Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.

1a,25(OH)
2
D
3
and 24R,25(OH)
2
D
3
; (4) systemic
transport of the dihydroxylated metabolites
24R,25(OH)
2
D
3
and 1a,25(OH)
2
D
3
to distal target
organs; (5) binding of the dihydroxylated metabol-
ites, particularly 1,25(OH)
2
D
3
, to either a nuclear
receptor or membrane receptor at the target organs
followed by the subsequent generation of appropriate
biological responses (see also Figure 3). An additional
key component in the operation of the vitamin D
endocrine system is the plasma vitamin D binding
protein, which carries vitamin D
3
and all its metabol-
ites to their various target organs.
0011 The three enzymes responsible for the conversion of
vitamin D
3
into its two key daughter metabolites
include the hepatic vitamin D
3
-25-hydroxylase and
the two kidney enzymes, the 25(OH)D
3
-1a-hydroxyl-
ase and the 25(OH)D
3
-24R-hydroxylase. All three
enzymes have been demonstrated to be cytochrome
P450 mixed-function oxidases. Both renal enzymes
are localized in mitochondria of the proximal
tubules of the kidney. The 25(OH)D
3
-1a-hydroxylase
has been cloned and the specific site of mutations
(which result in the appearance of vitamin D-resistant
rickets, type I) identified. Also, the 25(OH)D
3
-24R-
hydrodroxylase and vitamin D
3
-25-hydroxylase have
been cloned.
0012The most important point of regulation of the
vitamin D endocrine system occurs through the
stringent control of the activity of the renal
25(OH)D
3
-1a-hydroxylase. In this way, the produc-
tion of the hormone 1a,25(OH)
2
D
3
can be modulated
according to the calcium and other endocrine needs of
the organism. The chief regulatory factors are
1a,25(OH)
2
D
3
itself, parathyroid hormone (PTH),
and the serum concentrations of calcium and phos-
phate. Probably the most important determinant of
the 1a-hydroxylase is the vitamin D status of the
animal. When the circulating concentration of
1a,25(OH)
2
D
3
is low, production of 1a,25(OH)
2
D
3
by the kidney is high; when the circulating concen-
tration of 1a,25(OH)
2
D
3
is high, the output of
1a,25(OH)
2
D
3
by the kidney is sharply reduced.
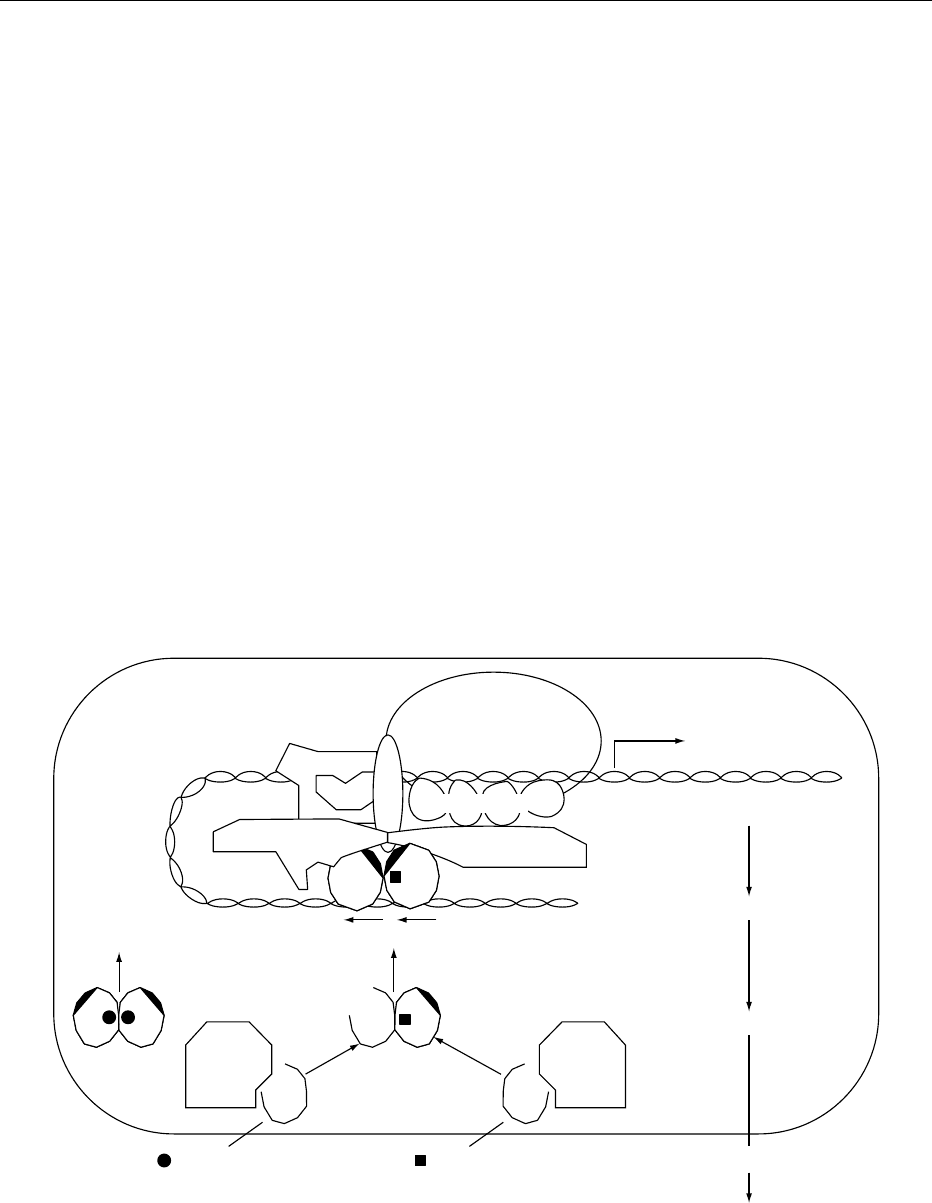
0013The process of nuclear receptor-mediated regula-
tion of gene transcription is exquisitely dependent
upon the complimentary relationship between the
unoccupied receptor and its cognate ligand. Thus,
the unoccupied receptor is largely incompetent
to engage in a productive fashion with the
Cell
nucleus
V
D
R
R
X
R
R
X
R
R
X
R
R
X
R
R
X
R
T
F
I
I
B
TATA
TBP
RXR Coactivator
DNA
MRNAs
hnRNAs
Transcription
initiation
Vitamin D
induced genes
Translation
Proteins
VDRE
RXRE
Corepressor
9-cis Retinoic acid 1,25(OH)
2
D
3
Corepressor
D R I P S
General
transcription
apparatus
V
D
R
VDR Coactivator
V
D
R
fig0003 Figure 3 Model of 1a,25(OH)
2
D
3
and VDR
nuc
activation of transcription. The VDR, after binding its cognate ligand 1a,25(OH)
2
D
3
, forms
a heterodimer with the retinoid X receptor. This heterodimer complex then interacts with the appropriate vitamin D response element
on the promoter of genes (in specific target cells) which are destined to be up- or downregulated. The heterodimer – DNA complex then
recruits the necessary coactivator proteins, such as TATA, TBP, TFIIB and other proteins to generate a competent transcriptional
complex capable of modulating mRNA production.
