Wallace J.M., Hobbs P.V. Atmospheric Science. An Introductory Survey
Подождите немного. Документ загружается.


164 Atmospheric Chemistry
b. The nitrate radical
Because OH is produced primarily by photochemical
reactions and has a very short lifetime, it is present
in the atmosphere at currently measurable con-
centrations only during the day. At night, the nitrate
radical NO
3
takes over from OH as the major
reactive oxidant in the troposphere. The nitrate radi-
cal is formed by Eq. (5.4). During the day, NO
3
is
photolyzed rapidly by solar radiation via two path-
ways
(5.10)
and
(5.11)
The resulting atmospheric residence time of NO
3
at noon in sunlight is 5s.NO
3
also reacts with NO
(5.12)
Although NO
3
is much less reactive than OH, at
night it is present in much higher concentrations than
OH is during the day so it can serve as an effective
oxidant.
c. Odd nitrogen and other “chemical families”
Odd nitrogen (NO
y
), or total reactive nitrogen as it is
sometimes called, refers to the sum of NO
x
plus all
compounds that are the products of the atmospheric
oxidation of NO
x
, including HNO
3
,NO
3
, dinitrogen
pentoxide (N
2
O
5
), and peroxyacetyl nitrate (PAN for
short). Grouping the nitrogen-containing species in
this manner is useful in considering their budgets in
the atmosphere.
The main sources of NO
y
are anthropogenic emis-
sions (see Section 5.6.1) but, away from pollution
sources, soils and lightning can play dominant roles.
Interactions between NO
y
and NMHC can lead to
photochemical smogs in cities (see Section 5.5.2).
On regional and global scales, interactions of NO
y
with odd hydrogen have a strong influence on OH
concentrations.
NO
x
and NO
y
are examples of “chemical families.”
Other useful chemical families are odd oxygen
(e.g., O, O*, O
3
and NO
2
), odd hydrogen (HO
x
, where
x 0, 1 or 2), and odd chlorine (ClO
x
,where x 0, 1
or 2). Note that odd hydrogen includes the OH radi-
cal and NO
y
includes the NO
3
radical.
NO
3
NO : 2NO
2
NO
3
h
(
0.580 mm) : NO
2
O
NO
3
h
(
0.700 mm) : NO O
2
d. Ammonia
Ammonia (NH
3
) originates from soils, animal waste,
fertilizers, and industrial emissions. It is the principal
basic gas in the atmosphere. Ammonia neutralizes
acid species by reactions of the form
(5.13)
The primary removal mechanisms for NH
3
involve
its conversion to ammonium-containing aerosols
by reactions such as (5.13). The aerosols are then
transported to the ground by wet and dry deposition.
The residence time of NH
3
in the lower troposphere
is 10 days.
5.3.3 Organic Compounds
Organic compounds contain carbon atoms. The four
electrons in the outer orbital of the carbon atom can
form bonds with up to four other elements: hydrogen,
oxygen, nitrogen, sulfur, halogens, etc. Hydrocarbons
are organics composed of carbon and hydrogen.
There are large natural and anthropogenic sources of
atmospheric hydrocarbons, gases that play key roles
in many aspects of the chemistry of the troposphere.
Methane is the most abundant and ubiquitous
hydrocarbon in the atmosphere. The present concen-
tration of CH
4
in the northern hemisphere is
1.7 ppmv; it has a residence time in the atmosphere
of 9 years. Sources of CH
4
include wetlands, land-
fills, domestic animals, termites, biomass burning,
leakages from natural gas lines, and coal mines. The
primary sink for tropospheric CH
4
is its oxidation by
OH to form formaldehyde (HCHO); HCHO then
photodissociates into CO (see Section 5.3.6) or, in air
with sufficient NO
x
, OH oxidizes CO to produce O
3
(see Section 5.3.5).
There are numerous nonmethane hydrocarbons
(NMHC) in the Earth’s atmosphere, and many of
them play important roles in tropospheric chemistry.
Based on their molecular structures, NMHC can be
grouped into several classes. Examples include alkanes
(C
n
H
2n 2
), which include ethane (CH
3
!CH
3
) and
propane (CH
3
!CH
2
!CH
3
); alkenes, which have a
double bond, such as ethene (CH
2
"CH
2
) and
propene (CH
3
CH"CH
2
); and aromatics, such as ben-
zene (C
6
H
6
) and toluene (C
7
H
8
). Oxygenated hydro-
carbons, which contain one or more oxygen atoms,
such as acetone (CH
3
COCH
3
), may provide an
important source of HO
x
in the upper troposphere,
thereby influencing O
3
chemistry in this region.
