Boggs S. Principles of Sedimentology and Stratigraphy
Подождите немного. Документ загружается.


7. 3 Siliceous Sedimentary Rocks (Cherts)
215
fibrous quartz, can also form at lower temperatus in small amounts as cavity
and fracture fillings.
The rates of diagenetic evolution of silica from biogenic opal-A to opai
CT and finally to quartz chert are controlled by several physicochemical fac
tors. Te mperature is commonly consided to be a particularly important
control, with increasing temperature promoting an increased rate of transfor
mation (Siever, 1983). The transformation is fastest where rates of sedimenta
tion
and burial are high (sediment is rapidly buried to depths where
high
temperatures prevail} or where a high geothermal gradient exists in a region.
In the deep ocean environment, conversion of opal-A to opal-CT apparently
takes place at burial depths corresponding to a temperature of about 45°C and
transformation of opal-CT to microquartz occurs at temperatures of about
80°C. Kastner and Gieskes (1983) demonstrated that the rate of transforma
tion of opal-A to quartz chert depends also upon the nature of the opal start
ing material and the presence of magnesium hydroxide compounds, which
serve as a nucleus for the crystallization of opal-CT. Williams, Parks, and
Crerar (1985) conclude that increasing surface-to-volume ratio of siliceous
particles, a ratio that increases with decreasing particle size, increases solu
bility of opal and the rate of transformation. Because these other factors also
affect the rate of transformation of opal-A to opal-CT, Knauth (1994) suggests
that some transformations may take place at shallower bual depths and
lower temperatures (e.g., path Bin Fig. 7.1.1). Bohrman et al. (1994) report the
formation of opal-CT nodules and layers (porcellanites) at very shallow burial
depths and low temperatures in cores of Antarctic deep-sea sediment. The
porcellanites are present only in sediments rich in opal-A with extremely low
levels of detrital minerals. This finding suggests that the absence of detrital im
purities allows the transformation of opal-A to opal-CT to proceed faster, as
previously reported by Isaacs (1982}. It has also been suggested (e.g., Williams,
Parks, and Crerar, 1985) that under some conditions opal-A may transform di
rectly to quartz chert without going through an intermediate opal-CT stage
{path C in Figure 7.1.1).
Ogin of Nonfossilifous Ch
The origin of chert that does not contain siliceous organic remains is poorly un
derstood. rect, inorganic precipitation of amohous silica has bn reported in
some ephemeral Australian lakes (Peterson and von der Borch, 1965). Pleistocene
cherts from alkaline Lake Magadi, Kenya, also apparently formed inorganically
by alteration of sodium silicate precursors such as magadiite owing to removal of
Na by meteoric waters; the residual silica crystallized to quartz chert (Schubel and
Simonson, 1990). No similar occurrences have been reported in the open marine
environment that could help explain the presence of nonfossiliferous bedded
chert deposits. The scarcity of radiolarians and sponge spicules in Phanerozoic
cherts does not preclude the possibility that these cherts were formed from the re
mains of siliceous organisms. They could have been derived from siliceous oozes
that we subsequently almost completely dissolved and recrystallized, leaving
w recognizable siliceous organic remains (Weaver and Wise, 1974). Murray,
Jones, and Brink (1992) suggest that some bedded chert-shale couplets may form
by diagenetic processes. According to these authors, biogenic Si02 in shales dis
solves, migrates out of the shales, and then reprecipitates adjacent to the shale to
form bedded chert.
216
Chapter 7 I Other Chemical/Biochemical and Carbonaceous Sedimentary Rocks
Ledesma-Vazquez et al. (1997) describe a 14-m-thick, shallow-water chert (El
Mono chert) from Pliocene deposits of Baja California that contains no recogniz
able fossil remains. They suggest that this chert formed when silica-rich geother
mal solutions reacted with Pliocene sediments to deposit secondary silica in the
form of Opal-A. Some reported nonfossiliferous chert may simply be the result of
inadequate examination. Etching with hydrofluoric acid might reveal siliceous or
ganisms in such cherts.
