Boggs S. Principles of Sedimentology and Stratigraphy
Подождите немного. Документ загружается.


7. 5 Sedimentary Phosphorites
225
chlorapatite [Ca5(P04)3Cl], and hydroxyapatite [Ca5(P04)30H]. Most are carbon-
ate hydroxyl fluorapatites in which up to 10 percent carbonate ions can be substi-
tuted for phosphate ions to yield the general formula Ca10(P04, C03)6 These
carbonate hydroxyl fluorapatites are commonly called francolite. The wastebasket
term collophane is often used for sedimentary apatites for which the exact chemi-
cal composition has not been determined. Detrital quartz, authigenic chert (micro-
crystalline quartz), opal-CT, calcite, and dolomite are also common constituents of
many phosphorites. Glauconite, illite, montmorillonite, and zeolites may also be
present in some deposits; moderately abundant organic matter is a characteristic
constituent of many phosphorites (Nathan, 1984).
The chemistry of phosphorites is dominated by phosphorus, silicon (present
in minerals other than apatite), and calcium. Slansky (1986, p. 70) shows that the
abundance of these elements in 20 phosphorites ranging in age from Precambrian
to Holocene is P205 = 22-39 percent; Si02 = <1-25 percent, and CaO = 43-53
percent. Other common constituents include Al203 ( <1-5 percent); Fe203
(<1-4 percent); MgO ( <1-6 percent); NazO ( <1 percent); K20 ( <1 percent);
F (1-4 percent); Cl ( <1 percent); 503 (0-11 percent); and organic carbon (0-2 per
cent).
Many trace elements, such as Ag, Cd, Mo, Se, Sr, U, Yu, and , as well as
the rare earth elements may also be present in phosphorites in amounts exceeding
their average compositions in seawater, the crust, and the average shale (Nathan,
1984). See also Notholt, Sheldon, and Davidson (1989) and McClellan and Van
Kauwenbergh (1990).
Distinguishing Characteristics
Phosphate-rich sedimentary rocks may occur in layers ranging from in lami
nae a few millimeters thick to beds a few meters thick. Some phosphate succes
sions such as the Phosphoria Formation of the Idaho-Wyoming area may reach
several hundred meters in thickness, although such successions are not com
posed entirely of phosphate-rich rocks. Phosphorites are generally interbedded
with shales, cherts, limestones, dolomites, and, more rarely, sandstones. Phos
phatic rocks commonly grade regionally into nonphosphatic sedimentary rocks
of the same age.
Phosphorites have textures that resemble those in limestones. Thus, they
may be made up of peloids, ooids, fossils (bioclasts), and clasts that are now com
posed of apatite. Some phosphorites lack distinctive granular textures and are
composed instead of fine, micrite-like, textureless collophane. The phosphatic
grains may contain inclusions of organic matter, clay minerals, silt-size detrital
gra ins, and pyrite. Peloidal or pelletal phosphorites are particularly common;
oolitic phosphorites are somewhat less so. Phosphatized fossils or fragments of
original phosphatic shells are important constituents of some deposits. Most
phosphorite grains are sand size, although particles greater than 2 mm may be
present. These larger grains, referred to as nodules, can range in size to several
ts of centimeters.
Because the textures of phosphorites have such close resemblance to those of
limestones, some geologists suggest using modified limestone classifications to
distinguish different kinds of phosphorites. For example, Slansky (1986) advo
cates using a classification system based to some extent on Folk's (1962) limestone
classication, and Cook and Shergold (1986b) and Trappe (2001) suggest adapting
Dunham's 1962 carbonate classification (modified by Embry and Klovan, 1971) for
use in describing phosphorites. Using these modified classifications thus yields
names such as wackestone phosphorite (Cook and Shergold) and phosdast
wackestone (Trappe).
