Boggs S. Principles of Sedimentology and Stratigraphy
Подождите немного. Документ загружается.

12m
NORTH
Fire 6.11
I
,
6.7 Origin of carbonate Rocks
185
SOUTH
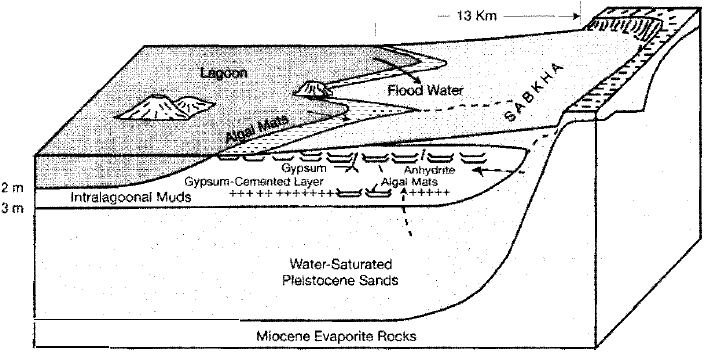
Abu Dhabi sabkha, Persian Gulf. Schematic representation of a typical sabkha environ
ment in which penecontemporaneous dolomite forms, commonly in association with gyp
sum and anhydrite, owing to evaporitic concentration of Mg. [After Butler, G. P., 1969,
Modern evaporite deposition and geochemistry of coexisting brines, the sabkha, Trucial
coast, Arabian Gulf: jour. Sed. Petrology, v. 39, Fig. 2, p. 72, reprinted by permission.]
10:1, dolomite is believed to form. One mechanism by which brines are concen
ated involves evaporation of capillary water in the sediments of the sabkhas.
Upward flow of water from the saturated groundwater zone replaces the water
lost by capillary evaporation, a process called evaporative pumping. Brines may
also be concentrated in surface ponds or bays by surface evaporation of water.
These concentrated brines have higher density than that of normal seawater,
causing them to sink downward. Flushing of large volumes of Mg-rich brine
downward through calcium carbonate sediment can putatively bring about
dolomitization, a process referred to as seepage refluxion (Fig. 6.10A).
The overall volume of dolomite that forms in sabkha environments is be
lieved to be relatively small, and there is still considerable controversy with regard
to the exact mechanism by which the dolomite forms. It is not definitely known if
it forms by replacement of aragonite or high-magnesian calcite (dolomitization) or
it forms as a primary precipitate of disordered protodolomite, which presum
ably later develops better ordering to become true dolomite. See Hardie (1987)
and Purser, Tucker, and Zenger (1994a) for additional discussion of this topic.
Mixing-Zone Model. Several studies published since the early 1970s (e.g., Han
shaw, Back, a
nd
Deike, 1971; Badiozamani, 1973; Folk and Land, 1975) have sug
gested that brackish ground waters produced by mixing of seawater with meteoric
water
could be saturated with respect to dolomite
at Mg
2+
/Ca
2+
ratios much
lower than those required under hypersaline conditions. Mixing of fresh water
and saline water in environments such as the subsurface zones of coastal areas
where meteoric waters come in contact with seawater (Fig. 6.10B) is suggested to
