Boggs S. Principles of Sedimentology and Stratigraphy
Подождите немного. Документ загружается.


6.7 Origin of Carbonate Rocks
175
Note by the presence of double arrows that this reaction is reversible. If equilibri-
conditions are disturbed by loss of carbon dioxide, the concentration of hy-
dgen ions decreases and the pH increases. The reaction shifts toward the left,
resulting in precipitation of solid CaC03. The partial pressure of carbon dioxide
thus clearly exerts a major control on calcium carbonate precipitation. Anything
that causes loss of carbon dioxide (Table 6.4) should theoretically trigger the onset
of precipitation because dissolved carbon dioxide increases the acidity of water by
releasing H" ions, as mentioned, although we shall see subsequently that inorgan-
pcipitation of calcium carbonate caused by loss of C02 may not be as impor-
tant under natural conditions in the open ocean as suggested by Equation 6.4.
Two principal inorganic mechanisms that can cause loss of carbon dioxide
from water are (1) increase in temperature and (2) decrease in water pressure. An
increase in temperature causes a decrease in the solubility of carbon dioxide (and
other gases) in water; that is, an increase in temperature reduces the capacity of
water to dissolve and retain carbon dioxide, resulting in escape of carbon dioxide.
Decrease in water pressure can also allow carbon dioxide to escape. Under natur
al conditions, pressure may be lowered and more C02 absorbed by the atmo
here, by wave agitation caused by storm activity or breaking of waves in the
surf zone or over shallow banks. Circulation of deep, pressurized waters to the
surface can likewise release carbon dioxide, and even lowering of atmospheric
pressure may cause slight loss of carbon dioxide from ocean water.
In addition to its effect on C02 solubility, increase in temperature causes a
decrease in the solubility of calcium carbonate minerals; that is, the calcium car
bonate solubility product decreases with increasing temperature. Decrease in sol
ubility means that a mineral will be more likely to precipitate under a given set of
condions. Thus, calcium carbonate deposition is favored in the more tropical
aas of the ocean, where surface water temperatures may reach almost 30°C,
compared to about one in the polar regions.
The solubility of chemical constituents is affected also by salinity and the
ionic strength of water (Table 6.4). Ionic strength is a function of the concentration
of ions in solution and the charges on these ions; thus, ionic strength increases as
salinity increases. The solubility of calcium carbonate minerals is markedly de
cased at lower values of salinity because decrease in ionic strength decreases the
concentration of foreign ions (e.g., Mg2+) other than Ca
2
+ and C03 . Foreign ions
terfere with the formation of the calcium carbonate crystal structure, making it
mo difficult for calcite or aragonite minerals to grow and precipitate. Therefore,
e solubility of calcium carbonate is several orders of magnitude lower in fresh
water than in seawater (e.g., Degens, 1965), meaning that calcium carbonate pre
cipitates more readily in fresh water than in seawater. On the other hand, the in
uence of salinity on the solubility of calcium carbonate in the surface waters of
e open ocean may be slight because these waters range in salinities only from
about thirty-two to thirty-six parts per thousand (o/oo).
This elementary discussion of carbonate solubility relationships is intended
only to provide a very basic understanding of carbonate solubility and the factors
Water
condition
Tempera ture
Pssure
lity
Direction
of change
Increase
Decrease
Decrease
Directed effect
Loss of C02, increase in pH
Loss of C02, increase in pH
Decrease in activity of "foreign" cations
"a� in CaC0.1 oluhility = in<rease ln tcndny to pn•dpita.
Effect on
CaC03 solub
i
lity
Decrease•
Decrease
Decrease
Kind of
CaC03 precipitated
Micrite or ooids
Miite or ooids
Micrite or ooids

176 Chapter 6 I Carbonate Sedimenta Rocks
that govern the precipitation of carbonate minerals. See Morse and Mackenzie
(1990) for a more rigorous discussion of carbonate geochemistry.
Im portance of Inorganic Precipitation
According to the theoretical considerations illustrated in Equation 6.4, significant
loss of carbon dioxide by any mechanism should lead to precipitation of calcium
carbonate minerals. Does loss of carbon dioxide cause precipitation of calcium
carbonate on an important scale in the open ocean environment today? Available
data show that near-surface water in the mode ocean is oversaturated by more
than six times with respect to calcite and more than four times with respect to arag
onite (Morse and Mackenzie, 1990, p. 217). Such gross oversaturation indicates a re
luctance of calcium carbonate minerals to precipitate. Why? There appear to be at
least two reasons why calcium carbonate minerals may not precipitate in e mod
e ocean as readily as suggested by the reaction in Equation 6.4.
