Bongard Frederic , Darryl Sue. Diagnosis and Treatment Critical Care
Подождите немного. Документ загружается.


247
0012
Respiratory Failure
Darryl Y. Sue, MD
Janine R. E. Vintch, MD
PATHOPHYSIOLOGY OF RESPIRATORY FAILURE
Respiratory failure is inability of the respiratory system to
maintain a normal state of gas exchange from the atmos-
phere to the cells as required by the body. Simply, the role of
the respiratory system is to maintain normal arterial blood
P
O
2
,P
CO
2
, and pH. Respiratory failure can result from disor-
ders of the lungs, heart, chest wall, respiratory muscles, and
central ventilatory control mechanisms. In addition, dys-
functions of the heart, the pulmonary and systemic circula-
tions, the oxygen-carrying capacity of the blood, and
systemic capillaries have important implications for patients
with respiratory failure.
Definition
Respiratory failure is present (1) if arterial P
O
2
(Pa
O
2
) is less
than 60 mm Hg or (2) if arterial P
CO
2
(Pa
CO
2
) is greater than
45 mm Hg. A Pa
O
2
of less than 60 mm Hg, indicating hypox-
emic respiratory failure, is valid during room air breathing
(inspired O
2
fraction [F
IO
2
] = 0.21), but hypoxemia while
breathing supplemental oxygen also indicates respiratory
failure. An exception to the rule that Pa
CO
2
greater than
45 mm Hg defines hypercapnic respiratory failure is meta-
bolic acidosis. Patients with metabolic acidosis normally
decrease Pa
CO
2
to compensate for low pH, but if Pa
CO
2
is
abnormally elevated even though below 45 mm Hg during
metabolic acidosis, respiratory failure is present.
Effectiveness and Efficiency of the Respiratory
System
It is useful to distinguish between the effectiveness and the effi-
ciency of the respiratory system in maintaining arterial blood
gases. An arterial P
O
2
of 100 mm Hg, for example, indicates
effective oxygenation (more than adequate). Effective elimi-
nation of CO
2
is evidenced by an arterial P
CO
2
of 40 mm Hg
if this Pa
CO
2
is consistent with an acceptable acid-base status.
On the other hand, there is an obvious difference between two
patients who each have a Pa
O
2
of 100 mm Hg if the first
patient is breathing room air (F
IO
2
= 0.21) and the other is
breathing 100% O
2
(F
IO
2
= 1.0). The first patient is exchanging
oxygen more efficiently from the atmosphere to the arterial
blood than the latter. Pa
O
2
determines the effectiveness of oxy-
genation; the relationship between inspired oxygen concentra-
tion and Pa
O
2
is a marker of efficiency.
Pa
CO
2
measures the effectiveness of ventilation. Two
patients with Pa
CO
2
of 40 mm Hg have equally effective ven-
tilation. If one of them needs a higher minute ventilation
(respired gas volume in 1 minute) than the other, however,
the patient requiring the higher minute ventilation is less
efficiently eliminating CO
2
than the one with the lower
minute ventilation. Thus the relationship between Pa
CO
2
and
minute ventilation (
.
V
E
) reflects the efficiency of ventilation.
Measurement of the degree of inefficiency of oxygenation
and ventilation is discussed below.
Classification of Respiratory Failure
One classification of respiratory failure separates disorders
that affect the lungs (airways, alveolar spaces, interstitium,
and pulmonary circulation) from those that affect primarily
the nonlung components of the respiratory system.
Respiratory failure from diseases that directly affect the
lungs almost always has hypoxemia, but these patients may
or may not have hypercapnia depending on the type of dis-
ease and its severity. Examples include pneumonia, aspira-
tion of gastric contents, acute respiratory distress syndrome
(ARDS), pulmonary embolism, asthma, and interstitial lung
diseases.
Disorders of the nonpulmonary respiratory system usu-
ally cause hypercapnia plus hypoxemia. Examples include
diseases that cause weakness of the respiratory muscles, CNS
diseases that disrupt ventilatory control, and conditions that
affect chest wall shape or size, such as kyphoscoliosis. In
hypercapnic respiratory failure, the lungs may in fact be nor-
mal, so hypoxemia out of proportion to hypercapnia most
Copyright © 2008 by The McGraw-Hill Companies, Inc. Click here for terms of use.
CHAPTER 12
likely indicates additional lung disease. An example might be
a patient with neuromuscular weakness from myasthenia
gravis who initially presents with hypercapnic respiratory
failure but who subsequently develops pneumonia from an
inability to clear tracheal secretions. At this point, the patient
may be considered to have hypoxemic respiratory failure in
addition to hypercapnia.
Hypercapnic Respiratory Failure
Patients with hypercapnic respiratory failure have an
abnormally high Pa
CO
2
. Because CO
2
is elevated in the alve-
olar spaces, O
2
is displaced from the alveoli, and Pa
O
2
decreases. Thus these patients usually have both hypercap-
nia and hypoxemia unless the inspired gas is enriched with
oxygen. The lungs themselves may or may not be abnormal,
especially if the primary disease affects nonlung parts of the
respiratory system such as the chest wall, respiratory mus-
cles, or brain stem. On the other hand, chronic obstructive
lung disease not infrequently leads to hypercapnic respira-
tory failure, and some patients with severe asthma, end-
stage pulmonary fibrosis, and severe ARDS can develop
hypercapnia.
Physiologic Considerations
A. Alveolar Hypoventilation—In the steady state, a patient
produces CO
2
from metabolic processes each minute and
must eliminate that CO
2
from the lungs each minute. If the
minute output of CO
2
(
.
V
CO
2
) exchanges into the gas-
exchanging spaces of the lungs, the fractional concentration
of CO
2
in the alveolar space is:
where F
ACO
2
is the fractional concentration of CO
2
in the
alveoli,
.
V
A
is the volume of air exchanging in the alveoli dur-
ing that minute (alveolar ventilation), and 0.826 adjusts for
temperature and water vapor. The sum of partial pressures of
individual gases equals the total pressure, so the fraction of
alveolar gas that is CO
2
also can be written as
where P
ACO
2
is the alveolar partial pressure of CO
2
and P
B
is
barometric pressure. Because P
ACO
2
cannot be measured,
Pa
CO
2
is usually substituted. Substituting and rearranging
the preceding equation gives
where 863 includes factors that adjust for
.
V
CO
2
at standard
temperature and pressure, dry (STPD); for
.
V
A
at body tem-
perature and pressure, saturated (BTPS); and for Pa
CO
2
in mil-
limeters of mercury. For constant CO
2
output, the relationship
between Pa
CO
2
and
.
V
A
describes a “ventilatory hyperbola” in
which Pa
CO
2
and
.
V
A
are inversely related. Thus hypercapnia is
always means alveolar hypoventilation, and hypocapnia is syn-
onymous with alveolar hyperventilation. Because alveolar ven-
tilation cannot be measured, estimation of alveolar ventilation
can be made only by using Pa
CO
2
and this formula.
