Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.


7a-hydroxylase, acyl CoA cholesterol acyl trans-
ferase (ACAT) and lecithin cholesterol acyl transfer-
ase (LCAT). The 7a-hydroxylase catalyzes the first
step in the conversion of cholesterol to bile acids,
while ACAT and LCAT are responsible for esterifying
cholesterol in cells and plasma, respectively.
Cholesterol and Lipoprotein Transport
0006 Cholesterol is transported along with other lipids
by the plasma lipoproteins. These are classified
according to their size and density, which are func-
tions of their compositions (Table 2). Each class is
characterized by specific proteins (apoproteins), some
of which have known important functions (Table 3).
Mutations in apoproteins are involved in several
types of hyperlipidemia. Although the liver contains
a large amount of cholesterol because of immense
amounts of membraneous organelles, it does not
store extra cholesterol. Normal nascent (newly se-
creted by the liver) very-low-density lipoprotein
(VLDL) has a cholesterol ester (CE)/triacylglycerol
(TG) ratio of < 1:4, but CE-rich VLDL may have a
ratio of 1:1. CE-rich VLDL is synthesized by the liver
in some experimental animal models fed very high
levels of dietary cholesterol. Such VLDL has extra
apoenzyme E (apoE). This may be important because
macrophages have not only the apoenzyme (apoB)/
LDL receptor, but also a B/E receptor, which will bind
apoB and/or apoE lipoproteins, as discussed below.
0007Circulating VLDL loses much of its triglyceride to
tissues and some of its other components to high-
density lipoprotein (HDL); in the process, VLDL
becomes transformed to the cholesterol-rich LDL
particle. LDL binds to its specific receptor on cells
and is carried into the cell by receptor-mediated endo-
cytosis. The major function of the LDL receptor is
delivery of lipids to cells, but in the liver, it helps
remove LDL and small VLDL from the bloodstream.
The receptor is subject to transcriptional control in
which high [cholesterol] leads to decreased receptor
synthesis, thus downregulating the entry of apoB-
containing lipoproteins. The LDL receptor is ex-
pressed in most cells and is basically the same in all
tissues, but the number of receptors varies greatly
between tissues. The greatest density of receptors
occurs in the adrenal cortex, which requires enor-
mous amounts of cholesterol for steroid hormone
synthesis. It is expressed at low levels in nondividing
cells, presumably because there is less need for chol-
esterol for membrane synthesis. Cholesterol that is
not immediately used in the cell is esterified by
tbl0002 Table 2 Classification and composition of major human plasma lipoproteins
Class Density (g ml
1
) Percentage
protein
Percentage
cholesterol
Percentage
cholesterolester
Percentage
triacylglycerol
Chylomicrons <1.00 2 1 3 85
Very-low-density lipoprotein 0.95–1.006 10 7 12 50
Low-density lipoprotein 1.006–1.063 23 8 37 10
High-density lipoprotein 1.063–1.210 55 2 15 4
The other major constituents of these lipoproteins are phospholipids; minor components are sphingolipids, carotenoids, and other hydrophobic
compounds.
tbl0003 Table 3 Major human apolipoproteins
Apoprotein Associatedlipoprotein Function
ApoA-1 HDL LCAT activation; possibly involved with reverse cholesterol transport
ApoA-II HDL Transport of HDL lipids; unknown
ApoA-IV HDL, chylo Unknown; possibly antiatherogenic
ApoB-48 Chylo Lipid transport
ApoB-100 VLDL, LDL Lipid transport; LDL receptor interaction
ApoC-1 VLDL, HDL Unknown; may function with A-I to activate LCAT
ApoC-II VLDL, HDL, chylo Activatation of lipoprotein lipase
ApoC-1II VLDL, HDL, chylo Inhibition of lipoprotein lipase
ApoD HDL Unknown
ApoE VLDL, HDL, Chylo Clearance of VLDL and chylomicrons from plasma;
abundant in brain and cerebrospinal fluid, modifies risk
of Alzheimer dementia
Apo, apoenzyme; chylo, chylomicron; HDL, high-density lipoprotein; LDL, low-density lipoprotein; VLDL, very-low-density lipoprotein.
1246 CHOLESTEROL/Role of Cholesterol in Heart Disease

ACAT, but normally, there are only small amounts of
the ester within cells. Among the other factors
thought to regulate LDL receptor expression are thy-
roid hormone, insulin, and platelet-derived and other
types of growth factors.
0008 Once LDL is delivered to cells, no degradation of
cholesterol can occur in peripheral cells, and removal
of cholesterol appears to be a function of another
lipoprotein. HDL seems to function in ‘reverse chol-
esterol transport,’ in which it helps remove choles-
terol from cells and return it to the liver for excretion
in the bile. The exact mechanism and sequence of
events are not yet understood. High levels of HDL
cholesterol appear to indicate that more reverse chol-
esterol transport is occurring. This topic is discussed
elsewhere in this volume.
Oxidation of LDL
0009 The term oxidized LDL (oxLDL) encompasses a var-
iety of particles, ranging from slight oxidation of LDL
fatty acids to particles with oxidation of fatty acids,
cholesterol and even apoB. LDL that has undergone
certain modifications such as peroxidation or chem-
ical acetylation or maleylation of apoB is taken up
avidly by the modified-LDL receptor, also called the
scavenger receptor. The expression of this receptor is
independent of cell [cholesterol], and there is no
downregulation of receptor number. Cholesterol ac-
cumulation can occur in macrophages and other cells
that have this receptor. In addition, fatty acid oxida-
tion fragments such as malondialdehyde may react
with lysine residues of apoB, which may increase its
ability to accumulate in macrophages. OxLDL is not
a good ligand for the native LDL receptor, but it is
more atherogenic than native LDL. After the discov-
ery of the LDL receptor by Mark Brown and Joseph
Goldstein in the mid-1970s, it gradually became evi-
dent that cholesterol accumulation in arterial lesions
involves some processes other than uptake of native
LDL by its receptors, since subjects with homozygous
familial hypercholesterolemia, whose cells lack the
receptors, accumulate cholesterol in ‘foam’ cells. Fur-
thermore, ‘foam’ cells develop from macrophages/
monocytes and smooth muscle cells, but in vitro
experiments show that neither of these cell types
accumulates much cholesterol from native LDL.