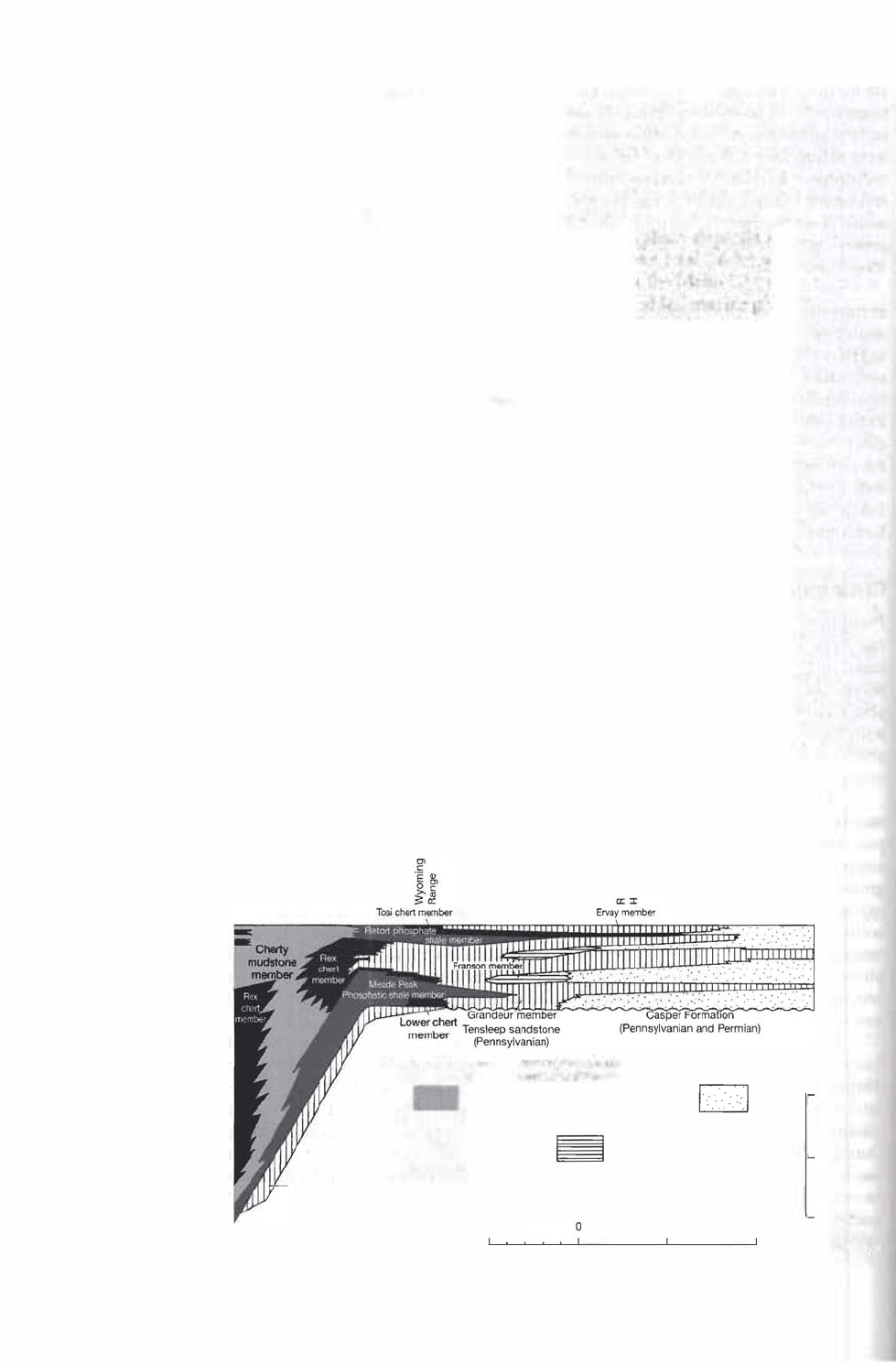
226
Chapter 7 I Other Chemical/Biochemical and Carbonaceous Sedimenta Rocks
Figure 79
Stratigraphic relations of the
phosphatic Phosphoria For
mation (Permian), Park City
Formation (Permian), and
Chugwater Formation (Trias
sic) of Idaho and Wyoming.
Note in particular the Retort
phosphate shale member
and the Meade Peak phos
phatic shale member. The
section runs from west to
east. [From Sheldon, R. P. ,
1986, Phosphorite deposits
of the Phosphoria Forma
tion, western United States,
in Notholt, A. ]. G., R. P.
Sheldon, and D. F. Davidson
(eds.), Phosphate deposits of
the world, v. 2, Fig. 8.1, p.
54, Cambridge University
Press, Cambridge.]
Principal Kinds of Phosphorite Deposits
Fo nds of phosphorite deposits are recognized: bedded, nodular, pebble bed, and
guano. TI1e major phosphorite deposits are maly bedded marine deposits. Bedded
phosphorites form distct beds of variable thickness, commonly terbedded and
interfingering with carbonaceous mudrocks, cherts, and carbonate rocks. e phos
phorite in bedded deposits occurs as peloids, ooids, pisoids, phosphatized bra
chiopods and other skeletal fragments, micrite-like apatite mud, and cements.
Perhaps the best-studied example of bedded phosphate deposits is the Pennian
Phosphoria Formation (Fig. 7.19). formation has a otal thickness of 420 m
extends throughout an area of about 350,000 km
2
the Idaho-Wyoming area (Mc
Kelvey et al., 1959; Sheldon, 1989; Herring, 1995). Bedded mare phosphorites are
also common in the Precambrian and Cambrian rocks of Australia� the Cretaceous
Te rtiary rocks of North Africa, and many other parts of the world (Cook and Shergold,
1986a; Notholt, Sheldon, and Davidson, 1989; Brnnett and Riggs, 1990; Soudry, 1992).
Bioclastic phosphorites are a special type of bedded phosphate deposit
composed largely of vertebrate skeletal fragments such as fish bones, shark teeth,
fish scales, and coprolites. The Rhaetic Bone Bed (Upper Triassic) of western Eng
land (Greensmith, 1989, p. 213) provides an example. Deposits composed mainly
of invertebrate fossil remains such as phosphatized brachiopod shells are also
known. These phosphate-bearg organic materials commonly become further en
riched P
2
05 during diagenesis and may be cemented by phosphate minerals.