B. Minute Ventilation—In a patient with alveolar hypoven-
tilation,
.
V
A
is reduced and Pa
CO
2
is increased. Although
.
V
A
cannot be measured directly, the total amount of gas moving
into and out of the lungs each minute can be measured eas-
ily. This is defined as the minute ventilation (
.
V
E
, L/min).
.
V
E
is
the sum of the
.
V
A
(the portion participating in gas exchange)
and any wasted or dead-space ventilation (
.
V
D
):
.
V
E
=
.
V
A
+
.
V
D
and
.
V
A
=
.
V
E
–
.
V
D
Substituting,
.
V
A
= f × V
A
;
.
V
E
= f × V
T
; and
.
V
D
= f × V
D
—
where f is the respiratory frequency, V
T
is tidal volume, and
V
D
is dead-space volume:
where V
D
/V
T
is the dead space:tidal volume ratio.
Substituting and rearranging gives
V
D
/V
T
reflects the degree of ventilatory inefficiency of
the lungs. In a normal subject, V
D
/V
T
is about 0.30, meaning
that about 30% of the minute ventilation is not participating
in gas exchange. In most lung diseases, the wasted proportion
of
.
V
E
increases, so V
D
/V
T
rises. From the preceding formula
for a constant V
D
/V
T
and
.
V
CO
2
, the relationship of Pa
CO
2
and
.
V
E
is described by a hyperbola transposed upward from
the hyperbola described by the relationship between Pa
CO
2
and
.
V
A
. For different values of V
D
/V
T
, these relationships are
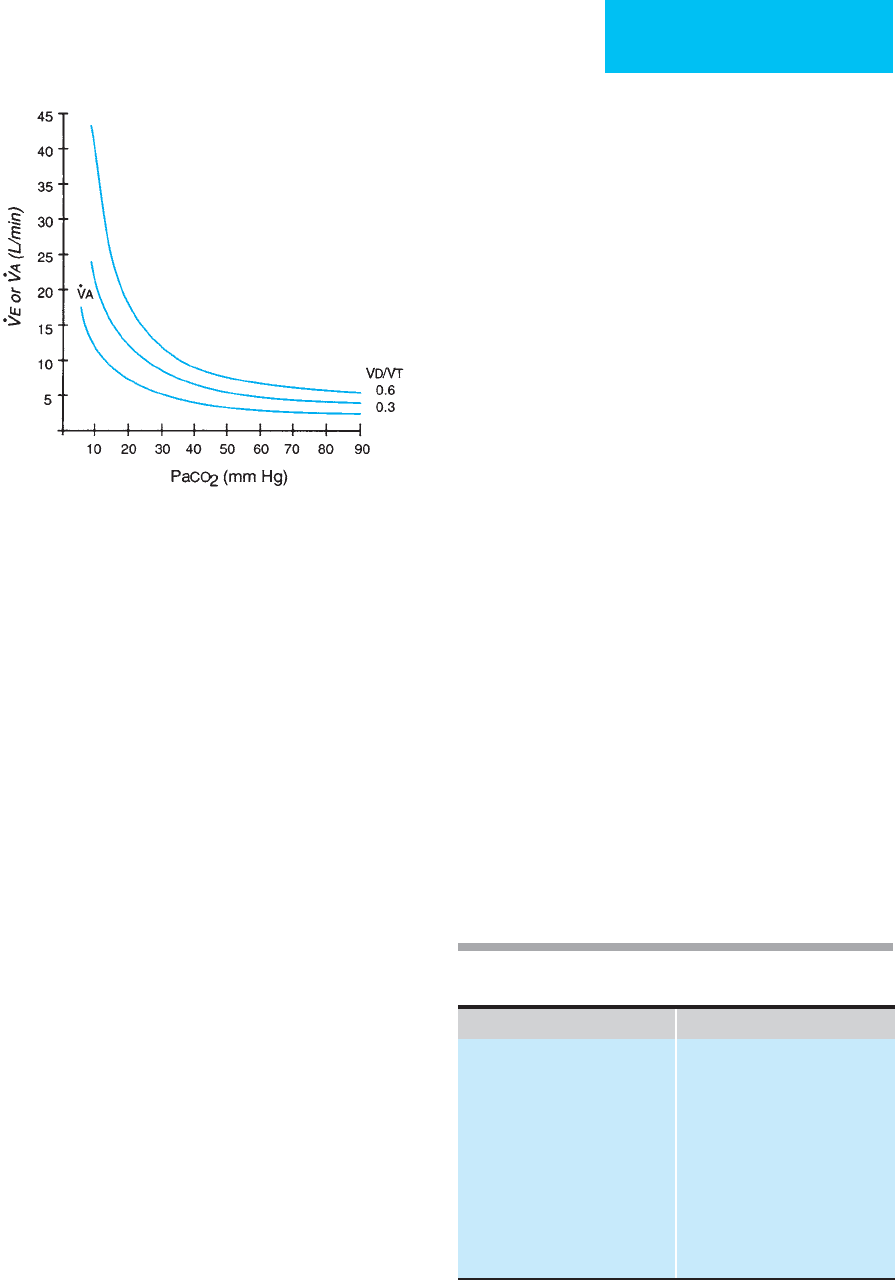
described by a family of hyperbolic curves (Figure 12–1).
These curves are useful in estimating V
D
/V
T
from measure-
ment of Pa
CO
2
and
.
V
E
, or they can be used to determine the
change in
.
V
E
needed to cause a desired change in Pa
CO
2
.
C. Mechanisms of Hypercapnia—Hypercapnia (alveolar
hypoventilation) occurs when
.
V
E
is abnormally low or when
.
V
E
is normal or high but V
D
/V
T
is abnormally increased. It is
emphasized that hypoventilation refers to alveolar
V
V
Pa
V
V
E
CO
CO
D
T
2
2
=
×
×−
⎛
⎝
⎜
⎞
⎠
⎟
863
1
VV 1
V
V
AE
D
T
=× −
⎛
⎝
⎜
⎞
⎠
⎟
V L/min Pa mm Hg V (L/min)
CO CO A
22
1
863
() ( )=××
F
P(mmHg)
P(mmHg)
ACO
ACO
B
2
2
=
F
VL/minSTPD)
V(L/minBTPS) 0.
ACO
CO
A
2
2
=
×
(
8826
248
RESPIRATORY FAILURE
249
hypoventilation, and for this reason, hypercapnia may be
present even though the patient’s
.
V
E
is greater than normal if
V
D
/V
T
is abnormally high or CO
2
output is increased (exer-
cise or other increased metabolic rate).
Alveolar dead space and V
D
/V
T
are useful physiologic
concepts that may or may not have anatomic counterparts.