First, the maitude of the pH changes that occur in the open ocean owing to
loss of carbon dioxide is relatively small because seawater is a well-buffered solu
tion. Buffering occurs because a considerable portion of the carbon dioxide dis
solved in seawater forms undissociated H
2
C03 rather than dissociating to H+
ions, HC03- ions, and co/- ions as predicted by Equations 6.2 and 6.3. Ts
buffering reaction is caused by the high alkalinity of ocean water; that is, the high
conctrations of bicarbonate and carbonate ions already present in surface wa
ters of the ocean inbit breakdown of H
2
C03 to form still more of these ions.
Therefore, the actual change in pH in seawater caused by either gain or loss of car
bon dioxide is comparatively small, and the pH values of seawater in the open
ocean rarely fall outside the range of 7.8 to 8.3 (Bathurst, 1975).
Second, the presence of Mg2+ ions at the concentrations found in seawater
has been shown experimentally to strongly inhibit the precipitation of calcite
(CaC03). Experiments by Berner (1975) show that Mg2 is readily adsorbed onto
the surface of calcite crystals and incorporated into eir crystal structu. Ts
nonequilibum incorporaon of Mg2+ into growing calcite crystals was interpret
ed by Beer as decreasing their stability, resulting in an increase in calcite solul
ity. Thus, calcite crystals do not readily nucleate and grow in the presence of Mg2+
in seawater concenations. See also Mucci and Morse (1983). Aragonite is also
composed of CaC03 but has a different crystal structure (orthorhombic) from that
of calcite (rhombohedral). Mg2+ ions appear to be less prone to sorb to aragonite
nuclei and disrupt crystal growth. Therefore, aragonite is less affected by Mg2+
and has a tendency in the presence of Mg2 to precipitate in preference to calcite.
Nonetheless, aragonite does not precipitate completely freely in ocean water, even
in surface waters supersaturated with respect to calcium carbonate, possibly
owing to formation of thin organophosphatic coatings on aragonite seed nucl
that inhibit their growth (Beer et a!., 1978).
Although calcite does not precipitate freely in the mode ocean owing to
the presence of abundant Mg2 ions, accumulating evidence suggests that calcite
was
precipitated in preference to aragonite at times in the geologic past (referred
to as "calcite seas"} when the concentration of Mg2+ ions in the ocean was low
(Sandberg, 1983; Stanley and Hardie, 1999). Stanley and Hardie link these times of
calcite precipitation to high rates of seaoor spreading, which increases removal
of Mg2+ om seawater by absorption into hot seafloor basalts. Thus, skeletal and
nonskeletal carbonates deposited during early Cambrian-middle Mississippian
and middle Jurassic-late T ary were dominantly low-magnesium calcite,
whereas those deposited during middle Mississippian-middle Jurassic and
Tertiary-Quaternary were dominantly aragonite and high-magnesium calcite
(Fig. 6.9). Seas that preferentially precipitate aragonite are referred to as "arago
nite seas" (Stanley and Hardie, 1999).

Cretacus
0
5
N
0
Jurassic
Tr iassic
Permian
Pennsylvanian
Mississippian
0
Devonian
'5
N
g
Silurian
.
Ordovician
Cambrian
Aonite (A)
+high-
magnasian
ccite (H MC)
�e
OM a
50
100
150
200
250
300
350
400
450
500
550
Figure 6.9
6.7 Origin of Carbonate RQoks
177
The most favorable times for precipitating aragonite + high-magnesian
calcite skeletal and nonskeletal carbonates, and low-magnesian calcite
skeletal and nonskeletal carbonates in the Phanerozoic (post
Precambrian) ocean. The ocean during times of dom inantly aragonite
precipitation is referred to as an "aragonite sea" and during times of
dominantly low-magnesian calcite precipitation as a "calcite sea." [Based
on Stanley and Hardie, 1999, and Sandberg, 1983.]
The generation of ooids provides an example of what appears to be largely
Iorganic precipitation of CaC03. Ooids in modern environments consist mainly
of aragonite, whereas many ancient ooids may have precipitated as calcite. Ooids
form mainly under high-energy, agitated-water conditions in warm waters that
a supersaturated with calcium carbonate. Wa rming and evaporation of cold
ean water driven onto shallow banks by tidal currents result in supersaturation
of the water. Currents and waves keep the grains moving and intermittently sus
p
ended, �llowing more or less even precipitation of calcium carbonate on all sides
of the grains. Both supersaturation of the water and intermittent burial and resus
pension of the ooids owing to agitation appear to be necessary for most ooids to
form, although some ooids are known to form in quiet water. Cyanobacteria m
other microorganisms may influence the formation of ooids-possibly by rap
ping carbonate grains on organic films or by mediating carbonate precipitation
rough removal of C02. The quantitative importance of organic influences on the
formation of ooids is not well understood (Tucker and Wright, 1990, p. 6).
