ASM Metals HandBook Vol. 14 - Forming and Forging
Подождите немного. Документ загружается.

Carbon
Steels containing up to 0.25% C can readily be flame cut; higher-carbon steels should be preheated to prevent
hardening and cracking; graphitic carbon makes flame cutting of cast iron difficult; cast iron containing up to 4%
C can be flame cut when a powder, flux, or filler rod is used as a supplemental oxidizing agent
Chromium
Steels containing up to 5% Cr can be flame cut without difficulty; steels with chromium content of 10% or more
require metal powder, chemical flux, or plasma arc cutting
Cobalt
When present in the amounts normally used in steelmaking, cobalt has no noticeable effect on flame cutting
Copper
Up to 3% Cu has no effect on flame cutting
Manganese
Has no effect on flame cutting of carbon steels; steel containing 14% Mn and 1.5% C are difficult to cut and must
be preheated
Molybdenum
Steels with up to 5% Mo can be cut easily; this is true of AISI 41XX steels; high molybdenum-tungsten steels
require metal powder or plasma arc cutting
Nickel
Steels with up to 3% Ni and less than 0.25% C may be readily cut by OFC; up to 7% Ni requires flux additions to
the oxygen stream; stainless steels, from 18-8 to 35-15 types, require chemical flux, metal powder, or plasma arc
cutting
Phosphorus
The amount usually found in steel has no effect on flame cutting
Silicon
No effect in steels with up to 4% Si; in higher-silicon steels with high carbon and manganese contents, preheating
and postannealing are usually needed to avoid hardening and cracking
Sulfur
Amounts usually found in steel have no effect; higher sulfur content slows cutting speed and emits sulfur dioxide
fumes
Tungsten
Steels containing up to 14% W are readily flame cut, but cutting is more difficult with a higher percentage; high
red-hardness tungsten steels are difficult to flame cut and require preheating
Vanadium The amounts normally found in steel do not interfere with flame cutting
Preheating may consist of merely warming a cold workpiece with a torch or it may require furnace heating of the work
beyond 540 °C (1000 °F). For some alloy steels, preheat temperatures are 200 to 315 °C (400 to 600 °F). Carbon steel
billets and other sections occasionally are cut at 870 °C (1600 °F) and higher.
In oxyfuel gas cutting, preheating is accomplished by means of the oxyfuel gas flame, which surrounds the cutting
oxygen stream. At cut initiation, the preheat flame, the result of oxygen and fuel gas combustion, brings a small amount
of material to ignition temperature so that combustion can proceed. After cutting begins, the preheat flame merely adds
heat to compensate for heat lost by convection and radiation or through gas exhausted during cutting. The flame also
helps to remove or burn off scale and dirt on the plate surface; the hot, combusted gases protect the stream of cutting
oxygen from the atmosphere.
Preheating may also be applied over a broader area of the work. It may include soaking the entire workpiece in a furnace
to bring it up to 100 to 200 °C (200 to 400 °F), or a simple overall warm-up with a torch to bring cold plate to room
temperature. A preheat significantly improves cutting speed, allowing faster torch travel for greater productivity and
reduced consumption of fuel gas. Broader preheat smooths the temperature gradient between the base metal and the cut
edge, possibly reducing thermal stress and minimizing hardening effects in some steels.
Combustion of Gases
Each cutting job entails a different type or volume of work to be completed. Consequently, the best gas for all cutting in a
fabricating plant is found through experimentation. Evaluating a gas for a single job requires a test run that monitors fuel
gas and oxygen flow rate, labor costs, overhead, and the amount of work performed. If plant production varies from week
to week, gas performance should be measured over a long enough period to achieve an accurate cost analysis. Any of the
fuel gases may perform well over a range of flow rates. When comparing gases, performance should be rated at the lowest
flow rate that gives acceptable results for each gas. The most important preheat fuel gases are acetylene, natural gas,
propane, propylene, and Mapp. Their properties are given in Table 2. These gases are hydrocarbons, which give off
carbon dioxide and water vapor as the products of complete combustion. Flames of hydrocarbon gases are complex,
displaying successive cones as a result of stepped chemical reactions. With acetylene, the products of complete
combustion cannot exist at the temperature of the inner cone. Combustion is completed in the cooler, outer sheath of the
flame. Chemical equations for combustion reactions of hydrocarbon gases often are simplified by treating the reactions as
though the products were formed in only one step.