Also, when native LDL delivers cholesterol and
other lipids to cells via receptor-mediated endocyto-
sis, feedback mechanisms decrease the number of
receptors on the cell surface. This is called downre-
gulation of the receptor, a process that normally helps
maintain cholesterol homeostasis. Further experi-
mentation has shown that the macrophages take up
chemically modified LDL by a separate, nonregulated
mechanism that allows accumulation and cellular
damage (Table 4).
0010It was soon learned that in vitro incubation of LDL
with cultured endothelial cells creates oxidatively
modified LDL that is readily bound and accumulated
by the macrophages. Addition of antioxidants in vitro
prevents this modification of LDL. We now know
that the uptake of oxidized LDL occurs via a distinct
receptor, called scavenger receptor A (SRA), on
macrophages/monocytes and smooth muscle cells.
Because this receptor is not downregulated, and
LDL lipids accumulate in these cells, eventually they
become ‘foam cells.’ Even slightly oxidized LDL,
often called ‘minimally modified,’ can be recognized
and taken up by the SRA. More than one type of
scavenger receptor is present on macrophages, and
other types of modification of LDL may also contrib-
ute to increased uptake by the macrophage. The
sequence of events thought to be involved in athero-
genesis is discussed below. Studies with experimental
animals and in vitro cell-culture studies suggest that
the use of antioxidants may help reduce or inhibit
production of oxLDL and therefore may help inhibit
atherogenesis. However, although epidemiologic data
offer evidence of an inverse relationship between diet-
ary antioxidant intake and risk of coronary heart
disease (CHD), effectiveness in preventing athero-
sclerosis has not yet been conclusively demonstrated
in humans.
Atherogenesis
0011Atherosclerosis (Greek athe
¯
re
¯
‘gruel’ þskle
¯
ro
¯
sis ‘hard-
ness’) occurs within major arteries when cholesterol-
laden lesions build up in the lining and wall, eventu-
ally resulting in narrowing and sometimes occlusion
of the lumen. In addition to decreasing the flow
through the narrowed vessel, the lesions, called
fibrous plaques, can cause thrombosis (blood clots),
which may lead to CHD (also called myocardial in-
farction (MI) or heart attack), decreased oxygen de-
livery to the brain or other organs, or thrombotic
tbl0004Table 4 Detrimental effects attributed to oxidized LDL
Avidly binds scavenger receptors, leading to accumulation of
LDL lipids in arterial macrophages
Cytotoxic to arterial endothelial cells in vitro and possibly in vivo
Stimulates release of mitogenic factor(s) for macrophages and
smooth muscle cells
Stimulates recruitment of additional monocytes/macrophages
from bloodstream into intima
Is immunogenic and leads to autoantibody production
Antagonizes normal nitric oxide-induced vasodilation of
arteries
CHOLESTEROL/Role of Cholesterol in Heart Disease 1247

stroke. Atherosclerosis is usually described as a multi-
factorial disease with numerous risk factors (Table 5).
Although genetics seems to be a predominant risk
factor, attention has focused on those factors that
can be changed, namely hypertension and blood
cholesterol levels. Risk factors appear to be multi-
plicative rather than merely additive. Risk varies be-
tween races, and in America, blacks have a higher
incidence of CHD than whites of the same age.
Blood cholesterol levels do not differ between the
two groups, and the higher incidence of CHD is at-
tributed to the greater incidence of hypertension
among blacks.
0012Theories on the etiology of atherosclerosis have
gradually coalesced into the prevailing ‘endothelial
injury’ hypothesis, which encompasses most in vivo
and in vitro observations (Table 6). The endothelium
lining of arteries plays a major role in the develop-
ment of atherosclerosis. The response to endothelial
injury is similar to the inflammatory response to in-
juries elsewhere. This may occur at the bifurcation of
blood vessels where flow is turbulent; it may be in
response to the constant insult of hypertension or
to chemicals such as benzo(a)pyrene in cigarette
smoke. White blood cells invade the area. If high
levels of LDL are present, oxidation of LDL, possibly
by endothelial cells, results in the formation of a
modified (oxidized) LDL. OxLDL triggers a series
of events: it causes more endothelial injury, and
endothelial cells synthesize endothelial-leukocyte ad-
hesion molecules (ELAMs). The latter attract mono-
cytes and T lymphocytes that adhere to the
endothelium. These move below the endothelium
and not only engulf more oxLDL but also secrete
cytokines and growth factors that stimulate move-
ment of smooth muscle cells and macrophages to
the area where all cells begin to accumulate increasing
amounts of lipid, particularly cholesterol esters. Over
a number of years, the process results in pronounced
lesions (Table 7).
tbl0005 Table 5 Primary and secondary risk factors for coronary
heart disease
Primary risk factors for CHD
Serum [cholesterol] >5.2 mmol l
1
[HDL cholesterol] <0.9 mmol l
1
Hypertriglyceridemia
Heredity
Hypertension
Smoking
Age (risk increases at 45 þ years age for men, 55 þ for women)
Diabetes mellitus
Obesity
Physical inactivity
Stress
Secondary risk factors for hyperlipidemia
Nephrotic syndrome
Alcoholism
Various drugs
Certain hormonal disorders (e.g., Cushing’s disease)
Systemic lupus erythematosis
Pregnancy
a
a
A normal physiologic response to pregnancy.