2NH
3
H
2
SO
4
: (NH
4
)
2
SO
4
P732951-Ch05.qxd 12/09/2005 09:05 PM Page 164

5.3 Some Important Tropospheric Trace Gases 165
5.3.4 Carbon Monoxide
Carbon monoxide is produced by the oxidation of
CH
4
or NMHC, such as isoprene. Other important
sources of CO are biomass burning and the com-
bustion of fossil fuels. The dominant sink of CO is
oxidation by OH
(5.14)
Because (5.14) is generally the major sink for OH
in nonurban and nonforested locations, the concen-
tration and distribution of OH are often determined
by the ambient concentrations of CO, although CH
4
,
NO
x
,H
2
O, etc. can also be determining factors. An
important feature of CO in extratropical latitudes is
its seasonal cycle; it accumulates in the atmosphere
during winter when OH concentrations are low, but
in spring CO is depleted rapidly due to reaction (5.14).
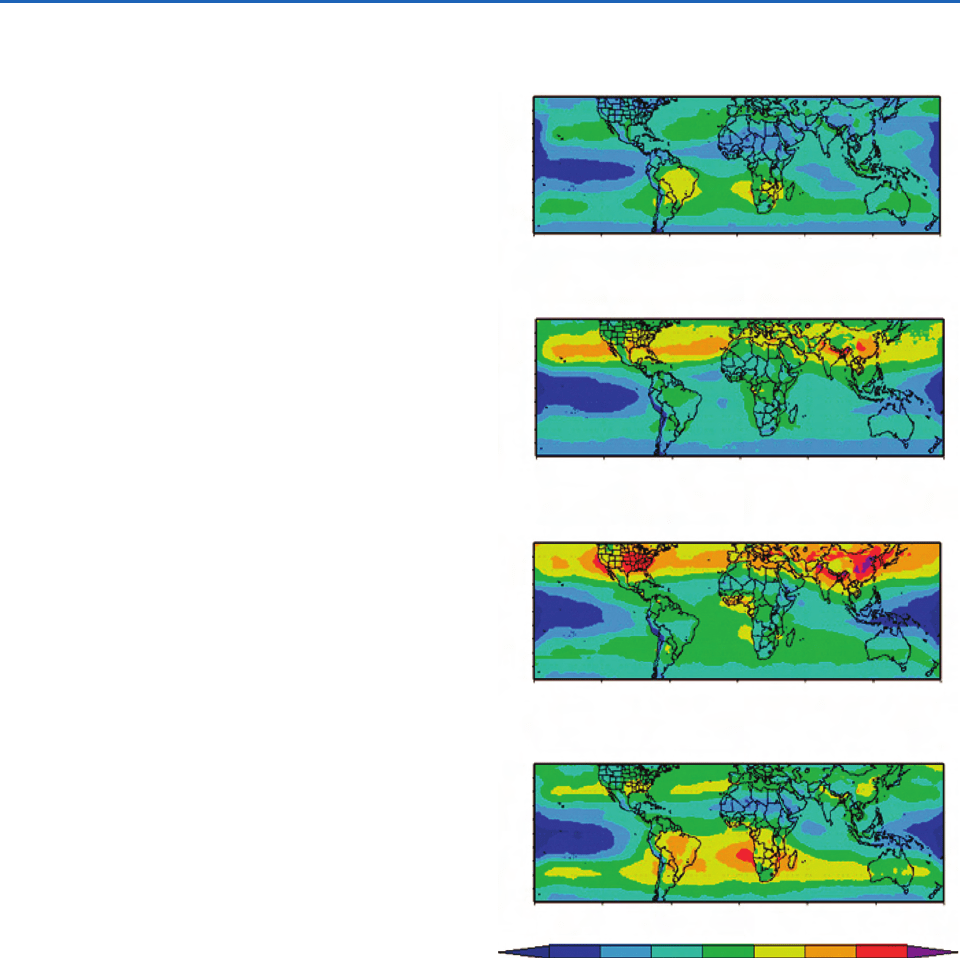
Figure 5.5 shows satellite measurements of CO.
The high concentration of CO emanating from South
America and Africa are due to biomass burning. The
plumes travel slowly across the southern hemisphere
and can be detected in Australia during the dry
season. A strong source of CO, due to industrial
emissions, is also apparent in southeast Asia; this
plume can sometimes reach North America.
