Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.


characterized by bad smells, dripping out, and
hygienic and preparation problems at the consumer
level. For processed fish (smoked, salted, pickled),
vacuum packaging is commonly utilized because it
limits the spoilage factors of this kind of product
(mainly fat oxidation and mold growth). The storage
life of vacuum-packaged processed fish depends upon
the water activity level, ranging between 2 and 3
weeks for slightly salted fish and a few months for
highly salted fish.
Prepared Foods
0078 Vacuum packaging is also utilized for a wide range of
prepared foods (delicatessen products, cooked meats
and poultry, prepared meals, salads).
0079 The spoilage mechanism and perishability of these
products are related to their chemical composition
(pH, additives) and heat treatment (pasteurization),
and therefore, their storage life is very variable,
ranging from 2 weeks to several months.
0080 An interesting application of vacuum packaging to
prepared foods is the cook-in-the-package technique,
which implies packaging under vacuum of raw or
partially cooked foods that are cooked inside the
package, pasteurized (when required), chilled and
stored, and reheated and unpackaged at the time of
consumption. This technology allows the rationaliza-
tion of meal preparation in central units (e.g., in
institutional kitchens and restaurant chains) because
of the storage life of the prepared meal (1 week or
more) and it has also been introduced to food-pro-
cessing plants to prepare meals to be distributed
chilled at the retail level.
See also: Cheeses: Cheeses with ‘Eyes’; Controlled-
atmosphere Storage: Applications for Bulk Storage of
Foodstuffs; Lactic Acid Bacteria; Meat: Structure;
Oxidation of Food Components; Spoilage: Bacterial
Spoilage; Molds in Spoilage
Further Reading
Brody AL (ed.) (1989) Controlled/Modified Atmosphere/
Vacuum Packaging of Foods. Trumball, CT: Food and
Nutrition Press.
Farber JM and Dodds KL (ed.) (1995) Principles of Modi-
fied Atmosphere and Sous Vide Product Packaging.
Lancaster, PA: Technomic.
Kadoya T (ed.) (1990) Food Packaging. San Diego, CA:
Academic Press.
Mathlouthi M (1994) Food Packaging and Preservation.
London: Blackie Academic & Professional.
Renerre M and Labadie J (1993) Fresh Meat Packaging and
Meat Quality. Proceedings of the 39th International
Congress of Meat Science and Technology. Calgara.
Robertson GL (1992) Food Packaging – Principles and
Practice. New York: Marcel Dekker.
CHLOROPHYL
H G Daood, Central Food Research Institute,
Budapest, Hungary
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Introduction
0001 The green color in nature results from the presence of
chlorophyll pigments that occur in the chloroplasts of
plant tissues. The chloroplasts hold chlorophylls close
to the cell wall and carry out photosynthesis on bio-
membranes. In coordination with other pigments like
carotenoids, chlorophylls play an important role in
the metabolism of light energy and catalyze synthesis
of carbohydrates, the key metabolites of the metabol-
ism of living cells.
0002 Because of their widespread occurrence in a variety
of plant products, chlorophylls play a vital role in
the acceptability of food commodities. Chlorophyll-
containing products have the potential to be used as a
natural food colorant and nutritional supplement.
The changes in chlorophyll content and composition
can be used as an indication for some physiological
process and orders. Recently, biological studies have
postulated the anticarcinogenic and antimutagenetic
effect of chlorophyll-related compounds. This article
covers topics concerning biosynthesis, biochemical
conversion, response to technological factors and
the antioxidant–oxidant role of food chlorophylls.
Biosynthesis
0003Chlorophylls in plant foods are synthesized from
d-aminolevulinic acid (ALA), whose role is demon-
strated in the biosynthesis of the tetrapyrrole nucleus.
Once ALA is formed, two molecules condense to
form porphobilinogen (PBG) by converting an ali-
phatic compound into an aromatic one. The head-
to-tail condensation of four molecules of PBG results
in the formation of the first tetrapyrrole intermediate,
1196 CHLOROPHYL
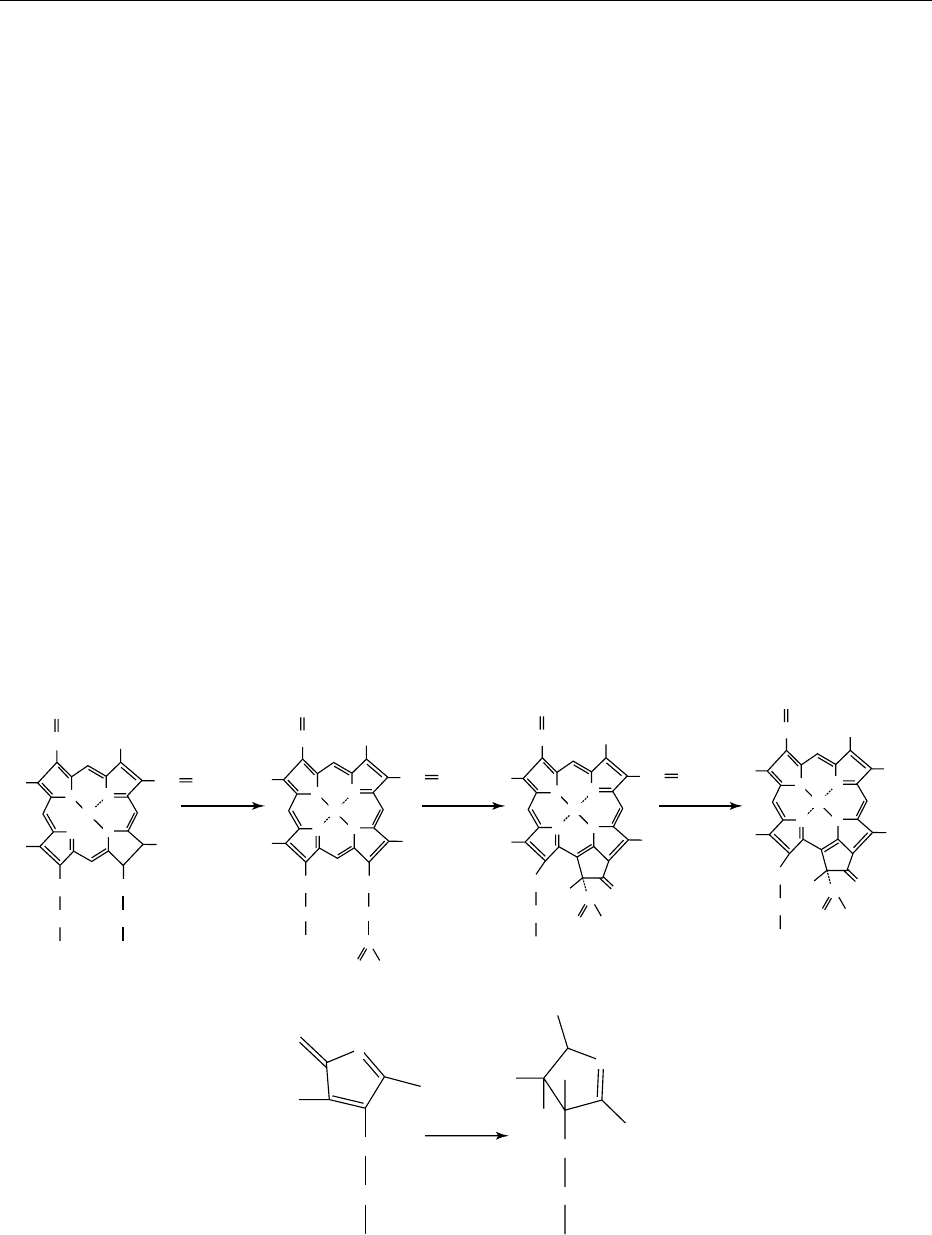
a linear hydroxymethylbilane porphyrin. This linear
molecule is enzymatically closed to form the first
cyclic tetrapyrrole, uroporphyrinogen III. By the
action of decarboxylase-catalyzed decarboxylation
of the acetic acid groups on the pyrrole rings A, B,
C, and D, uroporphyrinogen III is converted to copro-
porphyrinogen III. Propionic acid group oxidation
and aromatization of the oxidized intermediate
forming protoporphyrin IX follow the last step.
