Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.

Chemical Properties
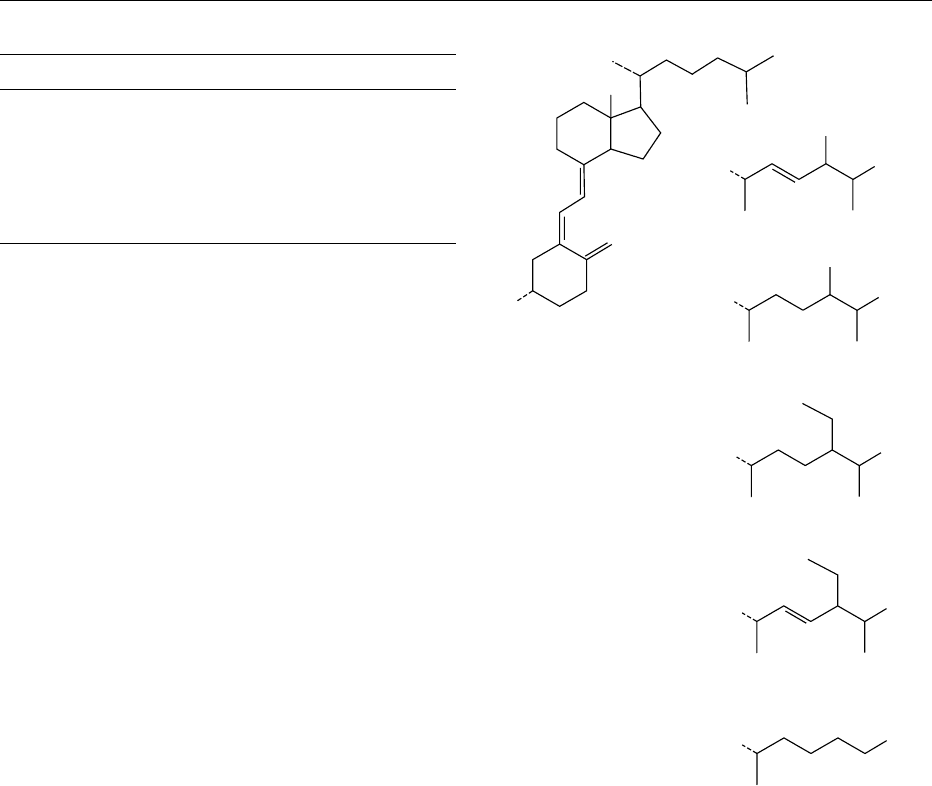
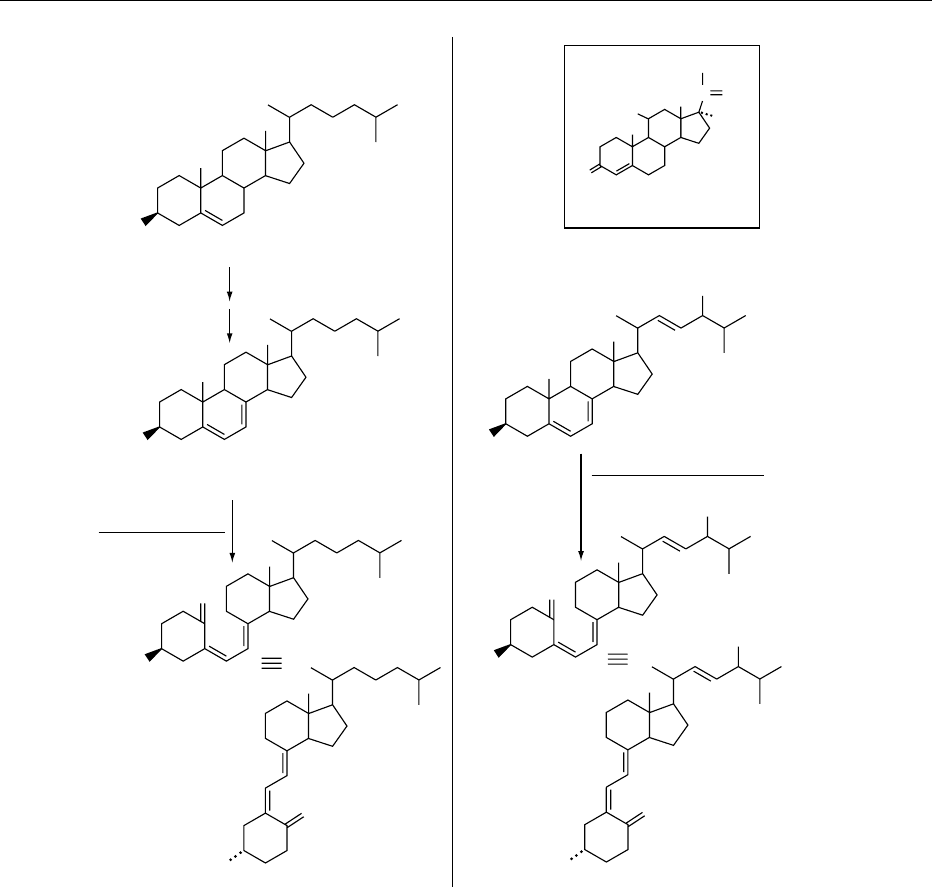
0002 Cholecalciferol is described in terms of steroid
nomenclature and numbering (Figure 1). The ‘9,10-
seco’ prefix is added to denote the bond cleavage and
the opening of the typical steroidal ring structure, an
essential feature necessary to impart antirachitic ac-
tivity. This cleavage is generally induced by ultravio-
let (UV) irradiation of the precursor compound,
7-dehydrocholesterol (provitamin D
3
), and occurs in
the skin during exposure to sunlight. The previtamin
D
3
so formed subsequently undergoes temperature-
dependent equilibration to vitamin D
3
. This pro-
cess, along with the requirement for further hydro-
xylation challenges its original classification as
vitamin; it is currently more accurately regarded as a
prohormone.
0003 Any homolog which possesses antirachitic activity
is referred to as a D vitamin and each of the several
compounds sharing this property elicits a unique
and selective biological response (Figure 1). Common
structural features of these substances are the b
stereochemistry of the 3-hydroxy substituent and the
cis conformation of the double bond at C5. While the
3-hydroxy substituent does not have an overwhelm-
ing influence on biological activity, other structural
features, such as the A ring configuration and side-
chain length, appear to be more critical. Thus, while
alterations to the side chain result in diverse activity,
only vitamins D
2
and D
3
are of prominence therapeut-
ically and commercially. They are usually obtained via
chemical synthesis, although vitamin D
3
has trad-
itionally been extracted from fish liver oils.