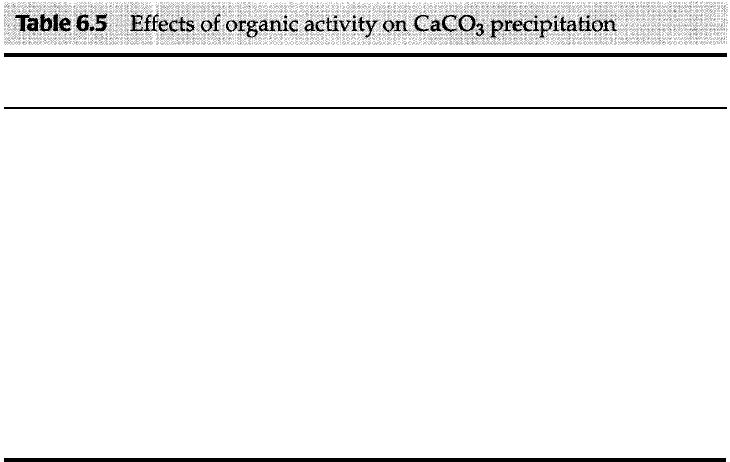
e Role of Organisms in Precipitation of Calcium Carbonate
Precipitation of minerals from water is fundamentally a chemical process; howev
er, chemical processes can be aided in a variety of ways by organisms. Although
purely inorganic precipitation of calcium carbonate minerals from normal-salinity
seawater or fresh water apparently can occur, it may be less common today than
pripita tion aided in some way by organic processes (Table 6.5). Furthermore, ge
ologic evidence suggests tlat organisms may have played a significant role in car
bonate sedimentation throughout most of Phanerozoic (post-Precambrian) time,
and some organisms (e.g., bacteria) may also have mediated carbonate precipita
tion during Precambrian time. The various ways that organisms may influence
carbon
a
te sedimentation are discussed below.
Dir Extraction of CaC03 from Water to form Skeletal Elements. The most impor
tant role that organisms play in forming carbonate sediment is probably the direct
178
Chapter 6 1 Carbonate Sedimentary Rocks
�ble 6.
$
Effe cts of or ganic ac
vity on CaC03 ppitaon
Kind of
organic activity
Extraction of CaC03
from seawater
or fresh water
Photosynthesis
Decay of soft tissue
Feeding, sediment
ingestion
Bacterial activity
Immediate effect
Promotes skeletal growth:
Shells or tests
Internal "stiffeners"
Removes C02 from
water; pH increases
May increase alkalinity;
pH increases
Reshapes sediment
Promotes CaC03
precipitation
Ultimate effect
Forms silt- to gravel-size allochems
upon death of organism
Forms micrite (carbonate mud)
upon death of organism
Promotes precipitation of micrite
or ooids
Promotes precipitation of CaC03
Generates pellets
Promotes precipitation of micrite,
generates peloids, calcifies
microbial mats
removal of dissolved carbonate constituents to build skeletal structures. The exact
mechanisms by which organisms remove dissolved substances to build their
shells or tests is not well understood, but the process is very common. Marine in
vertebrates that build protective shells or other skeletal structures of calcium car
bonate range from freely drifting, planktonic species such as foraminifers and
pteropods (winged marine snails) to bottom-dwelling benthonic organisms such
as calcareous algae, corals, molluscs, and echinoderms. They can remove CaC03
not only from calcium-carbonate-saturated surface waters in tropical regions, but
also from less saturated waters in temperate and colder regions. For example,
shell sands and gravels are important deposits of the modern seafloor in shallow,
cool water at high latitudes (e.g., Farrow, Allen, and Akpan, 1984; James and
Clarke, 1997). Some organisms build skeletal materials from (low-magnesian) cal
cite, whereas others build skeletal material from high-magnesian calcite or arag
onite. The importance of biologic removal of calcium carbonate from the oceans
is demonstrated by the fact that most Phanerozoic limestones contain some rec
ognizable calcium carbonate fossils, and many are composed dominantly of such
remains. Also, large areas of the modern ocean floor are covered by calcareous
oozes composed dominantly of the tests of foraminifers, one-celled algal coccol
ithophores, and pteropods.
