Bhushan B. Nanotribology and Nanomechanics: An Introduction
Подождите немного. Документ загружается.

1234 Bharat Bhushan
750
500
250
0
100
80
5.00
2.50
0
2.50
0
5.00
μm
60
40
20
nm
750
500
250
0
100
80
5.00
2.50
0
2.50
0
5.00
μm
μN
60
40
20
nm
750
500
250
0
100
80
5.00
2.50
0
2.50
0
5.00
μm
60
40
20 μN
nm
750
500
250
0
100
80
5.00
2.50
0
2.50
0
5.00
μm
μN
60
40
20
nm
5.00
200
0
–200
100 80 60 40 20
2.500 μm
nm
5.00
200
0
–200
100 80 60 40 20
2.500 μm
μN
nm
5.00
5.00
200
0
–200
80 60 40 20
2.500 μm
μN
nm
200
0
–200
100 80 60 40 20
2.500 μm
μN
nm
100
μN
μN
Undoped Si(100)
Undoped polysilicon film
n
+
–type polysilicon film
SiC film
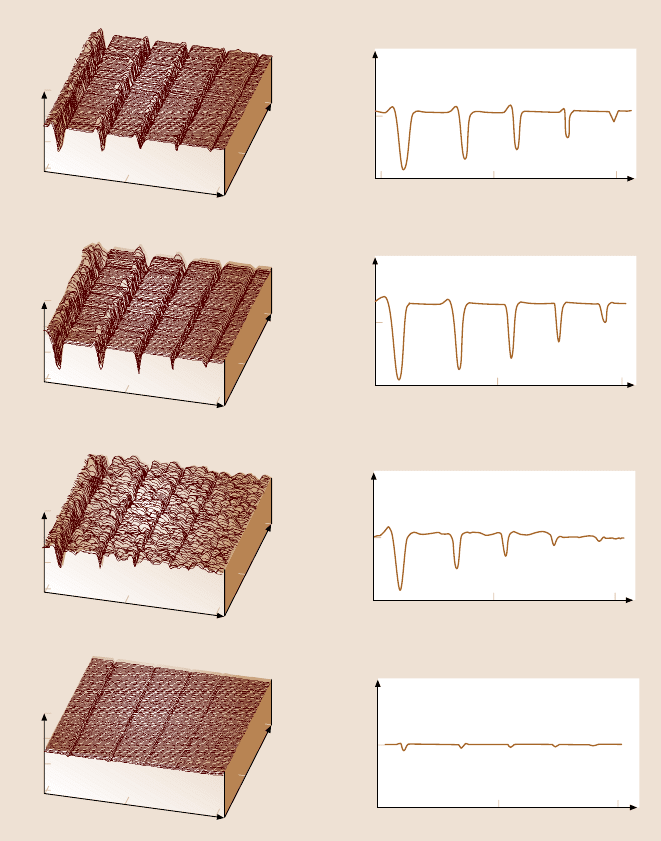
Fig. 22.19. AFM 3D maps and averaged 2D profiles of scratch marks on various sam-
ples [155]
results from microscale wear tests on the various films. For all the materials, wear
depth increases almost linearly with increasing number of cycles. This suggests that
the material is removed layer by layer in all the materials. Here also, SiC film
exhibits lower wear depths than the other samples. Doped polysilicon film wears
less than the undoped film. The higher fracture toughness and higher hardness of
22 Characterization of MEMS/NEMS and BioMEMS/BioNEMS 1235
SiC compared to Si(100) is responsible for its lower wear. Also the higher ther-
mal conductivity of SiC (see Table 22.2 compared to the other materials leads to
lower interface temperatures, which generally results in less degradation of the sur-
face [34,46,81]). Doping of the polysilicon does not affect the scratch/wear resis-
tanceand hardness much.The measurementsmade on the dopedsample areaffected
by the presenceof grain boundaries.These studies indicate that SiCfilm exhibitsde-
sirable tribological properties for use in MEMS devices.
22.3 Lubrication Studies for MEMS/NEMS
Several studies of liquid perfluoropolyether (PFPE) lubricant films, self-assembled
monolayers (SAMs), and hard diamond-like carbon (DLC) coatings have been car-
ried out for the purpose of minimizing adhesion, friction, and wear [46,47,82,102,
122–125,154,158–166]. Many variations of these films are hydrophobic (low sur-
face tension and high contact angle)and have low shear strength, which providelow
adhesion, friction, and wear. Relevant details are presented here.