tbl0006 Table 6 Likely sequence of events and relationship of LDL and atherogenesis
Dietary, genetic, and environmental factors
#
Increased blood levels of LDL lipids (cholesterol, triglycerides)
#
Greater adherence of monocytes to endothelial lining of arteries and more LDL entering lining of arteries
#
Oxidative modification of LDL to MM-LDL by cells in the intima (lining)
#
Stimulation of release of MCP-1 from endothelial cells by oxLDL; and simultaneous stimulation of release of MCSF
#
Movement of more monocytes into the area from the bloodstream; differentiation of these cells into macrophages with increased SRAs
#
Increased uptake of modified LDL by SRA and accumulation of cholesterol esters
#
Recruitment of smooth muscle cells from arterial media to intima; induction of SRA expression in these cells
#
Formation of lipid-laden foam cells from macrophages and smooth muscle cells
#
Formation of fatty streaks
#
Continuation of above sequence, leading eventually to localized cell necrosis, atheroma, and finally plaque formation
MCP-1, monocyte chemoattractant protein-1; MCSF, macrophage colony-stimulating factor; MM-LDL, minimally modified low-density lipoprotein; oxLDL,
oxidized LDL; SRA, scavenger receptor-1.
1248 CHOLESTEROL/Role of Cholesterol in Heart Disease
0013 Studies in molecular genetics are proving helpful in
establishing the relationships between lipoproteins,
lipids, apolipoproteins, membrane proteins, and nu-
merous other factors involved in cholesterol metabol-
ism and atherogenesis. Also, the use of transgenic
technology has provided animal models for studying
atherogenesis. There now exist transgenic animals
(mice or rabbits) for overexpression of most of the
apolipoproteins, as well as LCAT, cholesterol ester
transfer protein, and scavenger receptor B-1 protein.
In addition, ‘knockout’ animal models, in which cer-
tain apolipoprotein genes are missing, are being stud-
ied to help determine the functions of the proteins.
Intervention Trials and Current Status
0014 While epidemiologic studies long ago suggested the
relationship between high blood cholesterol levels
and increased incidence of atherosclerosis and CHD,
much of the progress in understanding the etiology,
pathology, and relationships has been made in
the last two to three decades. The positive
correlation between blood cholesterol levels and risk
of CHD-related deaths was established in the 1970–
80s by the Framingham Heart Study and the Lipid
Research Clinics Cornary Primary Prevention Trial.
In the mid- to-late 1980s, health agencies in many
countries published ranges of ‘desirable’ vs. ‘undesir-
able’ cholesterol levels, along with recommendations
on modifying high levels of total and LDL-cholesterol
levels (Table 8). But whether deliberately lowering
cholesterol levels could decrease the risk of CHD-
related events remained questionable until recent
years. Several intervention studies have now provided
good evidence that reducing plasma cholesterol levels
in patients with established CHD with drugs and/or
diet does in fact reduce the incidence of both fatal and
nonfatal heart attacks. The efficacy of early studies
with some of the statin drugs appeared questionable
because, although the incidence of CHD deaths de-
clined, overall mortality did not. Newer studies, how-
ever, have shown no increase in noncardiovascular
disease deaths. Table 9 summarizes some of these
studies.
0015Current thinking is that there should be aggressive
cholesterol management in most patients with CHD,
irrespective of the etiology of the condition. Very-
low-fat diets diets help to reduce total plasma choles-
terol levels in some hypercholesterolemic individuals,
but recent studies have shown that HDL cholesterol is
lowered by such diets, particularly in women. This is
of concern and deserves further work. Cholesterol
levels increase in older people, but the correlation of
high [blood cholesterol] and CHD appears to be less
prominent in the elderly population. Nevertheless, it
has been reported recently that high total cholesterol
levels do increase CHD risk in the elderly population.
In the USA, current guidelines, supported by the
American Heart Association and the American Col-
lege of Cardiology, and based on experimental evi-
dence and clinical trials, suggest that LDL cholesterol
should be reduced to 100 mg dl
1
. Since LDL chol-
esterol is a calculated value and since the measure-
ment of total and HDL cholesterol may be subject to
some error, the consensus opinion is that two or three
fasting lipoprotein measurements on consecutive days
should be made for an accurate baseline. All patients
with CHD and LDL cholesterol > 100 mg dl
1
should
immediately reduce the daily intake of saturated fats
to 7% of total calories and of cholesterol to
< 200 mg per day. In addition, weight loss is advised
for those who are overweight, and appropriate phys-
ical activity is suggested. If baseline LDL cholesterol
in those with CHD is > 130 mg dl
1
, drug therapy in
addition to the diet is normally advised immediately.
tbl0008 Table 8 Classification of cholesterol levels
Type Desirable Borderline high Highrisk
Total serum cholesterol < 5.2 mM (< 200 mg dl
1
) 5.2–6.2 mM (200–239 mg dl
1
) > 6.2 mM (> 240 mg dl
1
)
Low-density lipoprotein cholesterol < 3.35 mM (< 130 mg dl
1
) 3.35–4.1 mM (130–159 mg dl
1
) > 4.15 mM (> 160 mg dl
1
)
High-density lipoprotein cholesterol > 0.9 mM (> 35 mg dl
1
) < 0.9 mM (< 35 mg dl
1
Based on recommendations of the USA National Cholesterol Education Program.