0004 It is widespread practice to express vitamin D con-
centration in food in international units (IU), rather
than on a weight basis (1 IU is equivalent to 0.025 mg
of either calciferol, although this equivalence in
humans is occasionally challenged). This can be a
useful concept because it reflects the nutritional status
where several components of different biopotency
coexist within a product. Nevertheless, there is some
move back to mass units, particularly in supple-
mented foods where the contribution of chole-
calciferol is dominant.
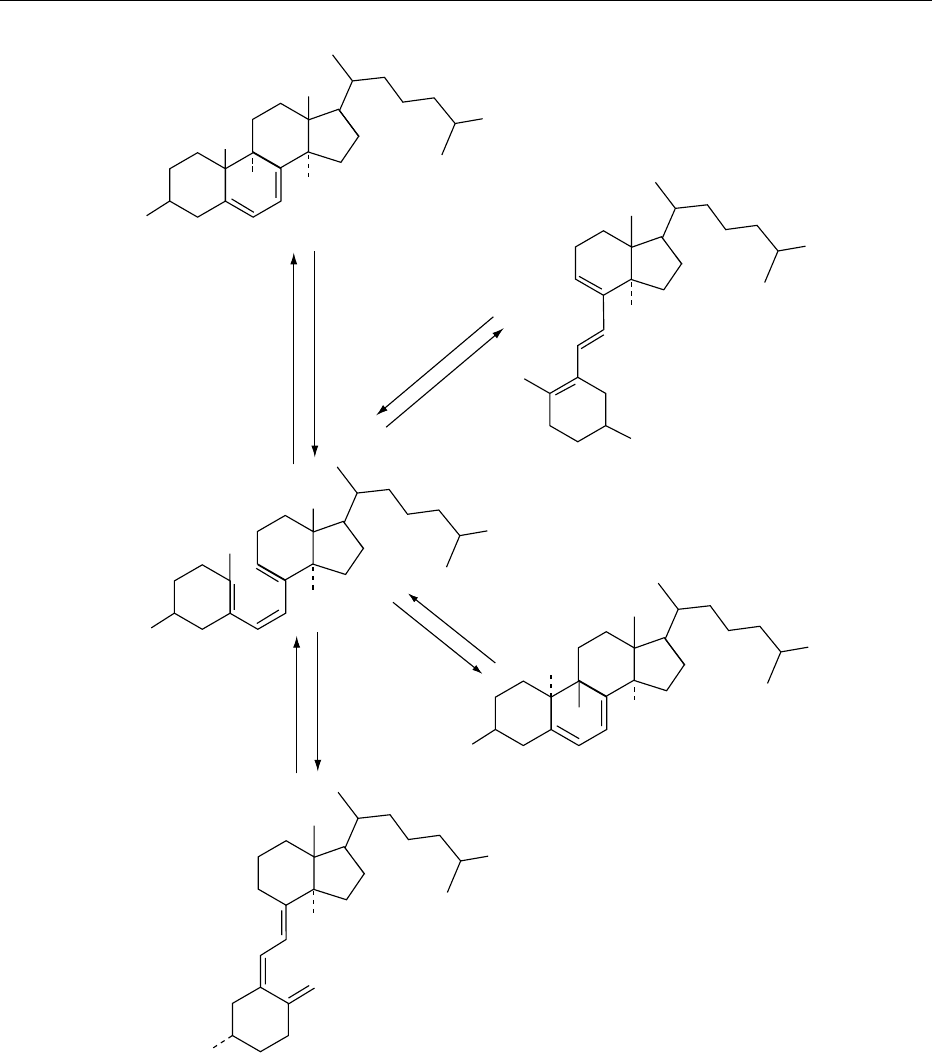
0005The stability characteristics of cholecalciferol have
received considerable attention because they play a
major part in the shelf-life of marketable products.
Under conditions of low thermal, photochemical, and
oxidative stresses, cholecalciferol is stable for several
years. When added to foods and exposed to industrial
and domestic processes, degradation is minimal.
However, excessive light (UV) causes significant
losses through the production of inactive substances
such as toxisterol, suprasterol, lumisterol, and tachy-
sterol, in a process which is accelerated by heating
(Figure 2). Cooking temperatures above 100
C, even
in the absence of light and air, will cause isomeriza-
tion through ring closure to the pyrocholecalciferols.
Cholecalciferol is also sensitive to low pH and, if
subjected to an acidic environment, will irreversibly
tbl0001 Table 1 Physical properties of the calciferols
Cholecalciferol Ergocalciferol
Molecular weight 384.62 396.63
Empirical formula C
27
H
44
OC
28
H
44
O
Melting point (
C) 84–85 115–118
l
max
(nm) 264.5 264.5
Extinction coefficient, E
1%
1cm
in hexane
485 459
Optical rotation in chloroform þ52
þ52
1
2
3
4
5
10
HO
6
19
7
8
9
11
12
13
14
15
16
17
18
21
20
22
23
24
25
26
27
28
R
R
R
R
R
D
2
D
3
D
4
D
5
fig0001Figure 1 The chemical structure of cholecalciferol (vitamin D
3
),
indicating the carbon-numbering system of the molecule. Related
calciferols with different side-chain configurations are also given,
including ergocalciferol (vitamin D
2
).
1206 CHOLECALCIFEROL/Properties and Determination
rearrange to the inactive isotachysterol via the 5,6-
trans isomer. These reactions (illustrated in Figure 3)
are complex and will occur to an extent determined
by the overall environment to which cholecalciferol is
exposed. The reactions largely involve alterations
to ring structure and so affect ergocalciferol and
other D vitamins in a similar manner. It is generally
acknowledged that ergocalciferol is less stable than
cholecalciferol. This may imply that the double bond
in the side chain of ergocalciferol imparts additional
lability to the molecule.
0006Losses during storage are also known to occur in
foods, and vary considerably between food types and
conditions of storage. Thus, low temperatures and
HO
H
H
HO
HO
H
HO
H
H
H
H
7-Dehydrocholesterol
(provitamin D
3
)
Previtamin D
3
Light
Light
Light
Heat
Cholecalciferol
(vitamin D
3
)
Lumisterol
3
Tachysterol
3
HO
fig0002 Figure 2 The major photochemical reactions of cholecalciferol. Ergocalciferol undergoes similar reactions. Overirradiation
products are not shown.