The trachea and airways are conduits for gas moving into
and out of the lungs during the respiratory cycle but do not
participate in gas exchange with the pulmonary capillary
blood. These spaces make up the anatomic dead space. An
artificial airway or part of a mechanical ventilator circuit that
is common to both inspiratory and expiratory pathways also
contributes to anatomic dead space. However, in patients
with lung disease, most of the increase in total dead space
consists of “physiologic dead space,” in which regional venti-
lation exceeds regional blood flow (ventilation-perfusion
[
.
V/
.
Q] mismatching). While
.
V/
.
Q mismatching usually is con-
sidered as a mechanism of hypoxemia rather than hypercap-
nia, it theoretically should cause elevated Pa
CO
2
as well.
However, in all but severe instances of
.
V/
.
Q mismatching,
hypercapnia stimulates increased ventilation, returning
Pa
CO
2
to normal. Thus
.
V/
.
Q mismatching does not usually
result in hypercapnia but in normocapnia with increased
.
V
E
.
As can be seen in Figure 12–1, increased
.
V
E
in the face of nor-
mal Pa
CO
2
indicates increased V
D
/V
T
—in this case an
increase in physiologic dead space.
Clinical Features
Acute hypercapnia acts largely on the CNS (Table 12–1).
Increased Pa
CO
2
depresses the CNS, and the mechanism is
primarily through a fall of pH in the cerebrospinal fluid
(CSF) resulting from acute elevation of Pa
CO
2
. Because CO
2
rapidly diffuses into the CSF, pH falls rapidly and severely
with acute hypercapnia. With chronic elevation of Pa
CO
2
,
however, the increase in Pa
CO
2
is present long enough for
plasma and CSF bicarbonate to increase in compensation for
the chronic respiratory acidosis. This explains why low pH
rather than absolute level of Pa
CO
2
best correlates with
altered mental status and other clinical changes.
Symptoms of hypercapnia may overlap those of hypox-
emia. In addition, while hypercapnia stimulates ventilation in
normal subjects, hypercapnic patients may have either
decreased or increased minute ventilation depending on the
primary disorder leading to respiratory failure. Dyspnea,
tachypnea, and hyperpnea may be associated with hypercapnic
respiratory failure just as often as bradypnea and hypopnea.
The major differential distinguishing point is between
hypercapnic respiratory failure owing to lung disease and that
resulting from nonlung disorders. Patients with lung disease
often will have hypoxemia out of proportion to the degree of
hypercapnia. This can be assessed using the alveolar-arterial
P
O
2
difference. However, patients with nonlung problems
may have secondary hypoxemia because the effects of neuro-
muscular weakness, for example, contribute to atelectasis or
aspiration pneumonia. Disorders of the lungs—in contrast to
disorders of other components of the respiratory system—are
associated with increased V
D
/V
T
, elevated
.
V
E
, and respira-
tory frequency. Patients with respiratory muscle weakness also
may be tachypneic. The effects of hypercapnia and hypoxemia
may mask neurologic disorders, overmedication with seda-
tives, myxedema, or head trauma. Alteration of mental status
may make it difficult to assess muscle strength, and the
strength of muscles in the extremities may not correlate with
respiratory muscle strength.
Figure 12–1. Alveolar ventilation (
.
V
A
) or minute ventila-
tion (
.
V
E
) as a function of Pa
CO
2
for a constant CO
2
output of
200 mL/min. Increased Pa
CO
2
(hypercapnia) is due to alve-
olar hypoventilation; decreased Pa
CO
2
(hypocapnia) is the
same as alveolar hyperventilation. The other two curves
show the relationship of
.
V
E
and Pa
CO
2
for normal dead
space:tidal volume ratio (V
D
/V
T
= 0.3) and abnormal
dead space:tidal volume ratio (V
D
/V
T
= 0.6).
Hypercapnia Hypoxemia
Somnolence
Lethargy
Coma
Asterixis
Restlessness
Tremor
Slurred speech
Headache
Papilledema
Anxiety
Tachycardia
Tachypnea
Diaphoresis
Arrhythmias
Altered mental status
Confusion
Cyanosis
Hypertension
Hypotension
Seizures
Lactic acidosis
Table 12–1. Clinical manifestations of hypercapnia and
hypoxemia.
CHAPTER 12
250
Hypoxemic Respiratory Failure
Hypoxemic respiratory failure is much more commonly
encountered than hypercapnic respiratory failure. These
patients have abnormally low Pa
O
2
but normal or low Pa
CO
2
.
The latter distinguishes them from hypercapnic respiratory
failure, in which the primary problem is alveolar hypoventila-
tion. Outside of unusual environments in which the atmos-
phere has a severely reduced amount of oxygen, such as high
altitude or when oxygen has been replaced with other gases,
hypoxemic respiratory failure indicates disease affecting
the lung parenchyma or pulmonary circulation. Common
situations in which hypoxemia without elevated Pa
CO
2
are
seen include pneumonia, aspiration of gastric contents, pul-
monary embolism, asthma, and acute respiratory distress
syndrome (ARDS).
Physiologic Considerations
A. Hypoxemia and Hypoxia—The term hypoxemia most
often denotes low P
O
2
in arterial blood (Pa
O
2
), but it can be
used to mean low capillary, venous, or pulmonary capillary
P
O
2
as well. It also may be used to signify low blood O
2
con-
tent or reduced saturation of hemoglobin with oxygen.
Hypoxemia should be distinguished from hypoxia, which is
decreased O
2
delivery to the tissues or the effects of
decreased tissue O
2
delivery. While hypoxia will result from
severe hypoxemia, hypoxia also can be a consequence of
decreased O
2
delivery owing to low cardiac output, anemia,
septic shock, or carbon monoxide poisoning, in which the
Pa
O
2
may be normal or even elevated.
B. Mechanisms of Hypoxemia—The physiologic mecha-
nism of arterial hypoxemia has important implications for
identifying the type of lung disease and the response to ther-
apy with oxygen or other treatment. Five distinct mecha-
nisms of hypoxemia can be identified, but these five can be
divided conceptually into two major groups (Table 12–2):
(1) decreased alveolar P
O
2
(P
AO
2
) and (2) increased influence
of venous admixture.
Mechanisms of arterial hypoxemia can be demonstrated
by analysis of the possible extremes of oxygenation of the
arterial blood. If desaturated systemic venous blood return-
ing to the lungs gained no oxygen during transit through the
lungs, the arterial blood would have the same oxygen con-
tent and partial pressure as the systemic venous blood (obvi-
ously a situation incompatible with life). Systemic venous
blood P
O
2
(P
–
v
O
2
) determines the lower limit for arterial P
O
2
.
On the other hand, if all the desaturated venous blood pass-
ing through the lungs reaches equilibrium with gases in the
alveolar space, then Pa
O
2
equals P
AO
2
. Thus alveolar P
AO
2
determines the hypothetical upper limit for arterial P
O
2
.
Therefore, all possible values of Pa
O
2
must be between P
–
v
O
2
and P
AO
2
.
Arterial hypoxemia is always the result either of decreased
P
AO
2
or of a greater quantity of desaturated venous blood
(venous admixture) mixing with pulmonary capillary blood
(see Table 12–2). In some patients with hypoxemic respira-
tory failure, both these mechanisms play a role.