5.3.5 Ozone
Because about 90% of the O
3
in the Earth’s atmos-
phere is in the stratosphere (see Section 5.7.1), it was
suggested in the middle of the 20th century that the
CO OH : H CO
2
stratosphere was a primary source for tropospheric
O
3
and that a balance existed between this source
and surface sinks. It has subsequently been recog-
nized that trace gases, such as NO, CO, and organic
compounds, which are emitted by human activities,
lead to the formation of O
3
through photochemical
reactions. In addition, various natural sources, such
as hydrocarbons from vegetation and NO from light-
ning, produce O
3
precursors.
Ozone plays a controlling role in the oxidizing
capacity of the troposphere. Much of the O
3
in the
troposphere is produced by in situ homogeneous
gas-phase reactions involving the oxidation of CO,
CH
4
, and NMHC by OH in the presence of NO
x
,as
outlined later.
Fig. 5.5 Concentrations of CO at an altitude of 4.5 km
measured from a satellite. Concentrations range from back-
ground values 50 pptv in regions shaded blue to as high
as 450 ppbv in the regions shaded red. The CO can be
transported upward and also be carried over large horizontal
distances. [Courtesy of NASA.]
17
Christian Friedrich Schönbein (1799–1868) German chemist. Discovered nitrocellulose (an explosive) when he used his wife’s cotton
apron to wipe up some spilled nitric and sulfuric acid and the apron disintegrated. Taught in England before joining the faculty at the
University of Basel.
18
Arie Jan Haagen-Smit (1900–1977) Dutch biologist and chemist. Joined the Biology Department, California Institute of Technology,
in 1937, where he worked on terpenes and plant hormones. Carried out pioneering work on analysis of Los Angeles’ smogs and the
emissions responsible for them.
Schönbein
17
discovered ozone by its odor follow-
ing an electrical discharge (it can sometimes be
smelled after a thunderstorm). He called it ozone,
after the Greek word ozein, meaning to smell.
Ozone is an irritating, pale blue gas that is toxic
and explosive. Because of its high reactivity, ozone
is an extremely powerful oxidizing agent that
damages rubber and plastics and is harmful to
humans and plants even at low concentrations
(several tens of ppbv). Haagen-Smit
18
suggested
that the formation of O
3
in cities is due to photo-
chemical reactions involving nitrogen oxides and
5.2 “Bad” and “Good” Ozone
Continued on next page
P732951-Ch05.qxd 12/09/2005 09:05 PM Page 165

166 Atmospheric Chemistry
At wavelengths 0.430
m, NO
2
is photolyzed
(5.15)
which is followed quickly by
(5.16)
where j and k
1
are rate coefficients.
19
However, much
of the O
3
produced by (5.16) is removed rapidly by
(5.17)
In combination, (5.15)–(5.17) constitute a null
cycle that neither creates nor destroys ozone.
Exercise 5.5 Derive an expression for the steady-
state concentration of O
3
given by Eqs. (5.15)–(5.17) in
terms of the concentrations of NO and NO
2
, j and k
2
.
Solution: All three reactions are fast: NO is formed
by (5.15) and NO
2
is formed by (5.17) as rapidly as it
is depleted by (5.15). Therefore, from the definition
of a rate coefficient,
where the square brackets indicate species concen-
tration. Therefore,
(5.18)[O
3
]
j
k
2
[NO
2
]
[NO]
j [NO
2
] k
2
[O
3
] [NO]
O
3
NO :
k
2
NO
2
O
2
O O
2
M :
k
1
O
3
M
NO
2
h
:
j
NO O
Expression (5.18) is called the Leighton
20
relationship.
It is an example of a photostationary state relation. ■
If typical concentrations of NO
2
and NO are
substituted into (5.18), together with values for j and
k
2
, the concentrations of O
3
obtained are far below
observed concentrations even in the free troposphere.
Hence, reactions other than (5.15)–(5.17) must be
involved in the control of tropospheric O
3
.This con-
clusion led to the suggestion that HO
x
, and related
radicals derived from organic species, are involved in
determining atmospheric concentrations of O
3
.The
hydroxyl radical can be produced from small amounts
of highly reactive atomic oxygen by (5.6b). Then, in
unpolluted air, OH is rapidly transformed to HO
2
by
(5.19a)
(5.19b)
and, in the absence of NO
x
,HO
2
is mostly con-
verted back to OH through reactions with NO or
O
3
,e.g.,
(5.19c)
In sunlight, a photostationary steady state is
established quickly between OH and HO
2
.HO
x
([OH] [HO
2
]) is lost by
(5.20)2HO
2
: H
2
O
2
O
2
HO
2
O
3
: O
2
OH
H O
2
M : HO
2
M
OH CO : H CO
2
hydrocarbons released by cars and oil refineries.
