Bhushan B. Nanotribology and Nanomechanics: An Introduction
Подождите немного. Документ загружается.


1244 Bharat Bhushan
Decrease in surface height (nm)
0
5
10
15
20
Si substrate
Wear tests
Si
PFTS
Decrease in surface height (nm)
0
5
10
15
20
Si substrate
Si
ODMS
Decrease in surface height (nm)
Normal load (μN)
0
0
5
10
15
20
10 20 30 40 50 60
Al substrate
OP
Al
ODDMS
ODP
a)
0
ODDMSODMSPFTSSi Al OP ODP
Critical load (μN)
20
40
60
b)
Fig. 22.27. Decrease of sur-
face height as a function of
normal load after one scan
cycle for various SAMs on Si
and Al substrates, and com-
parison of critical loads for
failure during wear tests for
various SAMs
22 Characterization of MEMS/NEMS and BioMEMS/BioNEMS 1245
normal loadfor variousSAMs and correspondingsubstrates [124,164].As shown in
the figure, the SAMs exhibit a critical normal load beyond which the surface height
drastically decreases. Unlike SAMs, the substrates show a monotonic decrease in
surface height with increasing normal load, with wear initiating fromthe very begin-
ning, i.e., even for low normal loads. The critical loads correspondingto the sudden
failure are shown in Fig. 22.27b. Amongst all the SAMs, ODDMS and ODP show
the best performance in the wear tests. Out of the two alkyl SAMs, ODDMS/Si and
ODP/Al showed better wear resistance than ODMS/Si and OD/Al due to the effect
of chain length. Wear behavior of the SAMs is reported to be mostly determined by
the molecule–substrate bond strengths.
Bhushan et al. [122] and Lee et al. [125] studied various fluoropolymer mul-
tilayers and fluorosilane monolayers on Si and a selected fluorosilane on PDMS
surfaces. For nanoscale devices such as in nanochannels, monolayers are preferred.
They reported that all fluorosilane films increased the contact angle. The fluorosi-
lane monolayer 1H, 1H, 2H, 2H–perfluorodecyltriethoxysilane (PFDTES) resulted
in a contact angle of about 100
◦
.
Based on these studies, a perfluoro SAM with a compliant layer should have
optimized tribological performance for MEMS/NEMS and BioMEMS/BioNEMS
applications.
22.3.3 Hard Diamond-Like Carbon (DLC) Coatings
Hard amorphous carbon (a-C), commonlyknown as DLC (implying high hardness)
coatings are deposited by a variety of deposition techniques including filtered ca-
thodic arc (FCA), ion beam, electron cyclotron resonance chemical vapor deposi-
tion (ECR-CVD), plasma-enhanced chemical vapor deposition (PECVD), and sput-
tering [136,154]. These coatings are used in a wide range on applications including
tribological, optical, electronic, and biomedical applications. Ultrathin coatings (3–
10nm thick) are employedto protectagainst wear and corrosionin magnetic storage
applications– thin-film rigid disks, metal-evaporatedtapes, and thin-filmread/write
heads –, Gillette Mach 3 razor blades, glass windows, and sunglasses. The coatings
exhibit low friction, high hardness and wear resistance, chemical inertness to both
acids and alkalis, lack of magnetic response, and optical band gaps ranging from
zero to a few eV, depending upon the deposition technique and its conditions. Se-
lected data on DLC coatings relevant for MEMS/NEMS applications is presented
in the following section on adhesion measurements.
22.4 Tribological Studies of Biological Molecules
on Silicon-Based Surfaces and of Coated Polymer Surfaces
22.4.1 Adhesion, Friction, and Wear of Biomolecules on Si-Based Surfaces
Proteins on silicon-based surfaces are of extreme importance in various applica-
tions including silicon microimplants, various bioMEMS such as biosensors, and
1246 Bharat Bhushan
therapeutics. Silicon is a commonly used substrate in microimplants, but it can have
undesired interactions with the human immune system. Therefore, to mimic a bi-
ological surface, protein coatings are used on silicon-based surfaces as a passiva-
tion layer, so that these implants are compatible with the body and avoid rejection.
Whether this surface treatment is applied to a large implant or a bioMEMS, the
function of the protein passivation is obtained from the nanoscale 3D structural
conformation of the protein. Proteins are also used in bioMEMS because of their
function specificity. For biosensor applications, the extensive array of protein activ-
ities provides a rich supply of operations that may be performed at the nanoscale.
