Bongard Frederic , Darryl Sue. Diagnosis and Treatment Critical Care
Подождите немного. Документ загружается.


RENAL FAILURE
317
such as furosemide. A markedly diminished FE
urea
cannot
distinguish between a rapidly reversible prerenal azotemia
and more definitive ischemic damage, such as occurs in acute
tubular necrosis. Nonetheless, values below 35% are very
useful in identifying renal hypoperfusion in patients taking
diuretics, as is most often the case in decompensated conges-
tive heart failure. Osmotic diuresis resulting from adminis-
tration of mannitol or acetazolamide or from diabetic
ketoacidosis increases the fractional excretion of urea despite
the existence of volume depletion.
Twenty-four hour urine collections for determinations of
creatinine, urea, and protein are the most reliable means of
assessing renal function and determining nitrogen balance.
Creatinine or urea clearance can be calculated as follows:
Creatinine clearance (mL/min)
Urea clearance (mL/min)
When serum levels of creatinine or urea are increasing
rapidly, the mean of the pre- and postcollection values is
used in the denominator. Timed collections less than 24 hours
are reasonably accurate for creatinine but are less useful for
assessing proteinuria or urea production.
Modest amounts of proteinuria (<1 g/day) are common in
many forms of acute renal failure, but proteinuria in the
nephrotic range (>3.5 g/day) is most often related to glomeru-
lar disease, except when associated with low-molecular-weight
proteins such as Bence Jones protein in multiple myeloma.
Urine protein electrophoresis performed on a 24-hour urine
collection will distinguish between these two disorders.
6. Urinalysis—Routine urinalysis consists of rapid dipstick
tests and microscopic examination. Dipstick determinations
measure pH and can reveal the presence of hemoglobin
(positive for intact red blood cells, free hemoglobin, and
myoglobin), protein, glucose, and ketones. Microscopic
analysis can suggest infection (white blood cells, white blood
cell casts, and bacteria), nephritis (red and white blood cells,
with or without cellular casts), or nephrosis (granular casts
or oval fat bodies). Red blood cell casts are associated most
commonly with glomerulonephritis but may be seen occa-
sionally with other types of acute glomerular injury such as
cholesterol emboli or with malignant hypertension. White
blood cell casts are seen most often with infectious
pyelonephritis, but when associated with sterile urine, they
may be a sign of immunologically mediated interstitial
nephritis. Tubular epithelial cells can be found with intersti-
tial nephritis or acute tubular necrosis. When associated with
muddy-brown casts, acute tubular necrosis is a more likely
diagnosis. Eosinophiluria, demonstrable on smears of urine
sediment stained with Wright’s or Hansel’s stain, can occur
in drug-induced allergic interstitial nephritis.
The urine dipstick test for protein is most sensitive for
albumin, whereas sulfosalicylic acid added to the urine
causes precipitation of all proteins. When the urine dipstick
is negative or only modestly positive for protein and the sul-
fosalicylic acid precipitation is markedly positive, Bence
Jones proteinuria should be suspected.
Urine culture and Gram staining should be performed on
any urine containing white blood cells. Sterile pyuria is often
a sign of drug-induced interstitial nephritis, but renal tuber-
culosis also should be considered.
7. Assessment of intravascular volume—Central
venous pressure monitoring can be accomplished with sub-
clavian, internal jugular, or femoral catheterization. A low
central venous pressure is most compatible with decreased
intravascular volume; elevated central venous pressure may
be secondary to intravascular expansion or pulmonary
hypertension. When the volume status is unclear in the con-
text of dyspnea, elevated levels of B-type natriuretic peptide
(BNP) may help to identify an element of heart failure that
would support a diagnosis of intravascular volume overload.
Pulmonary artery catheterization is the most reliable means
of assessing optimal fluid status by determination of cardiac
output and left ventricular filling pressure but usually is not
necessary.
8. Renal biopsy—Renal biopsy is most often helpful when
inflammatory nephritis (ie, glomerulonephritis, allergic
interstitial nephritis, etc.) is suspected. However, in patients
suspected of having acute tubular necrosis in whom renal
failure fails to resolve in 6–8 weeks, renal biopsy may be indi-
cated to diagnose irreversible causes of renal failure (eg, cho-
lesterol emboli, cortical necrosis, etc.).
D. Imaging Studies—Renal ultrasound provides an accurate
means of measuring renal size (small kidneys are evidence of
chronic renal disease) and determining the existence of
hydronephrosis. Because potentially nephrotoxic contrast
agents are not required, ultrasound has become the first
choice in the evaluation of ureteral obstruction. In rare
cases, tumor infiltration or retroperitoneal or perirenal
fibrosis may inhibit the expansion of the renal pelvis, thus
yielding a falsely negative result.
The plain abdominal x-ray can demonstrate the presence
of radiopaque kidney stones but is often more valuable in
evaluation of associated disease processes. Intravenous pyel-
ography (IVP) most reliably identifies the site of renal
obstruction, provided that creatinine levels are not exces-
sively elevated (≤4 mg/dL). Retrograde pyelography can image
both the ureters and the bladder. Percutaneous pyelography
can determine the site of renal obstruction when retrograde
pyelography is unsuccessful. CT scanning can evaluate the
site of obstruction (extrinsic versus intrinsic obstruction)
and assess for associated morbidity. Selective renal angiogra-
phy with digital subtraction is the best way to assess the renal
vasculature for stenosis or hemorrhagic leak.
=
×urine Urea (mg/dL) urine volume (mL/min)
se
rrum Urea (mg/dL)
=
×urine Cr (mg/dL) urine volume (mL/min)
seru
mmCr(mg/dL)
CHAPTER 13
318
The potential for radiocontrast nephrotoxicity always
should be considered. Risk factors include volume deple-
tion, low cardiac output, preexisting renal disease, large
contrast load or multiple exposures to contrast agents, a
history of contrast-induced acute renal failure, multiple
myeloma, and most significantly, advanced diabetic
nephropathy. Patients with diabetic nephropathy and crea-
tinine levels above 4 mg/dL have a 90% probability of
developing some degree of renal dysfunction. In some of
these patients, renal failure may be irreversible. Patients at
increased risk should be well hydrated prior to and imme-
diately after exposure to radiocontrast dyes, and every
effort should be made to limit the amount of contrast
material administered. The newer low-osmolality agents
may be preferable. Other means of minimizing the risk of
contrast-induced renal damage include oral administration
of the antioxidant acetylcysteine and infusion of sodium
bicarbonate.
Radionuclide scanning provides the only noninvasive
means of assessing the relative percentage of renal function
from each kidney (split function studies). Renal blood flow
can be assessed with diethylenetriamine pentaacetic acid
(DTPA), which is excreted by glomerular filtration and may
demonstrate renal vascular stenosis. Repeat examination
after administration of captopril increases this test’s sensitiv-
ity. Gallium scans can identify any type of renal inflamma-
tion and are most valuable in assessing unilateral lesions or
identifying a renal origin of pyuria. Gallium scans also may
be positive in patients with nephrosis. Technetium-99m mer-
captoacetyltriglycine (MAG3) is both filtered and secreted
and is the radionuclide imaging agent of choice for patients
with substantial renal failure.
MRI is not yet widely employed in the evaluation of acute
renal failure, but it may be of value in the diagnosis of renal
vein thrombosis. Gadolinium-enhanced magnetic resonance
angiography (MRA) is an accurate means for assessing renal
artery stenosis without the risks of catheterization or the
contrast material used for percutaneous angiography.