Ozone reacts with hydrocarbons from automobile
exhausts and evaporated gasoline to form second-
ary organic pollutants such as aldehydes and
ketones. Ozone produced in urban areas can be
transported into rural areas far removed from
industrial regions. For example, during a summer
heat wave in 1988 the Acadia National Park in
Maine had dangerously high concentrations of
5.2 Continued
O
3
, which probably originated in New York City.
Ozone alone, or in combination with SO
2
and
NO
2
, accounts for 90% of the annual loss of
crops due to air pollution in the United States.
In contrast to the bad effects of O
3
in the
troposphere, the much greater concentrations
of O
3
in the stratosphere reduce the intensity
of dangerous UV radiation from the sun (see
Section 5.7.1).
19
It is usual to represent chemical rate coefficients for photolytic reactions, such as (5.15), by j and the rate coefficients for nonphotolytic
reactions, such as (5.16), by k.
20
Philip Albert Leighton (1897–1983) American chemist. Faculty member at Stanford University. Main research interest was photochemistry.
His monograph The Photochemistry of Air Pollution (1961) was particularly influential in promoting research on urban air pollution.
P732951-Ch05.qxd 12/09/2005 09:05 PM Page 166
5.3 Some Important Tropospheric Trace Gases 167
and
(5.21)
Because H
2
O
2
and HNO
3
are highly soluble, they are
removed quickly from the troposphere by wet depo-
sition. The aforementioned reactions, together with
values for the appropriate rate coefficients, suggest
that OH concentrations near the Earth’s surface in
sunlight should be 3 10
12
molecules m
3
, which
is close to the measured concentration of OH during
daylight hours.
In the presence of a sufficiently high mixing
ratio of NO (10 pptv), HO
2
formed by the oxida-
tion of VOCs converts NO to NO
2
, which then
forms O
3
by
(5.22a)
(5.22b)
(5.22c)
(5.22d)
Note that OH and NO are regenerated in cycle
(5.22), and that NO is converted to NO
2
without
consuming O
3
, providing a pathway for net ozone
production. Thus, increases in NO might be expected
to produce increases in O
3
.
The OH radical also oxidizes methane
(5.23)
Then, if the concentration of NO
x
is low,
(5.24)
where CH
3
O
2
is the methylperoxy radical and
CH
3
OOH is methyl hydroperoxide. CH
3
OOH is
removed by wet deposition, resulting in a net loss of
HO
x
. If NO
x
is present, NO
2
from the reaction
(5.25)
leads to O
3
formation by (5.15)–(5.16). These reac-
tions demonstrate the importance of photochemistry
and NO
x
in determining the concentration of O
3
in
the global troposphere, as well as the complexity of
tropospheric ozone chemistry.
Figure 5.6 shows the global distribution of O
3
in the troposphere obtained by subtracting satellite
CH
3
O
2
NO : CH
3
O O
2
HO
2
CH
3
O
2
: CH
3
OOH O
2
OH CH
4
O
2
: H
2
O CH
3
O
2
O O
2
M : O
3
O
2
h
: NO O
HO
2
NO : OH NO
2
OH CO O
2
: HO
2
CO
2
OH NO
2
M : HNO
3
M
measurements of O
3
in the stratosphere from the
total column ozone concentration. It is evident that
O
3
is generally low throughout the year over the
tropical oceans. Over middle latitudes O
3
exhibits
pronounced seasonal variations, with a pronounced
spring maximum. Over industrialized regions of the
northern hemisphere ozone concentrations also tend
to be high during summer.
Dobson Units (DU)
December–February
40N
30N
20N
10N
EQ
20S
10S
30S
40S
180 120W 60W 0 60E 120E 180
March–May
40N
30N
20N
10N
EQ
20S
10S
30S
40S
180 120W 60W 0 60E 120E 180
June–August
40N
30N
20N
10N
EQ
20S
10S
30S
40S
180 120W 60W 0 60E 120E 180
September–November
40N
30N
20N
10N
EQ
20S
10S
30S
40S
180 120W 60W 0 60E 120E 180
15 20 25 30 35 40 45 50
Fig. 5.6 Composite seasonal distribution of the tropospheric
ozone column (in Dobson units) determined from satellite
measurements from 1979 to 2000. [From Atmos. Chem. Phys. 3,
895 (2003).]