0004 The next phase of chlorophyll biosynthesis starts
with the chelation of protoporphyrin IX, mediated by
Mg chelatase. This is followed by methylation of one
of the propionic acid residues to form Mg-protopor-
phyrin-n-monomethyl ester (Figure 1). The last phase
is characterized by the conversion of Mg-protopor-
pyrin-Me to protochlorophyllide and the protochlor-
ophyllide to chlorophyllide through reduction of the
vinyl substitutuent in the side chain of the pyrrole ring
B, followed by oxidation and photoreduction of the D
ring. The final step is esterification of the propionate
substituent on pyrrole ring D with geranyl geraniol,
which is then reduced to phytyl.
0005 The first enzyme involved in tetrapyrrole synthesis,
aminolevulinic acid synthetase, regulates chlorophyll
biosynthesis. During maturation of some plant seeds
chlorophyll biosynthesis may be stimulated by
ethylene in the dark.
0006Concerning biosynthesis of chlorophyll b, old and
more recent investigations gave convincing evidence
that chlorophyll b is formed from chlorophyll a. The
kinetics of
14
C pulse labeling indicate that chloro-
phyll a is the precursor of chlorophyll b. In light
pulse experiments, chlorophyll b formation was
shown to take place stoiciometrically at the expense
of chlorophyll(ide) a. In homogenate of plant leaves,
in the presence of NADPþ , labelled chlorophyll b is
formed from [
14
C]chlorophyll a. The label locates in
the tetrapyrrole and the phytol portion of the chloro-
phyll b, ruling out the possibility that the only carbon
transfer is by transphytylation reaction.
Biochemical conversion
0007Degradation of chlorophyll usually happens all the
way from ripeness to processing and storage of plant-
derived foods. Although the mechanism of degrad-
ation is fragmentary, chlorophylls are degraded to
colorless products.
0008Initially, chlorophylls are degraded to the phytyl-
free chlorophyllides by the enzyme chlorophyllase
N
N
N
N
Mg
CH
2
CH
2
COOH
CH
2
CH
2
COOH
CH
CH
2
CH
2
CH
2
CH
3
CH
3
CH
3
CH
N
N
N
N
Mg
CH
2
CH
2
COOH
CH
2
CH
2
C
O
CH
CH
2
CH
3
CH
2
CH
3
CH
3
CH
3
CH
OCH
3
N
N
N
N
Mg
CH
2
CH
2
HOOC
C
O
CH
CH
2
CH
3
CH
2
CH
3
CH
3
CH
3
CH
OCH
3
H
O
N
N
N
N
Mg
CH
2
CH
2
HOOC
C
O
CH
CH
2
CH
3
CH
2
CH
3
CH
3
CH
3
CH
3
OCH
3
H
O
Mg-protoporphyrin IX Mg-protoporphyrin IX
monomethyl ester
Mg-2,4-divinyl-pheoporphyrin Protochlorophyllide
CH
2
CH
2
COOH
CH
2
CH
2
COOH
H
3
C
H
3
C
N
N
D
H
H
Protochlorophyllide Chlorophyllide
D
fig0001 Figure 1 Last steps of chlorophyll biosynthesis in higher plants. Adapted from Castelfranco PA and Beale SI (1981) Chlorophyll
biosynthesis. In: Hatch MD and Boardman NK (eds) The Biochemistry of Plants. New York: Academic Press.
CHLOROPHYL 1197

(chlorophyll chlorophyllide hydrolase, EC3.1.1.14).
The enzyme is located in the lipid envelope of the
thylakoid membranes as an intrinsic membrane glyco-
protein. Although chlorophyllase activity increases
during ripening of some fruits and vegetables and
parallels the respiratory climacteric, it is not affected
by the endogenous ethylene. The enzyme activity
remains even during storage and after processing of
some plant products such as green tea. Generally,
enzyme activity reaches a maximum at both the begin-
ning and end of the vegetative growth phase.
0009 Small amounts of chlorophyllide a and b can
be found during the initial growth period, which
coincides with a phase of great chlorophyll synthesis.
Later, the synthetic and degeneration mechanisms
may overlap, making the detection of phytyl-free
compounds impossible. In ripening fruits and vege-
tables, the presence of allomerized chlorophyll de-
rivatives suggests that, in addition to dephytylation
by chlorophyllase, chlorophylls can be degraded by
other oxidative systems involving active oxygen.
0010 A chlorophyllide a-degrading enzyme is present in
green tissues. It catalyzes the breakdown of chloro-
phyllide a in the presence of H
2
O
2
and 2,4-dichloro-
phenol.
0011 Biochemical conversion of chlorophylls to pheo-
phytins or pheophorbides is initiated by the coordin-
ating function of Mg-dechelatase (the enzyme
catalyzes dechelation of Mg ion from chlorophyllins
to form different pheophytins) and decarbometh-
oxylase. It is evident from the occurrence of 13-2-
hydroxy-chlorophylls, hydroxy-lactone-chlorophylls,
and unknown intermediates in fresh or stored green-
colored plant products that more than one enzyme
system is involved in the chlorophyll degradation
mechanism.