Complications
The complications of acute renal failure are listed in
Table 13–3. Cardiovascular problems are most often due to
fluid overload and electrolyte abnormalities. Pericarditis is
probably the result of retained uremic toxins. Insufficient
erythropoietin may cause decreased red blood cell produc-
tion and anemia, but this mechanism is seen more commonly
in patients with chronic renal failure. In contrast, platelet dys-
function, clinically diagnosed on the basis of prolonged
bleeding time, is a common consequence of acute renal fail-
ure. Infections represent an important cause of morbidity
and death in acute renal failure—especially urinary tract
infections, which are particularly difficult to eradicate
because of inadequate urine concentrations of antibiotics.
Electrolyte and acid-base abnormalities are common, the
most serious being hyperkalemia. Hypoglycemia may result
from decreased renal catabolism of exogenously adminis-
tered insulin. Neurologic abnormalities include somnolence,
coma, and convulsions and are often compelling indications
for initiation of dialysis. GI hemorrhage in acute renal failure
is due to the combination of uremic coagulopathy and gas-
tritis, but in chronic renal failure, arteriovenous malforma-
tions are the most common causes.
Briguori C, Marenzi G: Contrast-induced nephropathy:
Pharmacological prophylaxis. Kidney Int Suppl 2006;100:
S30–8. [PMID: 16612399]
Carvounis CP, Nisar S, Guro-Razuman S: Significance of the
fractional excretion of urea in the differential diagnosis of
acute renal failure. Kidney Int 2002;62:2223–9. [PMID:
12427149]
Goldenberg I, Matetsky S: Nephropathy induced by contrast
media: Pathogenesis, risk factors and preventive strategies. Can
Med Assoc J 2005;172:1461–71. [PMID 15911862]
Hilton R: Acute renal failure. Br Med J 2006;333:786–90. [PMID
17038736]
Lameire N et al: Acute renal failure. Lancet 2005;365:417–30.
[PMID 15680458]
Maisel AS et al : Rapid measurement of B-type natriuretic peptide
in the emergency diagnosis of heart failure. N Engl J Med
2002;347:161–7. [PMID: 12124404]
Merten GJ et al: Prevention of contrast-induced nephropathy with
sodium bicarbonate. JAMA 2004;291:2328–34. [PMID:
15150204]
O’Neill WC, Baumgarten DA: Imaging. Am J Kidney Dis
2003;42:601–4. [PMID: 12955693]
Venkataraman R, Kellum JA: Prevention of acute renal failure.
Chest 2007;131:300–8. [PMID: 17218591]
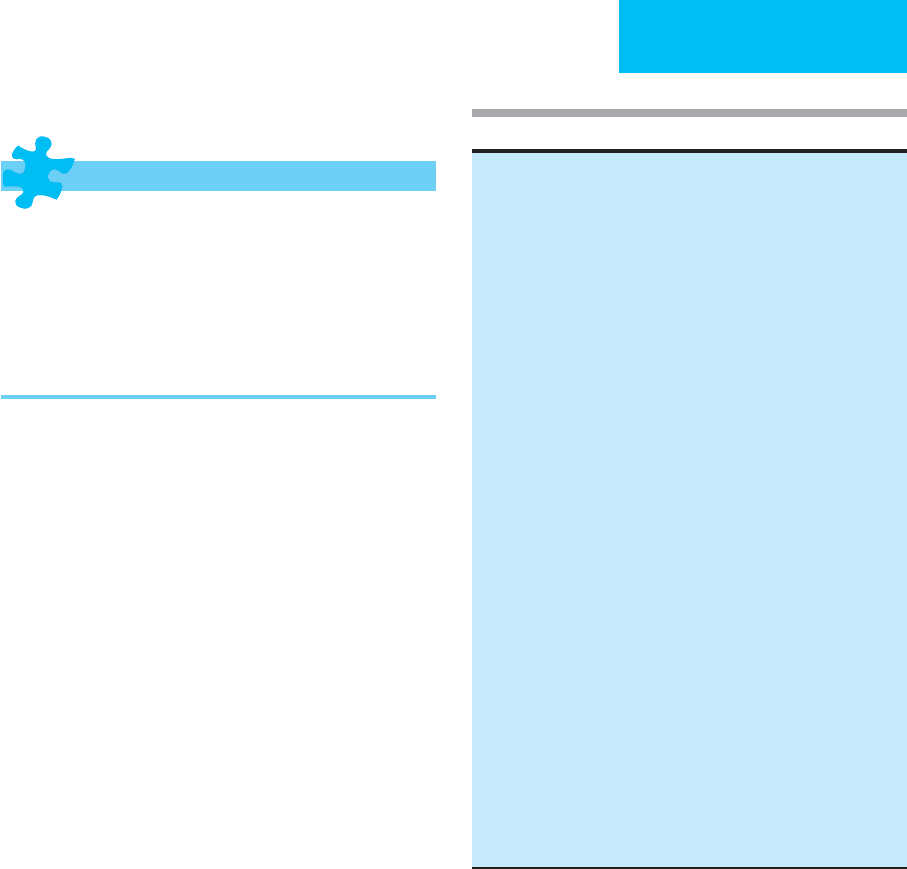
Fluid overload
Pulmonary edema
Anasarca
Pericarditis
Electrolyte disorders
Hyperkalemia
Hyperphosphatemia
Hypocalcemia (rarely, hypercalcemia)
Hypermagnesemia
Metabolic acidosis
Neurologic disorders
Altered sensorium
Peripheral neuropathy
Seizures
Others
Anorexia, nausea, vomiting
Platelet dysfunction
Anemia
Table 13-3. Acute renal failure—Common complications.
RENAL FAILURE
319
Prerenal Renal Failure
ESSENTIALS OF DIAGNOSIS
BUN:creatinine ratio >20:1.
Decreased urine output (unless renal losses are primary).
FE
Na
<1% and/or FE
urea
<35%.
Urine sediment may show a few granular casts but
absence of inflammatory cells and red and white blood
cell casts.
No evidence of urinary tract obstruction.
General Considerations
Prerenal azotemia is defined as a state of renal hypoperfu-
sion that can be rapidly reversed with proper management.
All causes of renal hypoperfusion stimulate renal autoregu-
latory mechanisms that function to maintain glomerular
filtration despite decreasing renal blood flow. Central in
this response is the balance between vasoconstricting
angiotensin II and vasodilating renal prostaglandins.
Angiotensin II causes vasoconstriction of both the afferent
and the efferent glomerular arterioles, but resistance in the
efferent arteriole rises more. As a result, glomerular hydro-
static pressure increases, filtration fraction rises, and unfil-
trable serum proteins are concentrated. The resulting
increase in efferent arteriolar oncotic pressure stimulates
water and urea reabsorption from the proximal tubule, thus
decreasing the fractional excretion of urea and increasing
the BUN:creatinine ratio. Furthermore, angiotensin II acts
directly on the proximal tubule to stimulate sodium reab-
sorption, thus decreasing the fractional excretion of
sodium.
The causes of prerenal failure can be classified as shown
in Table 13–4. A decrease in cardiac output can be the result
of primary cardiac disease or insufficient end-diastolic fill-
ing pressures. Volume depletion can occur along obvious
routes such as the GI or urinary system, via surgical drains,
or via more occult mechanisms, including diaphoresis and
retroperitoneal hemorrhage. Redistribution of fluid out of
the intravascular space with insufficient circulating vol-
ume can occur with hypoalbuminemia, vasodilatory shock
with capillary leak (sepsis), or intraabdominal accumula-
tion (eg, peritonitis, ascites, and pancreatitis). Crush injury
with massive tissue damage can cause sufficient localized
edema to result in intravascular depletion. Rarely,
overzealous administration of vasodilators decreases effec-
tive circulating volume and can mimic endogenous causes
of vasodilatory shock.