CHOLECALCIFEROL/Properties and Determination 1207
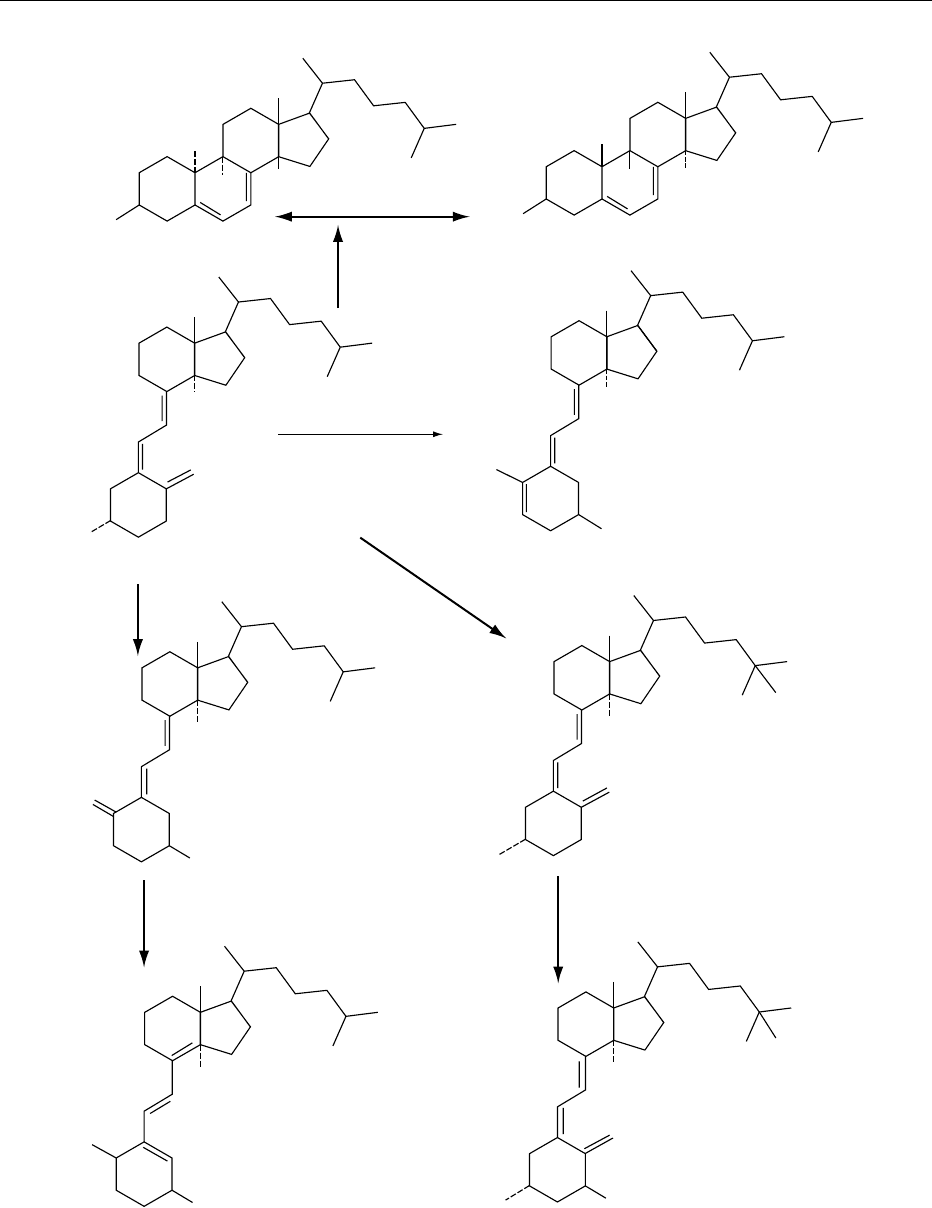
H
H
HO
HO
H
H
H
HO
>150 ⬚C
OH
H
OH
H
OH
H
HO
H
OH
OH
OH
H
HO
Pyrocholecalciferol
Isopyrocholecalciferol
Isocholecalciferol
Cholecalciferol
Isomerization
Acid
Metabolites
5,6-trans-cholecalciferol
Isotachysterol
3
1,25-Hydroxycholecalciferol
25-Hydroxycholecalciferol
fig0003 Figure 3 The major degradation reactions of cholecalciferol as relevant to foods.
1208 CHOLECALCIFEROL/Properties and Determination

absence of light will minimize losses in manufactured
foods, as will vacuum packaging or nitrogen flushing.
Foods containing natural, or added fat-soluble anti-
oxidants such as vitamin E and carotenoids will, in
general, exhibit superior cholecalciferol conservation.
(See Antioxidants: Natural Antioxidants; Antioxi-
dants: Synthetic Antioxidants.)
Occurrence and Forms in Foods
0007 Most natural foods are limited in their content of
active vitamin D components. 7-Dehydrocholesterol
(provitamin D
3
) and ergosterol (provitamin D
2
) are
widely distributed within the animal and plant
kingdoms and supply a potentially good dietary
source of vitamin D for humans. In particular, provi-
tamins are abundant in fish, eggs, yeast, liver, milk,
and some vegetables such as cabbage. It has been
suggested that ergosterol only enters the food
chain following fungal contamination. Consequently,
mushrooms are good sources of ergosterol, particu-
larly in the wild.
0008 Vitamin D itself is generally present at low levels in
unfortified foods derived from animal sources. Eggs,
fish, and fish liver oils possess significant amounts,
while meat and dairy products contain lesser quan-
tities. Plants and vegetable oils contain negligible levels
(< 2% recommended dietary allowance). Table 2 pro-
vides a list of the richest food sources of vitamin D
and their approximate concentrations.
0009 In addition, previtamin D isomers invariably coex-
ist with cholecalciferol and ergocalciferol. Previtamin
concentrations are proportional to the calciferols and
are influenced by thermal conditions during food
processing and storage. Higher temperatures increase
the previtamin-to-vitamin ratio. Although the pre-
vitamins are biologically active precursors, they are
not always accounted for in nutritional tables due to
analytical difficulties in their determination.