lower salinities sufficiently so that dolomites can form at Mg
2+
/Ca
2+
ratios rang
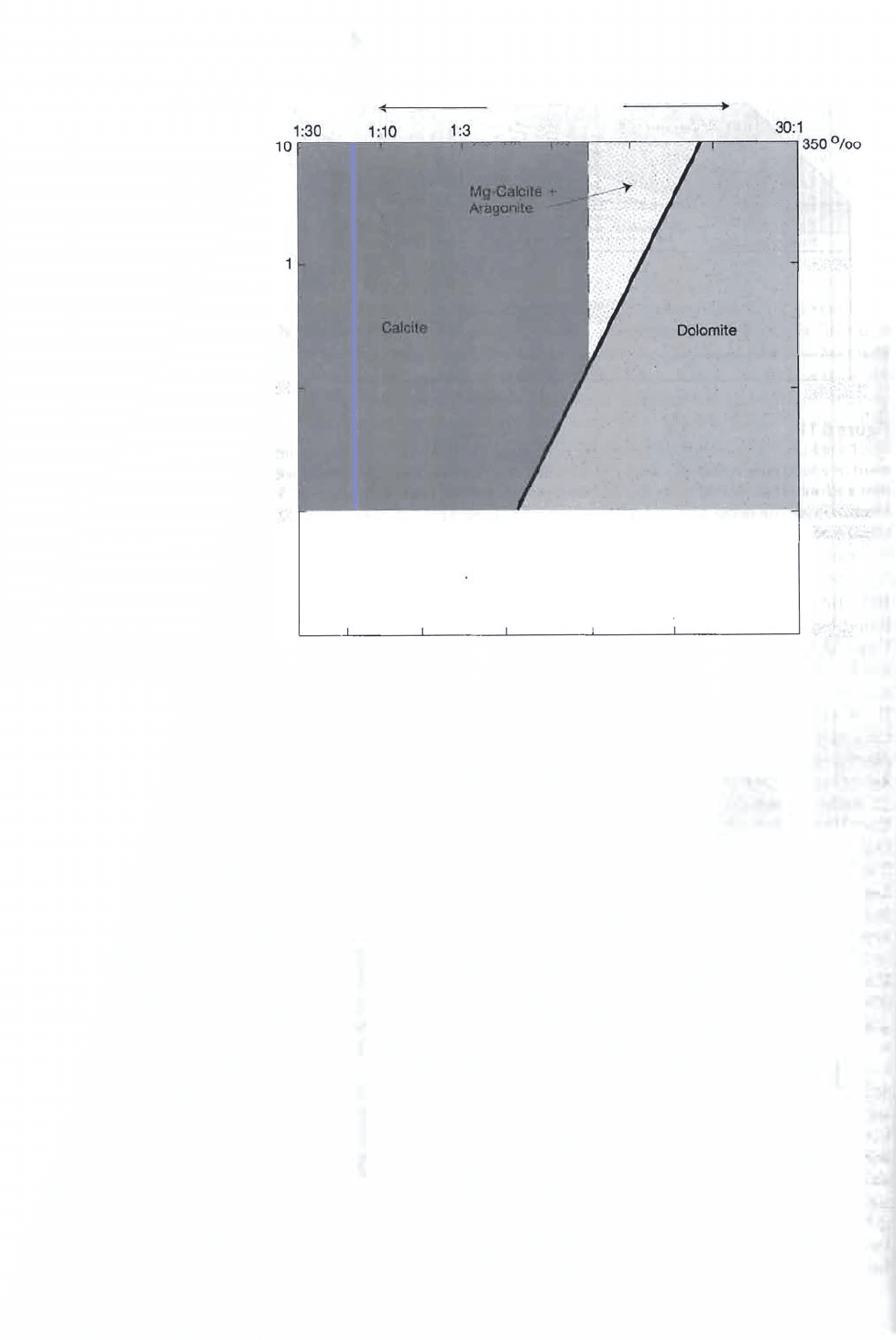
ing from normal seawater values of about 5:1 to as low as 1:1 (Fig. 6.12). Presum
ably, dolomite can form at lower Mg
2+
/Ca
2
+
ratios in these mixed waters
compared to seawater because of less competition by other ions in the less saline
water. The mixing-zone model, or variations thereof, has been referred to also as
the Dorag model (Badiozamani, 1973) and the schizohaline model (Folk and
Land, 1975).
186
Chapter 6 I Carbonate Sedimentary Rocks
Figure 6.12
The preferred fields of oc-
currence of dolomite, cal-
cite, magnesian-calcite, and
aragonite plotted as a tunc-
tion of salinity and Ca/Mg
ratios. Note that dolomite
can putatively form at pro-
gressively smaller Mg/Ca
ratios with decreasing
salinity owing to slower
crystallization rates and rei-
ative scarcity of competing
foreig n ions at low salini-
ties. [After Folk, R. L., and L.
S. Land, 19 75, Mg/Ca ratio
and salinity: Two controls
over crystallization of
dolomite: Am. Assoc. Petro-
leum Geologists Bull., v. 59,
Fig. 1, p. 61, reprinted by
permission.]
Mg/Ca (ppm)
Mg decreasing
Mg increasing
1:1
3:1
10:1
35 °/oo
w
�
�
w
0
�
w
0.1
3.5 °/oo
z
>
�
�
w
�
z
0.01
0.35 °/oo
(350 ppm)
0.001 35 ppm
1:10
1:3
1 :1
3:1
10:1
Mg/Ca (meq)
Although the mixing-zone dolomite model has attracted many proponents,
it has also come under some fairly devastating attacks. For example, Hardie
(1987) points out that Badiozamani, in his original (1973) calculations for the
model, used the solubility values of less soluble ordered dolomite when he
should have used the values of more soluble, less ordered, Ca-rich dolomite
(which is the kind of dolomite that actually forms under surface temperatures).
Hardie also maintains that there is no actual documentation that dolomite can
form at Mg/Ca ratios of 1:1, nor is there hard evidence that demonstrates the spe
cial power of low-salinity conditions to produce cation-ordered dolomite. Machel
and Moun�oy (1986) further point out that dolomite does not form in most mod
ern freshwater/seawater mixing zones, and where it does form, the volume of
dolomite is small. Purser, Tucker, and Zenger (1994b) observe that mixed waters
are potentially capable of dolomitization; however, the true significance of the
mixing zone may be more in its role of inducing fluid moveents in the marine
groundwater below.