tbl0007 Table 7 Stages in atherogenesis
Stage Age Characteristics
One Childhood Development of ‘fatty streaks’
in the aorta
Two Early and
Mid-adulthood
Fatty deposition under intima;
migration of smooth muscle
cells from arterial media to
intima; macrophages and
smooth muscle cells become
lipid-laden ‘foam cells’
Three Middle age and
seniors
Formation of frank plaques;
calcification of plaques
CHOLESTEROL/Role of Cholesterol in Heart Disease 1249

0016 The choice of drugs depends largely on the lipopro-
tein patterns (e.g., concomitant high [triglyceride],
low [HDL-C], and baseline [LDL-C]). Drugs most
often used individually or in combination are statins,
bile acid sequestrants, and nicotinic acid. Seques-
trants increase the excretion of bile acids in feces
and reduce blood cholesterol levels by conversion of
more cholesterol to bile acids. Statins inhibit HMG-
CoA reductase and thereby decrease the synthesis of
cholesterol and other products of the isoprenoid path-
way. Nicotinic acid appears to decrease the synthesis
of LDL and increase the synthesis of HDL. In patients
with a [LDL-C] of 100–129 mg dl
1
and low [HDL-
C] (< 35 mg dl
1
), nicotinic acid is probably the first-
choice drug. The efficiency, cost, and side-effects of
these drugs are usually important factors in decisions
about therapy. Each of these groups of drugs may
have undesirable side-effects in some patients, and
compliance is often a problem, but recent advances
in drug therapy have resulted in safer, but effective,
pharmocologic reductions in blood cholesterol levels.
Reports from many of the recent intervention trials
have indicated that in addition to the decreases in risk,
number of CHD deaths, and overall mortality, there
was also a reduction in the need for bypass surgery or
angioplasty. Furthermore, the interventions have
proven effective in reducing high blood cholesterol
levels in both genders, in middle-aged and elderly,
and in those with or without established CHD.
See also: Antioxidants: Natural Antioxidants;
Atherosclerosis; Coronary Heart Disease: Etiology and
Risk Factor; Antioxidant Status; Intervention Studies;
Prevention; Fats: Digestion, Absorption, and Transport;
Hypertension: Physiology; Hypertension and Diet;
Lipoproteins
Further Reading
American Heart Association Scientific Statement; AHA
Dietary Guidelines, Revision (2000) A statement for
healthcare professionals from the Nutrition Committee
of the American Heart Association. Circulation 102:
2284.
American Heart Association Task Force on Risk Reduction
(1997) When to start cholesterol-lowering therapy in
patients with coronary heart disease. Circulation 95:
1683–1685.
Blake GH and Triplett LC (1995) Management of hyper-
cholesterolemia. American Family Physician 51(5):
1157–1166.
Haber E (ed.) (1995) Molecular Cardiovascular Disease.
New York: Scientific American Medicine.
Jacobson TA (2000) ‘The lower the better’ in hypercholes-
terolemia therapy: a reliable clinical guideline? Annals of
Internal Medicine 133: 549–554.
Rye KA, Clay MA and Barter PJ (1999) Remodelling of
high density lipoproteins by plasma factors. Atheroscler-
osis 145: 227–238.
Silver DL, Jiang XC, Arai T, Bruce C and Tall A (2000)
Receptors and lipid transfer proteins in HDL metabol-
ism. Annals of New York Academy of Science 902:
103–112.
Steinberg D (1997) Low density lipoprotein oxidation and
its pathobiological significance. Journal of Biological
Chemistry 272: 20963–20966.
tbl0009 Table 9 Summary of recent intervention trials
Name of study Subjects, characteristics Treatment Outcome
WOSCOPS (1995) 6500 males, [Ch] ¼249–295 mg dl
1
,
no CHD
Low fat diet plus statin drug or placebo 20% #[total Ch]
31% #CHD risk
AFCAPS (1998) Healthy men and women with
average [Ch] * 221 mg dl
1
Low-fat diet plus statin drug or placebo 16% #[total Ch]
40% #MI risk
SSSS (1994) Hypercholesterolemic patients
with diagnosed CHD
Diet plus statin drug or placebo 24% #in MI
CLAS (1991) Middle-aged men with
atherosclerosis (4 years)
Colestipol þniacin þdiet vs. placebo þdiet 16.2% of subjects
had lesion regression
compared to 2.4% of
placebo group
AFCAPS, Airforce Coronary Atherosclerosis Prevention Study; Ch, cholesterol; CHD, coronary heart disease; CLAS, Cholesterol-lowering
Atherosclerosis Study; MI, myocardial infarction; SSSS, 4S, Scandinavian Simvastatin Survival Study; WOSCOPS, West of Scotland Coronary Prevention
Study.
1250 CHOLESTEROL/Role of Cholesterol in Heart Disease

CHOLINE
Contents
Properties and Determination
Physiology
Properties and Determination
S H Zeisel, University of North Carolina, Chapel Hill,
NC, USA
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Background
0001 Choline is a common constituent of foods and is
an essential component of the human diet. This
quaternary amine is important for the structural
integrity and signaling functions of cell membranes;
it is the major source of methyl groups in the diet;
it directly affects cholinergic neurotransmission;
and it is required for lipid transport/metabolism.
Most choline in human tissues is found in phospho-
lipids such as phosphatidylcholine and sphingomye-
lin. In some components, such as human milk, the
choline esters phosphocholine and glycerophospho-
choline are the predominant forms of choline.