Intrarenal vasoconstriction can be mediated by several
mechanisms, including an imbalance between vasoconstric-
tive angiotensin II and vasodilating prostaglandins (most
often seen when prostaglandin synthetase inhibitors are
given to patients with nephrosis, cirrhosis, congestive heart
failure, preexisting renal disease, or volume depletion). Renal
artery stenosis, preeclampsia, malignant hypertension, scle-
roderma with severe hypertension and renal failure, and
intravenous cyclosporine may be associated with intrarenal
vasoconstriction mediated by angiotensin II or endothelin.
Finally, obstruction to blood flow to or from the kidney can
result from renal artery stenosis or emboli to the renal arter-
ies, related to valvular heart disease, or in patients with atrial
or ventricular mural thrombi; can occur from valvular heart
disease or arrhythmias; or can occur as a complication of
percutaneous renal angioplasty. Renal vein thrombosis in
adults is seen most often in the context of nephrotic syn-
drome and is considered to be the result of its associated
hypercoagulable state.
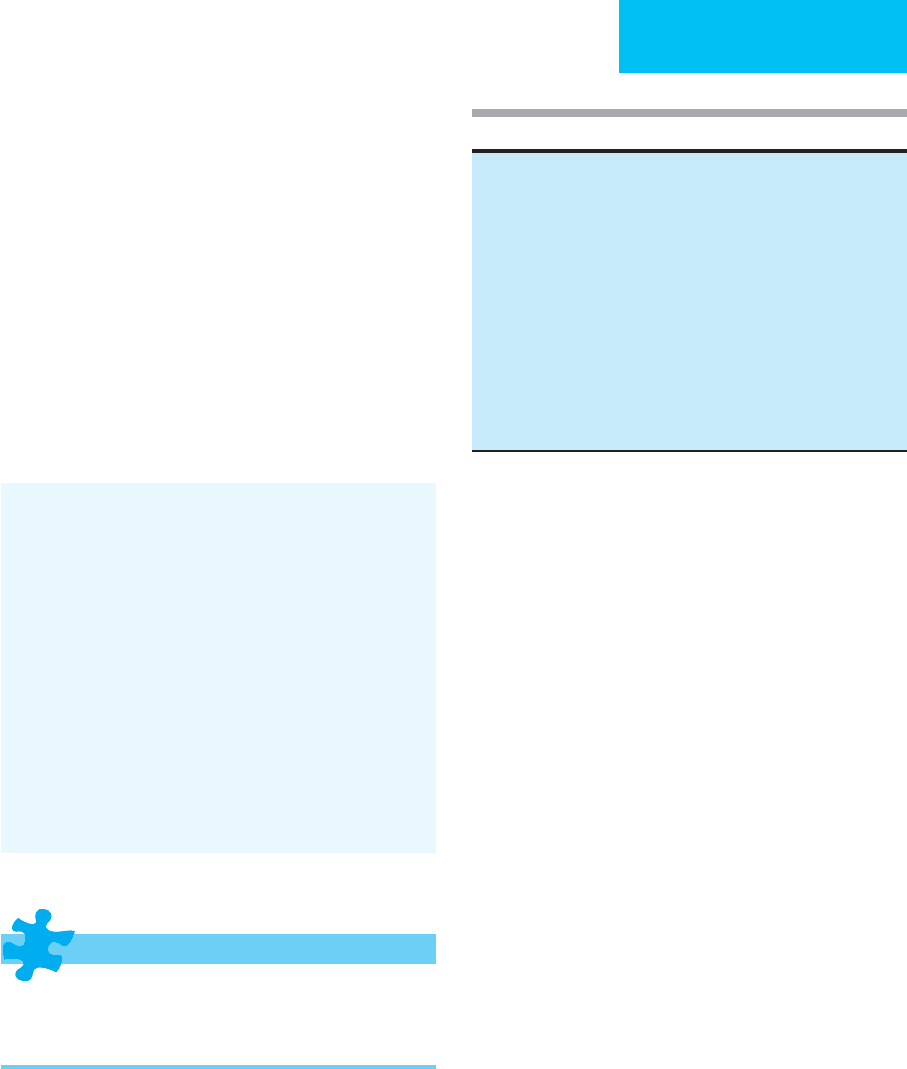
Table 13–4. Causes of prerenal renal failure.
Decreased cardiac output
Myocardial infarction
Cardiomyopathy
Pericarditis (constrictive or cardiac tamponade)
Arrhythmias
Valvular dysfunction
Pulmonary embolus
Pulmonary hypertension
Mechanical ventilation (especially with PEEP)
Trauma
Extracellular volume depletion
Dehydration
GI losses (vomiting, NG suction, diarrhea, ostomy output)
Renal losses (diuretics, osmotic diuresis, nonoliguric ATN, adrenal
insufficiency)
Peritoneal losses (surgical drains)
Skin losses (burns, diaphoresis)
Hemorrhage (gastrointestinal, intra- and retroperitoneal)
Redistribution of fluid
Hypoalbuminemia (nephrosis, cirrhosis, malnutrition)
Vasodilatory shock (sepsis, hepatic failure)
Peritonitis
Pancreatitis
Crush injury
Ascites
Vasodilators
Primary intrarenal vasoconstriction
NSAIDs (prostaglandin inhibition)
Hepatorenal syndrome
Preeclampsia
Malignant hypertension
Scleroderma
Cyclosporine
Renovascular obstruction
Renal artery (intravascular stenosis, embolus, laceration, thrombus)
Renal vein (intravascular thrombosis, tumor infiltration, extravascular
compression)

CHAPTER 13
320
Clinical Features
A. Symptoms and Signs—Patients may present with a his-
tory of excessive fluid losses from diarrhea, high urine out-
put, or sweating, and this may be compounded by low fluid
intake. They may complain of lightheadedness or syncope
and may have tachycardia, hypotension, and diaphoresis.
Often, postural decrease in blood pressure and increase in
heart rate are present in patients with significant volume
depletion. Although prerenal azotemia is associated most
commonly with volume depletion, patients with congestive
heart failure, systemic vasodilation, or hypoalbuminemia
may have substantial peripheral edema yet still have insuffi-
cient cardiac output or intravascular volume to maintain
adequate renal perfusion. Conversely, a severely hypoalbu-
minemic patient who appears euvolemic is likely to be
intravascularly depleted.
Thromboembolic diseases, including renal artery
embolization and renal vein thrombosis, decrease renal per-
fusion but are distinct in their symptomatology and diagnos-
tic workup. Renal artery embolization is most often
associated with cardiac valvular disease and can present with
nausea, vomiting, flank pain, and hematuria.
Renal vein thrombosis in adults is most often associated
with nephrotic syndrome, but it also may result from extrin-
sic compression by tumor. Acute thrombosis can present
with flank pain, hematuria, and increased proteinuria and
may be misdiagnosed as a kidney stone. Chronic thrombosis
may be asymptomatic except for an ipsilateral varicocele
when the thrombosis is in the left renal vein.
B. Laboratory Findings—Considering these varying clini-
cal presentations, evaluation of serum and urine
chemistries is required to confirm the diagnosis. Although
both serum creatinine and urea nitrogen increase, low
tubular flow results in increased reabsorption of urea.