Nodular phosphorites are brownish to black, spherical to irregularly shaped
nodules ranging in size from a few centimeters to a meter or more. Internal struc
ture of phosphate nodules ranges from homogeneous (structureless) to layered or
concentrically banded. Phosphatic grains, pellets, shark teeth, and other fossils
may occur within the nodules. Nodular phosphorites are particularly common
many Neogene to Holocene phosphatic deposits of the world (Burnett and Riggs,
1990). Phosphate nodules are also forming today in zones of upwelling in the
ocean, such as on the Peru continental margin (Buett and Froelich, 1988). Many
ancient nodular phosphorites may have had a similar origin under conditions of
marine upwellg; however, some ancient phosphorite nodules may be of diage
netic origin.
Southern
Idaho
-�
c
c
0
Grandeur tongue
Western
Wind River
Central
Southeastern
Wyoming
Mountains
Wyoming
Wyoming
� -�
c
c
5
.
c
-�
0
�
c
N
���
·
� � 0
=
� 0
I
i
Dinwy Foation risic)
Phosphoria Formation
of Permian age
D
Chey mudstone
-
Phosphorite
and mudstone
Chert
50
Park City Foati and its
equalent3 Ql Peian age
-
Carbonate rock
Greenish-gray shale
Chugwater Formation of Triassic
and Permian age
D
.
.
Red beds
50
100 miles
0
250
500 feet
Vertical ale
greatly exaggerat
Horizontal scale

7. 5 Sedimentary Phosphorites
227
Pebble-bed phosphorites are composed of phosphatic nodules, phospha
tized limestone fragments, or phosphatic fossils that have been mechanically con
centrated by reworking of earlier formed phosphate deposits. The Miocene and
Quaternary river-pebble and land-pebble deposits of Florida (Cathcart, 1989) pro
vide a good example of this type of deposit.
Guano deposits are composed of bird and bat excrement that has been
leached to form an insoluble residue of calcium phosphate. Guano occurs today
on small oceanic islands in the eastern Pacific and the West Indies. Guano deposits
a not important in the geologic record.
Origin of Phosphorites
Chemical/Biochemical Processes
As mentioned, e principal phosphate minerals sedimentary rocks are various
varieties of apatites, of which carbonate apatite [CawC03(P04)6] is particularly
important. Presumably, weathering of phosphorous-bearing rocks on land was
the principal process that furnished phosphorus to the oceans, through river
runoff, throughout geologic time. The average concentration of phosphorus in
ver water is 20 parts per billion (ppb), compared to 70 ppb in the ocean (Gul
brandsen and Roberson, 1973). Assuming approximately the same average con
tt of phosphorus the ancient ocean as in the modern ocean, how was the 70
ppb average phosphorus content of ancient oceans upgraded to form widespread
deposits of carbonate apatite containing as much as 40 percent P205, an enrich
ment of up to two millionfold?
Although a variety of inorganic mechanisms for extracting phosphorus from
ocean water have been considered by geologists, biologic utilization of phosphate
to build soft body tissue appears to provide the most feasible answer to the prob
lem of phosphate concentraon in sediments. Mode phosphate nodules are
forming in areas of oceanic upwelling where a steady supply of phosphate
brought from the large, deep-ocean reservoir allows continuous growth of organ
isms in large numbers. After death, organisms and organic debris not consumed
by
scavengers pile up on the ocean floor under reducing conditions where decay
is inhibited. These organic materials include the remains of phytoplankton and
zooplankton, coprolites (feces), and the bones and scales of fish. All contain phos
phorus; for example, phytoplankton contain about 0.4 percent phosphorus by dry
weight (Gross, 1982, p. 326). Under the reducing conditions of the seafloor, some
of e soft body tissue is thus pserved long enough to be buried and incorporat
ed into accumulating sediment. Perhaps about 1 to 2 percent of the total phos
phorus involved in primary productivity upwelling zones is ultimately
incorporated
into the sediments in this way (Baturin, 1982).
Slow decay of body tissue after burial releases phosphorus to the interstitial
waters of the sediment. Studies of the chemistry of interstitial waters in sediments
where mode phosphate nodules are forming and in other areas of the seafloor
where organic-rich sediments are accumulang under reducing conditions have
reported phosphorus concentrations ranging from 1400 ppb to as much as 7500
ppb (Bentor, 1980; Froelich et al., 1988). At such high phosphorus concentrations,
e interstitial waters are supersaturated with respect to calcium phosphate. The
phosphate thus begins to precipitate on the surfaces of siliceous organisms, car
bonate
grains, particles of organic matter, fish scales and bones, siliciclastic miner
grains, or older phosphate particles (Baturin, 1982). Phosphate may also replace
skeletal grains and carbonate grains, a process called phosphatation. Phosphorite
ains us form within the sediments by diagenec reactions between organic-rich
diments and their phosphate-enriched interstitial waters. Studies of phospho
rites on the Peru shelf indicate that some phosphorites form as thin (2-3 em) crusts

228
Chapter 7 I Other Chemical/Biochemical and Carbonaceous Sedimenta Rocks
beneath a few centimeters of organic-rich sediment (Burnett et. a!., 2000). Later ex
humation may expose these crusts at the sediment surface where they are re
worked by physical processes.