Though representing a smaller proportion of the
total choline pool, important metabolites of choline
include platelet-activating factor, acetylcholine,
choline plasmalogens, lysophosphatidylcholine, and
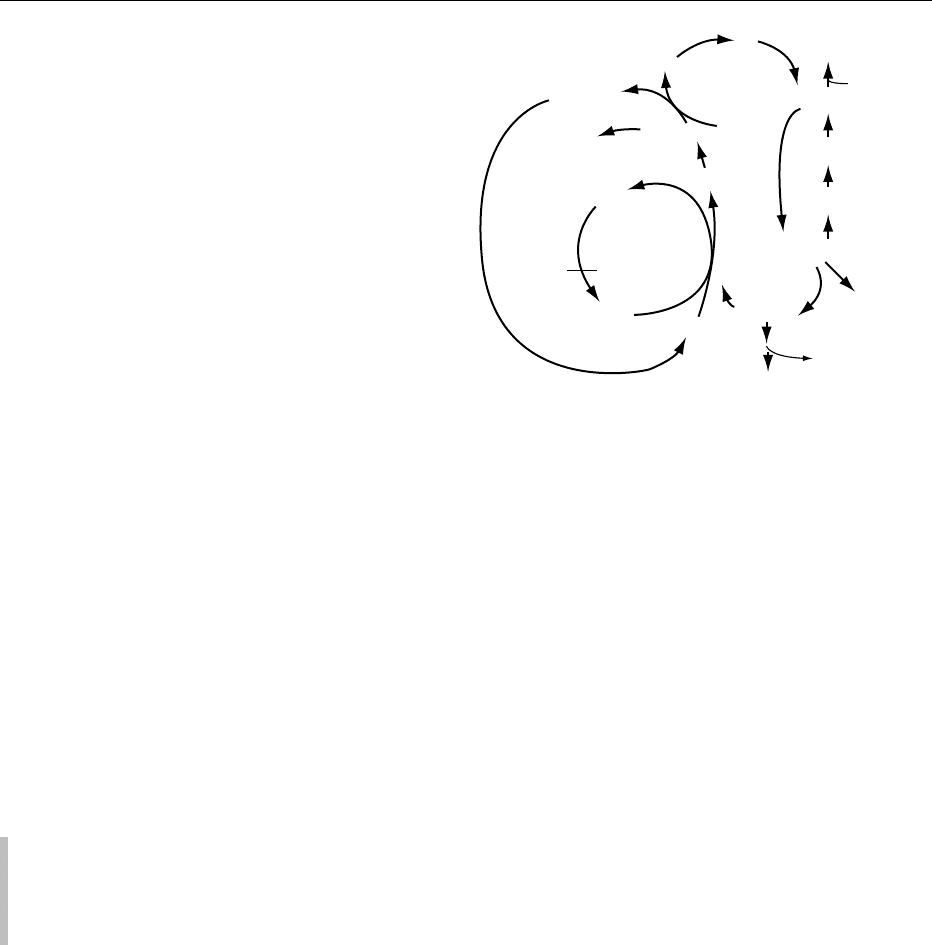
betaine (Figure 1).
Measurement of Choline
0002 Careful collection of biological samples to be ana-
lyzed for choline is exceedingly important. In blood
samples, choline concentrations double every 15 min
in the sample at room temperature, probably because
membrane phosphatidylcholine is hydrolyzed. There-
fore, it is important that blood be kept on ice until
plasma is separated by centrifugation. For similar
reasons, tissues and foods should be stored at
70
C. At this temperature, samples are stable for
at least one year. Acetylcholine is readily hydrolyzed
by basic conditions, or by cholinesterases present in
tissues. If acetylcholine is to be determined, rapid
deactivation of tissue enzymes is essential, and micro-
wave irradiation is commonly used. When acetylchol-
ine is not an important analyte (e.g., for tissues other
than brain), it is sufficient to freeze-clamp the tissue
using tongs cooled in liquid nitrogen.
0003There are multiple forms of choline in biological
tissues, as discussed above. Assay procedures are
available for the most common forms. For all the
assays, it is important to realize that purified choline
salts are very hygroscopic, and this makes assessing
the true weight of internal standards difficult. Unes-
terified choline can be measured in plasma and red
blood cells using a radioisotopic procedure, but this
difficult assay has largely been supplanted by a pro-
cedure that uses high-pressure liquid chromatography
(HPLC), an enzyme reactor, and electrochemical
detection after a simple sample pretreatment. An
alternative method using HPLC and fluorometric de-
tection has also been used. These methods are accur-
ate, reliable, and inexpensive, but are only suitable
for measurement of the choline (unesterified) and
acetylcholine, and they do not permit the use of
internal standards. A gas chromatography/mass
spectrometry method requires much more expensive
equipment, but has the advantage that it has been
adapted to measure other choline esters. The original
method has been enhanced by preseparation of the
various aqueous soluble choline metabolites using
HPLC. This procedure allows analysis of choline,
acetylcholine, phosphocholine, and glycerophospho-
choline in a single sample at minimum detection
levels of 5–10 pmol applied to the GC/MS (e.g., the
amount of choline present in 1 ml of plasma, but
owing to the complex extraction and derivatization
procedures, 25 ml of plasma is the minimum amount
that can be analyzed). It is a demanding procedure
and requires that the separate HPLC peaks be col-
lected, the metabolite in question be converted to
choline, and then this choline be injected on to the
gas chromatogram/mass spectrometer. It does allow
the inclusion of an isotopically labeled internal stand-
ard for each metabolite, thereby insuring a greater
accuracy and reliability when widely divergent matri-
ces are to be analyzed (e.g., tissues and foods).
0004Neither the electrochemical nor the mass spectro-
metric assays detect betaine. Betaine can be isolated
using HPLC and derivatized with 4
0
-bromo-phenacyl
triflate so that it can be detected and quantified using
UV absorbance. Phosphatidylcholine and sphingo-
myelin can be isolated by thin-layer chromatography
CHOLINE/Properties and Determination 1251

and quantified using choline or phosphorus determin-
ation, or isolated by HPLC and quantified with ultra-
violet or fluorometric detection. The advantage of
these last methods is the ability to differentiate phos-
pholipids by fatty acid composition.
