Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.

Detection
0039 Detection is generally carried out by measuring the
ultraviolet or visible absorbance at the end of the
capillary. Other detection systems such as laser-
induced fluorescence, mass spectrometry, and con-
ductivity detection have also been employed.
Fundamentals
0040 The basic phenomena in capillary electrophoresis are
electroosmotic flow (EOF) and electrophoretic flow
(m
ep
). Electroosmotic flow is bulk flow of a solvent in
a capillary under an applied potential. Electrophor-
etic flow is the flow of ions due to a certain charge,
with cations being attracted to the cathode and
anions being attracted to the anode.
0041 The magnitude of the EOF can expressed by
m
EOF
¼ðezÞ=, ð9Þ
where m
EOF
¼ EOF mobility; e ¼ dielectric constant;
z ¼zeta potential; Z ¼bulk viscosity.
0042According to eqn (9), the mobility is independent
of the applied electric field. The zeta potential is
essentially determined by the surface charge on the
capillary wall. Since it is strongly pH-dependent, the
magnitude of the EOF varies with pH. Depending on
the specific conditions, the EOF can vary by more
than an order of magnitude between pH 2 and 12.
The zeta potential is also dependent on the ionic
strength of the buffer.
0043The separation parameters of capillary electro-
phoresis can be described in similar terms to those
for GC and HPLC.
0044The time required for a solute to migrate to the
point of detection is called the migration time, and is
given by the quotient of migration and velocity. The
migration time and other experimental parameters can
be used to calculate the apparent solute mobility, using
m
a
¼ 1=tE ¼ 1L=tV ð10Þ
and
m
a
¼ m
e
þ m
EOF
, ð11Þ
(a)
Capillary zone electrophoresis (CZE)
(b)
Capillary gel electrophoresis
(c)
Micellar electrokinetic chromotography (MEKC)
(d)
Capillary isotachophoresis
(e)
Capillary isoelectric focusing
t = 0
t = t'
t = 2t'
t = 0
t = t'
t = 2t'
−
+
+
X
+
+
+
+
+
−
+
−
+
−
V
ep
V
x
V
eo
+ V
ep
V
eo
V
eo
V
mc
−
−
−
F
F
C H
A
I
I
K
K
D
D
J
J
M
M
B
B
L
L
E
E
G
G
F
C H
A
I
K
D
J
M
B
L
E
G
=
V
x
=
V
eo
+
[K
*
/(1
+
K
*
)]V
mc
CH
A
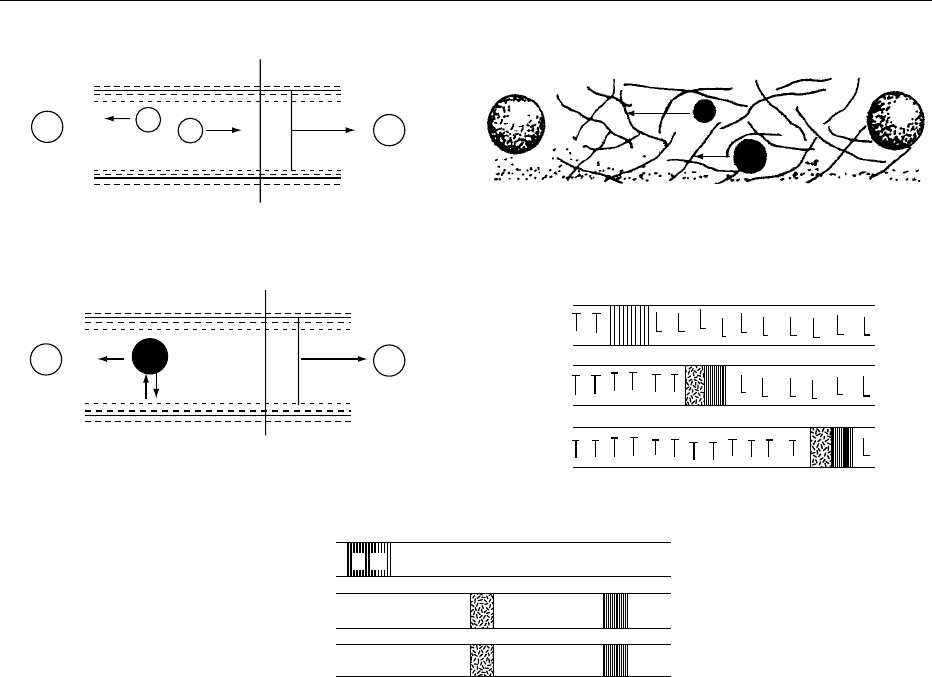
fig0009 Figure 9 Different modes of capillary electrophoresis. In (a), V
x
is the electromigration velocity, and V
eo
is the electroosmotic
velocity. In (b), there are obstructing strands of gel, and large ions move more slowly through the pores than the small ions. In (c), V
x
is
the electromigration velocity of the solute, V
eo
is the electroosmotic velocity, V
mc
is the migration velocity of the micelle, and K
*
is the
phase capacity ratio. In (d), L is the leading electrolyte, and T is the terminating electrolyte.
1266 CHROMATOGRAPHY/Principles

where l is the effective capillary length (to the de-
tector), L is the total length, t is the migration time,
and E is the electric field. In the presence of EOF, the
measured mobility is called the apparent mobility
(m
a
). The effective mobility (m
e
) can be calculated
from the apparent mobility by independently measur-
ing the EOF using a neutral marker that moves at a
velocity equal to the EOF.
See also: Chromatography: Thin-layer Chromatography;
High-performance Liquid Chromatography; Gas
Chromatography; Combined Chromatography and Mass
Spectrometry
Further Reading
Cserha
´
ti T and Forga
´
cs E (1999) Chromatography in Food
Science and Technology. Lancaster, PA: Technomic.