0012 Of the biological oxidants, lipoxygenase
(EC1.12.13.11) contributes to the oxidative degrad-
ation of chlorophyll. The enzyme is well known to
have pigment-bleaching activity of its isoenzyme-
1 and isoenzyme-2, with the former being more
active. Lipoxygenase cooxidizes chlorophylls through
the oxidation of 1,2-pentadiene containing unsatur-
ated fatty acids and results in the rapid formation
of free radicals and, in the presence of molecular
oxygen, in the creation of very reactive peroxy rad-
icals. The results of many biochemical studies con-
firm that the chlorophyll-bleaching reaction requires
an intermediate formed during peroxidation of poly-
unsaturated fatty acids by the isoenzyme. This type
of degradation is responsible for the low storage
stability of a variety of plant products. Furthermore,
lipoxygenase-catalyzed chlorophyll bleaching is a
characteristic change in some legumes and cereal
products.
0013Due to various bioconversions of chlorophylls, the
dephytylated polar intermediates, chlorophyllides,
pheophorbides, and probably hydroxy chlorophylls
appear and disappear during the ripening and storage
of food commodities. The results of several investi-
gations suggest that chlorophyll is first degraded to
chlorophyllide by chlorophyllase, followed by oxida-
tive degradation by peroxidase, lipoxygenase, or
chlorophyll oxidase. The simultaneous action of
dechelase may raise the diversity of chlorophyll ex-
tract by producing the corresponding Mg-free deriva-
tives of the aforementioned chlorophyll degradation
products. It should be noted that further degradation
of chlorophylls and their derivatives to colorless com-
pounds of low molecular weight is a function of
oxidation processes which is not yet well described.
Degradation during Processing
0014Except for enzymatic conversions, chlorophylls
undergo light-, heat-, and acid-catalyzed alterations
that lead to a marked shift in the greenness of stored
and processed foods.
0015Acid-catalyzed removal of Mg ion upon release of
endogenous acids (in case of mechanical injuries and
processes), acid formation via fermentation (brining,
pickling, etc.), and dressing with an acidic ingredient
are responsible for chlorophyll-to-pheophytin con-
version. The acids produced in the fermentation that
occurs naturally and spontaneously in brined or
pickled fruits induce degradation of chlorophylls
and chlorophyllides to the corresponding pheophy-
tins and pheophorbides. Although physically acid-
catalyzed conversion occurs inside the chloroplast,
the media in which it takes place is the fermentor,
since it is the diffusion across the membranes by
osmosis that leads to fermentation. The intracellular
pH is thus altered by the pH of the brining solution.
The change in content of chlorophylls and pheophy-
tins as a function of fermentation time and pH of the
brine of green olive fermentation is shown in Table 1.
The kinetic equation describing the degradation of
the pigments is:
dc=dt ¼ k½H
þ
n
½pigment
n
ð1Þ
In brining starting with alkaline treatment, when the
interior of the fruit reaches a pH of 8, the concen-
tration of chlorophylls is greater than that of hydro-
gen ions. In this case, if pheophytinization occurs, its
kinetics would be of second order. When the con-
centration of hydrogen ions in the fermentation
media, compared with the existing chlorophyll, is in
excess and can thus be considered to be constant, the
kinetic reaction is of pseudoorder and can be ex-
pressed as:
1198 CHLOROPHYL

dc=dt ¼ k
0
½pigment
n
ð2Þ
where k
0
¼ k½H
þ
n
.
0016 At the first step of brining, when the pH is suitable
for chlorophyllase activity a portion of the chloro-
phyll is converted to chlorophyllides, followed by
acid-catalyzed formation of the corresponding Mg-
free derivatives (pheophytins and pheophorbides).
The decrease in the concentration of chloropyll a
and b as well as the accumulation of Mg-free forms
as a function of time follows first-order kinetics. A
rapid accumulation of pheophytins in cooked or
canned foods is a result of the combined action of
heat and acid release after cell rupture.
001 7 At neutral or alkaline pH values, high-temperature
short-time heat treatment, as in blanching, often
results in an epimerization on carbon-10 of chloro-
phyll molecule, giving rise to the formation of the so-
called a
0
and b
0
epimers that are a brighter green color
than their original pigments. With an extended period
of thermal treatment the rate and final products of
chlorophyll degradation are changed. In canning the
function of time, temperature, and pressure deter-
mines the kinetics of chlorophyll degradation.
Chlorophyll degradation during thermal processing
is frequently described as a first-order reaction. For
such kinetics, the integrated form of eqn (2) for a
decay process at constant temperature and pressure
is given by:
X=X
0
¼ expðktÞð3Þ
where X
0
is the response value at t ¼ 0 (i.e., the
concentration of chlorophyll at t ¼ 0) and X is
the residual response value after treatment (i.e., the
residual concentration of chlorophyll).
0018The temperature dependence of the degradation
rate constant (k) at atmospheric pressure can be
adequately described using activation energy E
a
,as
given in the Arrhenius relationship:
k ¼ k
ref
exp½E
a
=Rð1=T
ref
1=TÞ ð4Þ
where k
ref
is the rate constant at reference tempera-
ture T
ref
,E
a
is the activation energy, and R is the
universal gas constant.
0019The pressure dependence of the rate constant (k)at
a certain temperature is commonly described as an
activation volume V
a
, as given in the Eyring relation:
k ¼ k
ref
exp½V
a
=RTðP P
ref
Þ ð5Þ
where k
ref
is the rate constant at reference pressure
P
ref
, V
a
is the activation volume at a certain tempera-
ture, T is the absolute temperature, and R is the
universal gas constant.
0020In order to interpret the validity of a first-order
reaction of chlorophyll degradation, eqn (3) is linear-
ized using a logarithmic data transformation [ln (X/
X
0
) ]. A log linear plot of relative chlorophyll a and
b retention versus degradation time is depicted in
Figure 2. These plots show that the rate of
chlorophyll degradation follows a first-order kinetic
model.
tbl0001 Table 1 Rate constant (k) for degradation of chlorophyll a, chlorophyll b, and total chlorophyll content in broccoli juice due to a
thermal or a combined pressure–temperature treatment
Pressure (MPa) Temperature (
C) Chlorophyll a k (10
2
min
1
) Chlorophyll b k (10
2
min
1
) Total chlorophyll k (10
2
min
1
)
0.1 80 2.51 + 0.26
a
0.57 + 0.03
a
1.59 + 0.19
a
90 4.41 + 0.24 1.20 + 0.05 2.95 + 0.20
100 8.84 + 0.24 2.46 + 0.11 6.15 + 0.31
110 14.13 + 0.95 4.73 + 0.27 10.29 + 0.65
120 23.60 + 0.35 9.07 + 0.78 17.66 + 0.36
200 60 0.45 + 0.02 ND 0.33 + 0.02
70 1.60 + 0.15 0.57 + 0.01 1.13 + 0.09
80 4.11 + 0.43 1.59 + 0.05 2.76 + 0.19
500 60 0.52 + 0.02 0.10 + 0.01 0.38 + 0.02
70 1.48 + 0.09 0.56 + 0.03 1.13 + 0.04
80 4.04 + 0.24 2.91 + 0.07 3.45 + 0.13
700 60 0.64 + 0.03 0.23 + 0.01 0.50 + 0.02
70 1.67 + 0.06 0.84 + 0.06 1.37 + 0.05
80 3.99 + 0.41 2.78 + 0.16 3.36 + 0.23
800 50 0.20 + 0.02 ND 0.16 + 0.02
60 0.57 + 0.04 0.19 + 0.02 0.44 + 0.03
70 1.91 + 0.05 0.96 + 0.04 1.45 + 0.04
80 3.48 + 0.02 3.67 + 0.14 3.94 + 0.27
a
Asymptotic standard error of regression.