22.3.1 Perfluoropolyether Lubricants
The classical approach to lubrication uses freely supported multimolecular layers
of liquid lubricants [46,47,79,81]. The liquid lubricants are sometimes chemically
bonded to improve their wear resistance. Partially chemically bonded, molecularly
thick perfluoropolyether (PFPE) lubricants are widely used for lubrication of mag-
netic storage media because of their thermal stability and extremely low vapor pres-
sure [34], and are found to be suitable for MEMS/NEMS devices.
Adhesion, friction, and durability experiments have been performed on virgin
Si (100) surfaces and silicon surfaces lubricated with two commonly used PFPE lu-
bricants – Z-15 (with −CF
3
nonpolar end groups) and Z-DOL (with −OH polar end
groups) [46,47,158,160,161,165].Z-DOL film was thermally bonded at 150
◦
Cfor
30minutes and the unbonded fraction was removed by a solvent (bonded washed,
BW) [34]. The thicknesses of the Z-15 and Z-DOL (BW) films were 2.8nm and
2.3nm, respectively. Nanoscale measurements were made using an AFM. The ad-
hesive forces of Si(100), Z-15 and Z-DOL (BW) measured by plots of forcecalibra-
tion and friction force versus normal load are summarized in Fig. 22.20. The results
measured by these two methods are in good agreement. Figure 22.20 shows that
the presence of mobile Z-15 lubricant film increases the adhesive force compared to
that of Si(100)by meniscus formation[79,81,167].In contrast,the presence of solid
phase Z-DOL (BW) film reduces the adhesive force as compared that of Si(100) be-
cause of the absence of mobile liquid. The schematic (bottom) in Fig. 22.20 shows
the relative size and sources of meniscus. It is well known that the native oxide
layer (SiO
2
) on the top of Si(100) wafers exhibits hydrophilic properties, and some
water molecules can be adsorbed on this surface. The condensed water will form
a meniscus as the tip approaches the sample surface. The larger adhesive force in
Z-15 is not only caused by the Z-15 meniscus, the nonpolarized Z-15 liquid does
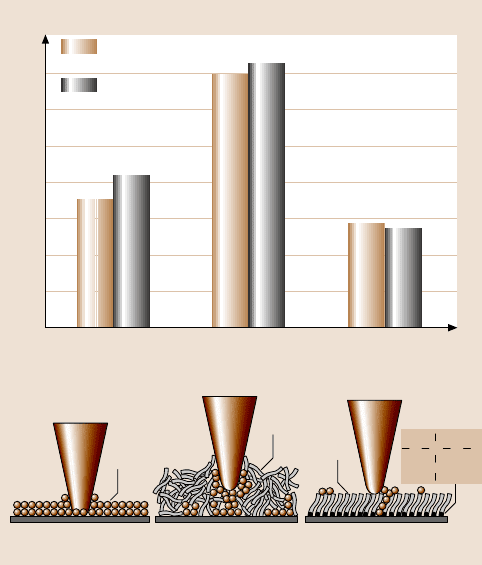
1236 Bharat Bhushan
100
75
50
25
0
Z-DOL (BW)Z-15Si(100)
Z-DOL
Z-15
Z-15 Z-DOL (BW)Si(100)
H
2
O
22 °C, RH 45– 55%
O
Si
O
O
Adhesive force (nN)
Force
calibration plot
Friction
force plot
Fig. 22.20. Summary of the adhesive forces of Si(100) and Z-15 and Z-DOL (BW) films
measured by plots of force calibration and friction force versus normal load in ambient air.
The schematic (bottom) showing the effect of meniscus, formed between AFM tip and the
surface sample, on the adhesive and friction forces [158]
not have good wettability and strong bonding with Si(100). Consequently, in the
ambient environment,the condensed water molecules from the environmentperme-
ate through the liquid Z-15 lubricant film and compete with the lubricant molecules
present on the substrate. The interaction of the liquid lubricant with the substrate is
weakened, and a boundary layer of the liquid lubricant forms puddles [160,161].