Because calcium-carbonate-secreting organisms exist in huge numbers
some parts of the ocean, disintegration of their skeletal remains after death has the
potential to supply large quantities of carbonate sediment of various sizes to the
ocean floor. Some skeletal material consists of the shells of large invertebrate or
ganisms such as pelecypods, gastropods, brachiopods, and corals. These large
shells can become fragmented into sand-size or smaller pieces owing to biogenic
activity or physical breakage. The calcareous tests of some invertebrates such as
foraminifers and pteropods are sand size, and the tests of nannofossils such as
coccoliths are fine-silt size. Some calcareous algae disintegrate to form sand-size
carbonate grains (e.g., Hillis, 1991; Hudson, 1985), whereas others yield mud-size
grains. For example, some red and green algae, such as some species of Halimeda,
Penicillis, and Udotea, have calcareous skeletal elements composed of tiny, needle
like aragonite crystals that are deposited within intercelluar spaces (e.g., Macin
tyre and Reid, 1995) and act as stiffeners for soft tissue. When these organisms die,
6.7 Origin of Carbonate Rocks
179
bacterial and chemical decomposition of the binding tissue releases the skeletal
particles. This decay process yields a fine lime mud (micrite) composed of elon-
gated aragonite crystals, 3-10 mm long, and very small ( <1 mm} equant crystals
(Macintyre and Reid, 1992, 1995).
Early quantitative studies of the rate of production of aragonite by disinte
ation of calcareous algae in the Florida Reef Tract (Stockman, Ginsburg, and
S, 1967) and the Bahamas (Neumann and Land, 1975) led to the conclusion
that much or all of the aragonite mud deposited in these areas in the recent geo
logic past could have been supplied by skeletal disintegraon of calcareous algae.
the oer hand, subsequent observations by Shinn et al. (1989), suggest that only
10-20 percent of the carbonate mud in the Bahamas is algal carbonate, a suggestion
supported by observations on crystal shape by Macintyre and Reid (1992) and the
biochemical studies of Robbin and Blackwelder (1992). Nonetheless, many work
ers agree that disintegration of the skeletal elements of calcareous algae into fine
detritus constitutes a major process for forming carbonate sediments in lagoons,
reefs, and fore-reef slopes (e.g., Hillis, 1991; Hudson, 1985; Multer, 1988). Unfortu
nately, it does not appear possible at this time to provide quantitative estimates of
the relative importance, in the modern ocean as a whole, of algal disintegration vs.
precipitation of CaC03 owing to C02 loss. Such estimates become even more ten
uous when we consider ancient carbonate rocks in the stratigraphic record.
Removal of C from Water by Photosynthesis. Another type of organic activity
that may important to the formation of carbonate rocks is removal of carbon
dioxide from water by photosynesizing plants. As mentioned, any process that
moves carbon dioxide from the water facilitates carbonate precipitation by in
creasing the pH. Aquatic plants remove carbon dioxide from water during photo
synthesis as shown by the followg relationship:
6H20 + 6C02
C
6
H
1206
+ 602
(water + carbon dioxide carbohydrates + oxygen}
(6.5)
Blue-green algae (cyanobacteria), photosynthesizing bacteria, and small phyto
plankton such as diatoms, dinoflagellates, and coccoliths are the most important
ers of carbon dioxide in e marine realm. The activities of photosynthesizing
organisms are at a peak in sunlight and at a minimum in the dark; therefore, the
rbon dioxide content of water in which active photosynthesis is taking place can
vary measurably from day to night. Removal of
C02
by organisms thus decreases
the acidy of the water (increases pH).
cterial Mediation of Pcipitation. Bacteria may play an indict role in precipi
tation of some carbonate sediment. For example, Chafetz (1986) suggests that
some marine peloids originated as a fine-grained precipitate of high-magnesian
calcite within and around active clumps of bacteria. Bacteria may also promote
precipitation of calcium carbonate on dead cyanobacteria, leading to lithification
of microbial mats to stromatolites (Buczynski and Chafetz, 1993; Chafetz, 1994).
Microbial-mediated calcium carbonate precipitation is related to photosynthesis
and ion transport through cell walls. Calcification occurs just outside cell walls in
an alkaline microenvironment, which is generated as Ca
2
+ is exported from the
in exchange for uptake of 2H+. Calcification results from either uptake of
C02
(microalgae) or HC03- (cyanobacteria). Excess organic carbon within the cell
wall can be absorbed from the cell into the microalkaline enviroent, pviding
additional source of carbon for calcification (Yates d Robbins, 2001). The
overall importance of bacterial mediation of carbonate production throughout ge
ologic time is not known; however, some microbiologists (e.g., Castainer, Le Me
tayer-Levrel, and Perthuisot, 1997; 1999) suggest that it may have been significant
(see
also Camoin, 1999).