Forga
´
cs E and Cserha
´
ti T (1997) Molecular Basis of Chro-
matographic Separation. Boca Raton, FL: CRC Press.
Grob RL (1995) Modern Practice of Gas Chromatography.
New York: John Wiley.
Grossmann PD and Colburn JC (eds) (1992) Capillary
Electrophoresis – Theory and Practice. San Diego, CA:
Academic Press.
Hill HH and McMinn DG (1995) Detectors for Capillary
Chromatography. New York: John Wiley.
Jennings W (1987) Analytical Gas Chromatography.
Orlando, FL: Academic Press.
Jinno K (1992) Hyphenated Techniques in SFC and Extrac-
tion. New York: Elsevier Science.
Li SFY (1992) Capillary Electrophoresis – Principles, Prac-
tice and Applications, Journal of Chromatography
Library. Amsterdam: Elsevier Scientific.
Maguire KL and Denyszyn RB (1985) In: White CM (ed.)
Modern Supercritical Fluid Chromatography, p. 45.
New York: Springer.
Sherma J and Fried B (eds) (1991) Handbook of Thin Layer
Chromatography, vol. 55. New York: Marcel Dekker.
Snyder LR (1968) Principles of Adsorption Chromatog-
raphy. New York: Marcel Dekker.
Snyder LR, Glajch JL and Kirkland JJ (1988) Practical
HPLC Method Development. New York: John Wiley.
Vindevogel J and Sandra P (1992) Introduction to Micellar
Electrokinetic Chromatography. Heidelberg: Hu
¨
rtig.
Thin-layer Chromatography
D R Tocher, University of Stirling, Stirling, UK
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Introduction
0001 Thin-layer chromatography (TLC) is a type of liquid
chromatography performed on a stationary phase in
the form of a thin layer usually on a glass, plastic, or
aluminum support. A solution of sample is applied as
a small spot or narrow band to the thin layer of
adsorbent that has been spread uniformly over the
support. The mobile phase (a solvent mixture) then
passes through the adsorbent by capillary action, and
the sample is resolved into discrete components. The
separated components are then located by either
physical methods or chemical staining reagents after
the solvent has evaporated. Quantitation by methods
such as densitometry may also then be possible,
depending upon the precise conditions utilized.
Although high-performance liquid chromatography
(HPLC) and gas chromatography (GC) are often
regarded as superior, especially with regard to quan-
titation, TLC has many advantages including low
capital costs, simplicity of operation, the availability
of many sensitive and selective reagents for detection
and confirmation without interference of the mobile
phase, and in-system calibration for quantitative
analysis. Therefore, TLC should be regarded as
highly complementary to the other techniques and
particularly suited to low-cost screening of many
samples.
Apparatus
0002TLC is very cost-effective as the basic apparatus
required is simple and relatively cheap, and many
samples can be analyzed on a single plate with low
solvent usage. However, more sophisticated and
expensive apparatus are available particularly for
automated sample application and quantitation.
Adsorbents and Supports
0003The predominant adsorbent used in TLC is silicic
acid, a partially hydrated silicon dioxide, often
termed silica gel, but others are also available, includ-
ing cellulose, aluminum oxide, celite (kieselguhr),
magnesium oxide, and zeolites. Modified versions
of these include C
8
-orC
18
- (reverse-phase) and
3-aminopropyl-silica gel, polyamide- and polyethyl-
ene imine (PEI)-cellulose. The particle size of the
adsorbents is usually in the range 1–25 mm.
0004TLC plates were often made in the laboratory
using specific apparatus. However, home-made plates
could be variable, and commercially produced pre-
coated plates, which are available in various sizes and
supports with almost every adsorbent, are now the
first choice due to their much greater reproducibility
and consistent quality. Adherence of the adsorbent
to the support is often enhanced in precoated plates
by the inclusion of binders such as calcium phos-
phate (10% w/w) or organic binders like poly-
acrylic acid.
CHROMATOGRAPHY/Thin-layer Chromatography 1267

0005 Silica gel is the most commonly employed adsorb-
ent in TLC, particularly that with a pore size of 6 nm.
Precoated analytical plates frequently use silica gel of
particle size 10–12 mm coated in a layer of 0.25 mm
thickness. Plates coated with this type of silica pro-
vide 1000–2000 theoretical plates per 5 cm of migra-
tion. Modern high-performance TLC (HPTLC) uses
silica gel of particle size 5–7 mm in layers of 0.20 mm
thickness. The reduced particle size provides 5000–
10 000 theoretical plates per 5 cm of migration dis-
tance and increases the separation efficiency. In com-
parison with conventional TLC, HPTLC is a more
efficient, instrumentalized, and quantitative method.
The narrower particle size distribution, thinner layers,
and shorter development distances of HPTLC lead to
a greater separation efficiency, faster separations, and
enhanced sensitivity through improved detection
limits such that quantitative HPTLC can produce
results comparable with GC and HPLC when opti-
mally performed. HPTLC plates cannot be prepared
easily in the laboratory and are usually purchased
ready-made.
0006 The adsorption properties of silica gel can be
modified by impregnation with various complexing
reagents, including silver nitrate, urea, boric acid,
oxalic acid, and ethylenediaminetetraacetic acid
(EDTA) to aid the separation of particular classes of
compounds. Reverse-phase plates can be produced by
using silanized silica gel in which the surface silanol
groups of the silica are silylated with chlorosilanes.