0005 A method has recently been devised, using liquid
chromatography/electrospray ionization/mass spec-
trometry (LC/ESI/MS), which greatly reduces the
number of steps in the analysis (eliminates the trans-
fer to the gas chromatograph), but the equipment
costs still remain very high. This method requires no
derivatization and allows the use of internal stand-
ards. After sample extraction, the aqueous and or-
ganic phases are separated and analyzed separately
Compound Chemical structure Biologic function
Choline
Betaine
Acetylcholine
Phosphatidylcholine
Platelet-activating factor
Sphingomyelin
Glycerophosphocholine
Phosphocholine
Methyl group donor;
renal osmolyte
Neurotransmitter
Necessary building
block of biomembranes;
very-low−density
lipoprotein component
necessary for hepatic
very-low−density
lipoprotein secretion
Hormone
Necessary building
block of biomembranes
Intracellular storage
pool of choline
Intracellular storage
pool of choline
HO
NCH
3
CH
3
CH
3
+
+
O
O
NCH
3
CH
3
CH
3
NCH
3
CH
3
CH
3
NCH
3
CH
3
CH
3
NCH
3
CH
3
CH
3
NCH
3
CH
3
CH
3
NCH
3
CH
3
CH
3
NCH
3
CH
3
CH
3
+
+
+
+
+
+
+
O
O
OO
O
OH
OH
OH
OH
OH
OH
P
P
P
P
O
O
O
O
O
O
O
O
OO
O
O
O
O
O
O
O
OH
O
P
OH
O
O
NH
fig0001 Figure 1 Biologically important metabolites of choline.
1252 CHOLINE/Properties and Determination

using straight-phase liquid chromatography. This
method requires 2 pmol of choline to be applied to
the LC/MS (e.g., the amount of choline in 0.2 mlof
plasma).
Dietary Sources of Choline
0006 Many foods eaten by humans contain significant
amounts of choline and esters of choline, but there
is currently no comprehensive database of the choline
content of foods. Some of this choline is added during
food processing (especially when preparing infant
formula). The average dietary intake of choline and
choline esters in the adult human is estimated to be 7–
10 mmol per day. When humans are switched from a
diet of normal foods to a defined diet containing
5 mmol per day, plasma choline and phosphatidyl-
choline concentrations decrease in most subjects.
Thus, the average dietary intake of choline seems to
exceed this level in adults. Human, commercially
available infant formulas and bovine and rat milk
contain approximately 1–2 mmol per liter of choline
and choline esters. Human milk has a significantly
higher phosphocholine concentration, the same or
lower glycerophosphocholine concentration, and
similar phosphatidylcholine and sphingomyelin
concentrations compared with either bovine milk or
bovine-derived infant formulas. Soy-derived infant
formulas have lower glycerophosphocholine and
sphingomyelin concentrations and higher phosphati-
dylcholine concentrations than do either human milk
or bovine milk-derived formulas. By assuming that a
newborn infant drinks 150 ml of milk per kilogram
per day, their choline intake would be approximately
200–250 mmol per kilogram per day, two to three
times that ingested by the adult human.
Intestinal Absorption
0007 The extent to which dietary choline is bioavailable
depends upon the efficiency of its absorption from the
intestine. In adults, some ingested choline is metabol-
ized before it can be absorbed from the gut. Gut
bacteria degrade it to form betaine and to make
methylamines. The free choline surviving these fates
is absorbed all along the small intestine. No other
component of the diet has been identified as compet-
ing with choline for transport by intestinal carriers.
Both pancreatic secretions and intestinal mucosal
cells contain enzymes (phospholipases A
1
,A
2
, and
B) capable of hydrolyzing phosphatidylcholine in
the diet. The free choline that is formed enters the
portal circulation of the liver.
0008 In infants, there are differences in the bioavailabil-
ity of the water soluble, choline-derived compounds
(choline, phosphocholine, and glycerophosphocho-
line) and the lipid-soluble compounds (phosphatidyl-
choline and sphingomyelin) present in milk.
See also: Phospholipids: Properties and Occurrence
Further Reading
Ceder G and Schuberth J (1977) In vivo formation and
post-mortem changes of choline and acetylcholine in
the brain of mice. Brain Research 128: 580–584.
Cheng W-L, Holmes-McNary MQ, Mar M-H, Lien EL and
Zeisel SH (1996) Bioavailability of choline and choline
esters from milk in rat pups. Journal of Nutritional
Biochemistry 7: 457–464.
Damsma G and Flentge F (1988) Liquid chromatography
with electrochemical detection for the determination of
choline and acetylcholine in plasma and red blood cells.
Failure to detect acetylcholine in blood of humans and
mice. Journal of Chromatography 428: 1–8.
FASEB Life Sciences Research Office (1981) Effects of
Consumption of Choline and Lecithin on Neurological
and Cardiovascular Systems, Report # PB-82-133257.
Washington, DC: Bureau of Foods, Food and Drug
Administration, Department of Health, Education, and
Welfare.
Fox JM, Betzing H and Lekim D (1979) Pharmacokinetics
of orally ingested phosphatidylcholine. In: Barbeau A,
Growdon JH and Wurtman RJ (eds) Nutrition and the
Brain, vol. 5, pp. 95–108. New York: Raven Press.
Goldberg AM and McCaman RE (1973) The determination
of picomole amounts of acetylcholine in mammalian
brain. Journal of Neurochemistry 20: 1–8.
Holmes-McNary M, Cheng WL, Mar MH, Fussell S and
Zeisel SH (1996) Choline and choline esters in human
and rat milk and infant formulas. American Journal of
Clinical Nutrition 64: 572–576.
Ikarashi Y, Sasahara T and Maruyama Y (1985) Determin-
ation of choline and acetylcholine levels in rat brain
regions by liquid chromatography with electrochemical
detection. Journal of Chromatography 322: 191–199.