180
Chapter 6 I Carbonate Sedimentary Rocks
Decay of Dead Organisms. Decay of dead organisms also affects pH. Decay can
release various organic acids and carbon dioxide to the water, causing acidity to
increase (pH decreases). On the other hand, some decay products can be alkaline
(pH increases). Alkalinity may be increased because organic matter is degraded
through sulfate reduction by bacteria (e.g., Bernasconi, 1994). Increase in alkalini
ty favors CaC03 precipitation.
Generation of Pellets. As mentioned in Section 6.4, many carbonate peloids a
fecal pellets, generated by organisms such as sea cucumbers, mollusks, and
worms. These organisms ingest calcium carbonate muds to obtain nutrients and
extrude the remains as pellets. This process does not generate new carbonate sed
iments; it merely reshapes the sediment into a few form.
Relative Importance of Inorganic and Organic Precipitation
of Calcium Carbonate
The organic production of sand- and gravel-size skeletal debris has unquestion
ably made a significant contribution to the overall budget of carbonate sediment
throughout Phanerozoic time. The most controversial carbonate deposits, how
ever, are the huge volumes of nonfossiliferous carbonate mud (micrites) present in
both the Precambrian and Phanerozoic stratigraphic record. Did these thick suc
cessions of carbonate muds originate through inorganic processes or did org
isms
participate some way?
interesting phenomenon at may have a bearing on this question is the
formation of whitings in such warm-water areas as the Bahamas, the Persian Gulf,
and the Dead Sea. The sudden appearance of these whitings, which are milky
patches of surface and near-surface water caused by dense concentrations of sus
pended aragonite crystals, has been suggested to result from spontaneous, large
scale, instantaneous physico-chemical nucleation of aragonite crystals in waters
supersaturated with calcium bicarbonate, i.e., inorganic precipitation. This view
has been challenged by other workers who propose that mechanisms such as
suspension of aragonite mud from the shallow seafloor by wave action, turbulent
tidal flow, turbulent boundary flow, or stirring up of mud by bottom-feeding fish
are responsible for whitings, rather than spontaneous nucleation and precipitation
of
aragonite. Recent isotopic studies by Shinn, Holmes, and Marot (2000) indicate,
however, that whitings are probably not the result of resuspension mechanisms,
leaving open the question of exactly how they do form. The weight of opinion ap
pears to be shifting toward microbially mediated precipitation by photosynthesiz
ing microalgae or cyanobacteria as the likely origin of whitings (e.g., Robbins, Tao,
and Evans, 1997; Yates and Robbins, 2001).
Does this mean that most lime mud precipitation was organically mediated?
Let's consider carbonate deposition during the Precambrian. Judging from the
abundance of calcareous skeletal fragments and whole fossils in Phanerozoic
limestones, the removal of calcium carbonate from seawater owing to some aspect
of organic activity may have been an important mechanism for forming carbonate
sediments since at least early Paleozoic timealthough the relative importance
of biotic and abiotic precipitation of carbonates may have varied throughout this
time. We have a more diicult time explaing the formation of Precambrian
limestones. The Precambrian record contains impressive thicknesses of carbonate
rocks (e.g., as much as 1000 m in Glacier National Park, Montana and Alberta)
that,
as far as we know, were deposited befo the widespread appearance of
calcium-carbonate-secreting organisms. Thus, it does not seem likely, on the
basis of available evidence, that shelled organisms were directly responsible for
deposition of large volumes of Precambrian limestone. Few, if any, Precambrian
organisms could extract CaC03 to build skeletal elements. Blue-green algae
(cyanobacteria) and other photosynesizing bacteria may have played an indirect

6.7 Origin of Carbonate Rocks
181
role in the precipitation of calcium carbonate through photosynthetic removal of
carbon dioxide and by trapping and binding of fine carbonate sediment to form
stromatolites. Cyanobacteria appear to have been particularly abundant in Pre-
camban time, possibly owing to fewer numbers of grazing organisms that fed on
the algal mats.
The problem of Precambrian carbonate deposition is considered in detail in a
cent monograph entitled "Carbonate sedentation and diagenesis in the evolv
ing Precambrian world," edited by Grotzinger and James (2000a). Early Precambri
an (Archean) carbonate deposition was particularly characterized by precipitation
of aragonite and high-maesian calcite directly onto the seafloor as encrustations
of
both inorganic and microbial origin. Carbonate facies include large (up to
meter-scale), upward-divergent "crystal fans" of calcite and dolomite, which re
place original aragonite and high-magnesian calcite. Other facies include carbon
ate muds, stromatolites, and ooid-intraclast grainstones. Grotzinger and James
(20b) suggest that the common precipitation of aragonite and calcite directly on
the seafloor in Archean time took place because the Precambrian surface seawater
was substantially oversaturated with respect to calcium carbonate, well above the
factor of 2-5 that is typical of the oceans today. Abiotic (inorganic) precipitation
appears to have tapered off in later Precambrian (Proterozoic) time, as microbially
mediated precipitation increased, suggesting a gradual depletion of the highly
oversaturated Archean seawater.
