Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.


Chiral Separations
0012 These can be approached mainly in two ways; by
transforming enantiomers into chromatographically
separable diasteroisomers, which does not necessitate
the use of chiral phases, or by preparing chiral phases.
Chiral phases can be obtained by covering the pack-
ings by making a solution pass through the column,
or by ionic or covalent bonding of the chiral reagent
with the column packing material.
Instrumental Configurations
0013 The basic equipment consists of a column packed
with a stationary phase, a driving force to propel the
solvent through the column (pump), a system (in-
jector) for introducing the sample on to the column,
a system (detector) for measuring a physical property
of the solutes being analyzed that differs from the
properties of the solvent of a property of the mobile
phase which is altered by the presence of the solute,
and a system for recording the detector signals and
converting them into graphic traces or chromato-
grams.
0014 A single solvent is often used to carry out the
separation (isocratic elution), but differing propor-
tions of various solvents are also often used (gradient
elution), in which case a gradient device is needed. A
variety of accessories, such as pressure controllers,
valves for switching solvents, valves for switching
column, and ovens for heating the columns, are also
commonly employed. Today most chromatographs
are controlled by a computer, which is also used for
data collection. This provides greater quality of quan-
titative data and enables automation of the system.
Solvents
0015 The nature of the solvent will depend upon the mode
of chromatographic separation employed, but a series
of precautions that are common to all types of HPLC
must be taken when preparing solvents. Because the
columns have frits at the ends to hold the packing in
place, the solvents must be devoid of particles and
consequently must be filtered through membranes
with a pore size of 0.5 mm or smaller, prior to use.
Bubble formation must also be avoided, since bubbles
may cause variations in the flow rate if they reach the
pump or perturbations in the chromatogram if they
reach or form in the detector cell. Solvents must
therefore be degassed by immersing the bottle con-
taining the solvent in an ultrasonic bath or by flushing
the solvent with a stream of helium or nitrogen before
delivering it to the chromatograph. A small stream of
helium is commonly bubbled through the solvent
during the chromatographic procedure to prevent
uptake of air. To prevent bubble formation in the
detector cell with depressurization, a restrictor is
sometimes attached to the outlet of the detector cell.
Pumps
0016The pump is the system for delivering the solvent
from the solvent reservoir to the column through the
injector. Basically, two types of pumps are available:
constant-pressure pumps and constant-flow-rate
pumps. The latter are more frequently used in HPLC.
0017Constant-pressure pumps are less expensive and
easy to operate, but the flow rate may vary with
changes in the viscosity of the mobile phase caused
by temperature fluctuations or by the accumulation
of undissolved sample components in the column.
These variations in flow rate affect retention times,
and they may also affect resolution, increasing
the difficulty of both qualitative and quantitative
analysis.
0018Constant-flow-rate pumps afford the advantage of
maintaining retention times irrespective of changes in
solvent viscosity. This type of pump includes syringe
pumps, which consist of a cylinder containing the
mobile phase, which is expelled by a piston. The
piston is driven by a motor, so as to supply a constant
flow devoid of pulses. This type of pump can achieve
relatively high pressures, but maintenance and chang-
ing solvents are complicated.
0019Reciprocating pumps, also a type of constant-flow-
rate pump, are most commonly employed. Their price
varies depending on their complexity of design, and
their main drawback is that they generate pulses that
may cause noise in the detector. Single-piston pumps
are the least expensive and have a rotating eccentric
cam, which drives the plunger, discharging the liquid
through a one-way valve. Duplex pumps have two
plungers driven by a single motor by means of a
shared cam. This arrangement means that, while
one of the plungers is in the discharge phase, the
other is in the intake phase, thereby superimposing
the two flow-rate profiles and considerably reducing
pulsation. In this type of pump, delivery from the
solvent reservoir is steepled and changing solvents is
rapid.
Injectors
0020Introducing the sample on to the column is one of the
most critical steps in HPLC. Ideally, the sample
should reach the column in the form of a tiny droplet
that does not undergo diffusion, which would
broaden the chromatographic bandwidth and thus
lower resolution.
0021Several methods are employed to deliver the
sample on to the column. In on-column injectors the
1276 CHROMATOGRAPHY/High-performance Liquid Chromatography

sample is introduced through a syringe which tra-
verses a septum and enables the desired quantity of
sample to be deposited at the column inlet. In this
method the mobile phase flows continuously through
the column. The stop-flow injector is a variation of
this type of injector. In this method the pump is
stopped before the syringe is inserted in the injector,
and injection is effected when column pressure has
dropped to atmospheric pressure. The advantage of
these injectors is that they are inexpensive and of
simple construction, yet they do not lower efficiency.
However, reproducibility is poor, they are not suitable
for high working pressures, and they are complicated
to operate.
0022 Valve injectors are most commonly used. In these
injectors the sample is delivered on to a pressurized
column with no appreciable interruption in flow. The
sample is deposited by a syringe into an external loop,
the valve selector is turned, and the mobile phase
passes through the loop on its way to the column.
The valve thus has two positions, a load position and
an injection position. High injection reproducibility
can be achieved with these injectors. The band-
broadening effect is comparable to or somewhat
higher than that obtained using on-column syringe
injectors. The drawback afforded by these injectors
is that there is a split-second interruption in mobile-
phase flow that may damage the column. To avoid
this problem, a bypass may be attached, such that
there is always a constant flow of mobile phase
from the pump to the column.
0023 Automatic injectors that are capable of running
analyses on up to 100 samples without operator at-
tention have been designed on the basis of the same
mechanism as valve injectors. There are also injectors
equipped with a system of valves connecting them to
several columns, which enables columns to be
switched without stopping the flow.
Columns
0024 The columns most commonly utilized in HPLC con-
sist of stainless-steel, plastic, or glass tubes, measuring
15–25 cm in length and packed with small-diameter
particles (3–20 mm). Internal column diameter is
normally between 2 and 5 mm.
0025 The use of small-internal-diameter (microbore)
columns has become increasingly important from
the early 1980s; they are similar to the columns de-
scribed above, but their internal diameter is between
0.5 and 2 mm, while they range in length from 10 to
25 cm. Packing particle size normally ranges from 3
to 5 mm. These columns are suitable for use when only
small samples are available or when low solvent con-
sumption is required.
0026The length of both conventional and microbore
columns is mainly limited by the pressure needed
to drive the solvent through the column, which is
inversely proportional to the size of the particles
used in the packing. Minimum column diameter is
limited by column-wall effects that cause solute mol-
ecules flowing next to the wall to move more slowly
than those flowing through the center of the column,
resulting in an increase in chromatographic band-
width.
