Bongard Frederic , Darryl Sue. Diagnosis and Treatment Critical Care
Подождите немного. Документ загружается.


RENAL FAILURE
327
B. Laboratory Findings—In the absence of detectable red
blood cells in the urine, a urinalysis dipstick positive for
heme is virtually diagnostic of hemoglobinuria or myoglo-
binuria. Similarly, a highly positive heme reaction (3+ or 4+)
in the face of minimal hematuria (3–5 red cells/high-power
field [hpf]) is equally suspicious. Since myoglobin is suffi-
ciently small to be filtered, patients with myoglobinuria have
brown urine, but the serum is clear. In contrast, hemoglobin-
uria also may produce a dark brown urine, but the spun
serum sample is pink because haptoglobin-bound hemoglo-
bin is a complex that is too large to be filtered. Further con-
firmation of the diagnosis is seen in serum chemistry values.
Rhabdomyolysis is associated with elevated creatine kinase
(CK) and other muscle-derived enzymes released into the
circulation, including aspartate aminotransferase (AST), lac-
tate dehydrogenase (LDH), and aldolase. Of these, aldolase is
specific for muscle damage. Occasionally, creatinine released
from damaged muscle leads to an abnormally low BUN:cre-
atinine ratio (<10:1). Severe hemolysis capable of producing
renal failure is usually associated with detectable free hemo-
globin levels above 25 mg/dL and a marked decrease in free
haptoglobin. As with rhabdomyolysis, elevated LDH and
AST may be present; thus these abnormalities do not permit
distinction between the two disorders.
C. Imaging Studies—Imaging studies are not helpful in
diagnosis of pigment nephropathy other than in evaluating
the extent of injury in patients with traumatic rhabdomyol-
ysis. In patients with serious trauma with renal failure, how-
ever, injury to the kidneys, ureters, bladder, or urethra should
be considered, and ultrasound, CT scan, or IVP may be help-
ful in distinguishing direct injury from rhabdomyolysis.
Treatment
The single most important therapeutic maneuver is to
restore circulating volume rapidly and initiate diuresis. If
oliguria is present, a single dose of mannitol, 12.5 g intra-
venously, can be given to promote diuresis. If successful,
mannitol can be continued as a 5% infusion. Alternatively,
furosemide, 40–200 mg intravenously, can be given and
repeated every 6 hours if necessary. Forced diuresis with
aggressive hydration should strive for a urine output of at
least 100 mL/h and should be maintained until levels of cre-
atine kinase or haptoglobin begin to normalize.
Alkalinization of the urine is recommended by some, but
there is no definitive evidence that it is necessary.
Rhabdomyolysis is commonly associated with several
potentially troublesome electrolyte disorders. Hypocalcemia
may be seen early, probably as a consequence of precipitation
of calcium salts owing to associated hyperphosphatemia.
Despite extremely low levels of serum calcium, tetany is
uncommon, perhaps owing to the associated acidosis. Severe
hyperuricemia also may be encountered. Hyperkalemia,
sometimes extremely difficult to control, may be encoun-
tered with both rhabdomyolysis and hemolysis. In contrast,
crush victims who have received early and vigorous fluid
resuscitation and who present with polyuria may develop
hypokalemia. Hypercalcemia during the recovery phase may
be the result of calcium release from healing muscle and an
increase in 1-25 dihydroxyvitamin D.
Better OS, Stein JH: Early management of shock and prophylaxis
of acute renal failure in traumatic rhabdomyolysis. N Engl J
Med 1990;322:825–9. [PMID: 2407958]
Gunal AI et al: Early and vigorous fluid resuscitation prevents
acute renal failure in the crush victims of catastrophic earth-
quakes. J Am Soc Nephrol 2004;15:1862–7. [PMID: 15213274]
Malinoski DJ, Slater MS, Mullins RJ: Crush injury and rhabdomy-
olysis. Crit Care Clin 2004;20:171–92. [PMID: 14979336]
2. Pulmonary Renal Syndromes
Any type of acute renal failure can present with fluid over-
load and pulmonary edema, but there are several conditions
in which simultaneous involvement of both lung and kidneys
is a common presentation or an intrinsic part of the basic
pathogenesis (Table 13–8). Of these, two are of particular
importance because their rapid identification and subse-
quent treatment can be lifesaving: Goodpasture’s syndrome
and paraquat intoxication.
Goodpasture’s Syndrome
Goodpasture’s syndrome is an autoimmune disease charac-
terized by the formation of anti-GBM antibodies that attack
Disease Renal Involvement
Goodpasture’s syndrome RPGN
Wegener’s granulomatosis RPGN
Systemic lupus erythematosus RPGN
Churg-Strauss syndrome RPGN
Sarcoidosis Interstitial nephritis
Scleroderma Hypertensive renal crisis
Pulmonary embolism Prerenal azotemia
Pneumonia Prerenal azotemia
Poisons (paraquat) Actue tubular necrosis
Congestive heart failure Prerenal azotemia
Adult respiratory distress
syndrome
Acute tubular necrosis, prerenal
azotemia
Key: RPGN = rapidly progressive glomerulonephritis; ATN = acute
tubular necrosis
Table 13–8. Pulmonary-renal syndromes causing acute
renal failure.
CHAPTER 13
328
both the pulmonary capillary and the glomerulus. The clas-
sic clinical presentation is that of a young male smoker with
signs of acute glomerulonephritis (ie, hematuria, protein-
uria, and red blood cell casts) and hemoptysis associated
with bilateral pulmonary infiltrates. Iron deficiency anemia
is also a frequent finding.
The diagnosis of Goodpasture’s syndrome can be made
by detecting serum levels of anti-GBM antibodies or by lin-
ear immunofluorescence of the GBM on renal biopsy.
Unfortunately, availability of these confirmatory tests may be
delayed, and rapid initiation of treatment often depends on a
high degree of suspicion. Early initiation of treatment is
essential because therapeutic success in reversing renal fail-
ure is rare if the therapy is started after the onset of oliguria.
Aside from smoking, genetic predisposition and hydrocar-
bon exposure have been identified as associated risk factors.
Both life-threatening hemoptysis and rapidly progressive
renal failure can be treated successfully with a combination
of corticosteroids, cyclophosphamide, and 2 weeks of daily
plasma exchange.
Pusey CD: Anti-glomerular basement membrane disease. Kidney
Int 2003;64:1535–50. [PMID: 12969182]
Pusey CD: The continuing challenge of anti-neutrophil cytoplasm
antibody-associated systemic vasculitis and glomerulonephritis.
J Am Soc Nephrol 2006;17:1221–3. [PMID: 16624927]
Paraquat Poisoning
Paraquat is a herbicide used in concentrated solutions.