Silanized silica has a low carrying capacity that can be
increased by impregnating the silanized silica plates
with hexadecane or paraffin oil. It is now more
common to utilize ready-made reverse-phase plates
with C
2
,C
8
,orC
18
hydrocarbon chains, or diphenyl
groups chemically bonded to the silica. Aminopropyl
bonded silica gel plates are also available. Kieselguhr
or celite, a diatomous earth, is used less often than
synthetic silica gel on account of its natural variabil-
ity. Other adsorbents include cellulose and its deriva-
tives, such as diethylaminoethyl (DEAE)-cellulose for
ion-exchange chromatography, magnesium oxide,
and alumina (aluminum hydroxide), which is manu-
factured in three types, acidic, basic, and neutral,
according to its pH.
0007Glass is the support employed most commonly for
adsorbents on grounds of rigidity, flatness, and inert-
ness. Layers of adsorbents can also be obtained pre-
coated on flexible aluminum or plastic (polyester)
sheets, which have the advantage of being able to be
cut to smaller size, but care must be taken in insuring
their compatibility with solvent systems and subse-
quent detection reagents. The standard sizes of TLC
and HPTLC plates are 20 20 cm and 10 10 cm,
respectively, although other sizes such as 10 20 cm
are available (Figure 1).
Sample Applicators and Development Chambers
0008The application of the sample solution to the adsorb-
ent layer is usually performed manually using micro-
syringes or disposable glass capillaries. However, a
wide range of instruments, including microproces-
sor-controlled instruments, are available for the
automated application of samples either as spots or
streaks. Rectangular glass chambers with lids are
usually employed for the linear development of
chromatograms in solvent mixtures. Sizes are avail-
able for standard 20 20 cm plates and 10 10
A
B
C
D
E
F
G
H
I
fig0001 Figure 1 Typical apparatus used in TLC. (A) Applicator with micrometer-controlled syringe containing 10 20 cm HPTLC plate;
(B) drying/storage rack containing standard 20 20 cm TLC plates; (C)10 10 cm HPTLC plates in drying/storage rack; (D) atomizer
for spraying plates; (E)20 20 cm TLC plate being developed in a standard chamber; (F)10 10 cm HPTLC plate being developed in
HPTLC chamber; (G) glass microsyringe; (H) sample application template/R
f
calculator overlayed on the standard plate; (I) HPTLC
plate contained in the sample application template.
1268 CHROMATOGRAPHY/Thin-layer Chromatography

HPTLC plates. Chambers can be purchased with
vertical grooves in the side and end walls to allow
the simultaneous development of several plates
(Figure 1).
Equipment for Detection and Quantification of
Components
0009 Atomizers operated by compressed air or hand
bellows are required for spraying developed chro-
matograms evenly with detection reagents. An oven
is necessary for heating plates sprayed with certain
reagents. For the quantitation of separated compon-
ents on developed chromatograms, modern computer-
controlled scanning densitometers are available along
with sophisticated software packages for evaluation
and data storage. Densitometry is particularly useful
for one-dimensional chromatography, but programs
can be adapted for two-dimensional chromatograms.
The location and quantitation of radiolabelled com-
pounds can be achieved using specialized radioactiv-
ity detectors that can scan the whole chromatogram,
although these instruments are rather expensive.
However, less elaborate equipment is required for
the location of radiolabelled compounds on chro-
matograms by autoradiography. Fluorography can
be performed with similar equipment. New detection
apparatus that may prove useful include the charge
coupled device array detector for HPTLC and
matrix-assisted laser desorption/ionization mass
spectrometry.
Specialized Systems
0010 Several techniques that rely on the same chromato-
graphic principle as conventional TLC, but which
require the use of specialized equipment, have been
developed. The most durable of these specialized
systems is probably TLC with flame ionization detec-
tion (TLC–FID), which was developed to ally the
quantitative benefits of FID to TLC. In the ‘Iatroscan’
system, samples are applied to silica gel fused to
thin quartz rods, which are then developed conven-
tionally in mobile phase. After drying, the developed
‘chromarods’ are passed through a flame ionization
detector to determine the mass of individual compon-
ents by quantifying the amount of ionizable carbon.
The ‘Iatroscan’ has been relatively successful, particu-
larly in certain areas such as lipid metabolism.
Another reasonably successful system is automated
multiple development (AMD), which permits
the multiple development of TLC plates in the same
or different solvent systems. This repeated develop-
ment of TLC plates can improve the resolution of
components.
0011 Other specialized systems have not had such a
durable or widespread appeal but have had limited
use. In over-pressured TLC (OPTLC), the TLC plate
is held beneath a flexible membrane under hydro-
static pressure, and solvent is forced through the ad-
sorbent using a pump. This system allows compounds
to be eluted completely from the TLC plate for col-
lection or detection using detectors normally associ-
ated with HPLC. Centrifugal TLC also involves the
forced flow of solvent. In this case, solvent is fed into
the center of a rapidly rotating TLC or HPTLC plate
and is forced through the adsorbent by centrifugal
force. In radial-development TLC, the sample is ap-
plied to the center of a plate and the solvent supplied
through a hole in the plate via a wick that dips into
a solvent reservoir. Another system allowed the
application of solvent under pressure to HPTLC
plates allowing very short development times. (See
Chromatography: High-performance Liquid Chro-
matography.)
Methodology
Sample Application
0012In conventional TLC and HPTLC, the sample is
applied as a small volume of solution on to the
adsorbent near the bottom of the plate. The point
of application is termed the origin. A maximum of
about 80 or 10 mg for TLC and HPTLC, respectively,
can be applied as a spot on adsorbent layers of silica
gel G 0.20–0.25 mm thick. Samples can also be ap-
plied as a narrow streak along the origin. Standards
are applied as separate spots on the same plate.