Seawater (Shallow Subtidal) Model. In the hypersaline model, seawater modified
by evaporation processes is required for dolomitization. A few workers have pro
posed that early dolomitization can also take place in normal, unmodified sea
water. According to the concept embodied in this model, dolomitization can occur
normal seawater if a sufficient volume of seawater is forced through the sediment
so that each pore volume of water the sediment is constantly being renewed with
new seawater (e.g., Carballo, Land, and Miser, 1987; Land, 1991). Thus, new Mg2+ is
constantly being supplied while replaced Ca2+ ions and other ions that might "poi
son" the dolomite crystal structure are removed. As an example, Carballo, Land,
6.7 Origin of Carbonate Rocks
187
and Miser (1987) report an area of Sugarloaf Key, Florida, where seawater is forced
up
ward and downward through Holocene carbonate mud during rise and fall of
seawater accompanyg sprg tides, a process they call tidal pumping. Owing to
the large volume of seawater driven through e sediment by this mechanism,
lae quantities of Mg
2
+
are imported into the sediment, and pore fluids are con-
sntly being replaced by new fluids. Under these conditions, dolomite is forming
in the sediment even though little or no evaporation of the seawater has oc-
curred. Carballo, Land, and Miser suggest that dolomite forms both by precipi-
tation as a cement and by later replacement of preexisting crystallites.
Dolomitization might also occur during a sea level rise (Fig. 6.10C) as marine
porewaters move landward within a platform (e.g., Tucker, 1993). Although the
seawater model has some problems, many geologists are apparently convinced
at normal seawater has been a major dolomitizing medium in the past (Purser,
Tucker, and Zenger, 1994b ).
Other Factors Affecting Early Dolomitization
Experimental work on the formation of dolomite at 200°C by Baker and Kaser
(1981) demonstrated that the presence of dissolved sol- inhibits the formation of
dolomite. Extrapolating their experimental results to lower temperatures, they
suggest that the reason for the scarcity of dolomite in open-marine environments
dissolved Sol- in seawater. Dissolved Sol- ions can allegedly inhibit the
dolomitization of calcite at sol- values as low as 5 percent of their seawater
value. Thus, according to these authors, any process that removes S04
2
- om sea
war (e.g., bacterial reduction of /-; precipitation of calcium sulfate
(Ca504 • 2Hz0]) favors the formation of dolomite . Subsequent experimental work
by Morrow and Abercrombie (1994) confirms that dissolved sulfate at a concen
traon of 0.5M retards, but does not prevent, dolomitization of calcite at high
temperatures. These authors suggest that the observed rates of dolomitization
may be due to dissolution of calcite at a more rapid rate in a sulfate-free environ
ment at high temperatures because of its greater degree of undersaturation.
Bacteria may play a role in precipitation of dolomite under some conditions
(e.g., Beasconi, 1994; Gouma Folk, and Kirkland, 1997; Vasconcelos and
McKenzie, 1997; Wright, 2000). For example, Vasconcelos and McKenzie (1997) re
port precipitation of dolomite at normal earth-surface temperatures in black, or
ga nic-rich sediments in a shallow-water coastal lagoon (Lagoa Vermelha) near Rio
. de Janeiro, Brazil. They attribute precipitation to the activities of sul.fate-reducing
anaerobic bacteria. Precipitation apparently occurs owing to the release of excess
Mg along with other by-products of sulfate reduction. Saturation of Mg on the
submicron scale in microenvironments around the cell bodies creates conditions
favorable for preferential precipitation of dolomite. The precipitate is a Ca-rich
dolomite that undergoes ageing with time to increase ordering. In addition to ob
rvations in Lagoa Vermelha, dolomite was produced in the laboratory by using
sulfate-reducing bacteria cultured from Lagoa Vermelha (Vasconcelos and
McKenzie, 1995; Warthma et aL, 2000; Van Lith et al., 2003).
Changes in Climate and Ocean Chemist
discussed, the concentration of Mg
2+
ions in the ocean was higher dung peri
ods of "aragonite seas," when rates of seafloor spreading and sea levels were low,
an
during periods of "calcite seas" when substantial amounts of Mg
2+
were
g absorbed onto hot seafloor basalts. Thus, dolomite precipitation may have
been favored in aragonite seas.
Also, ocean temperature appears to have an effect on dolomite precipitation.
Dug times of rapid seafloor spreading (and high sea level), C0
2
levels in the
atmosphere are high owing to increased rates of C0
2
outgassing related to high

188
Chapter 6 I Carbonate Sedimentary Rocks
rates of seafloor spreading. High concentrations of C02 in the atmosphere gener
ate a "greenhouse" (hothouse) effect because C02 prevents heat Joss from Earth
and leads to global waing. Lower concentrations are present during low rates
of seafloor spreading and lower sea level, producing so-called "icehouse" condi
tions. Some earlier observers (e.g. Givens and Wilkinson, 1987) reported that
dolomite is more common in rocks deposited during greenhouse conditions than
during icehouse conditions. Arvidson, Mackenzie, and Guidry (2000) suggest that
increased atmospheric temperature results in increased rates of terrestrial weath
ering
of siliciclastic and carbonate rocks and correspondingly incases rates of
transfer of dissolved carbon, mainly as bicarbonate (HCo3·), to the ocean. This
increase in ocean bicarbonate apparently causes greater supersaturation of ocean
water ith respect to dolomite than to calcite. Thus, dolomite precipitation is fa
vored during warm, greenhouse conditions.
