Bongard Frederic , Darryl Sue. Diagnosis and Treatment Critical Care
Подождите немного. Документ загружается.


POISONINGS & INGESTIONS
767
patient has severe serotonin syndrome or neuroleptic malig-
nant syndrome. Cyproheptadine and chlorpromazine poten-
tially antagonize serotonin in the CNS, but there are no
controlled trials of these agents. However, chlorpromazine
may be contraindicated in neuroleptic malignant syndrome
because of its antidopaminergic properties. On the other
hand, bromocriptine, a central dopaminergic agonist thought
to be useful in neuroleptic malignant syndrome, may cause
the serotonin syndrome and therefore is contraindicated.
One approach is to treat with benzodiazepines as neces-
sary while carefully supporting the patient and considering
the need for dantrolene and cyproheptadine. When the clin-
ical picture becomes clearer, then other treatment may be
added.
Boyer EW, Shannon M: The serotonin syndrome. N Engl J Med
2005;352:1112–20. [PMID: 15784664]
Kaufman KR et al: Neuroleptic malignant syndrome and serotonin
syndrome in the critical care setting: Case analysis. Ann Clin
Psychiatry 2006;18:201–4. [PMID: 16923659]
Rusyniak DE, Sprague JE. Toxin-induced hyperthermic syndromes.
Med Clin North Am 2005;89:1277–96. [PMID: 16227063]
Antihypertensives
ESSENTIALS OF DIAGNOSIS
β-Blockers:
Bradycardia.
Conduction blocks.
Hypotension.
Cardiogenic shock.
Depressed mental status.
Calcium channel blockers:
Bradycardia.
Hypotension.
Heart block.
Drowsiness.
General Considerations
Acute overdose of β-blockers and calcium channel blockers
can be potentially life-threatening and poses significant
treatment challenges for the intensivist. Clinical manifesta-
tions of β-blocker overdose are due to the effects of systemic
β-adrenergic blockade. Toxic effects involve mainly the car-
diovascular system, but CNS effects are seen as well. β-
Blockers are absorbed rapidly from the GI tract, and clinical
effects may appear as rapidly as 20–60 minutes after inges-
tion. Half-life depends on the specific drug but ranges from
2–12 hours; excessive overdose may prolong this half-life.
Clinical effects of calcium channel blocker overdose are
caused by actions on the myocardium and on the smooth
muscle of blood vessels. This produces vasodilation and neg-
ative inotropic, dromotropic, and chronotropic activity. The
most commonly used calcium channel blockers are vera-
pamil, diltiazem, and nifedipine, and each has slightly differ-
ent effects. All are well absorbed from the GI tract, but
diltiazem and verapamil both undergo a significant hepatic
first-pass effect. Metabolism is primarily in the liver.
Clinical Features
Although toxicity is seen most frequently with oral ingestion,
significant β-blocker toxicity also can be seen in patients
being treating with β-blocker eyedrops for conditions such as
glaucoma. Patients with significant β-blockade toxicity pres-
ent with bradycardia, conduction blocks, hypotension,
decreased cardiac output, and cardiogenic shock; depressed
mental status also may be seen. Bradycardia can be severe
and appears to be more common with ingestions of propra-
nolol than with other drugs. Overdose with atenolol, nadolol,
carvedilol, and metoprolol tend to present with hypotension
and a heart rate that may be within normal limits. Pindolol
and practolol overdoses may present with tachycardia owing
to their partial agonist activity. First-degree atrioventricular
block is common with propranolol overdoses, and junctional
rhythms, bundle branch block, complete atrioventricular
block, and asystole all have been observed with β-blocker
ingestions. Hypotension is common and may be profound.
Depressed mental status is also seen frequently and is more
common in patients with significant hypotension. Seizures
are uncommon but do occur with propranolol ingestion.
Significant bronchospasm is surprisingly rare.
Significant calcium channel blocker overdose commonly
presents with bradycardia, hypotension, and significant heart
block (including third-degree heart block), which can be life-
threatening. Hypotension is due to both decreased cardiac
output and peripheral vasodilation. These patients also may
be somewhat drowsy, although markedly altered mental sta-
tus is rare.
Differential Diagnosis
β-Blocker overdoses present with bradycardia and hypoten-
sion; these findings also can occur with barbiturate intoxica-
tion and in cases of ingestion of some antiarrhythmics such
as mexiletine.
Treatment
A. Decontamination—In both β-blocker and calcium
channel blocker overdoses, patients who present soon after
ingestion of a significant amount of the drug should be con-
sidered for gastric lavage. Activated charcoal should be given;
repeat doses may be helpful. Patients who are initially stable
and who remain so need only be observed and monitored for
12–24 hours.
CHAPTER 36
768
B. Specific Therapy—
1. β-Blocker overdose—
a. Glucagon—Glucagon has been used with significant
success in patients with symptomatic overdoses of β-blockers
and is considered to be the drug of choice. It is effective
because its effects are independent of β-receptors, and it has
both inotropic and chronotropic effects. The dose used is
higher than that used for stimulating gluconeogenesis. The
recommended initial dose is 0.05 mg/kg intravenously, fol-
lowed by an infusion of up to 0.07 mg/kg per hour as needed.
It is important to be certain that the preparation used for
these doses does not contain phenol as a diluent because even
small amounts of phenol may be toxic. The most common
side effects of this dose of glucagon are nausea and vomiting.
b.
β
-Agonists—β-Adrenergic agonists have been used to
treat β-blocker overdoses with varying success. Since use of
these agents requires quantities sufficient to overcome the
competitive blockade at the receptor, the necessary doses
may be prohibitively high. Agonists may be tried empirically,
but if excessive doses are used with minimal effect, the drug
should be discontinued.
c. Atropine—Atropine, the agent usually first used to treat
symptomatic bradycardia, may have little effect in cases of β-
blocker toxicity because these rhythms are not vagally medi-
ated. When used, a dose of not more than 1 mg intravenously
should be administered. Use of external or transvenous pac-
ing has been reported, but the heart is often refractory to
normal pacing potentials, and the pacemaker may not cap-
ture despite the use of high voltage settings.
2. Calcium channel blocker overdose—
a. Calcium—Calcium channel blocker overdoses have
been treated with a variety of medications with varying suc-
cess. Calcium intuitively would seem to be appropriate ther-
apy, but its use has been disappointing. This result is not
surprising, however, because the calcium channels are
blocked, and this effect is not easily overcome with additional
calcium. Calcium is relatively nontoxic, however, and admin-
istration of calcium chloride, 5–10 mL of a 10% solution, is
probably indicated in most cases of symptomatic calcium
channel blocker toxicity.
b. Glucagon—As with β-blocker overdoses, glucagon is
useful in managing patients with calcium channel blocker
ingestion, although results have been less impressive. Dosing
is the same as that used in treating β-blocker toxicity.
c. Atropine—Atropine has been used to treat bradydys-
rhythmias and heart block but has proved to be relatively
ineffective. If the patient does not respond to 1 mg intra-
venously, use of atropine should be discontinued.