1216 CHOLECALCIFEROL/Physiology

transcriptional machinery to effect meaningful regu-
lation of gene transcription. It is only after the ligand–
receptor complex has formed (which results in con-
formational changes in the receptor protein) that a
functional receptor protein is generated. Thus acqui-
sition of a detailed understanding of the complemen-
tarity of the ligand shape with that of the interior
surface of the nuclear VDR receptor ligand binding
domain is believed to be the key not only to under-
standing the structural basis of receptor action and its
formation of heterodimers and interactions with
coactivators (see Figure 3), but also to designing
new drug forms of the various hormones, including
1a,25(OH)
2
D
3
.
0014 The receptors for all steroid hormones (estrogen,
progesterone, testosterone, cortisol, and aldosterone)
and the nuclear receptors for 1a,25(OH)
2
D
3
, retinoic
acid, and thyroid hormone are all members of the
same super gene family (See Hormones: Steroid Hor-
mones, Figure 5); accordingly, there is a high level of
conservation in their amino acid sequences, particu-
larly in their DNA and ligand binding domains. The
X-ray crystallographic structures of the ligand-bind-
ing domains (LBD) of the thyroid hormone receptor,
the retinoic acid receptor, the estrogen receptor, the
ligand binding and coactivator assembly of the per-
oxisome proliferator-activated receptor-g, and the
progesterone receptor have all been determined with
their respective ligands bound. Also, the crystal struc-
ture of the nuclear receptor for vitamin D bound to its
natural ligand 1a,25(OH)
2
D
3
has been determined at
a 1.8-A
˚
resolution. The VDR
nuc
LBD structure, like
the other nuclear receptors, consists of 12 a-helices
that are arranged to create a three-layer sandwich
that completely encompasses the ligand
1a,25(OH)
2
D
3
in a hydrophobic core. The secondary
and tertiary structural features of this group of
proteins have been found to be remarkably similar.
Nutritional Aspects
Recommended Dietary Allowance
0015 The World Health Organization (WHO) has respon-
sibility for defining the International Unit (IU) of
vitamin D
3
. Their most recent definition, provided
in 1950, stated that ‘the International Unit of vitamin
D recommended for adoption is the vitamin D activ-
ity of 0.025 mg of the international standard prepar-
ation of crystalline vitamin D
3
.’ Thus, 1.0 IU of
vitamin D
3
is 0.025 mg, which is equivalent to
65.0 pmol. With the discovery of the metabolism of
vitamin D
3
to other active seco-steroids, particularly
1a,25(OH)
2
D
3
, it was recommended that 1.0 Unit
of 1a,25(OH)
2
D
3
be set equivalent in molar terms
to that of the parent vitamin D
3
. Thus, 1.0 Unit of
1a,25(OH)
2
D
3
has been operationally defined to be
equivalent to 65 pmol.
0016The vitamin D requirement for healthy adults has
never been precisely defined. Since vitamin D
3
is
produced in the skin after exposure to sunlight, the
human does not have a requirement for vitamin D
when sufficient sunlight is available. Man’s tendency
to wear clothes, to live in cities where tall buildings
block adequate sunlight from reaching the ground, to
live indoors, to use synthetic sunscreens that block
ultraviolet rays, and to live in geographical regions of
the world that do not receive adequate sunlight all
contribute to the inability of the skin to biosynthesize
sufficient amounts of vitamin D
3
. Thus, vitamin D
does become an important nutritional factor in the
absence of sunlight. It is known that a substantial
proportion of the US population is exposed to sub-
optimal levels of sunlight. This is particularly true
during the winter months. Under these conditions,
vitamin D becomes a true vitamin, and therefore
must be supplied in the diet on a regular basis.
0017Since vitamin D
3
can be endogenously produced by
the body and since it is retained for long periods of
time by vertebrate tissue, it is difficult to determine
with precision the minimum daily requirements for
this seco-steroid. The requirement for vitamin D is
also known to be dependent on age, sex, degree of
exposure to the sun, season, and the amount of
pigmentation in the skin.
0018In the USA, adequate amounts of vitamin D can be
readily obtained from the diet and/or from casual
exposure to sunlight. The UV exposure can be as little
as three 20-min periods of exposure of the face and
hands to ambient sunlight per week. However, in
some parts of the world where food is not routinely
fortified and sunlight is often limited during some
periods of the year, obtaining adequate amounts of
vitamin D becomes more of a problem. As a result,
the incidence of rickets in these countries is higher
than in the United States.
0019Many countries as well as the WHO have prepared
recommendations on the daily intake of essential nu-
trients, including vitamin D. The current ‘adequate
intake’ allowance of vitamin D recommended in 1998
by the Food and Nutrition Board of the US Institute
of Medicine is 200 IU per day (5 mg per day) for
infants, children, and adult males and females up to
age 51. For males and females aged 51–70 or >70, the
adequate indicated level is set at 400 IU per day (10 mg
per day) or 600 IU (15 mg per day), respectively. The
adequate allowance for pregnancy and lactation is set
at 200 IU per day (5 mg per day). The recommenda-
tions of the UK and Canada for adults are about 50%
lower than of the USA. Australia, which is generally
CHOLECALCIFEROL/Physiology 1217

sunny, does not recommend daily supplements of
vitamin D.
Food Sources
0020 Animal products constitute the bulk source of
vitamin D that occurs naturally in unfortified foods.
Marine fish, such as herring, salmon, and sardines,
and fish liver oils are good sources of vitamin D
3
.
Small quantities of vitamin D
3
are also derived from
eggs, veal, beef, butter, and vegetable oils, while
plants, fruits, and nuts are extremely poor sources
of vitamin D. In the USA, artificial vitamin D
3
fortification of foods such as milk (both fresh and
evaporated), margarine and butter, cereals, and choc-
olate mixes help in meeting the recommended dietary
allowance. Vitamin D
2
was used in the period of
1940–60 as a food supplement for vitamin D activity.
Functions of Vitamin D
0021 Classical target tissues In the classical target tissues
such as intestine, bone, and kidney, 1,25(OH)
2
D
3
,
largely in conjunction with PTH, serves to regulate
mineral homeostasis such that serum calcium and
phosphorus levels are maintained within a physio-
logical range that can support normal mineralization
of bone.
0022 In the intestine, 1,25(OH)
2
D
3
primarily stimulates
the active transport of Ca
2þ
and inorganic phosphate,
P
i
, via mechanisms that involve a calcium-binding
protein, termed calbindin D. Available evidence sug-
gests that calbindin D may be involved in protecting
the cell (as a buffer) against large fluxes of Ca
2þ
which result from active transport induced by
1,25(OH)
2
D
3
.