This dewetting allows water molecules to be adsorbed on the Si(100) surface as ag-
gregates along with Z-15 molecules. And both of them can form a meniscus while
the tip approaches the surface. Thus, the dewetting of the liquid Z-15 film results
in a higher adhesive force and poorer lubrication performance. In addition, as the
Z-15 film is fairly soft compared to the solid Si(100) surface, penetration of the tip
in the film occurs while pushing the tip down. This leads to the large area of the tip
involved to form the meniscus at the tip–liquid (mixture of Z-15 and water) inter-
face. It should also be noted that Z-15 has a higher viscosity than water, therefore
Z-15 film provides higher resistance to lateral motion and coefficient of friction. In
the case of Z-DOL (BW) film, the active groups of Z-DOL molecules are mostly
22 Characterization of MEMS/NEMS and BioMEMS/BioNEMS 1237
bonded on Si(100) substrate, thus the Z-DOL (BW) film has low free surface en-
ergy and cannot be displaced readily by water molecules or readily adsorb water
molecules. Thus, the use of Z-DOL (BW) can reduce the adhesive force.
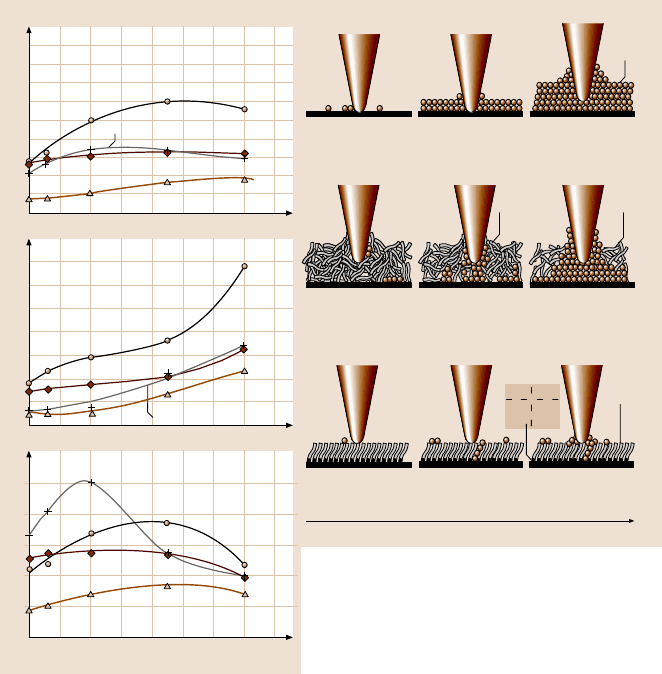
To study the effect of relative humidity on friction and adhesion, the variation
of friction force, adhesive force, and coefficient of friction of Si(100), Z-15, and Z-
DOL (BW) as a function of relative humidity are shown in Fig. 22.21. This shows
that, for Si(100) and Z-15 film, the friction force increases with a relative humid-
ity increase up to 45%, and then shows a slight decrease with further increases in
25
20
15
10
5
0
200
175
150
125
100
75
50
25
0
0.15
0.10
0.05
0
20 8060400
Z-15
Z-DOL (BW)
O
Si
O
O
Si(100)
Increasing relative humidity
0%
Z-DOL
70 %
Z-15
Z-15
70 nN, 2 μm/s, 22°C
Friction force (nN)
Si(100)
Z-DOL (BW)
Z-15
Relative humidity (%)
From friction force plot
Adhesive force (nN)
Si(100)
Z-DOL (BW)
Z-15
Coefficient of friction
Si(100)
Z-DOL (BW)
Z-15
Thermally treated Si(100)
Thermally treated Si(100)
Thermally treated Si(100)
H
2
O
Fig. 22.21. The influence of relative humidity of the friction force, adhesive force, and coef-
ficient of friction of Si(100) and Z-15 and Z-DOL (BW) films at 70 nN, 2 µm/s, and in 22
◦
C
air. Schematic (right) shows the change of meniscus while increasing the relativehumidity. In
this figure, the thermal treated Si(100) represents the Si(100) wafer that was baked at 150
◦
C
for 1h in an oven (in order to remove the adsorbed water) just before it was placed in the
0% RH chamber [158]
1238 Bharat Bhushan
the relative humidity. Z-DOL (BW) has a smaller friction force than Si(100) and
Z-15 over the whole testing range, and its friction force shows a relative appar-
ent increase when the relative humidity is higher than 45%. For Si(100), Z-15 and
Z-DOL (BW), their adhesive forces increase with relative humidity. And their co-
efficients of friction increase with a relative humidity up to 45%, after which they
decrease with further increasing of the relative humidity. It is also observed that the
effect of humidity on Si(100) really depends on the history of the Si(100) sample.