Transvenous pacing may be necessary and may require high
voltage settings outputs for capture.
d. Pressors—Dobutamine and dopamine infusions have
been used in these ingestions with varying results. These
agents may be tried in patients whose hypotension does not
respond to fluid administration and use of glucagon.
Norepinephrine also may be used.
DeWitt CR, Waksman JC: Pharmacology, pathophysiology and
management of calcium channel blocker and beta-blocker
toxicity. Toxicol Rev 2004;23:223–38. [PMID: 15898828]
Kerns W 2
nd
: Management of beta-adrenergic blocker and calcium
channel antagonist toxicity. Emerg Med Clin North Am
2007;25:309–31. [PMID: 17482022]
Love JN et al: Acute beta blocker overdose: Factors associated with
the development of cardiovascular morbidity. J Toxicol Clin
Toxicol 2000;38:275–81. [PMID: 10866327]
Newton CR, Delgado JH, Gomez HF: Calcium and beta receptor
antagonist overdose: A review and update of pharmacological
principles and management. Semin Respir Crit Care Med
2002;23:19–25. [PMID: 16088594]
Digoxin
ESSENTIALS OF DIAGNOSIS
Weakness, fatigue.
Palpitations.
Nausea, anorexia.
Visual complaints.
General Considerations
Digitalis is found in commercially prepared medications and
is also naturally occurring in plants such as oleander; toxic-
ity may be seen in patients exposed to medications or with
plant toxicity.
Digitalis preparations (eg, digoxin and digitoxin) have
two therapeutic effects. The first is to increase vagal tone,
which leads to conduction blockade at the atrioventricular
node and results in decreased chronotropy. The second is to
inhibit the myocardial Na
+
,K
+
-ATPase pump, which nor-
mally pumps calcium and sodium out of the cell and potas-
sium into it. Inhibition causes the intracellular calcium
concentration to rise, resulting in increased contractile force
(ie, positive inotropic effect). In the patient suffering from
digitalis toxicity, inhibition of the pump leads to excessive
extracellular potassium and intracellular sodium and cal-
cium, causing exaggerations of the therapeutic effects of the
drug. Toxicity is manifested by cardiac, GI, neurologic, and
electrolyte abnormalities.
Many of the symptoms of digitalis toxicity are nonspe-
cific. Patients may develop digitalis toxicity even when they
have normal blood levels of the drug if they have an under-
lying condition that sensitizes them to its effects (eg,
hypokalemia). Conversely, patients with elevated levels may
show no evidence of toxicity. The diagnosis of digitalis toxi-
city, therefore, often requires that the treating physician
know the patient groups at risk and suspect the diagnosis in
the appropriate setting. Table 36–9 summarizes the factors
that predispose to the development of digitalis toxicity.
Patients with any of these factors who are also taking digitalis

POISONINGS & INGESTIONS
769
should be evaluated for possible digitalis toxicity when the
clinical setting is suggestive.
Clinical Features
A. Symptoms and Signs—Over 80% of patients with digi-
talis toxicity will complain of weakness, nausea, anorexia,
fatigue, and visual complaints. The visual complaints may be
a clue to making this diagnosis. The patient may complain of
yellow or green vision or of halos around objects—in addi-
tion to other visual disturbances such as photophobia or
transient amblyopia. Other complaints include vomiting,
headache, diarrhea, and dizziness.
B. Electrocardiography—Patients with significant cardiac
toxicity may not manifest either GI or neurologic effects.
Cardiac toxicity is due both to the increased vagal effects of
the drug and to the effects on the Na
+
,K
+
-ATPase pump. In
general, cardiac toxicity results from depression of impulse
formation or conduction (vagally mediated) and from
enhancement of automaticity (caused by blocking the
Na
+
,K
+
-ATPase pump). Virtually any rhythm disturbance
can be observed in digitalis-toxic patients.
Effects on the sinus node lead to bradycardia, sinoatrial
block, and sinus arrest. Increased irritability and automatic-
ity in the atria cause atrial tachycardia and can produce
atrial fibrillation or flutter. The ventricular rate is often nor-
mal or slow as a result of the conduction block caused by
the drug. Digitalis effect in the atrioventricular node can
cause atrioventricular block or junctional rhythm. In fact,
patients with digitalis toxicity may show “regularized”
atrial fibrillation—that is, a regular junctional rhythm with
a background of atrial fibrillation owing to the high degree
of atrioventricular block. The effect in the ventricles may
cause premature ventricular contractions (the most common
digitalis toxic rhythm), ventricular tachycardia, and ventricular
fibrillation.
C. Laboratory Findings—Hyperkalemia may be observed in
acute ingestions owing to the effect of digitalis on the
Na
+
,K
+
-ATPase pump and the resulting shift of potassium to
the extracellular space. This effect is not prominent in
patients who are maintained on digitalis on a chronic basis
and develop toxicity.
Evaluation of the patient with suspected digitalis toxicity
includes a serum digitalis level, serum electrolytes (including
magnesium and calcium), BUN, and serum creatinine. An
arterial blood gas analysis or pulse oximetry should be per-
formed to ensure that the patient is not hypoxic. An ECG
also should be obtained.
Differential Diagnosis
Symptoms of digitalis intoxication are nonspecific and are
not infrequently misdiagnosed as gastroenteritis or a viral
syndrome. The bradydysrhythmias may be associated with
other medications, including β-blockers and calcium chan-
nel blockers. The observed ectopy also can occur with elec-
trolyte disorders, particularly hypokalemia, and in patients
who are hypoxic or in those with cardiac ischemia.
Treatment
A. Decontamination—In the patient with suspected digitalis
toxicity, it is crucial to determine whether the toxicity is acute,
chronic, or acute on chronic. Although the same general care
principles apply to all three situations, patients with an acute
overdose may need several additional measures such as gastric
emptying, which should be considered if the patient presents
within 1 hour of the ingestion. If performed, lavage should be
done carefully because all gastric emptying techniques cause
increased vagal tone and may lead to worsening of any brady-
dysrhythmias or blocks. Pretreatment with atropine may have
minimal effect owing to digoxin’s blocking effect on the atri-
oventricular node. Whether lavage is performed or not, char-
coal, 50–100 g orally, should be given to adsorb any digitalis
remaining in the GI tract. Repeat-dose activated charcoal may
be effective in enhancing elimination of the drug.
Cholestyramine, which binds digitalis in the gut, also has
been used for this purpose in doses of 4–8 g orally, but it has
no particular advantage over charcoal. Drug levels should not
be drawn until at least 6 hours after the acute ingestion
because it takes that long for the drug level to equilibrate, and
levels obtained sooner may be misleadingly high.