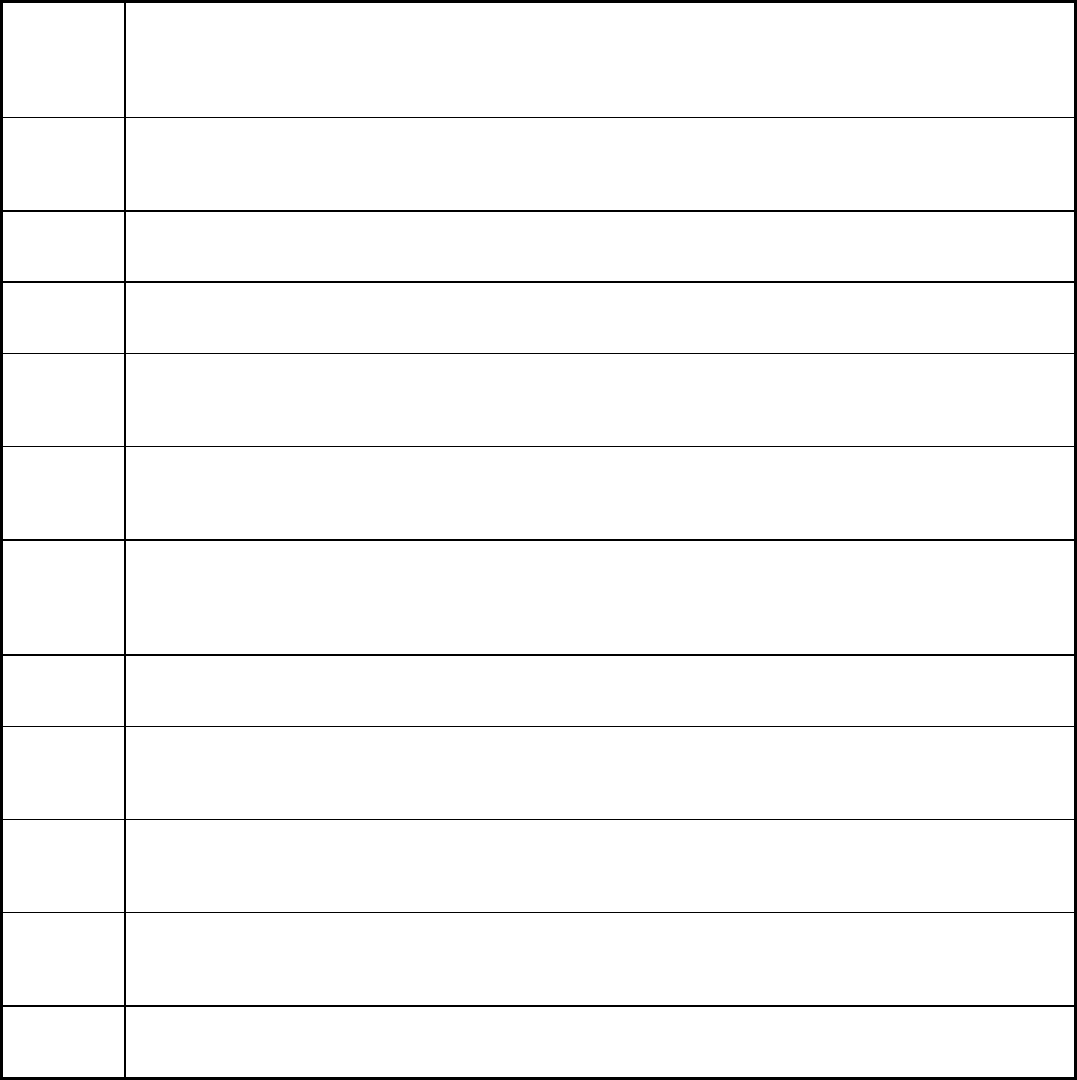
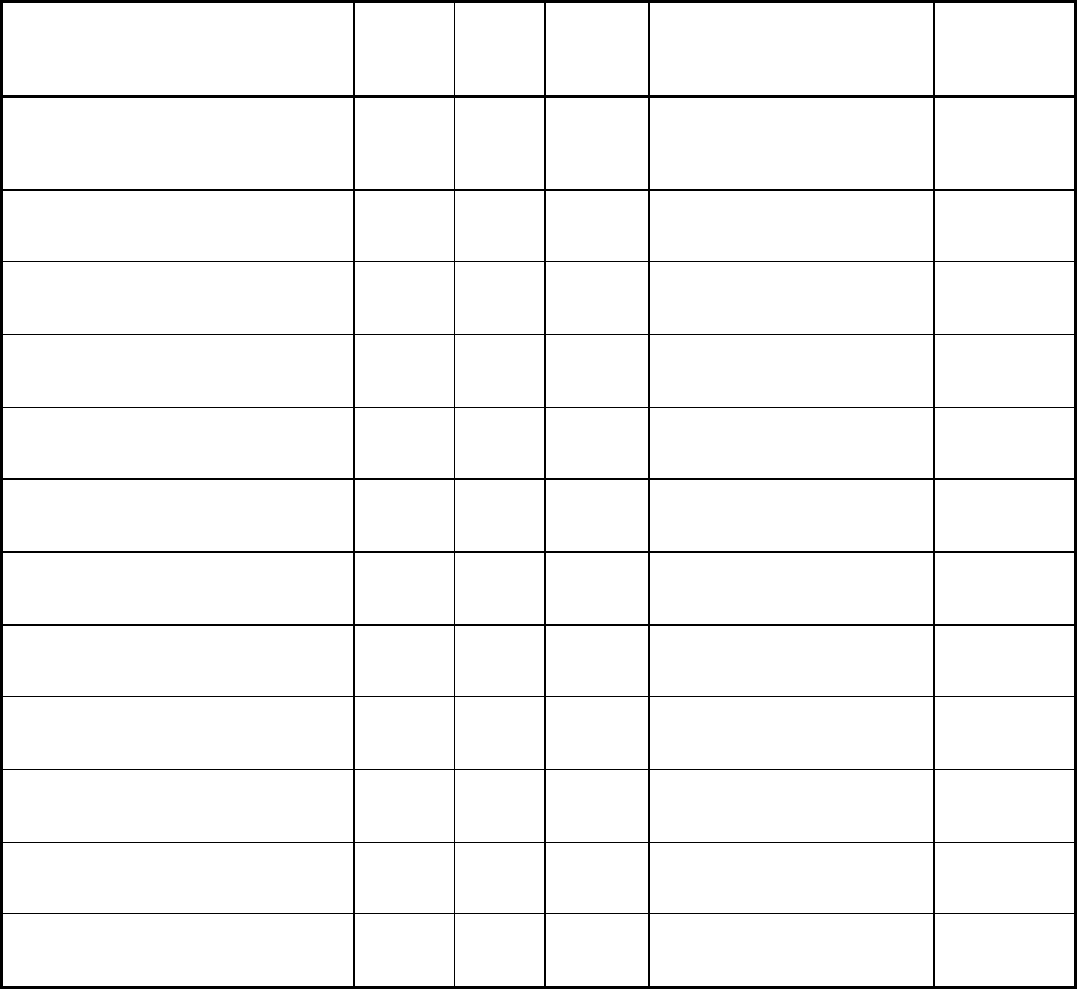
Table 2 Properties of common fuel gases
Acetylene
Propane
Propylene
Methylacetylene-propadiene
(Mapp)
Natural gas
Chemical formula C
2
H
2
C
3
H
8
C
3
H
6
C
3
H
4
(Methylacetylene,
propadiene)
CH
4
(Methane)
Neutral flame temperature
°F
5,600 4,580 5,200 5,200
4,600
°C
3,100 2,520 2,870 2,870
2,540
Primary flame heat emission
Btu/ft
3
507 255 433 517
11
MJ/m
3
19 10 16 20
0.4
Secondary flame heat emission
Btu/ft
3
963 2,243 1,938 1,889
989
MJ/m
3
36 94 72 70
37
Total heat value (after vaporization)
Btu/ft
3
1,470 2,498 2,371 2,406
1,000
MJ/m
3
55 104 88 90
37
Total heat value (after vaporization)
Btu/lb
21,500 21,800 21,100 21,000
23,900
kJ/kg
50,000 51,000 49,000 49,000
56,000
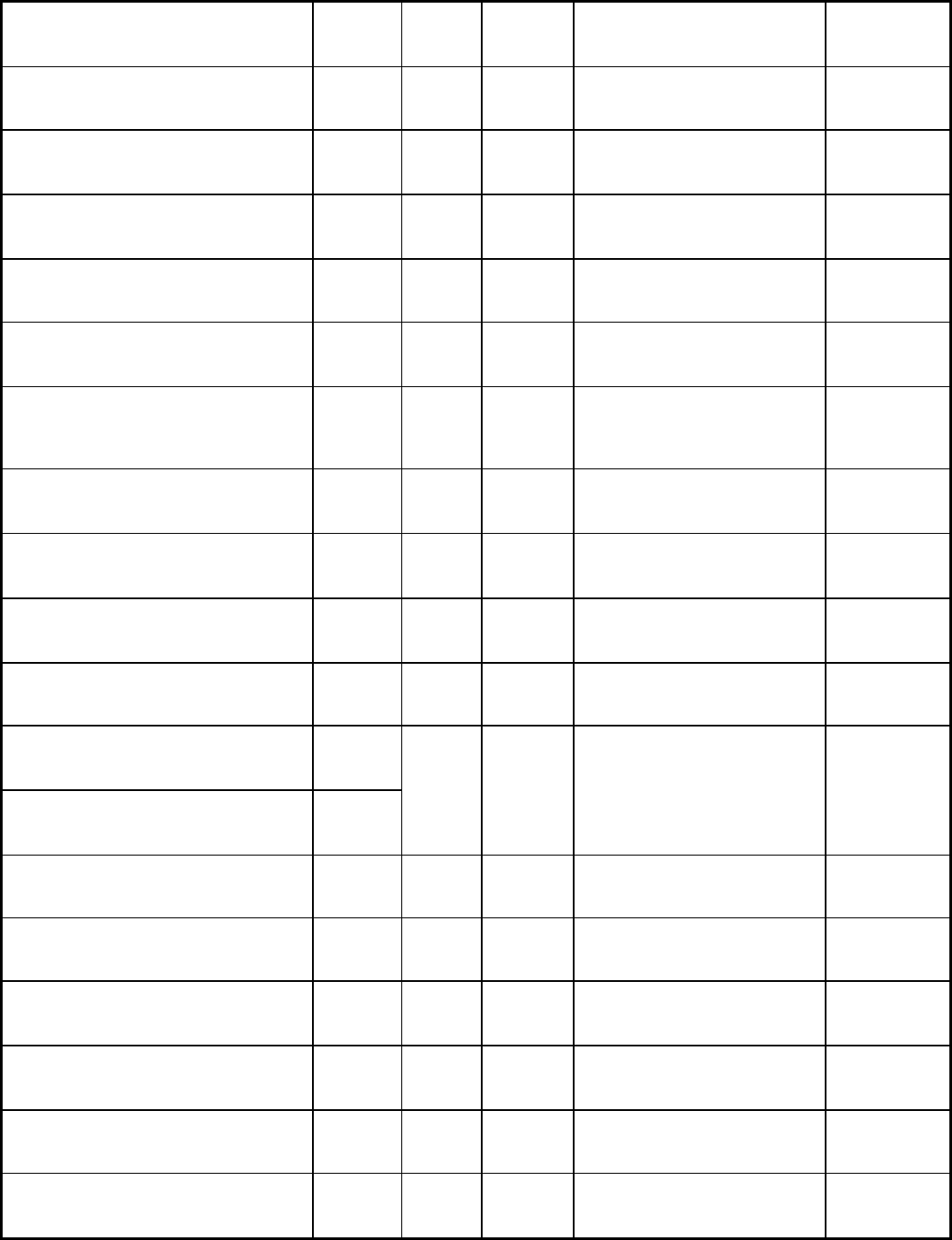
Total oxygen required (neutral flame)
vol O
2
/vol fuel
2.5 5.0 4.5 4.0
2.0
Oxygen supplied through torch (neutral
flame)
vol O
2
/vol fuel
1.1 3.5 2.6 2.5
1.5
ft
3
oxygen/lb fuel (60 °F)
16.0 30.3 23.0 22.1
35.4
m
3
oxygen/kg (15.6 °C)
1.0 1.9 1.4 1.4
2.2
Maximum allowable regulator pressure
psi
15
kPa
103
Cylinder
Cylinder Cylinder
Line
Explosive limits in air, % 2.5-80 2.3-9.5 2.0-10 3.4-10.8
5.3-14
Volume-to-weight ratio
ft
3
/lb (60 °F)
14.6 8.66 8.9 8.85
23.6
m
3
/kg (15.6 °C)
0.91 0.54 0.55 0.55
1.4
Specific gravity of gas (60 °F, 15.6 °C)
Air = 1
0.906 1.52 1.48 1.48 0.62
Source: American Welding Society

Acetylene (C
2
H
2
) combustion produces a hot, short flame with a bright inner cone at each cutting-tip port; the hottest
point is at the tip of this inner cone. Combustion starts in the inner cone and is brought to completion in a cooler, blue,
outer flame. The sharp distinction between the two flames helps to adjust the ratio of oxygen to acetylene.