Ingestion is highly corrosive for the oral and esophageal
mucosa, and net absorption results in pulmonary edema and
anuria. Effective treatment involves gastric lavage and aggres-
sive use of charcoal adsorbents. Daily treatments with char-
coal hemoperfusion or high-efficiency hemodialysis may be
helpful in lowering paraquat levels. There is also reason to
believe that low oxygen tension may limit lung injury.
Consultation with a poison control expert should be sought.
Web site information can be obtained from the national pes-
ticide information network at http://ace.orst.edu/info/nptn/
Van Vleet TR, Schnellmann RG: Toxic nephropathy: Environmental
chemicals. Semin Nephrol 2003;23:500–8. [PMID: 13680539]
3. Hepatorenal Syndrome
Conditions associated with combined hepatic and renal fail-
ure include infections (eg, sepsis, hepatitis, and leptospiro-
sis), drug toxicity (eg, acetaminophen, allopurinol, rifampin,
and methoxyflurane), poisons (eg, rodenticide and carbon
tetrachloride), and autoimmune disorders (eg, systemic
lupus erythematosus, vasculitis, and cryoglobulinemia).
More common, however, is prerenal azotemia associated
with cirrhosis. Patients presenting with this combination
often have signs of advanced liver disease, hypoalbuminemia,
and ascites. Under these conditions, if prerenal azotemia
becomes unresponsive to intravascular volume replacement,
a diagnosis of hepatorenal syndrome becomes appropriate.
This syndrome is a condition of severe renal vasoconstriction
presenting with extremely low urinary sodium (<10 meq/L),
decreased FE
urea
(<20%), an extremely high urine:plasma
creatinine ratio, and a relatively unimpressive urine sediment
showing bile-pigmented casts.
Until recently, hepatorenal syndrome was considered to
be almost universally fatal, rendering preventive measures as
the most important management option. Most patients with
hepatorenal syndrome develop the problem during hospital-
ization. GI hemorrhage, bacterial peritonitis, and the
overzealous use of diuretics to control ascites all have been
shown to be associated with the precipitation of the hepa-
torenal syndrome. Thus it is crucial that all patients present-
ing with liver disease and prerenal azotemia receive
aggressive volume repletion. In many cases, pulmonary
edema and ascites may be limiting factors in repletion; cen-
tral venous pressure or pulmonary artery catheterization
may be required to adequately judge how much fluid can be
administered safely. With increasing central venous pressure,
there also will be the increased risk of variceal hemorrhage.
In patients with peripheral edema associated with hypoalbu-
minemia, albumin infusions can successfully mobilize fluid
into the intravascular space, but ascites formation increases
in approximately one-third of patients. In the same vein,
high-volume paracentesis should be performed in conjunc-
tion with intravenous albumin infusions to minimize the
tendency for intravascular volume depletion.
Successful reversal of well-established hepatorenal syn-
drome is rare, but several new treatment strategies appear to
offer some success. The combined use of midodrine (an α
1
-
adrenergic agonist) and octreotide (a somatostatin analogue)
may improve renal hemodynamics and subsequent survival.
The use of vasopressin analogues in conjunction with albu-
min infusions also may improve renal perfusion, but there is
also the risk of ischemia. Interestingly, since the hepatorenal
syndrome is a result of a severe renal vasoconstriction, the
renal parenchyma may remain intact, and liver transplanta-
tion has been associated with return of renal function.
Recently, there have been reports in Europe of successful
treatment of hepatorenal syndrome with albumin-based
dialysis (molecular adsorbent recycling system [MARS]).
Cardenas A, Gines P: Hepatorenal syndrome. Clin Liver Dis
2006;10:371–85. [PMID: 16971267]
Gluud LL et al: Terlipressin for hepatorenal syndrome. Cochrane
Database Syst Rev 2006;4:CD005162. [PMID: 17054242].
Mitzner SR et al: Improvement of hepatorenal syndrome with
extracorporeal albumin dialysis MARS: Results of a prospective,
randomized, controlled clinical trial. Liver Transplant
2000;6:277–86. [PMID: 10827226]
Moreau R, Lebrec D: The use of vasoconstrictors in patients with
cirrhosis: Type 1 HRS and beyond. Hepatology 2006;3:385–94.
[PMID: 16496352]
RENAL FAILURE
329
Moreau R, Lebrec D: Diagnosis and treatment of acute renal fail-
ure in patients with cirrhosis. Best Pract Res Clin Gastroenterol
2007;21:111–23. [PMID: 17223500]
Wong F, Pantea L, Sniderman K: Midodrine, octreotide, albumin,
and TIPS in selected patients with cirrhosis and type 1 hepatore-
nal syndrome. Hepatology 2004;40:55–64. [PMID: 15239086]
4. Renal Failure in AIDS
Acute renal failure occurs in more than 50% of hospitalized
patients with AIDS. The combination of volume depletion,
nephrotoxic medications, and sepsis accounts for most cases
(Table 13–9), but most AIDS patients presenting with acute
renal failure can recover renal function and be discharged
from the hospital.
Intravascular volume depletion can result from poor fluid
intake, fever, and GI disturbances or may be secondary to
hypoalbuminemia owing to nephrotic syndrome or malnutri-
tion. Enlarged periaortic lymph nodes can obstruct lower
extremity venous return, resulting in severe leg edema but
insufficient central venous pressure. Careful attention to
restoring intravascular volume not only reverses prerenal
azotemia but also will serve to reduce substantially the risk of
nephrotoxicity associated with medications and radiocontrast
agents. In many cases of drug-induced renal failure, renal
function may return rapidly after termination of exposure to
the drug, such as can be seen with NSAIDs and the crystalluria
associated with indinavir and acyclovir. In other cases, a full-
blown syndrome of acute tubular necrosis may develop; this
disorder may require dialytic therapy before recovery.
A renal syndrome that seems to be unique to
patients with AIDS is rapidly progressive focal segmental
glomerulosclerosis. This syndrome is most common in
patients of African descent and is associated with nephrotic-
range proteinuria (>3 g/day), enlarged, hyperechogenic kid-
neys on renal ultrasound, and—in contrast to focal
segmental glomerulosclerosis in the non-AIDS population—
normotension. Progression of this type of renal disease can
be explosive, reaching a stage of irreversible renal failure in
weeks or months. Recent experience, however, suggests that
the new highly active antiretroviral therapy (HAART) regi-
mens can slow the progression of this type of AIDS
nephropathy.
Standard precautions during hemodialysis are employed
to limit transmission of HIV and hepatitis B. In addition,
HIV has been identified in peritoneal dialysate from patients
with AIDS, and this effluent should be handled with appro-
priate precautions.
Hyun G, Lowe FC:. AIDS and the urologist. Urol Clin North Am
2003:30;101–9. [PMID: 12580562]
Khan S, Haraqsim L, Laszik ZG et al: HIV-associated nephropathy.