Many antibodies (proteins) have an affinity to specific protein antigens. For exam-
ple, pathogens(disease causingagents, e.g., virus or bacteria)trigger the production
of antigens which can be detected when bound to a specific antibody on the biosen-
sor. The specific binding behavior of proteins that has been applied to laboratory
assays may also be redesigned for in vivo use as sensing elements of a bioMEMS.
The epitope-specific binding properties of proteins to various antigens are useful
in therapeutics. Adhesion between the protein and substrate affects the reliability
of an application. Among other things, the morphology of the substrate affects the
adhesion. Furthermore, for in vivo environments, the proteins on the biosensor sur-
face should exhibit high wear resistance during direct contact with the tissue and
circulatory blood flow without washing off.
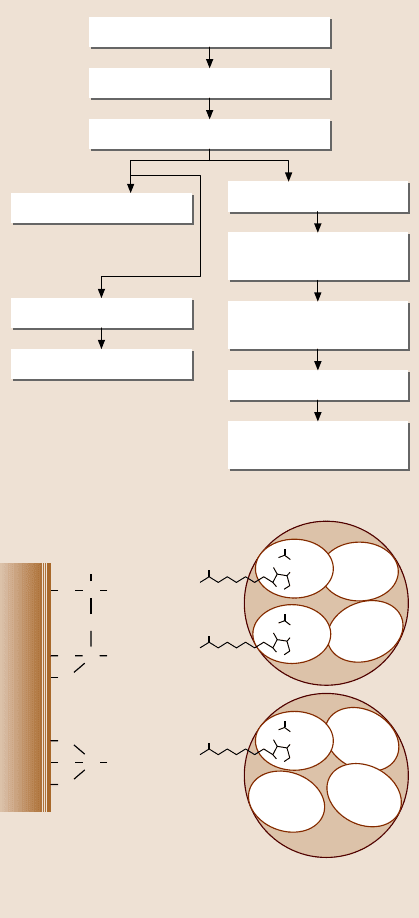
Bhushan et al. [72] studied the step-by-step morphological changes and the ad-
hesion of a model protein – streptavidin (STA) – on silicon-based surfaces. Fig-
ure 22.28a presents a flowchart showing the sequential modification of a silicon sur-
face. In addition to physical adsorption, they also used nanopatterning and chemical
linker methods to improve adhesion. Nanopatterned surfaces contain a large edge
surface area, leading to high surface energy, which results in high adhesion. In the
chemical linker method, sulfo-NHS-biotin was used as a cross linker because the
bonds between the STA and the biotin molecule are some of the strongest nonco-
valent bonds known (Fig. 22.28b). It was connected to the silica surface through
a silane linker, 3-aminopropyltriethoxysilane (3-APTES). In order to make a bond
between the silane linker and the silica surface, the silica surface was hydroxylated.
Bovine serum albumin (BSA) was used before STA in order to block nonspecific
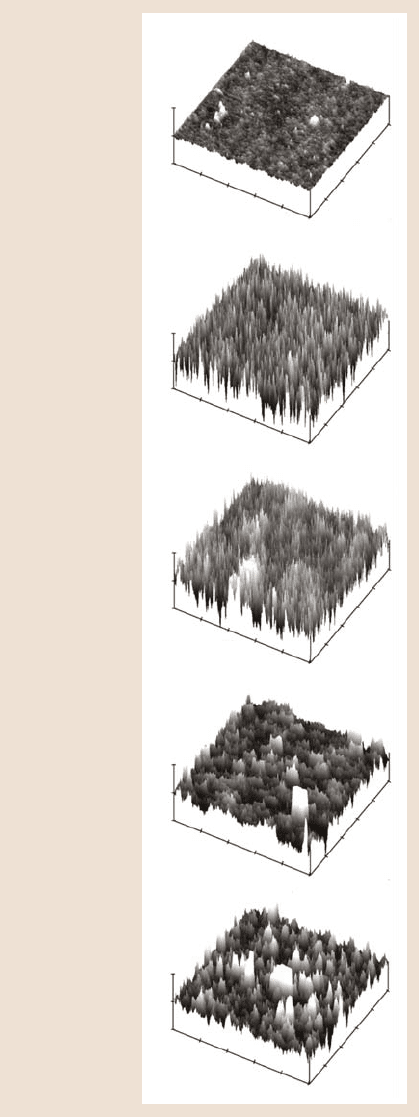
binding sites of the STA protein with silica surface. Figure 22.29 shows the step-by-