0010Some foods also contain small but significant quan-
tities of hydroxylated metabolites. These compounds
are highly bioactive and are found in edible tissues
(meat, liver) and fluids (milk) as a result of their
biosynthesis within the live animal. The most pre-
dominant and biologically important compounds
are 25-hydroxyvitamin D and 1,25-dihydroxyvita-
min D, while other trihydroxy metabolites may also
be present.
Use in Food Fortification
0011The supplementation of food products with vitamin
D is a contentious issue because of the toxicological
consequences of overdose. While adequate exposure
to sunlight will generally negate the need for this
practice, modern western lifestyles (including the
recent trend to reduce the intake of dietary fat) and
climatic variables justify its continuation. (See Food
Fortification.)
0012As many diets will not supply the 10 mg (400 IU) of
vitamin D required per day, some additional source is
generally recommended by nutritionists. The balance
of vitamin can be obtained from specially prepared
natural sources (concentrated fish oils) or from con-
sumption of artifically fortified food products. In this
way, the onset of diet-induced bone disease is minim-
ized, particularly in infants, the sick, and the elderly.
Legislation in many countries governs fortification
strategies.
0013Cholecalciferol is the most common D vitamin
additive, ergocalciferol being less frequently used for
human nutrition. Difficulties potentially arise in
delivery of the vitamin to the consumer as a conse-
quence of its intrinsic hydrophobic and labile nature.
Common vehicles are margarine, milk, and milk
powders, while a number of cereal products and vari-
ous dietetic formulations contain vitamin D as part of
their primary purpose.
0014Cholecalciferol can be easily incorporated into fat
and oil-based foods by simple dissolution. It is
common to add both vitamin D and vitamin A (ret-
inol) to these foods. A protective phenolic antioxidant
(e.g., 3-tert-butyl-4-hydroxyanisole, tert-butylhydro-
quinone, tocopherol) is usually incorporated at the
same time. For powdered foods, cholecalciferol is
added in an encapsulated ‘beadlet’ form, protected
by a gelatine or acacia barrier and incorporating
tbl0002 Table 2 Typical vitamin D content of various foods
Vitamin D content
of edible portion
(mgper100g)
a
Natural food products
Fish liver oils 150–3800
Fish 2–30
Egg yolk 5–8
Mammalian liver 0.5–4
Mushrooms (cultivated) 1–3
Mushrooms (wild) 10–30
Butter 1.5–2
Meat 0.2–2
Cheese 0.1–1
Liquid whole milk (raw) 0.05–0.15
Green vegetables (typical) 0.005 (approx.)
Supplemented food products
Dietary formulae (milk- and soya-based) 3–14
Whole-milk powders 6–12
Margarine 8–10
Infant formulae 5–9
Liquid milk 0.75–1.25
a
To convert to IU per 100 g, multiply by 40.
CHOLECALCIFEROL/Properties and Determination 1209

stabilizers and carrier. If the blending of the vitamin
and food is performed by dry mixing, then cholecal-
ciferol is maintained in its protected environment,
ensuring enhanced shelf-life. However, attaining uni-
form dispersion remains a problem and particle sizes
must be carefully controlled to avoid redistribution
during packaging, transport and storage. Alterna-
tively, these difficulties can be overcome using ‘wet-
blending’ procedures, but the vitamin is inevitably
released from within its stabilized form into direct
contact with the bulk food. Consequently, the receiv-
ing environment must be designed to minimize the
potentially rapid degradation of the cholecalciferol
supplement. (See Retinol: Physiology.)
Analytical Considerations
0015 While it is clinically important to measure the
hydroxylated metabolites, food scientists have been
largely concerned with vitamin D itself, at both
endogenous and supplemental levels.
0016 The quantification of the parent secosteroid in
foods is complicated by several factors, including
low concentration, overwhelming excesses of other
lipophilic components, limiting spectral properties
(nonselective l
max
, low absorptivity), absence of
native fluorescence, thermal instability, and the
requirement to differentiate cholecalciferol from
ergocalciferol.
0017 Historically, curative and prophylactic bioassay
techniques, based on the antirachitic quality of vita-
min D-containing food, have been used extensively
and have the advantage of estimating the true
(species-specific) physiological response. Although
sensitive, disadvantages of time, cost, and poor preci-
sion moderate against the use of bioassays in routine
food analysis.
0018 Competitive protein-binding radioassay tech-
niques, utilizing a vitamin D receptor, have been
exploited more recently, especially in clinical assays.
Such biochemical recognition methods offer advan-
tages in sensitivity and specificity, but extensive
sample purification is mandatory in order to avoid
endpoint interference from food artifacts. Lack of
binding discrimination between vitamins D
2
and D
3
as well as the need for radioactive tracers and lengthy
incubation periods remain problematic within the
food industry. (See Immunoassays: Radioimmuno-
assay and Enzyme Immunoassay.)
0019 Physicochemical determination by UV spectropho-
tometry or colorimetry without prior separation is
clearly impracticable except for highly simplified
food matrices, since the spectral properties of the
parent or derivative calciferol species are insufficiently
characteristic. Even high-potency pharmaceutical
preparations still require scrupulous clean-up proced-
ures in order to minimize spectral interference.
0020The development of instrumental chromatographic
techniques has revolutionized the task of vitamin D
estimation in both food and clinical samples. Al-
though novel detection methods exploiting selective
UV, fluorescence derivatization, or electrochemical
techniques are presently under investigation, contem-
porary UV approaches still require the inclusion of
rigorous purification procedures, since this detection
mode remains inherently nonspecific.
Isolation, Extraction, and Clean-up
0021Vitamin D is vulnerable to oxygen, light, low pH and
is also subject to reversible isomerization when
heated. Therefore, adequate precautions are essential
throughout any analytical procedure to avoid loss of
the target analytes.
0022All chemical extraction techniques exploit the
inherent lipophilic property of this vitamin and a
vitamin-rich fraction is separated from other food
components either by saponification or by total lipid
extraction.
Saponification
0023Alkaline hydrolysis is a convenient way to eliminate
the bulk of neutral lipids and is particularly favored in
high-fat foods and those of an intractable nature. This
popular technique is also advantageous for products
containing encapsulated supplements and when rela-
tively large sample quantities are needed for reasons
of assay sensitivity or analyte heterogeneity.
0024While some authors report the use of high-
temperature saponification, strategies such as direct
measurement or use of conversion factors are then
needed to account for the consequent elevated levels
of previtamin D. These concerns can significantly
complicate the assay and may be avoided through
the use of overnight hydrolysis at ambient tempera-
ture, which additionally offers operational simplicity.