C. Decreased P
A
o
2
—The total pressure in the alveolar space
is the sum of P
O
2
,P
CO
2
,P
H
2
O
, and P
N
2
. Because P
H
2
O
and
P
N
2
do not change appreciably, any increase in P
ACO
2
must
cause a fall in P
AO
2
. Thus alveolar hypoventilation causes
decreased P
AO
2
, which must result in decreased Pa
O
2
.The
alveolar gas equation, in simplified form, shows the relation-
ship between alveolar P
O
2
and P
CO
2
:
Mechanism
Pa
CO
2
(P
ACO
2
) P
AO
2
P(
A
–a)
O
2
*
P
O
2
on 100%
O
2
(mm Hg)
†
Example
Alveolar P
O
2
↓ Inspired P
O
2
Hypoventilation
↓
↑
↓
↓
Normal
‡
Normal
>550
>550
High altitude.
Neuromuscular disease, obesity-
hypoventilation syndrome.
Venous admixture
Right-to-left shunt
.
V/
.
Q mismatching
Diffusion limitation
Normal or low
Normal or low
Normal or low
Normal
Normal
Normal
Increased
Increased
Increased
<550
>550
>550
ARDS, septal defect.
Pneumonia, asthma, COPD.
Alveolar proteinosis.
∗
P(
A
–a)o
2
on room air. Normal <15–20 mm Hg.
†
Pao
2
while breathing 100% O
2
distinguishes right-to-left shunt from other mechanisms.
‡
P(
A
–a)o
2
calculated using P
AO
2
from alveolar gas equation.
Table 12–2. Mechanisms of hypoxemia.

RESPIRATORY FAILURE
251
where F
IO
2
is the oxygen fraction of the inspired gas, P
B
is the
barometric pressure, and R is the respiratory gas exchange
ratio, the steady-state ratio of CO
2
entering and O
2
leaving
the alveolar space. In practice, Pa
CO
2
substitutes for P
ACO
2
.
Because P
AO
2
decreases with increased P
ACO
2
, alveolar
hypoventilation is a cause of hypoxemia (reduced Pa
O
2
).
The alveolar gas equation also indicates that hypoxemia
occurs if total barometric pressure is reduced, such as at high
altitude or if F
IO
2
is low (such as when breathing a gas in
which some of the oxygen has been replaced by another gas).
In hypoxemia owing to reduced P
AO
2
alone, the decrease in
Pa
O
2
approximately equals the fall in P
AO
2
, and the alveolar-
arterial P
O
2
difference is normal.
D. Venous Admixture—The other causes of hypoxemia
result from increased amounts of deoxygenated venous
blood reaching the arterial blood without becoming fully
oxygenated by exposure to the alveolar gas. The alveolar-
arterial P
O
2
difference (P[
A
–a]
O
2
) is increased because
of increased venous admixture. During room air breath-
ing, P(
A
–a)
O
2
is normally between 10 and 20 mm Hg,
increasing with age and when the subject is in the
upright position.
During room air breathing, F
IO
2
= 0.21; if R = 0.8, Pa
CO
2
= 40 mm Hg, and Pa
O
2
= 55 mm Hg, then
P
AO
2
= (0.21 × 713) – (40/0.8)
= 150 – 50 = 100 mm Hg
and
P(
A
–a)
O
2
= 100 – 55 = 45 mm Hg
In this example, arterial hypoxemia is present (Pa
O
2
<60
mm Hg), and P(
A
–a)
O
2
is increased (>20 mm Hg). It should
be concluded that hypoxemia is due to one of the causes of
increased venous admixture.
1. Right-to-left shunt—If some systemic venous blood
bypasses the alveoli and then mixes with blood that did go
through the lungs, the resulting arterial mixture must have a
P
O
2
between P
AO
2
and P
–
v
O
2
. The exact P
O
2
depends on the
proportion of blood that bypassed the lungs and the values of
P
AO
2
and P
–
v
O
2
. This mechanism of hypoxemia is known as
right-to-left shunt, one of the mechanisms of hypoxemia
owing to increased venous admixture. Right-to-left shunt may
occur because of complete atelectasis of a lung or lobe in
which blood flow is maintained, or may be seen in patients
with congenital heart disease in which there is a right-to-left
shunt through a cardiac septal defect. Patients with ARDS may
have such severe pulmonary edema, localized atelectasis, or
alveolar collapse that right-to-left shunt occurs. Clues to the
presence of right-to-left shunt include severe hypoxemia while
breathing room air, only very small increases in Pa
O
2
when
supplemental oxygen is administered, a need to give an F
IO
2
greater than 0.6 to achieve an acceptable Pa
O
2
, and a Pa
O
2
of
less than 550 mm Hg while breathing 100% O
2
.By conven-
tion, when Pa
O
2
is less than 550 mm Hg while breathing 100%
O
2
, right-to-left shunt is confirmed.
2. Ventilation-perfusion mismatching—A second cause
of hypoxemia owing to venous admixture is ventilation-
perfusion (
.
V/
.
Q) mismatching. This mechanism, sometimes
termed
.
V/
.
Q inequality, is the most frequent cause of hypox-
emia. In contrast to right-to-left shunt, hypoxemia in
.
V/
.
Q
mismatching does not result from venous blood completely
bypassing ventilated areas of lung. Rather, some regions of
the lungs have insufficient ventilation for the amount of
blood flow, whereas others have excessive ventilation for the
amount of regional blood flow. Pulmonary capillary blood
draining those parts of the lungs that have relative “hypoven-
tilation” is less well oxygenated and contributes to hypox-
emia. The effects of
.
V/
.
Q mismatching on gas exchange are
often quite complicated, but practically any lung disease that
alters the distribution of ventilation or blood flow can result
in
.
V/
.
Q mismatching. Thus hypoxemia owing to
.
V/
.
Q mis-
matching is seen in asthma and other chronic obstructive
lung diseases in which variations in airway resistance distrib-
ute ventilation unevenly.
.
V/
.
Q mismatching also contributes
to hypoxemia in pulmonary vascular diseases such as pul-
monary thromboembolism, in which the distribution of per-
fusion is altered. In contrast to right-to-left shunt, most
patients with
.
V/
.
Q mismatching respond dramatically to sup-
plemental oxygen therapy. A clue to
.
V/
.
Q mismatching, there-
fore, is that the Pa
O
2
can be relatively easily brought up to
acceptable values with the administration of supplemental
oxygen.