ND, not determined.
Reproduced from Van Loey A, Ooms V, Weemaes C et al. (1998) Thermal and pressure degradation of chlorophyll in broccoli (Brassica oleraecea) juice.
A kinetic study. Journal of Agriculture and Food Chemistry 46: 5289, with permission.
CHLOROPHYL 1199
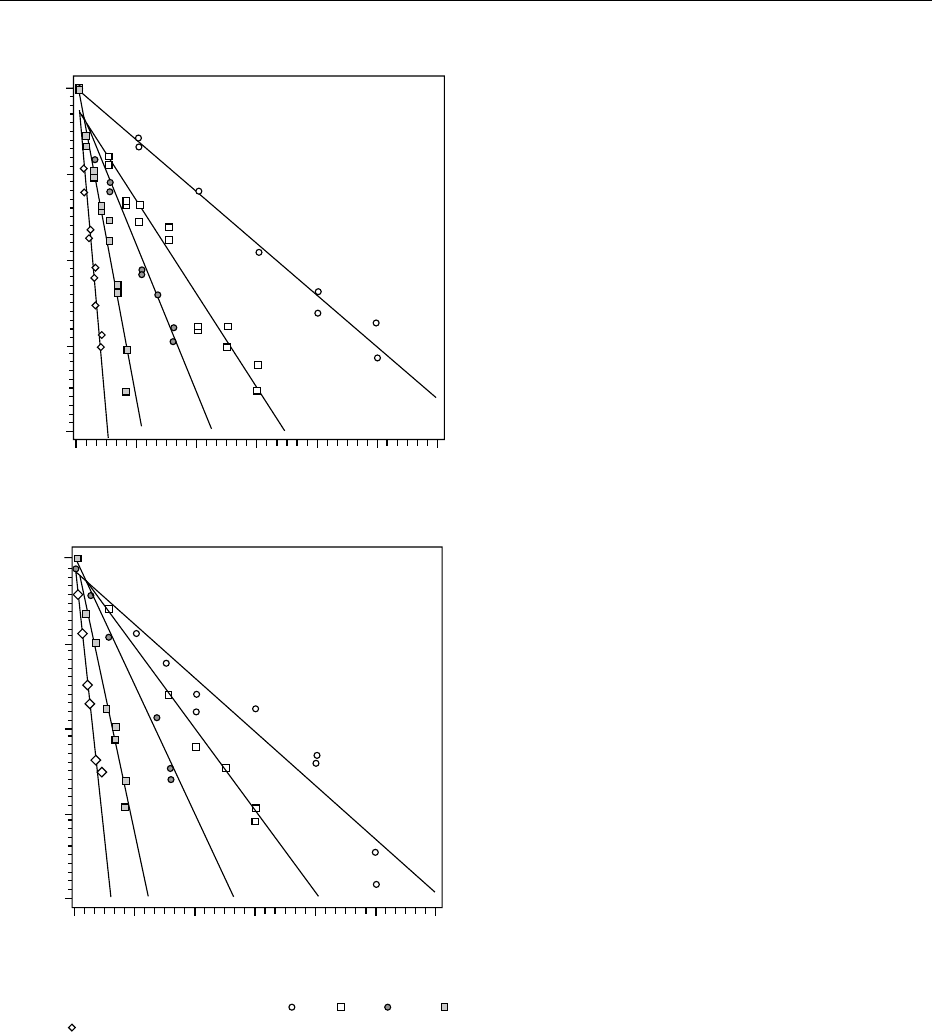
0021 With regard to the ranking in heat stability of
chlorophylls a and b, it is evident that chlorophyll a
is less heat-stable than chlorophyll b, and hence the
former degrades more quickly. The higher thermal
stability of chlorophyll b is attributable to the
electron-withdrawing effect of its C-3 formyl group.
0022 Chlorophyll exhibits an extreme stability toward
pressure processing at ambient or relatively low
temperatures (not higher than 50
C). This can be
ascribed to the stability of the covalent structure of
chlorophyll to high pressure and to the slight com-
pressibility of covalent bonds.
0023From semilogarithmic plots of chlorophyll reten-
tion in broccoli juice as a function of treatment time
at constant pressure and temperature, it can be seen
that the pressure–temperature-induced degradation
process follows a first-order kinetics. Rate constants
for chlorophyll degradation due to thermal or com-
bined pressure–temperature treatment are shown in
Table 2.
0024Severe heat processing enhances the decarbo-
methoxylation reaction, leading to formation of
pyro derivatives of chlorophylls. Heat treatment of
121
C for more than 15 min is required for the
appearance of pyropheophytin a and b. The rate
of decarbomethoxylation reaction as a function of
time and temperature follows first-order degradation
kinetics.
0025Chlorophyll to pheophytin conversion has been
recognized for more than 50 years in frozen foods
stored at lower than 18
C. This conversion is re-
sponsible for color change from a bright green to a
dull olive green in freeze-stored fruits and vegetables.
The rate of such a conversion is shown to be a first-
order model with respect to acid concentration. A
linear relationship is expected between the appear-
ance and pheophytin formation for frozen plant
products.
0026In drying processing, the rate of chlorophyll deg-
radation is affected by some factors, including drying
temperature and time, water activity (a
w
), and the
storage conditions of dried foods. At a
w
values greater
than 0.32 and ambient storage with nitrogen gas,
pheophytins are expected to be the predominant pig-
ment of green-colored dry vegetables. It is well dem-
onstrated that chlorophyll a undergoes degradation
more rapidly than does chlorophyll b by a factor of
2.5–3.0. A linear relationship between a
w
and log
time for a 20% loss of chlorophyll is recognized for
dry fruit and vegetables. Recent studies indicate that
the main reason for chlorophyll degradation in heat-
dried foods (sweet peppers, kidney beans, Chinese
onions, etc.) is the free radicals initiated in the food
system, particularly in the presence of unsaturated
fatty acids. It is recommended to increase the content
of effective scavengers in the product before drying
to neutralize free radicals and prevent pigment
degradation.