Adv Chron Kidney Dis 2006;13:307–13. [PMID: 16815235]
Kimmel PL, Barisoni L, Kopp JB: Pathogenesis and treatment of
HIV-associated renal diseases: Lessons from clinical and animal
studies, molecular pathologic correlations, and genetic investi-
gations. Ann Intern Med 2003;139:214–26. [PMID: 12899589]
Wyatt CM, Klotman PE: HIV-associated nephropathy in the era of
antiretroviral therapy. Am J Med 2007;120:488–92. [PMID:
17524746]
5. Renal Failure in the Renal Transplant
Recipient
Acute renal failure in the transplant recipient can be arbitrar-
ily defined as being early (<10 days after transplantation) or
late (>10 days). In the early period, acute renal failure may be
due to ischemic acute tubular necrosis of the transplanted
graft, acute or hyperacute immunologic rejection, or techni-
cal problems related to the surgery (eg, obstruction, leak, or
infection of the renal artery or vein or of the ureter). Acute
drug toxicity from cyclosporine can present as prerenal
azotemia associated with hypertension and is encountered
most often with relatively elevated intravenous dosing.
Acute renal failure in the late period may be the result of
acute rejection, cyclosporine toxicity, ureteral obstruction,
renal artery stenosis, or recurrence of the original renal dis-
ease. Acute rejection may present with fever, graft tenderness,
and swelling where the kidney is implanted into the iliac
fossa, but concurrent immunosuppressive therapy with
steroids or cyclosporine may mask these signs. If the history,
physical examination, and imaging studies (ie, ultrasound
and nuclear flow scans) cannot distinguish the cause of renal
failure, renal biopsy may be required. In any event, prompt
and continued consultations with the patient’s transplant sur-
geon and nephrologist are essential to proper management.
The intensivist is often in a position to identify potential
organ donors. Acceptable donors for renal transplantation
Table 13–9. Causes of acute renal failure associated with
AIDS.
Prerenal azotemia
Volume depletion, hypoalbuminemia, NSAIDs
Acute tubular necrosis
Pentamidine, amphotericin B, aminoglycosides, foscarnet, acyclovir,
radiocontrast agents, sepsis, shock
Allergic interstitial nephritis
Trimethoprim-sulfamethoxazole, phenytoin
Rapidly progressive focal segmental glomerulosclerosis
Obstructive uropathy
Sulfadiazine-related crystalluria, lymphoma, retroperitoneal fibrosis
Nephrolithiasis
Indinavir-induced crystalluria
Thrombotic thrombocytopenic purpura/hemolytic uremic syndrome
Renal edema
Hypoalbuminemia with massive proteinuria
Multiple myeloma
Acute glomerulonephritis
CHAPTER 13
330
are aged 6 months to 60 years with no identifiable renal dis-
ease, no active infection, and no malignancy (except brain
tumors). Physicians working in the ICU should familiarize
themselves with local laws and policies regarding the pro-
curement of organs and the criteria for brain death.
Kasiske BL et al: Recommendations for the outpatient surveillance
of renal transplant recipients. American Society of
Transplantation. J Am Soc Nephrol 2000;11:S1–86. [PMID:
11044969]
Silkensen JR: Long-term complications in renal transplantation. J
Am Soc Nephrol 2000;11:582–8. [PMID: 10703683]
Venkat KK, Venkat A: Care of the renal transplant recipient in the
emergency department. Ann Emerg Med 2004;44:330–41.
[PMID: 15459617].
NONDIALYTIC THERAPY FOR ACUTE RENAL
FAILURE
Fluid Balance
Achieving the appropriate fluid balance in the setting of an
ICU involves two potentially conflicting goals: providing suf-
ficient volume to ensure adequate renal perfusion and avoid-
ing volume overload with resulting pulmonary congestion.
In some patients, when decreased renal perfusion is sus-
pected in the context of pulmonary compromise, only a pul-
monary artery catheter will yield sufficient information to
guide appropriate therapy. Optimal fluid management for
acute renal failure can be divided arbitrarily into three peri-
ods: (1) the prevention phase, during the initial onset of olig-
uria, (2) the oliguric phase, once renal failure is well
established, and (3) the recuperative phase, often heralded by
relative polyuria. Not all episodes of acute renal failure pass
through each of these phases, but a discussion of their man-
agement is a useful guide to overall treatment goals.
Prevention Phase
On initial presentation, the onset of oliguria should call for
prompt evaluation of the type of renal dysfunction. If vol-
ume depletion is suspected, rapid restoration of circulating
volume may prevent ischemic damage. Restoration of ade-
quate circulating volume can be achieved by administration
of crystalloid or colloid. If simple fluid repletion is required,
normal saline usually suffices. In the context of hypoalbu-
minemia and edema, intravenous albumin can serve to draw
excess extravascular fluid into the circulation.
If oliguria persists despite adequate fluid replacement and
acute tubular necrosis is suspected, a short trial of diuretic
therapy may offer some benefit in limiting renal damage. A
single intravenous 12.5-g dose of mannitol may initiate
diuresis and has the theoretical advantage of limiting epithe-
lial cell swelling and intratubular precipitation of cellular
debris. If initially unsuccessful, further mannitol administra-
tion is potentially harmful in that it may lead to poorly
tolerated intravascular expansion. Alternatively, furosemide
may be given intravenously at a dose of 200 mg (1-hour infu-
sions are preferable to more rapid injection) and may be
repeated safely within 6 hours. Dosages above 1 g/day have
been associated with ototoxicity. If, despite the preceding
maneuvers, oliguria persists more than 24 hours, diuretics
should be discontinued, and the physician should be pre-
pared to manage a potentially prolonged period of oliguria.
Oliguric Phase
The oliguric phase of acute renal failure may persist for sev-
eral weeks. During this period, especially in the critical care
setting, the patient may require enormous amounts of intra-
venous fluids, including hyperalimentation, vasopressors,
and antibiotics. Every effort should be made to minimize the
volume of these infusions. Continuous infusions of vaso-
pressors should be maximally concentrated, and antibiotics
should be given in minimum volumes of fluid. When given
in adequate amounts, parenteral hyperalimentation always
requires 1–2 L/day. Thus enteral alimentation is always pre-
ferred when possible.
Proper maintenance of fluid balance requires careful
attention to all avenues of fluid intake and output, including
surgical drains, nasogastric suction, and diarrhea. Normal
insensible losses can equal 1000 mL/day but can increase
substantially in the presence of fever, burns, or exfoliative
dermatitis. Increased minute ventilation enhances water loss
from the lungs even if the inspired gas is humidified. On the
other hand, metabolism of carbohydrate and fat yields
approximately 500 mL/day of metabolically produced water.
Thus, unless insensible losses are increased, an anuric patient
requires only 500 mL/day of water. Finally, despite meticu-
lous monitoring of fluid input and output, there can be no
substitute for daily weight measurements.