As the surface of Si(100) wafer readily adsorb water in air, without any pretreat-
ment the Si(100) used in our study almost reaches its saturated stage of adsorbed
water, and shows less effect during increasing relative humidity. However, once the
Si(100) wafer was thermally treated by baking at 150
◦
Cfor1h,alargereffect was
observed.
The schematic (right) in Fig. 22.21 shows that Si(100), because of its high free
surface energy, can adsorb more water molecules with increasing relative humidity.
As discussed earlier, for the Z-15 film in the humid environment, the condensed
water from the humid environment competes with the lubricant film present on the
sample surface, and the interaction of the liquid lubricant film with the silicon sub-
strate is weakened and a boundary layer of the liquid lubricant forms puddles. This
dewetting allows water molecules to be adsorbed on the Si(100) substrate mixed
with Z-15 molecules [160,161]. Obviously, more water molecules can be adsorbed
on theZ-15surfacewith increasingrelativehumidity.The largeramountof adsorbed
water in the case of Si(100), along with the lubricant molecules in the case of the
Z-15 film, forms a larger water meniscus, which leads to an increase of the friction
force, adhesive force, and coefficient of friction of Si(100) and Z-15 with humid-
ity. However, at a very high humidity of 70%, large quantities of adsorbed water
can form a continuous water layer that separates the tip and sample surface, acting
as a kind of lubricant, which causes a decrease in the friction force and coefficient
of friction. For Z-DOL (BW) film, because of their hydrophobic surface proper-
ties, water molecules can be adsorbed at humidity higher than 45%, and causes an
increase in the adhesive force and friction force.
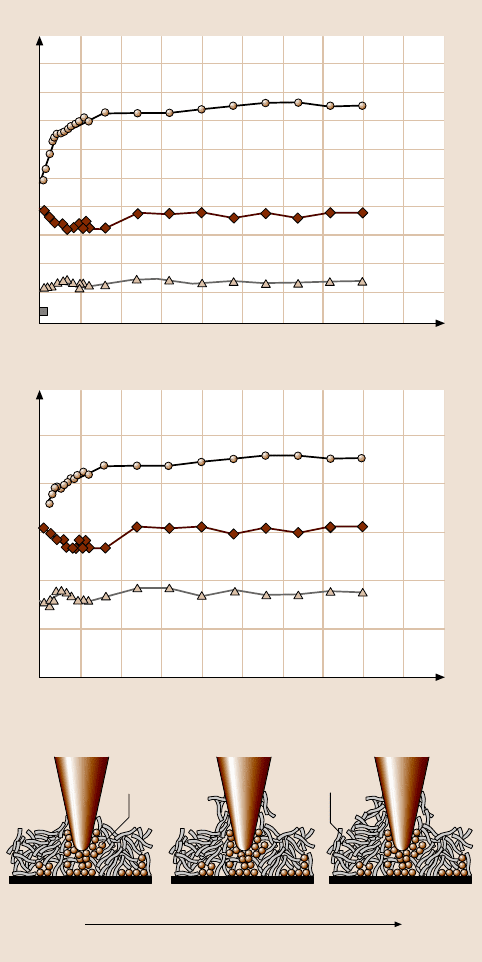
To study the durability of lubricant films at nanoscale, the friction of Si(100),
Z-15, and Z-DOL (BW) as a function of the number of scanning cycles are shown
in Fig. 22.22. As observed earlier, the friction force and coefficient of friction of
Z-15 are higher than that of Si(100) with the lowest values for Z-DOL(BW). During
cycling,thefrictionforce andcoefficientof frictionof Si(100)showa slightdecrease
during the initial few cycles then remain constant. This is related to the removal of
the top adsorbed layer. In the case ofZ-15film,thefrictionforceandcoefficient
of friction show an increase during the initial few cycles and then approach higher
stable values. This is believed to be caused by the attachment of the Z-15 molecules
onto the tip. The molecular interaction between these attached molecules to the
tip and molecules on the film surface is responsible for an increase in the friction.