step morphological changes in the silica surface during the deposition process using
the chemical linker method. There is an increase in roughness of the silica sur-
face boiled in de-ionized (DI) water compared to the bare silica surface. After the
silanization process, there are many free silane links on the surface which caused
higher roughness. Once biotin was coated on the silanized surface, the surface be-
came smoother. Finally, after the deposition of STA, surface shows large and small
clumps. Presumably, the large clumps represent BSA and the smaller ones repre-
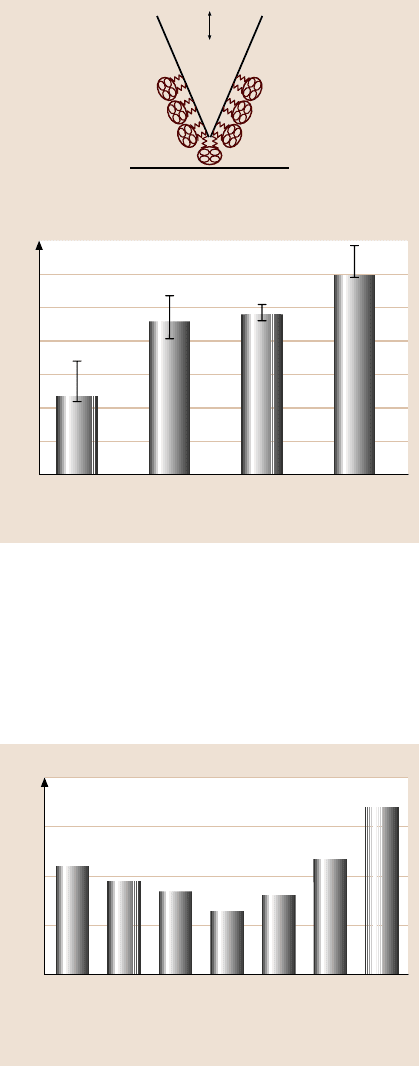
sent STA. To measure adhesion between STA and the corresponding substrates, an
STA-coated tip (or functionalized tip) was used and all measurements were made
in phosphate buffered saline (PBS) solution, a medium commonly used in protein
analysis and to simulate body fluid. Figure 22.30 shows the adhesion values of var-
22 Characterization of MEMS/NEMS and BioMEMS/BioNEMS 1247
a)
Silicon (cleaned)
Silica (thermal oxidation)
Pre-cycle cleaned
STA coated (adsorption)
Boiled in DI water
Silanized
(3-APTES monolayer)
Sulfo-NHS-biotin coated
(bonded to silane)
BSA coated
STA coated
(bonded to biotin)
Method II
Method I A
STA coated (adsorption)
Method I B
Patterned
Si
SiO
2
CH
2
CH
2
CH
2
NHO
O
NH
S
HN
O
Si CH
2
CH
2
CH
2
NHO
O
NH
S
HN
O
O
O
Si CH
2
CH
2
CH
2
NHO
O
NH
S
HN
O
O
O
b)
Streptavidin has four biotin-
binding pockets. Two or one
may be attached to the biotin on the surface, with the
remaining 2 or 3 available to bind the biotin analyte.
Fig. 22.28. (a)Flowchart
showing the samples used and
their preparation technique,
and (b) a chemical structure
showing streptavidin protein
binding to the silica sub-
strate by the chemical linker
method
ious surfaces. The adhesion value between biotin and STA was higher than that for
other samples, which is expected.Edges of patterned silica also exhibited high adhe-
sion values. It appears that both nanopatterned surfaces and chemical linker method
increase adhesion with STA.