0027Open capillary columns similar to those employed
in gas chromatography are the most recent to have
come into use. The column tube is made of glass and
is 20–50 mm in diameter and several meters long. The
stationary phase is chemically bonded to the wall of
the tube. Capillary columns are also prepared by
packing a tube with particles 5–30 mminsizeand
subsequently heating and drawing the tube to an
internal diameter of 50–125 mm.
0028In addition to the column on which the separation
is carried out, two other types of column are also uti-
lized in HPLC to protect the analytical column. These
are precolumns and guard columns. Precolumns are
placed between the pump and the injector to saturate
the mobile phase with the stationary phase and thus
prevent dissolution of the stationary phase in the
analytical column. Guard columns are placed be-
tween the injector and the main column in order to
retain components in the sample that might otherwise
become permanently adsorbed on the analytical
column, thereby affecting column efficiency and per-
meability. Both precolumns and guard columns are
normally made of a material similar to that used in
the analytical column.
0029Chromatography is customarily performed at am-
bient temperature, but it may be necessary to regulate
the column temperature or to carry out the separation
at a temperature other than room temperature.
In such cases thermostat-equipped compartments
(ovens) and systems for heating or cooling the
columns are required.
Detectors
0030In order to be suitable for use in HPLC, detectors
must meet a number of requirements. First and
foremost, detector design must prevent broadening
of chromatographic bandwidth to insure that the
separations achieved on the column do not deterior-
ate in the detector. In addition, response time must be
short and the response must be linear over a suffi-
ciently broad range of concentrations.
0031The detectors most frequently employed are the
refractive index detector, the photometric detector,
and the fluorescence detector.
CHROMATOGRAPHY/High-performance Liquid Chromatography 1277

0032 Refractive index detectors measure the difference
between the refractive index of the mobile phase and
that of the column eluate. They are universal detect-
ors that are highly sensitive to small changes in the
mobile phase and even to small variations in tempera-
ture or pressure. This sensitivity means that to achieve
a suitable signal-to-noise ratio they are only capable
of detecting solute concentrations in the order of
micromoles. In addition, they are unsuitable for
working with gradient conditions.
0033 Photometric detectors measure absorbance in the
ultraviolet (UV) or visible regions of all the compon-
ents in the column eluate. They are less universal than
refractive index detectors but by the same token are
more specific. This type of detector is normally
capable of detecting nanomoles, provided that the
compound contains a strong chromophore. The three
types of photometric detector most frequently used
are fixed-wavelength detectors, variable-wavelength
detectors, and diode array detectors. This last type of
detector is capable of performing complete spectral
analysis of the column eluate on a continuous basis,
i.e., without stopping the flow.
0034 Fluorimeter detectors are more specific and more
sensitive than photometric detectors, but their linear
range is smaller. Detection limits are in the order of
picomoles for suitably fluorescent compounds, and
they are very useful in trace component analysis.
0035 Electrochemical detectors are also widely employed
in HPLC. These come in two types: amperometric
and conductometric detectors. Amperometric detect-
ors are highly sensitive but are only applicable to
analyses that can be oxidized or reduced; conducto-
metric detectors are moderately sensitive and are
applicable for detecting anions and cations. This
type of detector is the detector most commonly used
in ion exchange chromatography.
0036 Derivatization of the components being analyzed
may sometimes be employed to increase the detection
limit or specificity.
0037 Mass spectrometry (MS) is being used more and
more as an online detection system in HPLC. The use
of HPLC-MS coupling has spread since 1980 due to
the improvements introduced in the different types of
interface used. Several coupled MS-HPLC systems are
available commercially, and improvements are con-
tinually being introduced in existing equipment. At
the same time, new equipment is appearing all the
time. MS has been considered to be the ideal detector,
since it furnishes information on component struct-
ure. Microbore HPLC is particularly useful for online
HPLC-MS, which requires low sample volumes. MS
in combination with HPLC may become a standard
technique in a few years’ time. (See Mass Spectrom-
etry: Principles and Instrumentation.)
Selected Applications
0038The use of HPLC in food analysis is growing daily,
and it is now routinely applied in many laboratories.
The many different types of columns and detectors
that are currently available commercially make it
possible to apply HPLC in analyzing nearly all the
nonvolatile components in foods, be they present
naturally or added artificially. The techniques
employed for certain groups of food components are
summarized below by way of example.
Carbohydrates
0039Nearly all chromatographic modes may be used in
separating carbohydrates. For example, ion exchange
on strongly or weakly basic anionic resins or on
cationic resins, and partition chromatography on
ion exchange resins, on chemically bonded cyano,
amino, propyl-amino, or combined amino-cyano
phases, or on silica gel or gel permeation are all
possible. Differential refractometry is the conven-
tional detection system, although direct detection,
using short UV wavelengths or by forming derivatives
detectable at longer wavelengths or fluorescent
derivatives, is also employed. (See Carbohydrates:
Determination.)
Acids
0040A variety of chromatographic modes are also applied
to this group of components. Certain workers have
employed ion exchange chromatography on strongly
acid cationic resins or strongly or weakly basic
anionic resins. Reversed-phase chromatography and
ion pair chromatography have also been used. Detec-
tion is carried out by refractometry, photometry using
UV, or visible wavelengths, as in the case of carbohy-
drates. (See Acids: Properties and Determination.)
Amino Acids and Amines
0041Most separations of amino acids and amines are per-
formed using reversed-phase chromatography of dan-
syl chloride, orthophthaldialdehyde (OPA), or phenyl
dithioisocyanate derivatives. 9-Fluorenylmethyl
chloroformate (FMOC) is a suitable reagent for the
analysis of secondary amino acids. Detection is
carried out by means of fluorescence or UV absorp-
tion. The derivatizing, highly fluorescent, reagent
6-aminoquinolyl-N-hydroxysuccinimidyl carbamate
(AQC) reacts rapidly and easily with both primary
and secondary amines and its use is currently spread-
ing. (See Amines; Amino Acids: Determination.)