It is considered mandatory during extraction to
include a protective antioxidant and to purge with
inert gas. Vitamin D, along with the other fat-soluble
vitamins, sterols, carotenoids and hydrocarbons,
remains in the nonsaponifiable fraction.
0025Enzymatic hydrolysis with lipase has been sug-
gested as an alternative to saponification, thereby
minimizing possible degradation and facilitating
concurrent recovery of the alkali-unstable vitamin
K, if required.
0026The nonsaponifiable fraction containing the calcif-
erol is rapidly and conveniently partitioned into a
solvent mixture of hexane and diethylether with
good recovery, although other solvent systems have
1210 CHOLECALCIFEROL/Properties and Determination

also been cited in the literature. Following washing to
remove hydrophilic remnants and excess alkali, the
organic phase is dried and evaporated to recover an
enriched crude extract. This will generally require
further clean-up prior to quantification, dependent
on the vitamin D concentration and level of coextrac-
tives.
Total Lipid Extraction
0027 The risks of thermal equilibration with previtamin D
and potential degradation of vitamin D may be
avoided through an initial total lipid extraction. A
variety of solvent systems have been recommended
depending upon sample type and fat content. A con-
sequence of employing such a technique for foods is
the need to isolate the vitamin components from
high-molecular-weight fractions (triglycerides, phos-
pholipids) by gel permeation and/or adsorption
chromatography. Various protocols have been advo-
cated which, while successful, contribute complex-
ities to the assays which are difficult for most
quality control laboratories to manage.
0028 This extraction route has been commonly applied
to low-fat clinical specimens (such as plasma), and
fat-reduced fortified foods (e.g., skim milk). It is also
useful when vitamin D and its metabolites are to be
estimated concurrently. The literature consensus
seems to support the view that saponification is
more appropriate when complex and poorly charac-
terized foods are to be assayed.
0029 The concentrations of cholecalciferol are generally
low, even after isolation of the crude extracts. Further
clean-up to remove substantial quantities of sterols
and other unsaponifiable material is usually incorpor-
ated prior to analysis. Such strategies may be as
simple as cholesterol precipitation or as complex
as multistage semipreparative chromatography,
depending on both sample type and the sophistication
of subsequent analytical techniques. Often at this
stage, analysts may expediently utilize the conveni-
ence of prepacked solid-phase extraction cartridges.
Usually, the adsorption mode with activated silica is
selected, producing an extract of higher vitamin D
content and fewer potential interferences. These dis-
posable cartridges are now widely replacing the
earlier techniques of open-column or thin-layer chro-
matography (although the latter is still occasionally
used for clean-up or qualitative ‘spot testing’).
Chromatography
0030 Quantification of enriched vitamin extracts is achiev-
able by applying gas–liquid chromatography (GLC)
techniques, although the thermal instability of vita-
min D at operating temperatures results in formation
of pyro and isopyro peaks of both parent or derivative
forms. This problem has been successfully avoided by
prior conversion to the thermostable isotachysterol
isomers. Modern GLC now benefits from the use of
capillary columns and ultrasensitive mass-selective
spectrometric detectors, but the extensive manipula-
tive procedures still make GLC less attractive than
other technologies for routine food analysis. (See
Chromatography: Gas Chromatography.)
0031High-performance liquid chromatography (HPLC)
has led to continuing improvements in the assay of
vitamin D (and its metabolites) in foods and has now
superseded GLC. While additional clean-up is not
always unavoidable, derivatization procedures are
unnecessary in the majority of schemes. Furthermore,
the ambient and nondestructive features inherent in
HPLC are more compatible with the lability proper-
ties of this, and other, vitamins. Recently, variants
of micellar capillary electrophoresis (CE) have been
applied to fat-soluble vitamin separations. Although
representing an alternative to HPLC techniques, the
application of CE to the hydrophobic vitamins is
currently in its infancy. (See Chromatography: High-
performance Liquid Chromatography.)
0032In the absence of useful native fluorescence or
stable electrochemical viewing modes and the current
infancy of commercial online liquid chromatography–
mass spectroscopy (LCMS) interfaces, the use of UV
spectrophotometric detection is universal.
0033The modest spectral properties of vitamin D often
require a semipreparative HPLC fractionation step
before an analytical HPLC stage can be undertaken.
Spectral selectivity is further gained by the judicious
use of either wavelength rationing or full-spectrum
(e.g., diode array) detection. Alternative choices to
attain additional specificity and selectivity include
the use of offline competitive protein-binding assay
or the precolumn conversion of cholecalciferol to its
bathochromically shifted isotachysterol.