Recuperative Phase
In many patients with acute renal failure, the oliguric phase
is followed by a period of relative polyuria, heralding the
beginning of tubular recuperation. During this period,
serum urea nitrogen and creatinine may remain elevated,
and renal dysfunction persists. One must bear in mind that
the urine produced is not appropriately concentrated and
that reabsorption of sodium and other ions is inadequate.
During this phase, it is the goal of management to replace
lost volume and electrolytes. Urinary sodium and potassium
should be measured and replaced in appropriate amounts.
Abnormalities of magnesium, calcium, and phosphate also
should be anticipated.
Monitoring Fluid Balance
In the presence of active pulmonary disease such as pneumo-
nia, acute respiratory distress syndrome (ARDS), or pul-
monary edema and suspected prerenal azotemia, aggressive
fluid replacement may worsen pulmonary gas exchange.

RENAL FAILURE
331
Furthermore, in patients with vasodilatory shock or hypoal-
buminemia, substantial edema may be present, and still the
patient has insufficient circulating volume. In these settings,
intravascular pressure monitoring becomes invaluable.
Although central venous pressure can be monitored easily
via subclavian, internal jugular, or femoral vein catheteriza-
tion, a more definitive assessment of optimal cardiac filling
pressures can be obtained with pulmonary artery catheteri-
zation. This is because central venous pressure can be
increased by pulmonary hypertension or central venous
thrombosis or occlusion even in a volume-depleted patient.
Similarly, positive-pressure ventilation and, especially, posi-
tive end-expiratory pressure may elevate pulmonary capil-
lary wedge pressure spuriously.
Acid-Base Balance
Uremic acidosis occurs as a result of the slow accumulation
of phosphates and sulfates and is related to protein catabo-
lism. Approximately 1 meq acid is retained for every gram of
protein catabolism. Thus a 70-g protein diet yields approxi-
mately 70 meq acid, which, when distributed into the total
body water compartment, consumes approximately 2 meq
bicarbonate per liter per day. Replacement of this amount of
bicarbonate is relatively easy and can be in the form of intra-
venous sodium bicarbonate, orally administered sodium or
potassium citrate, or as acetate in hyperalimentation solu-
tion (1 meq acetate converts to 1 meq bicarbonate plus some
energy). The patient’s tolerance for the associated sodium
and potassium load should be considered.
Patients with prolonged uremia may present with a sub-
stantial buffer deficit (serum bicarbonate <15 meq/L),
Kussmaul respirations, and associated hypocalcemia. In this
situation, bicarbonate replacement relieves the dyspnea by
correction of acidosis, but the rapid increase in serum pH
may precipitate tetany. Under these conditions, after deter-
mination of serum calcium and phosphate, it may be pru-
dent to administer 1 ampule of calcium gluconate after the
first 50–100 meq of bicarbonate. However, if phosphate lev-
els are particularly elevated, there is a theoretical risk of
metastatic calcifications with calcium administration.
Lactic acidosis is potentially a more difficult problem. In
the presence of insufficient tissue oxygenation, lactic acid
production can exceed 50 meq/h. This amount of acid low-
ers serum bicarbonate at a rate of 1–2 meq/L per hour. In the
face of oliguria, replacement of this amount of bicarbonate
would risk intravascular fluid overload and hypernatremia
and may require dialytic therapy. Therefore, in lactic acido-
sis, an aggressive effort to restore adequate tissue perfusion is
the mainstay of treatment.
Metabolic alkalosis is an uncommon complication of
acute renal failure, but it may occur as a result of massive
losses from gastric suctioning. Decreasing net acid loss can
be achieved by raising gastric pH with H
2
blockers such as
cimetidine or ranitidine or with proton pump inhibitors
such as omeprazole or lansoprazole. If these efforts fail, acid
can be given carefully as arginine hydrochloride or dilute
hydrochloric acid. These treatments can produce severe life-
threatening hyperkalemia and must be given with vigilant
monitoring of potassium levels.
Electrolytes
Sodium
Sodium balance in acute renal failure is maintained by eval-
uation and matching of sodium losses. Urinary losses are
best measured with 24-hour collections but can be reason-
ably assessed by random sampling and multiplication of
sodium concentration by the day’s total urine volume. After
diuretic administration, urinary sodium will be increased for
several hours and may not reflect a constant excretion rate.
GI losses must be either measured (eg, nasogastric suction)
or estimated (eg, diarrhea)(Table 13–10).
In oliguric renal failure, hyponatremia is the most com-
mon electrolyte abnormality and is almost always the result
of excessive free water administration. Since most fluid losses
are lower in sodium when compared with serum (see Table
13–10), a reasonable initial approach is to add 50 meq
sodium to each liter of infused fluid, most notably the hyper-
alimentation fluid. Daily monitoring of serum sodium
allows for readjustment of water and sodium administered as
necessary.
Potassium
Hyperkalemia is the most serious electrolyte abnormality
associated with acute renal failure. Cardiotoxicity, however,
does not correlate strictly with the measured serum potas-
sium and may be encouraged by acidosis, serum calcium
concentration, and medications. The most rapid and reliable
means for assessing cardiotoxicity is to obtain an ECG, which
may show hyperkalemia in the form of peaked T waves, a
prolonged PR interval, diminished to absent P waves, widen-
ing of the QRS complex, prolongation of the QT interval,
Sodium Content of Intravenous Sodium Content of
Infusion Fluids Body Fluids
1 g Na
+
= 43 meq Na
+
Gastric fluid = 30–90 meq
1 g NaCl = 17 meq Na
+
Na+ per liter
1 L of 0.9% NaCl = Diarrhea = 50–110 meq
154 meq Na
+
Na+ per liter
1 L of 0.45% NaCl = Small bowel ostomy = 70–150
77 meq Na
+
meq Na
+
per liter
1 L of Ringer’s lactate = Biliary drainage = 120–170 meq
130 meq Na
+
Na
+
per liter
50 mL of 7.5% NaHCO
3
= Sweat = 20–100 meq Na
+
per liter
44 meq Na
+
Table 13–10. Sodium content: intake and output.

CHAPTER 13
332
and ultimately, a sine wave pattern. Immediate management
requires medications designed to stabilize the myocardial
membrane and promote intracellular movement of potas-
sium. Infusion of calcium, bicarbonate, and insulin with glu-
cose temporarily improves the electrocardiographic
abnormalities. Definitive procedures for removing potas-
sium should be initiated as soon as possible.
In oliguric patients unresponsive to diuretic therapy,
exchange resins of sodium polystyrene sulfonate are the most
efficient means of extracorporeal potassium removal.