But after several scans, this molecular interaction reaches equilibrium and after that
the friction force and coefficient of friction remain constant. In the case of Z-DOL
(BW) film, the friction force and coefficient of friction start out low and remain low
22 Characterization of MEMS/NEMS and BioMEMS/BioNEMS 1239
0 50 75 10025 125
0.15
0.10
0.05
0
25
20
15
10
5
0
70 nN, 0.4 μm/s, 22°C, RH 45– 55 %
Friction force (nN)
Si(100)
Z-DOL (BW)
Z-15
Number of cycles
Coefficient of friction
Si(100)
Z-DOL (BW)
Z-15
Increasing scan number
Z-15
Z-15
H
2
O
Fig. 22.22. Friction force and coefficient of friction versus number of sliding cycles for
Si(100) and Z-15 and Z-DOL (BW) films at 70 nN, 0.4 µm/s, and in ambient air. Schematic
(bottom) shows that some liquid Z-15 molecules can be attached onto the tip. The molecular
interaction between the attached molecules onto the tip with the Z-15 molecules in the film
results in an increase of the friction force with multiple scanning [158]
1240 Bharat Bhushan
during the entire test for 100 cycles. This suggests that Z-DOL (BW) molecules do
not become attached or displaced as readily as Z-15.
22.3.2 Self-Assembled Monolayers (SAMs)
For lubrication of MEMS/NEMS, another effective approach involves the deposi-
tion of organizedand dense molecularlayers of long-chainmolecules.Two common
methods to produce monolayers and thin films are Langmuir–Blodgett(LB) deposi-
tion and self-assembled monolayers (SAMs) by chemical grafting of molecules.
LB films are physically bonded to the substrate by weak van der Waals attrac-
tion, while SAMs are chemically bonded via covalent bonds to the substrate.
Because of the choice of chain length and terminal linking group that SAMs of-
fer, they hold great promise for boundary lubrication of MEMS/NEMS. A num-
ber of studies have been conducted to study the tribological properties of various
SAMs [122–125,159,162–164,166,168].
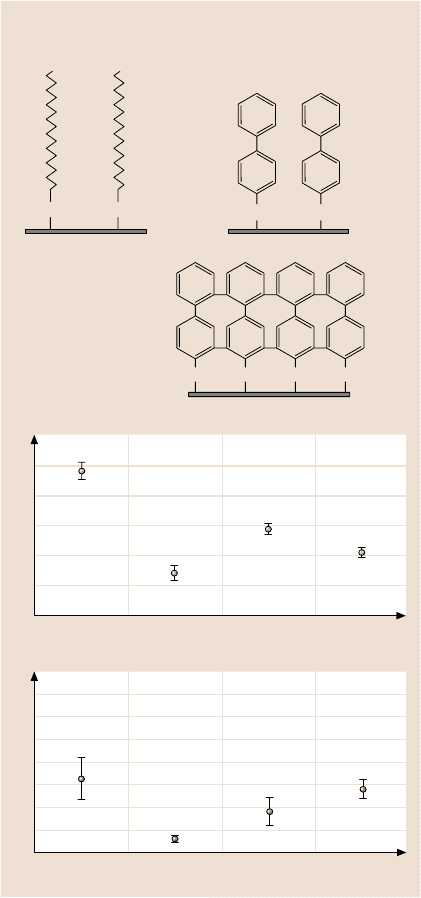
Bhushan and Liu [162] studied the effect of film compliance on adhesion
and friction. They used hexadecane thiol (HDT), 1,1,biphenyl-4-thiol (BPT), and
crosslinked BPT (BPTC) solvent deposited on an Au(111) substrate, Fig. 22.23a.
The average values and standard duration of the adhesive force and coefficient of
friction are presented in Fig. 22.23b.Based on the data, the adhesiveforce and coef-
ficient of friction of SAMs are lower than correspondingsubstrates. Among various
films, HDT exhibits the lowest values. Based on stiffness measurements of various
SAMs, HDT wasthe most compliant,followedby BPT and BPTC. Based on friction
and stiffnessmeasurements,SAMs withhigh-compliancelongcarbon chainsexhibit
low friction; chain compliance is desirable for low friction. Friction mechanism of
SAMs is explained by a so-called molecular spring model Fig. 22.24. According
to this model, the chemically adsorbed self-assembled molecules on a substrate are
just like assembled molecular springs anchored to the substrate. An asperity sliding
on the surface of SAMs is like a tip sliding on the top of molecular springs or brush.