0027Formation of pheophytins in edible oils during ex-
traction and refining is a well-known photo-catalyzed
degradation. At different temperature and luminous
energies, this conversion follows first-order kinetics.
From the Arrhenius plot display, it appears that the
In(C/Co)
In(C/Co)
−0.5
0.0
−1.0
−1.5
−2.0
−0.25
0.00
−0.50
−0.75
−1.00
0 180150120906030
0 180150120906030
(a)
(b)
Time (min)
Time (min)
fig0002 Figure 2 First-order thermal degradation of (a) chlorophyll a
and (b) chlorophyll b in broccoli juice at (
) 80, ( ) 90, ( ) 100, ( )
110, and (
) 120
C. Reproduced from Weemaes CA, Ooms V, Van
Loey M, Handrickx ME (1999) Kinetics of chlorophyll degradation
and color loss in heated broccoli. Journal of Agriculture and Food
Chemistry 47: 2404, with permission.
1200 CHLOROPHYL
incident luminous energy does not change activation
energy, but increases the reaction frequency factor.
Effect of Controlled Storage and
Atmosphere
0028 Storage in controlled or ethylene-containing atmos-
phere can affect, to a considerable extent, the chloro-
phyll content of food commodities. Recently, such
storage has been widely applied in food technology
either to speed up chlorophyll loss in some fruit
(citrus fruits, bananas, mangoes, pears, etc.) or to
maintain the greenness of stored crops.
0029 Ethylene can be used directly as gas to treat the
product in the package or in closed stores. Also it is
indirectly applicable either by treating trees or im-
mersing fruit in diluted solutions of ethylene, releas-
ing plant growth regulators such as ethrel and hydrel
(150–250 p.p.m.). To accelerate the ripening of some
pears, a combination of 500 p.p.m. ethylene and
100 p.p.m. acetylene gas is recommended.
0030 Ethylene treatment enhances degreening of the peel
of some fruit via de novo synthesis of chlorophyllase
and chloroplast-dependent enzyme that regulates
chlorophyllase activity. However, in spinach leaves
accelerated color loss by ethylene is not associated
with increased content of dephytylated derivatives.
This difference may be due to differences in the deg-
radation pathway in the different products. It is stated
in the literature that ethylene treatment can also en-
hance the synthesis of chlorophyll-oxidative enzymes
in cotyledons held in the dark. An inverse correlation
between the content of chlorophyll of ethylene-stored
radish cotyledons and the activity of chlorophyll-
oxidizing enzymes indicates that chlorophyll oxida-
tion is an important step in the degreening process.
0031Storage under controlled atmosphere (CA) retards
chlorophyll decomposition in a wide variety of agri-
cultural crops. CA of 10% O
2
and 10% CO
2
is very
effective in reducing the rate of chlorophyll degrad-
ation to the extent that the shelf-life would be
extended about 20% longer than that in air-held
samples (Figure 3).
0032Low O
2
(3%) and high CO
2
(20%) treatment, as in
CA of tomato storage, maintains firmness, reduces
chlorophyll loss, and slows lycopene and carotenoid
development. In CA storage, ethylene-mediated re-
sponses are impaired by low O
2
and high CO
2
and exogenous ethylene and low O
2
/high CO
2
con-
centrations act antagonistically. Controlled storage
may include vacuum cooling and prestorage treat-
ment with ethylene-reducing and respiration rate-
lowering agents. Of these agents, ethanol vapor
is used to maintain freshness and keep the chlorophyll
tbl0002 Table 2 Qualitative and quantitative changes in chlorophylls during table olive processing as a function of pH
Time (days) Pigment concentration (mmol kg
1
)
pH a series b series
Fruit Brine Chl Phy Chl þphy Chld Pho Chld þpho Chl Phy Chl þphy Chld Pho Chld þpho
0 6.08 49.97 49.97 12.37 12.37
4 8.44 8.28 40.73 40.73 7.04 7.04 7.62 7.62 4.52 4.52
6 7.28 6.93 33.09 8.47 41.56 4.70 2.70 7.40 7.29 0.37 7.66 3.86 0.85 4.71
8 6.67 5.49 26.18 15.44 41.62 3.18 3.90 7.08 6.61 0.72 7.33 3.28 1.43 4.71
10 5.17 5.49 25.01 17.20 42.21 2.60 4.95 7.55 6.58 1.06 7.64 2.58 2.12 4.70
14 5.40 4.56 15.12 26.52 41.64 1.40 5.61 7.01 5.95 1.43 7.38 2.11 2.35 4.46
19 5.41 4.76 13.91 27.63 41.54 0.70 6.10 6.80 4.86 2.43 7.29 1.41 3.10 4.51
20 5.08 4.65 12.59 29.01 41.60 0.28 6.80 7.08 4.83 2.48 7.31 1.05 3.40 4.45
26 4.83 4.57 11.76 29.53 41.29 7.02 7.02 3.79 2.74 6.53 4.14 5.10
30 4.69 4.60 6.16 35.40 41.56 7.05 7.05 3.99 3.65 7.64 0.35 4.36 4.71
33 4.55 4.52 5.78 35.32 41.10 7.23 7.23 3.66 3.17 6.83 4.61 4.61
40 4.61 4.42 3.69 36.99 40.68 7.16 7.16 3.14 4.42 7.56 4.60 4.60
50 4.53 4.46 1.52 38.50 40.02 7.18 7.18 2.42 5.20 7.62 4.66 4.66
54 4.44 4.35 1.54 38.24 39.78 7.18 7.18 2.24 5.70 7.97 4.70 4.70
60 4.43 4.29 0.90 39.10 40.00 7.15 7.15 1.88 6.18 8.06 4.70 4.70
70 4.41 4.32 0.37 40.53 40.90 7.20 7.20 1.47 6.30 7.77 4.71 4.71
89 4.33 4.21 0.23 40.82 41.05 7.12 7.12 0.95 6.30 7.25 4.78 4.78
104 4.45 4.25 40.77 40.77 7.14 7.14 0.61 6.59 7.20 4.58 4.58
117 4.37 4.26 40.69 40.69 7.15 7.15 6.57 6.57 4.90 4.90
161 4.21 4.10 40.85 40.85 6.94 6.94 6.41 6.41 4.80 4.80
203 4.34 4.19 40.81 40.81 6.97 6.97 6.68 6.68 4.51 4.51
287 4.27 4.08 40.76 40.76 7.17 7.17 6.59 6.59 4.67 4.67
a series: chlorophyll a and derivatives; b series: chlorophyll b and derivatives; Chl, chlorophyllide; Phy, pheophytin; Pho, pheophorbide.