Administration can be either orally or by retention enema
for at least 30 minutes (orally: 50 g resin in 150 mL 20% sor-
bitol; by enema: 50 g resin in 200 mL tap water to avoid
sorbitol-induced colonic irritation). These doses can be
repeated every hour as necessary. Each potassium ion
removed will be exchanged for a sodium ion—a situation
that may be poorly tolerated in the hypernatremic or fluid-
overloaded patient. In addition, to avoid the possibility of
forming intraluminal concretions, sodium polystyrene sul-
fonate never should be given concurrently with aluminum
hydroxide (used as a phosphate binder or antacid). When
these measures are poorly tolerated because of fluid overload
or hypernatremia—or when the GI tract is not available for
use—dialytic therapy should be considered.
Maintaining potassium balance requires evaluation of
renal and extrarenal losses (Table 13–11). A normal diet con-
tains approximately 70–100 meq potassium per day, and
dietary potassium restriction to 2 g (50 meq) per day is rea-
sonable for patients with oliguric renal failure.
Calcium
Hypocalcemia often accompanies chronic renal failure and is
the result of hyperphosphatemia and altered vitamin D
metabolism. In acute renal failure, hypocalcemia may be
associated with the hyperphosphatemic phase of rhabdomy-
olysis or may occur during administration of the antiviral
agent foscarnet. Hypercalcemia is seen in multiple myeloma
or hyperparathyroidism. Urinary calcium losses are minimal
during acute renal failure. Frank tetany is rare and is usually
the result of overly aggressive correction of acidosis. Since
hypoalbuminemia and acid-base disturbances are common
in the critically ill patient, ionized calcium levels, when avail-
able, are preferred to total serum calcium.
Magnesium
Magnesium excretion is limited during renal failure, and
magnesium-containing antacids such as Maalox and
Mylanta should be avoided. Magnesium wasting may
occur with amphotericin- or cisplatin-induced renal fail-
ure or during the polyuric recuperative phase of acute
tubular necrosis.
Phosphate
Hyperphosphatemia results from insufficient renal excretion
and secondary hyperparathyroidism. Avoiding hyperphos-
phatemia limits the risk of metastatic calcifications and helps
to maintain normal levels of ionized calcium. Diets should
be limited to 800 mg/day of phosphorus, but even at this
level, oral phosphate binders are required to limit GI absorp-
tion. When serum phosphorus exceeds 6 mg/dL, aluminum
hydroxide antacid can be given at a dosage of 30 mL with
each meal and at night. Sevelamer at a dose of 800–1600 mg
with each meal also can be used as an effective phosphate
binder and has the advantage of not presenting the potential
for aluminum toxicity or the constipation associated with
aluminum hydroxide.
When levels are controlled below 6 mg/dL, calcium car-
bonate at 500 mg with each meal or calcium acetate at 667
mg with each meal can be initiated. Intravenous hyperali-
mentation should be prepared without phosphate until levels
are normalized. However, once normal phosphorus levels are
reached, intravenous hyperalimentation solutions should
contain approximately 100–250 mg phosphorus per day
(5–10 meq sodium or potassium phosphate). In the presence
of renal failure, hypophosphatemia is almost always the result
of prolonged parenteral nutrition devoid of phosphate.
Nutrition
Nitrogen Balance
Several studies have suggested that appropriate nutritional
support can promote renal recovery and improve overall
survival in patients with acute renal failure. Unfortunately,
there is often conflict between giving adequate protein
replacement while at the same time limiting the production
of nitrogenous wastes. Under ideal conditions, an adequate
diet should include 1 g/kg per day of protein and 35–40
kcal/kg per day of carbohydrates and fats. There is no defin-
itive evidence that diets with substantially more than 1.2 g/kg
per day of protein enhance survival rates or reduce morbidity,
Table 13–11. Potassium content: intake and output.
Intake
2 g K
+
in diet = 50 meq
Output
Gastric fluid = 4–12 meq K
+
/L
Abdominal drainage = approximately the serum level
Diarrhea = 10–30 meq K
+
/L
Sorbitol-induced diarrhea = 30–40 meq K
+
/L
Sodium polystyrene sulfonate (Kayexalate) = 1 meq K
+
/g of retained
absorbent per hour
Urine = 5–150 meq K
+
/L
Peritoneal dialysis (2 L/h), CAVH (1 L/h) = 5–10 meq K
+
/h
∗
Hemodialysis = 40–60 meq K
+
/h
∗
∗
Assuming serum [K
+
] = 5 meq/L.
Key: CAVH = continuous arteriovenous hemofiltration

RENAL FAILURE
333
with the possible exception of the patient with extensive
burns. Overly aggressive protein feeding (>2 g/kg per day) is
unwarranted and can lead to abnormally high amino acid
levels and an unnecessary increase in retained nitrogen
wastes. In the very stable patient with minimal net negative
nitrogen balance (<5 g/day), protein intake can be limited to
0.6 g/kg per day. In the nonoliguric patient, this may reduce
the need for dialysis, but careful attention to nitrogen bal-
ance is required.
Nitrogen balance can be evaluated easily by measuring
the rate of urea production, commonly referred to as the urea
nitrogen appearance (UNA). The daily UNA can be measured
by obtaining a 24-hour urine for urea nitrogen measurement
and evaluating the change in BUN occurring at the begin-
ning and end of the 24-hour collection. The UNA then can
be calculated using the following formula:
where UNA equals the daily urea nitrogen appearance in
grams per day, BUN
1
and BUN
2
are the levels of blood urea
nitrogen in milligrams per deciliter at the beginning and end
of the 24-hour urine collection, total body water in liters is
estimated as 60% of lean body mass plus the amount of any
extra edema fluid, and UUN is the urine urea nitrogen
expressed as grams per day.
Under conditions of nitrogen balance, UNA depends on
protein ingestion and can be calculated as follows:
It is assumed that every 6.25 g of protein contains 1 g of
nitrogen and that the production of nonurea nitrogen is
30 mg/day per kilogram of lean body mass. The minimum
amount of urea clearance required to remove a given amount
of UNA can be calculated using the following formula:
When the preceding formulas are used, a 70-kg patient
receiving 1 g/kg per day of protein will receive 11 g nitrogen
(70 g ÷ 6.25). On balance, approximately 2 g nitrogen will
become nitrogenous wastes other than urea; the remaining
9 g will form urea.
On the other hand, many patients with acute renal failure
present with a degree of hypercatabolism. Under these con-
ditions, urea production is greatly enhanced owing to the
catabolism of endogenous proteins, and UNA can greatly
exceed that predicted from exogenous protein administra-
tion alone. For example, under conditions of severe stress,
endogenous protein breakdown can generate 30 g or more of
urea nitrogen per day, representing the catabolism of approx-
imately 200 g protein. In addition to enhanced proteolysis
accompanying hypercatabolism, increased urea production
is often the result of GI bleeding, with the ultimate break-
down and absorption of the blood and its proteins. There are
approximately 200 g protein per liter of whole blood.