Peptides
0042Diverse techniques are used to separate peptides on
account of the broad range of molecular weights
1278 CHROMATOGRAPHY/High-performance Liquid Chromatography
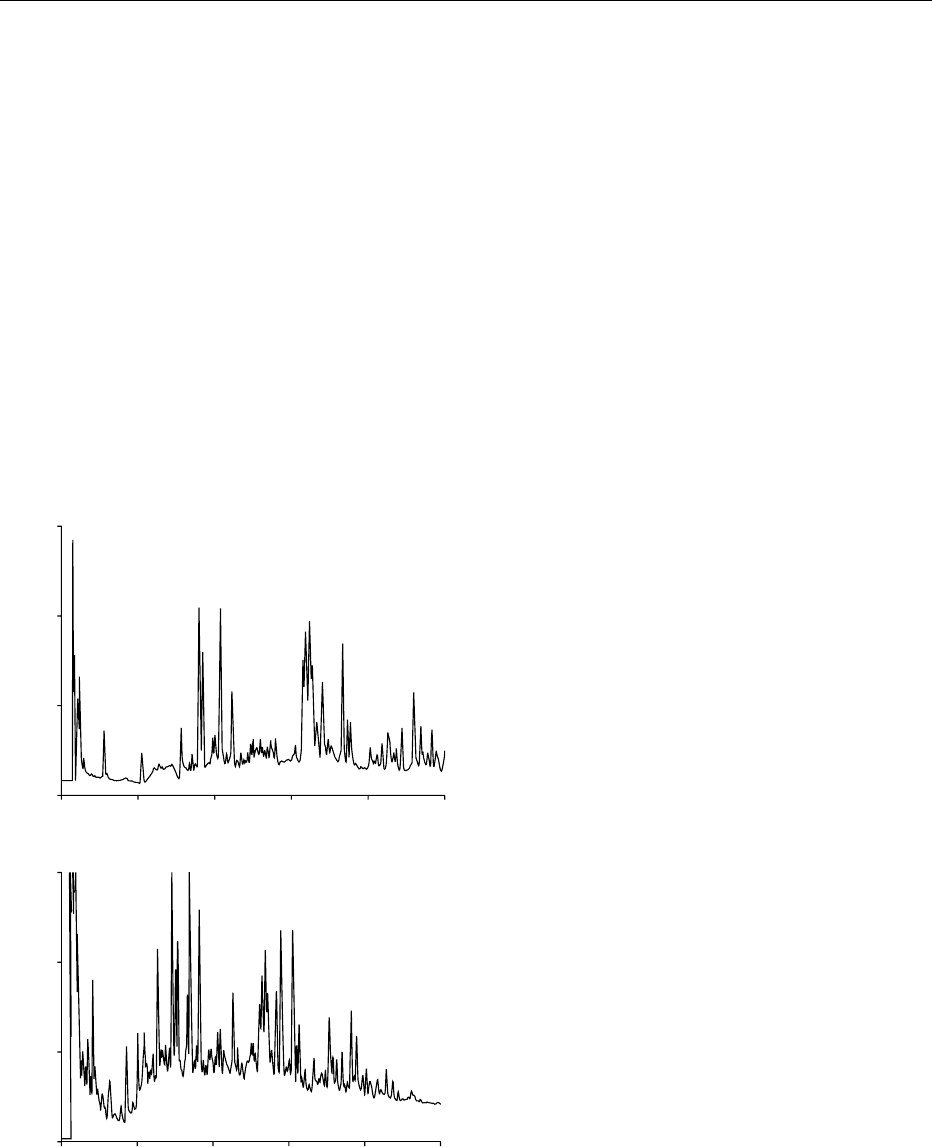
of these components. Conventional reversed-phase
columns are applied for peptides with molecular
weights of less than 3 kDa, while reversed-phase
columns packed with particles with large pore sizes
or size exclusion columns are used for larger peptides.
Detection is performed at 214 nm. Figure 1 shows a
chromatogram of the peptides with molecular weights
higher and lower than 700 from a sparkling wine, as an
example of this type of analysis. (See Peptides.)
Proteins
0043 Practically all known modes have been used in
the separation of proteins, e.g., separations based
on molecule size (gel permeation chromatography),
on charge (ion exchange chromatography), on
hydrophobicity (reversed-phase chromatography
and hydrophobic–interaction chromatography), and
even combinations of these mechanisms. The combin-
ation of HPLC and MS analysis is commonly used for
the sequence analysis of glycoproteins. Detection is
carried out at 214 or 280 nm. (See Protein: Determin-
ation and Characterization.)
Other Compounds
0044In addition to the major groups of food components
mentioned above, HPLC has found application in
many other areas of food analysis, and details
may be found in the relevant articles for the follow-
ing compounds or groups of compounds: lipid
components, phospholipids, triglycerides, vitamins,
colors, pesticides, drug residues, polycyclic aromatic
hydrocarbons, and nitrosamines. (See Colorants
(Colourants): Properties and Determination of
Natural Pigments; Properties and Determinants
of Synthetic Pigments; Fatty Acids: Analysis; Nitro-
samines; Pesticides and Herbicides: Types, Uses,
and Determination of Herbicides; Phospholipids:
Determination; Polycyclic Aromatic Hydrocarbons;
Triglycerides: Characterization and Determination;
Vitamins: Determination.)
Related Techniques
0045FPLC designates a fast chromatographic method, de-
veloped by Pharmacia, which is similar to HPLC and
yields high resolution. Since FPLC needs only a rela-
tively low backpressure to drive the high flow rates at
which the separations are performed, the risk of de-
naturation caused by shearing forces is reduced.
Moreover, the mechanical components are resistant
to corrosive buffers, and there is no contamination or
inactivation or the components of interest.
0046Given the range of columns available in the market,
a variety of separation modes can be applied using
this technique: size exclusion, hydrophobic inter-
action, chromatofocusing, ion exchange, and re-
versed-phase chromatography.
0047This method was developed to separate and purify
biomolecules and is very useful in separating isoen-
zymes and molecular species with similar charge char-
acteristics. It is also used to distinguish between
different types of meat or grains.
0048SFC is another technique, related to HPLC, which
uses as the mobile phase a supercritical fluid, i.e., a
fluid at a pressure and temperature above the critical
point. The properties of supercritical fluids are inter-
mediate between those of gases and those of liquid.
Thanks to their higher diffusivity and lower viscosity
as compared to liquids, high efficiencies are achiev-
able with shorter analysis times than those customar-
ily employed using HPLC.
0049The basic advantage of SFC with respect to gas
chromatography (GC) is the possibility of analyzing
0
Absorbance at 214 nm
Absorbance at 214 nm
0.00
0.05
0.10
0.15
10 20
Time (min)(a)
(b)
Time
(
min
)
30 40 50
0
0.00
0.05
0.10
0.15
10 20 30 40 50
fig0001 Figure 1 Chromatogram of peptides with molecular weights (a)
higher and (b) lower than 700 Da from a sparkling wine. Column:
Nova Pak C
18
. Solvents: (a) TFA/water; (b) TFA/acetonitrile.