Therefore, the serum BUN:creatinine ratio increases usu-
ally to greater than 20:1, and the FE
urea
is less than 35%.
Because renal tubular function is normal in early prerenal
azotemia, avid sodium reabsorption in the face of volume
depletion causes FE
Na
to be very low, usually less than 1%.
Urinary sediment is usually normal except for the finding
of a few granular casts; white blood cells and red and white
blood cell casts are absent.
C. Imaging Studies—The diagnosis of thromboembolic
disease may be suspected with the demonstration of
decreased function on IVP or absent flow on radionuclide
scan. The definitive diagnosis and a judgment about the
advisability of operation are best arrived at by renal
angiography (the presence of collateral circulation suggests
a better surgical outcome). Renal venography is the most
definitive diagnostic test for renal vein thrombosis but
risks dislodging the thrombus, with resulting pulmonary
embolization. Digital subtraction renal angiography with
evaluation of the venous phase, CT scan, and MRI are safer
alternatives.
Treatment
Prerenal azotemia caused by volume depletion should be
treated by correction of decreased extracellular fluid volume.
Most often, normal saline is given intravenously in an
amount sufficient to replete volume, but in some patients,
blood or colloid solutions are needed. The total volume of
replacement should be estimated in most cases as 10–25% of
extracellular fluid volume (ie, 2–4 L), but the amount actu-
ally given should be carefully titrated by monitoring urine
output, blood pressure, and heart rate. Elderly patients and
those with heart failure should be given fluid cautiously, usu-
ally with central venous pressure or pulmonary artery pres-
sure monitoring. Fluid replacement also should take into
account continued losses from the GI tract and other sources
of fluid loss.
Prerenal azotemia associated with cardiac failure usually
responds to standard drugs and procedures for improving
cardiac output, including loop diuretics, vasodilators,
inotropic agents, and oxygen. Low-dose dopamine (<5 μg/kg
per minute) has become a popular means of initiating diure-
sis in resistant cases. However, its use is unsupported by large
randomized studies. Potential secondary effects include
tachycardia and ischemia. Heart failure and prerenal
azotemia associated with substantial fluid overload also may
respond to infusions of nesiritide, with or without diuretics.
This approach has shown a remarkable success in allowing
for aggressive diuresis without worsening of renal perfusion.
If diuresis is associated with increasing BUN, ACE inhibitors
may be beneficial in decreasing renal resistance. When suc-
cessful, this approach yields an increase in the fractional
excretion of urea, which can be followed on a daily basis and
used as a guide to further treatment. The management of
pericarditis, arrhythmias, pulmonary emboli, and pul-
monary hypertension is discussed elsewhere. One should
note that positive end-expiratory pressure for treatment of
respiratory failure can spuriously elevate pulmonary wedge
pressure, thus making it a misleading measure of intravascu-
lar volume.
Volume repletion should be tailored to the patient’s car-
diopulmonary reserve. The hypoalbuminemic states associ-
ated with cirrhosis or malnutrition can be corrected with
intravenous albumin, but worsening of associated ascites
may occur in a third of patients. In contrast, albumin infu-
sions are futile in patients with ongoing nephrotic syndrome.
Treatment of vasodilatory shock often requires a combi-
nation of fluids and vasoconstrictors. Crush injury repre-
sents a particular case where intravascular volume depletion
combines with the potential nephrotoxicity of myoglobin to
yield a toxic acute tubular necrosis. Proper prevention of
myoglobin nephrotoxicity can be accomplished with aggres-
sive intravascular volume expansion (see below).
Primary intrarenal vasoconstriction by prostaglandin
synthetase inhibitors reverses spontaneously after cessation
of these drugs. Prevention of hepatorenal syndrome involves
restoration of intravascular volume despite increasing ascites
RENAL FAILURE
321
or edema (see below). Preeclamptic renal failure must be
treated by delivery or termination of pregnancy. Malignant
hypertension and renal crises associated with scleroderma
can be treated successfully with ACE inhibitors. Patients with
refractory hypertension may require nitroprusside, but pro-
longed use of this agent in renal failure may predispose to
thiocyanate toxicity. Intrarenal vasoconstriction resulting
from high doses of intravenous cyclosporine is reversed by
decreasing or discontinuing the drug.
Renal artery stenosis can be treated by percutaneous
angioplasty or surgery. Massive renal artery embolization
resulting from cardiac sources can be managed with anti-
coagulation and thrombolytic therapy but may require
operative management. Renal vein thrombosis is treated
with long-term anticoagulation with heparin and warfarin
but may respond to either intravenous or intraarterial
thrombolytic therapy.
Carvounis CP, Nisar S, Guro-Razuman S: Significance of the frac-
tional excretion of urea in the differential diagnosis of acute
renal failure. Kidney Int 2002;62:2223–9. [PMID: 12427149]
de Denus S, Pharand C, Williamson DR: Brain natriuretic peptide
in the management of heart failure: The versatile neurohor-
mone. Chest 2004;125:652–68. [PMID: 14769750]
Friedrich JO et al: Meta-analysis: Low-dose dopamine increases
urine output but does not prevent renal dysfunction or death.
Ann Intern Med 2005;142:510–24. [PMID 15809463]
Ichai C et al: Comparison of the renal effects of low to high doses
of dopamine and dobutamine in critically ill patients: A single-
blind, randomized study. Crit Care Med 2000;28:921–8. [PMID:
10809260]
Jones D, Bellomo R: Renal-dose dopamine: From hypothesis to
paradigm to dogma to myth and, finally, superstition? J
Intensive Care Med 2005;20:199–211. [PMID: 16061903]
Kaplan AA, Kohn OF: Fractional excretion of urea as a guide to
renal dysfunction. Am J Nephrol 1992;12:49–54. [PMID:
1415365]
Postrenal Renal Failure
ESSENTIALS OF DIAGNOSIS
Dilated renal pelvis on ultrasound.
Enlarged, palpable bladder (if obstruction to bladder
outflow).
General Considerations
Demonstrable changes in renal function occur only if both
kidneys are involved or if there is preexisting kidney disease
involving the contralateral kidney. It is important to keep in
mind that obstruction to urine flow decreases tubular sensi-
tivity to antidiuretic hormone, leading to nephrogenic dia-
betes insipidus. Thus the presence of a high urine volume
does not rule out the possibility of partial obstruction.
Obstruction to urine flow can occur anywhere along the
urinary tract and may be secondary to extrinsic compression
or intrinsic blockage (Table 13–5). Causes include tumors,
stones, blood clots, and sloughed renal papillae—the result
of papillary necrosis, a condition known to occur with anal-
gesic abuse, diabetes, recurrent infections, and sickle cell ane-
mia. Although sloughed renal papillae usually cause
unilateral obstruction, blockage of the bladder neck can
cause bilateral obstruction and acute-onset oliguria. In men,
the most common cause of postrenal renal failure is prosta-
tic hypertrophy.
Clinical Features
A. Symptoms and Signs—Obstruction to urine flow result-
ing in renal failure may be accompanied by symptoms and
signs related to unilateral ureteral obstruction or urethral or
bladder obstruction. Occasionally, obstruction is not associ-
ated with any symptoms or signs.
Acute ureteral obstruction may be painful, with the
patient complaining of severe flank and midback pain, espe-
cially when caused by renal stones or blood clots.