Reproduced from Mı
´
nguez-Mosquera M, Candul-Rojas B, Mı
´
nguez-Mosquera J (1994) Mechanism and kinetics of the degradation of chlorophyll during
the processing of green table olives. Journal of Agriculture and Food Chemistry 42: 1089, with permission.
CHLOROPHYL 1201
content at high levels in some horticultural crops. A
prestorage treatment of special interest is the short-
term immersion of broccoli florets in ozonated water
(10 p.p.m.)for 10–50 min. Although the treatment
significantly reduces ethylene formation and total
soluble proteins by the end of cold storage, it causes
the vegetables to lose their green color rapidly.
Antioxidant–Oxidant Role
0033 Due to their chemical nature and hydrophobic prop-
erties, chlorophylls are able to interfere with the
chains of lipid oxidation in foods. From industrial
practices and in vitro experiments, chlorophylls and
their degraded products can play the role of either
antioxidants or prooxidants. Factors which are most
likely to determine the antioxidative or oxidative
activity of chlorophylls include variety, ripeness
stage, and chemical composition of food where they
exist, the presence or absence of effective oxidants
or antioxidant, and the climate.
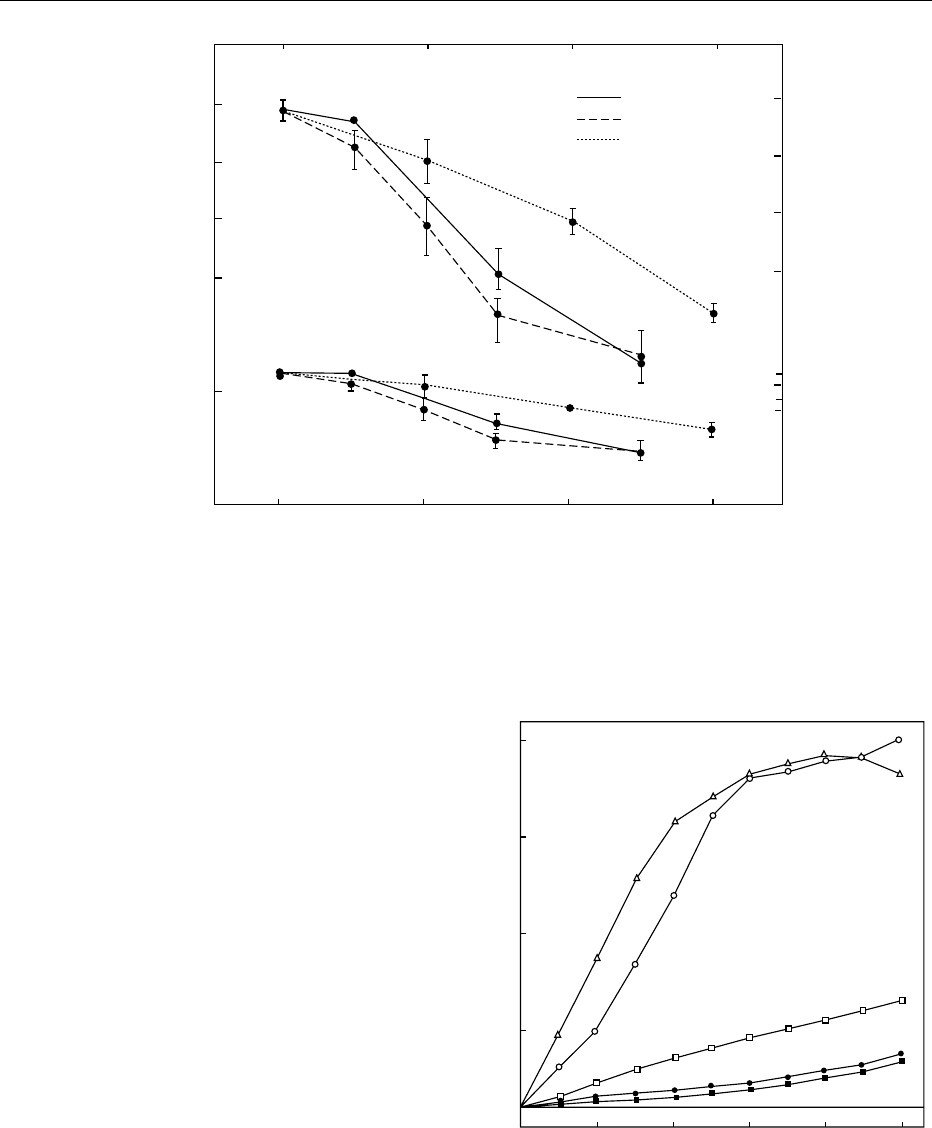
0034 When tested by ferric thiocyanate and other assays,
chlorophylls and their derivatives exhibit marked
antioxidant activity. Recent studies show that acidic
fractions from plant containing pheophytins and re-
lated compounds have an antioxidant effect which is
higher than that of a-tocopherol and comparable to
that of BHT (butylated hydroxytoluene) (Figure 4).
50
100
150
200
0
0246
Days in storage
Chl b
Chl a
Air
Ethylene
CA
Chlorophyll content (mg 100g
−1
f. wt)
fig0003 Figure 3 Change in content of chlorophylls of parsley leaves stored in air with or without 10 p.p.m. C
2
H
4
or controlled atmosphere of
10% O
2
and 10% CO
2
. Reproduced from Yamauchi N and Watada AE (1993) Pigment changes in parsley leaves during storage in
controlled and ethylene containing atmosphere. Journal of Food Science 58: 616, with permission.
0246810
OD 500 nm
2.0
1.5
1.0
0.5
Days
fig0004Figure 4 Antioxidative effect of fractionated extracts from
marine algae by the ferric thiocyanate method. Sample concen-
tration 0.02%; BHT concentration 0.01%. h, neutral fraction;
.
,
weakly acidic fraction; &, strongly acidic fraction; n, basic frac-
tion; continuous line, BHT; s, control. Reproduced from Cahyana
AH, Shuto Y, Kinoshita Y (1992) Pyropheophytin a as antioxidative
substance from the marine alga, arame (Eisenia bicyclis).
Bioscience Biotechnology Biochemistry 56: 1533, with permission.
1202 CHLOROPHYL

0035 The antioxidant activity of chlorophyll-derived
compounds is attributable to their interference with
free radical cycles as scavengers to neutralize highly
reactive oxygen-free forms such as superoxides. The
porphyrin ring system of chlorophylls seems to be
important for the antioxidant activity.
0036 Treating some foods with oil containing chloro-
phyll between 200 and 800 mg% significantly re-
duces peroxide value during storage in the dark.