3. Diffusion limitation—The third mechanism of hypox-
emia owing to venous admixture is diffusion limitation of O
2
transfer. This is an unusual cause of hypoxemia, and the basis
for this mechanism is often misunderstood. Normally, there
is more than sufficient time for blood passing through the
lungs to become fully equilibrated with the gases in the alve-
oli. Rarely, however, pulmonary capillary blood passes so
quickly through the lungs that there is insufficient time for
pulmonary capillary P
O
2
to equilibrate with P
AO
2
, resulting
in hypoxemia. This is a form of venous admixture because
the hypoxemia results from the influence of deoxygenated
venous blood. Diffusion limitation resulting in hypoxemia
theoretically can occur if P
AO
2
is very low—so that the diffu-
sion of oxygen is slowed—or if the transit time for pul-
monary capillary blood is very short. There are few diseases
in which diffusion limitation for oxygen transfer is thought
to be a major cause of hypoxemia. For example, hypoxemia
in patients with pulmonary vascular disease may be due to
diffusion limitation because of decreased mean transit time,
especially if cardiac output is increased. Pulmonary alveolar
proteinosis—a disease in which the alveolar spaces are filled
with a homogeneous protein and lipid fluid—may slow dif-
fusion of oxygen sufficiently to cause hypoxemia. In
PFP
P
R
AO IO B
ACO
2
22
=×−

CHAPTER 12
252
most other lung diseases presenting with hypoxemia,
.
V/
.
Q mismatching and right-to-left shunt explain hypoxemia
more often than diffusion limitation.
Clinical Features
Manifestations of hypoxemic respiratory failure are the result
of a combination of features of arterial hypoxemia and tissue
hypoxia (see Table 12–1). Arterial hypoxemia increases ven-
tilation by stimulation of carotid body chemoreceptors, lead-
ing to dyspnea, tachypnea, hyperpnea, and usually,
hyperventilation. The degree of ventilatory response depends
on the ability to sense hypoxemia and the capacity of the res-
piratory system to respond. In hypoxemic patients with
severe lung disease or ventilatory limitation, there may be lit-
tle or no increase in ventilation and absence of hyperventila-
tion. In patients who lack carotid body function, there will be
no ventilatory response to hypoxemia. There may be
cyanosis, especially marked in the distal extremities but also
centrally prominent around the mucous membranes and
lips. The degree of cyanosis depends on the hemoglobin con-
centration and the patient’s state of perfusion.
Other effects attributable to hypoxemia are due to inade-
quate supply of oxygen to the tissues, or hypoxia. Hypoxia
causes a shift to anaerobic metabolism, which is accompa-
nied by generation of lactic acid. Increased blood lactic acid
may further stimulate ventilation. Mild early hypoxia may
cause impaired mental performance, especially for complex
tasks or abstract thinking. More severe hypoxia can cause
much more severe alteration of mental status, including
somnolence, coma, seizures, and permanent hypoxic brain
damage. Sympathetic nervous system activity is increased,
and this contributes to tachycardia, diaphoresis, and systemic
vasoconstriction, leading to hypertension. More severe
hypoxia, however, can lead to bradycardia, vasodilation, and
hypotension as well as myocardial ischemia, infarction,
arrhythmias, and cardiac failure.
Manifestations of hypoxemic respiratory failure are mag-
nified in the presence of impaired tissue oxygen delivery.
Patients with reduced cardiac output, anemia, or circulatory
abnormalities can be expected to have global and regional
tissue hypoxia at less severe degrees of hypoxemia. Examples
include the increased risk of myocardial ischemia from
hypoxemia in a patient with coexisting coronary atheroscle-
rosis or a patient with hypovolemic shock who shows evi-
dence of lactic acidosis in the presence of mild arterial
hypoxemia.
Oxygen Delivery & Tissue Hypoxia
Adequate O
2
delivery to the tissues is the most important
function of the respiratory system, and this aspect requires
normal function of the lungs, heart, and circulation.
Recognition and treatment of compromised systemic O
2
delivery should be primary goals in management of respira-
tory failure in addition to correcting abnormalities of arterial
blood gases.
Physiologic Considerations
A. Oxygen Delivery—Systemic O
2
delivery is the product of
arterial O
2
concentration (mL O
2
/L blood) and cardiac out-
put (L/min). This calculation does not help to determine
whether the blood and O
2
are distributed to organs in pro-
portion to their needs, so even normal or high O
2
delivery
may be insufficient under certain conditions such as shock,
sepsis, or end-stage liver disease.
O
2
delivery (mL/min) = arterial O
2
content (Ca
O
2
,
mL O
2
/L blood) × cardiac output (
.
Q, L/min)
where Ca
O
2
, mL O
2
/L blood = [O
2
saturation × hemoglobin
(g/dL)
× 1.34 mL O
2
/g hemoglobin + Pa
O
2
(mm Hg) × 0.003
mL O
2
/mm Hg/dL] × 10.
In normal subjects at rest, normal arterial O
2
concentra-
tion is about 200 mL O
2
/L blood (O
2
saturation 97%, hemo-
globin 15 g/dL of blood, Pa
O
2
100 mm Hg). Resting cardiac
output is about 5 L/min, resulting in normal O
2
delivery =
1000 mL O
2
/min.
B. Causes of Decreased Oxygen Delivery—Factors
included in the formula for O
2
delivery can be examined to
identify pathologic states that result in potentially decreased
O
2
delivery. First, arterial O
2
concentration can be reduced as
a result of decreased O
2
saturation of hemoglobin from arte-
rial hypoxemia (decreased Pa
O
2
) or a rightward-shifted oxy-
hemoglobin dissociation curve (eg, acidemia, hyperthermia,
or hemoglobinopathy). Anemia is an important factor
because O
2
concentration is largely the product of hemoglo-
bin concentration and O
2
saturation. A decrease in hemoglo-
bin from 12 to 8 g/dL decreases O
2
concentration and O
2
delivery by 33%—considerably more than most changes in
Pa
O
2
or O
2
saturation. Carbon monoxide, because of its high
affinity for hemoglobin, displaces O
2
and reduces arterial O
2
concentration. In addition, carbon monoxide shifts the oxy-
hemoglobin curve leftward, which, although it tends to
increase O
2
concentration at any given Pa
O
2
, causes problems
in unloading O
2
at the tissue level.
Cardiac output depends on multiple factors, including
adequate systemic venous return, right and left ventricular
function, pulmonary and systemic resistance, and heart rate.
Even in the absence of underlying intrinsic heart disease,
patients with respiratory failure may have impaired or
reduced cardiac output. Hypoxemia and acidosis have
adverse effects on myocardial contractility or may cause
tachycardia, bradycardia, or myocardial infarction. There is
evidence that myocardial depression can be seen in conjunc-
tion with sepsis and septic shock, mediated through products
of microorganisms, patient-produced cytokines, or other
factors. Mechanical ventilation with positive pressure inter-
acts in a number of ways with the heart and circulation.
Although much of the decrease in cardiac output during
positive-pressure ventilation is due to diminished systemic
venous return, left ventricular diastolic compliance is
impaired, pulmonary vascular resistance is increased, and

RESPIRATORY FAILURE
253
right ventricular afterload increases. The degree and signifi-
cance of interaction vary with the type and severity of respi-
ratory failure and the type of mechanical ventilation.
