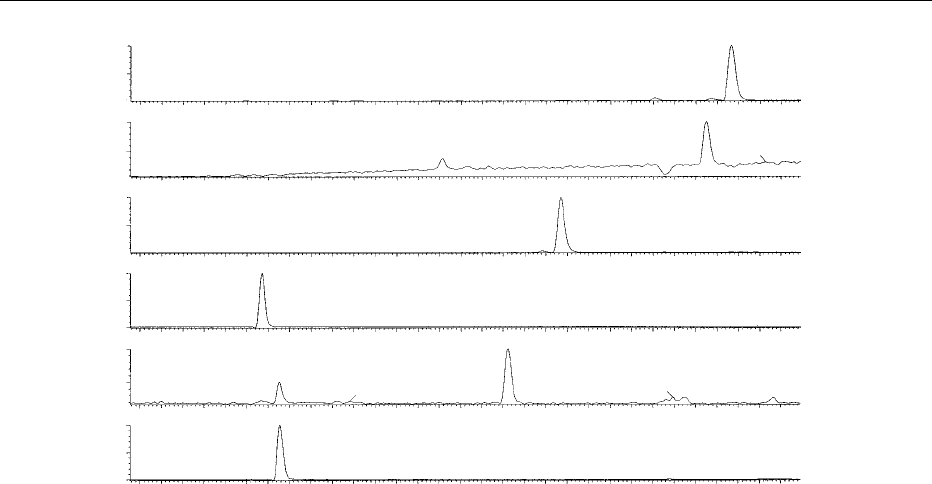
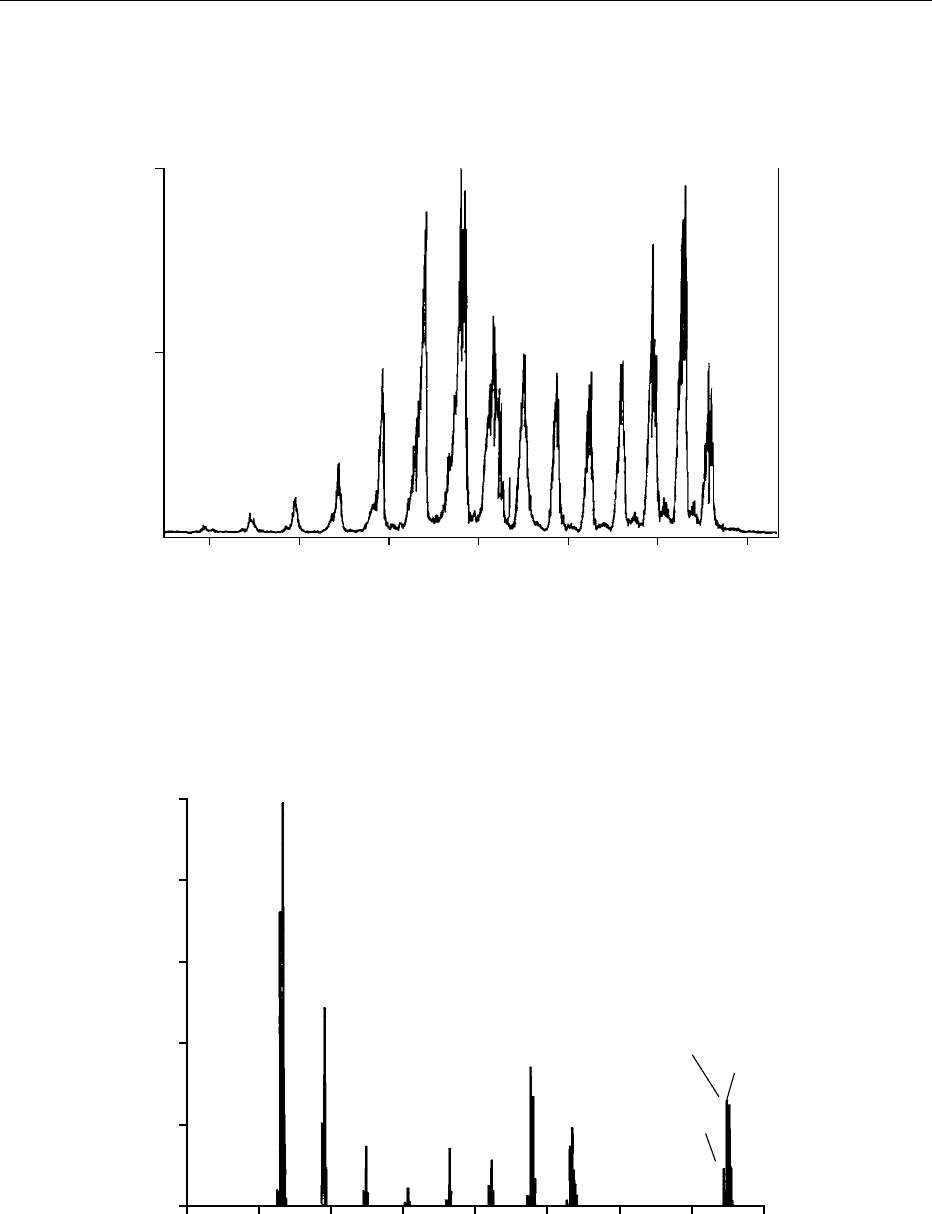
quantifying analytes. Figure 3 shows an LC-MS SIM
chromatogram of the molecular anions of the gluco-
sinolates glucoraphanin, glucoerucin, glucobrassicin,
4-hydoxyglucobrassicin, 4-methoxyglucobrassicin,
and neoglucobrassicin extracted from broccoli.
0007 Sinigrin (a glucosinolate not present in broccoli) is
used as a quantitative spike. Peak areas are integrated
and the ratios of these areas against that of the sini-
grin internal standard are used to generate quantita-
tive data by comparison with a calibration curve.