0034In view of the differing selectivities of normal-
phase and reversed-phase HPLC, many researchers
have concluded that the assay benefits by combining
the two modes. This multidimensional technique ex-
ploits the fact that cholecalciferol and ergocalciferol
are unresolved on silica columns yet are completely
separated using C
18
reversed-phase columns. There
are a number of reported methods which employ
reversed-phase chromatography during clean-up,
either low-pressure or HPLC, followed by normal-
phase quantitation. This regimen is generally of use
where both calciferols are known to coexist in the
sample or where the sample is not well defined
in terms of its vitamin D content. Alternatively,
normal-phase silica chromatography can be used
during the preparative step and the two vitamins
CHOLECALCIFEROL/Properties and Determination 1211
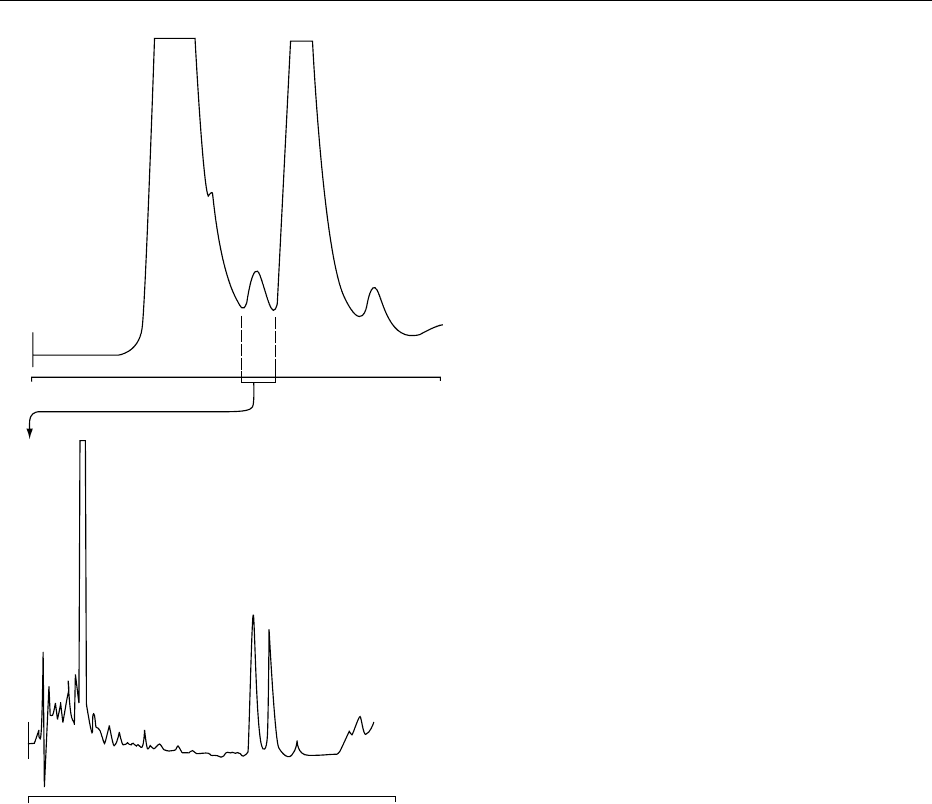
collected in a single fraction before application to a
reversed-phase column. This allows one vitamin to be
used as an internal standard for the other, greatly
decreasing the cumulative assay errors introduced
though potential isomerization and manipulative
losses. The method can be used where only one vita-
min is present, as is usually encountered in the food
industry. Chromatograms of a fish oil taken through
this analytical procedure are shown in Figure 4.
The endogenous vitamin is cholecalciferol, allowing
internal standardization with ergocalciferol.
0035The elution positions of previtamin D
3
and pro-
vitamin D
3
are shown in Figure 4a. They are well
separated from vitamin D
3
, thus allowing potential
differentiation of the species. Other calciferols shown
in Figures 2 and 3 can be similarly resolved. On silica
columns, using hydrocarbon/alcohol mobile phases,
the major isomers elute in the sequence previtamin
D
3
< trans-vitamin D
3
< lumisterol
3
< isotachysterol
3
< vitamin D
3
< tachysterol
3
< provitamin D
3
<
hydroxymetabolites. The pyrocalciferols elute close
to lumisterol
3
. The most likely interfering isomer is
tachysterol
3
which elutes close to vitamin D
3
in both
normal and reversed-phase analyses. Interestingly, the
retention sequence of previtamin D
3
, vitamin D
3
,
and provitamin D
3
is the same, regardless of which
separation mode is used.
See also: Antioxidants: Natural Antioxidants; Synthetic
Antioxidants; Chromatography: High-performance
Liquid Chromatography; Gas Chromatography; Food
Fortification; Immunoassays: Radioimmunoassay and
Enzyme Immunoassay; Retinol: Physiology
Further Reading
Ball GFM (1988) Fat-Soluble Vitamin Assays in Food
Analysis. A Comprehensive Review. London: Elsevier.
Bates CJ (2000) Vitamins: fat and water soluble: analysis.
In: Meyers RA (ed.) Encyclopedia of Analytical Chemis-
try, pp. 1–35. Chichester: John Wiley.
Belitz H-D and Grosch W (1987) Food Chemistry, pp.
305–319. Berlin: Springer-Verlag.
Collins ED and Norman AW (1991) Vitamin D. In: Machlin
LJ (ed.) Handbook of Vitamins, pp. 59–98. New York:
Marcel Dekker.
Eitenmiller RR and Landon WO (1999) Vitamin D. In:
Eitenmiller R and Landon WO (eds) Vitamin Analysis
for the Health and Food Sciences, pp. 77–107. Boca
Raton, FL: CRC Press.
Friedrich W (1988) Vitamin D. In: Vitamins, pp. 143–
216.Berlin: de Gruyter.
Indyk H and Woollard DC (1984) The determination of
vitamin D by high performance liquid chromatography.
New Zealand Journal of Dairy Science and Technology
19: 1–6.
Jones G, Seamark DA, Trafford DJH and Makin HLJ
(1985) Vitamin D: cholecalciferol, ergocalciferol and
hydroxylated metabolises. In: De Leenheer AP, Lambert
WE and De Ruyter MGM (eds) Modern Chromato-
graphic Analysis of the Vitamins, pp. 73–128. New
York: Marcel Dekker.
+
*
Fraction
collected
for analysis
(a)
020
(b)
0
40
Time (min)
D
2
D
3
fig0004 Figure 4 High-performance liquid chromatography (HPLC)
chromatograms showing the analysis of cholecalciferol in halibut
liver oil. An internal standard of ergocalciferol (D
2
) was used
throughout the analysis. (a) The semipreparative clean-up
stage. A Brownlee (25 cm 10 mm) silica column was used with
a mobile phase of 1% 2-propanol in hexane, flowing at 3 ml
min
1
. Detection was by ultraviolet at 280 nm (0.04 aufs). The
unsaponifiable fraction from 2.5 g of fish oil was dissolved in
1.0 ml of mobile phase and injected. The fraction collected and
evaporated for quantitative analysis is shown. The elution pos-
itions of previtamin D
3
(þ) and provitamin D
3
(*) are shown. (b) A
reversed-phase C
18
column (Waters 5 mm Radial-PAK) was used
for analyte quantification. A mobile phase of methanol:water:te-
trahydrofuran (90:8:2 v/v/v) at a flow rate of 2.0 ml min
1
was
used. The injection volume was 100 ml, representing the chole-
calciferol (D
3
) content of 0.25 g of oil. Detection was at 280 nm
(0.005 aufs).
1212 CHOLECALCIFEROL/Properties and Determination

Lawson DEM (1985) Vitamin D. In: Diplock AT (ed.) Fat
Soluble Vitamins. Their Biochemistry and Applications,
Chapter 2, pp. 76–153. London: Heinemann.
Luque de Castro MD, F-Romero JM, O-Boyer F and
Quesada JM (1999) Determination of vitamin D
3
me-
tabolites: state-of-the-art and trends. A review. Journal
of Pharmaceutical and Biomedical Analysis 20: 1–17.