Gradient elution. Detection at 214 nm. Reproduced from Moreno-
Arribas MV, Bartolome
´
B, Pueyo E and Polo MC (1998) Isolation
and characterization of individual peptides from wine. Journal of
Agriculture and Food Chemistry 46: 3422–3425, with permission.
CHROMATOGRAPHY/High-performance Liquid Chromatography 1279

components that span a broad range of volatilities as
well as heat-labile components. At the same time,
SFC is also compatible with many of the detectors
commonly used in GC or HPLC and SFC-MS
coupling is easy to carry out.
0050 The critical temperature of the supercritical fluids
employed as the mobile phases between 0
C and
200
C and the critical pressure of these fluids should
not be too high. Carbon dioxide, nitrous oxide,
alkanes (such as n-pentane), and xenon, all of which
are nonpolar, are most often used. Ammonia can be
used to elute polar solutes, but mixtures of phases,
e.g., a nonpolar mobile phase containing a small
quantity of a polar organic solvent, known as a
modifier are normally employed.
0051 SFC can be performed on capillary, packed, and
micropacked columns. Stationary phases should be
cross-linked; otherwise the supercritical fluids,
which are excellent solvents for polymers, could
extract the stationary phase. Enantiomers can be
resolved using chiral phases.
0052 The equipment used in SFC is similar to that used
in HPLC and basically consists of a high-pressure
syringe pump, an injector, and a restrictor or post-
column valve to keep the mobile phase in a supercrit-
ical condition inside the chromatographic column.
0053 Fluid density is commonly programmed to adjust
mobile-phase selectivity, in as much as the physico-
chemical properties of supercritical fluids (solvation
strength, viscosity, diffusion) are all dependent upon
density.
0054 This method has been applied in food analysis (oils,
cheeses, coffee, etc.) Some of the most interesting
applications include separations of acids, alcohols,
lipids, carbohydrates, vitamins, and terpenes. (See
Acids: Properties and Determination; Alcohol: Prop-
erties and Determination; Analysis of Food; Carbo-
hydrates: Determination; Coffee: Analysis of Coffee
Products; Vitamins: Determination.)
See also: Acids: Properties and Determination; Alcohol:
Properties and Determination; Amino Acids:
Determination; Chromatography: Principles; Coffee:
Analysis of Coffee Products; Colorants (Colourants):
Properties and Determination of Natural Pigments;
Properties and Determinants of Synthetic Pigments; Fatty
Acids: Analysis; Nitrosamines; Peptides;
Phospholipids: Determination; Polycyclic Aromatic
Hydrocarbons; Protein: Determination and
Characterization; Triglycerides: Characterization and
Determination; Vitamins: Determination
Further Reading
Charalambous G (1984) Analysis of Foods and Beverages.
Modern Techniques. Orlando: Academic Press.
Dabrio MV, Blanch GP, Cifuentes A et al. (2000) Cromato-
grafia y Electroforesis en Columna. Barcelona: Springer-
Verlag Ibe
´
rica.
Gonzalez-Llano D, Polo C and Ramos M (1990) Update on
HPLC and FPLC analysis of nitrogen compounds in
dairy products. Lait 70: 255–277.
Gruewedel D and Whitaker JR (1987) Food Analysis. Prin-
ciples and Techniques. New York: Marcel Dekker.
Henschen A, Hupe KP, Lottspeich F and Voelter W (1985)
High Performance Liquid Chromatography in Bio-
chemistry. Weinheim: VCH Press.
Macrae R (1987) HPLC in Food Analysis, 2nd edn.
London: Academic Press.
Moreno-Arribas MV, Bartolome
´
B, Pueyo E and Polo MC
(1998) Isolation and characterization of individual
peptides from wine. Journal of Agriculture and Food
Chemistry 46: 3422–3425.
Oliver RWA (1989) HPLC of Macromolecules. A Practical
Approach. Oxford: IRL Press.
Shaw LP (1988) Handbook of Sugar Separations in Foods
by HPLC. Boca Raton. FL: CRC Press.
Smith RM (1988) Supercritical Fluid Chromatography.
RSC Chromatography Monographs. Cambridge: Royal
Society of Chemistry.
Gas Chromatography
I Martı
´
nez-Castro, J Sanz and M V Dabrio,
Instituto de Quı
´
mica Orga
´
nica General (CSIC), Madrid,
Spain
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Background
0001Gas chromatography was born in 1951, when James
and Martin developed a new separation technique in
order to analyze a mixture of 17 fatty acids. This
technique includes different chromatographic modes
that use a gas as the mobile phase: the separation
process takes place in a chromatographic column.
Like other separation techniques, gas chromatog-
raphy (GC) can be employed on the preparative
scale, but more frequently, it is used as a powerful
analytical technique.