In most cases of chronic toxicity, withdrawal of the drug
and a period of observation are all that are required for treat-
ment. In cases of both acute and chronic toxicity, forced
diuresis, hemoperfusion, and hemodialysis have been shown
to be ineffective in removing digitalis owing to its high
degree of protein binding.
B. Management of Electrolyte Abnormalities—Patients
who develop hyperkalemia should be treated with the
standard treatment regimen. It is important to note, how-
ever, that some of these measures may be ineffective in the
presence of digitalis toxicity. Use of bicarbonate and the
Table 36–9. Predisposing factors in digitalis toxicity.
Increased Sensitivity Increased Drug Level
Electrolyte disturbances
Hypokalemia
Hypernatremia
Hypercalcemia
Hypomagnesemia
Cardiac abnormalities
Ischemia
Cardiomyopathy
Conduction abnormalities
Hypoxemia
Drugs
Verapamil
β-blockers
Diuretics
Renal disease
Drugs
Quinidine
Verapamil
Amiodarone
CHAPTER 36
770
administration of glucose and insulin intravenously require
an intact Na
+
,K
+
-ATPase pump to reduce the elevated potas-
sium and may not produce the desired effect of lowering the
potassium level. Intravenous calcium is contraindicated
because it sensitizes the patient further to the toxic effects of
the digitalis. Sodium-potassium exchange resins are effective
and probably constitute the first choice in treating hyper-
kalemia from digitalis toxicity (polystyrene sulfonate, 15 g in
20–100 mL of syrup orally one to four times daily or 30–50
g in 100 mL of water per rectum every 6 hours). Dialysis is
also effective and can be used if hyperkalemia is life-
threatening or refractory to exchange-resin treatment.
Digitalis antibodies (see below) are also effective in
treating hyperkalemia. A serum potassium level over 5
meq/L in the setting of an acute overdose is an indication for
treatment with antidigitalis antibodies.
C. Treatment of Arrhythmias—The major and most life-
threatening toxicity associated with digitalis is cardiotoxicity.
Bradydysrhythmias, owing to the increased vagal tone,
should be treated with atropine starting with doses of 0.5 mg
intravenously. Doses can be repeated at 5-minute intervals as
necessary to a total dose of about 2 mg (0.3 mg/kg). If the
bradydysrhythmias are refractory to atropine, electrical pac-
ing may be necessary.
Tachydysrhythmias or rhythms resulting from increased
automaticity should be treated in a stepwise approach. First,
if the patient is hypokalemic (serum potassium <3.5 meq/L),
particularly if the patient is suffering from chronic toxicity,
potassium should be gently replenished, with frequent moni-
toring of the serum potassium level. Magnesium should be
given to virtually all patients with tachydysrhythmias except
those with elevated magnesium levels or those with renal fail-
ure. The optimal dose is unknown, but 2 g of magnesium sul-
fate intravenously over 20–30 minutes appears effective. In
patients whose tachydysrhythmia does not respond to elec-
trolyte replacement or who have contraindications to elec-
trolyte replacement, lidocaine should be used. If the patient’s
tachydysrhythmias persist despite adequate lidocaine dosing,
phenytoin poses an effective alternative, aiming for a thera-
peutic level of 10–20 μg/mL. Cardioversion is safe when used
in patients who have normal digitalis levels and no evidence
of toxicity. However, cardioversion in patients with digitalis
toxicity can lead to refractory ventricular tachycardia, ventric-
ular fibrillation, or asystole. It should be avoided if possible in
this group of patients, and all attempts should be made to
treat dysrhythmias medically. However, in some cases, car-
dioversion may be necessary to regain a perfusing rhythm. If
needed, it is critical that the lowest possible energy level be
used to achieve cardioversion. If possible, pretreatment with
lidocaine may be prudent.
D. Antibodies—Digoxin-specific antibodies (digoxin immune
Fab [ovine]) are a vital addition to the armamentarium for
treating digitalis toxicity, but their use should be limited to very
specific situations. These antibodies are sheep serum Fab frag-
ments that have a high affinity for digoxin—higher than the
affinity of digoxin for Na
+
,K
+
-ATPase. They circulate in the
intravascular space and diffuse into the extracellular space,
where they bind to free digoxin. The complex formed has no
biologic activity and is excreted in the urine. The intracellular-
to-extracellular gradient produced by the binding of extracel-
lular free digoxin causes intracellular digoxin to diffuse from
within the cells to be bound to the antibody and subsequently
excreted. Indications for use of these antibodies are listed in
Table 36–10. In general, digitalis-specific antibody should be
used when there are life-threatening dysrhythmias that do not
respond to conventional therapy, in patients with an initial
potassium level of greater than 5 meq/L (particularly in acute
ingestions), in patients who have ingested more than 10 mg of
digoxin (4 mg in children), and in those who have a steady-
state digoxin level of greater than 10 ng/mL.
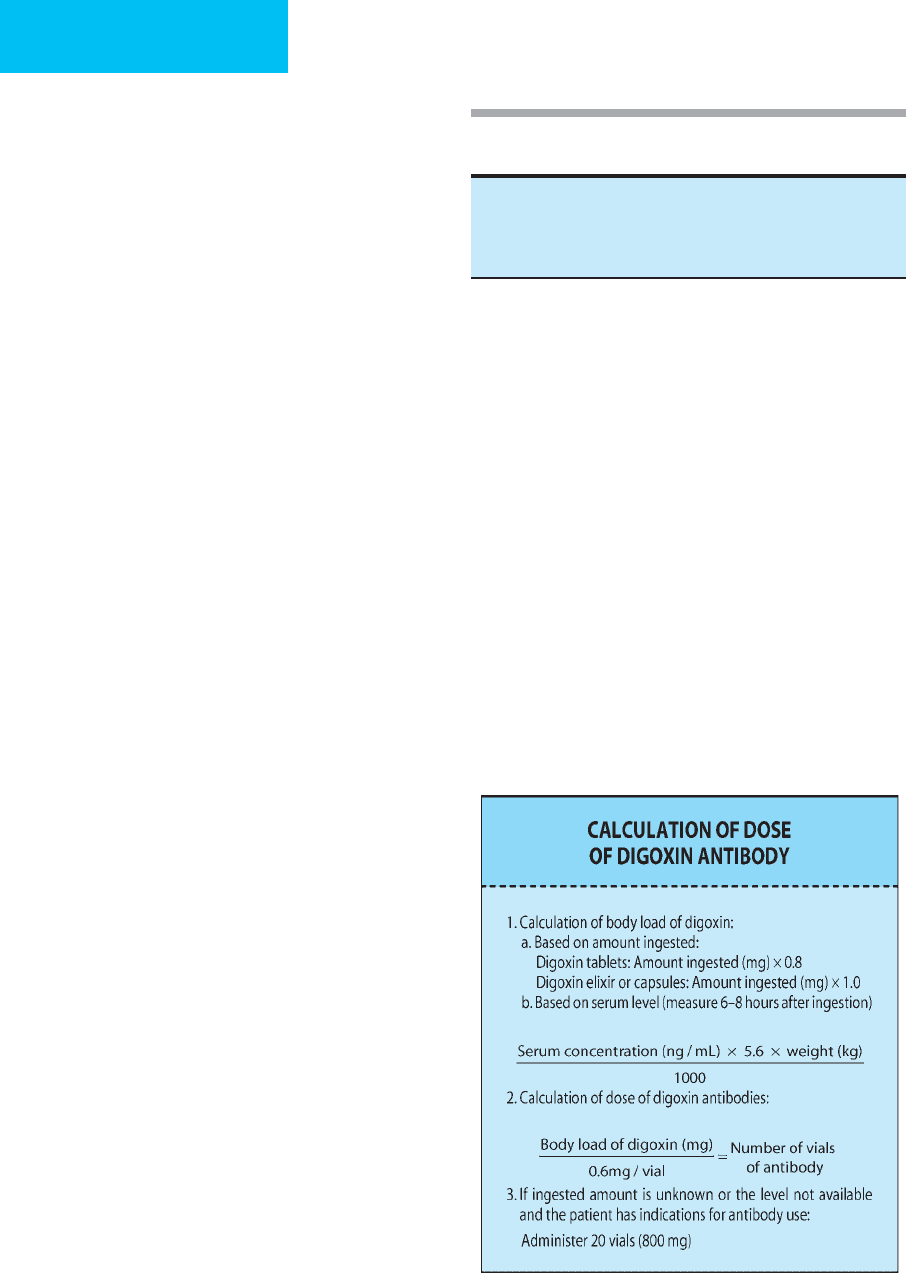
Dosing of digoxin antibodies is based on the fact that
each vial contains 40 mg antibody and will bind 0.6 mg
digoxin or digitoxin. The formula for calculating the dose of
antibody in a particular patient is shown below:
Life-threatening dysrhythmias
Serum potassium >5 meq/L
Acute ingestion of >10 mg of digoxin (>4 mg in children)
Steady-state digoxin level >10 ng/mL
Table 36–10. Indications for therapy with digoxin
antibodies.

POISONINGS & INGESTIONS
771
In patients with life-threatening complications who have
ingested an unknown amount, or if the blood level is
unavailable, a dose of 20 vials (800 mg) should be given.
Dysrhythmias are treated successfully with these antibod-
ies in about 70% of patients. The rhythms usually respond
within 20–60 minutes of administration of the antibody. Side
effects are generally mild. Up to 15% of patients may develop
minor allergic reactions, and some patients with preexisting
congestive heart failure may have an exacerbation owing to
the volume of fluid used to infuse the antibodies. It is impor-
tant to note that measuring digoxin levels after giving anti-
bodies is unreliable for up to 7 days after their
administration. Levels tend to rise to alarming levels because
so much of the drug ends up in the circulation bound as the
inactive antibody complex.
Current Controversies and Unresolved Issues
The treating physician must understand the indications for
digoxin antibodies and use them appropriately. Patients who
have minor manifestations of digitalis toxicity such as GI
complaints or visual changes and those with evidence of car-
diac toxicity that does not need intervention or responds to
conventional therapy do not need this treatment. In addition,
patients with elevated digitalis levels but without evidence
of toxicity do not need treatment unless their levels are over
10 ng/mL at steady state. The levels should be measured at least
6–8 hours after the ingestion or the last dose of the medication.
Critchley JA, Critchley LA: Digoxin toxicity in chronic renal fail-
ure: Treatment by multiple dose activated charcoal intestinal
dialysis. Hum Exp Toxicol 1997;16:733–5. [PMID: 9429088]
Dawson AH, Whyte IM: Therapeutic drug monitoring in drug
overdose. Br J Clin Pharmacol 2001;52:97–102S. [PMID:
11564057]
Eddleston M et al: Anti-digoxin Fab fragments in cardiotoxicity
induced by ingestion of yellow oleander: A randomised, con-
trolled trial. Lancet 2000;355:967–72. [PMID: 10768435]
Van Deusen SK, Birkhahn RH, Gaeta TJ: Treatment of hyper-
kalemia in a patient with unrecognized digitalis toxicity. J Toxicol
Clin Toxicol 2003;41:373–6. [PMID: 12870880]
Acetaminophen
ESSENTIALS OF DIAGNOSIS
Nausea and vomiting.
Jaundice.
Right upper quadrant pain.
Asterixis.
Lethargy and coma.
Bleeding.
Hypoglycemia.
General Considerations
Acetaminophen is an antipyretic and analgesic medication
available over the counter generically and in several brand-
name preparations. It is also used commonly in many com-
bination medications, both over the counter and available by
prescription. Overdose may occur inadvertently or inten-
tionally. Patients may ingest excessive doses of acetamino-
phen in an attempt to treat their own pain, being unaware of
the potential for toxicity. In the intentional overdose, treating
physicians must be aware that many medications contain
acetaminophen as a component of a combination prepara-
tion, and what might otherwise be a relatively benign inges-
tion from the other active ingredients in the medication
becomes a potentially lethal one in light of the amount of
acetaminophen consumed.
Acetaminophen is normally metabolized by the liver to
non-toxic compounds. If these pathways are saturated, a
toxic intermediate (N-acetyl-p-benzoquinoneimine) is
formed that is detoxified by glutathione. Excessive amounts
of acetaminophen deplete glutathione stores, leading to
accumulation of high levels of these toxic metabolites. The
major toxicity is hepatotoxicity, with hepatocyte necrosis
and, in severe cases, frank liver failure. N-Acetylcysteine, the
antidote for this toxicity, acts by enhancing glutathione stores
and providing a glutathione substitute to allow for detoxifi-
cation of the toxic metabolites.
Susceptibility to toxicity is variable. Patients with liver
disease or severe malnutrition are more sensitive, whereas
children under 9–12 years of age are more resistant than their
adult counterparts. Toxic doses vary; in adults, doses of less
than 125 mg/kg are rarely toxic unless the patient has preex-
isting liver disease or malnutrition. Doses of 125–250 mg/kg
produce variable toxicity, with some patients developing sig-
nificant liver damage at these levels. Doses over 250 mg/kg
commonly place the patient at risk to develop massive
hepatic necrosis and liver failure. Patients with severe hepa-
totoxicity eventually die from massive liver failure 4–18 days
after ingestion. Among patients who recover, liver enzymes
begin to normalize 5 days after ingestion, and full recovery
occurs within 3 months. Chronic liver disease after aceta-
minophen ingestion is extremely rare in patients who were
healthy prior to ingestion.
Clinical Features
A. History—The treating physician must be aware of the
potential for acetaminophen toxicity in virtually all patients
with intentional overdoses because of the widespread avail-
ability of this compound in combination preparations. In
addition, patients may unintentionally take excessive
amounts of acetaminophen to treat themselves for painful
conditions, not knowing that this drug may be toxic.