Precambrian Bedded Chert
Bedded cherts are also very common in stratigraphic successions of Precambri
an age, associated with stromatolitic carbonates, iron formations, Archean green
stones, and silicic volcanic successions. These cherts do not contain unequivocal
remains of silica-secreting organisms, although a few workers (e.g., LaBerge,
Robbins, and Han, 1987) reported possible siliceous organic remains in some Pre
cambrian cherts. The remains of cyanobacteria have also been reported in Precam
brian cherts (e.g., Fairchild et al., 1996). Whether or not cyanobacteria or other
bacteria may have aided in precipitation of silica gel (opal-A) remains to be estab
lished. Until the presence of silica-secreting Precambrian organisms is proven, or a
definite link between bacteria and silica precipitation established, we must as
sume that deposition took place by inorganic processes, probably along pathway
C in Figure 7.1.1. How these processes operated given the geochemical constraints
discussed above is not understood, nor is the immediate source of the silica
known. Perhaps the silica content of the Precambrian ocean was higher than that
of the Phanerozoic ocean, possibly related to volcanism; or perhaps it was high
er simply because silica concentrations could build in the absence of silica-se
creting organisms. Thus, the origin of Precambrian cherts remains something of
an enigma.
Nodular and Other Replacement Chert
In addition to occurrence as bedded chert, chert can occur in the form of small
nodules, lenses, or thin, discontinuous beds, e.g., Fig. 7.11. Nodular cherts are es
pecially common in limestones but may be present also in evaporites and silici
clastic sedimentary rocks, particularly shales. Relict textures in nodular cherts
suggest that most are formed by diagenetic replacement. Some replacement cherts
form in the open ocean where they replace carbonates and clays, as shown by
preservation of burrows and other sedimentary structures (Hein and Karl, 1983).
The silica that forms these so-called deep-sea cherts is fuished by dissolution of
local siliceous organisms, especially diatoms (in Cenozoic occurrences). Nodular
cherts are particularly common in shallow platform carbonate rocks. Silica is sup
plied in this environment by the dissolution of sponge spicules or other forms of
biogenic opal-A within the sediment pile. This dissolution process causes the pore
waters to become supersaturated in silica with respect to opal-CT and quartz.
Chertification then occurs, possibly controlled by force-of-crystallization replace
ment of the host carbonate, in turn controlled by nonhydrostatic stresses resulting
from opal-CT and quartz crystal growth that simultaneously causes calcite disso
lution (Maliva and Siever, 1989). Silica cement can also accumulate in voids. The
transformation from opal-A to quartz chert does not necessarily require an inter
mediate opal-CT stage and may be represented by path C in Figure 7.1.1. For ad
ditional insight into some of the geochemical conditions that favor the formation
of replacement chert and the mechanisms of replacement, see Maliva and Siever
(1988b) and Knauth (1994).

7.4 IRON-BEARING SEDIMENTA RY ROCKS
Introduction
4 Iron-Bearing Sedimenta RocKs 217
Some iron present in almost all sedimenta rocks. The average iron content of
siliciclastic shales, for example, is 4.8 percent. Sandstones conta 2.4 percent iron
on average, and limestones conta about 0.4 percent (Blatt, 1982). The term iron
rich is reserved for sedimentary rocks that contain much higher iron content-at
least 15 percent total iron. Most iron-rich sedimentary rocks were deposited dur
ing three time periods: the Precambrian, the early Paleozoic, and the middle to
late Mesozoic (Jurassic-Cretaceous). They make up only a minor fraction of the
total sedimentary record; however, they have great economic significance as iron
ores. They constitute the major source of iron mined for commercial purposes.
Economically important iron deposits are located on all major continents except
Antarctica, and at least one deposit of sedimentary iron characterized as very
large occurs in each of these continents: the Lake Superior region-Labrador
Tugh, North America; the Transvaal-Griquatown region, South Africa; the Ham
mersley Range, Australia; the Krivoy Rog-KMA, former USSR, Eurasia; and
Minas Gerais, Brazil, South America.
Kinds of Iron-Rich Sedimentary Rocks
The major sedimentary iron deposits of the world were divided by James (1966)
into two principal kinds: iron-formation (for dominantly Precambrian, cherty
iron-rich sediments) and ironstone (for dominantly Phanerozoic noncherty iron
rich sediments). Some subsequent workers (e.g., Trend all, 1983; Young, 1989) have
expressed dissatisfaction with this scheme, particularly labeling ironstones as
solely Phanerozoic deposits. Kimberley (1994) suggests than an ironstone is any
chemical sedimentary rock with > 15 percent iron and an iron formation is a strati
graphic unit composed largely of ironstone. us, according to Kimberley, an iron
formation can be either cherty or noncherty. More in keeping with previous usage,
I have chosen in this book to retain the term ironstone for the mainly non banded,
noncherty, commonly oolitic, iron-rich sedimentary rocks and the term iron for
mation for the mainly well-banded, cherty, iron-rich sedimentary rocks. Vo lumet
rically, ironstones are much less importt than iron formations. Other kinds of
iron-rich sedimentary rocks of minor importance include iron-rich shales and mis
ce aneous iron-rich deposits such as bog iron ores and iron-rich laterites (e.g.,
Dimroth, 1979).