Instrument
0002The analytical instrument consists of a mobile phase
supply (usually a gas cylinder) regulated by a con-
trol system, a sample introduction system (usually
called injector), a thermostatically controlled oven
1280 CHROMATOGRAPHY/Gas Chromatography
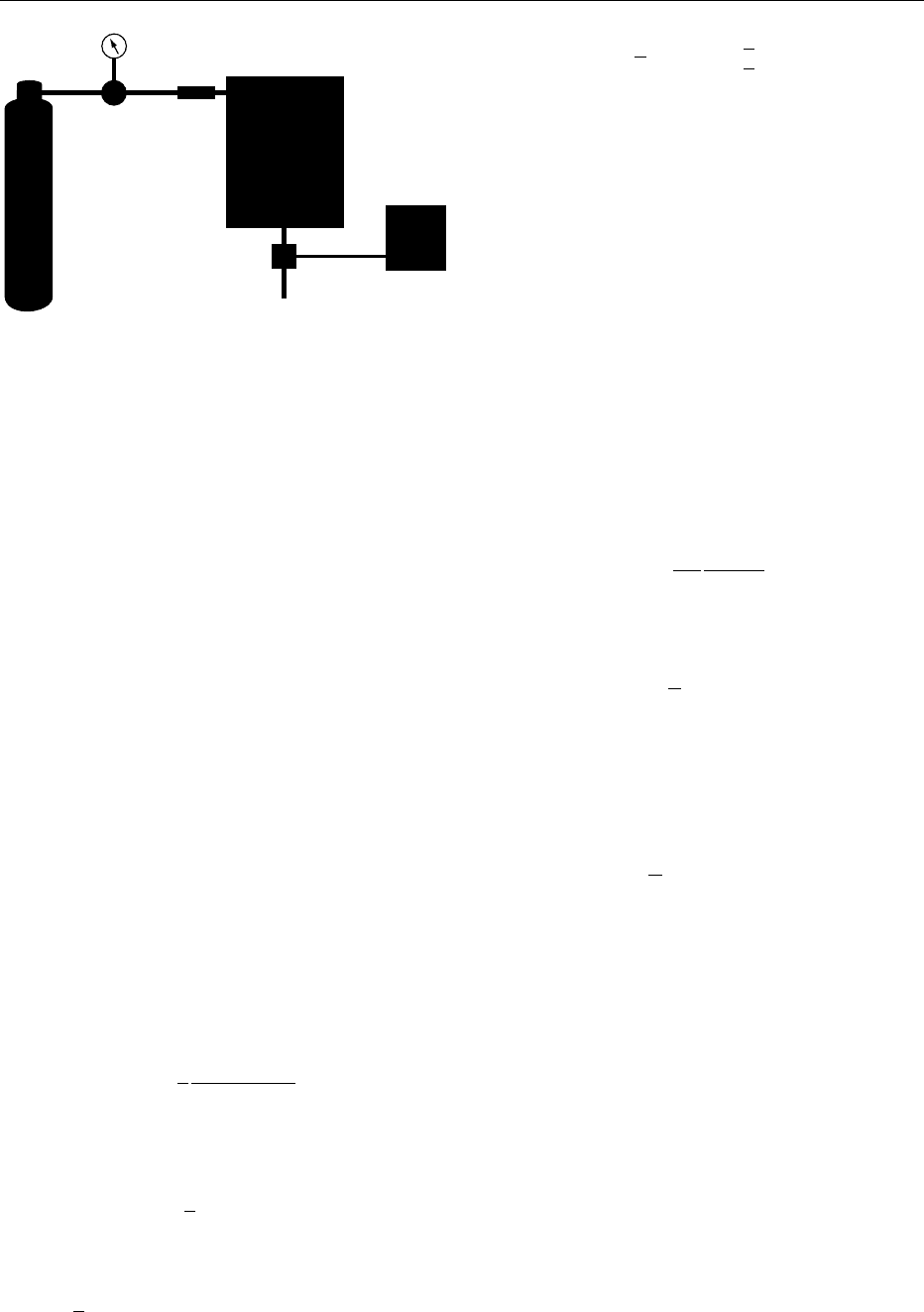
containing the column, a detector, and a data-acqui-
sition system (Figure 1).
Mobile Phase
0003 The separation process is mainly based on the inter-
action of the analytes with the stationary phase; the
mobile phase is called the ‘carrier gas,’ since its role is
merely to transport the analytes through the column.
Helium, nitrogen, hydrogen, and argon are usually
employed as mobile phases. High-purity gases are
necessary because traces of impurities, such as oxygen
or water, can spoil the stationary phase or disturb the
detector response. Helium is recommended to achieve
faster separations, and nitrogen is a less expensive
alternative, whereas the use of hydrogen requires
special care.
0004 Carrier gas pressure is between 5 and 700 kPa at
the inlet (p
i
), while the outlet pressure (p
o
) is usually
ambient pressure. The density, pressure, and velocity
(u) of the carrier gas vary nonlinearly through the
column, and it is necessary to specify at which column
point they are measured or calculated. A factor that
corrects for the mobile phase compressibility in the
column can be calculated as:
j ¼
3
2
ðp
i
=p
o
Þ
2
1
ðp
i
=p
o
Þ
3
1
: ð 1Þ
The average linear carrier gas velocity can be
obtained by dividing the column length (L) by the
retention time of an unretained compound:
u ¼ L=t
M
ð2Þ
t
M
is called the ‘hold-up time.’
0005 The flow rate at the column outlet (F
c
) can be easily
measured and related to the average flow rate
through
F ¼ jF
c
. It is also related to linear velocity:
F ¼ ku
o
¼ k
u
j
: ð3Þ
The retention time of a compound is defined as the
length of time between sample injection and the emer-
gence of the compound peak maximum. It is related
to the volume of mobile phase entering the column
during this time (V
R
, retention volume) by the
following equation:
t
R
¼ V
R
=F
c
: ð4Þ
The net retention volume, V
N
, takes into account the
compression correction factor and the hold-up time:
V
N
¼ jF
c
ðt
R
t
M
Þ: ð5Þ
The specific retention volume at the column tempera-
ture (V
y
g
) is the net retention volume per gram of
stationary phase (W
S
):
V
y
g
¼ V
N
=W
S
: ð6Þ
The specific retention volume at 0
C is:
V
g
¼
V
N
W
S
273:17
T
c
: ð7Þ
The variation of efficiency with the carrier-gas vel-
ocity is described by the Van Deemter equation:
H ¼ A þ
B
u
þðC
S
þ C
M
Þu; ð8Þ
where C
S
and C
M
, respectively, represent the contri-
bution of the stationary and mobile phases to the zone
spreading caused by the resistance to mass transfer
and other diffusion phenomena. When the column is
an open capillary, Golay’s equation can be used:
H ¼
B
u
þðC
S
þ C
M
Þu: ð9Þ
In most cases, C
S
is negligible when compared with
C
M
.
Stationary Phase
0006Stationary phase type (solid or liquid) determines
the two main gas chromatographic modes: gas–solid
chromatography (GSC) and gas–liquid chromatog-
raphy (GLC). System selectivity, which depends on
the compounds to be separated and on the stationary
phase, is estimated by the separation factor, a:
¼ V
N2
=V
N1
¼ðt
R2
t
M
Þ=ðt
R1
t
M
Þ: ð10Þ
Solid Phases
0007These consist of adsorbent particles; the separation
mechanism is mainly physical adsorption. Since
2
1
6
3
5
7
fig0001 Figure 1 Scheme of a gas chromatograph. 1, gas cylinder;
2, pressure control system; 3, injector; 4, oven; 5, column; 6,
detector; 7, recorder/data system.
CHROMATOGRAPHY/Gas Chromatography 1281

sorption/desorption processes are usually faster than
those of solution/vaporization (occurring in GLC),
transfer kinetic constants are higher, and C
S
values
are lower in GSC, allowing better efficiencies. Selec-
tivities can be high enough to separate stereoisomers
and isotopes. Other characteristics of GSC are a wide
temperature operation range, isotherms with a nar-
row linearity range, and high retention values. These
characteristics make GSC the technique of choice for
the separation of permanent gases and small polar
molecules. Solid phases are formed by small particles
of uniform size, with a high specific surface area.