Development
0013The choice of solvent system depends on the compon-
ents to be resolved and the type of adsorbent utilized
(see Table 1). Regardless of the components, in
conventional TLC, the solvent mixture is placed in
the development chamber. In the case of solvent
systems containing large proportions of polar solv-
ents, the chamber can be lined with filter paper to aid
saturation of the atmosphere. The plate is placed
within the chamber so that the bottom edge is im-
mersed in the solvent mixture, and the chamber is
sealed with a lid (Figure 1). When the solvent has
migrated to within 1 cm of the top edge of the plate,
the plate is removed from the chamber. Although
ascending chromatography, as described above, is
the most common form of TLC, chromatograms can
also be developed by descending chromatography
using a wick to feed the solvent on to the adsorbent
layer.
0014TLC plates can also be subjected to multiple
development in which the plate is developed par-
tially in one solvent system and removed from the
CHROMATOGRAPHY/Thin-layer Chromatography 1269

tbl0001 Table 1 Substances separated by selected one-dimensional TLC systems
AdsorbentSubstancesseparatedSolventsystem
a
Detectionreagent
Alumina Alkaloids CHCl
3
Dragendorff reagent
Amino acids (dicarboxylic) 2
M HAc Ninhydrin
Aromatic hydrocarbons CCl
4
10% tetracyanoethylene in C
6
H
6
Carotenes Hexane/C
6
H
6
/EtOH (100:100:1) None required
Food dyes (fat-soluble) Hexane/EtAc (98:2) None required
Food dyes (water-soluble) H
2
O/EtOH/n-BuOH (1:1:5) None required
Neutral lipids Hexane/Et
2
O/HAc (94.5:5:0.5) 0.05% 2
0
,7
0
dichlorofluorescein in
MeOH
Sterols C
6
H
6
/EtOH (95:2) 10% phosphotungstic acid in 90%
EtOH
Steroids CHCl
3
/EtOH (99:1) Anisaldehyde/H
2
SO
4
/HAc
(1:2:100), UV
Vitamins (fat-soluble) Toluene 1.3% FeCl
3
in 2 M HCl/0.7%
K
3
FeCN
6
(1:1)
Cellulose Amino acids n-BuOH/Me
2
CO/Et
2
NH/H
2
O
(10:10:2:5)
Ninhydrin
Antibiotics (water-soluble) PrOH/pyridine/HAc/H
2
O
(15:10:3:12)
Ninhydrin
Food dyes (fat-soluble) n-PrOH/EtAc/H
2
O (6:1:3) None required
Nucleotides n-BuOH/Me
2
CO/HAc/5% NH
4
OH/
H
2
O (4.5:1.5:1:1:2)
20% SbCl
5
in CCl
4
Nucleosides and free bases H
2
O 20% SbCl
5
in CCl
4
DEAE cellulose Amino acids n-BuOH/HAc/H
2
O (4:1:5) Ninhydrin
Nucleotides Isobutyric acid/NH
4
OH/H
2
O
(33:1:16)
20% SbCl
5
in CCl
4
PEI cellulose Nucleotides 0.25, 1.0, 1.6 M LiCl in H
2
O (in
successive developments)
20% SbCl
5
in CCl
4
Celite (Kieselguhr) Sugars EtAc/iso-PrOH/H
2
O (130:57:23) 0.2% naphthoresorcinol in EtOH/
10% H
3
PO
4
Oligosaccharides Iso-PrOH/EtAc (65:35) 0.2% naphthoresorcinol in EtOH/
10% H
3
PO
4
Food dyes (fat-soluble) Cyclohexane None required
Magnesium oxide Carotenoids Pet. ether/C
6
H
6
(3:1) or pet. ether/
Me
2
CO (97:3)
None required
Polyamine Anthocyanins HAc/HCl/H
2
O (10:1:3) 10% oxalic acid in Me
2
CO/H
2
O
(1:1), UV
Antioxidants MeOH/Me
2
CO/H
2
O (6:1:3) 10% phosphomolybdic acid in
EtOH
Flavonoids Me
2
CO/95% EtOH/H
2
O (2:1:2) 25% PbAc in basic aqueous
solution
Sephadex Proteins 0.5 M NaCl 1% naphthalene black in MeOH/
H
2
O/HAc (5:4:1)
Silica gel Alkaloids CHCl
3
/Me
2
CO/Et
2
NH (5:4:1) Dragendorf reagent
Amino acids n-BuOH/HAc/H
2
O (4:1:1) Ninhydrin
Antibiotics
Macrolides CHCl
3
/MeOH/H
2
O (80:20:2.5) 20% phosphomolybdic acid in
EtOH
Penicillins Me
2
CO/MeOH (1:1) 20% phosphomolybdic acid in
EtOH
Streptomycins H
2
O/Na citrate/citric acid
(100:20:5)
20% phosphomolybdic acid in
EtOH
Tetracyclines BuOH/HAc/H
2
O (2:1:1) 20% phosphomolybdic acid in
EtOH
Antioxidants CHCl
3
20% phosphomolybdic acid in
EtOH
Bile acids Me
3
pentane/iso-PrOH/HAc
(60:20:0.5)
5% phosphomolybdic acid in
EtOH/Et
2
O (1:1)
Food dyes (fat-soluble) Hexane/Et
2
O/HAc (70:30:1) None required
Continued
1270 CHROMATOGRAPHY/Thin-layer Chromatography

chamber. After evaporation of solvent from the
developed plate, the plate is developed fully or
partially in another solvent system. Multiple devel-
opments enable improved resolution of compon-
ents. Development of the chromatogram with the
mobile phase in one direction only is known
as one-dimensional TLC (Figure 2). In instances
where one-dimensional TLC/HPTLC does not
allow the complete resolution of all the compon-
ents of a mixture, improved separation can be
achieved with two-dimensional TLC/HPTLC. In this
technique, the sample is applied as a spot at one
corner and developed fully in one direction in the
first solvent system. The plate is removed from
the chamber and, after evaporation of the solvent, is
then developed in a second solvent system in a
direction at 90
to that of the first development
(Figure 3).