Treatment of endogenous hypercatabolism and the nega-
tive nitrogen balance that results is controversial. Although
adequate nutrition is essential, it is often not sufficient to
match the ongoing catabolism. Several studies have demon-
strated that increased protein catabolism associated with
stress is mediated by hormones (eg, glucagon, cate-
cholamines, and cortisol) and cytokines (eg, interleukins,
tumor necrosis factor, etc.). For this reason, overly aggressive
protein administration is not only futile but yields unneces-
sary amounts of nitrogenous waste, thereby increasing the
need for dialysis therapy. Until specific therapy for cytokine
neutralization is available, the most successful strategy will
be to administer the majority of needed calories in the form
of carbohydrates, thus allowing for the maximum adminis-
tration of “anticatabolic” insulin.
Calories
Adequate caloric intake is essential to minimize negative
nitrogen balance and to improve overall survival. In the crit-
ically ill patient with acute renal failure, approximately
35 kcal/kg per day is a reasonable goal. Hyperglycemia
resulting from administration of large amounts of carbohydrate
can be managed with insulin.
Vitamins and Trace Elements
There is no evidence that patients with acute renal failure
have unique requirements for either vitamins or trace ele-
ments. Thus daily minimum requirements should be ade-
quate. Once dialysis is initiated, replacement of water-soluble
vitamins should be assured. In most situations, a standard
multivitamin preparation suffices with the possible excep-
tion of folic acid, which should be replaced at a dosage of at
least 1 mg/day.
Fournier A et al: The crossover comparative trial of calcium acetate
versus sevelamer hydrochloride (Renagel) as phosphate binders
in dialysis patients. Am J Kidney Dis 2000;35:1248–50. [PMID:
10877727]
Strejc JM: Considerations in the nutritional management of
patients with acute renal failure. Hemodial Int 2005;9:135–42.
[PMID: 16191061]
Van den Berghe G et al: Outcome benefit of intensive insulin ther-
apy in the critically ill: Insulin dose versus glycemic control. Crit
Care Med 2003;31:359–66. [PMID: 12576937]
Urea clearance (L/day)
UNA (g/day)
BUN (mg/dL
=
))
× 100
UNA (g/day)
Protei
=
nnintake(g/day)
Nonurea nitrogen (g/d
625.
− aay)
UNA (g/day)
BUN BUN
total body water
21
=
−
×+
100
UUUN
CHAPTER 13
334
DIALYTIC THERAPY FOR THE CRITICALLY
ILL PATIENT
Renal replacement therapy can provide homeostasis of fluid,
electrolyte, acid-base, and nitrogen balance. Consequently,
the initiation of renal replacement therapy should be consid-
ered whenever any of these factors cannot be controlled with
other therapy. At present, three types of renal replacement
treatment are available for the patient with acute renal fail-
ure: intermittent hemodialysis, peritoneal dialysis, and con-
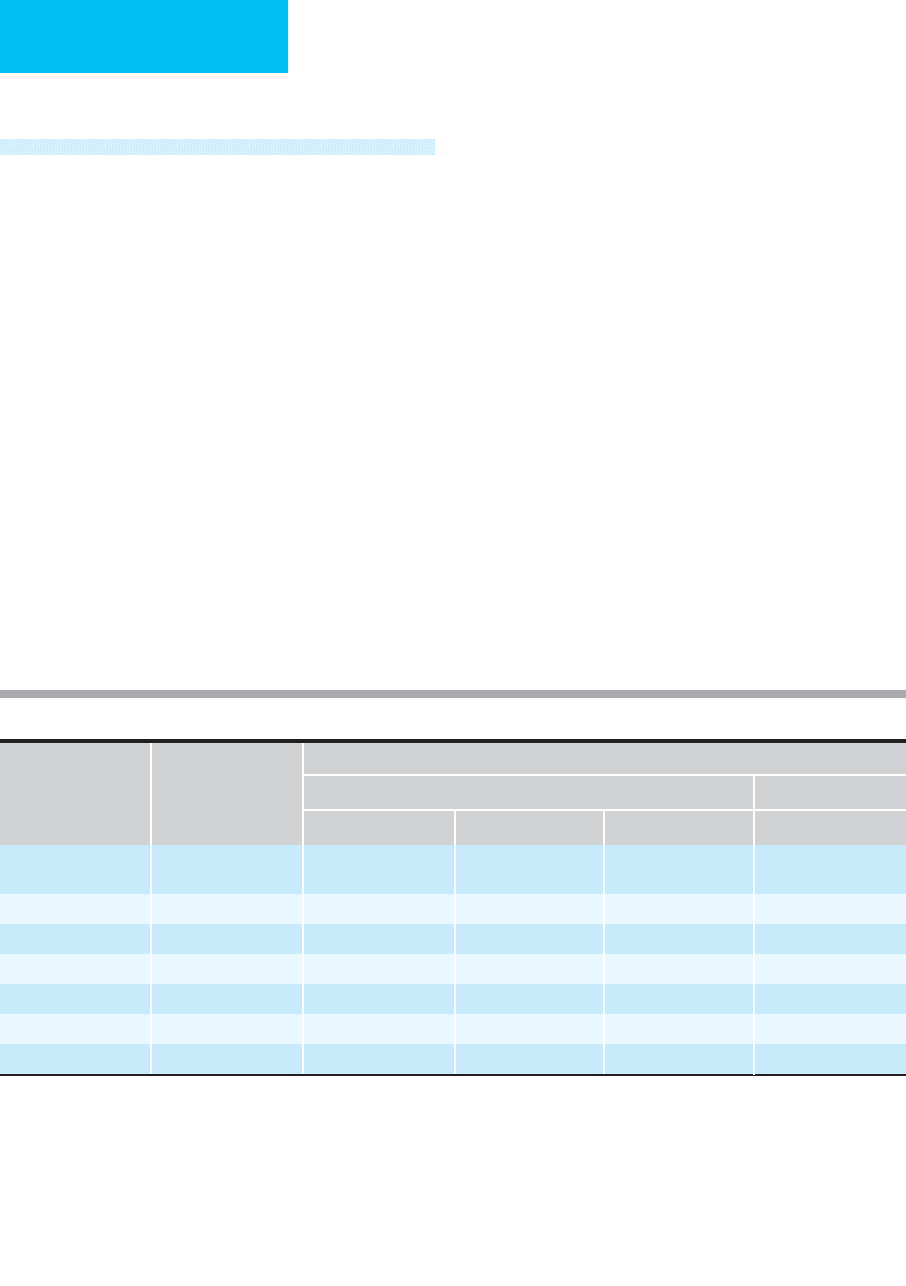
tinuous renal replacement therapy (CRRT) (Table 13–12).