Gas Chromatography–Mass Spectrometry
0008 GC-MS was the first combined chromatography–
mass spectrometry technique to enjoy widespread ac-
ceptance in food science (one of the earliest published
applications of GC-MS was in flavor analysis). GC-
MS is still used widely in food science, particularly in
flavor analysis. User-friendly and cheap benchtop in-
struments are now available for conducting these
types of analysis. Despite their compactness and sim-
plicity, these instruments are sensitive and equipped
with powerful data systems that are used not only to
control and acquire data from the gas chromatograph
and mass spectrometer, but also to identify flavor
components by automated matching against reference
libraries of spectra of known flavorants. More sophis-
ticated GC-MS instruments are also used in many
other areas of food analysis, especially in the identifi-
cation of toxic residues (e.g., dioxins). Advances in
column technology (particularly the widespread use
of flexible fused silica columns) and improved mass
spectrometer vacuum pumps mean that coupling a gas
chromatograph to a mass spectrometer is now routine
and does not require any special form of interface.
Capillary columns generally operate at helium gas
flows of a few milliliters per minute, and modern
turbomolecular vacuum pumps are easily able to
cope with these flow rates and still maintain ion
source pressures (for electron ionization (EI) ) of
<10
3
Pa. One of the main recent advances in GC-
MS technology has been the introduction of ‘fast’ GC-
MS techniques in which the speed of analysis available
from some types of modern GC capillary columns
have been complemented by fast-scanning mass spec-
trometers (time-of-flight instruments, for example).
GC-MS techniques are now routine and will not be
dwelt on at any length here. LC-MS is now providing
some of the most interesting and versatile new meth-
odologies in food analysis and food science generally
and this article will focus mainly on this technology.
Liquid Chromatography–Mass
Spectrometry
0009LC-MS presents far more technical problems than
GC-MS because of the incompatibility of flowing
100
%
0
100
%
9
100
%
1
100
%
−2
100
%
17
100
%
−1
m/z 463.05
m/z 447.05
m/z 436.04
m/z 420.05
m/z 358.03
m/z 477.06
11.0010.009.008.007.006.005.00 12.00
13.00 14.00 15.00 16.00 17.00
18.00 19.00
18.33
17.85
17.75
16.54
7.16
9.40
11.57
12.17 12.65 13.45 13.94
14.57 15.91
19.14
19.55
16.37
17.09
Neoglucobrassicin
4-Methoxyglucobrassicin
4-Hydroxyglucobrassicin
14.35
Glucobrassicin
Glucoerucin
Glucoraphanin
7.34
5.00
6.70
7.33
7.77
9.08
9.41
13.12
16.96
17.23
19.32
Time
7.77
Sinigrin
fig0003 Figure 3 Negative-ion electrospray liquid chromatography–mass spectrometry (LC-MS) selected ion-monitoring (SIM) chromato-
gram of intact glucosinolates extracted from broccoli. Copyright Institute of Food Research, reproduced with permission.
1296 CHROMATOGRAPHY/Combined Chromotography and Mass Spectrometry
liquids with the high vacuum necessary for mass spec-
trometers to function. A typical liquid chromatograph
eluent flow of 1 ml min
1
becomes over 1000 times this
flow of gas when converted to a vapor, far in excess of
the gas flow that a conventional mass spectrometer ion
source can cope with and still maintain vacuum. Fur-
thermore, the expansion from liquid to gas results in
dilution of analyte molecules entrained in the solvent,
potentially reducing sensitivity by a very large factor.
Consequently, special interfaces and ion sources have
been developed to remove excess solvent vapor and
insure that the analytes are not diluted excessively.
LC-MS Interfaces and Ion Sources
0010 Several different techniques, or interfaces, for com-