Depending on this ratio, the flame may be carburizing (reducing), neutral, or oxidizing. A neutral flame results when just
enough oxygen is supplied for primary combustion, yielding carbon monoxide (CO) and hydrogen (H
2
). These products
then combine with oxygen in ambient air to form the blue, outer flame, yielding carbon dioxide (CO
2
) and water (H
2
O).
The neutral ratio of oxygen to acetylene is about 1 to 1, and the flame temperature at the tip of the inner cone is about
3040 °C (5500 °F). This flame is used for manual cutting.
When the oxygen-to-acetylene ratio is reduced to about 0.9 to 1, a bright streamer begins to appear, and the flame
becomes carburizing, or reducing. A carburizing flame is sometimes used for rough cutting of cast iron.
When the oxygen-to-acetylene ratio is increased to more than 1 to 1, the inner cones are shorter, "necked in" at the sides,
and more sharply defined; this flame is oxidizing. Flame temperature increases until, at a ratio of about 1.7 to 1, the
temperature is maximum, or somewhat over 3095 °C (5600 °F) at the tip of the cones. An oxidizing flame can be used for
preheating at the start of the cut, and for cutting very thick sections.
According to the equation:
2C
2
H
2
+ 5O
2
4CO
2
+ 2H
2
O
an oxygen-to-acetylene ratio of 2.5 to 1 is required for a complete reaction. For complete combustion, however, as much
as 1.5 parts of oxygen is taken from ambient air. In oxyacetylene cutting, part of this oxygen may be supplied from the
cutting oxygen, but total oxygen consumption is relatively low, an advantage of acetylene over all other fuel gases.
Operation of oxyacetylene equipment in confined spaces, such as the inside of a closed tank or vessel, requires forced
ventilation to supply the additional air needed for breathing and for flame combustion.
Acetylene must be used at pressures below 105 kPa (15 psi), which is a stable operating range. Safety codes specify
equipment and handling practices for acetylene. When supplied in special cylinders, acetylene is dissolved in acetone,
which is contained in a porous mass that fills the cylinder. This technique eliminates the sensitivity of acetylene at
pressures over 105 kPa (15 psi). Such cylinders can be filled to pressures exceeding 105 kPa (15 psi), but not greater than
1725 kPa (250 psi). Acetylene may also be supplied from generators. With either means of supply, safety regulations
must be observed to avoid sudden decomposition and explosion.
Despite some disadvantages, acetylene has been used for cutting for a longer time than any other gas. Its performance is
well understood, equipment for it is perfected and widely marketed, and it is readily available. It has become the standard
against which other gases are compared.
Natural gas is a mixture of gases, but consists principally of methane, and therefore is usually given the chemical
symbol for methane (CH
4
). One source defines the most widely used mixture as 85% methane (CH
4
), 4% ethane (C
2
H
6
),
and 11% (N
2
, H
2
, O
2
, H
2
O). Some wells produce natural gas with large proportions of ethane and propane.
The chemical equation for complete combustion:
CH
4
+ 2O
2
CO
2
+ 2H
2
O
indicates an oxygen-to-methane ratio of 2 to 1; this ratio is used for the preheat flame. Maximum flame temperature at the
tip of the inner cones is about 2760 °C (5000 °F). Both higher and lower temperatures have been reported; also, the
optimum oxygen-to-gas ratio is about 2 to 1. The flame is more diffuse than with acetylene; heat intensity is lower; and
adjustment for carburizing, neutral, and oxidizing flame is less clearly defined. Initial cutting speeds are slower, and
oxygen consumption is greater. Also, more time is required for preheating with natural gas than with acetylene. An excess
of oxygen shortens preheat time, but increases consumption of oxygen. Furthermore, natural gas cannot be used for
welding of steel, so extra installations are needed if this operation is to be performed.
Despite these disadvantages, the use of natural gas for cutting has increased. It is the lowest-cost commercial fuel gas and,
with careful torch adjustment, produces excellent cuts in light-to-heavy-gage material.
Neither acetylene nor natural gas accumulates in low pockets. When burned alone in air, the flame of natural gas does not
produce soot.
Propane (C
3
H
8
) is a petroleum-base fuel usually supplied as a liquid in storage tanks from which it is drawn off as a gas.