The molecular spring assembly has compliant features and can experience orienta-
tion and compression under load. The orientation of the molecular springs or brush
under normal load reduces the shearing force at the interface, which in turn reduces
the friction force. The orientation is determined by the spring constant of a single
molecule as well as the interaction between the neighboring molecules, which can
be reflected by packing density or packing energy. It should be noted that the orien-
tation can lead to conformational defects along the molecular chains, which lead to
energy dissipation.
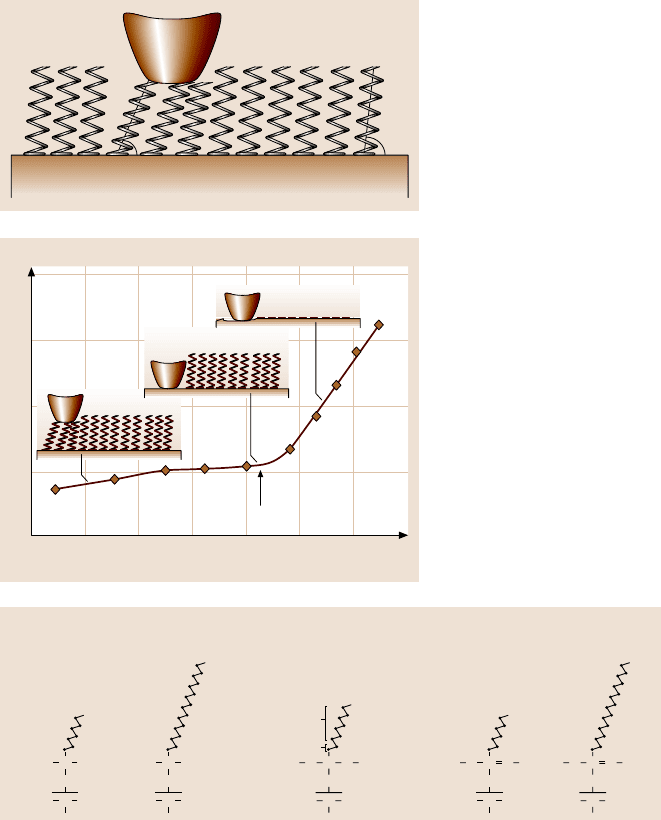
SAMs with high-compliancelong carbon chains also exhibitthe best wear resis-
tance [162,163]. In wear experiments, curves of wear depth as a function of normal
load show a critical normal load. A representative curve is shown in Fig. 22.25. Be-
low the critical normal load, SAMs undergo orientation, at the critical load SAMs
wear away from the substrate due to weak interface bond strengths, while above the
critical normal load severe wear takes place on the substrate.