Identification of Separated Components
0015Colored compounds are visible on developed chro-
matograms, but colorless compounds require detec-
tion by physical or chemical means. Detection by
chemical means usually involves spraying the adsorb-
ent layer of the developed chromatogram with a deri-
vatizing reagent that reacts with the separated
components to produce a colored derivative in situ.
Nonspecific reagents such as sulfuric acid and iodine
will detect a wide range of compounds. Specific re-
agents react with specific functional groups and will
AdsorbentSubstancesseparatedSolventsystem
a
Detectionreagent
Food dyes (water-soluble) HAc/iso-BuOH/H
2
O (2:5:2) None required
Gangliosides CHCl
3
/MeOH/0.3% KCl (30:18:4)
to purify CHCl
3
/MeOH/0.25%
CaCl
2
(55:45:10) to resolve
classes
0.5% orcinol in 20% sulfuric acid
0.5% orcinol in 20% sulfuric
acid
Insecticide (Imidacloprid) CHCl
3
/Me
2
CO/MeOH (23:1:1) UV
Lipids (neutral) Hexane/Et
2
O/HAc (80:20:2) 3% CuAc in 8% H
3
PO
4
Lipids (polar) MeAc/iso-PrOH/CHCl
3
/MeOH/
0.25% aq. KCl (25:25:25:10:9)
3% CuAc in 8% H
3
PO
4
Lipopolysaccharides iso-PrOH/H
2
O/CHCl
3
/NH
4
OH
Et
3
NH (120:60:16:4:1)
0.1% orcinol in 30% aq. MeOH
Mycotoxins CHCl
3
/Me
2
CO (90:10) p-Anisaldehyde/MeOH/HAc/
H
2
SO
4
(0.5:70:10:5)
Opiates (acetate or methoximine
derivatives)
CH
2
Cl
2
/iso-PrOH (88:12) þ 1.5%
NH
4
OH
Conc. Sulfuric acid
Plasticizers CH
2
Cl
2
4 M H
2
SO
4
/20% resorcinol (1:1)
Mono-, di-, and trisaccharides MeCN/H
2
O (85:15) 0.5% KMnO
4
Polysaccharides n-BuOH/MeOH/H
2
O (50:25:20) 0.5% KMnO
4
Shellfish toxin (domoic acid) BuOH/HAc/H
2
O (3:1:1) UV and ninhydrin
Steroids CHCl
3
/EtOH (92:8) 10% phosphomolybdic acid in
EtOH
Synthetic sweeteners CHCl
3
/HAc (90:10) 0.2% 2
0
,7
0
dichlorofluorescein in
EtOH
Te r p e n e a l c o h ol s CH
2
Cl
2
0.06% diphenylpicryl hydrazyl in
CHCl
3
Terpene aldehydes CHCl
3
10% SbCl
5
in CCl
4
Vitamins (fat-soluble) Cyclohexane/EtAc (75:25) UV
Vitamins (water-soluble) HAc/Me
2
CO/MeOH/C
6
H
6
(5:5:20:70)
UV
3-Aminopropyl silica Oligosaccharides MeCN/10 m
M Me
3
NH
2
Ac (3:2) Fluorography after spraying with
4-methylenaphthalene
enhancer
C
8/18
-silica (reverse-phase) Alkaloids
Antifungals MeOH/H
2
O (90:10) UV
Carotenoids Pet.ether/MeCN/MeOH (2:4:4) None required
Quinones Me
2
CO/H
2
O (19:1) 10% phosphomolybdic acid in
EtOH
Vitamin K CH
2
Cl
2
/MeOH(70:30) UV
a
Solvent proportions by volume.
Ac, sub>/sub>CO
2
; Bu, C
4
H
9
-;Et, C
2
H
5
-; Me, CH
3
-; Pr, C
3
H
7
-; DEAE cellulose, diethylaminoethyl cellulose; PEI cellulose, polyethylene imine cellulose;
UV, ultraviolet. For health and safety reasons, C
6
H
6
can usually be replaced with toluene, and hexane can be replaced with iso-hexane.
Table 1 Continued
CHROMATOGRAPHY/Thin-layer Chromatography 1271
only detect compounds containing the group, e.g.,
ninhydrin for amino groups. The use of specific re-
agents can be used as an aid to identification and
characterization of components.
0016 Physical methods of location commonly involve
ultraviolet light. Commercial plates are available
coated with adsorbents containing an indicator that
absorbs light at 254 nm and reemits or fluoresces light
at the green end of the spectrum. When separated
on these plates, compounds that absorb ultraviolet
light appear as dark spots on a green fluorescent
background. Plates with adsorbents not containing
fluorescent indicator can be sprayed with 2
0
,7
0
-
dichlorofluorescein with similar results when viewed
under ultraviolet light. Radiolabelled compounds can
be located on TLC plates physically by autoradiog-
raphy or in situ measurement of radioactivity using a
radioscanner.
0017 A useful aid to the identification of separated com-
ponents is the R
f
value, which is defined as the ratio of
the distance traveled by the compound to the distance
traveled by the solvent. Although the R
f
value of
a given compound in a defined mobile phase and
adsorbent is very characteristic, many factors,
including the thickness and moisture content of the
adsorbent and development distance, affect the repro-
ducibility of the value. For this reason, the R
f
value is
only an indication of the identity of a compound, and
confirmation of identity should be obtained by other
means. Identification of separated components can be
aided by comparison with authentic standards run
alongside the samples, if such standards are available,
and by staining patterns to different reagents.
Quantification of Separated Components
0018Once located, it is often useful and necessary to quan-
tify the components in a mixture, and this has been
the most difficult aspect of TLC. However, various
methods can be employed for the quantitation of
separated components.