0023 In addition to the genomic effects of 1,25(OH)
2
D
3
,
recent studies have shown that 1,25(OH)
2
D
3
can
stimulate the rapid (2–4 min) transport of calcium,
termed transcaltachia, via a receptor-mediated pro-
cess, albeit one independent of gene activation.
0024 In the kidney, 1,25(OH)
2
D
3
functions in concert
with PTH to enhance renal Ca
2þ
reabsorption,
besides regulating its own biosynthesis by feedback
inhibition of renal 1-hydroxylase.
0025 1a,25(OH)
2
D
3
plays an important role in bone
growth, development, and differentiation, and sup-
ports bone mineralization indirectly by supplying the
minerals calcium and phosphorus via their enhanced
intestinal absorption. In addition, 24,25(OH)
2
D
3
has
been shown to promote the bone-mineralization pro-
cess and to participate in the process of fracture-
healing.
0026 Nonclassical target tissues The 1,25(OH)
2
D
3
hor-
mone promotes differentiation of cells in the
hemopoietic system. Such effects of 1,25(OH)
2
D
3
offer a therapeutic prospect for leukemia.
0027In the immune system, 1,25(OH)
2
D
3
acts as an
immunomodulator, regulating the functional per-
formance of cells involved in the immune response.
0028Insulin production by the endocrine pancreas is
influenced by vitamin D status in that the blunted
secretion of insulin and impaired glucose tolerance
seen in vitamin D-deficient conditions are corrected
by treatment with vitamin D
3
and/or 1,25(OH)
2
D
3
.
0029Myopathy and abnormalities in muscle contraction
seen in patients afflicted with metabolic bone disease
are amenable to correction with vitamin D therapy.
00301a,25(OH)
2
D
3
induces differentiation of keratino-
cytes in skin and exerts antiproliferative effects on
these epithelial cells. Such an effect of 1,25(OH)
2
D
3
in the skin has prompted the use of 1,25(OH)
2
D
3
analogs for treatment of psoriasis, which is a hyper-
proliferative disease of the skin.
Disease States in Humans Related to
Vitamin D
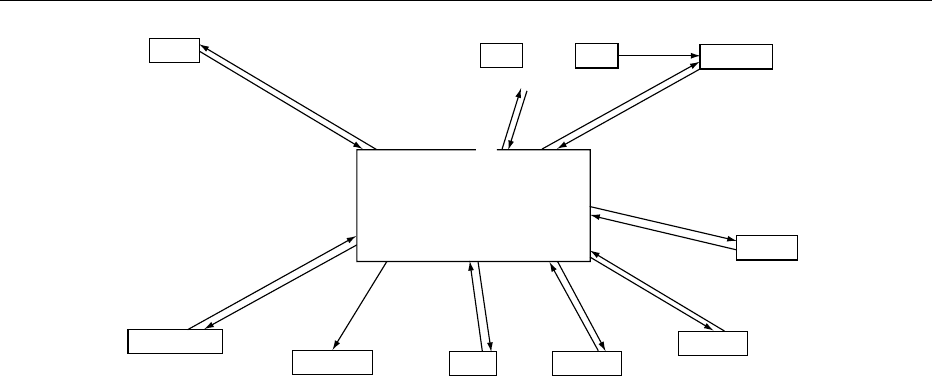
0031In humans, diseases related to vitamin D can arise
because of (1) altered availability of vitamin D; (2)
altered hepatic conversion of vitamin D
3
; (3)
impaired renal metabolism of 25(OH)D
3
, or (4) vari-
ation in end organ responsiveness to 1,25(OH)
2
D
3
(see Figure 4).
Renal Disorders
0032Chronic renal failure, also known as renal osteody-
strophy, is characterized by impaired renal produc-
tion of 1,25(OH)
2
D
3
and intestinal malabsorption
of calcium, which can often lead to derangements
in skeletal metabolism and hyperparathyroidism.
These symptoms are alleviated by 1,25(OH)
2
D
3
ad-
ministration. In 1977, in the USA, the Food and Drug
Administration approved the prescription use of
1,25(OH)
2
D
3
(calcitriol or Rocaltrol
1
) for renal
osteodystrophy.
Vitamin D-resistant Rickets
0033Also known as familial X-linked hypophosphatemic
rickets, vitamin D-resistant rickets is characterized
by a primary phosphate leak in the kidney, skeletal
deformities, and hypophosphatemia. A combination
of oral phosphate and 1,25(OH)
2
D
3
is effective in
treating these patients.
Vitamin D-dependent Rickets
0034Vitamin D-dependent rickets is also referred to as
hereditary hypocalcemic vitamin D-resistant rickets,
and is classified into Type I and Type II disease states.
1218 CHOLECALCIFEROL/Physiology
0035 Type I rickets is believed to arise as a result of an
inborn error in the renal 1-hydroxylase enzyme. The
clinical features include hypocalcemia, hypophos-
phatemia, and several rachitic lesions. These symp-
toms can be treated with pharmacological doses of
vitamin D
3
or low doses of 1,25(OH)
2
D
3
.
0036 Point mutations in the vitamin D receptor gene
have been shown to be responsible for the defective
receptors seen in these children with Type II rickets.
The clinical manifestations are defective bone miner-
alization, decreased intestinal calcium absorption,
hypocalcemia, and increased serum 1,25(OH)
2
D
3
levels. To date, a point mutation in a steroid receptor
gene resulting in the loss of functional activity has
been demonstrated only for the vitamin D receptor
and is therefore unique in this respect.
Diseases of Parathyroid
0037 Hypoparathyroidism Hypoparathoidism exhibits
hypocalcemia as a major clinical consequence and is
corrected with large doses of vitamin D or physio-
logical doses of 1,25(OH)
2
D
3
.
0038 Hyperparathyroidism Increased 1,25(OH)
2
D
3
levels, enhanced intestinal absorption of calcium
(contributing to hypercalciuria), and nephrolithiasis
are typical of this disorder.
0039 Pseudohypoparathyroidism Pseudohypoparathyroid-
ism results from a state of resistance to PTH. The
biochemical abnormalities are hypocalcemia, hyper-
phosphatemia, elevated serum PTH, and decreased
serum 1,25(OH)
2
D
3
levels.
0040Bone disorders Clinically, a deficiency in vitamin D
manifests as rickets in children and osteomalacia in
adults. Hypocalcemia, hypophosphatemia, increased
serum alkaline phosphatase, and decreased serum
25(OH)D
3
levels are some of the salient biochemical
abnormalities, all of which can be ameliorated by
vitamin D administration.