Examination may indicate localized tenderness, especially to
percussion. Urethral obstruction, especially in prostatic
hypertrophy, will cause bladder distention and inability to
urinate. Although urine output is very often low or absent,
partial obstruction may cause renal failure without oliguria
or anuria.
B. Laboratory Findings—Elevated serum creatinine and
BUN are indicative of renal insufficiency, and serum elec-
trolytes reflect the severity of renal failure. In patients with
renal stones or blood clots, hematuria without red blood cell
casts is a prominent feature, but ureteral or urethral obstruc-
tion from extrinsic compression such as tumors will not be
associated with abnormal urinary sediment.
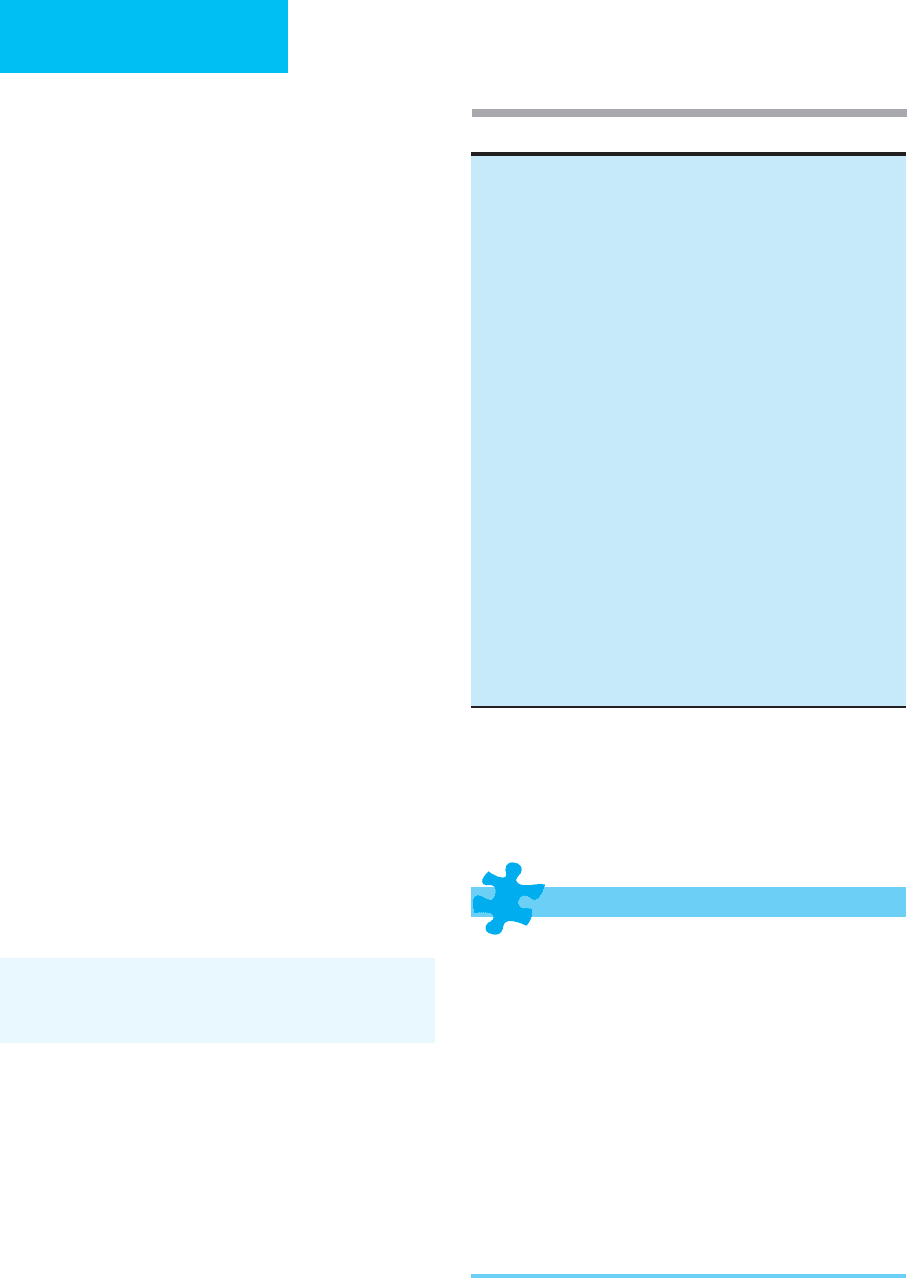
Table 13-5. Causes of postrenal renal failure.
Ureteral obstruction (bilateral or unilateral single kidney)
Extrinsic
Tumors (endometrial, cervix, lymphoma, metastatic)
Retroperitoneal fibrosis or hemorrhage
Accidental surgical ligation
Intrinsic
Stones, blood clots, sloughed papillae (papillary necrosis), tumors
(transitional cell, etc.)
Bladder or urethral obstruction
Prostatic hypertrophy or tumor
Bladder carcinoma
Uterine prolapse
Stones, blood clots, sloughed papillae
Neurogenic bladder (functional or iatrogenic)
Obstructed Foley catheter
CHAPTER 13
322
C. Imaging Studies—The existence of postrenal obstruc-
tion, regardless of the site or cause, almost always can be
determined by renal ultrasound examination. In rare cases,
however, tumor infiltration of the renal parenchyma or
perirenal fibrosis may inhibit dilation of the renal pelvis and
yield a falsely negative ultrasound examination. Minimal or
absent caliceal dilation also may occur during the first few
hours after an acute obstruction. Radiologic procedures
requiring potentially nephrotoxic radiocontrast agents are
most helpful in determining the site and nature of the
obstruction. IVP is employed most often in the context of
suspected kidney stone, but imaging is poor if serum creati-
nine levels are elevated. Retrograde pyelography performed
through a cystoscope avoids intravenous contrast adminis-
tration and offers a direct view of the bladder and the possi-
bility of a corrective urologic procedure. Percutaneous
pyelography demonstrates the site of obstruction and offers
rapid relief of obstruction if the catheter is left in situ. CT
scans and MRI are most helpful for determining the extrin-
sic or intrinsic nature of the obstruction. Radionuclide scan-
ning can be valuable in cases of suspected obstruction when
dilation of the renal pelvis can be identified, but the severity
of the blockage is in doubt.
Treatment
Acute ureteral obstruction with a kidney stone or sloughed
papillae is most often managed with aggressive hydration
and control of pain. Treatment of other causes of ureteral
obstruction, such as tumors, depends on the location and
extent of obstruction and may require surgical relief of the
obstruction. Percutaneous nephrostomy is a reasonable
alternative for short-term correction of obstruction of the
kidney. Bladder obstruction or dysfunction can be treated
initially with insertion of a Foley catheter, but in some
patients this may be difficult to perform without special
urologic instruments. An infected, obstructed kidney is a
medical emergency that cannot be managed adequately
with antibiotic therapy alone. Rapid relief of obstruction is
essential.
Klahr S: Urinary tract obstruction. Semin Nephrol 2001;21:
133–45. [PMID: 11245776]
O’Neill WC: B-mode sonography in acute renal failure. Nephron
Clin Pract 2006;103:C19–23. [PMID: 16543751]
Intrinsic Renal Failure
Intrinsic renal failure is often a diagnosis of exclusion after
prerenal and postrenal causes have been eliminated or
treated. Cases of intrinsic acute renal failure can be catego-
rized as follows (Table 13–6): (1) those involving the
glomeruli (glomerulonephritis), (2) those involving the
interstitium (interstitial nephritis), (3) those leading to
microcapillary or glomerular occlusion, (4) acute tubular
necrosis, and (5) cortical necrosis.