This gives convincing evidence on the antioxidant
effect of chlorophylls. In dry, oiled, and toasted
laver, for example, the high lipid oxidative stability
is ascribed to the presence of chlorophylls in the oil
used. A positive correlation between oil stability and
chlorophyll content is recognized in the processing of
virgin oil.
0037 The antioxidant activity of chlorophylls can be
enhanced by ascorbic acid and a-tocopherol which
exists at low level as 10
6
mol l
1
for each in the food
system. This suggests that synergism between chlor-
ophylls and other effective bioantioxidants is possible
and of great importance from the point of view of oil
oxidative stability.
0038 Cooling green colored vegetables modifies their
antioxidative ability due to chlorophylls. In cooked
juices from broccoli, cabbage, green pepper, and leek,
the superoxide scavenging ability is higher than that
of the original raw materials. Moreover, the antioxi-
dant effect of cooked spinach containing considerable
amounts of chlorophyll a and b is much lower than
that estimated for other vegetable extracts having
no chlorophyll remains. It is, therefore, convincingly
evident that Mg-free derivatives of chlorophylls
have greater chemical capacity and ability as oxida-
tion barriers than their origins. Also, as many
recent in vitro studies have indicated, the antioxidant
effect is more marked for chlorophyll a than chloro-
phyll b.
0039 In oil-in-water food emulsions which have been
stored in the dark, the addition of chlorophylls from
plant sources such as dried leaves insures oxidation
prevention equal to that provided by 80 p.p.m. of
propyl gallate. However, the effect of green plant
extract on the stability of oil-in-water emulsions is
not the same when the emulsions are exposed to light.
In contrast, under various conditions the prooxidant
effect of chlorophylls is recognized.
0040 As a practice in the processing of rapeseed oil, the
chlorophyll content of raw oil should not exceed
25 mg kg
1
to maintain top-grade oil and extend
shelf-life. The oxidation stability of the refined oil is
reduced as the preprocessing content of pheopytins
exceeds 30 p.p.m. The prooxidant effect of chloro-
phyll-derived compounds is increased, to a high
extent, when the oil is stored in the light.
0041The degree of unsaturation of oil is another factor
which modifies the prooxidant ability of chloro-
phylls. In highly unsaturated marine oil, the addition
of chlorophylls from green tea extracts at less than
25 p.p.m. impairs oil oxidative stability and, more-
over, removing chlorophylls from the tea extract
gives it an antioxidant effect which is even higher
than that of synthetic antioxidants at the same
concentration. It can therefore be concluded that, in
highly unsaturated oils, the prooxidant capacity of
chlorophyll-related compounds is so high that oxida-
tion is scarcely inhibited by spontaneous antioxidants
like flavonoids and tocopherols.
0042Another type of chemical decay in foods is the
photo-catalyzed oxidation of unsaturated lipids.
Chlorophylls contribute to photooxidation of oils
exposed to light or irradiation via a photosensitiza-
tion reaction. The mechanism of light-induced photo-
sensitization may involve free radical initiation and
incorporation of singlet oxygen.
0043Fat-soluble oxygen quenchers and free radical
scavengers such as carotenoids, ascorbyl palmitate,
and tocopherols can interfere to minimize chloro-
phyll-sensitized oxidation of fatty acids and oils.
Ascorbyl palmitate is recommended to be applied
as an antioxidant with photo-oxidation-preventive
properties (total quenching rate ¼ 10
8
mol s
1
)in
fatty foodstuffs. Tocopherols are efficiently used to
minimize chlorophyll-sensitized oxidation. The activ-
ity of tocopherol analogs to prevent photosensitiza-
tion is in the order of d > g > b > a. Carotenoid-type
pigments actively interfere with photosensitized
oxidation in food systems. The antiphotosensitization
ability of carotenoids is attributed to the rapid trans-
fer of radicals from chlorophyll to carotenoid mol-
ecules as well as the ability of carotenoids to quench
oxygen free forms. In photosensitized reactions,
carotenoid pigments undergo trans-to-cis isomeriza-
tion on their structure. This may explain the presence
of cis carotenoids in photosynthetic tissues.
0044It should be noted that all chlorophyll-derived
compounds are able to induce photoisomerization of
carotenoids in light-exposed food systems. Different
carotenoids vary in their rate and capacity of inter-
action with photosensitizing agents. When tested by
the headspace and rancimat methods, capsanthin has
the highest rate and ability to minimize chlorophyll-
induced photosensitization, followed by b-carotene
and lutein.
Metal–Chlorophyll Interaction
0045Bright green color in drastically heat-processed vege-
tables is associated with the formation of metal–
chlorophyll complexes. Copper and zinc, present as
CHLOROPHYL 1203
contaminants in processing solutions, are the most
familiar metals reacting with chlorophylls during the
thermal processing and storage of processed foods.
Such metal complexes can be found in canned green
beans, Brussel sprouts, spinach and pea pure
´
es, table
olives, candies, and chewing gum. The formation
of Zn–chlorophyll complexes is of greater interest
because of the toxicity nature of copper complexes.
0046 The formation of green-colored metal complexes
of chlorophyll derivatives is the basis of efforts by
processors to preserve the desired green color of
canned vegetables. This process is called regreening.
Spontaneous regreening of vegetables during process-
ing, which has long been known by canners as a
nonuniform defect, is attributed to Zn-containing
pigments.
0047 Copper–chlorophyll complexes are formed more
rapidly than Zn complexes. Clorophyll a-related
derivatives form metallocomplexes more rapidly
than chlorophyll b derivatives. Furthermore,
pyropheophytins and pheophorbides have a mark-
edly high tendency to react with metal ions. Steric
hindrance due to the lack of a carbomethoxy group
at C-10 is one possible reason for the rapid inter-
action of pyropheophorbides and metals. The other
explanation is that the distribution of charge in the
aromatic system is affected by the carbomethoxy
group, which is strongly electron-withdrawing. In
the absence of a carbomethoxy group, the pyrrole
nucleus could become slightly more negatively
charged, resulting in an increased reaction rate with
positively charged ions. The kinetic parameters of
Zn–chlorophyll reaction are shown in Table 3.
0048Food additives such as sugars, salts, and other pre-
servatives may influence metal complexes of chloro-
phylls. While sucrose, glucose fructose, chlorides,
sulfates, lactates, acetates, and propionates have no
influence on metal–chlorophyll complex formation,
malate, tartarate, citrate phosphate, and other metal-
chelating agents such as ethylenediaminetetraacetic
acid significantly decrease the rate of metal–chloro-
phyll reaction. In contrast, thiocyanate, benzoate,
oleate, and caprylate ions accelerate the chemical
interaction of chlorophyll with Cu and Zn.