Macrae R (1988) HPLC in Food Analysis, 2nd edn.
London: Academic Press.
Mattila PH, Piironen VI, Uusi-Rauva EJ and Koivistoinen
PE (1996) New analytical aspects of vitamin D in foods.
Food Chemistry 57: 95–99.
Parrish DB (1979) Determination of vitamin D in foods.
CRC Critical Reviews in Food Science and Nutrition 12:
29–57.
Strohecker R and Henning HM (eds) (1965) Vitamin D. In:
Vitamin Assay, Tested Methods, pp. 254–282. Verlag
Chemie.
Physiology
A W Norman, University of California-Riverside,
Riverside, CA, USA
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Background
0001 Vitamin D is essential for life in higher animals. Clas-
sically, it has been shown to be one of the most
important biological regulators of calcium homeo-
stasis. It has been established that these important
biological effects are only achieved as a consequence
of the metabolism of vitamin D into a family of
daughter metabolites, including the two key kidney-
produced metabolites 1a,25(OH)
2
-vitamin D
3
(1a,25(OH)
2
D
3
) and 24R,25(OH)
2
-vitamin D
3
(24R,25(OH)
2
D
3
). 1a,25(OH)
2
D
3
is considered to
be a steroid hormone and there is increasing evidence
that 24R,25(OH)
2
D
3
is also a steroid hormone.
0002 It has become increasingly apparent since the
1980s that 1a,25(OH)
2
D
3
also plays an important
multidisciplinary role in tissues not primarily related
to mineral metabolism, e.g., the hematopoietic
system, effects on cell differentiation and prolifer-
ation including important interactions with keratino-
cytes and cancer cells, and participation in the
processes of parathyroid hormone and insulin secre-
tion. The purpose of this chapter is to provide a
succinct overview of our current understanding of
the important nutritional substance vitamin D and
the mechanisms by which its daughter hormone
1a,25(OH)
2
D
3
mediates biological responses.
Historical Review
0003The first scientific description of rickets, which is the
hallmark of a vitamin D-deficiency, was provided in
the seventeenth century by both Dr. Daniel Whistler
(1645) and Professor Francis Glisson (1650). The
major breakthrough in understanding the causative
factors of rickets was the development of nutrition as
an experimental science and the appreciation of the
existence of vitamins. Considering the fact that now
we accept that the biologically active form of vitamin
D is a steroid hormone, it is somewhat ironic that
vitamin D, through a historical accident, became clas-
sified as a vitamin. It was in 1919/20 that Sir Edward
Mellanby, working with dogs raised exclusively in-
doors (in the absence of sunlight or ultraviolet light),
devised a diet which allowed him to establish un-
equivocally that rickets was caused by a deficiency
of a trace component present in the diet. In 1921, he
wrote, ‘The action of fats in rickets is due to a vitamin
or accessory food factor which they contain, probably
identical with the fat-soluble vitamin.’ Furthermore,
he established that cod-liver oil was an excellent anti-
rachitic agent; this ultimately led to the antirachitic
factor being classified as a vitamin.
0004The chemical structures of the vitamins D were
determined in the 1930s in the laboratory of Profes-
sor A. Windaus at the University of Gottingen. Vita-
min D
2
, which could be produced by ultraviolet
irradiation of ergosterol, was chemically character-
ized in 1932. Vitamin D
3
was not chemically charac-
terized until 1936, when it was shown to result from
the ultraviolet irradiation of 7-dehydrocholesterol.
Virtually simultaneously, the elusive antirachitic com-
ponent of cod-liver oil was shown to be identical to
the newly characterized vitamin D
3
. These results
clearly established that the antirachitic substance
vitamin D was chemically a steroid, more specifically
a seco-steroid (see below).
0005The modern era of vitamin D began during the
period of 1965–70 with the discovery and chemical
characterization of 1a,25(OH)
2
D
3
and its nuclear
receptor, the VDR
nuc
.
Chemistry of Vitamin D
0006Vitamin D
3
is the naturally occurring form of vitamin
D and is produced from 7-dehydrocholesterol (see
Figure 1). Vitamin D
2
is a synthetic form of vitamin
D that is produced by irradiation of the plant yeast
steroid, ergosterol.
0007The structures of vitamin D
3
(cholecalciferol) and
its provitamin 7-dehydrocholesterol are presented in
Figure 1. Vitamin D is a generic term and indicates a
molecule of the general structure shown for rings A, B,
CHOLECALCIFEROL/Physiology 1213
C, and D with differing side-chain structures. The A,
B, C, and D ring structure is derived from the cyclo-
pentanoperhydrophenanthrene ring structure for ster-
oids. Technically, the steroid vitamin D is classified as
a seco-steroid. Seco-steroids are those in which one of
the rings has been broken; in vitamin D, the 9,10
carbon–carbon bond of ring B is broken, and it is
indicated by the inclusion of ‘9,10-seco’ in the official
nomenclature.
0008 Vitamin D (synonym calciferol) is named
according to the revised rules of the International
Union of Pure and Applied Chemistry (IUPAC).
Because vitamin D is derived from a steroid, the
structure retains its numbering from the parent com-
pound cholesterol (see Figure 1). Asymmetric centers
are designated by using the R,S notation; the config-
uration of the double bonds is indicated as E for
‘entgegen’ or trans, and Z for ‘zusammen’ or cis.