1248 Bharat Bhushan
Silica boiled
in DI water
σ = 0.12 nm
P–V = 3.0 nm
10
5
0
nm
0
1 μm
10
5
0
nm
0
1 μm
10
5
0
nm
0
1 μm
10
5
0
nm
0
1 μm
10
5
0
nm
0
1 μm
Silanized
(3-APTES
monolayers)
silica
σ = 1.1 nm
P–V = 17.0 nm
After coated
with sulpho-
NHS-biotin
(bonded to
silane)
σ = 0.96 nm
P–V = 15.0 nm
After coated
with BSA
σ = 0.62 nm
P–V = 14.0 nm
After coated
with streptavidin
(bonded to biotin)
at 10 μg/ml
σ = 0.78 nm
P–V = 15.0 nm
Fig. 22.29. Morphological
changes in silica surface dur-
ing functionalization of silica
surface by chemical linker
imaged in PBS. Streptavidin
is covalently bonded at a con-
centration of 10 µg/ml [72]
22 Characterization of MEMS/NEMS and BioMEMS/BioNEMS 1249
Adhesive force (nN)
Unpatterned
silica
Edge of
patterned
silica
Silica boiled
in DI water
Silica coated
with
sulfo-NHS-biotin
3.5
3.0
2.5
2.0
1.5
1
0.5
0
Streptavidin
Sample surface
Adhesion measurement in PBS with functionalized tip
AFM tip
Fig. 22.30. Adhesion meas-
urements of silica, patterned
silicon, silica boiled in DI
water, and sulfo-NHS-biotin
using functionalized (with
streptavidin) tips obtained
from force–distance curves,
captured in PBS
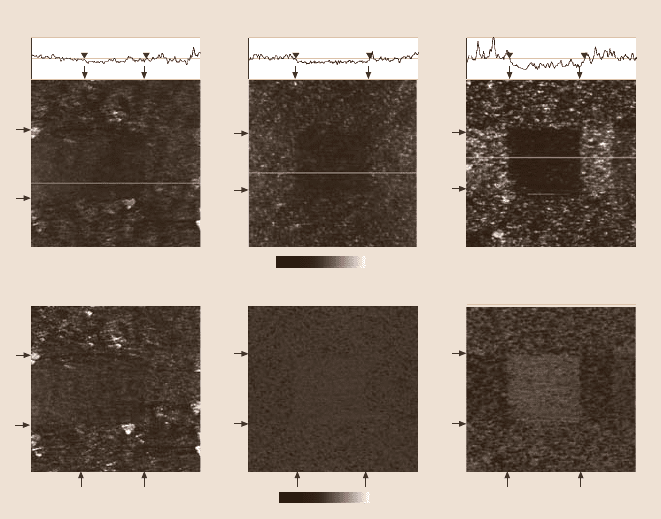
Tokach ichu et al. [120] studied friction and wear of STA deposited by physical
adsorption and the chemical linker method. Figure 22.31 shows the coefficient of
friction between the Si
3
N
4
tip and various samples. The coefficient of friction is
less for STA-coated silica samples compared to uncoated sample. The streptavidin
coating acts as a lubricant film. The coefficient of friction is found to be depen-
Coefficient of friction
Pre-cycle
cleaned
silica
0
1 10 100
STA by adsorption
at μg/ml
Silanized
silica
NHS-
biotin
coated
silica
STA by
chemical
linker
at
10 μg/ml
0.01
0.02
0.03
0.04
Fig. 22.31. Coefficient of fric-
tion for various surfaces with
and without biomolecules
1250 Bharat Bhushan
dent upon the concentration of STA, and decreases with an increase in the concen-
tration. Bhushan et al. [72] have reported that the density and distribution of the
biomolecules vary with concentration. At higher concentration of the solution, the
coated layer is more uniform and the silica substrate surface is highly covered with
biomolecules than at lower concentration. This means that the surface forms a con-
tinuous lubricant film at higher concentration.
In the case of samples prepared by the chemical linker method, the coefficient
of friction increases with an increase in the biomolecular chain length due to in-
creased compliance. When normal load is applied on the surface, the surface be-
comes compressed, resulting in a larger contact area between the AFM tip and the
biomolecules. Besides that, the size of STA is much larger than that of APTES and
biotin. This results in a tightly packed surface with the biomolecules, which results
in very little lateral deflection of the linker in the case of STA-coated biotin. Due to
this high contact area and low lateral deflection the friction force increases for the
same applied normal load compared to directly adsorbed surface. These tests reveal
that surfaces coated with biomolecules reduce the friction, but if the biomolecular
coating of the surface is too thick or the surface has some cushioning effect, as seen
in the chemical linker method, that increases the coefficient of friction.