C. Assessment of Oxygen Delivery—The assessment of
adequacy of O
2
delivery remains a topic of debate. In normal
subjects, total O
2
consumption of the body is independent of
O
2
delivery over a wide range. Increasing or decreasing O
2
delivery (except at extremely low rates) by changing cardiac
output or hemoglobin does not result in a parallel increase or
decrease in O
2
consumption. However, in some patients with
septic shock, ARDS, or other critical illness, O
2
consumption
may become functionally dependent on O
2
delivery even
when O
2
delivery is in the normal range. This finding has
been taken to indicate that adequate O
2
delivery in a given
patient cannot be assumed even when O
2
delivery is normal;
an increase in O
2
consumption in response to increased O
2
delivery above normal indicates that the original O
2
delivery
was in fact inadequate. To explain this finding, investigators
have proposed that distribution of blood flow in the periph-
eral circulation is poorly matched to O
2
requirements of indi-
vidual organs—a form of “distributive shock.” In some
studies, lactic acidosis has been associated with O
2
delivery
dependency; this supports the concept of inadequate tissue
oxygenation. In other studies, organ dysfunction is cited as
evidence of hypoxia when O
2
consumption is no longer inde-
pendent of O
2
delivery. On the other hand, some investigators
believe that these findings are artifacts of measurements or do
not reflect the responses of most patients with these disor-
ders. There have been a few studies suggesting that increasing
O
2
delivery has resulted in improved outcome from septic
shock. Several other investigators, however, have been unable
to find any difference in patient survival in critical illness by
empirically increasing O
2
delivery. It is highly likely that this
dependence of O
2
consumption on O
2
delivery is patient-
specific. Therefore, attention should be paid to trying to iden-
tify evidence of inadequate O
2
delivery by monitoring renal,
hepatic, cardiac, and other organ system functions.
Temperature & Blood Gases
The blood gas analyzer maintains the sample of blood at
37°C while Pa
O
2
,Pa
CO
2
, and pH are determined. For a given
quantity of O
2
and CO
2
in an aliquot of blood, Pa
O
2
and
Pa
CO
2
will change if the temperature of the blood changes.
When the sample is cooled, Pa
O
2
and Pa
CO
2
decrease; when it
is warmed, Pa
O
2
and Pa
CO
2
increase.
In patients with hypothermia or hyperthermia, some lab-
oratories report “temperature-corrected” blood gases, that is,
what the Pa
O
2
and Pa
CO
2
would be if measured at the
patient’s actual temperature. These corrections are deter-
mined empirically and are derived easily from tables and
nomograms or are displayed automatically by the analyzer.
However, temperature correction of a patient’s blood gas
results may lead to an incorrect clinical interpretation unless
they are compared with temperature-corrected normal blood
gas values. For example, in a normal animal made hypothermic,
temperature-corrected Pa
O
2
and Pa
CO
2
decrease, and because
HCO
3
–
remains constant, pH increases. If compared with
customary normal values measured at 37°C, interpretation
of temperature-corrected Pao
2
,Pa
CO
2
, and pH would lead to
an erroneous conclusion of hypoxemia and respiratory alka-
losis. One approach to avoiding this problem is to use refer-
ence normal values at each temperature for comparison.
A preferable method is to report all blood gas values at
the 37°C at which the blood is analyzed regardless of the
patient’s actual temperature. These results can then be com-
pared with normal values for Pa
O
2
,Pa
CO
2
, and pH deter-
mined at 37°C. Interpretation will be correct for both
hypoxemia and acid-base status. Temperature correction of
blood gases is unnecessarily complex and may be misleading
if steps are not taken to provide corrected normal values.
Glenny R et al: Gas exchange in health: Rest, exercise, aging. In:
Roca J, Rodriguez-Roisin R, Wagner PD (eds), Pulmonary and
Peripheral Gas Exchange in Health and Disease. New York:
Marcel Dekker, 2000.
Levy MM: Pathophysiology of oxygen delivery in respiratory fail-
ure. Chest 2005;128:547S–53S. [PMID: 16306052]
Pierson DJ: Indications for mechanical ventilation in adults with
acute respiratory failure. Respir Care 2002;47:249–6. [PMID:
11874605]
Rice TW et al: Comparison of the Sp
O
2
/F
IO
2
ratio and the
Pa
O
2
/F
IO
2
ratio in patients with acute lung injury or acute res-
piratory distress syndrome. Chest 2007;132:410–7. [PMID:
17573487]
West JB, Wagner PD: Ventilation, blood flow, and gas exchange. In:
Murray JF, Nadel JA (eds), Textbook of Respiratory Medicine,
3rd ed. Philadelphia: Saunders, 2000.
TREATMENT OF ACUTE RESPIRATORY FAILURE
Respiratory failure is treated by a combination of specific
treatment directed at the underlying compromise of the res-
piratory system plus supportive care of oxygenation and ven-
tilation. The general principles of support are similar
regardless of the type of respiratory system disorder.
Physiologic Basis Of Treatment
Hypercapnic Respiratory Failure
Because hypercapnia is synonymous with alveolar hypoven-
tilation, supportive care restores alveolar ventilation to nor-
mal until the underlying disorder can be corrected. Alveolar
ventilation sometimes can be improved by establishing an
effective airway—suctioning to remove secretions, stimula-
tion of cough, postural drainage, or chest percussion—or by
establishing an artificial airway with an endotracheal tube or
tracheostomy.
Mechanical assistance may be necessary to maintain the
desired alveolar ventilation until the primary problem is cor-
rected. Although the mechanical ventilator theoretically can
provide any desired amount of ventilation, care should be

CHAPTER 12
254
taken to correct hypercapnia judiciously in patients with
chronic hypercapnia. This is because correction of Pa
CO
2
to
normal in these patients can result in severe, life-threatening
alkalemia owing to their elevated plasma bicarbonate levels
as compensation.
Hypoxemia is seen often in patients with hypercapnic res-
piratory failure—especially those with lung disease—and
administration of supplemental oxygen is often necessary. In
some patients with hypercapnia, however, supplemental oxy-
gen may be hazardous if not titrated carefully. This group of
patients with chronic lung disease (either obstructive or
restrictive) or chest wall compromise (eg, kyphoscoliosis)
appears to have particular insensitivity to hypercapnia and
depend on hypoxemia to stimulate ventilation. If sufficient
oxygen is given to overcome hypoxemia, ventilatory drive
may be blunted subsequently, and the patient’s hypercapnia
may worsen.
Patients with hypercapnic respiratory failure owing to
sedative drug overdose or botulism—and most patients with
chest trauma—will improve with time, and treatment is
largely supportive. Some primary diseases associated with
hypercapnia require specific treatment, including myasthe-
nia gravis, electrolyte abnormalities, obstructive lung disease,
obstructive sleep apnea, and myxedema.