1. Glomerulonephritis
ESSENTIALS OF DIAGNOSIS
Nephritic urine sediment with red blood cells, white
blood cells, and red blood cell casts.
Proteinuria (variable, sometimes in nephrotic range:
≥ 3.5 g/day).
Kidney size normal or increased; no evidence of
obstruction.
Hypertension (variable).
Evidence for immunologic disease: antinuclear anti-
bodies, antistreptolysin O, cryoglobulins, antineutrophil
cytoplasmic antibodies (ANCAs), anti–glomerular base-
ment membrane (anti-GBM) antibodies, IgA levels,
hepatitis B antigen, hepatitis C antibody, HIV, decreased
C3 and C4.
Renal biopsy to confirm diagnosis.
Table 13–6. Causes of intrinsic acute renal failure.
Acute glomerulonephritis
Postinfectious: streptococcal, bacteria, hepatitis B, HIV, visceral
abscess
Systemic vasculitides: systemic lupus erythematosus,
Wegener’s granulomatosis, polyarteritis nodosa, Henoch-Schönlein
purpura, IgA nephritis, Goodpasture’s syndrome
Membranoproliferative glomerulonephritis
Idiopathic
Acute interstitial nephritis
Drugs: penicillins, NSAIDs, ACE inhibitors, allopurinol, cimetidine, H
2
blockers, proton pump inhibitors
Infectious: streptococcal infections, diphtheria, leptospirosis
Metabolic: hyperuricemia, nephrocalcinosis
Poisons: ethylene glycol (calcium oxalate)
Autoimmune disease: systemic lupus erythematosus, cryoglobulinemia
Microcapillary/glomerular occlusion
Thrombotic thrombocytopenic purpura, hemolytic uremic syndrome,
disseminated intravascular coagulation, cryoglobulinemia,
cholesterol emboli
Acute tubular necrosis
Drugs: aminoglycosides, cisplatin, amphotericin B
Ischemia
Septic shock
Intratubular obstruction: rhabdomyolysis, hemolysis, multiple
myeloma, uric acid, calcium oxalate
Poisons: radiopaque contrast media, carbon tetrachloride,
ethylene glycol, heavy metals
Cortical necrosis
Key: ACE = angiotensin-converting enzyme; NSAID = nonsteroidal
anti-inflammatory drug
RENAL FAILURE
323
General Considerations
Table 13–6 lists several glomerulonephritides that are most
commonly associated with acute renal failure. Most—
perhaps all—are immunologically mediated as a result of
recent or ongoing infections, systemic vasculitis, or idiosyn-
cratic autoimmune reactions. Histologic evaluation is most
often positive for immune deposits in varying locations
within the glomerular structures. In pauci-immune
glomerulonephritis, such as is found with Wegener’s granu-
lomatosis or polyarteritis, no immune deposits are found,
but the presence of antineutrophil cytoplasmic antibodies
(ANCAs) may stimulate leukocytes to release their enzymes,
thus mediating an intraglomerular inflammatory reaction.
Clinical Features
A. Symptoms and Signs—Patients with acute glomeru-
lonephritis may present with edema (especially in periorbital
distribution), hypertension, fatigue, and possibly pulmonary
congestion. They may note smoky, rust-colored, or grossly
bloody urine. These findings are common to glomerulonephri-
tis owing to any cause. Other clinical features reflect the under-
lying disease causing glomerulonephritis. Postinfectious
glomerulonephritis presents about 10 days after acute infec-
tion, usually a streptococcal pharyngitis or skin infection.
Symptoms and signs of systemic vasculitis are highly variable
and depend on the distribution of involvement and the size of
the vessels involved. They may include arthralgias and arthritis,
fever, rash, myalgias and muscle tenderness, hypertension,
abdominal pain, nausea, vomiting, diarrhea, headache, and
other features that are manifestations of organ system involve-
ment, including the heart, GI tract, kidneys, CNS, peripheral
nerves, lungs, pleura, and pericardium. In some situations, the
clinical presentation may strongly suggest a given diagnosis,
such as one of the pulmonary-renal syndromes.
B. Laboratory Findings—The most compelling finding sug-
gestive of acute glomerulonephritis is the presence of red
blood cell casts in the urine. These casts are the result of leak-
age of red blood cells past damaged glomeruli into the tubu-
lar lumen, and they must be carefully distinguished from
granular pigmented casts. The absence of red blood cell casts
does not rule out the diagnosis of glomerulonephritis, how-
ever, and they are found occasionally in other disorders such
as acute interstitial nephritis and cholesterol emboli. Other
urinary abnormalities include varying degrees of proteinuria
and pyuria in the absence of urinary infection. A history of
symptoms suggestive of systemic vasculitis can be confirmed
by serologic testing, including antinuclear antibodies, anti-
GBM antibodies, IgA levels, antistreptolysin O antibodies,
cryoglobulins, ANCAs, antibodies and markers for hepatitis
B, hepatitis C, and HIV, and decreased levels of the third and
fourth components of complement (C3 and C4).
Since therapy of glomerulonephritis often entails long-
term risk, renal biopsy usually should be performed to
confirm the diagnosis.
Treatment
Most cases of acute glomerulonephritis are treated with
aggressive immunosuppressive medications, including high-
dose corticosteroids and cyclophosphamide. Recent reports
suggest that mycophenolate mofetil may be an effective alter-
native to cyclophosphamide in some cases. Since therapy is
likely to be prolonged, with the possibility of substantial
morbidity, consultation with a nephrologist is strongly sug-
gested prior to initiation of treatment. On the other hand,
poststreptococcal glomerulonephritis is treated with sup-
portive therapy alone, including prevention of fluid overload
and control of hypertension.
Glomerulonephritis associated with infective endocardi-
tis responds to appropriate antibiotics. Goodpasture’s syn-
drome and cryoglobulinemia often require plasma exchange.
ANCA-positive glomerulonephritis commonly requires
treatment with cyclophosphamide. Patients presenting with
dialysis-requiring renal failure also may benefit from plasma
exchange.
Chadban SJ, Atkins RC: Glomerulonephritis. Lancet
2005;365:1797–806. [PMID 15910953]
Kaplan AA: The use of apheresis in immune renal disorders. Ther
Apher Dial 2003;7:165–72. [PMID: 12918939]
2. Acute Interstitial Nephritis
ESSENTIALS OF DIAGNOSIS
Interstitial nephritis:
History of drug sensitivity, infection, or toxin ingestion.
Flank pain (occasionally).
Kidney size normal or increased.
Allergic interstitial nephritis:
Fever, rash, peripheral blood eosinophilia.
Sterile pyuria, hematuria, white blood cell casts, tubular
epithelial cells, eosinophiluria.
Positive renal gallium scan with prolonged renal uptake.
General Considerations
Several drugs are capable of eliciting an autoimmune attack
on the renal interstitium, resulting in allergic interstitial
nephritis (see Table 13–2). Direct invasion by infection is less
common. Acute urate nephropathy results from the
intratubular precipitation of uric acid and is most often asso-
ciated with the chemotherapeutic treatment of large lym-
phomas or leukemias, with serum levels of uric acid often
above 20 mg/dL. The metabolism of ethylene glycol, ingested
accidentally or deliberately, results in calcium oxalate deposi-
tion within the interstitium and may result in irreversible
CHAPTER 13
324
renal damage. Autoimmune diseases such as systemic lupus
erythematosus or cryoglobulinemia may cause a “pure”
interstitial nephritis but more often present with concomi-
tant glomerular disease.