The particular therapy chosen is often dictated by the
patient’s condition (eg, massive fluid overload, hypercatabo-
lism, or vascular instability) and associated morbid states
(eg, respiratory compromise, hemorrhagic risks, or abdomi-
nal surgery). Aside from these needs, the patient’s baseline
requirements for fluid and solute removal depend on nutri-
tional intake and residual renal function.
Using conventional techniques, machine-driven
hemodialysis is best suited for the hemodynamically stable
patient in whom solute balance is the major concern and
rapid fluid removal is well tolerated. Peritoneal dialysis is pre-
ferred in the patient with significant hemorrhagic risk and in
whom vascular access is difficult to obtain. Continuous
hemofiltration and its related techniques are best for provid-
ing fluid removal in the patient with vascular instability or
massive fluid overload. Despite these generalizations, with
appropriate technical modifications, adequate renal replace-
ment therapy can be provided by any of these methods.
Indications for Dialytic Therapy
Fluid Overload
Poorly tolerated volume overload is the most evident indica-
tion for initiating renal replacement therapy. In general, the
need to relieve pulmonary vascular congestion is the most
pressing issue. It should be noted that even massive amounts
of peripheral edema may be appropriate for the patient’s
condition (ie. hypoalbuminemia or vasodilatory shock), and
fluid removal may cause intravascular volume depletion,
hampering the return of endogenous renal function. When
hypotension is associated with edema and apparent pul-
monary congestion, pulmonary artery catheter monitoring
can be invaluable in determining the amount of fluid that
can be removed safely.
Time-Averaged
Urea Clearance Protein Loss
Treatment Prescription mL/min L/day L/week g/day
∗
Hemodialysis
†
3 × 4 h/week
7 × 4 h/week
14.3
33.3
21
48
144
336
6
15
Peritoneal dialysis 2 L/h 26.7 24 168 30
CAPD
2 L/6 h
‡
6.9 10 70 10
CAVH 14 L/day 9.7 14 98 15
CAVHD 1–2 L/h 19–35 29–51 189–357 11
CVVH 1–3 L/h 17–50 24–72 168–504 18–36
CVVHD 1–3 L/h 19–52 27–75 189–525 18–36
∗
Includes amino acids and peptides. Published data have been adjusted to account for increased porosity of currently available dialyzers.
†
Assumes average urea clearance of 200 mL/min.
‡
Assumes 3 L/d net filtrate.
Key: CAPD = chronic ambulatory peritoneal dialysis
CAVH = continuous arteriorvenous hemofiltration
CAVHD = continuous arteriorvenous hemodialysis
CVVH = continuous venovenous hemofiltration
CVVHD = continuous venovenous hemodialysis/hemodiafiltration
Modified from Kaplan AA: Dialysis and other extracorporeal therapy for acute renal failure. In: Current Therapy in Nephrology and
Hypertension, 4th ed. Glassock RJ (editor). Mosby, 1998. Copyright 1998 Elsevier.
Table 13–12. Renal replacement therapies: urea clearance and protein losses.

RENAL FAILURE
335
In the hemodynamically stable patient, intermittent
hemodialysis can provide the most rapid removal of fluid by
easily removing 1–2 L of fluid per hour by ultrafiltration. In
patients with vascular instability, one of the continuous ther-
apies is more appropriate because modest rates of fluid
removal can proceed steadily throughout the day. For exam-
ple, peritoneal dialysis can provide for the gentle removal of
the 2–3 L/day necessitated by intravenous medications and
hyperalimentation. Excessive net fluid removal (>5–10
L/day), however, may lead to hypernatremia because fluid
removed by peritoneal dialysis is hyponatric when compared
with plasma. In patients presenting with massive fluid over-
load, continuous hemofiltration offers the best-tolerated
treatment because the ultrafiltrate is isosmotic. A reasonable
combination of treatments would employ several days of con-
tinuous hemofiltration to achieve normovolemia, followed by
intermittent hemodialysis to provide maintenance therapy.
Electrolyte Abnormalities
Electrocardiographic changes caused by hyperkalemia
should be treated initially with nondialytic therapy (eg, cal-
cium, glucose, and insulin). The only renal replacement ther-
apy capable of rapid potassium removal is machine-driven
hemodialysis, providing clearance rates of 150–250 mL/min
or more (see Table 13–11). Neither continuous hemofiltra-
tion nor peritoneal dialysis can achieve potassium clearance
rates much above 20–40 mL/min, and both these continuous
therapies are best reserved for normalization of modest lev-
els of hyperkalemia or for maintaining potassium balance.
Toxic serum levels of calcium, magnesium, or phosphate
are also most rapidly corrected with machine-driven hemodial-
ysis. Once normal levels are achieved, any of the renal replace-
ment therapies can maintain homeostasis if nutritional intake
is limited and magnesium-containing phosphate binders are
avoided. Renal replacement therapies using dialysate (ie,
hemodialysis, peritoneal dialysis, or continuous hemodialysis)
may contain calcium concentrations of 3.5 meq/L (1.75 mmol/L
of ionized calcium) in the dialysate. Therefore, successful and
rapid treatment of hypercalcemia requires lower dialysate cal-
cium concentrations of 2.5 meq/L (1.25 mmol/L) or less.
Severe hypophosphatemia may complicate all renal replace-
ment therapies, especially in patients being maintained on
intravenous hyperalimentation devoid of phosphate.
Acid-Base Abnormalities
Uremic acidosis rarely generates more than 50–100 mmol
acid per day and can be easily corrected by any of the renal
replacement techniques. Severe uremic acidosis is encoun-
tered most often as a presenting abnormality of unattended
chronic renal failure. Under these conditions, hemodialysis
can provide the most rapid correction of acidosis, but aggres-
sive hemodialysis may precipitate a dysequilibrium syn-
drome. Despite associated hyperkalemia, the dialysate bath
composition should include at least 2 mmol/L potassium
because correction of acidosis causes a substantial lowering
of serum potassium concentration. Consideration also
should be given to a relatively low-calcium bath (2.5 meq/L)
because overly aggressive correction of long-standing
hypocalcemia may precipitate nausea, vomiting, muscle
cramping, and hypertension.
Lactic acid may be produced at rates of up to 50 mmol/h
and usually is associated with severe hemodynamic instabil-
ity. Although daily hemodialysis can provide adequate
replacement of lost bicarbonate, the patient may be left with
rapidly worsening acidosis during the interdialytic period.
Continuous hemofiltration can provide continuous correc-
tion of acidosis and may be best for managing the fluid over-
load often associated with shock and its treatment.
Replacement solutions containing 150 mmol/L bicarbonate
can provide as much as 100 mmol/h of continuous buffer
replacement, but required calcium replacement must be
administered in a separate solution. Peritoneal dialysis, with
exchanges at 2 L/h, can provide approximately 25 mmol
buffer per hour. Dialytic solutions containing lactate should
be avoided because conversion to bicarbonate may be slowed
in patients with circulatory impairment.