Institute of Medicine, and National Academy of Sciences
USA (1998) Dietary Reference Intakes for Folate, Thia-
min, Riboflavin, Niacin, Vitamin B
12
, Panthothenic
Acid, Biotin, and Choline, vol. 1. Washington DC:
National Academy Press.
Jenden DJ, Roch M and Booth RA (1973) Simultaneous
measurement of endogenous and deuterium-labeled
tracer variants of choline and acetylcholine in subpico-
mole quantities by gas chromatography–mass spectrom-
etry. Analytical Biochemistry 55: 438–448.
Koc H, Mar M-H, Swenberg JA and Zeisel S (2001) Quan-
titation of choline and its metabolites in various tissues
and food items by liquid chromatography/electrospray
ionization/isotope dilution mass spectrometry. Analyt-
ical Chemistry, in press.
Mar MH, Ridky TW, Garner SC and Zeisel SH (1995) A
method for the determination of betaine in tissues using
high performance liquid chromatography. Journal of
Nutritional Biochemistry 6: 392–398.
CHOLINE/Properties and Determination 1253
Ou Z, Ogamo A, Guo L, Konda Y, Harigaya Y and Naka-
gawa Y (1995) Identification and quantitation of choline
glycerophospholipids that contain aldehyde residues by
fluorometric high-performance liquid chromatography.
Analytical Biochemistry 227: 289–294.
Pomfret EA, daCosta K, Schurman LL and Zeisel SH (1989)
Measurement of choline and choline metabolite concen-
trations using high-pressure liquid chromatography and
gas chromatography–mass spectrometry. Analytical Bio-
chemistry 180: 85–90.
Postle A, Al M, Burdge G and Hornstra G (1995) The
composition of individual molecular species of plasma
phosphatidylcholine in human pregnancy. Early Human
Development 43: 47–58.
Riccny J, Tucek S and Vins I (1992) Sensitive method for
HPLC determination of acetylcholine, choline and their
analogues using fluorometric detection. Journal of
Neuroscience Methods 41: 11–17.
Sheard NF and Zeisel SH (1986) An in vitro study of
choline uptake by intestine from neonatal and adult
rats. Pediatric Research 20: 768–772.
Zeisel SH, daCosta K-A, Franklin PD et al. (1991) Choline,
an essential nutrient for humans. FASEB Journal 5:
2093–2098.
Zeisel SH, daCosta KA, Youssef M and Hensey S (1989)
Conversion of dietary choline to trimethylamine and
dimethylamine in rats: dose–response relationship. Jour-
nal of Nutrition 119: 800–804.
Zeisel SH, Story DL, Wurtman RJ and Brunengraber H
(1980) Uptake of free choline by isolated perfused rat
liver. Proceedings of the National Academy of Sciences,
of the USA 77: 4417–4419.
Physiology
S H Zeisel, University of North Carolina, Chapel Hill,
NC, USA
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Metabolism of Choline
0001 There have been several comprehensive reviews of
the metabolism and functions of choline that describe
its role in the synthesis of the phospholipids in cell
membranes, methyl metabolism, cholinergic neuro-
transmission, transmembrane signaling, and lipid-
cholesterol transport and metabolism. Choline can
be acetylated, phosphorylated, or oxidized.
0002 Choline, methionine, and folate metabolism inter-
act at the point that homocysteine is converted to
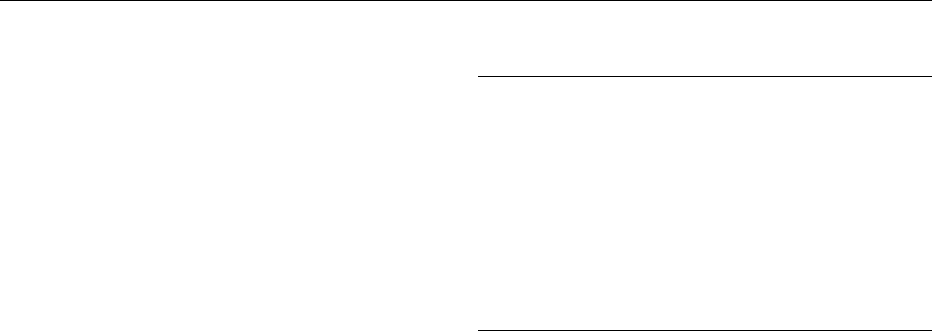
methionine (Figure 1). Betaine:homocysteine methyl-
transferase catalyzes the methylation of homocys-
teine using the choline metabolite betaine as the
methyl donor. Elevated plasma homocysteine is an
independent risk factor for cardiovascular disease
and stroke in humans. Treatment with betaine or
choline also lowers elevated plasma homocysteine in
humans. In an alternative pathway, 5-methyl-tetra-
hydrofolate:homocysteine methyltransferase regener-
ates methionine. In addition, tetrahydrofolate is
needed to scavenge one-carbon groups when betaine
is metabolized. Perturbing metabolism of one of the
methyl donors results in compensatory changes in the
other methyl donors owing to the intermingling of
these metabolic pathways. Rats ingesting a choline-
deficient diet have diminished tissue concentrations
of methionine and S-adenosylmethionine, doubled
plasma homocysteine concentrations, and diminished
hepatic methyl-folate content. These effects are re-
versible by refeeding choline. Rats fed diets deficient
in both methionine and choline for 5 weeks have
hepatic folate concentrations that are half of those
present in controls. Rats treated with the antifolate,
methotrexate, have diminished pools of choline me-
tabolites in liver. Severe folate deficiency, induced in
rats by feeding an amino acid-defined diet containing
no folate and succinylsulfathiazole for 4 weeks have
resulted in hepatic choline and phosphocholine con-
centrations that were 65 and 80% lower, respectively
than in controls.