Subsuace (Burial) Dolomite
As mentioned, much dolomite in the geologic record has relict textures that in
dicate the dolomite was formed by replacement (dolomitization) of a precursor
limestone. Such dolomitization appears to have taken place in the subsurface
much later (perhaps millions to hundreds of millions of years later) than the
time of formation of penecontemporaneous dolomite. For example, Mounoy
and Amthor (1994) report that massive replacement dolomites form 50-90 per
cent of all Devonian dolomites in the Western Canada Sedimentary Basin and
that dolomitization took place both in the intermediate (500-1500 m) to deep
(1500-3000 m) subsurface.
Reasoning from the concepts presented in the seawater model above, e
problem of understanding late-stage, large-scale subsurface dolomitization may re
duce mainly to finding a mechanism for circulating large volumes of Mg-rich water
(normal seawater, modified seawater, basin brines, evaporite brines) deep into the
subsurface. At the higher temperatures present in the subsurface, dolomitization
can apparently take place readily in any buried limestone that has sufficient poros
ity and permeability to allow circulation of large volumes of Mg-bearing water.
Several mechanisms have been suggested to drive circulation of fluids down or up
through buried limestones in a basin, including (1) gravity-driven flow owing to
the presence of a hydraulic head, the maitude of which is determined by the el
evation of the meteoric recharge area for the subsurface formations (e.g., Carven
and Freeze, 1984), (2) thermal convection resulting from a geothermal heat source
below the basin (e.g., Kohout, Henry, and Banks, 1977), and (3) buoyant circulation
caused by circulation within freshwater lenses along the mixing zone with saline
waters; the resulng discharge of brackish water at the coast causes a compensat
ing inflow of saline waters at depth (e.g., Whitaker and Smart, 1990).
6.8 DIAGENESIS
Most carbonate sediments are deposited under marine condions, although car
bonate rocks can also form under some nonmarine conditions (Chapter 11). After
deposition, carbonate sediments a subjected to a variety of diagenetic processes
that bring about changes in porosity, mineralogy, and chemistry. Carbonate min
erals are generally more susceptible to dissolution, recrystallization, and replace
ment than are most silicate minerals. Thus, the mineralogy of carbonate sediments
may be pervasively altered. For example, an original aragonitic mud may alter en
tirely to calcite during early diagenesis or burial. ln turn, the calcite may be re
placed completely or nearly completely by dolomite at a later time. Such changes
may also destroy or modify original depositional textures such as carbonate
grains and micrite. Porosity of carbonate sediments may be either reduced by
compaction and cementaon or enhanced by dissolution.
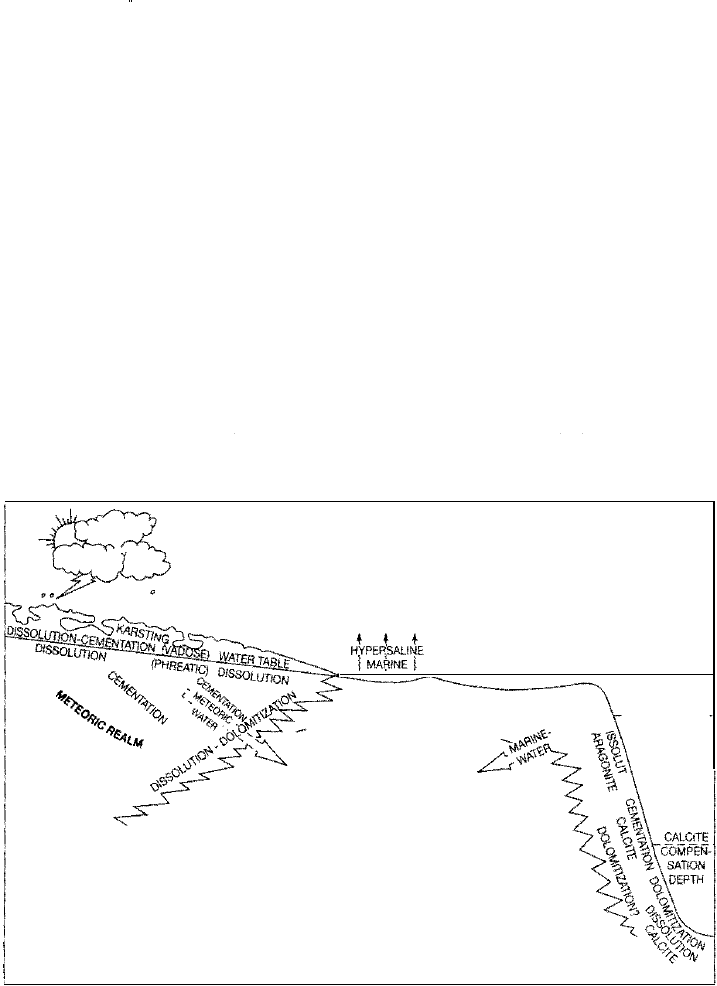
Regimes of Carbonate Diagenesis
Carbonate sediments may go through the same general stages of diagenesis as sili
ciclastic
sediments, that
is, shallow burial (eogenesis), deep
burial (mesogenesis),
and uplift and unroofing (telogenesis). Diagenesis takes place in three major
regimes or realms (Fig. 6.13): the marine, the meteoric, and the subsurface.