The gas is dispensed from bulk storage tanks through pipelines. It has a narrow range of flammability and is relatively
stable, but is heavier than air. Complete combustion requires an oxygen-to-propane ratio of 5 to 1. However, about 30%
of the oxygen needed is taken from the ambient air. When the ratio of oxygen to propane is 4.5 to 1, the flame
temperature is about 2760 °C (5000 °F) at the tip of the inner cones. At 4.25 to 1, the flame temperature is about 2650 °C
(4800 °F). Flame properties are similar to those of natural gas, with respect to diffuseness, heat intensity, flame
adjustment, and cutting speed. When burned alone in air, the flame is soot-free.
Propylene is a liquefied gas similar to propane. It has a higher flame temperature than propane. The flame temperature
of propylene is about equal to Mapp gas, although its heat content is slightly less. On a volume basis, propylene is usually
less expensive than acetylene; it does, however, consume more oxygen during combustion. The combustion equation for
propylene is:
2C
3
H
6
+ 9O
2
6CO
2
+ 6H
2
O
The combustion ratio for propylene is 4.5 to 1. Line oxygen for a neutral flame is about 3.5 to 1. Distributors sell
propylene under various trade names, either pure or as improved mixtures with propane and other hydrocarbon additives.
Mapp gas (stabilized methylacetylene-propadiene) is a proprietary gas mixture; it is shipped and stored as a liquid,
either in bulk storage tanks or in portable cylinders.
Both methylacetylene and propadiene have the chemical symbol C
3
H
4
and by themselves are unstable, giving off their
heat of formation during decomposition. As with acetylene, this heat is in addition to the heat of combustion. However,
the methylacetylene-propadiene mixture in Mapp gas is stabilized by the addition of other hydrocarbons. The composition
of Mapp gas is not disclosed, so the chemical equation for complete combustion in oxygen is not given. However, when
the flame is neutral, the ratio of oxygen to fuel gas is about 2.3 to 1; the normal operating ratio for cutting varies from 2.5
to 1 to 4 to 1, depending on speed and thickness. Maximum flame temperature at the tips of the inner cones, reported as
2925 °C (5300 °F), occurs at oxygen-to-fuel ratios from 3.5 to 1 to 4 to 1. Flames can be adjusted for carburizing, neutral,
or oxidizing conditions.
Mapp gas is heavier than air, but it has a strong odor to reveal its presence in case it leaks or has collected in low pockets.
At low temperatures, Mapp gas withdrawal rates from the cylinder are reduced. At about 0 °C (32 °F), methylacetylene
has a vapor pressure of only 14 kPa (2 psi).
Effect of Oxyfuel Cutting on Base Metal
During the cutting of steel, the temperature of a narrow zone adjacent to the cut face is raised considerably above the
transformation range. As the cut progresses, the steel cools through this range. The cooling rate depends on the heat
conductivity and mass of the surrounding material, on loss of heat by radiation and convection, and on speed of cutting.
When steel is at room temperature, the rate of cooling at the cut is sufficient to produce a quenching effect on the cut
edges, particularly in heavier cuts in large masses of cold metal. Depending on the amount of carbon and alloying
elements present and on the rate of cooling, pearlitic steel transforms into structures ranging from spheroidized carbides
in ferrite to harder constituents. The heat-affected zone (HAZ) may be 0.8 to 6.4 mm ( to in.) deep for steels 9.5 to
150 mm ( to 6 in.) thick. Approximate depths of the HAZ in oxyfuel gas cut carbon steels are given in Table 3. Some
increase in hardness usually occurs at the outer margin of the HAZ of nearly all steels.
Table 3 Approximate depths of HAZ in gas-cut carbon steels
Plate thickness
HAZ depth
mm in. mm
in.
Low-carbon steels
<13
<
<0.8
<
13
0.8
150 6 1.4
High-carbon steels
<13
<
<0.8
<
13
0.8-1.6
-
150 6 1.4-6.0
-
Note: The depth of the fully hardened zone is considerably less than the depth of the HAZ.
For most applications of gas cutting, the
affected metal does not have to be removed.
Low-Carbon Steel. For steels containing 0.25% C or less, cut at room temperature, the hardening effect is usually
negligible, although at the upper carbon limit it may be significant if subsequent machining is required. Short of
preheating or annealing the workpiece, hardening may be lessened by ensuring that the cutting flame is neutral to slightly
oxidizing, the flame is burning cleanly, and the inner cones of the flame are at the correct height. By increasing the
machining allowance slightly, the first cut usually can be made deep enough to penetrate below the hardened zone in most
steels. Mechanical properties of low-carbon steels generally are not adversely affected by oxyfuel gas cutting.
Medium-Carbon Steels. Steels having carbon contents of 0.25 to 0.45% are affected only slightly by hardening
caused by oxyfuel gas cutting. Up to 0.30% C, steels with very low alloy content show some hardening of the cut edges,
but generally not enough to cause cracking. Over 0.35% C, preheating to 260 to 315 °C (500 to 600 °F) is needed to avoid
cracking. All medium-carbon steels should be preheated if the gas cut edges are to be machined.
High-Carbon and Alloy Steels. Gas cutting of higher-carbon (over 0.45% C) and hardenable alloy steels at room
temperature may produce, on the cut surface, a thin layer of hard, brittle material that is susceptible to cracking from the
stress of cooling. The cooling stress that causes cracking is similar to the stress that causes distortion.
Microcracks, or even incipient cracks, can be dangerous, because in service under tension they can develop into large
fractures. The problems of hardening and the formation of residual stress can be alleviated by preheating and annealing.
Preheating serves three purposes. It:
• Reduces the temperature gradient near the cut during cutting.