B. Symptoms and Signs—Despite ingesting potentially
lethal amounts of the agent, patients with acetaminophen
overdose often are asymptomatic or minimally symptomatic
CHAPTER 36
772
in the first 24 hours. GI complaints such as nausea and
vomiting are common in large overdoses but may not be
seen in all serious overdoses. Patients also may be somewhat
lethargic and diaphoretic. Twenty-four to forty-eight hours
after ingestion, the patient feels well; however, hepatotoxicity
begins during this time, and levels of hepatic enzymes begin
to rise. Three to four days after significant ingestion, the
patient presents with progressive hepatic damage, nausea,
vomiting, jaundice, right upper quadrant pain, asterixis,
lethargy, coma, and bleeding, and hypoglycemia may develop.
C. Laboratory Findings—The single most important labo-
ratory test in patients who present after acetaminophen
ingestion is the acetaminophen level. A serum sample
should be drawn at least 4 hours after an acute single inges-
tion; levels drawn before this 4-hour time delay are unreli-
able in predicting toxicity. The acetaminophen treatment
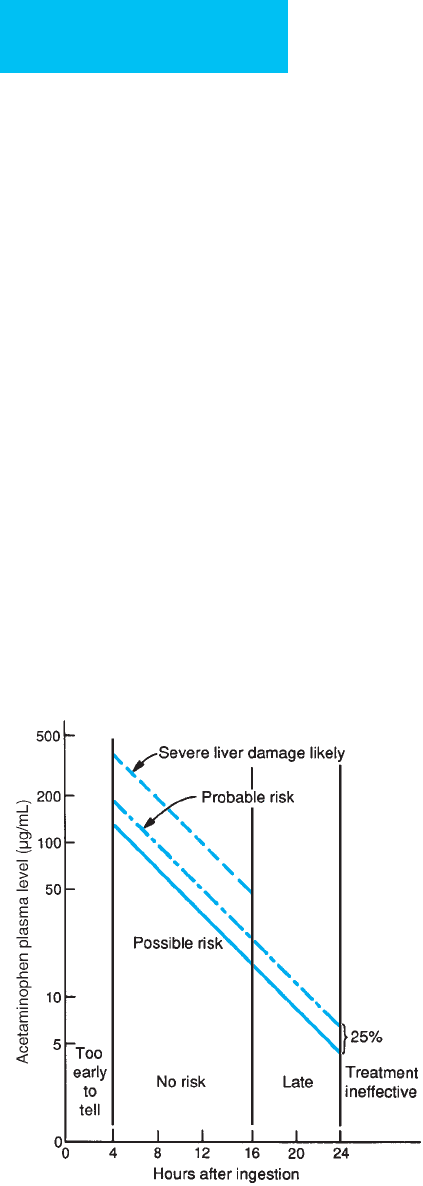
protocol nomogram (Figure 36–1) is used to ascertain
patient risk. In general, patients who have a 4-hour level of
over 150 μg/mL should be regarded as toxic and treated
accordingly.
Patients with significant ingestions should have their liver
enzymes and coagulation studies checked at least once every
12–24 hours and should be monitored closely for hypo-
glycemia, which is common among those with hepatotoxicity.
Differential Diagnosis
Liver failure may be caused by a number of other toxins, most
commonly chronic ethanol abuse. Acute ingestions such as
cyclopeptide toxicity following mushroom ingestion also
cause liver damage. Shock liver and massive hepatic necrosis
from hepatitis also may produce a similar clinical picture.
Treatment
A. Decontamination—All patients who present within 1 hour
of ingesting a potentially lethal amount of acetaminophen-
containing medications should be considered for gastric
emptying. Use of activated charcoal is somewhat controver-
sial because activated charcoal binds and prevents the
absorption of both acetaminophen and its antidote, acetyl-
cysteine (N-acetylcysteine). The charcoal may bind up to
40% of the acetylcysteine. However, increasing the acetylcys-
teine dose can provide adequate absorption despite the use of
charcoal. Therefore, if charcoal is used, the acetylcysteine dose
should be adjusted accordingly.
Patients who present more than 4–6 hours after inges-
tion already have absorbed the acetaminophen, making
gastric decontamination and charcoal administration rela-
tively ineffective. It should be noted, however, that patients
may have ingested other medications along with the aceta-
minophen that may respond to repeated-dose charcoal
treatment if the ingested agent is one that can be recovered
in this way.
B. Acetylcysteine—To date, there are no reliably effective
methods for enhancing the elimination of acetaminophen
already absorbed from the gut. Therefore, antidote therapy
is the mainstay of treatment for patients with potentially
toxic ingestions. Acetylcysteine is indicated in patients who
have toxic levels, as determined by the treatment nomo-
gram, and in those who may have ingested toxic amounts
of acetaminophen and present 8 hours or more after inges-
tion (see Figure 36–1). Acetylcysteine is most effective
when given within the first 8 hours after ingestion; after
that time, efficacy decreases with increasing delay. There is
no benefit to giving acetylcysteine in the first 4 hours after
ingestion, so treating the patient with acetylcysteine can
wait until the acetaminophen level is known if this infor-
mation will be available within 8 hours of ingestion. If the
result will be delayed beyond the 8-hour window and the
patient ingested a significant amount of acetaminophen,
acetylcysteine therapy should be started empirically and
can be discontinued if the level obtained falls in the non-
toxic range.
Acetylcysteine usually is given orally. The loading dose is
140 mg/kg, and subsequent doses of 70 mg/kg then are given
every 4 hours, typically for a total of 17 doses. A 2-day regi-
men also has been used successfully in selected patients.
Patients with acetaminophen toxicity may have significant
nausea and vomiting, which may make oral administration
of acetylcysteine difficult. As a rule, patients who vomit
Figure 36–1. Acetaminophen treatment protocol.
(Adapted from Rumack BH et al: Acetaminophen overdose:
662 cases with evaluation of oral acetylcysteine treatment.
Arch Intern Med 1981;141:382 [PMID: 7469629].)
POISONINGS & INGESTIONS
773
within 1 hour of receiving their oral acetylcysteine dose
should have that dose repeated. In addition, several measures
can be taken to minimize this difficulty. Antiemetics (eg,
prochlorperazine, metaclopramide, or ondansetron) should
be used. If they prove inadequate, the acetylcysteine may be
administered via an NG tube.
Some patients with toxic acetaminophen levels may have
persistent vomiting despite the preceding measures, preclud-
ing enteric administration of acetylcysteine. In this situation,
intravenous acetylcysteine is indicated and can be lifesaving.
Recently, an intravenous formulation of N-acetylcysteine was
approved for use in the United States. Dosing is the same as
for the oral route.
Common complications of intravenous acetylcysteine
treatment are rashes and urticaria. Serious reactions are rare.