0049The presence of active anionic compounds in
canning solutions facilitates the formation of metal
complexes of chlorophyll-derived compounds by
adsorbing on to chloroplast membranes, thereby
increasing the negative surface charge and giving
rise to a higher affinity to bind positively charged
ions. Increasing cation concentration in the food pro-
motes the complex formation of chlorophylls. The
mechanism for this promotion includes a direct
common ion effect, which increases the rate constant
Table 3 Reaction rate constants (k), half-life values (t
1/2
) and energy activation (E
a
) for the reaction of chlorophyll a and derivatives
with zinc (II) ion at 20, 25, 30, and 35
C
Pigment Temp. (
C) Slope + SD (10
3
)(min
1
) k + SD (min
1
m
1
) Correlation coefficient t
1/2
+ SD (min) E
a
(kcalmol
1
)
Pheophytin 20 0.99 + 0.02 0.035 + 0.001 0.998 305 + 7 22.09
25 1.91 + 0.05 0.067 + 0.002 0.999 157 + 3
30 3.37 + 0.16 0.12 + 0.01 0.995 89 + 4
35 6.36 + 0.16 0.22 + 0.01 0.998 47 + 1
Pyropheophytin 20 1.73 + 0.01 0.061 + 0.004 0.998 174 + 1 22.60
25 3.39 + 0.01 0.119 + 0.002 0.999 89 + 1
30 6.41 + 0.01 0.23 + 0.01 0.998 47 + 1
35 11.45 + 0.01 0.40 + 0.01 0.999 26 + 1
Ethyl pheophorbide 20 1.76 + 0.01 0.062 + 0.001 0.998 171 + 1 21.40
25 3.12 + 0.01 0.110 + 0.001 0.998 96 + 1
30 5.65 + 0.01 0.20 + 0.01 0.998 53 + 1
35 10.55 + 0.01 0.37 + 0.01 0.998 29 + 1
Methyl pheophorbide 20 1.81 + 0.01 0.064 + 0.001 0.999 166 + 1 21.36
25 3.28 + 0.01 0.115 + 0.001 0.999 92 + 1
30 6.11 + 0.01 0.21 + 0.01 0.998 49 + 1
35 10.72 + 0.01 0.38 + 0.01 0.995 28 + 1
Pheophorbide 20 3.36 + 0.01 0.118 + 0.001 0.998 90 + 1 21.52
25 5.73 + 0.01 0.20 + 0.01 0.999 53 + 1
30 10.31 + 0.01 0.36 + 0.01 0.997 29 + 1
35 20.47 + 0.01 0.72 + 0.02 0.998 15 + 0.5
Pyropheophorbide 20 5.57 + 0.01 0.20 + 0.01 0.998 54 + 1 22.06
25 10.68 + 0.01 0.38 + 0.01 0.998 28 + 1
30 19.33 + 0.01 0.69 + 0.01 0.998 16 + 0.5
35 35.50 + 0.01 1.25 + 0.01 0.998 9 + 0.5
SD, standard deviation for duplicate determination.
Reproduced from Tonucci LH and von Elbe H (1992) Kinetics of zinc complexes of chlorophyll derivatives. Journal of Agriculture and Food Chemistry 40:
2341, with permission.
1204 CHLOROPHYL
of the reaction of cations and chlorophylls, and an
indirect pH-lowering effect of cation.
0050 The pH of the media is an important factor
affecting chlorophyll–metal complex formation. The
rate of Zn–complex formation increases between
pH 4.0 and 8.0, reaching a maximum between 6.0
and 8.0. Raising the pH to values higher than 8.0
decreases the amount of metal–chlorophyll com-
plexes formed. At high pH values, chlorophyll a and
b are very stable and the amount of derivatives avail-
able for the reaction is reduced.
See also: Antioxidants: Natural Antioxidants; Colorants
(Colourants): Properties and Determination of Natural
Pigments; Copper: Physiology; Freezing: Structural and
Flavor (Flavour) Changes; Oxidation of Food
Components; Storage Stability: Mechanisms of
Degradation; Zinc: Physiology
Further Reading
Castelfranco PA and Beale SI (1981) Chlorophyll biosyn-
thesis. In: Hatch MD and Boardman NK (eds) The Bio-
chemistry of Plants. New York: Academic Press.
Cross J (1987) Pigments in Fruits. London: Academic Press.
Czygan FC (1980) Pigments in Plants. New York: Gustav
Fischer.
Eskin NAM (1990) Biochemistry of Foods. San Diego:
Academic Press
Fennema OR (1985) Food Chemistry. New York: Marcel
Dekker.
Goodmin TW (1976) Chemistry and Biochemistry of Plant
Pigments, 2nd edn. New York: Academic Press.
Heaton JW and Marangoni AG (1999) Chlorophyll degrad-
ation in processed foods and senescent plant tissues.
Trends in Food Science and Technology 7: 8–15.
Hendry GAF (1996) NERC Unit of Comparative Plant
Ecology. Sheffield: Press of Sheffield University.
Hulme AC (1970) The Biochemistry of Fruits and Their
Products. London: Academic Press.
Jen JJ (1989) Quality Factors of Fruits and Vegetables.
Chemistry and Technology. Washington: Press of Ameri-
can Chemical Society.
Murata N (1992) Progress in Photosynthesis Research.
Dordrecht: Kluwer Academic.
Schwartz SJ and Lorenzo TV (1990) Chlorophylls in foods.
In: Clydesdale FM (ed.) Critical Reviews in Food Science
and Nutrition. Boca Raton: CRC Press.
Chocolate See Cocoa: Chemistry of Processing; Production, Products, and Use
CHOLECALCIFEROL
Contents
Properties and Determination
Physiology
Properties and Determination
D C Woollard, AgriQuality NZ, Auckland 1,
New Zealand
H E Indyk, Anchor Products, Waitoa, New Zealand
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Physical Properties
0001 Cholecalciferol (9,10-seco(5Z,7E)-5,7,10(19)-chol-
estatriene-3b-ol), commonly referred to as vitamin
D
3
, exists in the pure form as white crystalline needles.
Referred to as a ‘fat-soluble’ vitamin, it is insoluble in
water but is readily soluble in most organic solvents,
notably hydrocarbons, chlorinated hydrocarbons,
and alcohols. A closely related substance, ergo-
calciferol (9,10-seco(5Z,7E)-5,7,10(19),22-ergosta-
tetraene-3b-ol), will also be occasionally referred to
in the text. Described more simply as vitamin D
2
,itis
physically, chemically, and nutritionally similar to
cholecalciferol. The term ‘vitamin D’ usually implies
collectively both cholecalciferol and ergocalciferol to-
gether with any other active isomers and metabolites.
Relevant physical properties are listed in Table 1.
CHOLECALCIFEROL/Properties and Determination 1205