bining liquid chromatography and mass spectrometry
were developed in the 1970s and 1980s. These in-
cluded the direct liquid introduction (DLI) interface,
the moving belt interface, dynamic FAB (fast atom
bombardment) or dynamic LSIMS (liquid secondary
ion mass spectrometry), and thermospray ion source.
All of these techniques had some disadvantages in
terms of the range of compounds amenable to analy-
sis, robustness and reliability, sensitivity and, in some
cases, limited flow rates.
Electrospray and Atmospheric Pressure Chemical
Ionization
0011 The interfaces mentioned above have been super-
seded by electrospray (ESI) and atmospheric pressure
chemical ionization (APCI) ion sources. Both of these
ion sources can be coupled directly to liquid chro-
matographs, with very few restrictions on flow rate
or solvent composition (See Mass Spectrometry: Prin-
ciples and Instrumentation). Both techniques have
been used very widely in food and nutrition studies.
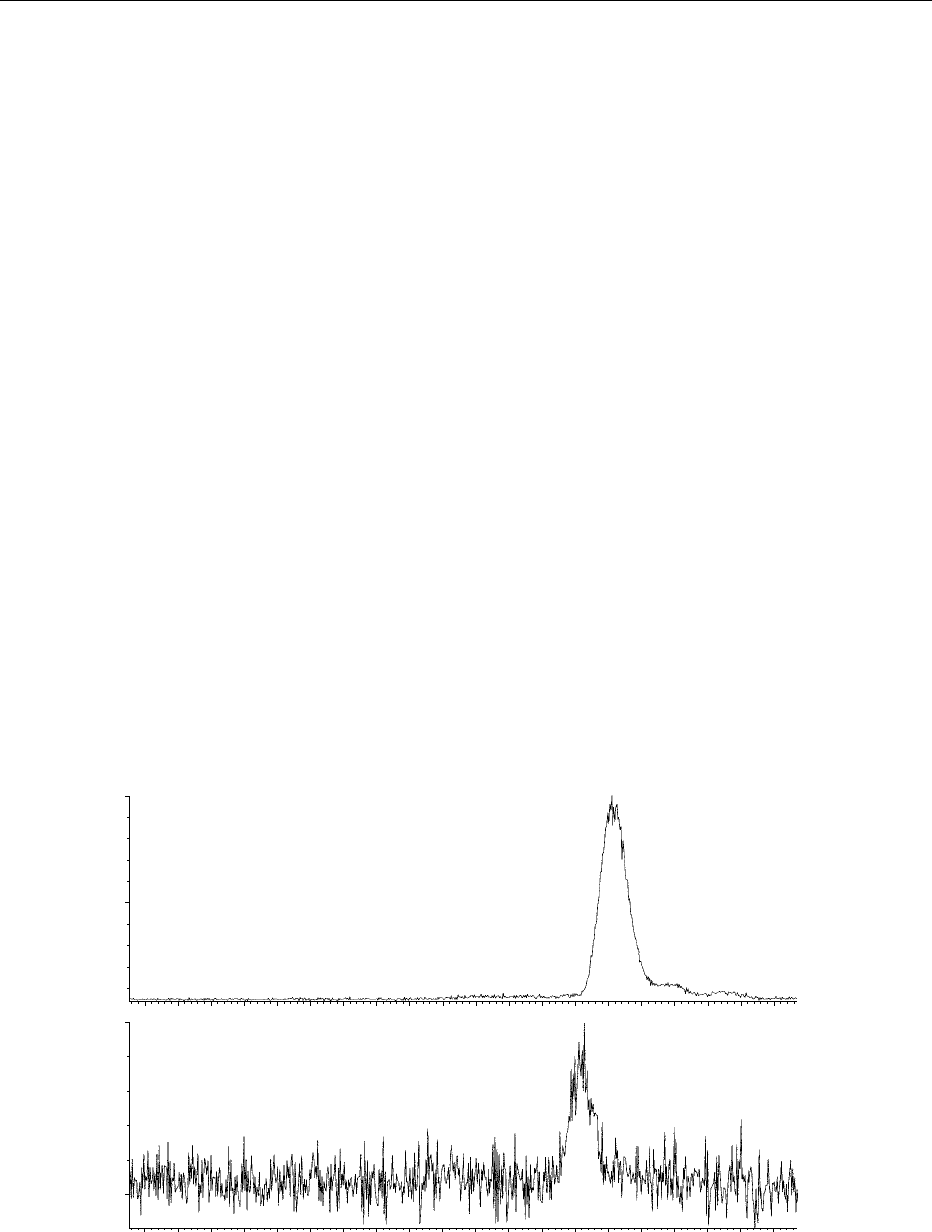
Examples of SIM chromatograms of 1 and 20 pmol of
b-carotene (Figure 4) and a mass chromatogram
(Figure 5a) and spectrum (Figure 5b) of quercetin 3
0
glucoside are shown. These types of data are useful in
quantitative and qualitative metabolic studies.
The Particle Beam Interface
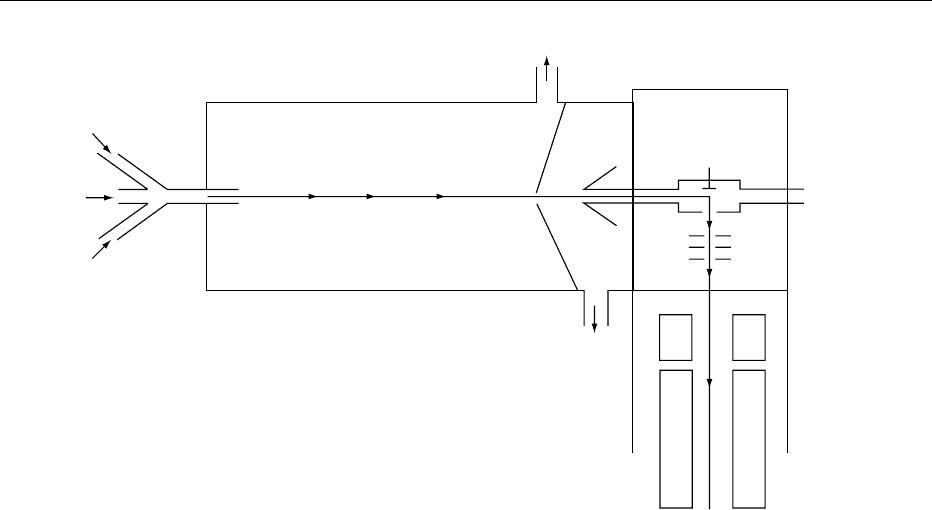
0012A third type of interface, the particle beam, completes
the trio of modern LC-MS technology, providing a
suite of techniques capable of handling nearly all types
of analysis. A schematic diagram of a particle beam
interface is shown in Figure 6.
0013The incoming liquid stream (i.e., the high-perform-
ance liquid chromatography (HPLC) eluent) enters
the device on the left of the diagram. The liquid is
nebulized by helium gas flowing, forming a fast-
moving spray of droplets that enter the heated deso-
lvation chamber. Solvent begins to evaporate from the
spray droplets which shrink, thus concentrating any
dissolved sample molecules. The partially evaporated
spray then passes through a narrow orifice (skimmer
1 in the diagram) and across a region that is main-
tained under vacuum by a rotary pump. A supersonic
molecular beam is formed in this region and, because
100
%
40
100
%
4
20 pmol beta-carotene
1 pmol beta-carotene
Peak area 61625
Peak area 5292
11.00 12.00 13.00 14.00 15.00 16.00 17.00 18.00 19.00 20.00
Time
fig0004 Figure 4 Positive-ion atmospheric pressure chemical ionization liquid chromatography–mass spectrometry selected ion-
monitoring chromatogram of the molecular ion of b-carotene (1 and 20 pmol injections, respectively). The slight retention time shift
between the two traces is caused by temperature drift affecting the chromatography. These types of data are useful for quantifying
carotenoids in food extracts and in metabolic experiments. Copyright Institute of Food Research, reproduced with permission.
CHROMATOGRAPHY/Combined Chromotography and Mass Spectrometry 1297
the solvent molecules and helium atoms are signifi-
cantly lighter than sample molecules, they diffuse
away more rapidly and are pumped out of the system
preferentially. This leaves the heavier solute mol-
ecules concentrated in the beam. A second skimmer
removes yet more solvent molecules and allows par-
ticles of sample to enter the EI or chemical ionization
(CI) ion source. The interface can handle flows of
between 0.1 and 1 ml min
1
, depending on the
volatility of the solvent.
0014The great advantage of the particle beam system is
that it can generate CI and, especially, EI spectra that
are highly reproducible and comparable with spectra
obtained by other types of EI and CI inlet systems.