Complications are minimized when acetylcysteine is deliv-
ered in at least 250 mL of diluent and infused over 1 hour.
C. Other Measures—Supportive care, including administra-
tion of vitamin K and lactulose, is indicated in patients with
coagulopathy or encephalopathy, respectively. In severe cases
refractory to supportive care, liver transplantation may be
necessary.
Current Controversies and Unresolved Issues
Administration of acetylcysteine beyond 24 hours after acet-
aminophen ingestion has been shown to have little effect.
Despite this information, and because there are few treat-
ment options other than supportive care and liver transplan-
tation in severely toxic cases, acetylcysteine probably should
be used in these situations. The duration of treatment is also
controversial. Currently, a 72-hour course is considered the
standard of care, but studies have shown that a 48-hour
course may be just as effective.
American College of Emergency Physicians Clinical Policies
Subcommittee (Writing Committee) on Critical Issues in the
Management of Patients Presenting to the Emergency
Department with Acetaminophen Overdose, Wolf SJ et al:
Clinical policy: critical issues in the management of patients pre-
senting to the emergency department with acetaminophen over-
dose. Ann Emerg Med 2007;50:292–313. [PMID: 17709050]
Broughan TA, Soloway RD: Acetaminophen hematoxicity. Dig Dis
Sci 2000;45:1553–8. [PMID: 11007105]
Dart RC et al. Acetaminophen poisoning: An evidence-based con-
sensus guideline for out-of-hospital management. Clin Toxicol
(Phila) 2006;44:1–18. [PMID: 16496488]
Dawson AH, Whyte IM: Therapeutic drug monitoring in drug over-
dose. Br J Clin Pharmacol 2001;52:97–102S. [PMID: 11564057]
Gunn VL et al: Toxicity of over-the-counter cough and cold med-
ications. Pediatrics 2001;108:E52. [PMID: 11533370]
Gyamlani GG, Parikh CR: Acetaminophen toxicity: suicidal vs
accidental. Crit Care 2002;6:155–9. [PMID: 11983042]
Larson AM et al. Acetaminophen-induced acute liver failure:
Results of a United States multicenter, prospective study.
Hepatology 2005;42:1364–72. [PMID: 16317692]
Salicylates
ESSENTIALS OF DIAGNOSIS
Nausea and vomiting.
Tinnitus.
Diaphoresis.
Hyperventilation.
Confusion and lethargy.
Convulsions and coma.
Cardiovascular failure.
General Considerations
Salicylates are used widely as antipyretics, analgesics,
antiplatelet agents, and anti-inflammatories; salicylates are
also found in topical preparations used to treat sore joints
and muscles. Not only are they available as aspirin preparations,
but they also constitute a common component of other com-
bination medications readily available over the counter.
Another source of salicylates is oil of wintergreen; this for-
mulation contains very large amounts of the drug, with con-
centrations as high as 7 g per teaspoon (compared with
325–650 mg per tablet in most aspirin preparations).
When taken orally, salicylates are absorbed rapidly from
both the stomach and the small bowel. Peak blood levels
occur 2 hours after ingestion of a normal dose. In therapeu-
tic doses, salicylates undergo hepatic metabolism and renal
excretion with a half-life of 4–6 hours. In the event of an
overdose, the hepatic enzymes become saturated, and metab-
olism changes from first-order (concentration-dependent)
to zero-order (concentration-independent) kinetics. Under
these circumstances, the drug’s half-life increases dramati-
cally to 18–36 hours. In the event of an overdose, renal excre-
tion of the unchanged salicylate becomes the major pathway
of drug elimination.
Salicylates produce respiratory alkalosis by directly stim-
ulating the CNS respiratory center and by increasing its sen-
sitivity to changes in CO
2
and oxygen concentrations.
Salicylates also uncouple oxidative phosphorylation, which
leads to an increased metabolic rate with a resulting increase
in glucose utilization, oxygen consumption, and heat pro-
duction. Clinical effects of this uncoupling include hypo-
glycemia and fever. Inhibition of enzymatic components of
the Krebs cycle occurs, leading to an increase in pyruvate and
lactate that causes a elevated anion gap metabolic acidosis. As
a result of their stimulatory effects on lipid metabolism, sal-
icylates increase ketone formation.
Clinically, it is important to divide patients with salicylate
toxicity into two groups: those who take the medication on a
chronic basis and those who have taken an acute overdose.
Patients who use aspirin chronically, such as the elderly or
CHAPTER 36
774
patients with arthritis, may present with unintentional over-
dose, and because they may have subtle clinical findings,
these patients are often misdiagnosed. As a result of this mis-
diagnosis, serious sequelae may develop, such as pulmonary
and CNS complications, with a mortality rate that
approaches 25%. The acute overdose group, on the other
hand, ingests this drug intentionally, and the acutely elevated
levels cause these patients to be more symptomatic and
therefore easier to diagnose. Acute ingestions of over 150
mg/kg are commonly associated with symptoms of toxicity.
Pulmonary and neurologic complications are less common
in this group, and the mortality rate is only 2%.
Clinical Features
A. Symptoms and Signs—Patients with mild to moderate sal-
icylate toxicity present with nausea, vomiting, tinnitus,
diaphoresis, and hyperventilation (eg, hyperpnea or tachypnea),
confusion, and lethargy. In cases of severe poisoning, convul-
sions, coma, and respiratory or cardiovascular failure may
occur. These symptoms of coma, seizures, hyperventilation, and
dehydration are more common in patients with chronic poison-
ing and are observed at lower salicylate levels (35–50 mg/dL).
Pulmonary edema, cerebral edema, gastritis with hematemesis,
and hyperpyrexia are observed occasionally.
B. Laboratory Findings—Salicylate levels are important in
the management of these patients. Peak levels after overdose
occur 4–6 hours after ingestion. This peak may be delayed or
prolonged if the patient ingested enteric-coated preparations
or if the patient develops gastric concretions of aspirin after
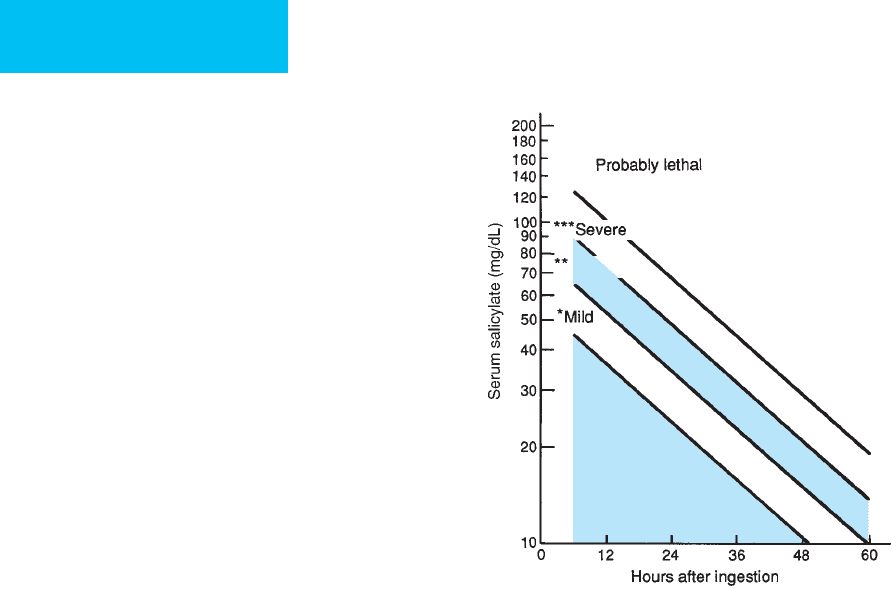
a massive ingestion. The Done nomogram (Figure 36–2)
estimates the severity of acute salicylate toxicity; it does not
apply in the patient with chronic toxicity. Levels obtained
6 hours or more after an acute ingestion can be plotted on the
nomogram and extrapolated to obtain the level of severity.