To allow phosphate precipitation to take place within sediments, Mg
2
+ ions
(which inhibit phosphate precipitation, as they also do in carbonate precipitation)
may be removed from pore waters owing to maesium replacements of iron in
day merals in the anoxic marine sediments (e.g., Drever, 1971). On the other
hand, phosphate minerals are reported to precipitate from some sediments in
which Mg concentrations are about that of seawater (e.g., Froelich et al., 1988).
How phosphate precipitation under these conditions is possible is poorly under
stood. It may be related in some way to the presence of filamentous bacteria
(cyanobacterial mats) and certa organic compounds within the pore waters
(Glenn and Arthur, 1988; Schwennicke et a!., 2000; Sisodia and Chauhan, 1990).
Physical Processes
The presence of clastic textures and primary depositional sedimentary structures
in many ancient phosphorite deposits seems inconsistent with a diagenetic con
centration mechanism. Therefore, Kolodny (1980) suggested a two-stage process
for the origin of ancient phosphorite deposits. In the first stage, apatite forms dia
genetically in reducing bass by mobilizg phosphorus interstitial waters in
the manner postulated for formation of modern phosphorites. The final stage in
volves reworking and enrichment of these diagenetically formed phosphorite
grains by mechanical concentration processes under oxidizing conditions. Con
centration presumably takes place in a high-energy environment, probably during
lower stands of sea level. During this stage, the phosphate grains may be trans
ported into a different depositional setting than that in which they formed. This
final stage of phosphorite formation, during which the original diagenetically
formed phosphorite sediments are mechanically reworked under shallow-water
conditions, accounts for the clastic textures and primary sedimentary structures in
many ancient phosphorites.
Summary of Phosphorite Deposition
In summary, most phosphorite workers propose that upwelling of phosphorus
rich waters from deeper parts of the ocean and biologic utilization of the phos
phate in soft body tissue are the important factors in the origin of phosphorite
deposits. Phosphorus is deposited on the seafloor in organic detritus and is buried
with accumulating sediment. Phosphate becomes concentrated in the pore waters
of sediment during slow decay of the phosphate-bearing, soft-bodied organisms
and other organic detritus. Carbonate apatite precipitates diagenetically from
these phosphate-enriched pore waters by some process not yet fully understood
to form phosphate grains and cements. Apatite may also replace skeletal grains or
other carbonate grains. Subsequently, these diagenetic deposits are reworked me
chanically, possibly owing to lowered sea levels, aowing final concentration and
deposition of phosphatic sediments by waves and currents. These processes are
summarized diagrammatically in Figure 7.20.
This postulated multistage process for formation of phosphorite deposits
has some limitations. It does not, for example, explain why phosphorites accumu
lated on a much vaster scale at some times in the geologic past than at present. A
possible explanaon for this phenomenon is that major episodes of phosphorite
deposition were tied to climate and sea level changes. For example, a period of
glaciation may produce a large volume of cold, nutrient-rich water that, after a
long residence time, will eventually be circulated into shallower areas during
rising sea level (transgression). Such an event would produce a major burst of
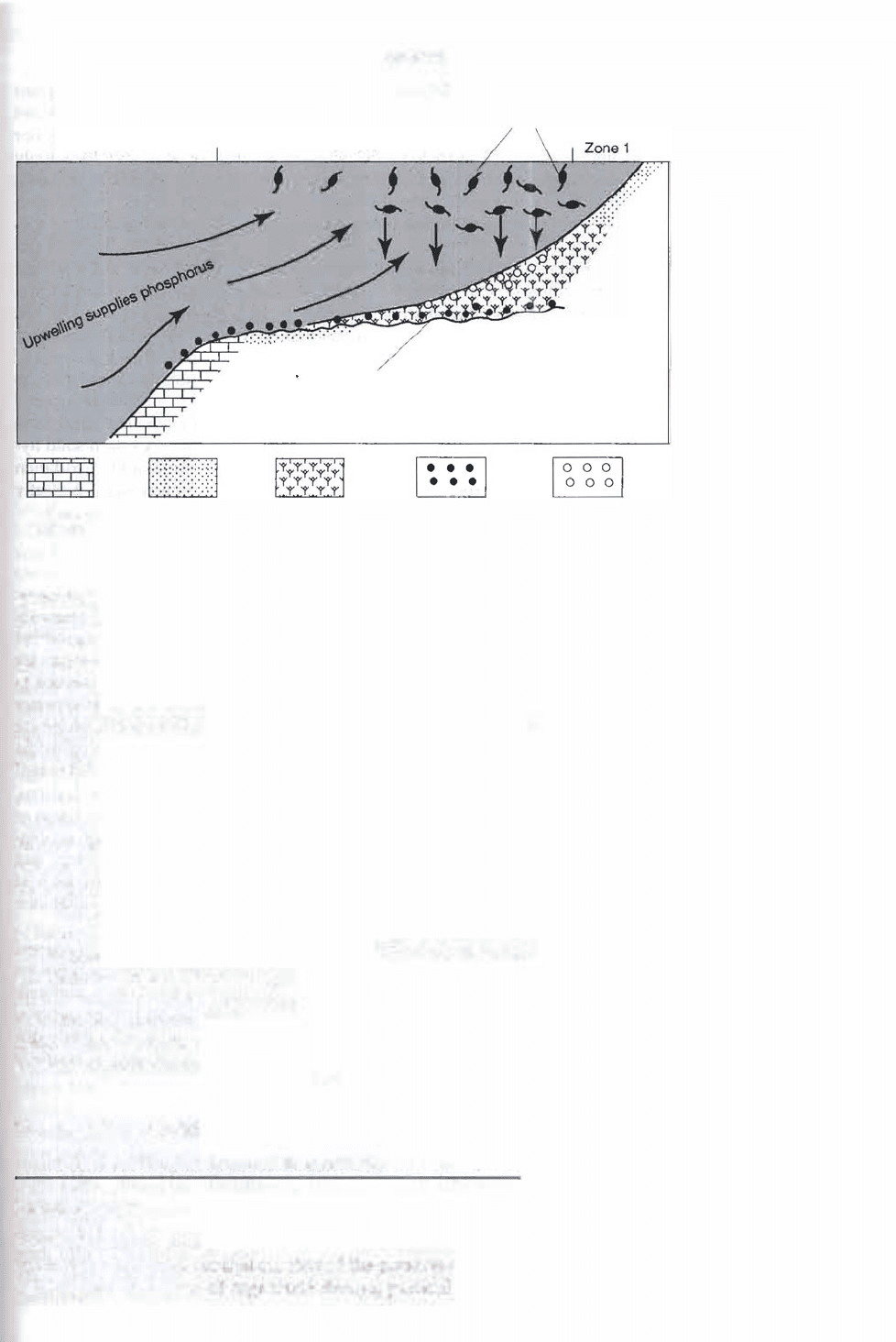
Zone 3
Carbonate
sediment
Figure 7. 20
Siliciclastic
sediment