Clinical Features
A. Symptoms and Signs—Drug-induced allergic intersti-
tial nephritis is often associated with a history of drug hyper-
sensitivity and classically presents with a triad of fever, rash,
and peripheral blood eosinophilia. Unfortunately, the triad is
complete in only one-third of patients. The rash is usually
symmetric and widespread, appears suddenly, and is usually
not pruritic. In the case of NSAID toxicity, nephrotic syn-
drome with glomerular involvement may be present in addi-
tion to evidence of interstitial involvement.
B. Laboratory Findings—Further evidence for interstitial
nephritis includes sterile pyuria, white blood cell casts, and
occasionally, eosinophiluria. Hematuria and varying degrees
of proteinuria usually are present as well. Infectious causes of
interstitial nephritis can be identified by blood or urine cul-
tures. Acute urate nephropathy should be anticipated when-
ever aggressive chemotherapy is initiated in a patient with a
large lymphoma or acute leukemia. In these patients, serum
uric acid is often above 20 mg/dL, and urinalysis reveals flat,
rhomboid crystals of uric acid or needle-shaped crystals of
sodium urate. Autoimmune diseases are best identified by
finding serologic evidence of specific diseases.
C. Imaging Studies—A gallium scan positive for prolonged
renal uptake (>72 hours) supports the diagnosis of allergic
interstitial nephritis.
Treatment
Drug-induced acute interstitial nephritis usually reverses
spontaneously after cessation of the offending agent, but
patients may benefit from a course of corticosteroids if renal
failure is severe or prolonged. In anticipation of aggressive
chemotherapy for lymphomas or leukemias, the risks of
acute urate nephropathy can be greatly reduced by pretreat-
ment with allopurinol and forced alkaline diuresis designed
to keep urine pH ≥ 7.0. Alkalinization of the urine can be
accomplished by administration of 5% dextrose in water to
which approximately 100–150 meq/L of sodium bicarbonate
has been added. Acetazolamide may be added to stimulate
bicarbonate excretion.
Infectious causes of interstitial nephritis are treated with
antibiotics selected for urinary tract bacterial pathogens and
adjusted on the basis of urine and blood cultures. Ethylene
glycol-induced renal failure may require hemodialysis.
Baker RJ, Pusey CD: The changing profile of acute tubulointersti-
tial nephritis. Nephrol Dial Transplant 2004;19:8–11. [PMID:
14671029]
Taber SS, Mueller BA: Drug-associated renal dysfunction. Crit
Care Clin 2006;22:357–74. [PMID 16678005]
3. Microcapillary & Glomerular Occlusion
ESSENTIALS OF DIAGNOSIS
Hematuria.
Proteinuria (variable).
Thrombotic thrombocytopenic purpura–hemolytic
uremic syndrome and disseminated intravascular
coagulation: thrombocytopenia and microangiopathic
hemolytic anemia.
Cryoglobulinemia: cryoglobulin present in serum, pur-
pura (variable), and hypertension (variable).
Cholesterol embolization: hypertension, signs of periph-
eral vascular occlusive disease (claudication), skin discol-
oration (livedo reticularis, purple toes), eosinophilia, and
a history of recent intraaortic catheterization.
General Considerations
These disorders have in common obstruction of the various
parts of the renal circulation or glomerular vascular pole.
Thrombotic thrombocytopenic purpura (TTP), hemolytic
uremic syndrome (HUS), and disseminated intravascular
coagulation (DIC) have the potential to deposit thrombi of
fibrin and platelets in the glomerular capillary lumen.
Cryoglobulins can precipitate in glomerular capillaries but
also may cause an immunologically mediated glomeru-
lonephritis. Microscopic cholesterol emboli may occur spon-
taneously in patients with severe atherosclerotic disease but
are seen most often after catheterization of the aorta for diag-
nostic purposes. Cholesterol emboli are of varying size and
may block intrarenal arteries, preglomerular arterioles, or
the vascular pole of the glomerulus.
Clinical Features
A. TTP and HUS—Thrombotic thrombocytopenic purpura
(TTP) and hemolytic uremic syndrome (HUS) can be sus-
pected when severe thrombocytopenia is associated with
microangiopathic hemolytic anemia in the presence of nor-
mal prothrombin time and partial thromboplastin time.
Symptoms include jaundice, renal disease, and varied neuro-
logic manifestations.
B. DIC—Disseminated intravascular coagulation (DIC) is
often associated with sepsis and is diagnosed by a combina-
tion of thrombocytopenia, increased fibrin split products,
elevated
D
-dimers, and elevated prothrombin time and par-
tial thromboplastin time. Owing to the ongoing coagulopa-
thy, renal biopsies are performed only rarely in these diseases.
C. Cryoglobulinemia—Cryoglobulinemia is often associ-
ated with purpura and hypertension and is confirmed by
identification of serum cryoglobulins. It has now been
RENAL FAILURE
325
established that many cases of cryoglobulinemia are associ-
ated with hepatitis C infection.
D. Cholesterol Embolization—A syndrome of microvascu-
lar embolization with livedo reticularis, purple toes, signs of
peripheral vascular disease, and occasionally eosinophilia
will lead to a suspicion of cholesterol embolization. Renal
biopsy, however, is the only confirmatory test.
Treatment
TTP requires aggressive plasma exchange with fresh-frozen
plasma. HUS may respond to intravenous immune globulin
or plasma exchange. DIC is best treated by management of
the inciting cause. Cryoglobulinemia may respond to
immunosuppressive therapy or plasma exchange. If there is
an associated hepatitis C infection, long-term management
may include interferon. Renal failure associated with choles-
terol embolization is irreversible. However, on occasion,
renal failure from cholesterol emboli may be associated with
an element of acute tubular necrosis owing to vascular occlu-
sion. In these patients, some improvement may occur. On
theoretical grounds, anticoagulation is contraindicated
because it may be a precipitating factor.
Fukumoto Y et al: The incidence and risk factors of cholesterol
embolization syndrome, a complication of cardiac catheteriza-
tion: A prospective study. J Am Coll Cardiol 2003;42:211–6.
[PMID: 12875753]
Gallosi A et al: Extrahepatic manifestations of chronic HCV infec-
tion. J Gastrointest Liver Dis 2007;16:65–73. [PMID: 17410291]
George JN: Clinical practice: Thrombotic thrombocytopenic pur-
pura. N Engl J Med 2006;354:1927–35. [PMID: 6672704]
Guillevin L, Pagnoux C: Indications of plasma exchanges for sys-
temic vasculitides. Ther Apher Dial 2003;7:155–60. [PMID:
12918937]
Kamar N et al: Treatment of hepatitis C-virus-related glomeru-
lonephritis. Kidney Int 2006;69:436–9. [PMID: 16514428]
Meyrier A: Cholesterol crystal embolism: Diagnosis and treatment.
Kidney Int 2006;69:1308–12. [PMID: 16614719]
Rock G: The management of thrombotic thrombocytopenic pur-
pura in 2005. Semin Thromb Hemost 2005;31:709–16. [PMID:
16388422]
4. Acute Tubular Necrosis
ESSENTIALS OF DIAGNOSIS
History of exposure to nephrotoxic drugs or hypotension.
Oliguria in ischemic ATN but absence of oliguria in toxic
ATN.
FE
Na
>1%.
Urinalysis: tubular epithelial cells, red blood cells, and
“muddy brown” casts.