Iron Fonnations
Iron formations are iron-rich deposits that range in age from early Precambrian to
Devonian, although they are primarily of Precambrian age (James and Trendall,
1982). They consist of distinctively banded successions (Fig. 7.14), 50-600 m thick,
composed of layers enriched in iron alteating with layers rich in chert. Bandg
occurs on a scale ranging from millimeters to tens of meters. Cherty iron forma
ons are associated with dolomite, quartz-rich sandstone, and black shale and can
ade locally into chert or dolomite. Iron formations can have a variety of textures
at may resemble those of limestones. Micritic, pelleted, intraclastic (rip-up
clasts), peloidal, oolitic, pisolitic, and stromatolic textures are recognized. Simon
son (2003) suggests that the term granular iron foation (GIF) be used for iron
formations that have (or originally had) coarse, granular textures and that the
term banded iron formation (BIF) be reserved for iron foations that have

218
Chapter 7 I Other Chemical/Biochemical and Carbonaceous Sedimentary Rocks
Figure 7.14
Banded iron formation, Helen Formation
(
Archean), Helen Mine, Algoma Steel Corp.,
Wawa, Ontario, Canada. The very dark layers
are iron oxides with quartz (chert); lighter
layers are iron carbonates (ankerite). [Photo
graph courtesy of Robert H. Hodder.]
Figure 75
much finer grained textures. Sedimentary structures reported from banded iron
formations include cross-bedding, graded bedding, load casts, ripple marks, erosion
channels, shrinkage cracks, and slump structures. These structures show that
many of the particles that make up iron formations, especially the granular iron
formations, have undergone mechanical transport and deposition.
Ironstones
Ironstones are dominantly Phanerozoic sedimentary deposits that occur on all
continents. They are mainly early Paleozoic, Jurassic-Cretaceous, and early Ceno
zoic in age, but they range in age from middle Precambrian to Holocene
(Petranek and Van Houten, 1997). They form bedded successions a few meeFs to
a few tens of meters thick (e.g., Fig. 7.15), which are poorly banded or nonband
ed, in sharp contrast to the much thicker, well-banded iron formations. They
commonly have an oolitic texture (Fig. 7.16), and they may contain fossils that
have been partly or completely replaced by iron minerals. Sedimentary strue
tures that include cross-bedding, ripple marks, scour-and-fill structures, dasts,
and burrows are present in many ironstones, indicating that mechanical transrt
of grains was involved in the origin of these rocks. Ironstones are commonly
terbedded with carbonates, particularly limestones; shales; and fine-grained sand
stones of shelf to shallow-marine origin. They may grade locally to siliciclastic
Oolitic ironstones of the Bel! Island Group (Ordovi
cian), at Wa bana, Bell Island, Newfoundland. The
succession shown also includes sandstones (light
colored), dark shales, and minor carbonates. [Pho
tograph courtesy of Robert H. Hodder.]

4 Iron-Bearing Sedimentary Rocks 219
Figure 7.16
Ironstone ooids with quartz nuclei, cement
with sparry calcite cement, Clinton For
mation (Silurian), New York. Ordinary light
photograph. Scale bar = 0.5 mm.
sedimentary rock units. See Van Houten and Bhattacharyya (1982) and Yo ung
and Taylor (1989) for more details.
Minalo and Chemist of Iron Formations and Ironstones
On the basis of relative abundance of major kinds of iron-bearing merals, James
(1966) defes four different mineral facies in iron-rich sedimentary rocks: oxides,
silicates, carbona tes, d sulfides. The principal minerals in each of these mineral
classes are shown in Ta ble 7.2. The oxides and silicates are commonly the most im
portant iron-bearing minerals; however, sulfide minerals may constitute the major
in mineral some thin beds.