These spectra are therefore searchable against data-
bases of known spectra. The major difference be-
tween the particle beam interface and other LC-MS
systems is that the device processes the HPLC eluent
in such a way that sample molecules can be ionized by
the conventional mass spectrometric techniques of EI
100
%
2
100
%
−2
Sum of intensities of m/z 300−800
Quercetin 3' glucoside
Diode array trace (190−450 nm)
2.00 4.00 6.00 8.00 10.00 12.00 14.00 16.00 18.00 20.00 22.00
Time
100
%
0
155.2
160 180 200 220 240 260 280 300 320 340 360 380 400 420 440 460 480 500 520
m/z
480.2
481.2
520.2
501.1
482.2
468.5
463.2
443.2
426.7
414.9
403.0
376.0
368.0
357.1
344.1
322.1
305.0
304.1
172.2
190.1
208.5
222.1
229.2
247.2
261.6
274.2
287.1
303.0
479.2
fig0005 Figure 5 Positive-ion electrospray ionization liquid chromatography–mass spectrometry (a) chromatogram and (b) mass spectrum
of the important food flavonoid quercetin 3
0
glucoside. Copyright Institute of Food Research, reproduced with permission.
1298 CHROMATOGRAPHY/Combined Chromotography and Mass Spectrometry
and CI. APCI and ESI are effectively ionization
techniques that generate anions or protonated mol-
ecules with few fragment ions under normal operat-
ing conditions. The particle beam method does not
have the versatility and sensitivity of APCI and ESI
but is useful for analyzing nonthermally labile
molecules that are insufficiently volatile for GC-MS
analysis.
Capillary Electrochromatography–Mass
Spectrometry
0015 CEC is a high-resolution separation technique that
has been described as a hybrid of HPLC and CE.
The high efficiency available from CEC makes it an
ideal partner for coupling to mass spectrometry as the
narrow chromatographic peaks result in increased
mass flow per unit time into the mass spectrometer
ion source, and thus greater sensitivity as well as
selectivity. Many of the technical problems associated
with coupling CEC to mass spectrometry (principally
Joule heating and consequent bubble formation) are
being tackled successfully, to the extent that auto-
mated sample introduction systems may be used
with CEC-MS systems. Although the technique
has not yet caught on in food science and nutrition,
CEC-MS has great potential in studies requiring
separation of complex mixtures and high-sensitivity/
high-selectivity analysis.
Capillary Electrophoresis–Mass
Spectrometry
0016Many of the difficulties associated with combining
the ultrahigh-resolution separation technique of CE
with MS have been overcome. However, a significant
number of publications devoted to interfacing CE
with MS still appear annually, indicating that an
ideal solution is still not available. Despite this, sev-
eral mass spectrometer manufacturers now supply
special probes for interfacing CE with MS, indicating
that the technology is now commercially viable. The
ionization technique most compatible with CE is elec-
trospray and the vast majority of CE-MS applications
reported make use of this ionization technique. Re-
cently the use of microfabricated devices (‘CE on a
chip’) with mass spectrometers has been reported and
promises significant advances in sensitivity and repro-
ducibility. As with CEC, there are few reported appli-
cations of CE-MS in the food and nutrition sciences;
nevertheless, the potential of technique in these fields
is very high.
Supercritical Fluid Chromatography–
Mass Spectrometry
0017Techniques for coupling SFC to MS were first
described in the late 1970s. Although a number of
different SFC-MS interfaces were developed in the
Quadrupole
analyzer
Vacuum pump 2
Vacuum pump 1
GC transfer line
EI/CI source
Skimmer 2
Skimmer 1
Desolvation chamber
Helium
Nebulizer
LC
fig0006 Figure 6 Schematic diagram of a particle beam liquid chromatography–mass spectrometry interface. LC, eluent exiting liquid
chromatograph; EI/CI, electron impact/chemical ionization; GC, gas chromatography. Reproduced from Mellon FA, Self R and Startin
JR (2000) Mass Spectrometry of Natural Substances in Foods. London: Royal Society of Chemistry, with permission.
CHROMATOGRAPHY/Combined Chromotography and Mass Spectrometry 1299
intervening years, APCI, with specially adapted SFC
probes, is currently the main method of choice. The
APCI source is particularly suited to SFC because it is
unaffected by the increasing flow rates resulting from
pressure programming of the supercritical mobile
phase and because of the ease of connection with
E+06
1,739
100
50
Relative abundance (%)
Time (min:s)
26:40 30:00 33:20 36:40 40:00 43:20 46:40
1
2
3
4
5
7
8
9
10
11
13
14
15
16
17
18
19
20
21
22
23
12
6
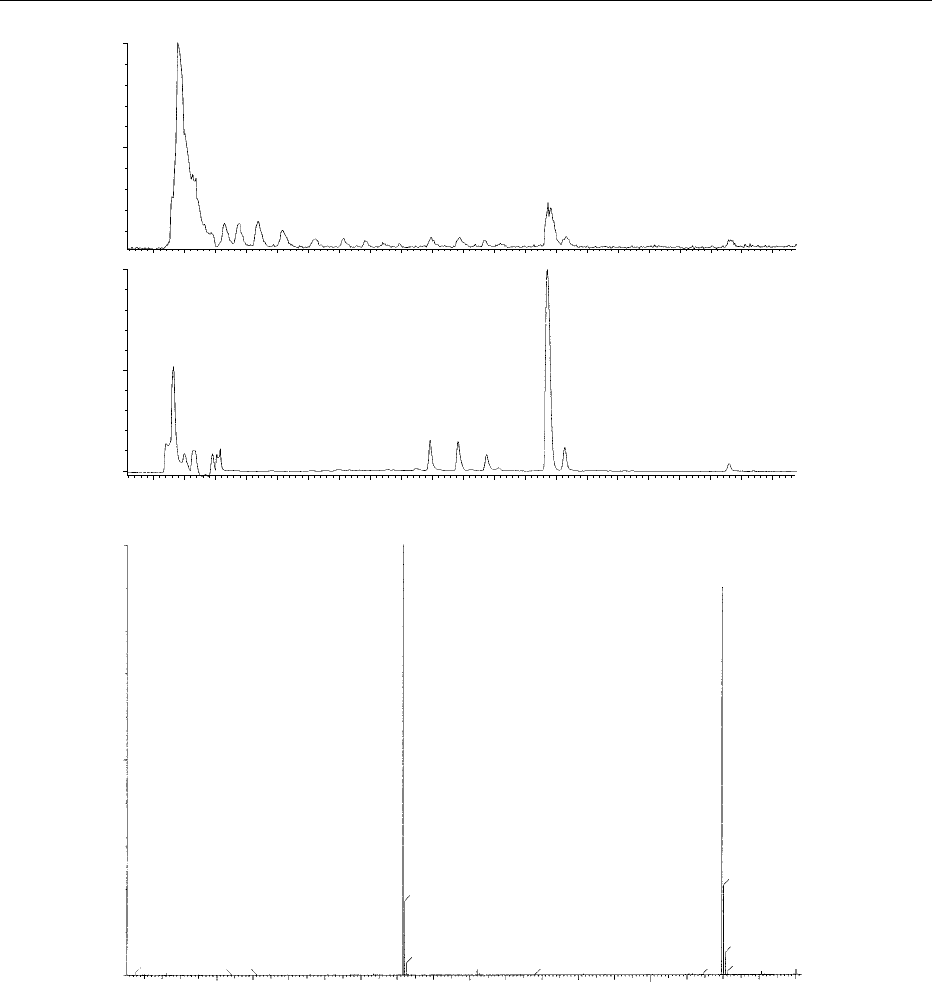
fig0007 Figure 7 Reconstructed ion chromatogram of milk fat triacylglycerols analyzed by capillary supercritical fluid chromatography–
mass spectrometry. Samples ionized by atmospheric pressure chemical ionization with ammonia as reactant ion. Reproduced from
Laakso P and Manninen P (1997) Identification of milk fat triacylglycerols by capillary supercritical fluid chromotagraphy atmospheric
pressure chemical ionization mass spectrometry. Lipids 32: 1285–1295, with permission.