Physical Processes in Carbonate Deposition
Calcium carbonate fossils, skeletal fragments, ooids, and other carbonate grains
are subject to the same physical transport processes in the ocean as terrigenous
grains. Thus, ultimate deposition of most limestones occurs through fluid-ow
and sediment gravity-flow processes. Limestones may, therefore, display many of
e same bedding characteristics and sedimentary structures as terrigenous sedi
mentary rocks.
Depth Control of Calcium Carbonate Production
When precipitation of a mineral phase just equals dissolution, the solution is in
equilibrium with the solid and is said to be saturated with this mineral phase. A
solution
that precipitates a mineral is supersaturated, and a solution that dis
solves the mineral is undersaturated. Much of the warm surface water of the
mode ocean is supersaturated with calcium carbonate. Little inorganic CaC03
may actually be precipitating in these waters, however, owing to Mg inhibition or
other factors discussed. This condition of supersaturation changes rapidly with
depth. The degree of calcium carbonate saturation drops off abruptly in waters
below the surface layer; at depths greater than a few hundred meters, seawater is
undersaturated. The saturation of surface waters likewise decreases in colder wa
ters of high latitudes.
The undersaturated state of deeper waters is a function of several factors, al
though increase in carbon dioxide partial pressure is one of the most important
variables. In shallower water, C02 production is increased closer to the ocean
floor by the respiration of benthonic organisms. Oxidation of organic matter on
e seafloor in both shallow and deeper water also in creases C02 production. Fur
eore, colder water found at depth can contain more dissolved C02 than
warmer surface waters. Both decrease in temperature and increase in hydrostatic
pres sure with depth cause an increase in the solubility of calcium carbonate and
thus the corrosiveness of seawater.
Because of decreasing calcium carbonate saturaon of seawater with depth,
calcium carbonate production is confined mainly to the very shallow water areas
of the ocean and to the supersaturated surface waters of the deeper ocean. These

182
Chapter 6 I Carbonate Sedimentary Rocks
are the waters in which most calcium-carbonate-secreting organisms live. Calcium
carbonate dissolution prevails in the deeper, undersaturated waters. The rate of
dissolution does not, however, crease in a linear fashion with depth. Experi
ments in which calcite spheres were suspended on moorings at different depths in
the ocean have demonstrated only slight corrosion of the spheres above a depth of
about 3500 m, but solution of the spheres increased abruptly at that depth (Peter
son, 1966). Effective solution of calcium carbonate thus occurs only at relatively
great depths in e ocean (and to some extent very cold, high-latitude surface
waters). The particular depth at any locality at which the rate of dissolution of cal
cium carbonate equals the rate of supply of calcium carbonate to the seafloor, so
that no net accumulation of carbonate takes place, is called the calcium carbonate
compensation depth (CCD). The position of the calcium carbonate compensation
depth has been compared to the snowline of mountain ranges. Where biogenic
oozes are accumulating in the modern ocean, white carbonate oozes cover elevat
ed areas of the seafloor above the ceo but give way to brown or gray pelagic
clays or siliceous oozes below. In different parts of the mode ocean, the CCD
ranges in depth from about 3500 to 5500 m, because of differences in rates of pro
duction of CaC03 in surface waters and variations in the factors that control car
bonate saturation. The average depth of the CCD in today' s ocean is about 4500 m.
(The average depth of the mode ocean is about 3800 m; depth ranges to slightly
more than 11,000 m.)
Dolome
General Statement
Dolomites are calcium carbonate rocks composed of more than 50 percent of the
mineral dolomite [CaMg(C03h]. To dierentiate the rock from the mineral,
dolomites are sometimes referred to as dolostones or dolomite rock. They are
abundant and widely distributed in the geologic record, rging in age from Pre
cambrian to Holocene, although the greatest volumes of dolomites are Paleozoic
and older. Dolomites occur in close association with limestones and in many
stratigraphic units as interbeds in the limestones; they are also commonly associ
ated with evaporites.
Because dolomites recur so frequently in the stratigraphic record, they must
have formed under environmental conditions that were relatively common and
that were repeated again and again in various localities. Dolomites have been
studied very extensively; therefore, in theo, we ought to understand their origin
quite well. the contrary, the origin of dolomites remains one of e most thor
oughly researched but poorly understood problems sedimentary geolog Al
though it is clear from the presence of relict limestone textures and structures that
many coarsely crystalline dolomites are secondary rocks, formed by diagenetic
placement of older limestones, many fine-crystalne dolomites lack such textural
evidence of replacement and cannot be proven to have originated by diagenetic
alteration of limestones. It is these fine-crystalline dolomites that have created the
so-called dolomite problem, which geologists have not been able to satisfactorily
solve sce dolomites were first recognized by the French naturalist Deodat de
Dolomieu more than 200 years ago in 1791 (Zenger, Bourroui-Le Jan, and
Carozzi, 1994; see also review by Warren, 2000).