The marine realm includes the seafloor and the very shallow marine subsur
face. The diagenetic environment here is characterized by seawater temperatures
and marine waters of normal salinity. The principal diagenetic processes in this
environment involve bioturbation of sediments, modification of carbonate shells
and other grains by boring organisms, and cementation of grains in warm-water
areas, particularly in reefs, platform-margin sand shoals, and carbonate beach de
posits (beachrock).
Marine carbonate sediments may be brought from the seaoor realm into the
meteoric realm in two ways: by falling sea level and by progressive sediment fill
ing of a shallow carbonate basin. Older carbonate rock can also be brought into the
meteoric realm by late-stage uplift and unroofing of a deeply buried carbonate
complex
(telogenesis). The meteoric realm is characterized by the presence of
freshwater; it includes the unsaturated (sediment pores not filled with water) va
dose zone above the water table and the phreatic zone, or water-saturated zone,
below the water table. Meteoric waters are typically highly charged with C02;
thus, they are chemically very aggressive (acidic). Because aragonite and high
magnesian calcite are more soluble than calcite, they dissolve readily in these cor
rosive waters. On the other hand, dissolution of aragonite and high-magnesian
calcite may saturate the waters in calcium carbonate with respect to calcite, caus
ing calcite to precipitate (a process called calcitization). This dissolution reprecip
itation process causes less stable aragonite and high-magnesian calcite to be
replaced by more stable calcite. Calcite may also precipitate into open spaces as a
cement. Thus, dissolution, alteration of aragonite and high-magnesian calcite to
�
. '
. . .
00L
OMITIZA0"
CEMEN
IARI£
R
0
_
G
---------- t�/) o COMPENTION
ME CHANICAL
�
�
�
'·
/ /
-� DEPT�
COMCTION
�
�
"'' s
BRFACE
%
-
REALM
CHEMICAL COMCTION
AO CEMENTATION
DISSOLUTON
BASINAL
BRINES
CEMENTATION
6.8 Diagenesis
189
Figure6.13
The principal environments
in which postdepositional
modification of carbonate
sediments occurs. The
dominant diagenetic
processes that occur in
each of the major diage
netic realms are also indi
cated. See text for details.
[From Moore, C. H., 1989,
Carbonate diagenesis and
porosity. Fig. 3.1, p. 44,
reprinted by permission of
Elsevier Science Publishers,
Amsterdam.]

190
Chapter 6 I Carbonate Sedimentary Rocks
calcite, and calcite cementation are the principal diagenetic processes in the mete
oric realm.
After an initial period of diagenesis on the seafloor, and possibly in the me
teoric realm, carbonate sediments are gradually buried and subjected to in
creased pressures, higher temperatures, and compositionally changed pore fluids
in the subsurface realm. Under these changed conditions, carbonate sediments
may undergo physical compaction, chemical compaction (dissolution at grain
boundaries), and additional chemical or mineralogical changes that may include
dissolution, cementation, aragonite-to-calcite transformation, and replacement of
calcite by another mineral such as dolomite. The exact nature of the changes that
take place during deep subsurface diagenesis depends upon the specific condi
tions (temperature, pore-fluid composition, pH) of the burial environment.
Major Diagenetic Processes and Changes
Biogenic Alteration
Organisms in carbonate depositional environments rework sediment by
boring,
burrowing, and sediment-ingesting activities, just as they do in siliciclastic envi
ronments. These activities may destroy primary sedimentary structures (e.g.,
Demicco and Hardie, 1994) in carbonate sediment and leave behind mottled bed
ding and various kinds of organic traces. In addition, many kinds of small organ
isms, such as fungi, bacteria, and algae, create microborings skeletal fragmts
and other carbonate grains. Fine-grained (micritic) aragonite or high-magnesian
calcite may then precipitate into these holes. This boring and micrite-precipitation
process may be so intensive in some warm-water environments that carbonate
grains are reduced almost completely to micrite, a process called micritization. If
boring is less intensive, only a thin micrite rim, or micrite envelope, may be pro
duced around the grain (Fig. 6.14A). Bacteria are suggested to affect carbonate
diagenesis in a variety of other ways, such as mediating precipitation of car
bonate
cements and diagenet formation of micrite (e.g., Camoin and Arnaud
Vanneau, 1997). Larger organisms, such as sponges and molluscs, create
macroborings in skeletal grains and carbonate substrate, and other organisms,
such as fish, sea cucumbers, and gastropods, may break down carbonate grains in
various ways to smaller pieces.
Cementation
Cementation is an important process in all diagenetic realms. the ocean floor,
cementation takes place mainly in warm-water areas within the pore spaces of
grain-rich sediments or in cavities. Reefs, carbonate sand shoals on the margins of
platforms, and carbonate beach sands are favored areas for early cementation.