General Considerations
The most common form of hospital-acquired intrinsic acute
renal failure is referred to as acute tubular necrosis (ATN).
This designation is somewhat misleading because in many
cases there is more tubular dysfunction than necrosis. In gen-
eral, the pathogenesis involves direct toxicity to the tubular
epithelium, resulting in inability to reabsorb glomerular fil-
trate properly and massive sodium wasting, intrarenal vaso-
constriction, and tubular blockage with necrotic cellular
debris and proteinaceous material. The most common
causes are ischemia, exogenous toxins including drugs (see
Table 13–2) and radiocontrast agents, and endogenous tox-
ins such as myoglobin, hemoglobin, light chains of
immunoglobulins, or crystals (see Table 13–6). Any cause of
prerenal azotemia (see Table 13–4), if sufficiently severe or
prolonged, can cause ischemic tubular damage and lead to
this disorder. Furthermore, all conditions of renal hypoper-
fusion increase the likelihood of toxicity from exogenous or
endogenous toxins. In many cases, a combination of noxious
stimuli can be identified.
Clinical Features
Ischemic ATN is encountered most often after an identifiable
episode of hemodynamic compromise or after prolonged
ischemia during aortic surgery. The presentation is that of
acute onset of oliguria with an FE
Na
greater than 1% and a
urine rich with cellular and proteinaceous debris, including
red blood cells, white blood cells, tubular epithelial cells, and
“muddy brown” casts. In this setting, correction of identifi-
able prerenal factors and a renal ultrasound examination
excluding obstruction are often sufficient to make the diag-
nosis on clinical grounds, and renal biopsy usually is reserved
for patients in whom renal failure is abnormally prolonged
(6–8 weeks).
ATN from toxic causes is often nonoliguric, and the urine
sediment may be unremarkable. A history of toxin exposure
(eg, aminoglycosides, radiocontrast agents, etc.) and the
exclusion of prerenal and postrenal causes are often suffi-
cient for a clinical diagnosis. Urinalysis with dipstick positive
for blood in the absence of identifiable red blood cells is sug-
gestive of rhabdomyolysis or hemolysis.
Treatment
Although several experimental treatments have been
designed to enhance recovery (eg, antiendothelin antibodies,
ATP, MgCl
2
, thyroxine, growth factors, etc.), none are cur-
rently applied in clinical practice. Experimentally, infusions
of atrial natriuretic peptide have been shown to increase cre-
atinine clearance and decrease the need for dialysis, but cur-
rently available treatment strategies are limited to prevention
and management. Preventive measures include ensuring ade-
quate hydration when nephrotoxic agents are unavoidable
(eg, radiocontrast agents or necessary nephrotoxic antibi-
otics) and proper dosing of nephrotoxic drugs with special

CHAPTER 13
326
attention to changing renal function and daily measurements
of toxic drug levels. Recent evidence suggests that pretreat-
ment with acetylcysteine or infusions of sodium bicarbonate
may help to prevent contrast-induced nephropathy.
In the early stages of oliguric ATN, a short course of man-
nitol or furosemide may decrease the tendency toward tubu-
lar obstruction. Mannitol has the theoretical advantage of
stimulating an osmotic diuresis originating in the proximal
tubule, with the possibility of increasing tubular flow
throughout the nephron. Unfortunately, when mannitol is
given without resulting diuresis, it may precipitate intravas-
cular volume expansion, which may be poorly tolerated. If
diuresis is not established after 24 hours of treatment with
furosemide or mannitol, further attempts at diuretic therapy
will be futile and potentially harmful.
Birck R et al: Acetylcysteine for prevention of contrast nephropathy:
Meta-analysis. Lancet 2003;362:598–603. [PMID: 12944058]
Gill N et al: Renal failure secondary to acute tubular necrosis:
Epidemiology, diagnosis, and management. Chest
2005;128:2847–63. [PMID: 16236963]
Merten GJ et al: Prevention of contrast-induced nephropathy with
sodium bicarbonate. JAMA 2004;291:2328–34. [PMID:
15150204]
Weisbord SD, Palevsky PM: Radiocontrast-induced acute renal
failure. J Intensive Care Med 2005;20:63–75. [PMID: 15855219]
5. Cortical Necrosis
Cortical necrosis is the result of severe and prolonged renal
hypoperfusion and has been most often associated with the
hemodynamic catastrophes of pregnancy, including eclamp-
sia, abruptio placentae, and postpartum hemorrhage. The
diagnosis is usually suspected when an episode of acute renal
failure fails to resolve in 6–8 weeks. Renal biopsy is often
required for definitive diagnosis. Renal damage is irre-
versible.
Common Syndromes Associated with
Acute Renal Failure
1. Pigment Nephropathy: Rhabdomyolysis
& Hemolysis
ESSENTIALS OF DIAGNOSIS
Urine dipstick positive for heme in the absence of red
blood cells.
Rhabdomyolysis: elevated creatine kinase and aldolase;
decreased BUN:creatinine ratio.
Hemolysis: elevated serum-free hemoglobin, decreased
haptoglobin, elevated lactate dehydrogenase (LDH).
General Considerations
Rhabdomyolysis is commonly associated with acute renal
failure, especially in the context of traumatic crush injury.
Hemolysis is usually more insidious and less likely to cause
renal failure except when severe, such as with major transfu-
sion incompatibilities.
In the case of rhabdomyolysis, intravascular volume deple-
tion promotes intratubular precipitation of myoglobin and is
probably the most important comorbid factor in the initiation
of nephrotoxicity. Hemoglobin-related toxicity seems to be
enhanced by the presence of fragmented red blood cell mem-
branes. Several etiologic factors in the development of rhab-
domyolysis and hemolysis are listed in Table 13–7.
Clinical Features
A. Symptoms and Signs—Patients may note dark-colored
red or brown urine. Symptoms and signs in rhabdomyolysis
may be due to crush injury and other associated trauma and
may be focal or diffuse. However, patients with rhabdomyol-
ysis owing to inflammatory muscle disorders such as
polymyositis may complain of muscle pain, tenderness, and
weakness. Patients with hemolysis-induced renal failure will
have symptoms related to severe anemia. Other clinical find-
ings are due to renal failure and associated complications.
Table 13–7. Causes of pigment nephropathy: hemolysis
and rhabdomyolysis.
Rhabdomyolysis
Physical trauma: crush injury, heat stress, electrocution, exercise,
hypothermia, malignant hyperthermia, neuroleptic malignant syndrome
Anoxic injury: arterial occlusion, seizures, tetanus, compartment
syndrome
Metabolic: hypokalemia, hypophosphatemia, diabetic ketoacidosis,
myxedemia, carnitine deficiency, hereditary muscle enzyme
deficiency
Infections: influenza-like viral infections, gas gangrene, pyomyositis
Inflammation: polymyositis, dermatomyositis
Poisons and toxins: ethanol, amphetamine, cocaine, snake and
spider venom.
Hemolysis
Transfusion reactions
Drug toxicities: quinine sulfate, hydralazine, etc.
Poisons and toxins: benzene, aniline, fava beans, snake and spider
venom, etc.
Mechanical trauma: valvular prosthesis, extracorporeal circulation,
march hemoglobinuria
Enzyme deficiencies: glucose-6-phosphate dehydrogenase deficiency
Osmotic stress: intoxication with hypotonic fluids as seen in drowning,
transuretheral prostatectomy, incorrect priming of extracorporeal
circulation
Infections: malaria
Autoimmune: drugs, systemic lupus erythematosus