6 Carbonaceous Sedimentary Rocks: Coal, Oil Shale, Bitumens 229
Phosphate-rich
pelagic organisms
Zone 2
Eros1on surface - mechanical
reworking and concentration
of phosphate nodules
Biogenic
Siliciclastic
& carbonate
sediment
�
�
Phosphate
nodules
consolidated
�
Phosphate
nodules
unconsolidated
Schematic illustration of the formation of phosphorites in areas of upwelling on open
ocean shelves. Near-shore, shallow-water siliciclastic deposits form in zone 1. Zone 2 is the
zone where high contents of phosphate-rich biogenic detritus accumulate in sediments
awing to rain-out of pelagic organisms; phosphate nodules form in this zone by diagenet
ic processes, followed by reworking of phosphate-rich sediments during lowered sea level.
Zone 3 is a deeper water zone of carbonate sediments with local phosphate nodules.
[After Baturin, G. N., 1982, Phosphorites on the sea floor: Origin, composition, and distri
bution, Fig. 5.4, p. 227, reprinted by perm ission of Elsevier Science Publishers.]
organic activity in the shallow zone (Cook and Shergold, 1986b), leading to
creased phosphorite deposition. For additional discussion of the possible influ
ence of climate on paleocirculation patterns (e.g., upwellg) and phosphorite
deposition, see Parrish (1990).
To my knowledge, the only serious challenge to the upwelling (biologic
utilization) hypothesis for phosphogenesis has been mounted by Kimberley
(1994). Kimberley suggests, as he suggested for iron-rich sedimentary rocks, that
phosphorite deposition is brought about by precipitation from concentrated so
lutions, which acquired their phosphorus content by high-temperature dissolu
tion of phosphorus-bearing minerals deep beneath the edge of a continental
block. Thus, phosphorus is furnished to the ocean, according to Kimberley, by
exhalative processes rather than by weathering and subsequent biologic-uptake.
7. 6 CARBONACEOUS SEDIMENTARY ROCKS:
COAL, OIL SHALE, BITUMENS
Introduction
Most sedimentary rocks, even some of Precambrian age, contain at least a small
amount of organic matter hat consists of the preserved residue of plant or animal
tissue. When the tissu of organisms decays, particularly in an oxygen-deficient

230
Chapter 7 I other Chemical/Biochemical and Carbonaceous Sedimentary Rocks
environment, organic degradation may not be complete; more decay-resistant
fractions of organic substances such as cellulose, fats, resins, and waxes are not
immediately decomposed. If a depositional basin happens to be an oxygen-poor
environment-such as a restricted basin, stagnant swamp, or bog-or if the supply
of organic matter is so great that it simply overwhelms all available oxidants,
decay-resistant organic matter may be preserved long enough to become incorpo
rated into accumulating sediment. ce buried, it may persist for hundreds of mil
lions of years.
The average content of organic matter in sedimentary rocks is 2.1 weight
percent in mudrocks, 0.29 percent in limestones, and 0.05 percent in sandstones
(Degens, 1965). The average in all sedimentary rocks is about 1.5 percent. Organic
matter contains about 50 to 60 percent carbon; therefore, the average sedimentary
rock contains about 1 percent by weight organic carbon. A few special types of
sedimentary rocks contain significantly more organic material than these average
rocks. Black shales typically contain 3-10 percent organic matter. Oil shale or kero
gen shale contains even higher percentages, ranging to 25 percent or more, and
coals may be composed of more than 70 percent organic matter. Certain solid hy
drocarbon accumulations-such as asphalt, formed from petroleum by oxidation
and loss of volatiles-constitute another example of a sedimentary deposit greatly
enriched in organic carbon.