100
80
60
40
20
0
350
400 450 500 550 600 650 700 750
m/z
[M-C
18
]
+
411.4
{
[M-C
16
]
+
439.3
{
[M-C
14
]
+
467.5
{
[M-C
12
]
+
495.5
{
[M-C
10
]
+
523.5
{
[M-8:0]
+
551.6
{
[M-6:0]
+
577.6
{
[M-4:0]
+
605.7
710.7(40:1)
712.7(40:0)
708.7(40:2)
{
[M+18]
+
{
fig0008 Figure 8 Mass spectrum of peak 9 in Figure 7. Samples ionized by atmospheric pressure chemical ionization with ammonia as
reactant ion. Reproduced from Laakso P and Manninen P (1997) Identification of milk fat triacylglycerols by capillary supercritical fluid
chromotagraphy atmosphere pressure chemical ionization mass spectrometry. Lipids 32: 1285–1295, with permission.
1300 CHROMATOGRAPHY/Combined Chromotography and Mass Spectrometry

SFC systems. Although SFC-MS is much less popular
than LC-MS in the food sciences, it is particularly
suited to certain types of analysis. A good example
is provided by the identification of milk fat triacylgly-
cerols by capillary SFC-MS. Samples were ionized, in
APCI mode, by admitting methanol or 0.5% ammo-
nia in methanol as reagent gas. A reconstructed ion
chromatogram of the milk fat triacylglycerols is
shown in Figure 7.
0018 Individual mass spectra yielded abundant [M þ
18]
þ
and [M RCOOH]
þ
ions when ammonia was
present in the reagent gas, thus defining molecular
weight and the nature of the fatty acid constituents
of the triacylglycerols. As can be seen in Figure 7,
complete chromatographic resolution of all triacyl-
glycerols was not achieved, However, the specificity
of the mass spectrometric data allowed identification
of different components of unresolved chromato-
graphic peaks where fatty acid chain lengths yielded
characteristic fragment ions. The mass spectrum of
peak number 9 in Figure 7 is shown in Figure 8.
0019 Three different chromatographically unresolved
triacylglycerols, 40:0, 40:1, and 40:2, are present.
This study demonstrated the value of SFC-MS in the
analysis of both saturated and unsaturated triacylgly-
cerols.
0020 The interpretation of the mass spectrum in Figure 8
was complicated by the occurrence of several differ-
ent-molecular-weight species in a single chromato-
graphic peak. However, the [M RCOO]
þ
ion
region contained ion clusters that indicated the pres-
ence of C
18
and C
16
fatty acid moieties in the triacyl-
glycerols.
See also: Chromatography: High-performance Liquid
Chromatography; Gas Chromatography; Supercritical
Fluid Chromatography; Mass Spectrometry: Principles
and Instrumentation; Applications
Further Reading
Chapman JR (1995) Practical Organic Mass Spectrometry:
A Guide for Chemical and Biochemical Analysis, 2nd
edn. Chichester: John Wiley.
Cole RB (1997) Electrospray Ionization Mass Spectrom-
etry. New York: John Wiley.
James P (2000) Proteome Research: Mass Spectrometry
(Principles and Practice). Berlin: Springer Verlag.
Johnstone RAW and Rose ME (1996) Mass Spectrometry
for Chemists and Biochemists, 2nd edn. Cambridge:
Cambridge University Press.
McMaster MC and McMaster C (1998) GC/MS: A
Practical User’s Guide. New York: John Wiley.
Mellon FA, Self R and Startin JR (2000) Mass Spectrometry
of Natural Substances in Food. London: Royal Society
of Chemistry.
Siuzdak G (1996) Mass Spectrometry for Biotechnology.
San Diego: Academic Press.
Willoughby R, Sheehan E and Mitrovich S (1998) A Global
View of LC/MS: How to Solve Your Most Challenging
Analytical Problems. Pittsburgh: Global View.
CHROMIUM
Contents
Properties and Determination
Physiology
Properties and Determination
R Farre
´
and M J Lagarda, University of Valencia,
Burjassot, Spain
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Physical and Chemical Properties
0001 Chromium is a hard, brittle, white metal of the first
transition series, with an atomic number of 24, a
relative atomic mass of 51.996 amu, an electronic
configuration [Ar]3d
5
4s
1
, and a relative density of
7.19. The melting and boiling points are 1857
C
and 2672
C, respectively.
0002The resistance of chromium to attack from a var-
iety of chemicals at normal temperature makes it
useful in protecting other more reactive metals, e.g.,
it is a component of stainless steel. However, at high
temperatures it reacts with many chemicals.