Iron formations consist maly of Si02 and Fe, but the chemical composition
of these rocks ranges widely depending upon the type of deposit. It is difficult to
ble 2
Principal iron-bearing minerals in iron-rich sedimentary rocks
Mineral class
Oxides
Silicates
Suldes
Caonates
Mineral
Goethite*
Hematite
Magnetite
Chamosite
Greenalite
Glauconite
Stilpnomelane
Minnesotaite (iron talc)
Pyrite
Marcasite
Siderite
Ankerite
Dolomite
Calcite
'Nol found in Preca mbrian iron formations
Chemical formula
3(Fe, Mg)O · (AI, Feh03 • 2Si02 · nH20
FeSi03 · nH20
Kg(Fe, Al) (Si03)6 • 3H20
2(Fe, Mg)O · (Fe, Alh03 · SSi02 ·3H20
(OHh(fe,
MghSi40
10
FeC03
Ca(Mg, Fe)(C03h
CaMg(C03h
CaC03

220
Chapter 7 I Other Chemical/Biochemical and Carbonaceous Sedimentary Rocks
establish a truly representative average composition; however, Gole and Klein
(1981) suggest that iron formations commonly conta 40-50 percent Si02; 29-32
percent total Fe; percent MgO; 2-7 percent CaO; 1-2 percent Al203; and less
than 1 percent each of Ti02, MnO, Na20, K20, P205, S, and C. Although iron, ex
pressed as Fe203, FeO, or FeS, is the dominant chemical constituent in some iron
rich sediments, the iron content of many iron-rich sedimentary rocks is commonly
exceeded by that of silica. Also, manganese concentration may reach considerable
percentages in some iron formations. The average iron content of ironstones
similar that of iron formations; however, ironstones typically have a higher con
centration of alumum and phosphorus and a lower concentration of silicon.
Appel and LaBerge (1987}, Melnik (1982), and Trendall and Morris (1983) provide
additional details.
Iron-Rich Shales
Pyritic black shales occur in association with both Precambrian iron formations
and Phanerozoic ironstones. They commonly form thin beds in which sulfide con
tent may range as high as 75 percent. Pyrite occurs disseminated in these black
carbonaceous shales and in some limestones. It may be present also as nodules, as
laminae, and as a replacement of fossil fragments and other iron minerals. Pyrite
rich layers have likewise been reported in some limestones. Siderite-rich shales
(clay ironstones) occur primarily association with other iron-rich deposits. They
are present also in the coal measures of both Great Britain and the United States.
Siderite (iron carbonate) occurs disseminated in the mudrocks or as attened nod
ules and more or less contuous beds.
Miscellaneous Iron-Rich Sediments
Bog iron ores a minor accumulations of iron-rich sediments that occur particu
larly in small freshwater lakes of high altitude. They range from hard, oolitic,
pisolitic, and concretionary forms to soft, earthy types. Iron-rich laterites are
residual iron-rich deposits that form as a product of intense chemical weathering.
They are basically highly weathered soils in which iron is enrhed. Manganese
crusts and nodules are widely distributed on the modern seafloor in deeper parts
of the Pacific, Atlantic, and Indian oceans in areas where sedimentation rates are
low.
They have been reported also from ancient sedimentary deposs in associa
tion with such oceanic sediments as red shales, cherts, and pelagic limestones.
Both iron-rich (15-20 percent Fe) and iron-poor ( < �6 percent Fe) varieties of
manganese
nodules are known. These nodules contain various amounts of Cu,
Co, Ni, Cr, and V in addition to manganese and iron oxide minerals. The presence
of these valuable metals in manganese nodules has caused considerable interest
the possibility of ming them from the seafloor. Recovery vessels are already
being plaed and designed, and political negotiations have been underway for
some time among the major nations of the world regarding undersea mining
rights to these potentially valuable deposits. They are not likely to be mined, how
ever, until cheaper land sources of iron are exhausted.
Iron-rich metalliferous sediments have been discovered several oceanic
settings, particularly near active mid-ocean spreading ridges. They form by pre
cipitation from metal-rich hydrothermal fluids that have become enriched
through contact and interaction with hot basaltic rocks. These sediments are en
riched in Fe, Mn, Cu, Pb, Zn, Co, Ni, Cr, and V. Metal-enriched sediments have
been reported also om some ancient sedimentary deposits in association wi
submarine pillow basalts and ophiolite sequences of ocean crustal rocks.