This lowers differential expansion, which may
cause distortion or upsetting of the metal. Meta
l upset during the heating cycle can produce excessive stress in
cooling
• Increases the cutting speed and improves the surface of the cut, especially in heavier sections and in the difficult-
to-cut steels
• Reduces the cooling rate in the annealing range for the heat-
affected portion of the cut during the cooling cycle.

By slower cooling, more ductile microstructures are obtained, and the formation of the hard martensitic structures
is suppressed
If the higher-carbon and alloy steels are adequately preheated (and, in certain instances, annealed afterward), no cracks
will occur. Ordinarily, a preheat temperature of 260 to 315 °C (500 to 600 °F) is sufficient for high-carbon steels; alloy
steels may require preheating as high as 540 °C (1000 °F). Preheat temperature should be maintained during cutting.
Thick preheated sections should be cut as soon as possible after the piece has been withdrawn from the furnace.
Local preheating involves heating that area of the workpiece that encloses what will become the HAZ of the cut. If the
area to be heated is small and the section is not too thick, the preheating flame of a cutting torch may be used, but usually
a special heating torch is required.
Local preheating is used when it is impossible or impractical to preheat the entire workpiece. It is important to heat the
workpiece uniformly through the section to be cut, without causing too steep a temperature gradient. A multiflame
heating torch is sometimes mounted ahead of the cutting torch in machine-guided cutting. Local preheating also can be
accomplished using a preheat adaptor.
Annealing serves two main purposes in controlling the effects of gas cutting in carbon and low-alloy steels. It restores
the original structure of the steel, whether it be predominantly pearlitic or predominantly ferritic with spheroidized
carbide, and it also provides stress relief. Many steels do not require annealing if they have been properly preheated. (See
Heat Treating, Volume 4 of the ASM Handbook, for annealing practices for specific steels.)
Local annealing, also called flame annealing, is a localized postheat treatment that can be used to prevent hardening or
to soften an already hardened cut surface. Either the preheating flame of the cutting torch or a special heating torch may
be used for local annealing, depending on the mass of the workpiece and the area to be covered. The heat-affected portion
of the workpiece should be heated uniformly, and the temperature gradient at the boundary of the heated mass should be
gradual enough to avoid distortion of the workpiece.
Local annealing is not a substitute for preheating; it cannot correct damage done during cutting, such as upsetting of the
metal or cracking at the cut edges. Local annealing is limited to steel plate up to 40 mm (1 in.) thick. From 40 to 75 mm
(1 to 3 in.) thick, heat should be applied to both sides of the plate. This method is not suitable for thicknesses over 75
mm (3 in.). If local annealing cannot be done simultaneously with cutting, the cut edges should be tempered after cutting
with a suitable heating torch.
Stainless steels do not support oxyfuel combustion and therefore require metal powder cutting, chemical flux cutting,
or plasma arc cutting processes. Except for stabilized types, stainless steels degrade under the heat of metal powder or
chemical flux processes. Carbide precipitation occurs in the HAZ about 3 mm ( in.) from the edge, where the metal has
been heated to 425 to 870 °C (800 to 1600 °F) long enough for dissolved carbon to migrate to the grain boundaries and
combine with the chromium to form chromium carbide. The chromium-poor (sensitized) regions near grain boundaries
are subject to corrosion in service. This type of corrosion can be prevented by a stabilizing anneal, which puts the carbon
back into solution. However, the required quench through the sensitizing temperature range may distort the material.
Water quenching of the cut edge directly behind the cutting torch may avert sensitization. Because it takes about 2 min at
sensitizing temperature for carbide precipitation to occur, water quenching must be done immediately. Distortion is more
likely with this method than with the stabilizing anneal. Still another procedure is to remove the sensitizing zone entirely
by chipping or machining.
Distortion, which is the result of heating by the gas flame, can cause considerable damage during cutting of thin plate
(<8 mm, or in., thick), cutting of long narrow widths, close-tolerance profile cutting, and cutting of plates that contain
high residual stresses. The heat may release some of the locked-in stress, or may add new stress. In either case,
deformation (warpage) may occur, thereby causing inaccurate finished cuts. Plates in the annealed condition have little or
no residual stress.
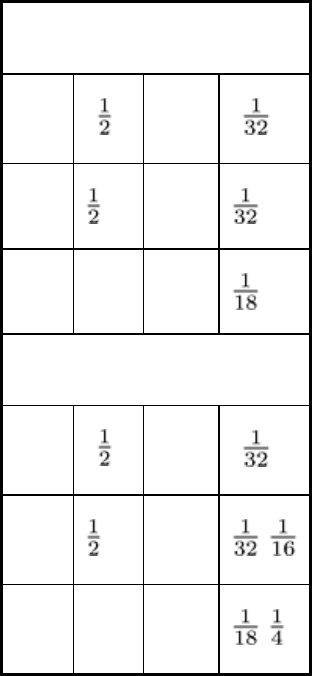
Deformation. In cuts made from large plates, the cutting thermal cycle changes the shape of narrow sections and leaves
residual stress in the large section (see Fig. 2). The temperature gradient near the cut is steep, ranging from melting point
at the cut to room temperature a short distance from it. The plate does not return to its original shape unless the entire
plate is uniformly heated and cooled.
Fig. 2 Effects of oxyfuel gas cutting thermal cycle on shape of sections.
(a) Plate with large restraint on one
side of kerf, little restraint on the other side. Phantom lines indicate
direction of residual stress that would
cause deformation except for restraint. (b) Plate with little restraint on either side.