0003As a typical transition metal element, chromium
forms many compounds that are colored and para-
magnetic. It has oxidation states from 2toþ6, but
CHROMIUM/Properties and Determination 1301

the most common and stable oxidation states are
þ2, þ3, and þ6. Since Cr(II) is a very strong redu-
cing agent, it is not found in biological systems. All
Cr(VI) compounds, except the hexafluoride (CrF
6
),
are oxo compounds; chromium occurs predomin-
antly as either chromate (CrO
2
4
) or dichromate
(Cr
2
O
2
7
). Cr(VI) compounds are strong oxidizing
agents and are therefore readily reduced to Cr(III) in
acidic solutions. The most stable and important oxi-
dation state is þ3.
0004 One of the outstanding features of Cr(III) che-
mistry is the ability of trivalent chromium to form
coordination complexes, most of which are hexacoor-
dinated. These coordination complexes are relatively
inert. In aqueous solution the hexahydrate ion
[Cr(H
2
O)
6
]
3þ
exists in an octahedral configuration.
In aqueous solution the ligand and displacement
reactions have half-lives of several hours.
0005 Chromium can form bridges with hydroxyl groups
(olation). Olation is enhanced by alkalis and tempera-
tures up to 120
C, but can be prevented and reversed
by oxalate ions and other strong ligands, whereas
weaker ligands can only prevent the reaction. In bio-
logical systems pyrophosphate, methionine, serine,
glycine, leucine, lysine, and proline therefore inhibit
the olation of Cr(III). In such systems chromium is
able to react because its solubility is maintained by
weaker organic and inorganic ligands.
Determination in Foods and Beverages
0006 There are a wide range of techniques available for
the analysis of chromium and other trace elements.
Chromium is one of the most difficult elements to
determine accurately because of the very low levels
in foods.
0007 The determination of chromium content in bio-
logical matrixes has long been a controversial prob-
lem because of the many errors that can arise and
invalidate the data. Therefore, much of the earlier
work should be viewed critically. Reasons for this
skepticism arise from a number of problem areas:
.
0008 The low chromium content of normal (nonconta-
minated) biological samples. In food samples the
concentration is on the order of nanograms per
gram and, therefore, it is essential to apply very
sensitive measurement techniques.
.
0009 Chromium losses by volatilization during sample
dry ashing above 450
C.
.
0010 Contamination at any point through sample collec-
tion and handling, before the content is measured.
.
0011 Errors inherent in the applied analytical method,
such as, for instance, matrix interferences in
electrothermal atomic absorption spectrometry
(ETA-AAS) and spectral in inductively coupled
plasma–atomic emission spectrometry (ICP-AES).
0012Studies should report precise data on all steps of the
analytical procedure, including sample collection,
homogenization, and matrix reduction. An accurate
determination is a challenge because of problems that
originate from the risk of chromium losses by volatil-
ization or adsorption and/or enrichment by contam-
ination. Interlaboratory assays therefore give rise,
in the case of chromium analysis, to very variable
results.
0013Of all of the proposed analytical methods, AAS is
the one most often used for chromium determination
in biological samples. When applied with a graphite
furnace (ETA-AAS) it is one of the most sensitive
techniques, but it is open to a wide range of interfer-
ences, which depend on the matrix and can lead to
erroneous results. The advances in background cor-
rection procedures have contributed to overcome
these limitations.
0014ICP-AES is also applied to determine chromium in
foods and beverages. Although the detection limits
are higher than those obtained with ETA-AAS, it
offers the advantages of simpler sample preparation
and the possibility of carrying out multielemental
analysis.
0015Determination of total chromium content may not
be a valid indicator of the nutritional benefit of a food
because not all chromium in foods has activity as a
glucose tolerance factor. Some authors point out that
the alcohol-extractable fraction of chromium seems
to be a more reliable index. The speciation of chro-
mium is of great interest because of the differences
in bioavailability, essentiality, and toxicity between
Cr(III) and Cr(VI).
Precautions to be Taken in Chromium
Determination in Foods
0016As mentioned above, contamination is one of the
main problems encountered in chromium determin-
ation in foods. Indeed, it is the explanation why
food chromium contents reported some years ago
are higher than those obtained recently, as better
measures have now been adopted to avoid contam-
ination.
0017The possibility that small amounts of chromium
can be retained by the surface of vessels makes it
necessary to decontaminate the material used,
whether glass or plastic. In order to decontaminate
the containers, all material used has to be soaked in
diluted nitric acid (10–20% v/v) for 12–24 h and then
rinsed several times with deionized water. A mixture
of 4 mol l
1
nitric acid and 4 mol l
1
perchloric acid
1302 CHROMIUM/Properties and Determination

(1:1, v/v) or a solution of 2,4-pentanedione or dithi-
zone/carbon tetrachloride has also been proposed.
0018 To avoid losses by adsorption on to container
walls, it is advisable to store digested samples and to
prepare standard solutions in diluted acid (nitric or
hydrochloric acid) below pH 1.5. However in acidic
conditions reduction of Cr(VI) to Cr(III) is favored.
0019 Since the acids used in chromium determination,
especially nitric acid, are a source of contamination, it
is advisable to use reagents of high quality or to distil
them previously. Reagent blanks must be used to
monitor such contamination.
0020 Contact with materials that can give up chromium,
i.e., metallic surfaces such as stainless steel, should be
avoided. Therefore, knives, mixers, homogenators, or
mills which are made from stainless steel should be
avoided and replaced by others made from polymeric
materials, quartz, or titanium. Colored polymeric
materials such as screw-caps for bottles, dispensers,
and pipette tips can contain chromium and should be
controlled for contamination.
0021 Chromium determinations below the 1 mgl
1
level
require strict contamination control and clean-room
facilities.
Sample Digestion
0022 The choice of the digestion method is important in
trace chromium determination. The use of dry
methods carries the risk of the formation of acid-insol-
uble Cr(III) oxide and can lead to chromium losses by
adsorption on the walls of the crucible or by volatil-
ization after formation of chromyl chloride at tem-
peratures higher than 550
C. These losses are
minimized when the temperature is increased grad-
ually and does not exceed 450–500
C. Nevertheless,
some authors have reported losses of chromium by
volatilization at 450
C and obtained better results at
lower temperatures together with oxygen plasma. In
the latter procedure, the amount of sample is limited
and relatively large amounts of hydrogen peroxide are
needed as an ashing aid. A dry-ashing in Pt crucibles
with a temperature ramp at a rate of 50
Ch
1
up to
450
C is the digestion method adopted by the Nordic
Committee on Food Analysis as its official method-
ology for chromium determination in foodstuffs.