0
2.5
–2.5
0
0
0
2.5
–2.5
0
0
0 5 nm
0
3
(μm)
2.5
–2.5
0
0
(nm) (nm) (nm)
3
(μm)
3
(μm)
75% of free amplitude 50% of free amplitude
Cross sectional profiles and heights
30% of free amplitude
Phase images
0
0
0
0
0
0
05°
3
(μm)
3
(μm)
3
(μm)
Fig. 22.32. Wear mark images and cross-sectional profiles of precycle cleaned silica coated
with streptavidin at 10 µg/ml by physical adsorption at three normal loads (increasing from
left to right)
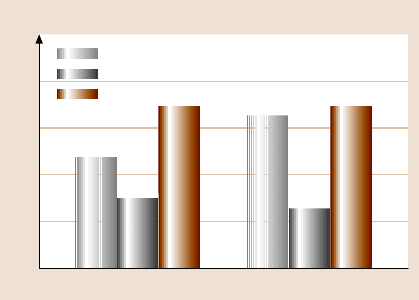
22 Characterization of MEMS/NEMS and BioMEMS/BioNEMS 1251
Figure 22.32 shows the wear maps of STA deposited by physical adsorption
at three normal loads. The wear depth increases with increasing normal load. An
increase in normal load causes partial damage to the folding structure of the strepta-
vidin molecules. It is unlikely that the chemical (covalent)bonds within the strepta-
vidin molecule are broken; instead, the folding structureis damagedleading to wear
mark. When the load is high, i.e., 30% of the free amplitude(≈ 8nN), the molecules
may have been removedby the AFM tip due to the effect of indentation/ Because of
this, there is a significant increase in the wear depth from 50% of the free amplitude
(≈ 6nN) to 30% of the free amplitude (≈ 8nN). The data show that biomolecules
will be damaged during sliding.
22.4.2 Adhesion of Coated Polymer Surfaces
As mentioned in Sect. 22.A, PMMA, PDMS, and other polymers are used in the
construction of micro/nanofluidic-basedbiodevices. Adhesion between the moving
partsneeds to be minimized.Furthermore,if the adhesionbetweenthe microchannel
surface and the biofluid is high, the biomolecules will stick to the microchannel
surface and restrict flow. In order to facilitate flow, surfaces with low bioadhesion
are required.
Tambe and Bhushan [169,170] and Bhushan and Burton [171] have reported
adhesive force data for PMMA and PDMS against an AFM Si
3
N
4
tip and a silicon
ball. Tokachichu and Bhushan [172] measured contact angle and adhesion of bare
PMMA and PDMS and coated with a perfluoro SAM of perfluorodecyltriethoxysi-
lane(PFDTES). Oxygenplasma treatmentwas usedfor hydroxylationof the surface
to enhance chemical bonding of the SAM to the polymer surface. They made meas-
urements in ambient and in PBS and fetal bovine serum (FBS); the latter is a blood
component. Figs. 22.33 and 22.34 show the contact angle and adhesion data. SAM-
coated surfaces have a high contact angle Fig. 22.33, as expected. The adhesion
valueof PDMS in ambient is high because of electrostatic charge present on the sur-
face. The adhesion values of PDMS are higher thanPMMA because PDMS is softer
Contact angle (deg)
PMMA
0
Virgin
Oxygen plasma treated
PFDTES coated
30
60
90
120
150
PDMS
Fig. 22.33. Sessile drop
contact-angle measurements
of virgin, oxygen-plasma-
treated and PFDTES-coated
PMMA and PDMS surfaces.