Heavy mineral placers are sedimentary deposits that form by mechanical
concentration of mineral particles of high specific gravity, commonly in beach or
alluvial environments. Magnetite, ilmenite, and hematite sands are common

7 Iron-Bearing Sedimenta Rks
221
constituents of placers, particularly beach and marine placers. Placers are local
accumulations, generally less than 1-2 min thickness, that occur mainly in Pleis-
tocene-Holocene sediments. Marine placer deposits containing about 5 percent
iron ore were mined off the southern tip of Kyushu, Japan, for many years (Mero,
1965). Offshore placers containing up to 10 percent magnetite and ilmenite have
been reported off the southeastern coast of Taiwan (Boggs, 1975). Beach placers
containing ilmenite have been exploited commercially in Australia since about
1965 (Hails, 1976). "Fossil" placer deposits are comparatively rare, although thin,
heavy-mineral laminae are common in some ancient beach deposits. Hails (1976)
re ports that outcrops of ilmenite- and magnetite-bearing placers of Cretaceous age
are exposed discontinuously through New Mexico, Colorado, Wyoming, and
Montana subparallel to the Rocky Mountains.
Origin of Iron Formations and Ironstones
Iron Deposition in Mode Environments
There are no modern counterparts to the ancient environments that presumably
favored widespread deposition of iron-rich sediments to produce iron formations
and ironstones. Iron-rich ooids and peloids have been reported off the northeast
coast of Venezuela, in muddy deposits of the Orinoco Delta, and off the Amazon
Delta (e.g., Va n Houten, 2000). On the whole, however, modern examples of iron
rich sediments are comparatively rare and provide few clues to the depositional
conditions that favored generation of ancient iron deposits.
Iron Deposition in Ancient Environments
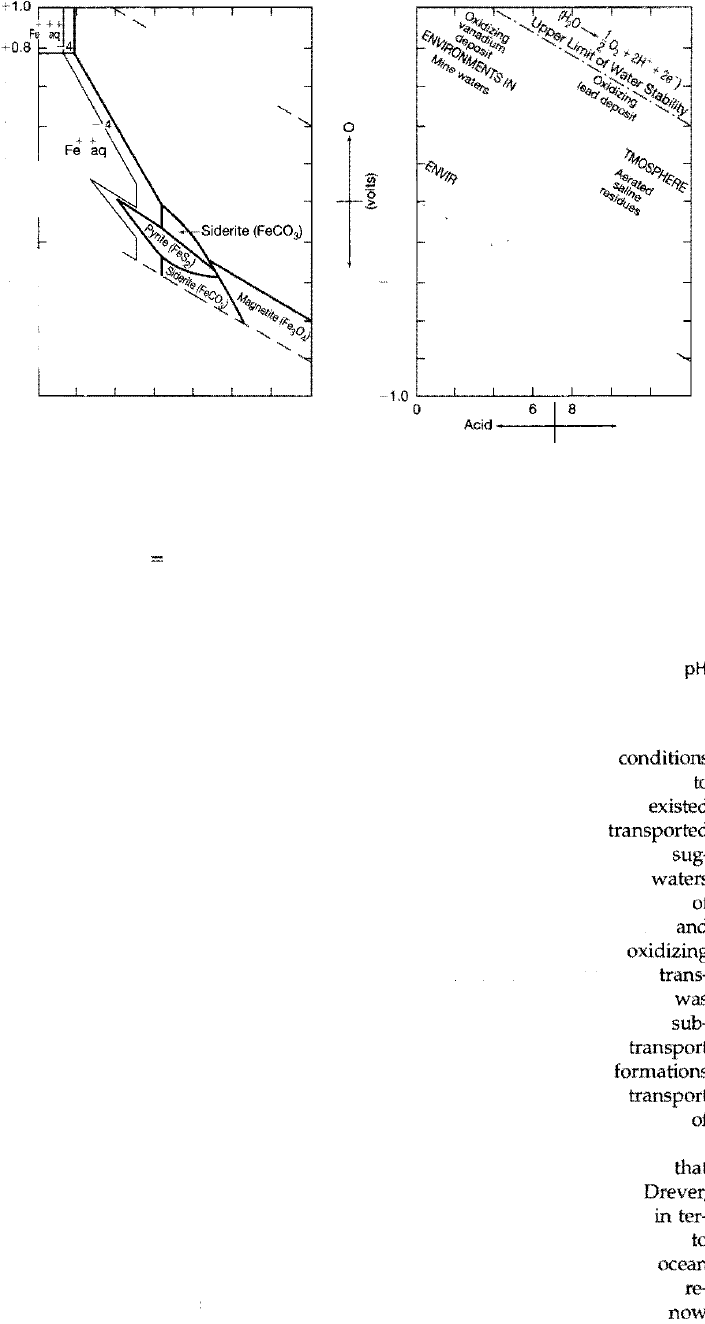
e transport and deposition of iron are governed by both the Eh and the pH of
the environment. Eh-pH diagrams such as Figure 7.17 A can be used to predict the
stability of iron-bearing minerals and serve to illustrate that Eh is commonly more
important than pH in determining
which iron-bearing mineral will be deposited.