Common laboratory findings in a patient suffering from
salicylism are an elevated anion gap metabolic acidosis and
respiratory alkalosis. Other laboratory abnormalities may
include a prolonged prothrombin time, thrombocytosis,
hypernatremia, hyper- or hypoglycemia, ketonemia, lactic
acidemia, hypokalemia, and elevated liver transaminases. A
urine Phenistix test is usually positive, as is the ferric chloride
test (5–10 drops of 10% ferric chloride solution added to
urine that has been boiled for 1–2 minutes will turn the solu-
tion a burgundy color). Because coingestions are common,
an acetaminophen level should be obtained.
Differential Diagnosis
Because salicylism often presents with altered mental status
and an increased metabolic state, other entities that cause
this combination should be considered in the differential
diagnosis. Stimulants are the primary toxicologic cause, and
meningitis, sepsis, or encephalitis are possible infectious
sources. Pneumonia, renal failure, diabetic ketoacidosis, and
alcoholic ketoacidosis also should be considered.
Treatment
A. Decontamination—Gastric lavage should be performed
in any patient with an ingestion of over 100 mg/kg within
1 hour before presentation. Gastric lavage also should be
considered in patients who have ingested massive amounts of
salicylates because they are prone to develop intragastric or
intraintestinal concretions; lavage may be helpful as late as
12–24 hours after ingestion in these cases. Activated charcoal
should be given to all patients with salicylate ingestion.
Repeated-dose activated charcoal administration should be
considered in patients with a significant exposure.
B. Alkaline Therapy—Alkalinization is the mainstay of
therapy for salicylate poisoning. It is indicated for patients
with significant acidemia and for those with blood salicylate
levels of over 35 mg/dL. In an alkaline environment, salicy-
lates remain in an ionized form and do not easily diffuse into
tissues. Alkalinization of the urine leads to trapping of the
salicylates in the renal tubules and facilitates excretion. The
goal of treatment is to achieve and maintain the urine pH at
8.0 or above. An adequate serum potassium level is required
before urinary alkalinization can be achieved, and patients
Asynotinatuc
Moderate
Figure 36–2. Nomogram for determining severity of
salicylate intoxication. Absorption kinetics assume acute
(one-time) ingestion of non-enteric-coated preparation.
(Redrawn and reproduced, with permission, from Done AK:
Salicylate intoxication: Significance of measurement of sali-
cylate in blood in cases of acute ingestion. Pediatrics
1960;26:800 [PMID: 13723722].)
POISONINGS & INGESTIONS
775
may require potassium supplementation to ensure adequate
blood levels. Patients receiving bicarbonate therapy should
be evaluated serially for the possible development of cerebral
or pulmonary edema.
C. Hemoperfusion and Hemodialysis—Hemoperfusion
and hemodialysis effectively remove salicylates from the
blood (Table 36–11). Hemodialysis may be preferable
because it also can be used to manage fluid and electrolyte
imbalances. Hemodialysis is specifically indicated for
patients who are deteriorating despite supportive and con-
ventional therapy, those with acute levels over 120 mg/dL if
drawn less than 6 hours after ingestion or 100 mg/dL if
drawn at 6 hours after ingestion despite the clinical presenta-
tion, those with chronic toxicity and levels of 60–70 mg/dL,
those with rising salicylate levels despite attempts at elimina-
tion, those with significant CNS effects, and those with pul-
monary edema or renal or hepatic failure.
D. Other Measures—Patients who develop seizures should
be treated with benzodiazepines or phenobarbital.
Hypotension should be treated with fluids and vasopressors.
Pulmonary edema may develop as a result of capillary dam-
age from salicylates and can be exacerbated by aggressive fluid
therapy. Development of pulmonary edema may require
intubation and mechanical ventilation with positive end-
expiratory pressures. Hemodialysis is frequently required in
these instances.
Chyka PA et al: Salicylate poisoning: An evidence-based consensus
guideline for out-of-hospital management. Clin Toxicol (Phila)
2007;45:95–131. [PMID: 17364628]
Hofman M, Diaz JE, Martella C: Oil of wintergreen overdose. Ann
Emerg Med 1998;31:793–4. [PMID: 9624330]
O’Malley GF: Emergency department management of the
salicylate-poisoned patient. Emerg Med Clin North Am
2007;25:333–46. [PMID: 17482023]
Skelton H et al: Drug screening of patients who deliberately harm
themselves admitted to the emergency department. Ther Drug
Monit 1998;20:98–103. [PMID: 9485563]
Theophylline
ESSENTIALS OF DIAGNOSIS
Nausea and vomiting.
Tachycardia with atrial or ventricular dysrhythmias.
Hypotension.
Agitation, hyperreflexia, seizures.
Hypokalemia.
General Considerations
Although inhalational agents are now the first-line therapy
for reactive airways disease, theophylline is a phosphodiesterase
inhibitor that is still used for the treatment of this disorder.
It comes in several forms, including an elixir and tablets that
are absorbed rapidly. Drug levels usually peak 2–4 hours after
ingestion. Theophylline is also available as a sustained-
release preparation whose serum level peaks anywhere from
6–24 hours after ingestion.
As a phosphodiesterase inhibitor, theophylline produces
an increase in intracellular cAMP, a mediator of β-adrenergic
effects. In addition, at toxic levels, theophylline causes cate-
cholamine release from the adrenal medulla. Therefore, the
result of toxic levels of theophylline is excessive β-stimulation,
and most of the toxic effects are a result of this excessive cat-
echolamine activity. Theophylline toxicity also causes CNS
effects, the mechanism of which is unclear.