Commercial solid phases are based on silica
(Spherosil, Porasil), graphitized carbon (Carbopack,
Graphpac), or porous organic polymers (Porapak,
Chromosorb Century series, Tenax).
Liquid Phases
0008 These are usually inert and thermally stable poly-
meric molecules. When the stationary phase is a
liquid, the main separation mechanism is partition,
although other mechanisms like chirality, mesomer-
ism, and complex formation may also contribute.
0009 Partition Compounds dissolved in the liquid phase
are in equilibrium (Henry’s law) with the vapor; the
elution order may be proportional to the boiling
points or very different, depending on the activity
coefficients.
0010 Chiral interactions Enantiomer pairs always coelute
when chromatographed on achiral phases. However,
liquid phases possessing chiral groups can show a
greater interaction with one of the pair members,
which is then relatively more retained. As chiral inter-
action energies are small, a values are usually close
to 1, and a high efficiency is required, such as that
provided by capillary columns. Chiral phases are
based on peptides, amide-substituted polysiloxanes,
or substituted cyclodextrins.
0011 Interactions with mesophases (liquid crystals) These
are formed by elongated or planar molecules. In a
temperature range from the melting point to the an-
isotropic transition, they are liquids ordered in a
mono- or bidimensional orientation. These phases
present a high selectivity for long or planar isomers.
0012 Complex formation Organometallic compounds
possessing metal ions are able to interact selectively
with solutes having electron-donor groups.
0013 Characteristics of liquid phases The liquids used as
stationary phases have to be thermally stable over a
wide temperature range. The lower limit is marked by
the melting point, and the so-called maximum allow-
able operation temperature (MAOT) is determined
by the increase in the detector signal caused either
by an appreciable value of vapor pressure or by ther-
mal degradation of the phase: in both cases, some
molecules from the phase arrive continuously at the
detector. The most important characteristic of a sta-
tionary phase is its polarity, which is usually defined
by various empirical scales, the most popular being
that of McReynolds, which uses as probes 10 solutes
with different polar groups and a series of saturated,
linear hydrocarbons. The molecular interactions are
supposed to be additive and are described by several
parameters (McReynolds constants) deduced from
the retention observed in the studied phase and in
squalane, which was defined as the zero on this
polarity scale. Five McReynolds constants, which
appear in most commercial catalogs, are directly
related to phase polarity. Cross-linking improves
the phase stability.
0014Table 1 shows some characteristics of the most
commonly used liquid phases. Hydrocarbons (like
squalane or apolane) are used to obtain reference
data. Polysiloxanes (with methyl, phenyl, trifluoro-
propyl, or cyanoalkyl substituents, showing a wide
polarity range) are commonly called ‘silicones’ and
are nowadays the most popular liquid phases, since
they have a good thermal stability and high permea-
bility to solutes. Polyethylene glycols with different
chain lengths (from a molecular weight of 150 to
around 4.1 10
6
) are another set of highly used
phases.
Columns
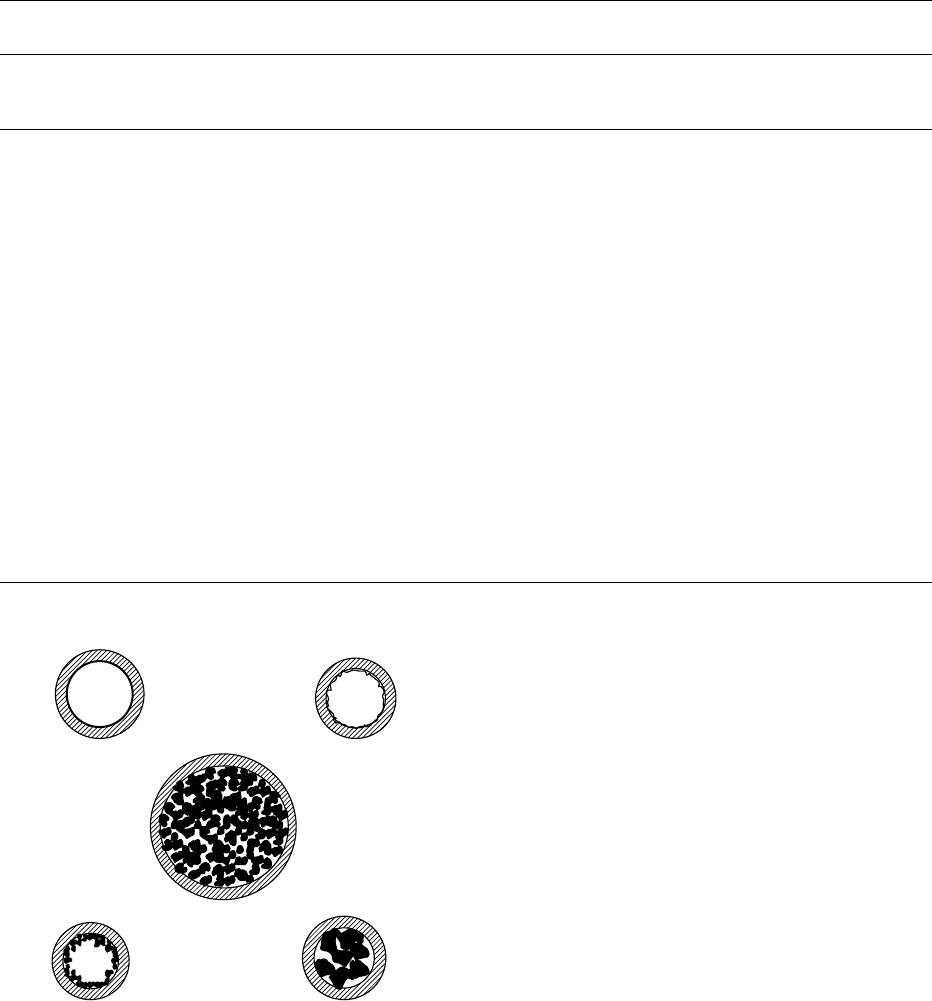
0015Columns are usually classified as open or packed.
Figure 2 shows a cross-section of the main types of
columns.
0016In open columns, the stationary phase is distributed
on the inner wall, and the mobile phase circulates
through a central channel. Packed columns consist
of a glass or metal tube packed with solid particles
(100–250 mm), whose surface adsorbs the analyzed
solutes (GSC) or is covered with a thin film of liquid
stationary phase (GLC). Table 2 lists the main
characteristics of the analytical columns.