For example, hematite (Fe203) is precipitated under oxidizing conditions at the
pHs commonly found in the ocean and most surface waters; siderite (FeC0
3
)
forms under moderately reducing conditions; and pyrite (Fe52) forms under
moderate to strong reducing conditions. Figure 7.178 shows the ranges of Eh and
Ph in some natural environments.
Because the iron geochemisy of natural systems is far more complex than the
simplified conditions assumed in constructing Eh-pH diagrams, such diagrams are
of only limited use in intereting e actual environment of iron deposition. Many
problems are associated with the formation of sedimentary iron deposits, and the
mechanisms by which iron was transported and deposited in the past to generate
iron formations and ironstones are still poorly derstood and very controversial.
ree problems have to be addressed to account for e formation of iron-rich rocks:
the source of the iron, transport of the iron to the depositional basin, and precipita
tion of the iron within the basin. Most workers now appear to agree that large iron
formations were deposited in continental shelf to upper slope mare environments.
Many workers originally assumed that the iron was derived by subaerial
weathering of iron silicate minerals; however, having the source on land creates a
major problem with respect to transport of iron in solution. Iron in the oxidized or
ferric (Fe
3
+) state is much less soluble than iron in the reduced or ferrous (Fe
2
+)
state (Fig. 7.17). Ferric iron is soluble only at a pH less than about 4; such values
rarely occur under natural conditions. Thus, under oxidizing conditions present
the weathering environment, iron tends to precipitate rather than undergo solu
tion. How, then, can large quantities of iron be taken into solution and transported
from subaerial weathering sites under the oxidizing conditions that commonly
prevail in streams and rivers?
222
Chapter 7 I Other Chemical/Biochemical and Carbonaceous Sedimenta Rocks
+0.6
Q.4
+0.2 �1
� 0.0 �
l
I
" �
-o.2
�
o
I
"
-oA L
i
-0.6
i
-0.8
0
' "
0
Hematite (Fe203)
-1.0
L
2 4 6 8 10 12 14
pH
A
7.17
:�
0
l
w
·8
�
+1.0
+0.8
+0.6
+0.4
+0.2
0.0
-0.2
0.4
-0.6
-0.8
B
,,
"
o
�·
.,
�& �0
� 1o
"'�t" ��
v�
�
<
"'�t. o,
S
;
o
� '�
o
. � ,.,,
o
�
�
o
"'�t
S
•; I� r
•. V
<
o
o "-·{o.0 .
�
�
� .1 . 0:�'c o Os
�
o
�� ol';;o��;l �
�
�
tr t �� �,�
s
,
.6 . .
,
.
o "-
7
.
2 4 10 12 14
Acid- Alaline
pH
A. Eh-pH diagram showing the stability fields of the common iron minerals, sulfides, and
carbonates in water at 25°C and 1 atm total pressure. Total dissolved sulfur = 1 o-
6
; total
dissolved carbonate 10°. B. Graph showing Eh and pH of waters in some natural envi
ronments. [A. from Garrels, R. M., and C. L. Crist, 1965, Solutions, minerals, and equilib
ria, Fig. 7.21, p. 224, reprinted by permission of Harper & Row, New York. B. after Blatt,
H., G. V. Middleton, and R. Murray, 1980, Origin of sedimentary rocks, 2nd ed., Fig. 6.1 2,
p. 241, reprinted by permission of Prentice-Hall, Englewood Cliffs, N.J., based on data
from
Baas Becking, L. G. M., et al., 1960, Limits of the natural environment in terms of
pH
and oxidation-reduction potentials: jour. Geology, v. 68, p. 24284.]
This problem of soluon and transport of iron under oxidizing
conditio
prompted
some workers (e.g., Lepp and Goldich, 1964; Cloud, 1973; Lepp, 1987)
postulate that e low aospheric oxygen concentrations that appartly
exisd
during the early Precambrian allowed great quantities of iron to be transported
from land in the soluble, reduced (Fe
2
+) state to marine basins. Lepp (1987)
sug·
gests that this transported ferrous iron was initially stored in basinal bottom
wate
in the ocean for long periods of time before finally precipitang. This argument oJ
low oxygen concentrations cannot, however, be used to explain the solution
and
transport of iron during later Precambrian and Phanerozoic time when an
oxidizing
atmosphere existed. Some workers have suggested that iron may have been
trans
ported as colloids by physical processes rather than in true solution or that it waE
sorbed to clay particles or organic materials and transported along with these sub
snces. It appears unlikely, however, that mechanisms such as colloidal ansport
can account for transport of the large quanties of iron at occur in iron
formations
or ironstones (Ewers, 1983). Reducing condions seem to be quired for transport
of large amounts of iron in solution; hce the dilemma with respect to ansport oJ
iron
by surface waters dung late Precambrian and Phanerozoic time.