Hypoxemic Respiratory Failure
Oxygen supplementation is the most important therapy for
hypoxemic respiratory failure. In severe disorders such as
ARDS, mechanical ventilation, positive end-expiratory pres-
sure (PEEP), and other types of respiratory therapy may be
necessary. Although not a feature of most cases, hypercapnia
may develop because the high work of breathing leads to res-
piratory muscle fatigue. Attention to oxygen transport is
important, and severe anemia should be corrected and ade-
quate cardiac output maintained. The underlying disease
leading to hypoxemic respiratory failure must be addressed,
especially if pneumonia, sepsis, or other cause is identified.
Treatment may include diuretics, antibiotics, and bron-
chodilators as well as other measures.
In some patients with nonuniform lung disease,
dependent positioning of uninvolved or less involved lung
areas may improve oxygenation. Gravity and the weight of
the lungs increase perfusion and ventilation to dependent
lung regions. Patients with hemoptysis or heavy respiratory
secretions, however, should not be placed in this position
because of the likelihood of aspiration of blood or secre-
tions into uninvolved areas. In ARDS with diffuse noncar-
diogenic pulmonary edema, there has been considerable
interest in placing the patient prone. Prone ARDS patients
appear to have less tendency of dependent lung regions to
collapse as well as smaller areas of the lungs compressed by
the heart or abdominal contents. In some patients,
improvement in arterial hypoxemia is transient after turn-
ing from supine to prone, but in others the effects persist
for at least several hours.
Airway
When upper airway obstruction is the patient’s only prob-
lem, prompt restoration of an adequate airway is all that is
required to reverse respiratory failure. In all patients, estab-
lishment of the airway is essential for ventilation, oxygena-
tion, and delivery of respiratory medications.
Upper Airway Obstruction
Primary upper airway obstruction should be considered in all
patients with respiratory difficulty—but especially if they
present with any of the following: head and neck trauma, sus-
pected malignancy of the larynx or trachea, acute dyspnea
with wheezing (ie, inspiratory, expiratory, or both), dyspha-
gia, neurologic disease affecting motor or sensory function,
speech difficulty, masses in the neck owing to thyroid enlarge-
ment or lymphadenopathy, or pain, infection, or inflamma-
tion of the pharynx, larynx, or trachea. Patients with asthma
or chronic obstructive pulmonary disease (COPD) also may
have upper airway obstruction from tracheal or subglottic
stenosis if there is a history of recent or remote endotracheal
intubation or tracheostomy. Acute respiratory distress, espe-
cially in the elderly and in children, should arouse suspicion
of a foreign body in the airway. A frequent intermittent cause
of upper airway obstruction is seen in obstructive sleep apnea
syndrome (see below), in which obstruction occurs during
certain stages of sleep. It is important to consider this not
uncommon problem as a complicating factor in patients with
respiratory failure from other causes.
Natural Airway
The normal natural airway permits speech, humidifies
inspired gas, and protects against aspiration and infection;
coughing is effective, and the mucociliary function of the
trachea is maintained. When a decision is made to insert an
artificial airway, the benefits and risks of the endotracheal
tube must outweigh the benefits of the natural airway
(Table 12–3). In patients with severe upper airway obstruc-
tion, the choice is usually easy. In other patients with acute
respiratory failure, the decision rests primarily on whether
oxygen, respiratory medications, and respiratory therapy
via the natural airway will be adequate or whether an arti-
ficial airway would be preferable. A trial of aggressive treat-
ment before intubation often provides useful information.
Guidelines (Table 12–4) for selecting patients who need
endotracheal intubation may be helpful, but clinical assess-
ment of the response to therapy is usually more so.
Noninvasive positive-pressure ventilation does not require
endotracheal intubation and is an important alternative in
patients who meet criteria.
Endotracheal Tubes
Endotracheal tubes are usually made of relatively stiff plastic,
with soft, low-pressure, easily deformable inflatable tracheal

RESPIRATORY FAILURE
255
cuffs. Skilled practitioners should insert these tubes with
attention to prevention of aspiration of gastric contents; ade-
quate oxygenation during the procedure; avoidance of
trauma to the mouth, tongue, nose, epiglottis, and vocal
cords; and selection of an tube of proper size for the patient.
Experience with sedation using opioid analgesics or rapid-
acting sedatives (eg, benzodiazepines such as midazolam)
and muscle relaxants should be available for intubation of
awake patients.
Nasotracheal intubation offers greater patient comfort,
less severe positioning of the head and neck during place-
ment, and better stabilization of the tube. However, the route
of the tube and its smaller size sometimes can complicate
suctioning and weaning from a mechanical ventilator. The
smaller radius of curvature of the nasotracheal route has
been linked to higher tube resistance compared with the
same tube passed orally, but this finding has been ques-
tioned. The nasotracheal route generally is appropriate in
patients who require intubation for reasons unrelated to lung
disease, for example, sedative drug overdose. Otherwise, oro-
tracheal intubation should be used, especially if a larger tube
diameter is needed to facilitate airway suctioning or for
fiberoptic bronchoscopy. Placing an orotracheal tube
requires a laryngoscope for inspection of the vocal cords.
Laryngoscopes with straight or curved blades to hold the
tongue and other structures away are used most often, but
fiberoptic laryngoscopes have proved extremely useful for
difficult intubations or special circumstances.
Smaller-diameter endotracheal tubes impose less risk of
trauma to the vocal cords and may be more comfortable for
the patient. Larger tubes provide better access for tracheal
suctioning, delivery of medications, and fiberoptic bron-
choscopy and generally will create an adequate seal between
the tube and the trachea with lower cuff pressure. Weaning
from mechanical ventilation may be easier with a larger tube
because of lower airflow resistance. Most women can accom-
modate endotracheal tubes of 7.5–8 mm in inner diameter;
men generally will accept tubes of 8.5 or 9 mm in inner
diameter. If fiberoptic bronchoscopy is a consideration, a tube
with at least 8 mm in inner diameter is required, and 8.5 mm
is preferable.
Care of the Artificial Airway
The intubated patient must be suctioned frequently because
both cough and the mucociliary clearance mechanism are
impaired. The frequency of suctioning depends on the
amount and nature of secretions. Although the artificial air-
way becomes rapidly colonized with bacteria, suctioning
should be done using sterile technique to prevent introduc-
tion of additional organisms. A sealed system (ie, suction
catheter contained within a sheath in-line with the endotra-
cheal tube) facilitates frequent suctioning and may minimize
nosocomial contamination. For some patients, disposable
suction catheters are more effective—especially catheters with
bent tips that can be directed into the right or left main
bronchus as desired. It has become common practice to instill
small aliquots of sterile normal saline into the endotracheal
tube to facilitate suctioning of secretions, but this should be
done only if increased amounts of secretions are obtained.
Hypoxemia and tracheal trauma are the most common
complications of suctioning. Minimal negative pressure
should be used, and the suction catheter should be intro-
duced gently. During suctioning, Pa
O
2
may fall rapidly, par-
ticularly if the patient is receiving high concentrations of
inspired O
2
and suctioning is performed for more than
10–15 seconds. In patients with focal infiltrates or known
collections of secretions in particular parts of the tracheo-
bronchial tree, selective bronchial suctioning can be tried. By
using bent-tip catheters and by positioning the patient’s
body or head properly, successful suctioning of the left main
bronchus, for example, often can be accomplished.