The dolomite problem arises from the fact at scientists have not yet been
successful in the laboratory in precipitating perfectly ordered dolomite at the
normal temperatures ( �25°C) and pressure ( -1 atm) that occur at Ear's sur
face. Perfectly ordered dolomite has 50 percent of the cation sites filled by Mg
and 50 peent filled by Ca (stoichiometric dolomite). Elevated temperatures, ex
ceeding 60°C, are required to produce stoichiometric dolomite in the laboratory

6.7 Origin of Carbonate Rocks
183
(e.g., Usdowski, 1994). In laboratory experiments carried out at the normal tem-
peratures found natural environments, only a dolomite-like material called
protodolomite forms. Protodolomite contains excess CaC03 in its stcture and is
not a true (stoichiometric) dolomite; see also discussion by Lumsden and Lloyd
(1997). Thus, geochemists have been unable to determine directly from low-tem-
perature experimental work what geochemical conditions favor the precipitation
of dolomite in natural environments. Nonetheless, geologic evidence suggests
that dolomite does form naturally at, or near, near-surface temperatures.
Since the mid-1940s, minor modern dolomite sediments have been reported
om numerous localities, including some in Russia, South Australia, the Persian
Gulf, the Bahamas, Bonaire Island off the Ve nezuela mainland, the Florida Keys,
the Canary Islands, and the Netherlands Antilles. Ages of these dolomites are es
mated by radiocarbon methods to range from a few years to about 4000 years.
Most are not perfectly ordered dolomites; mole percent MgC03 ranges from
about 30 to 50 percent but falls mainly between 40 and 46 percent. Discovery of
dolomite in modern environments was initially hailed as evidence by some
workers that dolomite can be precipitated naturally as a primary deposit. Others
suggested that these modern dolomites formed by replacement, that is, rapid al
teration of an initial precipitate of CaC03 to dolomite-a process called
dolomitization. Subsequent research has failed to establish unequivocally the
relative importance of dolomite precipitation versus dolomite replacement in the
igin of these early-formed, or penecontemporaneous, dolomites. Both mecha
nisms remain viable.
Early-formed dolomites include all those formed at or near the surface in
e unconsolidated state as opposed to diagenetic dolomites that formed during
burial and uplift by replacement of older, consolidated limestones. The volume
of dolomite in the modern environment is small. Thus, an additional aspect of
the dolomite problem has to do with the following question: Can the processes
responsible for generation of early-formed dolomites account for the vast
dolomite deposits of the past? That question is still being debated, and you will
not find the answer here; however, it may be useful to examine some of the
conditions that appear to favor the early formation of dolomite in modern en
vironments. By extension, we may be able to gain some insight into the forma
on of ancient dolomite deposits.
Requirements fo r Dolomite Formation
The chemical reactions of interest with respect to formation of dolomite are as
follows:
Ca2+(aq) + Mg2r(aq) + 2CO/-(aq) CaMg(C03)
2
(solid) (6.6)
2CaC03 (solid) + Mg2+(aq) CaMg(C03h (solid) + Ca21 (aq)
(6.7)
Equation 6.6 illustrates the direct precipitation of dolomite from aqueous solution;
Equation 6.7 illustrates replacement of calcite or aragonite by dolomite. As sug
gested at the beginning of this discussion, the problem with the reaction shown in
Equation 6.6 is that this reaction requires temperatures far in excess of normal sur
ce temperatures. The reasons why such high temperatures are necessary are far
om well understood, but the problem is certainly related to kinetics (reaction
rates). For example, it has been pointed out by a number of workers, such as Gains
(19), that the Mg2+ ion is strongly bound by water (hydrated) in solution and
must be separated from the attached water before it can be incorporated into the
solid dolomite crystal lattice. At low temperatures, Ca2+ ions, which are much less
songly bound by water, are more likely to enter the lattice and form CaC03 min
er als. At elevated temperatures, Mg2+ ions are less strongly hydrated and thus
184
Chapter 6 I Carbonate Sedimenta Rocks
more easily desolvated, allowing the naked Mg
2
+ ion to enter into the crystal lat
tice to form dolomite. The hily ordered state of dolomite also creates a kinetics
problem at low temperatures. The nucleation and growth of the highly ordered
dolomite lattice in a solution saturated in calcium bicarbonate are so slow that, in
competition for calcium ions and carbonate ions, well-ordered dolomite is pre
vented from forming, and minerals such as aragonite or caon-disordered, high
magnesium calcites form instead. For additional discussion of the ketics of
dolomite formation, see Machel and Mounljoy (1986).