As the metal heats, it expands, and its yield strength decreases; the weakened heated material is compressed by the
surrounding cooler, stronger metal. The hotter metal continues to expand elastically in all directions until its compressive
yield strength is reached, at which point it yields plastically (upsets) in directions not under restraint. The portion of this
upset metal at about 870 °C (1600 °F) is virtually stress-free; the remainder is under compressive stress that is equal to its
yield strength. Metal that expands but does not upset is under compressive strength below yield. The net stress on the
heated side of the neutral axis causes bowing of a narrow plate during cutting, as shown in Fig. 2.
As the heated metal begins to cool, it contracts, and its strength increases. First, the contraction reduces the compressive
stress in the still-expanded metal. When the compressive stress reaches zero and the plate regains its original shape,
previously upset metal also has regained strength. This metal is now in tension as it cools, and its tensile yield strength
increases. Tension increases until the metal reaches room temperature. Residual tensile stress in the cooling side of the
neutral axis causes the bowing of narrow plates after cooling (Fig. 2). Controlled upsetting is the basis of flame
straightening.
Control of Distortion. Preheating the workpiece can reduce distortion by reducing differential expansion, thereby
decreasing stress gradients. Careful planning of the cutting sequence also may help. For example, when trimming
opposite sides of a plate, both sides should be cut in the same direction at the same time. When cutting rings, the inside
diameter should be cut first; the remaining plate restrains the material for the outside-diameter cut. In general, the larger
portion of material should be used to retain a shape for as long as possible; the cutting sequence should be balanced to
maintain even-heat input and resultant residual stresses about the neutral axis of the plate or part.
Equipment
Commercial gases are usually stored in high-pressure cylinders. Natural gas--primarily methane--is supplied by pipeline
from gas wells. The user taps into local gas lines. Acetylene, dissolved in acetone, is available in clay-filled cylinders.
High-volume users often have acetylene generators on site. For heavy consumption or when many welding and cutting
stations use fuel gas, banks of gas cylinders are maintained at a central location in the plant, and the gas is manifolded and
piped to the point of use.
Manual gas cutting equipment consists of gas regulators, gas hoses, cutting torches, cutting tips, storage tanks, reverse
flow check valves, and flashback arrestors. Auxiliary equipment may include a hand truck, tip cleaners, torch ignitors,
and protective goggles. Machine cutting equipment varies from simple rail-mounted "bug" carriages to large bridge-
mounted torches that are driven by computer-directed drives.
Gas regulators reduce gas pressure and moderate gas flow rate between the source of gas and its entry into the cutting
torch to deliver gas to the cutting apparatus at the required operating pressure. Gas enters the regulating device at a wide
range of pressures. Gas flows through the regulator and is delivered to the hose-torch-tip system at the operating pressure,
which is preset by manual adjustment at the regulator and at the torch. When pressure at the regulator drops below the
preset pressure, regulator valves open to restore pressure to the required level. During cutting, the regulator maintains
pressure within a narrow range of the pressure setting.
Regulators should be selected for use with specific types of gas and for specific pressure ranges. Portable oxyacetylene
equipment requires an oxygen regulator on the oxygen cylinder and an acetylene regulator on the acetylene cylinder,
which are not interchangeable.
High-low regulators conserve preheat oxygen when natural gas or propane is the preheat fuel used in oxyfuel gas
cutting. These gases require a longer time to start a cut than do acetylene or Mapp gas. High-low regulators reduce
preheat flow to a predetermined level when the flow of cutting oxygen is initiated. When the regulator switches from high
to low, preheat cutback may range from 75 to 25% as plate thicknesses increase from 9.5 to 200 mm ( to 8 in.). High-
low regulators are used for manual and automatic cutting with natural gas propane and liquefied petroleum gas (LPG).
Hose. Flexible hose, usually 3 to 13 mm ( to in.) in diameter, rated at 1380 kPa (200 psig) maximum, carries gas
from the regulator to the cutting torch. Oxygen hoses are green; the fittings have right-hand threads. Fuel gas hoses are
red; the fittings have left-hand threads and a groove cut around the fitting. For heavy cutting, two oxygen hoses may be
necessary, one for preheat and one for cutting oxygen. Multiple-torch cutting machines often have three-hose torches.
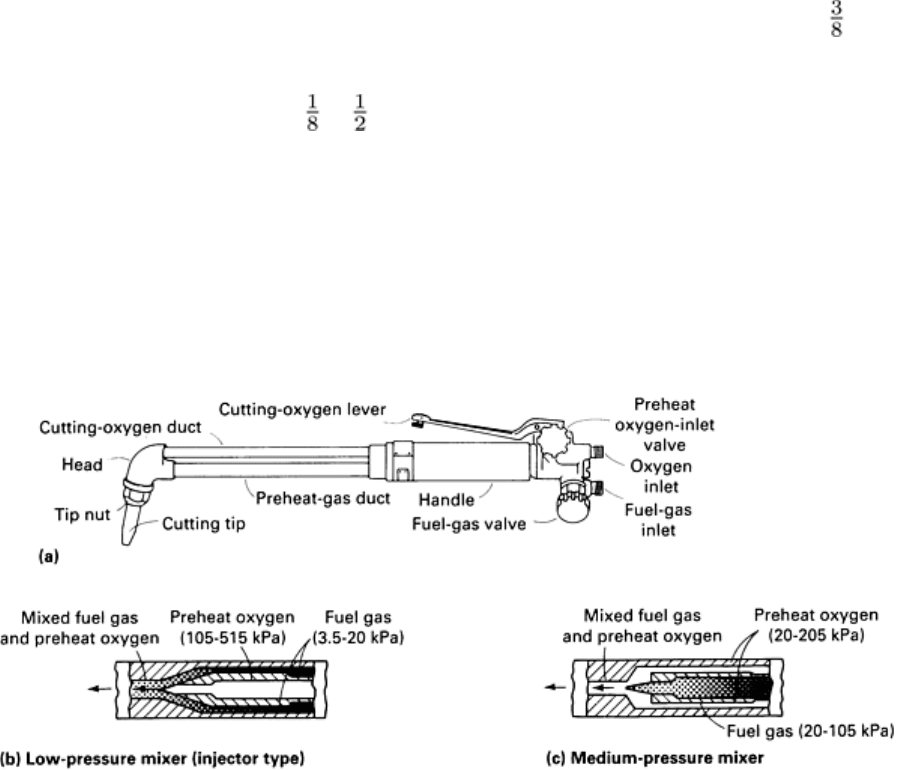
Cutting torches, such as the one shown in Fig. 3, control the mixture and flow of preheat oxygen and fuel gas and the
flow of cutting oxygen. The cutting torch discharges these gases through a cutting tip at the proper velocity and flow rate.