Theophylline toxicity, like that of salicylates, causes two
distinct clinical entities depending on whether the toxicity
results from acute ingestion or chronic intake. Acute toxicity
usually results from an intentional overdose in a patient not
already taking the medication. Chronic toxicity is usually an
inadvertent overmedication in a patient already maintained
on this drug. Patients with acute intoxications more com-
monly have metabolic abnormalities and tolerate higher lev-
els of the drug, often not demonstrating serious toxic effects
until the levels reach 80–100 mg/L. Patients with chronic tox-
icity, on the other hand, often do not demonstrate metabolic
abnormalities but may manifest serious toxicity at levels as
low as 40 mg/L. It is also important to note that in the patient
with chronic toxicity, the serum levels often do not correlate
with the severity of toxicity—that is, the patient with what
may appear to be a mildly elevated drug level may have life-
threatening toxic effects. This is particularly true in elderly
patients with chronic theophylline toxicity.
Clinical Features
A. Symptoms and Signs—Theophylline toxicity causes GI,
cardiovascular, CNS, and metabolic abnormalities. As a result
of local gastric irritation and central effects, patients often
complain of nausea and vomiting. This is not universal,
Acute ingestion with levels over 120 mg/dL if drawn <6 hours after
ingestion, or over 100 mg/dL if drawn ≥6 hours after ingestion
Chronic toxicity with a salicylate level of 60–70 mg/dL
Deterioration despite conventional therapy
Significant central nervous system toxicity
Renal failure
Hepatic failure
Pulmonary edema
Rising salicylate levels despite attempts at decontamination
and elimination
Table 36–11. Indications for hemodialysis in salicylate
therapy.

CHAPTER 36
776
however, and patients may have other life-threatening toxic
effects without GI complaints.
Owing to the excessive β-stimulation, patients are often
tachycardic and prone to ventricular tachydysrhythmias.
Atrial dysrhythmias, including atrial fibrillation and multifo-
cal atrial tachycardia, are also seen. As a result of the stimula-
tion of peripheral β
2
-adrenergic receptors and vasodilation,
these patients may develop hypotension. Decreased diastolic
pressure may be a warning sign that severe vasodilation is
developing.
Patients with theophylline toxicity demonstrate agitation,
hyperreflexia, and tremulousness. Seizures may develop and
are often the first CNS sign of toxicity; this is frequently the
case in patients with chronic toxicity. Seizures may be focal or
generalized and are often prolonged; status epilepticus is not
uncommon. Seizures may be refractory to anticonvulsant
therapy and can result in permanent brain damage or death.
B. Laboratory Findings—Hypokalemia is common with
acute ingestions; it occurs as a result of a theophylline-
induced intracellular shift of potassium. Theophylline toxic-
ity also causes hyperglycemia, metabolic acidosis, respiratory
alkalosis, and leukocytosis.
Differential Diagnosis
In addition to theophylline, toxic causes of altered mental
status, seizures, and cardiovascular abnormalities include tri-
cyclic antidepressants, anticholinergic agents, and phenoth-
iazines. Rarely, calcium channel blockers, β-blockers, and
overdoses of local anesthetic agents cause these findings.
Nontoxic causes include meningitis, sepsis, anaphylaxis, head
trauma, and hypoglycemia.
Treatment
A. General Measures—Basic supportive measures such as
intravenous access, hemodynamic monitoring, oxygen
administration, and airway management (as needed) should
be the first priority.
B. Correction of Hypotension—Hypotension should be
treated initially with infusion of crystalloid as a bolus of
250–500 mL over several minutes, repeated as necessary. If
infusions of balanced salt solution do not correct the
hypotension, or if the patient cannot tolerate the volume,
pressors may be necessary. Pure α-agonists such as phenyle-
phrine are preferable to pressors with beta-effects that may
exacerbate theophylline toxicity. Propranolol also may be
used to treat the hypotension in patients who do not have
contraindications to using this drug. The mechanism for the
efficacy of propranolol lies in the fact that it blocks the
peripheral β
2
-adrenergic receptors that participate in the
peripheral vasodilation of theophylline toxicity.
C. Antiarrhythmics—Patients who have severe supraven-
tricular dysrhythmias from theophylline toxicity (eg, severe
sinus tachycardia, supraventricular tachycardia, or multifocal
atrial tachycardia) may be treated with verapamil or β-
blockers if there are no contraindications to using these
drugs. Ventricular dysrhythmias should be treated by cor-
recting hypokalemia and administering lidocaine.
D. Anticonvulsants—Seizures should be treated with ben-
zodiazepines, phenobarbital, or phenytoin singly or in com-
bination. Seizures accompanying theophylline toxicity are
frequently refractory to treatment. It may be necessary to
either place the patient under general anesthesia or use neu-
romuscular blockade to prevent acidosis and rhabdomyoly-
sis and to facilitate ventilation. Patients still may have
electrical seizure activity despite being anesthetized or para-
lyzed. Electroencephalographic monitoring should be used
to make this determination.
E. Decontamination—Once the patient is stabilized hemo-
dynamically, prevention of further absorption of the drug is
the next goal. Patients who present within the first hour after
ingestion should be considered for gastric lavage; because
seizures may be precipitous, intubation is often indicated
before lavage if the patient manifests signs of significant tox-
icity. If the patient ingested a sustained-release form of theo-
phylline, lavage should be considered as long as 3–4 hours
after ingestion.
Charcoal administration is pivotal in treating patients
with theophylline toxicity. All patients, regardless of the time
since ingestion, should receive activated charcoal, in a dose of
either 1–2 g/kg or 10 g of charcoal for every gram of theo-
phylline ingested. This can be given orally or via the lavage
tube. For patients who cannot drink the charcoal slurry, an
NG tube should be placed and the charcoal delivered directly
into the stomach. Serial charcoal dosing is a mainstay in the
treatment of theophylline toxicity. Charcoal avidly binds
theophylline, making this treatment akin to “gastrointestinal
dialysis.” Doses of 0.5–1 g/kg of charcoal every 2 hours dra-
matically decrease the half-life of theophylline. To prevent GI
fluid and electrolyte losses, it is essential not to give cathartics
with each dose of the charcoal. Cathartics should be given
with the first dose of charcoal only, and subsequent doses
should be a slurry of the charcoal only. If the serial charcoal
needs to be continued for more than 24 hours, cathartics can
be given once or twice daily as needed. If the patient has per-
sistent vomiting that precludes charcoal administration,
metoclopramide or ondansetron can be given intravenously.
In these patients, the charcoal may be better tolerated when
given as a continuous administration of 0.25–0.5 g/kg per
hour via an NG tube.
Despite appropriate treatment, some patients with theo-
phylline toxicity continue to have dysrhythmias, hypoten-
sion, and seizures. Following an acute ingestion, serum levels
of more than 90–100 mg/L are associated with more serious
toxic effects. After chronic intoxication, this toxic level is
approximately 60 mg/L. When drug concentrations reach
these levels, hemoperfusion or hemodialysis may be indi-
cated. Table 36–12 lists the major indications for initiating
hemodialysis or hemoperfusion therapy in patients with