0017Packed columns are mainly used for permanent
gases analysis by GSC; they are cheap, robust, and
easy to handle. The most frequently used GLC
columns are open tubular and are usually referred to
as ‘capillary’ since their diameter is very small. Since
the system efficiency depends on the dimensions of
the analytical columns, they should be chosen in
order to achieve the desired resolution in the min-
imum time. Efficiency is always directly related to
1282 CHROMATOGRAPHY/Gas Chromatography
column length, as shown in eqn (2). The efficiency of
a packed column is mainly related to the particle
diameter, which appears in two terms (A and C
M
)of
the van Deemter equation (eqn (8)). Column diameter
has a large influence on the efficiency of capillary
columns, as Golay’s equation (eqn (9)) shows. Open
tubular capillary columns require more sophisticated
equipment than packed columns, but their efficiency
is very high, since it is possible to use very long
columns with a moderate pressure drop (between
0.1 and 7 kg cm
2
).
0018Capillary columns with the inner wall covered by a
porous layer open tubular (PLOT, Figure 2)orbya
support-coated open tubular layer (SCOT, Figure 2),
where the stationary phase is coated, have both a high
sample load capacity and a high efficiency.
Operating Conditions
0019The flow rate and column temperature can be easily
modified, enabling the best conditions to be selected
for an adequate separation.
0020The flow rate is usually controlled through either a
pressure regulator or a flow controller. According to
eqn (4), t
R
is inversely proportional to the flow rate
for a given compound. A linear velocity of carrier gas
close to the van Deemter optimum (eqn (8)) affords
the best efficiency, but higher flow rates reduce the
analysis time.
0021Temperature has a decisive influence on retention.
When the temperature increases, V
R
and t
R
decrease
(eqn (7)). The temperature operation should be
selected in such a way that the retention factor (k)
values lie between 1 and 15. When a mixture contains
WCOTPLOT
Packed
SCOTPacked capillary
fig00 02 Figure 2 Cross-sectionofcolumns.PLOT,porouslayeropen
tubular;SCOT,support-coatedopentubular;WCOT,wall-coated
opentubular.AdaptedfromDabrioetal.(2000)Cromatografiay
electroforesisencolumna,p.122.Barcelona:Springerwithper-
mission.
tbl00 01 Table 1 Commonliquidstationaryphases
CompositionCommercialnamesMcReynoldsconstants(120
C)Operating
temperature
range(
C)
XYZUS
2,6,10,15,19,23-Hexamethyl-
tetracosane
Squalane0000020/120
24,24-Diethyl-19,29-dioctadecyl-
heptatetracontane
Apolane-8721103122535/260
Poly(dimethylsiloxane)100%OV-1,SE-30,OV-101,SP-2100,CP-Sil5CB,
DB-1,SPB-1,BP-1
165544654230/330
Poly(94%methyl,5%phenyl,1%
vinylsiloxane)
SE-54,DB-5,CP-Sil8CB,SPB-5,BP-5,AT-5337266986750/300
Poly(86%methyl,7%phenyl,7%
cyanopropylsiloxane)
OV-1701,CP-Sil19CB,BP-10,DB-1701,
HP-17
8217015723616030/250
Poly(50%methyl,50%
phenylsiloxane)
OV-17,DB-17,SP-2250,HP-50,SPB-5011915816224320220/350
Poly(50%methyl,50%
trifluoropropylsiloxane)
OV-210,SP-2401,DB-21014623835846831020/275
Poly(50%methyl,25%phenyl,
25%cyanopropylsiloxane)
OV-225,XE-60,DB-225,CP-Sil43CB,BP-1522836933849238620/250
Poly (ethylene glycol) Carbowax, Superox, Supelcowax, DB-Wax,
CP-Wax 52CB, BP-20
322 536 368 572 510 60/225
Poly (ethylene glycol) modified
with nitroterephthalic acid
FFAP, SP-1000, AT-1000, OV-351, Nukol,
CP-Wax 58CB, BP-21
340 580 597 602 627 50/250
Poly(biscyanopropyl siloxane) OV-275, CP-Sil 88, SP-2340, Silar 10C 629 872 763 1106 849 30/250
CHROMATOGRAPHY/Gas Chromatography 1283
compounds with very different volatilities, it is not
possible to find an optimum temperature for separat-
ing all components. Since the oven temperature is
easily controlled, it can be changed during the
chromatographic run (‘programmed’) in such a way
that each compound traverses the column within an
optimum temperature range.
Sample Introduction
0022 The objective of injection is to introduce the sample
as a narrow band, whose composition should corres-
pond closely to that of the original mixture. No uni-
versal system is available to handle all the different
samples encountered. Permanent gases may be intro-
duced with gas-tight syringes or using multiport
valves. Liquid samples and solutions are usually
introduced by microsyringe at the injection port,
which is a heated chamber where the carrier gas
enters under controlled flow or pressure. The sample
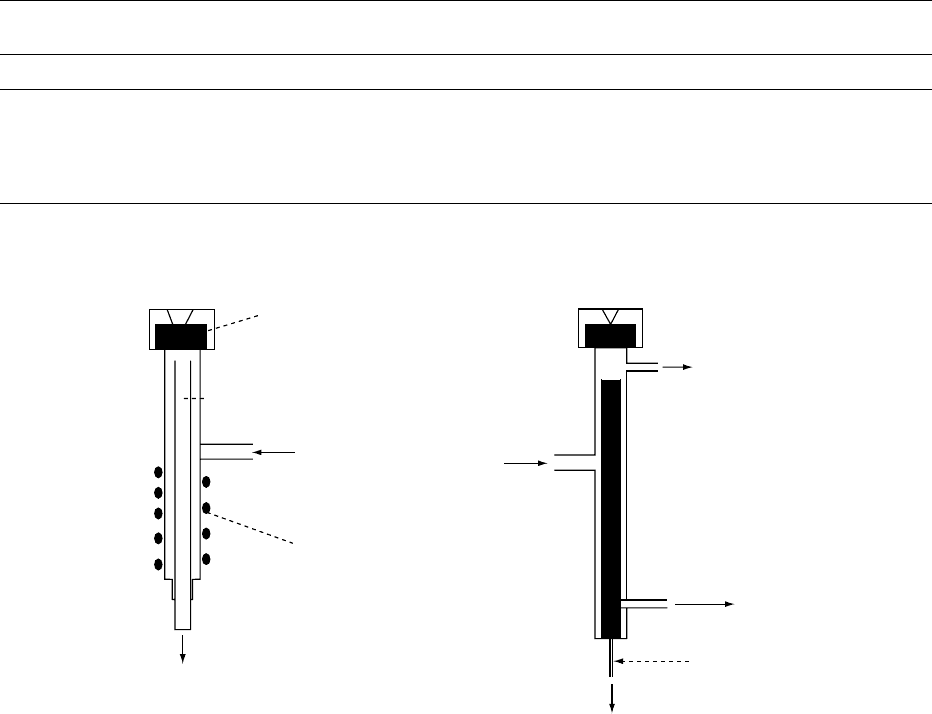
is vaporized and transferred to the column inlet by the
carrier gas stream. A schematic diagram of an injector
is shown in Figure 3.