0023 In wet digestion procedures, mixtures of acids and
oxidizing agents are used, the most commonly used
ones being nitric, sulfuric, and perchloric acids with
hydrogen peroxide or vanadium pentoxide. When
foods have a high fat content, mixtures of nitric,
perchloric, and sulfuric acids, or nitric and sulfuric
acids, are recommended.
0024 The high boiling point of sulfuric acid helps the
activity of oxidizing agents and, with the addition of
nitric acid, the disadvantage of forming sulfate com-
pounds of low solubility is overcome. Digestion,
however, with nitric and sulfuric acids requires good
refluxing to prevent losses.
0025Significant losses of chromium during wet ashing
occur when perchloric is present and the sample is
concentrated close to dryness because of chromyl
chloride (CrO
2
Cl
2
) formation, which boils at
117
C. Formation of chromyl chloride can be
minimized by the addition of sulfuric acid.
0026The main risk of wet digestion is contamination
through the reagents, especially when high volumes
are used. Digestions in closed systems and/or in micro-
wave systems require less time and reagents. There-
fore, the risk of contamination decreases and losses by
volatilization are also reduced to a minimum.
0027Microwave digestion is now widely used to meas-
ure chromium contents in foods, because it is faster,
safer, and provides more reproducible conditions
than conventional methods, resulting in better analyt-
ical precision.
0028In some vegetable species chromium is retained by
an insoluble residue of silica. When this occurs, treat-
ment with hydrofluoric acid is necessary to overcome
the problem of low recovery values.
0029Extreme care must be taken in all wet ashing
procedures where strong acids and strong oxidizing
agents are used. The formation of explosive nitro
compounds has been reported when fat-rich food
samples have been digested with nitric acid.
Determination
Molecular Absorption Spectroscopy
0030The traditional spectrophotometric method of
520 nm, based on the violet complex formed with
1,5-diphenyl-carbazide (DPC), is still widely used
for determining chromium in water, because of
its high sensitivity. Chromium must be present as
Cr(VI) and the diphenylcarbazide is oxidized to the
diphenylcarbazone. Iron, copper, molybdenum, and
vanadium can interfere. To remove interference and
to increase the sensitivity of the method, both cation
exchange resins (iron and copper) and cupferron
(iron, copper, molybdenum, and vanadium) have
been used.
0031This method has been applied to chromium deter-
mination in foods after destroying the organic matter
and removing the interferences. Detection limits of
10 ng g
1
have been reported.
0032The complex chromium hematoxiline, with a
maximum absorbance between 360 and 390 nm, has
also been applied to foods, although less often than
the diphenylcarbazide method.
CHROMIUM/Properties and Determination 1303

Flame Atomic Absorption Spectrophotometry
(FAAS)
0033 The low levels of chromium in foods and the com-
plexity of the matrix make it difficult to measure
chromium directly in digested samples by FAAS.
With an air-acetylene flame and at a wavelength of
357.9 nm it is possible to obtain detection limits of
0.1 mgg
1
. It has been well established that chromium
atomization is dependent on flame stoichiometry,
with higher sensitivity with reducing flames (rich in
acetylene). A 10-fold difference in sensitivity between
fuel-lean and fuel-rich flames has been reported.
However, the composition of the flame affects the
behavior of the interfering compounds, and often
the conditions for maximum signal-to-background
are quite different from those required for minimizing
interferences.
0034 This determination is not free of interelement
interferences. Iron depresses chromium absorbance
in fuel-rich air-acetylene flames. It can be separated
from the analyte by extraction with acetylacetone/
chloroform (1:1, v/v) at pH 1–2, or by extraction, as
Fe(III), from 5 mol l
1
hydrochloric acid into isobutyl
acetate or 4-methyl-2-pentanone (MIBK).
0035 Direct chromium determination by FAAS in
digested samples is not often applied, owing to inter-
ferences and the low concentration of chromium. For
this reason it is more usual to enrich and to remove
interference by chelating chromium and then
extracting with an organic solvent.
0036 Chromium can be chelated as Cr(III) with 2,4-
pentanedione at pH 7.5 and heating to nearly 80
C.
The chelate is then extracted with chloroform or
MIBK. With the former, a backextraction to an aque-
ous solution should be carried out before measuring
by FAAS. To remove metal interferences the same
chelating reagent or ammonium pyrrolidine dithio-
carbamate (APDC) is added at a lower pH value
and then extraction with chloroform is carried out.
0037 Alternatively, chromium can be chelated with
APDC as Cr(III) (pH 5 at 80–90
C) or as Cr(VI)
(pH 3–9 at room temperature). Therefore, to work
under less strict conditions it is advisable to oxidize
chromium to Cr(VI), and to eliminate the excess oxi-
dizing agent. The procedure is not free of interfer-
ences, because manganese and tin are also chelated.
In order to remove interfering substances and to
increase the stability of the element in solution,
some authors have proposed that backextraction
should be carried out.
0038 Another method that has been applied to chro-
mium determination in foods is based on the forma-
tion of a compound by ionic association between
Cr(VI) and the system hydrochloric acid/MIBK
(0
C). Prior to this, chromium is oxidized to Cr(VI)
with potassium permanganate. This procedure offers
the advantage that it is not necessary to remove the
excess oxidizing agent.
Electrothermal Atomization Atomic Absorption
Spectrophotometry
0039ETA-AAS is considered one of the techniques of
choice for chromium determination in biological
matrices and it is the technique most often used to
determine chromium in foods. It is not free of inter-
ferences, but it is simpler than FAAS solvent-extrac-
tion methods. In foods, detection limits of 1 mgkg
1
have been reported.
0040If organic matter is present, chromium is found
mainly in the form of Cr(III), and in this oxidation
state it easily forms refractory oxides during heating
in graphite tubes and then reacts with graphite to
form a carbide. To avoid the resulting decrease in
the signal, pyrolytically or specially coated graphite
tubes should be used. Pyrolytically coated tubes
increase sensitivity threefold with respect to uncoated
ones.