Risk Benefits
Trauma of insertion
Oro- or nasopharyngeal trauma
due to chronic pressure
Tracheal damage (erosion,
tracheomalacia)
Impaired cough response
Increased aspiration risk
Impaired mucociliary function
Increased infection risk
No speech
Increased resistance and work of
breathing
Bypasses upper airway
obstruction
Route for oxygen and other
medications
Facilitates positive-pressure
ventilation and PEEP
Route for respiratory medications
Facilitates suctioning of secretions
Fiberoptic bronchoscopy route
Table 12–3. Risks and benefits of the artificial airway.
Table 12–4. Indications for intubation and mechanical
ventilation.∗
Physiologic
Hypoxemia persists after oxygen administration
P
A
co
2
>55 mm Hg with pH <7.25
Vital capacity <15 mL/kg with neuromuscular disease
Clinical
Altered mental status with impaired airway protection
Respiratory distress with hemodynamic instability
Upper airway obstruction
†
High volume of secretions not cleared by patient, requiring
suctioning
∗
These are guidelines that must take into account the patient’s
clinical status and other factors.
†
Consider need for tracheostomy if obstruction is above trachea.

CHAPTER 12
256
In order to provide positive-pressure ventilation, the
inflated tracheal cuff of the endotracheal tube exerts pressure
on the interior of the trachea to create an effective seal.
Almost all endotracheal and tracheostomy tubes incorporate
low-pressure, high-volume cuffs made of highly compliant
rubber or plastic. These cuffs provide a tracheal tube seal
after inflation to a relatively low pressure. If higher than nor-
mal pressures are needed to achieve a seal, damage to the tra-
chea may occur, including erosion, inflammation, softening
of the cartilage rings with tracheal dilation (tracheomalacia),
and hemorrhage. Endotracheal cuff pressure must be moni-
tored to anticipate these complications and to make certain
that the smallest effective pressure and volume are used. The
best way to do this is to slowly inflate the cuff with air, using
a small syringe, until there is minimal leak around the cuff
with inspiration and adequate tidal volume and ventilation.
The pressure read on a manometer and the amount of air put
into the cuff are recorded. The desired cuff pressure is the
smallest possible that will maintain an adequate seal between
the tube cuff and the trachea, and the pressure is ideally less
than 15 cm H
2
O. The incidence of tracheal complications
rises when cuff pressures exceed 20–25 cm H
2
O for pro-
longed periods. An automated system for maintaining opti-
mal cuff pressure is under investigation.
Complications from Endotracheal Tubes
Complications from endotracheal tubes may be classified as
early and late. Early complications are due to the trauma of
tube insertion or to malpositioning of a tube into a main
bronchus or in the esophagus. Confirmation of the tube’s
position by physical examination and chest x-ray are essen-
tial. The tip of the tube should be in the center of the trachea
and 3–5 cm above the carina, but head flexion or extension
can cause 1–5 cm of movement of the endotracheal tube tip
on chest x-ray. If the carina is difficult to see, the position of
the tip of the tube relative to the aortic arch on chest x-ray is
helpful. Analysis of expired CO
2
gives reliable assurance of
tracheal rather than esophageal placement; fiberoptic bron-
choscopy also can be valuable for this purpose in selected
patients.
Unplanned extubations are a complication of endotra-
cheal intubation, occurring in 3–10% of placements. The
endotracheal tube should be secured carefully, and the
patient should be educated about the need for and impor-
tance of the tube. Agitation and patient movement are asso-
ciated with extubation, and sedation and physical restraints
should be used as necessary. Several studies have shown that
unplanned extubations led to reintubation in about half of
patients, but some patients can be observed carefully for res-
piratory distress and deterioration of arterial blood gases
rather than immediate reintubation. Factors predictive of a
high likelihood for reintubation include severity of the
underlying disease, a high minute ventilation requirement
during the preceding 24 hours, a high F
IO
2
requirement, and
altered mental status.
Aspiration of oral secretions or refluxed gastric secretions
is a common problem in intubated patients. Contrary to
common belief, the inflated tracheal cuff does not reliably
protect against aspiration of secretions around the tube.
Endotracheal tubes with a port above the cuff reduce the
incidence of ventilator-associated pneumonia when the port
is connected to continuous suction.
A randomized trial of orotracheal compared with nasotra-
cheal intubation showed that there was a slightly greater fre-
quency of radiographic sinusitis (by CT scan) with
nasotracheal intubation. The incidence was relatively high in
both groups (30% nasotracheal and 22% orotracheal), how-
ever, indicating that this was a frequent complication in all
patients needing endotracheal intubation for more than 7 days.
Most late complications arise from prolonged pressure of
the tube against anatomic structures. The curve of the tube
puts maximum pressure on the side of the mouth, the palate,
and the posterior pharynx (from oral intubation) or the
nasal turbinates and the posterior pharynx (nasal intuba-
tion). The greatest pressure is exerted on the vocal cords, the
narrowest part of the passage. Most late complications are
due to laryngeal trauma, followed by the development of
glottic injury and subglottic stenosis. The incidence of sub-
glottic stenosis is estimated to be less than 5% of patients
intubated for more than 10–14 days. A study of patients with
translaryngeal intubation (orotracheal or nasotracheal)
found that the extent of injury estimated by laryngoscopy
was not predictive of late complications. The duration of
translaryngeal intubation did not influence the ability of the
larynx to heal without future ill effects.
Despite the inability to correlate late complications with
the duration of intubation, early tracheostomy (within 5–7
days) is recommended if prolonged intubation is anticipated.
Translaryngeal intubation for up to 10 days does not usually
cause an appreciable increase in complications, and tra-
cheostomy is preferred if intubation for 21 days or more can
be anticipated. This conclusion is based on a similar inci-
dence of complications from translaryngeal intubation and
tracheostomy. Thus translaryngeal intubation for as long as
21 days may be acceptable unless a longer need for an artifi-
cial airway is expected or whenever there is reason to suspect
greater potential laryngeal trauma (eg, patient movement,
malnutrition, and local or systemic infection). In such cases,
earlier tracheostomy is advised, especially in view of studies
that demonstrate reduced complications when tracheostomy
is performed at 5 days.
Tracheostomy, of course, prevents or avoids laryngeal
injury but does not prevent tracheal injury from the tra-
cheostomy cuff. Attention to cuff pressure and cuff volume
is essential for tracheostomy tubes as well as endotracheal
tubes. Tracheostomy may be contraindicated if the patient
has a bleeding disorder, local infection, or neck mass.
Percutaneous tracheostomy with and without fiberoptic
bronchoscopic assistance potentially reduces complications.
Not only is the timing of tracheostomy controversial,
but so is the role of tracheostomy itself. This is based on