0019Areas of adsorbent that contain components can be
scraped from the support and the compounds eluted
from the adsorbent with suitable solvents. The
recovered compounds can be estimated gravimetri-
cally after evaporation of the solvent, or by using a
A
SF
WE
CE
FAME
TAG
FFA
DAG
St
MAG
PL
FAI
C
SF
Pigm-
MGDG
NL
C
B
CB
GL
S
DGDG
PE
PA
PI
PS
SQDG
PE
PG
U
PC
PC
SM
LPC
O
123
SF2
WE
FFA
FAI
St
MAG
SF1
CB
C
S
PE
PI
PS
PC
SM
LPC
O
SE
TAG
Pigm
MGDG
GL
DGDG
SQDG
U
PC
PS
PG
PE
12 3
C
21
O
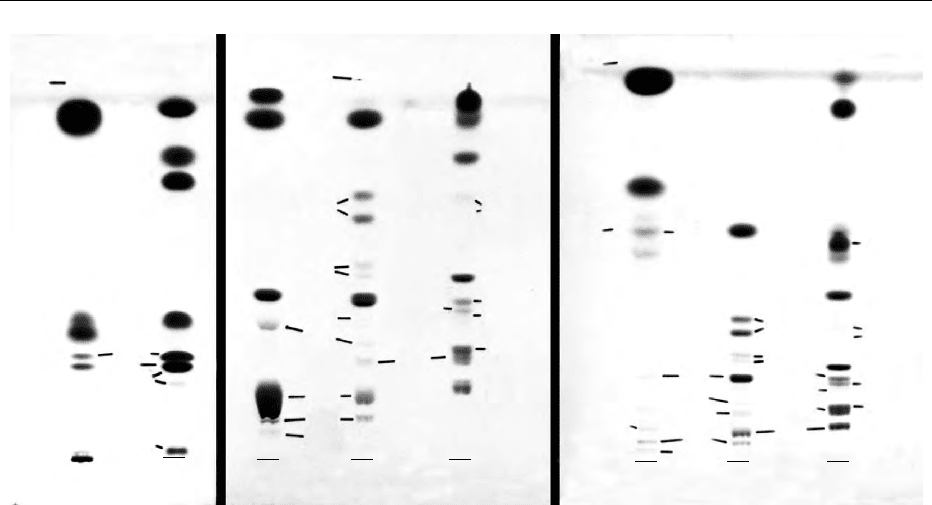
fig0002 Figure 2 HPTLC chromatograms of lipid mixtures developed in various systems. (A) Single development in hexane/Et
2
O/HAc
(80:20:2 by volume): 1, zooplankton total lipid; 2, standard authentic mixture, all based on oleic acid. (B) Single development in MeAc/
iso-PrOH/CHCl
3
/MeOH/0.25% aqueous KCl (25:25:25:10:9, by volume): 1, cod roe total lipid; 2, rat brain total lipid; 3, algal (Croomonas
salina) total lipid. (C) Double-development with development to half final distance in solvent system of (B) followed by full development
in solvent system of (A): 1, Zooplankton total lipid; 2, rat brain total lipid; 3, algal (C. salina) total lipid. All chromatograms were stained
by charring after spraying with 3% copper acetate in 8% orthophosphoric acid. C, cholesterol; CB, cerebrosides; CE, cholesteryl ester;
DAG, diacylglycerol; DGDG, digalactosyl diacylglycerol; FAI, fatty alcohol; FAME, fatty acid methyl ester; FFA, free fatty acid; GL,
glycolipid; LPC, lyso-phosphatidylcholine; MAG, monoacylglycerol; MGDG, monogalactosyl diacylglycerol; NL, neutral lipid; O, origin;
PA, phosphatidic acid; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PI, phosphatidylinositol; Pigm, pigment; PL, polar
lipid; PS, phosphatidylserine; SF, solvent front; SM, sphingomyelin; SQDG, sulfoquinovosyldiacylglycerol; St, sterol; TAG, triacylgly-
cerol; U, unknown; WE, wax ester.
1272 CHROMATOGRAPHY/Thin-layer Chromatography
specific assay such as the measurement of phosphorus
or sugar content.
0020 Developed chromatograms can be subjected to
scanning densitometry and the separated components
quantified on the basis of transmittance, reflectance
intensity, or fluorescence, depending on the nature of
the compounds and the detection reagent/stain used.
Modern techniques of imaging in conjunction with
sophisticated computer software packages for image
analysis and data processing have increased the accur-
acy of these techniques. As well as locating radio-
labeled compounds, radioscanners can also quantify
the amount of radioactivity present in individual com-
ponents. Alternatively, bands of adsorbent that con-
tain compounds can be scraped from the support into
scintillation vials and measured directly for radio-
active content by liquid scintillation counting after
addition of suitable scintillation fluid.
Preparative TLC
0021 Although TLC is mostly used as an analytical tech-
nique, one-dimensional TLC can be scaled up to a
preparative scale. To this end, sample can be applied
as a streak across the origin of an analytical plate, the
length of the streak being determined by the amount
of sample. Special preparative TLC plates coated with
thicker layers of adsorbent can be obtained, which
can accommodate relatively large amounts of sample.
However, these plates lack the resolving power of the
analytical plates. After nondestructive visualization
of the separated components, the bands of adsorbent
containing the compound or compounds of interest
are scraped from the support and eluted with suitable
solvents.
Modes of Chromatography
0022TLC conforms to the basic principles of liquid chro-
matography. The most common mode of chromatog-
raphy used in TLC is adsorption chromatography,
although other modes of chromatography can be
employed. With silica, celite, keiselghur, and cellu-
lose, the mechanism is by adsorption chromatog-
raphy if the adsorbent on the plate is completely free
of water, and the solvent system is a nonpolar mix-
ture. However, if water is present on the adsorbent or
if the mobile phase contains a highly polar solvent,
then separation will be by partition chromatography.