0041When the furnace temperature reaches 450
C,
breakdown products of chromium complexes are
formed. Some of these are volatile and, therefore,
there is a risk of losses of the element. Alternatively,
other breakdown products can prevent complete
atomization of chromium, because of the strong
bonding with oxygen, nitrogen, or carbon. To achieve
complete atomization of chromium, temperatures
higher than 2300
C should be used. The usual ranges
of temperatures are: drying (100–200
C), ashing
(600–1600
C), atomization (2300–2700
C).
0042Diammonium hydrogen phosphate, magnesium
nitrate, and palladium have been used successfully
as matrix modifiers. The use of magnesium nitrate
as a matrix modifier extends the possible charring
temperature to 1600
C, thus insuring a more com-
plete remove of inorganic salts.
0043Since chromium is measured at a wavelength of
357.9 nm, it is better to correct background absorp-
tion with the Zeeman effect or a tungsten lamp rather
than with a deuterium lamp. The emission intensity of
the deuterium arc is low at the chromium resonance
line of 357.9 nm. The hollow cathode lamp current
has to be reduced in order to balance the source and
background beams. The Zeeman effect can compen-
sate for high absorbances, such as those produced by
biological matrices.
0044The standard addition method is generally applied,
even when prior digestion of organic matter is carried
out. In the case of liquid samples, e.g., beer or wine, it
is possible to determine chromium by injecting the
1304 CHROMIUM/Properties and Determination

diluted sample directly into the graphite furnace and
then applying the standard addition method. This
direct procedure has also been applied to suspensions
of vegetables and milk samples (whole, low-fat, skim,
condensed, evaporated, and powdered). In these cases
slurries of the samples were prepared with Triton
X-100. Chromium contents below 0.2 mgg
1
are
measurable.
Inductively Coupled Plasma Spectrometry
0045 The detection limits of ICP with atomic emission
(ICP-AES) are lower than those obtained by direct
FAAS, but not commensurate with ETA-AAS. More-
over, they can be seriously affected by spectral inter-
ferences, which are much more usual in ICP-AES than
in AAS. A wavelength of 267.716 nm is usually
chosen, though some overlap with emission lines of
manganese and phosphorus can be observed. Other
emission lines used are 283–563, 284–325, and
357–869 nm, but all are prone to spectral interfer-
ence. Algorithms are generally used to correct for
such interferences.
0046 One of the main advantages of ICP is that sample
preparation is simpler than in ETA-AAS, because
elements can be measured when organic residues are
present and several elements can be measured simul-
taneously. This explains why it is used in chromium
determination in foods.
0047 Online coupling mass spectrometry (MS) with ICP
improves the detection limits 10–100-fold, obtaining,
in optimized conditions, values below 0.1 mg1
1
and
therefore close to those of ETA-AAS. ICP-MS has the
advantage of multielement capability. An ICP-MS
method was proposed as the Association of Official
Analytical Chemists (AOAC) official method for
chromium determination in drinking, groundwater
and waste waters. On the other hand, the lower back-
ground and greater sensitivity of the double-focusing
instruments at low-resolution mode make it possible
to obtain better detection limits than with quadru-
pole-based instruments.
0048 Chromium determination in food samples by flow-
injection ICP-MS has been described. The digests
obtained in a wet high-pressure microwave system
are delivered at a previously established flow rate to
the ICP-MS instrument. FIA gives good recovery
values and these are more reproducible than when a
conventional method is used.
Electrochemical Methods (Polarography,
Voltammetry)
0049 Differential pulse polarography (DPP) is used to
measure chromium in water and biological materials
(including foods). Chromium is only detectable as
Cr(VI). A reduction peak is observed at 0.80 V,
when sodium citrate is used as a buffer (pH 9–10.5)
and ammonia (ammonium chloride) as a support
electrolyte. Detection limits as low as 0.12 mgl
1
can
be obtained.
0050DPP is mainly used for chromium determination in
water. Preparation of the sample depends on its com-
plexity and the expected chromium concentration.
0051Nitric acid should not be used to acidify the
sample, since the formation of nitrous acid can par-
tially reduce Cr(VI) to Cr(III). If water contains sul-
fites, these can also reduce Cr(VI) to Cr(III), so Cr(VI)
must be chelated with sodium diethyldithiocarba-
mate (pH 4, diluted sulfuric acid/ potassium acid
phthalate) and extracted with MIBK. Sulfuric acid is
added and the solution is heated to remove the solv-
ent. Hydrogen peroxide and sulfuric acid are added to
insure that chromium is present entirely as Cr(VI).
0052In the case of samples with a high chloride or
organic matter content, sodium sulfite is added to
prevent the formation of chromyl chloride.
0053Samples having chromium levels lower than 1 mg
g
1
can be enriched by passing the solution through
an ion exchange resin.
0054Voltammetric chromium measurements have been
applied to wet digested biological samples after a
controlled adsorptive preconcentration step on a
hanging-mercury-drop electrode. Optimal conditions
were a preconcentration potential of 1 V, a precon-
centration period of 60 s, and a solution pH of 6.3. A
current peak was recorded at 1.1 V.
0055Electrochemical determinations can also be useful
to differentiate between chromium species.
Gas-Liquid Chromatography (GLC)
0056The great sensitivity of GLC allows the detection of
extremely small amounts of chromium. GLC has
been applied mainly to chromium determination in
biological fluids, such as blood and urine, and to
chromium determination in water, when speciation
is wanted. It is applied less frequently to foods, be-
cause this method requires prior destruction of the
organic matter and chromium levels are higher in
foods than in biological fluids.
0057Since GLC can only be applied to volatile com-
pounds, volatile chromium chelates are formed
with fluorine substitutes of acetylacetone derivatives.
Chromium complexes with b-diketones are volatile
and stable, and trifluoroacetyllactone (TFA) is the
chelating agent most frequently reported in practical
applications, because of the enhanced sensitivity of
halides using electron capture detection (ECD). This
determination requires a purification step to remove
the excess chelating reagent and other interfering
CHROMIUM/Properties and Determination 1305