Conclusion
0041Current evidence supports the concept that the clas-
sical biological actions of the nutritionally important
fat-soluble vitamin D in mediating calcium homeo-
stasis are supported by a complex vitamin D endo-
crine system which coordinates the metabolism
of vitamin D
3
into 1a,25(OH)
2
D
3
and
24R,25(OH)
2
D
3
. It is now clear that the vitamin D
endocrine system embraces many more target tissues
than simply the intestine, bone, and kidney. Notable
additions to this list include the pancreas, pituitary,
breast tissue, placenta, hematopoietic cells, skin, and
cancer cells of various origins. Key advances in under-
standing the mode of action of the 1a,25(OH)
2
D
3
have been made by a thorough study of nuclear recep-
tors as well as emerging studies describing a mem-
brane receptor for this steroid hormone. Integral to
Hyperparathyroidism
Diabetes Medullary
carcinoma
Chronic renal
disease
Hypophosphatemic
VDRR
Vitamin D-
dependent rickets
Cirrhosis
Drug induced
metabolism
Obstructive
jaundice
Hypoparathyroidism
Pseudohypo-
parathyroidism
Secondary hyper-
parathyroidism
Tuberculosis
Sarcoidosis
PTH
CT
Ca
2+
,P
i
D
3
, Ca
2+
,P
i
D
3
D
3
Ca
2+
Ca
2+
,1a,25(OH)
2
D
3
1a,25(OH)
2
D
3
and
24R,25(OH)
2
D
3
1a,25(OH)
2
D
3
25(OH)D
3
1a,25(OH)
2
D
3
1a,25(OH)
2
D
3
1a,25(OH)
2
D
3
Ca
2+
25(OH)D
3
25(OH)D
3
,
PTH Ca
2+
P
i
D
3
25(OH)D
3
Ca
2+
P
i
1a,25(OH)
2
D
3
24R,25(OH)
2
D
3
Psoriasis
Glucocorticoid
antagonism
Idiopathic
hypercalcemia
Malabsorption
syndrome
Steatorrhea
Tropical sprue
Anticonvulsant
treatment
Fibrogenesis
imperfecta ossium
Osteitis fibrosa cystrica
Osteomalacia
Osteoporosis
Osteopenia
Osteosclerosis
Renal osteodystrophy
Rickets
Bone
Parathyroid
Pancreas
Liver Thyroid
Kidney
Lung
Intestine
Diet
Skin
Blood
D
3
25(OH)D
3
PTH CT
P
i
Ca
2+
1a,25(OH)
2
D
3
/24R,25(OH)
2
D
3
fig0004 Figure 4 Human disease states related to the vitamin D endocrine system. Under the boxed headings (e.g., parathyroid, liver, bone,
etc.) are listed disease states occurring in man which have been shown, or are believed, to have some functional linkage between
some aspect of the vitamin D endocrine system and that particular organ. The information associated with the arrows indicates the
direction of flow of calcium, phosphate or the calcium-regulating hormones (CT (calcitonin), PTH (parathyroid hormone), vitamin D
3
,
1a, 25(OH)
2
D
3
, 24R, 25(OH)
2
D
3
). Ca
2þ
, calcium; P
i
, inorganic phosphate; VDRR, vitamin D-resistant rickets.
CHOLECALCIFEROL/Physiology 1219

these observations are efforts to define the signal
transduction systems, which are subservient to the
nuclear and membrane receptors for 1a,25(OH)
2
D
3
,
and to obtain a thorough study of the tissue distribu-
tion and subcellular localization of the gene products
induced by this steroid hormone. There are clinical
applications for 1a,25(OH)
2
D
3
or related analogs for
treatment of the bone diseases of renal osteodystro-
phy and osteoporosis, psoriasis, and hypopara-
thyroidism; other clinical targets for 1a,25(OH)
2
D
3
currently under investigation include its use in
leukemia, breast, prostate, and colon cancer as well
as use as an immunosuppressive agent.
See also: Cholecalciferol: Properties and Determination;
Dietary Reference Values; Hormones: Steroid
Hormones; Renal Function and Disorders: Nutritional
Management of Renal Disorders
Further Reading
Bikle DD (1995) Vitamin D: New actions, new analogs,
new therapeutic potential: update 1995. Endocrine
Reviews 4: 77–83.
Bouillon R, Okamura WH and Norman AW (1995) Struc-
ture–function relationships in the vitamin D endocrine
system. Endocrine Reviews 16: 200–257.
Haussler MR, Whitfield GK, Haussler CA et al. (1998) The
nuclear vitamin D receptor: Biological and molecular
regulatory properties revealed. Journal of Bone and
Mineral Research 13: 325–349.
Norman AW (2001) Vitamin D. In: Bowman B and Russell
R (eds) Present Knowledge in Nutrition (PKN), 8th edn.,
pp. 134–143. Washington, DC: International Life Sci-
ences Institute.
Norman AW and Litwack G (1997) Calcium-regulating
hormones: Vitamin D, parathyroid hormone, and calci-
tonin. In: Hormones, 2nd edn., pp. 251–280. San Diego,
CA: Academic Press.
Norman AW, Henry HL, Bishop JE et al. (2001) Different
shapes of the steroid hormone 1a,25(OH)
2
-vitamin D
3
act as agonists for two different receptors in the vitamin
D endocrine system to mediate genomic and rapid
responses. Steroids 66(3–5): 147–158.
Reichel H, Koeffler HP and Norman AW (1989) The role of
the vitamin D endocrine system in health and disease.
New England Journal of Medicine 320: 980–991.
Rochel N, Wurtz JM, Mitschler A, Klaholz B and Moras D
(2000) The crystal structure of the nuclear receptor for
vitamin D bound to its natural ligand. Molecular Cell
5(1): 173–179.
Weatherman RV, Fletterick RJ and Scanlon TS (1999)
Nuclear receptor ligands and ligand-binding domains.
Annual Review of Biochemistry 68: 559–582.
CHOLESTEROL
Contents
Properties and Determination
Absorption, Function, and Metabolism
Factors Determining Blood Cholesterol Levels
Role of Cholesterol in Heart Disease
Properties and Determination
A J Sheppard and R G O’Dell, United States Food and
Drug Administration, Washington DC, USA
J A T Pennington, National Institute of Health,
Bethesda, MD, USA
This article is reproduced from Encyclopaedia of Food Science,
Food Technology and Nutrition, Copyright 1993, Academic Press.
Background
0001 Cholesterol is a well-known and commonly deter-
mined lipid component and an important intermediate
in the synthesis of steroid hormones. It is a sterol
(C
27
H
45
OH) that occurs notably in animal fats and
oils, bile, gallstones, nerve tissues, blood, brain,
plasma, and egg yolk. Cholesterol is the most
common animal sterol and is also found in trace
amounts in vegetable fats and oils, seaweeds, and
green leaves. Cholesterol was first found in gall-
stones and derives its name from the Greek khole
´
(bile) and stereos (solid). The determination of chol-
esterol in serum and foods is of significance because
of the implication of cholesterol in the etiology of
arteriosclerosis and coronary heart disease. (See Ath-
erosclerosis; Coronary Heart Disease: Intervention
Studies.)