0023The chamber is provided with an inert glass or
quartz liner to minimize sample contact with hot
metallic surfaces. It is closed with a mechanical seal
or, more usually, with an elastomer membrane called
an septum, with can be pierced with a microsyringe to
introduce the sample. Depending on the column
dimensions, the quantity of sample can be varied
from nanoliters to a few microliters, containing nano-
grams or micrograms of analytes. There are special
devices capable of transferring several hundreds of
microliters of very diluted samples, the solvent excess
being discarded before it enters the analytical column.
Injection Modes
0024There are several different ways of injecting samples.
The more common modes are detailed below.
0025Direct injection The sample is introduced through
the septum in the hot injector, where the liquid
Septum
(a)
Liner
Carrier gas
Heater
Column
(b)
Carrier gas
Septum
purge
Splitter
Column
fig0003 Figure 3 Scheme of an injector. a) for direct injection; b) for split/splitless injection. Adapted from Dabrio MV et al. (2000) Cromato-
grafia y electroforesis en columna, p. 130. Barcelona: Springer with permission.
tbl0002 Table 2 Some characteristics of GC columns
Type Name Internaldiameter (mm) Length (m) d
f
(mm) Particle size (mm)
Open WCOT (Wall-coated open tubular) 0.05–0.75 10–100 0.1–0.5 –
PLOT (Porous layer open tubular) 0.5–1 10–100 0.1–5
a
–
SCOT (Support-coated open tubular) 0.5–1 10–100 0.1–5
a
–
Packed Micropacked 0.8–1 2–7 5–15
b
80–200
Packed 2–6 2–7 5–15
b
80–500
a
Bed thickness (particles þliquid phase).
b
percentage of liquid phase (referred to support þphase).
1284 CHROMATOGRAPHY/Gas Chromatography

rapidly vaporizes; carrier gas sweeps the vapor into
the column inlet.
0026 Split injection This was designed for the introduc-
tion of samples into capillary columns. The basic
design used for direct injection was modified as
shown in Figure 3. The carrier gas, controlled by a
pressure regulator or a combination of a flow con-
troller and a back-pressure regulator, is divided into
two streams: one is used to eliminate the possible
impurities from the septum membrane; the other
enters the vaporization chamber. This gas stream,
after sweeping the vaporized sample, is split before
it enters the column inlet to attain a high flow rate.
The sample is also proportionally divided, so only a
small part reaches the column. Since quantitative
analysis presents some problems related to the split
process, the use of internal standards is recom-
mended.
0027 Splitless injection When very dilute solutions need
to be injected, the amount of sample entering the
column can be increased, stopping the split process
by using a solenoid valve. The split valve is closed, the
sample is introduced, and after a controlled delay
(usually 15–60 s), the split valve is opened again.
Most of the sample enters the column while the split
is closed, while the rest is purged out when the valve
opens. Since the sample enters the column as a broad
band, it is a necessary focusing step. Usually, the
column is kept at a low temperature during injection,
and sample vapors condense at the column inlet in a
narrow band (cryofocusing); next, the temperature is
raised in order to elute the solutes correctly. Sample
solvent condensation can also be used (solvent effect)
to retain the solutes as a narrow band at the column
inlet.
0028 Programmed temperature vaporizer (PTV) With
the same basic design of a split/splitless detector, it
can be heated and cooled very fast. The injector is
cold when the sample is introduced and deposited on
inert packing; then, the injector is heated to the de-
sired temperature so that the vaporization is homo-
geneous, and sample discrimination is reduced.
0029 On-column injection A liquid sample is deposited
directly at the column head, without previous vapor-
ization. The injectors are very simple, having a
mechanical stop valve instead of a septum, and the
microsyringes usually have very thin needles (less
than 0.3 mm outer diameter). This system reduces
the discrimination, thermal decomposition, and deg-
radation of labile compounds. Its main drawback is
possible column contamination with nonvolatile
sample impurities.
External Sampling Devices
0030Pyrolysis A solid sample is thermally decomposed;
the volatile fragments resulting from its breakdown
can be chromatographically separated and detected,
and their chromatographic pattern related to that of
the original sample.
0031Headspace sampling The sample is thermostatized
in a closed vial, and the vapor is sampled after equi-
librium is attained. Usually, the process is automatic,
under either atmospheric or high-pressure conditions,
and the technique is frequently used for aroma
analysis.
0032Purge and trap A stream of inert gas passes over or
through the sample, and the stripped volatiles are
trapped on a solid sorbent. These can be recovered
either by a solvent or by thermal desorption (see
below). The enrichment factors obtained are very
high; matrix interferences are largely reduced. This
technique is useful when very low concentrations of
volatile substances have to be analyzed.
0033Thermal desorption The injector chamber can be
ballistically heated to the desired temperature while
it is purged with carrier gas. When a solid matrix is
placed in this chamber, the volatile substances present
are released and stripped towards the column; they
can then be cryofocused before arriving at the column
inlet. In the direct mode (DTD, direct thermal desorp-
tion), the solid matrix is a sample (e.g., a spice or a
herb). In the indirect mode, volatiles have been previ-
ously stripped from the sample and trapped on an
adsorbent.
Multidimensional Gas Chromatography
0034This usually refers to a combination of columns with
different selectivity, where a small fraction from a
complex sample is preseparated on the first column
and introduced into the second column for further
separation. Whereas the first column may be packed
or may be a capillary, the second column is always a
capillary and more selective. A special pneumatic
switching arrangement is necessary in order to
provide a sharp sample band.
Detectors
0035A high number of detectors have been designed
for GC. The most frequently used detectors can be
classified into four types.
CHROMATOGRAPHY/Gas Chromatography 1285