Uremia
Although urea is not universally accepted as a uremic toxin,
its levels are used most commonly to judge the degree of ure-
mic toxicity. Several studies suggest that maintaining predial-
ysis BUN at or below 120 mg/dL (43 mmol/L) is beneficial
for overall survival, and it is no longer acceptable to tolerate
excessively high urea levels prior to initiation of renal
replacement therapy. There is good or better evidence to sug-
gest that proper protein-calorie nutrition is also beneficial.
Thus it is inappropriate to withhold adequate nutrition in
order to avoid the associated increase in nitrogen and fluid
intake. On empirical grounds, when pericarditis,
encephalopathy, or hemorrhage is associated with BUN lev-
els above 100 mg/dL, it is hard to argue that such symptoms
are not at least partially the result of retained uremic toxins.
With the preceding considerations in mind, it is reasonable
to initiate renal replacement therapy when BUN is above
100 mg/dL (36 mmol/L). Nonetheless, if rapid return of
renal function is anticipated (eg, in the presence of prerenal
azotemia or obstructive uropathy), levels of 150 mg/dL
(54 mmol/L) or more may be tolerated for a limited period.
Specific indications for dialytic therapy for complications
of uremia include uremic encephalopathy, pericarditis, and
uremic platelet dysfunction. Slowed mentation, somnolence,
and convulsions are part of the uremic syndrome and are
usually associated with other neuromuscular manifestations,
including asterixis, myoclonus, and muscle twitching. In gen-
eral, these symptoms respond within several days after the
start of dialytic therapy.
Despite the existence of massive pericardial effusions,
patients with uremic pericarditis may present with hyper-
tension and pulmonary congestion. The treatment of ure-
mic pericarditis often presents two distinct problems:
CHAPTER 13
336
removal of fluid in the face of potentially compromised
hemodynamics and definitive treatment of the pericardial
inflammation. Initial rapid fluid removal with hemodialysis
may be well tolerated, but as volume removal proceeds,
intravascular pressures may become inadequate to maintain
intracardiac filling, and severe hypotension may result. Thus
normovolemia should be achieved with gentle fluid removal
and anticipation of rapid declines in blood pressure.
Although the toxins responsible for uremic pericarditis have
not been identified, it has been shown empirically that
aggressive solute removal can lead to resolution of pericardi-
tis. Hemodialysis performed five times weekly is recom-
mended, but means to limit anticoagulation should be
employed in order to minimize the risk of hemoperi-
cardium. In patients with large pericardial effusions, early
use of pericardiotomy may be recommended because
aggressive hemodialysis may be associated with a high mor-
tality rate. In one retrospective report, peritoneal dialysis
was found to be superior to hemodialysis in avoiding the
need for surgical drainage. Peritoneal dialysis also avoids the
risk of anticoagulation.
Uremic platelet dysfunction is identified most often with
prolongation of the bleeding time. In general, bleeding times
will normalize along with lowering of serum urea. Acutely,
rapid correction of platelet dysfunction can be achieved with
infusions of desmopressin at a single dose of 0.3 μg/kg.
Drug Dosing During Renal Replacement
Therapy
Many renally excreted medications require dosage modifica-
tion to account for the amount removed by a given renal
replacement therapy. Unfortunately, most of the published
data are of questionable accuracy as a result of variability
between patients and the great differences in clearance rates
achievable with each technique. Four factors govern the
removability of a given drug: molecular weight, degree of
protein binding, volume of distribution, and endogenous
plasma clearance. In general, hemodialysis can offer the
most rapid clearance rates for a low-molecular-weight drug
(<500 kDa). Continuous hemofiltration—but not continu-
ous hemodialysis—will efficiently remove drugs with
molecular weights as high as 10,000 kDa or more. Peritoneal
dialysis will eliminate drugs in the range of MW 500–10,000
kDa. Highly protein-bound medications are not substan-
tially removed by any of the renal replacement techniques,
with the possible exception of peritoneal dialysis. In addi-
tion, modifications of the major techniques may profoundly
alter clearance rates because most of the published data were
obtained with more conventional methodology. For exam-
ple, it has been shown recently that the most modern “high
flux” dialyzers can offer substantial removal of relatively
high-molecular-weight drugs (>1500 kDa), thus greatly
changing their dosing requirements as compared with pre-
vious recommendations.
Aronoff GR et al: Drug Prescribing in Renal Failure: Dosing
Guidelines for Adults, 4th ed. New York: American College of
Physicians, 1999.
Stopping Dialysis
Owing to the availability of chronic dialysis, irreversibility of
renal failure is not an acceptable indication for stopping
treatment. Instead, the patient’s wishes and overall clinical
status should be the only considerations in discontinuing
therapy. In general, withholding of dialysis may be consid-
ered when there is evidence of irreversible vital organ failure
or severe cerebral damage. Most forms of acute renal failure
reverse within 8 weeks. If renal failure persists beyond this
period, one should initiate plans for maintenance dialysis
therapy.
Specific Types of Renal Replacement
Therapy in Acute Renal Failure
1. Hemodialysis
Intermittent hemodialysis is the most widely used technique
for acute renal failure. The method of treatment chosen
varies depending on the rate of generation of nitrogenous
wastes and the patient’s tolerance for fluid overload. In gen-
eral, 4-hour treatments performed three times weekly are
sufficient to provide adequate replacement in the oliguric or
anuric patient. Patients with significant residual renal func-
tion may require fewer treatments per week, especially if
renal failure is nonoliguric. Conversely, the patient with
severe hypercatabolism and poorly tolerated fluid overload
may require daily treatments.
The major advantage of hemodialysis is its highly effi-
cient solute removal, thus limiting treatment time and mak-
ing the patient available for other procedures and treatments.
Disadvantages include relatively rapid fluid removal, which
may be poorly tolerated. Other disadvantages of hemodialy-
sis involve the need for large-bore hemoaccess and anticoag-
ulation of the extracorporeal circuit.
Effectiveness of Hemodialysis
Solute clearance and ultrafiltration rates are variable depend-
ing on the blood flows obtained and the dialyzers chosen.
Most modern dialyzers provide 150–250 mL/min of urea
clearance with blood flows between 200 and 300 mL/min.
More rapid solute clearance can be obtained with the newer
more porous filters, especially when operated at blood flows
of up to 400 mL/min or more. Currently available dialyzers
also can produce between 1 and 3 L of ultrafiltrate per hour,
usually limited by the patient’s hemodynamic stability. A rel-
atively gentler type of hemodialysis involves the prolonga-
tion of the treatment in a low-efficiency mode. These slow,
low-efficiency dialysis (SLED) treatments are applied from
8–18 hours at a time and allow for less aggressive fluid