1220 CHOLESTEROL/Properties and Determination
Structure
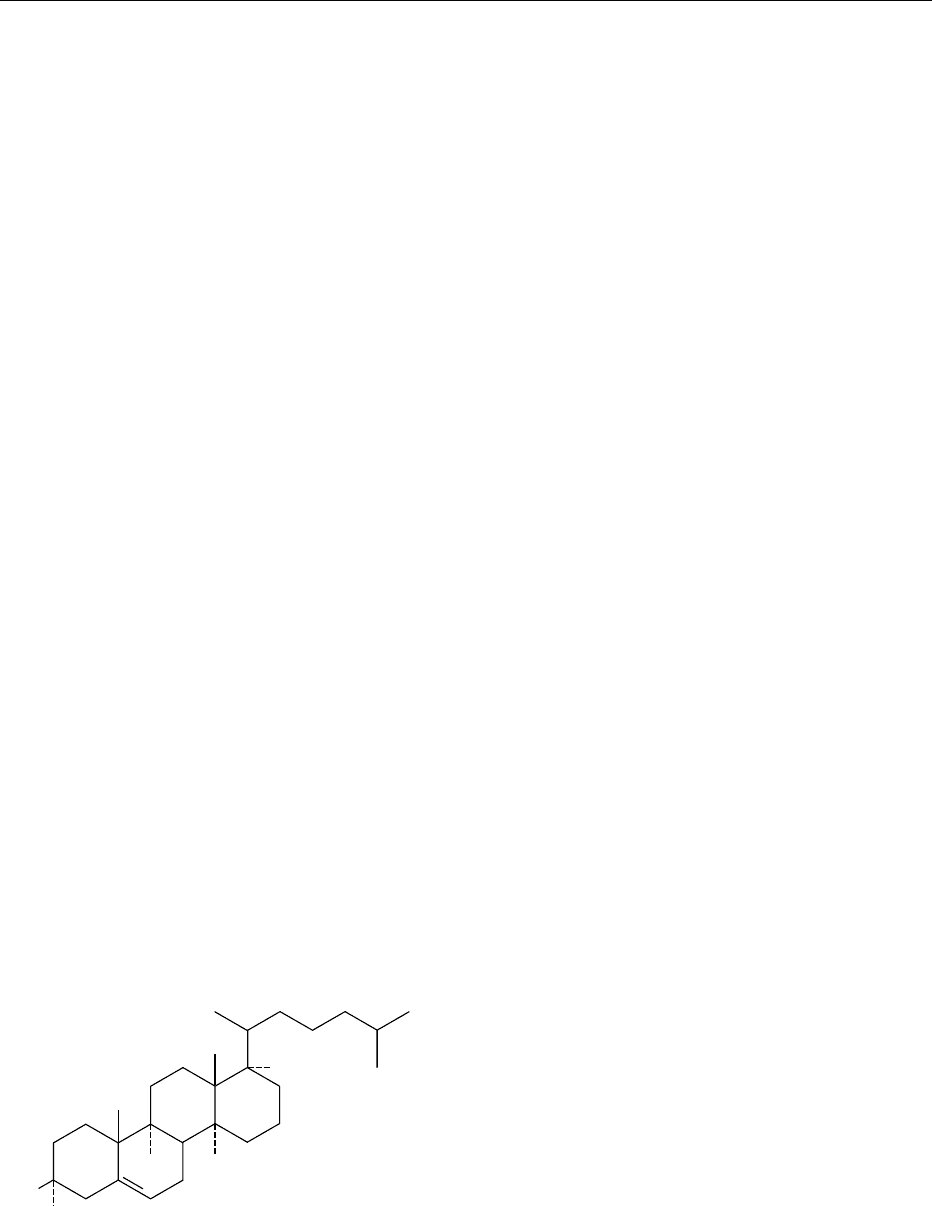
0002 A systematic study of the chemistry of cholesterol
began at the end of the nineteenth century. The classic
work of Wieland, Vindaus, Diels, Rosenheim, and
King led to the formulation of the structure of choles-
terol in 1932. The fundamental carbon skeleton of
the cholesterol molecule is the cyclopentanoperhydro-
phenanthrene ring. The structure of cholesterol is
shown in Figure 1. The hydroxyl group on C3 is
connected by a ‘solid’ bond (b orientation) and the
hydrogen by a ‘dashed’ bond (a orientation), depicting
the naturally occurring b-cholesterol. Atoms con-
nected by solid bonds (b orientation) are regarded as
projecting in front of the plane of the steroid ring, and
those connected by dashed bonds (a orientation) as
lying behind the plane. The molecular weight of chol-
esterol is 384.64. Cholesterol consists of 83.87%
carbon, 11.99% hydrogen, and 4.145% oxygen by
weight. The formal chemical name of the molecule is
cholest-5-en-3b-ol. Because the cholesterol nucleus
contains eight centers of asymmetry, approximately
240 isomers of the molecule are possible. However,
only two carbon centers (C3 and C5) appear to be
involved in naturally occurring cholesterol isomers. In
some of the earlier scientific literature, cholesterol is
referred to as ‘cholesterin.’
Chemical Characteristics
0003 Cholesterol is a glistening, white, soapy, crystalline
substance that is practically insoluble in water (about
0.2 mg per 100 ml). It is slightly soluble in alcohol
(1.29% w/w at 20
C) and more soluble in hot alco-
hol (100 g of saturated 96% alcoholic solution con-
tains 28 g of cholesterol at 80
C). One gram of the
compound dissolves in 2.8 ml of ether, 4.5 ml of
chloroform, or 1.5 ml of pyridine. Cholesterol is
also soluble in benzene, hexane, petroleum ether,
oils, fats, and aqueous solutions of bile salts. It crys-
tallizes easily from absolute alcohol, acetic acid,
ether, and similar solvents as colorless rhombic plates
with one or more characteristic notches in the
corners. Because cholesterol has an unsaturated
bond, it will accept up to two halogen atoms. Choles-
terol is not saponifiable.
0004Cholesterol gives a number of color reactions that
are useful to test for the molecule both qualitatively
and quantitatively. The Salkowski reaction produces
a series of colors when a chloroform solution of chol-
esterol is stratified over concentrated sulfuric acid.
The acid assumes a yellowish color with a green
fluorescence, whereas the chloroform layer first be-
comes bluish red and then gradually changes to a
violet–red. If the chloroform layer is decanted into a
porcelain evaporating dish, it changes from violet–red
to violet, to green, and then to yellow. Another test,
the Liebermann–Burchard reaction, which involves
adding acetic anhydride and concentrated sulfuric
acid (under conditions as nearly anhydrous as pos-
sible) to a chloroform solution of cholesterol, results
in an initial blue to violet color that changes to emer-
ald green. Under carefully controlled conditions, the
intensity of the green color produced is proportional
to the amount of cholesterol present.
0005Free cholesterol unites with digitonin, a glycosidic
saponin, to form cholesterol digitonide; cholesterol
esters do not form such compounds. Cholesterol digi-
tonide is insoluble in petroleum ether; cholesterol
esters are freely soluble in petroleum ether. This dif-
ference in solubilities is useful to test both qualita-
tively and quantitatively for free versus esterified
cholesterol.