Dietary Requirements
0003Though there is a pathway (in all tissues, but most
active in the liver) for the de novo biosynthesis of the
choline moiety via the sequential methylation of phos-
phatidylethanolamine using S-adenosylmethionine
AdoHcy
Sphingomyelin
ceramide
AdoMet
Phosphatidylcholine
Phosphorylcholine
CDP-Choline
Choline
Acetylcholine
Betaine
methyl-groups
for methyl-THF
Sarcosine
methyl-THF
Homocysteine
Vit. B12
DNA methylation
other methylations
PtdEtn
Methionine
Tetrahydrofolate
methylenetetra-
hydrofolate
reductase defect
fig0001Figure 1 Choline, folate, and homocysteine metabolism
are closely inter-related. The pathways for the metabolism of
these three nutrients intersect at the formation of methionine
from homocysteine. PtdEtn, phosphatidylethanolamine; AdoHcy,
S-adenosylhomocysteine; AdoMet, S-adenosylmethionine
1254 CHOLINE/Physiology
as the methyl donor (Figure 1), only some of the
demand for choline can be met by using methyl groups
derived from one carbon metabolism. Animals and
humans fed a choline deficient diet deplete choline
stores and develop liver dysfunction. Healthy male
humans with normal folate and vitamin B
12
status
fed a choline-deficient diet have diminished plasma
choline and phosphatidylcholine concentrations, and
develop liver damage (elevated plasma alanine ami-
notransferase). Liver cell death occurs in choline defi-
ciency, because hepatocytes initiate programmed cell
death (apoptosis) when deprived of choline. Because
methyl supplementation with betaine, methionine,
folate, or vitamin B
12
does not prevent apoptotic
death induced by choline deficiency in hepatocytes,
it must be that depletion of intracellular choline moi-
eties rather than depletion of methyl groups is the
critical parameter involved in induction of apoptosis.
Some humans (male and female) fed with total paren-
teral nutrition solutions devoid of choline, but ad-
equate for methionine and folate, develop fatty liver
and liver damage that resolves when a source of diet-
ary choline is provided. Fatty liver occurs because
choline is required to make the phosphatidylcholine
portion of the very-low-density lipoprotein particle.
Animals fed a choline-deficient diet may also develop
growth retardation, renal dysfunction and hemor-
rhage, or bone abnormalities. A metabolite of choline,
betaine, is used in the kidney glomerulus as an osmo-
lyte, and for this reason, choline-deficient animals
have trouble excreting concentrated urine.
0004 Human studies of choline requirements in women,
children, or infants have not been conducted. Thus,
we do not know whether choline is needed in the diet
of these groups. Female rats are less sensitive to
choline deficiency than are male rats perhaps because
estrogen enhances females’ capacity to form the
choline moiety de novo from S-adenosylmethionine.
Pregnancy may be a time when dietary supplies of
choline are especially limiting. Though female rats are
resistant to choline deficiency, pregnant rats are as
vulnerable to deficiency, as are males. During preg-
nancy, large amounts of choline are delivered to the
fetus across the placenta, and this depletes maternal
stores of the various forms of this nutrient. At birth,
humans and other mammals have plasma choline
concentrations that are much higher than those in
adults. Also, the need for choline is increased during
lactation, because so much must be secreted into
milk; lactating rats are more sensitive to choline
deficiency than are nonlactating rats.
0005 The Institute of Medicine (IOM) recently made
recommendations for choline intake in the diet.
There were insufficient data with which to derive an
estimated average requirement for choline, and so
only an adequate intake can be estimated. The IOM
report cautioned, ‘this amount will be influenced by
the availability of methionine and methyl-folate in the
diet. It may be influenced by gender, and it may
be influenced by pregnancy, lactation, and stage of
development. Although AIs are set for choline, it may
be that the choline requirement can be met by en-
dogenous synthesis at some of these stages.’ The
IOM recommendations are shown in Table 1.
0006The recent report by Jacob that folate deficiency in
humans exacerbates choline deficiency highlights
why studies of choline–folate–homocysteine inter-
relationships in humans are important for the future
refinement of these recommendations.
0007Plasma choline concentration varies in response to
diet and can rise as much as twofold after a two-egg
meal. Fasting plasma choline concentrations vary
from 7 to 20 mM, with most subjects having concen-
trations of 10 mM. Individuals that have starved for
up to 7 days have diminished plasma choline, but
levels never drop below 50% of normal. Plasma
phosphatidylcholine concentration also decreases in
choline deficiency, but these values are also influ-
enced by factors that change plasma lipoprotein
levels. Fasting plasma phosphatidylcholine concen-
trations are approximately 1–1.5 mM.
Choline and Brain Development
0008Choline availability during embryogenesis and peri-
natal development may be especially important. There
is a sensitive period in rodent brain development
during which treatment with choline (about three
times the dietary intake) produces long-lasting en-
hancement of spatial memory that is lifelong. This
period occurs during embryonic days 12–17 in the
rat (rats give birth on day 21; mice give birth one
day earlier and probably have a slightly different
period of susceptibility). Choline supplementation
tbl0001Table 1 Institute of Medicine recommendations for choline
intake in the diet
AI for infants 0–6 months 125 mg per day,
18 mg per kilogram
6–12 months 150 mg per day
AI for children 1–3 years 200 mg per day
4–8 years 250 mg per day
9–13 years 375 mg per day
AI for males 14–18 years 550 mg per day
19 and older 550 mg per day
AI for females 14–18 years 400 mg per day
19 years and older 425 mg per day
AI for pregnancy All ages 450 mg per day
AI for lactation All ages 550 mg per day
AI = adequate intake level.
CHOLINE/Physiology 1255