Thus, the official name of vitamin D
3
is 9,10-seco
(5Z, 7E)-5,7,10(19)cholestatriene-3b-ol. Vitamin D
2
differs from D
3
by virtue of the presence of a 22-ene
and 24-methyl group in the side-chain. The official
HO
2
1
3
4
5
6
7
8
9
10
19
11
12
18
13
15
14
16
17
20
2221
23
24 27
25
26
A
B
C
D
Cholesterol
HO
3
5
6
7
19
10
18
17
20
22
23
25
A
B
C
D
HO
3
5
6
7
19
1
10
18
20
22
28
27
23
25
26
A
B
C
D
HO
3
5
6
7
8
19
1
9
18 10
22
28
27
28
27
23 25
26
A
C
D
HO
HO
A
3
5
6
7
7
6
5
3
10
1
AAB
Cortisol
(A classic steroid hormone)
Ergosterol
(Pro-vitamin D
2
)
C
HO
OH
OC
CH
2
OH
D
O
19
8
8
19
9
9
11
1
18
20
22
23
25
A
B
C
D
18
11
20
22
23
25
C
D
7-dehydrocholesterol
(Pro-vitamin D
3
)
Sunlight
(Natural process)
Vitamin D
3
or
Cholecalciferol
Vitamin D
2
or
Ergocalciferol
UV Light
(Commercial preparation)
HO
A
7
6
5
3
1
19
8
9
18
20
22
23
25
C
D
fig0001 Figure 1 Structural relationship of vitamin D
3
(cholecalciferol) and vitamin D
2
(ergocalciferol) with their respective provitamins
(7-dehydrocholesterol and ergosterol), cholesterol, and a classic steroid hormone, cortisol (see inset box). The two structural
representations presented at the bottom for both vitamin D
3
and vitamin D
2
are equivalent; these are simply different ways of drawing
the same molecule. It should be noted that vitamin D
3
is the naturally occurring form of the vitamin; it is produced from
7-dehydrocholesterol, which is present in the skin, by the action of sunlight. Vitamin D
2
(which is equivalently potent to D
3
in humans
and many mammals, but not birds) is produced commercially by the irradiation of the plant sterol ergosterol with ultraviolet light.
1214 CHOLECALCIFEROL/Physiology
name of vitamin D
2
is 9,10-seco(5Z,7E)-
5,7,10(19),22-ergostatetraene-3b-ol.
0009 Vitamin D
3
can be produced photochemically by
the action of sunlight or ultraviolet light from the
precursor sterol 7-dehydrocholesterol that is present
in the epidermis or skin of most higher animals. The
chief structural prerequisite of a provitamin D is that
it be a sterol with a D5–7 diene double bond system
in ring B (see Figure 1). The conjugated double
bond system in this specific location of the molecule
allows the absorption of light quanta at certain wave-
lengths in the UV range; this can be readily provided
in most geographical locations by natural sunlight.
This initiates a complex series of transformations
(partially summarized in Figure 1) that ultimately
result in the transformation into vitamin D
3
. Thus,
it is important to appreciate that vitamin D
3
can be
endogenously produced and that as long as the
animal (or human) has access on a regular basis
to sunlight, there is no dietary requirement for this
vitamin.
Physiology and Biochemistry
Vitamin D Endocrine System
0010Vitamin D
3
is not known to have any intrinsic
biological activity itself. It is only after vitamin D
3
is metabolized first into 25(OH)D
3
in the liver
and then into 1a,25(OH)
2
D
3
and 24R,25(OH)
2
D
3
by the kidney, that biologically active molecules are
produced. In toto, some 37 vitamin D
3
metabolites
have been isolated and chemically characterized.
Figure 2 illustrates the concept of the vitamin D endo-
crine system. The elements of the vitamin D endocrine
system include the following: (1) in the skin, photo-
conversion of 7-dehydrocholesterol to vitamin D
3
or
dietary intake of vitamin D
3
; (2) metabolism of vita-
min D
3
by the liver to 25(OH)D
3
, which is the major
form of vitamin D circulating in the blood compart-
ment; (3) conversion of 25(OH)
2
D
3
by the kidney
(functioning as an endocrine gland) to produce the
two principal dihydroxylated metabolites,
OH
24R,25(OH)
2
D
3
1α,25(OH)
2
D
3
(−)(+)
(+)
(+)
1-Hydroxylase24-Hydroxylase
Endocrine modulators
Parathyroid
hormone
(−)
Short
feedback
loop
(−)
Long
feedback
loop
Ca
2+
, P
i
, H
+
Estrogen
Calcitonin
Growth hormone
Prolactin
Insulin
Glucocorticoid
Kidney
25(OH)D
3
25(OH) D
3
25
24
20
15
15
25
25
15
14
12
11
8
8
7
7
7
9
9
HO
HO
HO
HO HO
24R,25(OH)
2
D
3
OH
1α,25(OH)
2
D
3
7-dehydrocholesterol
Placental
production
37 Chemically
characterized
metabolites
Macrophages (activated)
Keratinocytes
Astrocytes (activated)
Paracrine production of
1α,25(OH)
2
D
3
1α,25(OH)
2
D
3
24R,25(OH)
2
D
3
Fetus
1α,25(OH)
2
D
3
Mediated
cellular growth and
defferentiation
1α,25(OH)
2
D
3
Rapid actions
1α,25(OH)
2
D
3
Nuclear receptors
Adipose
Adrenal
Bone
Bone marrow
Brain
Breast
Cancer cells (many)
Cartilage
Colon
Eggshell gland
Epididymis
Ganglion
Hair follicle
Intestine
Kidney
Liver (fetal)
Lung
Muscle, (cardiac)
Muscle, (smooth)
Osteoblast
Ovary
Pancreas β cell
Parathyroid
Parotid
Pituitary
Placenta
Prostate
Skin
Stomach
Testis
Thymus
Thyroid
Uterus
Yolk sac (birds)
Intestine
Bone
Parathyroid
Liver
Pancreas β cell
PKC (activation)
Development
Generation of
osteoclasts
Selected biological
responses
(present in skin)
(hormonal origin)
Hematopoietic cells
Skin
Brain
(Sunlight)
Vitamin D
3
Dietary
sources
∆ Heat
6
6
5
5
3
3
3
10
1
11
10
1
1
Blood Blood
Blood
Blood
1α,25(OH)
2
D
3
Blood
24R,25(OH)
2
D
3
24R,25(OH)
2
D
3
Receptors
Chondrocyte
Fracture-healing callus
Reabsorption of Ca
2+
and P
i
Mobilization / accretion of Ca
2+
and P
i
Absorption of Ca
2+
Classic target organs
Bone
Intestine
Kidney
Liver
HO
OH
OH
OH
Blood
P
i
Ca
fig0002 Figure 2 Summary of the vitamin D endocrine system. In this system, the biologically inactive vitamin D
3
is activated, first in the liver
to generate 25(OH)D
3
, which is then converted by the endocrine gland, the kidney, to the hormones 1a ,25(OH)
2
D
3
and 24R,25(OH)
2
D
3
.
P
i
, inorganic phosphate. There is currently much research being conducted to understand structure–function relationships with
respect to chemical synthesis of analogs of 1a,25(OH)
2
D
3
and their interaction with the vitamin D endocrine system. The objective is
to develop new drug forms of 1a,25(OH)
2
D
3
.
CHOLECALCIFEROL/Physiology 1215