0006Fieser showed that the melting point of cholesterol,
which had been purified by recrystallization from
acetic acid, was 149.5–150.0
C. Radin and Gramza
indicated that the acceptable melting point of re-
crystallized cholesterol was 149.3–151.3
C. The
Merck Index indicates that the melting point of
anhydrous cholesterol is 148.5
C, and the boiling
point is 233
C at 0.5 mmHg and 360
C at 1 atm
(760 mmHg). At 360
C, some decomposition occurs.
Primary Cholesterol Standard
0007The requirements for primary standards become pro-
gressively more stringent as methods are developed
that are more sensitive and more compound-specific;
such is the case for cholesterol. Primary standards are
chemical substances that, by virtue of their purity, can
be weighed directly for the preparation of solutions
with known concentrations. Primary cholesterol
standards are expected to be at least 99% pure.
H
1
2
3
4
5
10 9 8
7
6
H
H
14
13
12
11
18
CH
3
17
16
15
H
20
22
23
24
27
CH
3
25
CH
3
26
21
H
3
C
HO
19
CH
3
fig0001 Figure 1 Structure of b-cholesterol showing carbon atom
numbering. Reproduced from Cholesterol: Properties and Deter-
mination, Encyclopaedia of Food Science, Food Technology and Nu-
trition, Macrae R, Robinson RK and Sadler MJ (eds), 1993,
Academic Press.
CHOLESTEROL/Properties and Determination 1221

Cholesterol stored at room temperature, unprotected
by a nitrogen cover, will undergo autoxidation over
an extended period. In addition, ultraviolet light will
produce structural changes in cholesterol unless
amber glass containers are used. Consequently, vari-
ous amounts of cholesterol oxidation products may
be present in what was initially pure cholesterol.
Among the cholesterol oxides that have been identi-
fied in stored cholesterol are 7-ketocholesterol,
20-hydroxycholesterol, and 24-, 25-, and 26-hydro-
xycholesterol. Therefore, primary standards that
have been stored must be checked and may need to
undergo repurification before use. Crystalline stand-
ards should be stored in small amounts over a desic-
cant such as silica gel at 20
C in the dark.
0008 Cholesterol may be recrystallized from ethanol or
acetic acid, or as the dibromide. A cholesterol prepar-
ation may be added to absolute ethanol, which is
gently heated until the cholesterol dissolves and is
then cooled to room temperature. The precipitated
cholesterol is collected on a filter, washed with a small
volume of diethyl ether, dried overnight in air, and
dried in an oven at 90
C for 2 h. Cholesterol is re-
crystallized from boiling glacial acetic acid solution,
which is then cooled to room temperature in an ice
bath. The crystals are collected on a filter, washed
with acetic acid and methanol, and dried as described
above.
0009 The recrystallization of cholesterol by the dibro-
mide method (Schoenheimer) is a more arduous task
than the recrystallization from either ethanol or acetic
acid, but it produces a superior product. A bromine
solution is added to the cholesterol solution. A white
paste is produced that is then transferred to a filter
and washed with acetic acid until the filtrate is color-
less. Zinc dust is then added to a suspension of the
white material in diethyl ether and glacial acetic acid
(750:10 v/v); the reaction produces evolution of
hydrogen. The resultant white precipitate of zinc
salts is dissolved in water, and the ether solution is
decanted. The water contains any excess solid zinc.
The ether solution is washed in a separatory funnel
with acid solution and neutralized with sodium hy-
droxide solution. Methanol is added to the ether
solution, and most of the ether is evaporated on a
steam bath as the purified cholesterol begins to crys-
tallize. The crystallization proceeds more rapidly as
room temperature is approached. The product is col-
lected on a filter and dried as described above.
0010 The cholesterol purified by the above methods may
be characterized by the color reactions discussed
above (Salkowski and Liebermann–Burchard). Other
color tests, such as the formaldehyde–sulfuric acid
test, may be used. In this test, formaldehyde–sulfuric
acid solution is added to a solution of cholesterol
dissolved in chloroform. The solution, which turns
cherry red, is poured into another tube, and two to
three drops of acetic anhydride are added. A blue
color develops. These color tests have been adapted
to form the basis of spectrophotometric measure-
ments. The results of the purified products are deter-
mined by reference to a standard of known purity.
0011The purity of cholesterol standards may be assessed
by using melting point and boiling point determin-
ations, microscopic comparison of the crystals with a
pure reference material, and infrared and ultraviolet–
visible spectra. The spectrum of the cholesterol
standard is compared with that of a pure crystalline
reference material.
0012Classic colorimetric tests, microscopic examin-
ations, and melting point determinations have been
supplemented with more modern techniques, such
as mass spectrometry, nuclear magnetic resonance
spectrometry, gas chromatography (GC) and high-
performance liquid chromatography (HPLC) for
determining the purity of prepared crystalline choles-
terol. No one test in itself is sufficient to determine
purity. Confirmation of results requires a minimum of
two tests, which should preferably be chemically
or physically unrelated. (See Chromatography:
High-performance Liquid Chromatography; Gas
Chromatography; Mass Spectrometry: Principles
and Instrumentation; Spectroscopy: Nuclear Mag-
netic Resonance.)
0013A serum reference material for determining serum
cholesterol is normally used for both manual and
automated methods. A serum reference material
may be prepared by filtering pooled human serum
through clarifying and ‘sterilizing’ filters. Stable
serum preparations of cholesterol, with concentra-
tions ranging from 100 to 400 mg dl
1
, may be
made by adding an alcohol-precipitated cholesterol-
rich protein from human serum to bovine, horse, or
human serum. Aliquots of the preparation should be
stored in sealed vials or ampoules at a temperature of
20
C or below. The stability of these preparations
is similar to that of human serum. These sterile prep-
arations may be shipped at room temperature for
periods of up to 5 days. The concentrations of some
commercial reference sera may vary considerably if
the Abell–Kendall method is used as the reference
assay. The Abell–Kendall procedure includes an ini-
tial step in which the serum is treated with alcoholic
potassium hydroxide to liberate the cholesterol from
the lipoprotein complexes and to saponify the choles-
terol esters. The total cholesterol is extracted into a
measured volume of petroleum ether. The cholesterol
in an aliquot of the petroleum ether extract is meas-
ured by means of the Liebermann–Burchard color
reaction. All commercial reference sera should be
1222 CHOLESTEROL/Properties and Determination

checked against serum reference materials and stand-
ardized by the reference cholesterol method (Abell–
Kendall method).
Colorimetry-based Analytical Methods
0014 Routine lipid testing in clinical settings generally in-
cludes serum determination of triglycerides and chol-
esterol, and a more recent trend has been to include
lipoprotein–cholesterol determination. The literature
contains more than 200 methods or modifications for
measuring serum cholesterol. This number is perhaps
an indication of the difficulties of developing a reli-
able assay. Free cholesterol and cholesteryl esters may
be determined separately, but it is common practice to
determine the two together as ‘total cholesterol.’ (See
Spectroscopy: Visible Spectroscopy and Colorimetry.)