In addition, reverse-phase TLC plates also separate
by partition chromatography. TLC plates are also
available precoated with reverse-phase silica gel that
has been impregnated with a chiral reagent and
copper ions. These can be used to separate optically
active isomers, e.g., amino acids, by chiral chroma-
tography on the basis of ligand exchange.
0023Alumina separates components by adsorption but,
depending upon the nature of the surface and the
solvent system, can also function as an ion exchanger.
SF1
NL
SF2
2
PC
SM
O
PS
PA
PI
PE
S
CB
1
U
A
SF1
NL
SF2
MGDG
U
GL
B
1
DGDG
PG
U
U
PC
PS
PA
CL
SQDG
PE
O
2
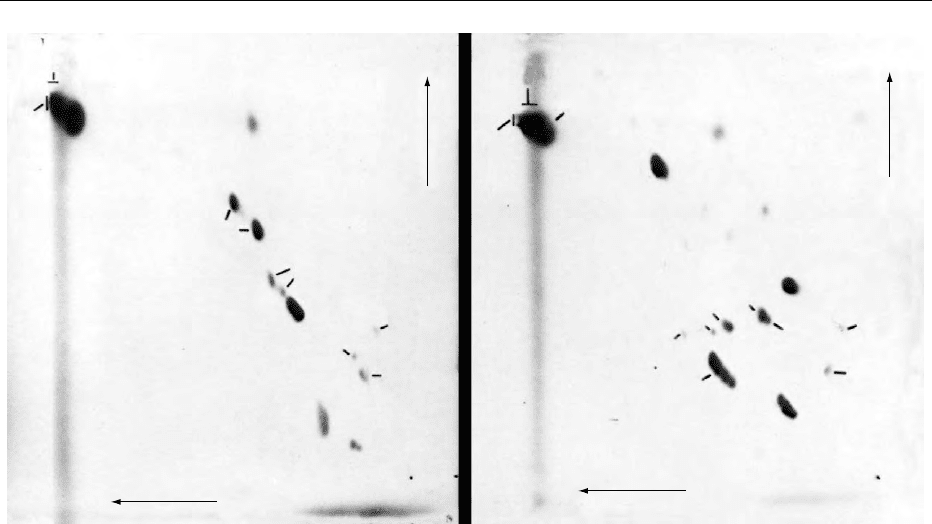
fig0003 Figure 3 Two-dimensional HPTLC of total lipid from (A) rat brain and (B) the alga C. salina. Plates were first developed in direction
1 with MeAc/iso-PrOH/CHCl
3
/MeOH/0.25% aqueous KCl (25:25:25:10:9, by volume) and then in direction 2 with CHCl
3
/MeOH/7M
NH
4
OH (65:35:5, by volume). Developed chromatograms were stained by charring after spraying with 3% copper acetate in 8%
orthophosphoricacid.Abbreviations:asin Figure 2.
CHROMATOGRAPHY/Thin-layer Chromatography 1273
Modified cellulose such as DEAE-cellulose can be
used for ion-exchange separations on TLC. Separ-
ation by size-exclusion chromatography can be
carried out with TLC plates coated with Sephadex
gel but is slower and significantly more difficult
than other forms of TLC. Polyamides such as
polyhexamethylenediaminoadipate can be used as
the adsorbent to separate components that interact
with it by hydrogen bonding. (See Chromatography:
Principles.)
Applications
0024 The actual method employed for preparing samples
from foodstuffs for analysis by TLC depends on the
nature of the substances being investigated. Many
methods involve the extraction of the food with a
suitable solvent followed by precipitation and filtra-
tion steps to remove classes of compounds that are
not of interest. (See Analysis of Food.)
0025 The choice of adsorbent and solvent system used in
TLC is dictated by the nature of the sample to be
analyzed. Examples of broad groups of substances
that have been successfully resolved by TLC methods
are shown in Table 1, along with suggested solvent
systems and detection reagents commonly used.
However, for most substances, there can be a variety
of different solvent systems and detection reagents
that are suitable, depending on the precise compos-
ition of the samples to be resolved.
See also: Chromatography: Principles; High-
performance Liquid Chromatography; Gas
Chromatography
Further Reading
Ackman RG (1982) Flame ionization detection applied to
thin-layer chromatography on coated quartz rods.
Methods in Enzymology 72: 205–252.
Henderson RJ and Tocher DR (1992) Thin-layer chroma-
tography. In: Hamilton RJ and Hamilton S (eds) Lipid
Analysis: A Practical Approach, pp. 65–111. Oxford:
Oxford University Press.
Jork J, Funk G, Fischer G and Wimmer CJ (1989) Thin-
layer Chromatography: Reagents and Detection
Methods. Cambridge: VCH.
Kirchner JG (1978) Thin-layer Chromatography. Tech-
niques of Chemistry, vol. XIV. New York: Wiley.
Sherma J (1996) Thin layer chromatography in food
analysis. Food Testing and Analysis 2: 12–15.
Touchstone JC (1988) Instrumentation for thin-layer chro-
matography: a review. Journal of Chromatographic
Science 26: 645–649.
Wilson ID (1996) Thin layer chromatography: a neglected
technique. Therapeutic Drug Monitoring 18: 484–492.
High-performance Liquid
Chromatography
M V Moreno-Arribas and M C Polo, Instituto de
Fermentaciones Industriales (CSIC), Madrid, Spain
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Introduction
0001High-performance liquid chromatography (HPLC) is
an instrumental form of liquid chromatography that
employs stationary phases consisting of small par-
ticles, thereby achieving more efficient separations
than those used in conventional liquid chromatog-
raphy. Since its origin in the late 1960s, it has been
known by several different names, including high-
pressure liquid chromatography, because of the
high pressures required to force the mobile phase or
solvent through the stationary phase, and high-
resolution liquid chromatography, because of the
good resolution achieved using this technique.