Kinds of Organic Matter in Sedimentary Rocks
Three basic kinds of organic matter are accumulating in subaerial and subaqueous
environments under present conditions: humus, peat, and sapropel. Soil humus is
plant organic matter that accumulates in soils to form a number of decay products
such as humic and fulvic acids (complex high molecular weight organic acids).
Most soil humus is eventually oxidized and destroyed, and little is preserved in
sedimentary rocks. Peat also consists of humic organic matter, but peat accumu
lates in freshwater or brackish-water swamps and bogs where staant, anaerobic
conditions prevent total oxidation and bacterial decay. Therefore, some of the
humus
that accumulates under these reducing conditions can be preserved in sed
iments. Sapropel refers to fine organic matter that accumulates in lakes, lagoons,
or marine basins where oxygen levels are low owing to poor water circulation or
where the supply of organic remas is high enough to suppress oxygen concen
tration levels. It consists of the remains of phytoplankton, zooplankton, and
spores and fragments of higher plants. Phytoplankton are tiny plants such as
algae that drift about in the upper water column owing to currents; zooplankton
are small drifting animals, such as foraminifers.
It is often difficult to differentiate accurately between the types of organic
matter found in ancient sediments; however, both humic and sapropelic types are
recognized. Humic organic matter is the chief constituent of most coals, although
a few are formed of sapropel. The organic matter in oil shales and other carbona
ceous mudrocks and limestones originated from sapropel, but it is so finely dis
seminated and altered that it is difficult to identify. This type of organic matter is
called kerogen [see "Oil Shale (Kerogen Shale)" below].
Classification of Carbonaceous Sedimentary Rocks
The predominant organic constituents of carbonaceous sediments are thus huc
and sapropelic organic matter. The nonorganic constituents are mainly either silici
clastic grains or carbonate materials. Carbonaceous sediments can be classified on
the basis of relative abundance of nonorganic constituents and the kind of organic
matter that composes the organic constituents (humic vs. sapropelic) into three
basic types of organic-rich rocks: coal, oil shale, and asphaltic substances (Fig. 7.21).
Each of these types of rocks contains at least 10 to 20 percent organic constituents.
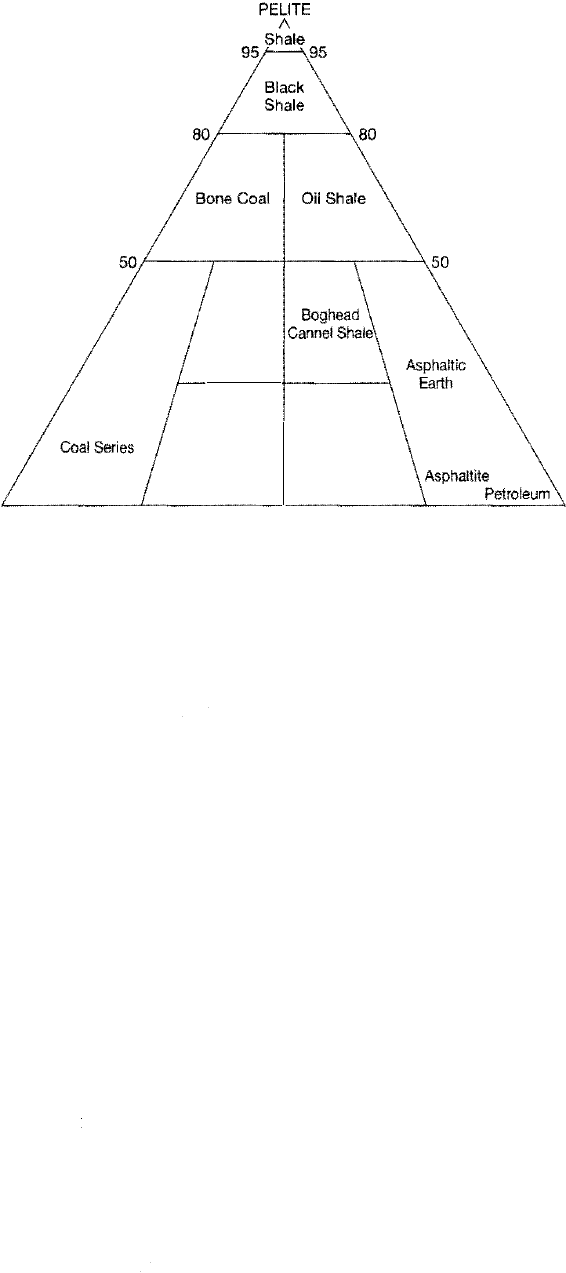
7.6 Carbonaceous Sedimentary Rocks: Coal, Oil Shale, Bitumens
231
HUMUTH
(coal series)
Coals
Cannel
Shale
Cannel
Boghead
Coal
Cannel
50
SAPROTH
(petroleum series)
Figure 7.21
Cl
assification and nomenclature of
carbonaceous sediments on the
basis of relative abundance of
humic organic constituents (Hu
mulith), sapropelic organic con
stituents (saprolith), and
fine-grained terrigenous con
stituents (pelite). [From Pettijohn,
F. ]., 1957, Sedimentary rocks, 3rd
ed., Fig. 11.37, p. 446. Copyright
1949, 1957 by Harper & Row, Pub
lishers, N. Copyright 1975 by ].