0015 The Liebermann–Burchard reagent has had a cen-
tral role in much of the methodological development
of cholesterol measurement systems. Since Grigant
introduced a procedure for the quantitative estima-
tion of cholesterol in 1910 by using the Liebermann–
Burchard reagent (developed between 1885 and
1890), numerous modifications of the method have
appeared in the literature. Most of the deviations
from earlier methods consist of changes in extraction
and color development.
0016 The reaction of cholesterol with sulfuric acid in
acetic anhydride (Liebermann–Burchard) to form a
colored product has provided the basis for many sub-
sequent methods. These methods may be categorized
into three groups: (1) direct, in which the serum is
added directly to the color reagent; (2) extraction,
in which cholesterol is extracted into an organic
solvent before it is added to the color reagent; and
(3) hydrolysis, in which the esterified cholesterol is
hydrolyzed before solvent extraction and color devel-
opment. Many methods utilize acetic anhydride,
acetic acid, or ferric chloride to develop color. Speci-
ficity is enhanced by prior extraction techniques.
Values for cholesterol are usually higher by direct
and automated methods than by manual, extraction,
or hydrolysis methods. The color development of
these various procedures obeys the Lambert–Beer
law, which states that the absorbance is directly pro-
portional to the concentration.
0017 The direct methods are simple and convenient, but
are subject to interference from compounds normally
present in serum, such as bilirubin and proteins.
Much of this interference is eliminated by extracting
the cholesterol with a solvent before the color devel-
opment reaction is initiated. Esterified cholesterol in
serum should be hydrolyzed before a total cholesterol
determination is made because more color is
produced by esterified cholesterol than by free
cholesterol. Failure to hydrolyze results in an over-
estimation of total cholesterol. Standard solutions of
cholesterol must be reacted with the color reagent to
determine when the color has developed its maximum
intensity. Color reagents should be added to test solu-
tions and standard solutions at fixed intervals, for
example, every 30 or 60 s. When the color intensity
of the standard solutions has reached its maximum,
the color intensities of the test solutions should be
read according to the preparation schedule, for
example, every 30 or 60 s. The time required for the
color to develop its maximum intensity is influenced
by the ambient temperature and the composition of
the individual batches of prepared reagents.
0018The American Association of Clinical Chemistry
has suggested three methods for small laboratories
that need a manual procedure for estimating total
serum cholesterol. These procedures are an enzymatic
method, the iron–uranyl acetate method (Parekh–
Jung), and the Liebermann–Burchard reagent
method. The modified Liebermann–Burchard reagent
method, in both manual and automated forms, is
widely used by small clinical laboratories. This dis-
cussion focuses on the manual manipulation of the
method for clarity of the chemistry. The Liebermann–
Burchard color reaction was discussed above with
regard to primary standards. In the modified method,
the cholesterol is extracted from serum test samples
into 2-propanol to eliminate any interfering sub-
stances. A measured aliquot of the extract is evapor-
ated before the Liebermann–Burchard color reagent
is added. If the reagents are added directly to the
extract, it is difficult to control the rate of color reac-
tion under manual conditions. The heat produced
from the exothermic reaction of 2-propanol with
sulfuric acid cannot be controlled sufficiently to
allow reproducible measurements. However, under
stringent automated conditions, this problem can be
circumvented. After a timed incubation and color-
development period, the absorbance is measured at
630 nm. In this method, reference serum and serum
test samples are treated in the same manner.
According to the American Association for Clinical
Chemistry, recoveries of cholesterol added to serum
were 98–101% over a linear range of cholesterol
concentrations of 0.8–4.0 g
1
. This method may be
used to determine cholesterol in food and tissue ex-
tracts that have been cleaned in a separating funnel
before being dried and redissolved in 2-propanol.
Crystalline cholesterol is used as the primary standard
for these test samples.
0019An estimation of the relationship of unesterified
cholesterol to cholesterol ester can be made with
another modification of the Liebermann–Burchard
method. Unesterified cholesterol in serum is
CHOLESTEROL/Properties and Determination 1223

precipitated as the digitonide from ethanol–ether so-
lution. The solvent is evaporated, and the esters are
extracted from the residue by adding petroleum ether,
bringing the solution to a boil, cooling and centrifu-
ging, and collecting the supernatant. The supernatant
is processed as in a total cholesterol determination.
The resultant value is a measure of the esterified
cholesterol in the serum test sample.
0020 The Parekh–Jung manual method is used in many
clinical laboratories. This method is based on the
precipitation of proteins and associated substances
with ferric acetate–uranyl acetate reagent. The mix-
ture is centrifuged, and the resultant supernatant is
treated with sulfuric acid–ferrous sulfate color re-
agent. After a 20-min incubation and color-develop-
ment period, the absorbance is measured at 560 nm.
0021 The enzymatic method for the determination of
total serum cholesterol is frequently used both manu-
ally and in an automated setting. The enzyme method
is of limited value when either aqueous or pure
alcohol standards are used. The method may be cali-
brated accurately with a homogeneous and stable
serum pool. The method provides a direct measure
of serum cholesterol. The enzymatic analytical deter-
mination is calibrated by using serum labeled with
a target value assigned by an accepted reference
method (e.g., the Abell–Kendall method). (See
Enzymes: Uses in Analysis.)
0022 Although cholesterol in serum is primarily free,
cholesterol associated with lipoproteins is esterified.
Cholesteryl esters are freed from the lipoproteins and
enzymatically hydrolyzed by cholesterol-ester hydro-
lase to free cholesterol and fatty acids. The free chol-
esterol is then oxidized by cholesterol oxidase to
cholest-4-en-3-one and hydrogen peroxidase. The per-
oxide, in the presence of peroxidase, oxidatively
couples with phenol and 4-aminoantipyrine to pro-
duce a quinoneimine dye. The absorbance values at
560 nm are proportional to the concentration of total
cholesterol in the serum test sample. The absorbance
obeys the Lambert–Beer law over a wide concentra-
tion of up to about 5.5 g of cholesterol per liter. An
estimate of the relationship of unesterified cholesterol
to cholesterol ester is made by subtracting the free
cholesterol value (the enzyme cholesterol esterase
is withheld from the working test reagent) from
the total cholesterol value. The enzymatic method
has also been used successfully to determine the chol-
esterol content of foods. For this purpose, the choles-
terol is extracted with a chloroform–methanol
solvent, and crystalline cholesterol is used for calibra-
tion. A survey of the literature indicates that the coef-
ficient of variation for this method is about 1–3%.
0023 The Abell–Kendall method is generally accepted
by clinical chemists as the total cholesterol reference
method. The results obtained by other methods
are nearly always compared with those obtained by
the Abell–Kendall method. The method, however,
does not lend itself well to analysis of large numbers
of test samples. A saponification step generally pre-
cludes the development of an automated version
of the method. The serum or plasma is saponified
with alcoholic potassium hydroxide. The free
cholesterol (from both unesterified and esterified
cholesterol) is extracted into petroleum ether and
dried; the cholesterol is determined photometrically
by a modified Liebermann–Burchard reagent (acetic
anhydride–sulfuric acid–acetic acid) at 620 nm. The
results of this method are in good agreement
with those obtained by the Schoenheimer–Sperry
method.