Pressure of the gases at the torch inlets, as well as size and design of the cutting tip, limits these functions, which are
operator controlled.
Fig. 3 (a) Typical manual cutting torch in which preheat gases are mixed before entering torch head.
(b) and
(c) Sections through preheat gas duct showing two types of mixers commonly used with the torch shown.
After
the workpiece is sufficiently preheated, the operator depresses the lever to st
art the flow of cutting oxygen.
Valves control the flow of oxygen and fuel gas to achieve the required flow and mixture at the cutting tip.
Oxygen inlet control valves and fuel gas inlet control valves permit operator adjustment of gas flow. Fuel gas flows
through a duct and mixes with the preheat oxygen; the mixed gases then flow to the preheating flame orifices in the
cutting tip. The oxygen flow is divided: A portion of the flow mixes with the fuel gas, and the remainder flows through
the cutting-oxygen orifice in the cutting tip. A lever-actuated valve on the manual torch starts the flow of cutting oxygen;
machine cutting starts the oxygen from a panel control.
Fuel gases supplied at low pressure (usually below 21 kPa, or 3 psi), such as natural gas tapped from a city line, require
an injector-mixer (Fig. 3b) to increase fuel gas flow above normal operating pressures. Optimum torch performance relies
on proper matching of the mixer to the available fuel gas pressure.
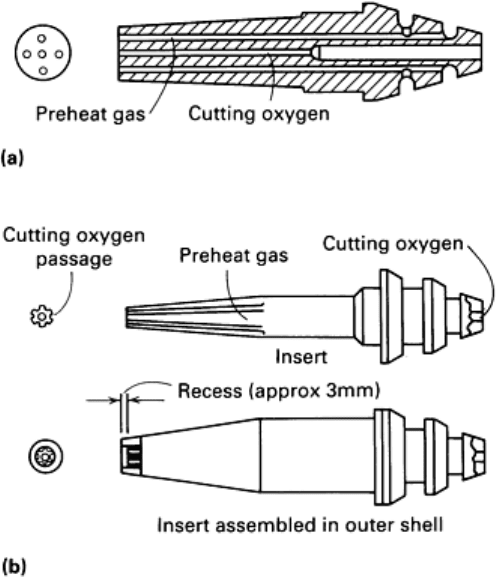
Cutting tips are precision-machined nozzles, produced in a range of sizes and types. Figure 4(a) shows a single-piece
acetylene cutting tip. A two-piece tip used for natural gas (methane) or LPG is shown in Fig. 4(b). A tip nut holds the tip
in the torch. For a given type of cutting tip, the diameters of the central hole, the cutting-oxygen orifice, and the preheat
ports increase with the thickness of the metal to be cut. Cutting tip selection should match the fuel gas; hole diameters
must be balanced to ensure an adequate preheat-to-cutting-oxygen ratio. Preheat gas flows through ports that surround the
cutting-oxygen orifice. Smoothness of bore and accuracy of size and shape of the oxygen orifice are important to
efficiency. Worn, dirty bores reduce cut quality by causing turbulence in the cutting-oxygen stream.
Fig. 4 Types of cutting tips. (a) Single-piece acetylene cutting tip. (b) Two-
piece tip for natural gas or LPG. Fuel
gas and preheat oxygen mix in tip. Recessed bore promotes laminar flow of gas and ancho
rs the flame when
natural gas or propane is used.
The size of the cutting-tip orifice determines the rate of flow and velocity of the preheat gases and cutting oxygen. Flow
to the cutting tip can be varied by adjustment at the torch inlet valve or at the regulator, or both.
Increasing cutting-oxygen flow solely by increasing the oxygen pressure results in turbulence and reduces cutting
efficiency. Turbulence in the cutting oxygen causes wide kerfs, slows cutting, increases oxygen consumption, and lowers
quality of cut. Consequently, larger cutting tips are required for making heavier cuts.
Standard tips, as shown in Fig. 5(a), have a straight-bore oxygen port. Oxygen pressures range from 200 to 400 kPa (30 to
60 psi) and are used for manual cutting. High-speed tips, or divergent cutting tips (Fig. 5b), use a converging, diverging
orifice to achieve high gas velocities. The oxygen orifice flares outward. High-speed tips operate at cutting-oxygen
pressures of about 700 kPa (100 psi) and provide cutting jets of supersonic velocity. These tips are precision made and are
more costly than straight-drilled tips, but they produce superior results: improved edge quality and cutting speeds 20%