To get around this difficulty, many recent workers have now suggested at
the source of the iron lay within the depositional basin itself. Some (e.g., Drever,
1974; Button et ., 1982) propose that dissolution of iron-bearing minerals
in ter
rigenous siliciclasc bottom sedents or other submarine rocks provided iron
to
bottom waters
.
other words, iron-bearing nerals were transported to the
ocean
where feic iron was reduced by anoxic (low-oxygen) bottom wars and the re
sulting ferrous iron (Fe
2
-) taken into solution. A considerable body of opion
now

5 Sedimentary Phosphorites
223
appears to be solidifying around the alternate possibility that the iron in major
iron formations was derived by "exhalation" from oceanic rocks (e.g., Gross, 1980;
Simonson, 1985, 2003; Kimberley, 1994; Isley, 1995), although not all geologists
agree with this idea (e.g., Petranek and Van Houten, 1997). Submarine reaction of
outpouring
lava and hydrothermal activity from hot springs located along mid-
ocean ridges furnished iron to ocean water (Chapter 1), or possibly iron-rich flu-
ids were also exhaled from source regions too deep within the crust or mantle for
seawater convection (Kimberley, 1994). Presumably the ocean at that time was
stratified into an upper, oxygen-rich layer and intermediate-deep oxygen-poor
(anoxic) layers (e.g., Simonson, 2003).
Deep, iron-rich, anoxic oceanic waters are postulated to move upward to
ward the surface along continental shelves because of (1) upwelling (e.g., Button
et al., 1982), (2) spreading laterally as a plume from high-standing mid-ocean
ridges (e.g., Isley, 1995), or (3) explosive exhalation through an ocean into the
atmosphere and subsequent raing of atmospheric precipitates onto the shelf
slope (e.g., Kimberley, 1994). The relative importance of each of these postulated
ansport mechanisms is not known. Once iron-rich waters have moved into the
upper slope-shelf environment, ferrous iron (Fe
2
-) is oxidized to ferric iron (Fe
3
+)
and precipitaon occurs. Oxidation can take place in the presence of molecular
oxygen or, putatively, by a photochemical process caused by ultraviolet radiation
in sunlight (e.g., Braterman et al., 1983; An bar and Holland, 1 992).
At ts time, it does not appear possible to apply a single depositional model
to all iron-rich sedimentary rocks of all ages. Although consensus seems to be
emerging that a major source of the iron in iron formations probably lay within
the ocean itself, some iron may have been derived from continental sources, par
ticularly during the early Precambrian. Many puzzling aspects of the formation of
sedimentary iron deposits, particularly banded iron formaons, still remain. Why,
for example, were chert and iron not deposited together as banded iron forma
tions after the Precambrian? Presumably, the silica concentration of Precambrian
seaw ater was much higher than in today's seawater, possibly as high as 60 ppm
(Siever, 1992). But how was the silica precipitated? Coprecipitation with iron
(Ewers, 1983), biogenic inducement (LaBerge, Robbins, and Han, 1987), or evapo
rative concentration and polymerization owing to electrolyte changes (Morris,
1993)? All of this is controversial. And what mechanism or mechanisms produced
e banding? Evaporation of water in restricted basins (Garrels, 1987), periodic
a-level changes that affected the interface between underlying iron-rich seawa
ter and overlying iron-poor seawater (Simonson and Hassler, 1996), or periodic
explosive exhalations through an ocean that subsequently rained iron precipitates
onto the shelf (Kimberley, 1994)? Again, these proposals are controversial. Also,
did low forms of life such as bacteria and algae catalyze or initiate precipitaon of
iron in some manner? If so, how did they cause precipitation, and how important
was such biologic activity? How did the iron-rich ooids that are common in iron
stones form (e.g., Yo ung, 1989)? The origin of iron-rich sedimentary deposits will
likely remain controversial for some time!