Areas of the seafloor along the platform margin where sediments become well
cemented are referred to as hardgrounds. Cemented carbonate beach sand is
called beachrock. Seafloor cement is commonly aragonite, less commonly high
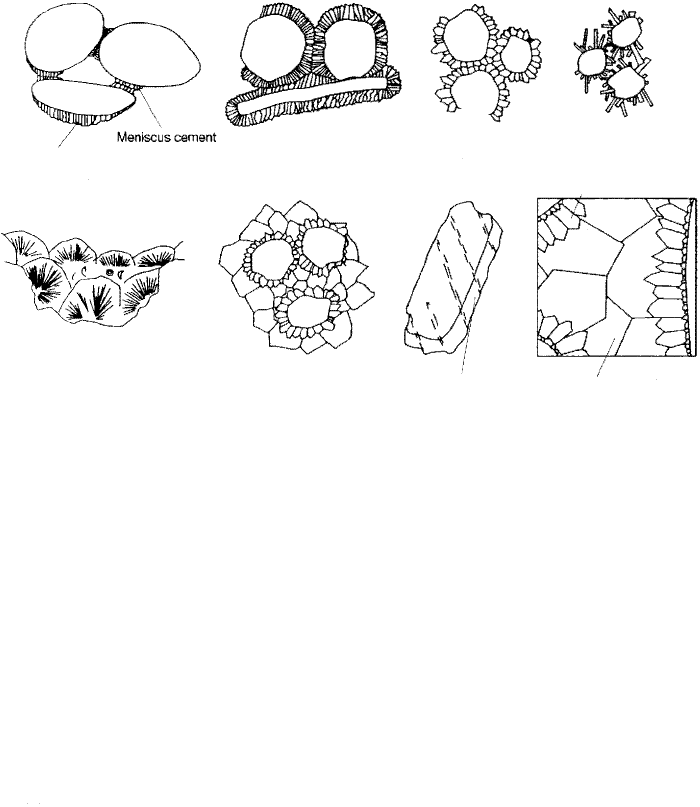
magnesian calcite. Seafloor cement can take several textural forms, as shown in
Figure 6.15. Beachrock may contain meniscus cements that form where water is
held by capillary forces as interstitial water drains from beaches during low tide.
Because beach sediments are not constantly bathed in water, pendant cements
may also form in beachrock along the bottoms of grains where drops of water are
held. Isopachous rinds, which completely surround grains, form under subaque
ous conditions where grains are constantly surrounded by water. Aragonite ce
ments may also occur as a mesh of needles or as fibrous radial crystals that have a
botryoidal form.
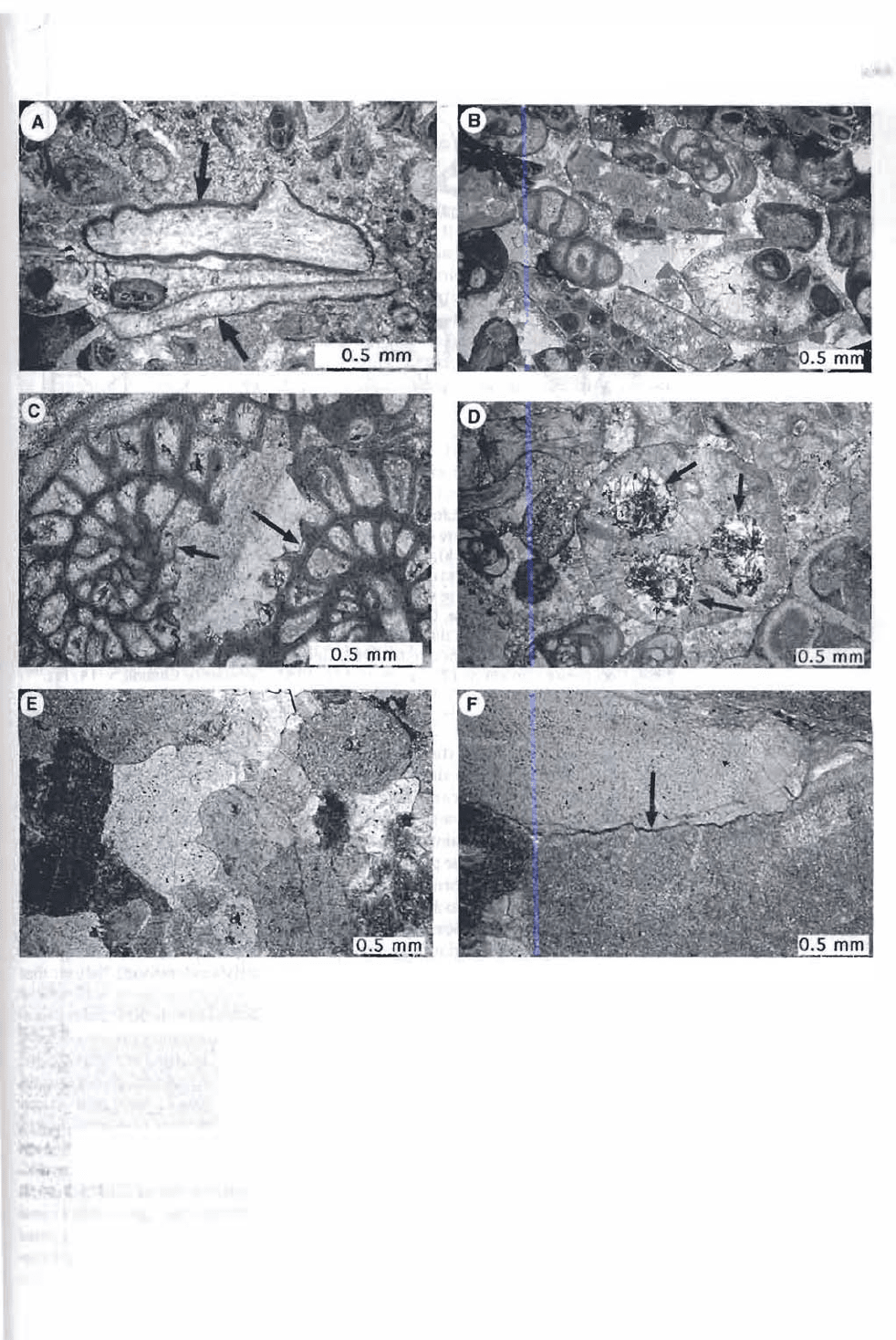
gure 6.14
Diagenetic fabrics in limestones: A. Dark, micrite rims or envelopes (arrows) around fossil
fragments, Renault Formation (Mississippian), Missouri. B. Sparry calcite (white) cement
ing fossils and fossil fragments, Salem Formation (Mississippian), Missouri. C. Recrystalliza
tion fabric that has partially destroyed fossil fusulinid foraminifers (arrows), Morgan
Formation (Pennsylvanian), Colorado. D. Patchy replacement of a fossil fragment by chert
(arrows), Salem Formation (Mississippian), Missouri. E. Grain fabric that is tightly packed
owing to physical compaction of echinoderm (crinoid) fragments, Kimswick Limestone
(Ordovician), Missouri. F. Irregular boundary (arrow) between two echinoderm fragments
f0med as a result of pressure solution (chemical compaction), middle Mississippian lime
stone, Oklahoma.
6.8 Diagenesis
191
192
Chapter 6 I Carbonate Sedimentary Rocks
Pendant
(gravitational)
cement
Botoidal
Figure 6.15
lsopachous
(fibrous to bladed)
Blocky
lsopachous
(bladed)
Syntaxial rim
Mesh of
needles
Bladed prismatic
Coarse mosaic
Principal kinds of cements that form in carbonate rocks during diagenesis. Seafloor
diagenetic environments are characterized particularly by aragonitic meniscus and
pendant cements (in beachrock), isopachous cement, needle cement, and botryoidal ce
ment. Meteoric-realm cements are composed dominantly of calcite and include menis