Pettijohn. Reprinted by permisssion
of Harper Collins Publishers, Inc.]
Coals are the best known kind of carbonaceous sediment. They are composed pre
dinantly of combustible organic matter but contain various amounts of impu
rities (ash), which are largely siliciclastic materials. The amount of ash that coals
can
contain and still retain the name of coal is not precisely fixed. Some very im
pure coals (bone coals) may contain 700 percent ash, but most coals have less
than 50 percent ash by weight. Most coals are humic, although a few are sapropelic
coals made up mostly of spores, algae, and fine plant debris. Cannel coals and
boghead coals (see below) are sapropelic coals. Coals are defined in various ways,
but a commonly accepted definition is that of Schopf (1956, p. 527):
Coal is a readily combustible rock containing more than 50 percent by weight
and more than 70 percent by volume of carbonaceous materiaL formed om
compaction or induration of variously altered plant rem ains similar to those of
peaty deposits. Differences in the kinds of plant materials (type), in degree of
metamorphism (rank), and range of impurities (grade), are characteristic of the
varieties of coal.
Char
acteristics and Classication. A common method of classiing coals is by
rank, which is based on the degree of coalification or carbonification (increase in
organic carbon content) attained by a given coal owing to burial d metamor
phism (Table 7.3). Peat is included in Table 7.3 but is actually not a true coal. Peat
consists of unconsolidated, semicarbonized plant remains with high moisture
content. Lignite or brown coal is the lowest ranked coal. Liites are brown to
brownish black coals that have high moisture content and commonly retain many
of the structures of the original woody plant fragments. They are dominantly Cre
taceous or Tertiary in age. Bituminous coals are hard, black coals that contain
fewer volatiles and less moisture than lignite and have a higher carbon content.
They commonly display tn layers consisting of alteating bright and dull
bands (Fig. 7.22). Sub bituminous coal has properties intermediate between those
of lignite and bituminous coal. Anthracite is a hard, black, dense coal commonly

232
Chapter 7 I Other Chemical/Biochemical and Carbonaceous Sedimentary Rocks
Figure 7. 22
. ble 7.3
Classification of coal on the basis of r
Fixed carbon Vo latile matter (wt.
Calorific value limits
limits (wt. percent),
percent), dry,
(Btullb ), moist,
Class
dry, mineral- and
mineral- and
mineral- and
(rank steps) matter-free basis matter-free basis
matter-free basis
Anthracite
86-98
2-14
Bituminous
69-86
22->31
10,500-14,000
Subbituminous
<
69
>31
8,300-10,500
Lignite
<69
>31
6,300-8,300
Peat
low
high
low
Source: Data from American Society for Te sting Materials (ASTM), 1981, A11111WI book of ASTM sta11dards, Part 26,
American Society for Te sting Materials.
Note: Vo latile matter is that part of coal that bus as a gas, mainly hydrogen.
containg more than 90 percent carbon. It is a bright, shiny rock that breaks with
conchoidal fracture, such as the fractures in broken glass. Bituminous coals and
anthracite are largely of Mississippian and Pennsylvanian (Carboniferous) age.
Cannel coal and boghead coal are nonbanded, dull black coals that also break
with conchoidal fracture; however, they have bituminous rank and much higher
volatile content than anthracite. Cannel coal is composed maly of spores. Bog
head coals are composed dominantly of nons pore algal remains. Bone coal is very
impure coal containing high ash content.
Coals are also classified on the basis of megascopic textural appearance
and recognizable petrographic or microscopic constituents. Stapes (1919) recog
nized four types of coal, now called lithotypes, on the basis of megascopic ap
pearance. Four lithotypes are recognized: vitrain, clarin, durain, and fusain.
These lithotypes, which comprise millimeter-thick bands or layers of humic coal,
are described Ta ble 7.4 and illustrated in Figure 7.23.
Layered and banded bituminous
coal, Cedar Grove Seam (Pennsyl
vanian), Logan County, West Vir
ginia. The thickness of the coal
seam is about 2.3 m. [Photograph
courtesy of Island Creek Coal Com
pany
.]

Lithotypes
7. 6 Carbonaceous Sedimentary Rocks: Coal, Oil Shale, Bitumens
233
Vitrain-brilliant, glossy, vitreous, black coal, bands 5 mm thick; breaks with a con
choidal fracture; clean to touch.
Clarin-smooth fracture with pronounced gloss; dull intercalations or striations; small
scale sublaminations within layers give surface a silky luster; the most common
macroscopic constituent of humic coals.
Durainccurs in bands a few em thick; firm, somewhat granular texture; broken sur
faces have a fine lumpy or matte texture; characterized by lack of luster, gray to brown
ish black color, and earthy appearance.