Models for Early-Foed Dolomite
As mentioned, e relative importance of dolomite precipitation vs. dolomitiza
tion (replacement) is not known; nonetheless, many geologists dearly believe at
dolomitization was an important process in forming ancient dolomites. Much of
the recent and current research on dolomites has thus focused on attempts to un
derstand the mechanisms of dolomitization. Theoretical considerations suggest
that dolomite formation is favored kinetically by high Mg
2
+ /Ca
2
7 ratios, low
Ca
2
+ /CO/- ratios, and low salinity {Machel and Mounoy, 1986). It is favored
also by higher temperatures, as mentioned. In fact, at temperatures exceeding
about lOO"C, most kinetic inhibitors, such as Mg
2
+ hydration, become ineective.
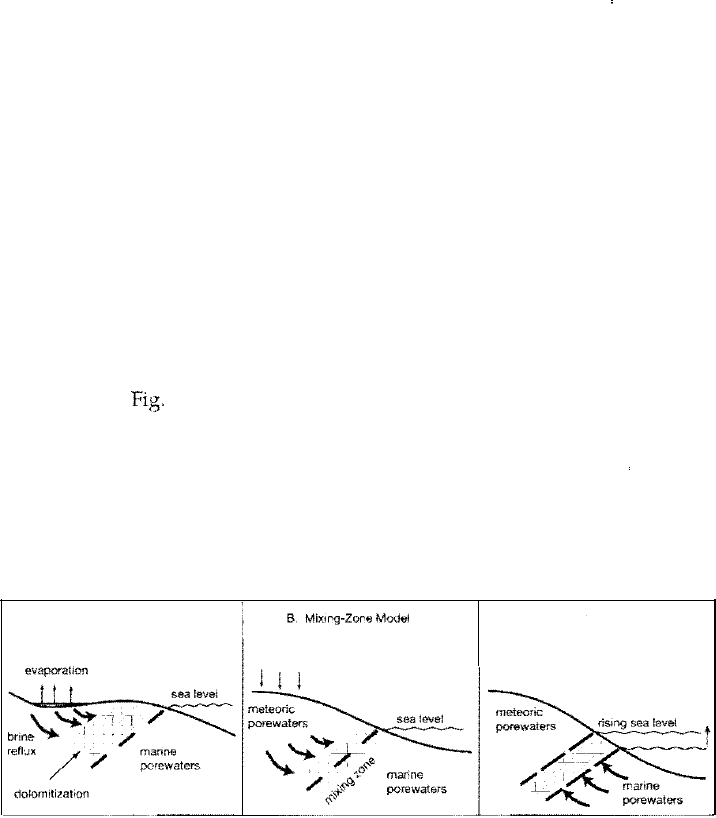
By examining the various conditions under which dolomites in modern en
vironments are forming, three principal models that meet, in one way or anoth
er, the conditions favorable for dolomite formation, particularly dolomitization
have been proposed: (1) the hypersaline (sabkha, evaporation, reflux) model, (2)
the mixed-water (mixing-zone) model, and (3) the seawater (shallow-subtidal)
model {Fig. 6.10). Models 1 and 3 invoke seawater, concentrated by evaporation
in the case of Modell, as the dolomitizing fluid. Model 2 requires mixing of sea
water and fresh (meteoric) water.
Heline Model. Many known occurrences of modern or Holocene dolomite
are in hypersaline environments such as the sabkhas (coastal plains characterized
by the presence of evaporites) of the Persian Gulf and the supratidal zones of arid
climates (e.g., 6.11). Under strongly evaporative conditions, where rates of
evaporation exceed rates of precipitation, seawater beneath the sediment surface
becomes concentrated by evaporation. This concentration process leads to precip
itation of aragonite and gypsum, which preferentially removes Ca
2
� from the
water and increases the Mg/Ca ratio. The Mg/Ca rao in normal seawater is
about 5:1. When this ratio rises to sufficiently high levels, possibly in excess of
A, Hypersaline Model
C. Seawater Model
rainfall
Figure 6.10
Dolomite models. Schematic representation of various conditions under which dolomitiza
tion may occur: A. Intense evaporation and brine reflux. B. Mixing of meteoric and marine
porewaters. C. Pumping or flushing normal seawater through carbonate sediments.
Arrows indicate the principal direction of fluid flow.