The maximum error in the
data is ±2
◦
[120]
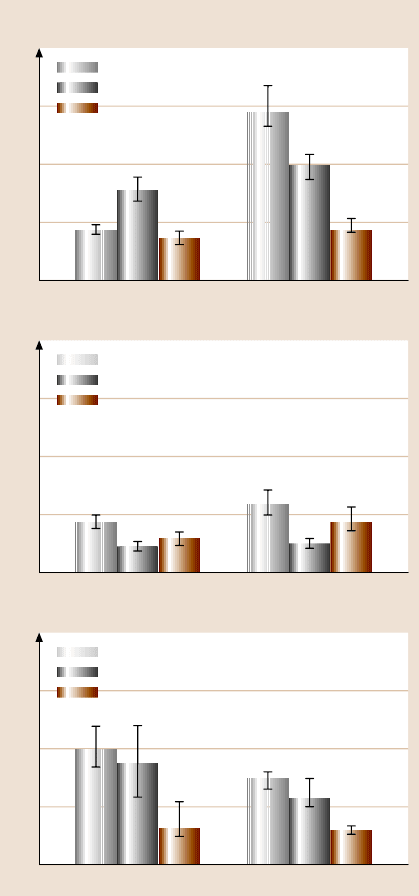
1252 Bharat Bhushan
Adhesive force (nN)
PMMA
Virgin
Oxygen plasma treated
PFDTES coated
80
PDMS
60
40
20
0
a)
Adhesive force (nN)
PMMA
Virgin
Oxygen plasma treated
PFDTES coated
80
PDMS
60
40
20
0
b)
Adhesive force (nN)
PMMA
Virgin
Oxygen plasma treated
PFDTES coated
80
PDMS
60
40
20
0
c)
Fig. 22.34. Adhesion meas-
urement of virgin, oxygen-
plasma-treated and PFDTES-
coated PMMA and PDMS
surfaces with bare silicon ni-
tride AFM tip (a)inambient,
and (b) in PBS environ-
ment, and (c) dip-coated tip
with FBS in a PBS environ-
ment [120]
than PMMA (elastic modulus = 5GPa and hardness = 410MPa [121]) and results
in a higher contact area between the PDMS surface and the AFM tip, and PMMA
does not develop electrostatic charge. When SAM is coated on PMMA and PDMS
surfaces, the adhesion values are similar, which shows that electrostatic charge on
virgin PDMS plays no role when the surface is coated. In the PBS solution, there

22 Characterization of MEMS/NEMS and BioMEMS/BioNEMS 1253
is a decrease in adhesion values because of the lack of a meniscus contribution.
The adhesion values in the FBS-coated tip in PBS are generally lower than for the
uncoated tip in PBS. In summary, the adhesion values of SAM-coated surfaces are
lower than bare surfaces in various environments.
22.5 Nanopatterned Surfaces
22.5.1 Analytical Model and Roughness Optimization
One of the crucial surface properties for surfaces in wet environmentsis nonwetting
or hydrophobicity.It is usually desirable to reduce wetting in fluid flow applications
and some conventional applications, such as glass windows and automotive wind-
shields, in order for liquid to flow away along their surfaces. Reduction of wetting
is also important in reducing meniscus formation, consequently reducing stiction,
friction, and wear. Wetting is characterized by the contact angle, which is the an-
gle between the solid and liquid surfaces. If the liquid wets the surface (referred
to as a wetting liquid or a hydrophilic surface), the value of the contact angle is
0 ≤ θ ≤ 90
◦
, whereas if the liquid does not wet the surface (referred to as a nonwet-
ting liquidor a hydrophobicsurface),the valueof the contactangleis 90
◦
<θ≤180
◦
.
A surface is considered superhydrophobic if θ is close to 180
◦
. Superhydrophobic
surfaces should also have very low water contact angle hysteresis. One of the ways
to increase the hydrophobic or hydrophilic properties of the surface is to increase
surface roughness. It has been demonstrated experimentally that roughness changes
contact angle. Some natural surfaces, includingleaves of water-repellentplants such
as lotus, are known to be superhydrophobic due to their high roughness and the
presence of a wax coating Fig. 22.35. This phenomenon is called in the literature
the lotus effect [173].
If a droplet of liquid is placed on a smooth surface, the liquid and solid surfaces
come together under equilibrium at a characteristic angle called the static contact
angle θ
0
; see Fig. 22.36. The contact angle can be determined from the condition of
the total energy of the system being minimized. It can be shown that
cosθ
0
= dA
LA
/ dA
SL
, (22.1)
where θ
0
is the contact angle for smooth surface, and A
SL
and A
LA
are the solid–
liquid and liquid–air contact areas. Next, let us consider a rough solid surface with
a typical size of roughness details smaller than the size of the droplet (on the order
of a few hundred microns or larger), Fig. 22.36. For a rough surface, the roughness
affects the contact angle due to the increased contact area A
SL
. For a droplet in
contact with a rough surface without air pockets, referred to as a homogeneous
interface, based on the minimization of the total surface energy of the system, the
contact angle is given as [174]
cosθ = dA
LA
/ dA
F
=
A
SL
A
F
(
dA
LA
/ dA
SL
)
= R
f
cosθ
0
, (22.2)