7. 5 SEDIMENTARY PHOSPHORITES
Intduction
The phosphorus content of rocks is usually expressed as percentage P2 05. The av
erage sedimentary rock contains less than one percent P2 05 one-half percent
phosphorus. Sedimentary phosphorites are rocks that are significantly enriched
phosphorus as compared with other types of rocks. Siificantly, in this context,
is commonly taken to mean that they contain more than about 15 percent P20s or
224
Chapter 7 I other Chemical/Biochemical and Carbonaceous Sedimentary Rocks
6.5 percent phosphorus. These phosphorus-rich sedimentary rocks are called by a
variety of other names-phosphate rock, rock phosphate, phosphates-in addi
tion to phosphorites. Sedimentary rocks that contain less than about 15 percent
P205 but considerably more than that in average sediment rocks are referred to as
phosphatic, e.g., phosphatic shale. The total volume of sedimentary phosphates in
the geologic record is small; however, like iron-rich sedimentary rocks, phospho
rites have a special economic interest. They contribute more than 80 percent of the
world's production of phosphate and make up about 96 percent of the world's
total resources of phosphate rock. Total world resources of sedimentary phos
phate rock are estimated to be about 158,000 million tons of all grades and types
(Notholt, Sheldon, and Davidson, 1989).
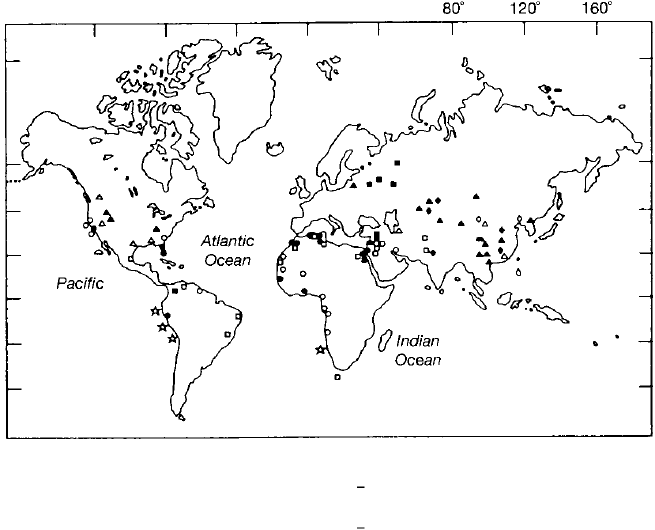
Sedimentary phosphates occur in rocks of all ages from Precambrian to
Holocene, but phosphorite deposition appears to have been particularly prevalent
during the Precambrian and Cambrian in central and southeast Asia (China,
USSR/MPR, Australia); the Permian in North America; the Jurassic and Early Cre
taceous in easte Europe; the Late Cretaceous to Eocene in the Te thyan province
of the Middle East and North Africa; and the Miocene of southeaste North
America (Fig. 7.18).
Phosphorite nodules and phosphatic sediments occur also on the present
ocean floor at shallow depths in the vicity of coastles. They are particularly
common off the coasts of Peru and Chile, southwest Africa, eastern United States,
southern and Baja California, the contental margins of India, and on some
seamounts and atolls the Pacific (see Glen, Prev6t-Lucas, and Lucas, 2000). Many
of these ocean-floor phosphate occurrences are older than the Holocene; however,
modern phosphate nodules are present on the ocean floor a few places.
Mineralo and Chemistry
Sedimentary phosphorites are composed of calcium phosphate minerals, all of
which are varieties of apatite. The principal varieties are fluorapatite [Ca5(P04)3F],
120° 80° 40°
00
40°
60° 60"
40° 40°
20°
20"
00
Pacific
00
0
Figure 7.18 20°
Ocean
Ocean
�q
r
� ... ··� .
20°
Worldwide distribution of
major sedimentary phos-
40°
phorite deposits. [After
Cook, P. )., 1976, Sedimen-
60°
tary phosphate deposits, in
Wolf, K. H. (ed.), Handbook
of strata-bound and strati-
form ore deposits, Fig. 1, p.
505, reprinted by permis-
sion of Elsevier Science Pub
lishers, Amsterdam.]
Age
*
Quaternary
•
•
Teiary
•
• Mesozoic
.
w
"
o
)
40°
60°
Paleozoic
Solid
Major deposits (being exploited or
Precambrian
symbol
likely to be exploited in near future)
Open Impoant occurrence (extensive deposits,
Unceain
symbol but unlikely to be exploited in near future)