cus and pendant cements in the vadose zone and isopachous, blocky, and syntaxial rim
cements in the phreatic zone. Cements of the subsurface burial realm are also main
ly calcite and include syntaxial rims, bladed prismatic, and coarse mosaic types. [Modified
from james, N. P., and P. W. Choquette, 1983, Geoscience Canada, v. 10, Fig. 3, p. 165;
1984, Geoscience Canada, v. 11, Fig. 24, p. 177; 1987, Geoscience Canada, v. 14, Fig.
21, p. 16.]
In the meteoric realm, dissolution is a more important process than cementa
tion; however, cementation does occur. The cement is almost exclusively calcite.
As mentioned, the calcium carbonate that forms this cement is derived by dissolu
tion of less stable aragonite and high-magnesian calcite. In the (water-) unsaturat
ed vadose zone, calcite cements are commonly meniscus and pendant cements.
the water-saturated phreatic zone, they are isopachous, blocky, or syntaxial rim
cements. Syntaxial rims form by precipitation of optically continuous calcite
around single-crystal fossil echinoderm fragments, in much the same way that ce
ment overgrowths form around quartz grains.
Calcite cementation may also take place during deep burial, although the
conditions that control cementation at depth are poorly understood. Factors that
have been cited to favor carbonate cementation during deep burial include unsta
ble mineralogy (aragonite and high-magnesian calcite favors solution and reprecr
pitation); pore waters highly oversaturated in calcium carbonate; high porosity
and permeability (which enable high rates of fluid flow); increase temperature;
and decrease carbon dioxide partial pressure. The calcium carbonate needed for
cementation at depth may be supplied, at least in part, by pressure solution of car
bonate sediment much the same way that pressure solution of quartz grains
supplies silica to pore waters in siliciclastic sediment. Coarse mosaic calcite d
bladed prismatic calcite (Fig. 6.15) are common kinds of deep-burial cements. The
combination of bladed prismatic and coarse mosaic cement shown in Figure 6.15
is called drusy cement (see Fig. 6.1A). These calcite cements are commonly coarse
grained and clear or white in appearance. They are usually referred to as sparry
calcite cement. Figure 6.148 provides an additional example of a skeletal lime
stone cemented by sparry calcite cement.