Bhushan et al. [122], Kasai et al. [124], and Tambe and Bhushan [164] stud-
ied perfluorodecyltricholorosilane(PFTS), n-octyldimethyl (dimethylamino) silane
22 Characterization of MEMS/NEMS and BioMEMS/BioNEMS 1241
a)
b)
Au(111)
SSSS
Adhesive force (nN)
60
40
20
0
Au
HDT BPT BPTC
Coefficient of friction
0.08
0.06
0.04
0.02
0
Materials
Au
HDT BPT BPTC
Hexadecane thiol
(HDT)
1,1'–biphenyl–4–thiol
(BPT)
Cross-linked 1,1'–
biphenyl–4–thiol
(BPTC)
Biphenyl
–(C
6
H
6
)
2
–
Au(111)
S
S
CH
3
S
Alkyl
–(CH
2
)
n
–
Au(111)
CH
3
S
Fig. 22.23. (a) Schematics of
structures of hexadecane thiol
and biphenyl thiol SAMs on
Au(111) substrates, and (b)
adhesive force and coefficient
of friction of Au(111) sub-
strate and various SAMs
(ODMS) (n = 7), and n-octadecylmethyl(dimethylamino)silane (ODDMS) (n = 17)
vapor-phase-deposited on a Si substrate, and octylphosphonate (OP) and octade-
cylphosphonate (ODP) on an Al substrate, Fig. 22.26a. Figure 22.26b presents the
contact angle, adhesive force, friction force, and coefficient of friction of the two
substrates and with various SAMs. Based on the data, PFTS/Si exhibits a higher
1242 Bharat Bhushan
α
2
α
1
Substrate
Tip
Fig. 22.24. Molecular spring
model of SAMs. In this fig-
ure, α
1
<α
2
, which is caused
by the further orientation un-
der the normal load applied
by an asperity tip [162]
Critical load
Decrease of surface height (nm)
Normal load (μN)
0
7
7
5
3
1
–1
123456
Fig. 22.25. Illustration of
the wear mechanism of
SAMs with increasing nor-
mal load [163]
CH
3
Si
O
SiCH
3
(CH
2
)
17
CH
3
Si
O
Si
(CF
2
)
7
CF
3
OO
(CF
2
)
2
Al
O
P
(CH
2
)
7
OO
CH
3
Al
O
P
(CH
2
)
17
OO
CH
3
CH
3
Si
O
SiCH
3
CH
3
(CH
2
)
7
n-Dimethyl(dimethylamino)silane Perfluorodecyltrichlorosilane n-Phosphonate
Octodecyl (ODDMS) Deca (PFTS) Octadecyl (ODP)Octa (ODMS) Octa (OP)
a)
Fig. 22.26. (a) Schematics of structures of perfluoroalkylsilane and alkylsilane SAMs on Si
with native oxide substrates, and alkylphosphonate SAMs on Al with native oxide
contact angle and lower adhesive force compared to of ODMS/Si and ODDMS/Si.
The data for ODMS and ODDMS on the Si substrate are comparable to those for
OP and ODP on the Al substrate. Thus the substrate had little effect. The coefficient
of friction of various SAMs were comparable.
For wear performance studies, experiments were conducted on various films.
Figure 22.27a shows the relationship between the decrease of surface height and the
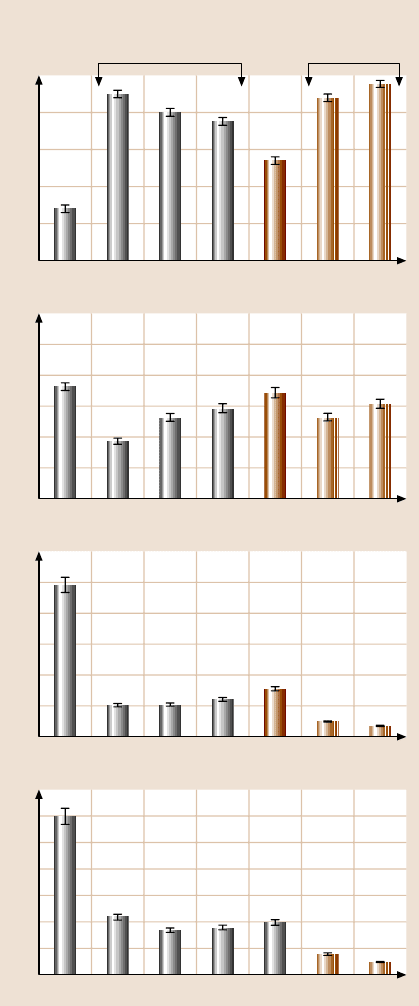
22 Characterization of MEMS/NEMS and BioMEMS/BioNEMS 1243
20
ODDMSODMSPFTSSi Al OP ODP
Contact angle
(deg)
40
60
80
100
120
0
ODDMSODMSPFTSSi Al OP ODP
Adhesive force (nN)
10
20
30
40
50
60
0
ODDMSODMSPFTSSi Al OP ODP
Friction force (nN)
0.5
1
1.5
2
2.5
3
At 5 nN normal load
0
ODDMSODMSPFTSSi Al OP ODP
Coefficient of friction
0.01
0.02
0.04
0.05
0.06
0.06
0.03
Si substrate Al substrate
b)
Fig. 22.26. (continued)
(b) contact angle, adhesive
force, friction force, and co-
efficient of friction of Si with
native oxide and Al with na-
tive oxide substrates and with
various SAMs