0002This article describes the equipment needed to
carry out HPLC and summarizes some of the app-
lications of this technique in food analysis. Two
techniques related to HPLC, fast-protein liquid chro-
matography (FPLC) and supercritical fluid chroma-
tography (SFC), are also considered. The basic theory
of the chromatographic process and the factors
that affect separation efficiency are discussed in
the section on Chromatography. (See Chromatog-
raphy: Principles; Thin-layer Chromatography;
High-performance Liquid Chromatography; Gas
Chromatography; Supercritical Fluid Chromatog-
raphy; Combined Chromatography and Mass Spec-
trometry.)
Separation Modes
0003There are many different stationary and mobile
phases that can be used in HPLC, and for this reason
there is a great variety of separation mechanisms.
The treatises on HPLC use different criteria in their
attempt to classify the modes of this technique:
type of stationary phase, predominant separation
mechanism, type of groups of compounds it is
aimed at. These criteria sometimes overlap; that is
to say, it is possible to work in several different chro-
matographic methods with the same stationary
phase, and the same group of compounds can be
separated using several different stationary phases,
and for this reason it is not easy to classify HPLC
techniques into specific groups. Generally speaking,
it can be said that there are three large groups
1274 CHROMATOGRAPHY/High-performance Liquid Chromatography

that dominate the field of chromatographic separ-
ations: reversed-phase chromatography, ion exchange
chromatography and size exclusion chromatography.
There are also other types of chromatography that
are rapidly gaining in importance and cannot be in-
cluded exactly in any of these three groups: ion pair
chromatography, affinity chromatography, and chiral
separations.
Reversed-Phase Chromatography
0004 This is the most commonly used method for chroma-
tographic separations, so it could in fact be called
‘normal-phase’ except that it was developed later
than the latter. In reversed-phase chromatography,
the stationary phase is of a nonpolar nature and the
mobile phase is more polar than the stationary one.
Among the most widely used stationary phases are
silica-based ones with cyano, phenyl, or alkyl func-
tionality and those of a polymeric nature, above all
polystyrene-divinylbenzene (PSDVB). The mobile
phase is usually water with an organic modifier
(methanol, acetonitrile, or tetrahydrofuran). Ternary
or quaternary mixtures of these components can be
used to improve selectivity.
0005 There is controversy over the mechanism that
controls reversed-phase separations. The three basic
models are the partitioning model, the solvophobic
effect, and the adsorption model. It is also necessary
to consider the possibility that a mixed mechanism
might exist.
0006 In the partitioning mechanism it is accepted that
the retention of a solute in reversed-phase liquid
chromatography is due to its partitioning between
the mobile phase and the stationary phase. This
mechanism assumes that the stationary phase behaves
in a similar way to a liquid, a situation that may occur
in certain conditions, such as when working with
monomeric phases and high percentages of water in
the mobile phase, and the size of the hydrocarbonated
chains is large enough for them to be associated. The
adsorption mechanism accepts that retention takes
place through the interaction of the solute to the
organic molecules bonded to the surface of the
packing matrix. The solvophobic effect theory
accepts that the solute is expelled from the mobile
phase towards the stationary phase.
Ion Exchange Chromatography
0007 This is used for the analysis of electrically charged
substances in working conditions. Stationary phases
with electrically charged functional groups are used
for this method of chromatography. The support can
be silica or a polymer. The mobile phase contains a
buffer which controls the loading of the solutes, and a
salt that competes with the solutes in their interaction
with the charges of the stationary phase. The charges
permanently bonded to the chromatographic support
are compensated by counterions, that is to say, by free
ions with the opposite charge. Passage through the
column of a sample or of the mobile phase with ions
of the same sign as the counterions of the column may
result in the displacement of the counterions and the
retention of the ions of the sample or of the stationary
phase.
0008The ion exchangers can be classified as cationics
and anionics. Within each group it is possible to
differentiate strong and weak exchangers. Strong
cation exchangers (SCX) usually contain sulfonic
groups, while weak ones have carboxylic groups.
Quaternary ammonium salts are the most usual func-
tional groups in strong anion exchangers (SAX), and
primary amines are used in weak anion exchangers.
Size Exclusion Chromatography
0009This separation method is based on the size of the
molecules. For many years it was known as gel
permeation chromatography (GPC) or gel filtration
chromatography (GFC). The retention of the mol-
ecules depends on their size: the larger molecules
that do not enter the pores of the packing elute first,
while the smaller molecules, whose diameter makes it
possible for them to enter and exit the pores of the
chromatographic packing, take a longer time.
Ion Pair Chromatography
0010This chromatographic method enables the separation
of solutes that are ionized in separation conditions.
On most occasions the separations are carried out on
the same columns as those used in reversed-phase
separations without the formation of ion pairs. The
mobile phase is made up of a water buffer and an
organic modifier, to which mixture is added a coun-
terion with an opposite charge to that of the solute.
Quaternary ammonium salts are often used as
counterions for the analysis of anion substances,
while n-alkyl sulfate salts are among the most com-
monly used for the analysis of cation substances.
Affinity Chromatography
0011This is based on the specific interaction between a
molecule, or group of molecules, and a ligand,
which is a molecule that is attached to the station-
ary phase to interact with those of the solute. The
growing importance of affinity chromatography is
due to the development of bioaffinity chromatog-
raphy in which the interactions between ligands and
molecules are based on recognitions of a biological
type.
CHROMATOGRAPHY/High-performance Liquid Chromatography 1275