0024No discussion of colorimetric cholesterol method-
ology is complete without a brief discussion of the
separate determination of blood cholesterol and chol-
esterol esters that was originally developed by Bloor
and Knudson in 1916. Practically all methods for
separating free and esterified cholesterol emulate
their work. Bloor and Knudson adapted the Windaus
cholesterol digitonin precipitation method to separ-
ate cholesterol from its esters in small amounts of
blood. The method consists of the determination of
total cholesterol in an aliquot of an alcohol–ether
extract of blood and the determination of cholesterol
esters in another aliquot after precipitation of the free
cholesterol by digitonin. The difference between the
two values represents free cholesterol. The color re-
agent and color development were previously used by
Liebermann–Burchard.
Gas Chromatographic (GC) Analysis
0025GC is used to measure cholesterol as free cholesterol,
as the trimethylsilyl ether derivative, or as cholesteryl
butyrate. Although colorimetric methods have been
used in the past to measure the cholesterol content of
foods, body fluids, and tissues, they have been, for the
most part, cumbersome and not compound-specific
in many applications. Agricultural chemists, bio-
chemists, nutritionists, and food scientists began to
explore the use of GC for measuring sterols in the
mid-1970s. Although blood cholesterol determin-
ations by GC can be fairly easy to accomplish, GC is
not widely used as a routine clinical technique for
determining cholesterol. The most probable reasons
for this are the difficulties of automating the prepara-
tive stages and the fact that adequate colorimetric
assays currently exist. Most chromatographic deter-
minations of cholesterol are found in the research
environment, in food and nutrition analytical labora-
tories, and in government regulatory laboratories.
1224 CHOLESTEROL/Properties and Determination

0026 All GC methods use a column stationary phase that
is classified as nonpolar, typically methyl silicone (SE-
30). In one method, the test sample is saponified, and
the unsaponifiable materials are analyzed by GC.
This method performs well for most products, but
not for those that contain measurable amounts of
a-tocopherol in addition to cholesterol. The retention
times of a-tocopherol and cholesterol are nearly
identical and, therefore, an analysis for either com-
pound is not feasible in the presence of the other.
The a-tocopherol may be present naturally, or may
be added as a nutritional supplement or antioxidant.
(See Tocopherols: Properties and Determination.)
0027 Another approach is to saponify the test sample,
extract the unsaponifiables, and derivatize the unsa-
ponifiable compounds before GC analysis. The offi-
cial method of the Association of Official Analytical
Chemists (AOAC) uses this approach to form
trimethylsilyl ethers (Punwar method). Another
well-established method uses the classic butyric an-
hydride–pyridine reaction to attach a C
4
ester at pos-
ition 3 on the A ring of the sterol to form cholesteryl
butyrate. During GC analyses, both derivatives func-
tion equally with respect to response, reproducibility,
and reliability. The trimethylsilyl ethers create prob-
lems over an extended series of analyses. The detector
sensitivity drops drastically, and the detector must be
disassembled, thoroughly cleaned, and reinstalled to
restore sensitivity. The butyrate derivatives burn
cleanly in the hydrogen flame ionization detector
and do not degrade detector sensitivity. The proced-
ure for preparing the trimethylsilyl derivative is some-
what less tedious than that for preparing the buyrates.
0028 The GC official method of the AOAC determines
cholesterol as the trimethylsilyl ether on a 2.4 m
3 mm internal diameter silanized glass column packed
with 0.5% Apiezon L on 80–100 mesh Gas-Chrom Q
(Alltech Associates/Applied Science, Deerfield, IL).
An alternative column may be used that consists
of a 1.8 m 4 mm internal diameter glass column
packed with 1% SE-30 on 100–120 mesh Gas-
Chrom Q (Alltech Associates/Applied Science, Deer-
field, IL). In this method, one column is maintained at
230
C, and the carrier gas flow is adjusted to elute
the trimethylsilyl ether of cholesterol in 9–11 min. An
internal standard of 5a-cholestane is used, and test
samples containing 0.5–1 g of fat are extracted with
chloroform–methanol–water (50:100:40 v/v/v).
0029 A GC method based on steryl butyrates has been
incorporated into a total-lipid analytical system
(Sheppard–Hubbard system) for determining total
lipids, fatty acid composition, and cholesterol in
foods from extraction of a single test portion. The
system is widely used in university, food quality
control, commercial, and regulatory laboratories to
determine the lipid components required for fatty
acid and cholesterol labeling. The system has also
been used to identify adulterated foods. This analyt-
ical system uses an aliquot of the fatty acid methyl
ester preparation to prepare cholesteryl butyrate and
other sterols. The fatty acid methyl esters are not
affected by the reaction that forms the butyrate de-
rivatives of the sterols. On the SE-30 column, the
fatty acid methyl esters elute near the solvent front
and before the appearance of the butyrates of the
tocopherols and the sterols. In this system, external
calibration is used. However, internal standards can
be used if the internal standard peak is situated very
close to the peak being measured and if there are no
interfering peaks from compounds such as squalane,
squalene, or cholestane in the test sample. Generally,
in this system, there are too many peaks from the
matrix to have a clear retention time available for
internal standards.
0030The relative retention times for some sterols are 1.0
for cholesteryl butyrate, 1.15 for brassicasteryl butyr-
ate, 1.3 for campesteryl butyrate, 1.4 for stigmasteryl
butyrate, and 1.6 for sitosteryl butyrate. Free choles-
terol and other sterols can be easily separated with a
15 m 0.242 mm, SE-54, wall-coated, fused-silica
capillary column operated at 250
C with a helium
flow rate of 0.74 ml min
1
. The sequence of com-
pound appearance is the same as that for SE-30
packed columns.
High-performance Liquid
Chromatography (HPLC)
0031HPLC is increasingly being used to determine choles-
terol and other sterols in foods and tissue extracts.
However, like GC, HPLC is not generally used in
routine clinical analyses performed with automated
clinical multiple analysis systems based on colorimet-
ric or fluorometric assays. Sterols that can be separ-
ated by GC usually cannot be separated by an HPLC
system. One HPLC system in widespread use deter-
mines the benzoate ester of cholesterol on a
300 3.9 mm internal diameter mBondapak C
18
column with a 100% methanol mobile phase and a
variable wavelength ultraviolet detector set at 230 nm.
GC and HPLC determinations of cholesterol in a var-
iety of foods show that the two techniques yield statis-
tically identical results. Amounts as low as 10 ng of
cholesterol benzoate can be determined using HPLC.
See also: Atherosclerosis; Chromatography: High-
performance Liquid Chromatography; Gas
Chromatography; Coronary Heart Disease: Intervention
Studies; Enzymes: Uses in Analysis; Mass
Spectrometry: Principles and Instrumentation;
CHOLESTEROL/Properties and Determination 1225