Dissolution
Cementation is a very common diagenetic process in carbonate rocks, yet, some
what paradoxically, so is dissolution. Dissolution of carbonate minerals requires
conditions essentially opposite to those that lead to cementation. Dissolution is fa
vored by unstable mineralogy (presence of aragonite or high-magnesian calcite),
cool temperatures, and low pH (acidic) pore waters that are undersaturated with
calcium carbonate. Dissolution takes place particularly in chemically aggressive
pore waters highly charged with C02 and/or organic acids. Dissolution is rela
tively unimportant on the seaoor but is particularly prevalent the meteoric
realm where chemically aggressive meteoric waters percolate or flow down
through the vadose zone into the phreatic zone. Extensive dissolution of aragonite
and high-magnesian calcite takes place in this environment and even calcite may
dissolved if pore waters are suiciently aggressive. Dissolution tends to be con
centrated particularly along the water table (the boundary between the vadose
and phreatic zones), which accounts for the common presence of caves in carbon
a rocks at the level of the water table. Dissolution is less intensive the deep
burial (subsurface) realm than in the meteoric realm for two reasons. First, most
aragonite and high-magnesian calcite may already have been converted to more
sble calcite the meteoric realm (see "Neomorphism" below). Second, increas
ing temperature at depth decreases the solubility of all carbonate minerals. Disso
lution may occur at depth if enough C02 is added to pore waters as a result of
burial decay of organic matter (decarboxylation) to overcome the decrease in solu
bility resulting from increased temperature. Likewise, mixing of subsurface wa
ters at depth may produce uids that are undersaturated with respect to calcite,
thus promoting destruction of carbonate cements or other carbonate elements
(Morse, Hanor, and He, 1997). Buried carbonate sediments that are brought back
into the meteoric zone after uplift may undergo extensive dissolution of both pre
viously formed cements and other carbonate minerals under the influence of
chemically aggressive, COrcharged meteoric waters.
Neomorphism
Neomorphism is a term used by Folk (1965) to cover the combined processes of in
version (e.g., transformation of aragonite to calcite) and recrystallization.
Inversion refers to the change of one mineral to its polymorph, such as aragonite
to calcite. Strictly speaking, inversion takes place only in the solid (dry) state.
When the transformation of aragonite to calcite takes place in the presence of
water, it occurs by means of dissolution of the less stable aragonite and nearly si
multaneous precipitation (replacement) by more stable calcite. Many geologists
refer to this process as calcitization, as mentioned. During diagenesis, most arag
onite is eventually calcitized. Recrystallization indicates a change in size or shape
of a crystal, with little or no change in chemical composition or mineralogy. Calci
tization and recrystallization commonly go hand in hand.
Neomorphism may occur in all three diagenetic realms but is particularly
important in the meteoric and subsurface diagenetic environments. Neomor
phism may affect both carbonate grains and micrite and commonly increases
crystal size. This process destroys original textures and fabrics and, when perva
sive, may cause the entire rock to become recrystallized. Thus, a fine-grained (mi
critic) limestone can be converted into a coarse-grained sparry rock. On a smaller
scale, recrystallization results in the formation of large, dear crystals of calcite
that closely resemble sparry calcite cement. In fact, one of the most difficult prob
lems in the microscopic study of carbonate rocks is to differentiate between spar
calcite cement and neomorphic spar. Figure 6.14C shows an example of
neomorphic spar.
6.8 Diagenesis
193

194
Chapter 6 I Carbonate Sedimentary Rocks
Replacement
As described under "Siliciclastic Diagenesis," replacement involves the dissolu
tion of one mineral and the nearly simultaneous precipitation of another mineral
of different composition in its place. Replacement of calcium carbonate minerals
by other minerals is a common diagenetic process. Dolomitization of CaC03 sedi
ment is one kind of replacement process. In addition, many other kinds of noncar
bonate minerals may replace carbonate minerals during diagenesis, includg
microcrystalline quartz (chert; Fig. 6.14D), pyrite (iron sulfide), hematite (iron
oxide), apatite (calcium phosphate), and anhydrite (calcium sulfate). Replacement
can occur in all diagenetic environents. We have already discussed the replace
ment of CaC03 by dolomite in seafloor and burial environments. In carbonate
evaporite sequences, replacement of carbonate minerals by anhydrite at depth is a
common process. Replacement of carbonates by microcrystalline quartz (chert) is
also common in the meteoric and deep-burial environments. For example, Maliva
and Siever (1989) report replacement of Paleozoic carbonates by chert at burial
depths ranging from 30 to 1000 m. Replacement of carbonate minerals by silica
may be very selective, with silica replacing fossils and other carbonate grains in
preference to micrite, as in Figure 6.14D.
Physical and Chemical Compaction
Newly deposited, watery carbonate sediments have initial porosities ranging
from 40 to 80 percent. As burial into the subsurface proceeds, the pressure of over
lying sediments brings about grain reorientation and tighter packing (Fig. 6.14E).
As with siliciclastic sediments, compaction results loss of porosity and thinning
of beds at fairly shallow burial depth. At deeper burial to depths of about 1000 ft
(305m) and at progressively higher overburden pressures, grains may also deform
Figure 6.16
Well-developed sutured stylolites in Cretaceous limestones,
Calcare Massicio, Tu scany, Italy. [Photograph courtesy of
. E. F. McBride.]