Fusain-soft, black; resembles common charcoal; occurs chiey as irregular wedges; fri
able and porous if not mineralized.
Macerals
Vitrinites-riginated as wood or bark; a major humic constituent of bright coals. Subtypes:
Collinite-structureless or nearly structureless; commonly occurs as a matrix or im
pregnating material for fragments of other macerals.
Te llinite-derived from cell-wall material of bark and wood and preserves some of
the cellular texture.
Inertinites-composed of woody tissues, fungal remains, or fine organic debris of uncer
tain origin; relatively high carbon content. Subtypes:
Fusunite-cell structures composed of carbonized or oxidized cell walls and hollow
lumens (the space bounded by the wall of an organ) that are commonly mineral
filled; characteristic of fusain.
Semifusinite-a transitional state between fusite and vitrinite.
Schlerotiniteomposed of the remains of fungal schlerotia (a hardened mass of
tubular filaments or threads) or altered resins; characterized by oval shape and
varying size.
Micronite ( <10 Jlm) and macronite (10-100 Jlm)-structureless, opaque, granular
macerals derived from fine-grained organic detritus.
Inertodetrinite-finely divided, structureless, clastic form of inertinite in which frag
ments of various kinds of inertinite macerals occur as dispersed particles.
Liptinites (exinites)riginate from spores, cuticles, resins, and algae; can be recognized
from shapes and structures unless original constituents are compacted and squashed.
Subtypes:
Sporinite-composed of the remains of yellow, translucent bodies (spore exines) that
are commonly flattened parallel to bedding.
Cutinite-formed from macerated fragments of cuticles (layers covering e outer
wall of a plant's epidermal cells).
Resinite-the remains of plant resins and waxes; occur as isolated rounded to oval
or spindle-shaped, reddish, translucent bodies, or as diffuse impregnations, or as
fillings in cell cavities.
Alginite-composed of the remains of algal bodies; serrated, oval shape; characteris
tic of bodhead coaL
Under the microscope, coal can be seen to consist of several kinds of organic
units that are single agments of plant debris or, in some cases, fragments consist
ing of more than one type of plant tissue. Stopes (1935) suggested the name mac
eral for these organic units as a parallel word for the term mineral used for the
constituents of inorganic rocks. The starting materials for macerals are woody
tissues, bark, fungi, spores, and so on; however, these materials are not always
recoizable in coals. Macerals are divided into three major groups: vitrinite, iner
tinite, and liptinite (Table 7.4).
Coal macerals are identified on the basis of several characteristics: (1) reflec
tivity-the extent to which they reflect light, (2) degree of anisotopy (differences
in reflectivity in different directions within a maceral) or isotopy as viewed under

234
Chapter 7 I Other Chemical/Biochemical and Carbonaceous Sedimentary Rocks
Figure 7. 23
c
c
v
c
Bituminous coal showing examples of three different litho
types: V, vitrain; C, clarain; and D, dura in. The small divisions
on the scale equal 1 em. [From Bustin, R. M., et al., 1985, Coal
petrology, its principles, methods, and applications: Geol.
Assoc. Canada Short Course Notes, v. 3, Pl. 6A, p. 51 , reprint
ed by permission.]
D
c
a petrographic microscope, (3) presence or absence of fluorescence when the
specimen is irradiated with blue (ultraviolet) light, (4) morphology (shape), (5)
relief, and (6) size. Study of macerals is referred to as coal petrology (e.g., Ting,
1982; Bustin et al., 1985; Ward, 1984).
·-
Origin. Coals occur in rocks ranging in age from Precambrian (algal coal) to Te r
tiary, and peat analogs of coal are present Quateary sediments. Coals origi
nate in climates that promote plant growth under depositional conditions that
favor preservation of organic matter. Although ancient coals accumulated at all
latitudes from the equator to polar regions, most were deposited in middle lati
tudes (McCabe, 1984; see also, Cobb and Cecil, 1993). For coal to be preserved, the
rate of accumulation of organic matter must exceed the rate of decomposition
owing to microbial and chemical processes. Accumulating organic matter is most
likely to be preserved in depositional environments where oxidation of organic
matter is inhibited owing to rapid burial, such as in swampy areas in which the
water table is close to the peat surface. For thick coal deposits to form, these con
ditions must last for a geologically long period of time. Although land plants were
moderately well established by Devonian time, swampy environments large
enough to form major coal deposits have existed only since Carboniferous time
(Mississippian and Pennsylvanian Periods). Since that time, only the Tr iassic Period
appears
to have been a time when coal-forming processes were at a minimum.
Compaction and loss of volatiles accompanying deep burial thins coal beds
by as much as 30 to 1 (Ryer and Langer, 1980); that is, 30 m of original peat may
produce only 1m of coal. The rank of coal tends to increase with depth owing to
increase in temperature with depth. The formation of anthracite, for example,
quires temperatures in excess of about 200°C (Daniels et al., 1990). Coals occur
predominantly in siliciclastic depositional sequences, although thin limestones
may be associated with some coals.
Oil Shale (Kerogen Shale)
Oil shale is fine-grained sedimentary rocks from which substantial quantities of
oil can be derived by heating. That is, sufficient oil is generated to produce more