P732951-Ch05.qxd 12/09/2005 09:05 PM Page 167

168 Atmospheric Chemistry
An increase in tropospheric O
3
has occurred glob-
ally over the past century, from 10–15 ppbv in the
preindustrial era to 30–40 ppbv in 2000 in remote
regions of the world. The increase is attributable to
the increase in NO
x
emissions associated with the
rapid increase in the use of fossil fuels since the
industrial revolution.
5.3.6 Hydrogen Compounds
Hydrogen compounds are the most important oxi-
dants for many chemicals in the atmosphere and are
involved in the cycles of many chemical families.
Hydrogen compounds include atomic hydrogen (H),
which is very short-lived because it combines quickly
with O
2
to form the hydroperoxyl radical (HO
2
);
molecular hydrogen (H
2
), which, next to CH
4
,is
the most abundant reactive trace gas in the tropo-
sphere; the hydroxyl radical OH (see Section 5.3.1);
the hydroperoxyl radical HO
2
; hydrogen peroxide
(H
2
O
2
), which is formed by the reaction of HO
2
radicals and is an important oxidant for SO
2
in
cloud droplets; and H
2
O, which plays multivarious
roles in atmospheric chemistry, including its reac-
tion with excited atomic oxygen to form OH (reac-
tion 5.6b).
The primary source of odd hydrogen species
(HO
x
), in the form of OH, in the lower troposphere
where water vapor is abundant, is (5.7). Another
source of HO
x
is the photolysis of formaldehyde
(5.26)
followed by (5.19b) and
(5.27)
both of which produce HO
2
.The simplest loss mech-
anisms for HO
x
are of the form (5.20), and
(5.28)
HO
x
and NO
x
can react to produce O
3
(see
Section 5.3.5). Consequently, there is considerable
interest in HO
x
chemistry in the upper troposphere
where NO
x
is emitted in significant quantities by jet
aircraft. Cycling between OH and HO
2
occurs on
a timescale of a few seconds and is controlled by
OO
2
: H
2
O O
2
CO O
2
: HO
2
CO
CHO h
: H HCO
reactions (5.22). These reactions account for 80%
of the measured concentrations of HO
x
in the upper
troposphere. NO
x
controls the cycling within HO
x
that leads to O
3
production. NO
x
also regulates the
loss of HO
x
through reactions of OH with HO
2
,NO
2
,
and HO
2
NO
2
.
5.3.7 Sulfur Gases
Sulfur is assimilated by living organisms and is then
released as an end product of metabolism. The most
important sulfur gases in the atmosphere are SO
2
,
H
2
S, dimethyl sulfide (CH
3
SCH
3
or DMS for short),
COS, and carbon disulfide (CS
2
).
Sulfur compounds exist in both reduced and oxi-
dized states, with oxidation numbers from 2 to 6.
After being emitted into the Earth’s oxidizing atmos-
phere, the reduced sulfur compounds (most of which
are biogenic in origin) are generally oxidized to the
4 oxidation state of SO
2
[i.e., S(IV)] and eventually
to the 6 oxidation state of H
2
SO
4
[i.e., S(VI)]. The
6 oxidation state is the stable form of sulfur in
the presence of oxygen. The oxidation of sulfur
compounds illustrates an effect that often applies to
other compounds, namely the more oxidized species
generally have a high affinity for water (e.g., sulfuric
acid). Consequently, the more oxidized species are
removed more readily from the atmosphere by wet
processes.
The principal natural sources of SO
2
are the oxida-
tion of DMS and H
2
S. For example,
(5.29a)
The HS then reacts with O
3
or NO
2
to form HSO
(5.29b)
(5.29c)
The HSO is then converted rapidly to SO
2
by
(5.29d)
(5.29e)
Volcanoes and biomass burning are also sources of
atmospheric SO
2
. However, the largest source of SO
2
is fossil fuel combustion (see Fig. 5.15 for sources and
sinks of sulfur compounds).
HSO
2
O
2
: HO
2
SO
2
HSO O
3
: HSO
2
O
2
HS NO
2
: HSO NO
HS O
3
: HSO O
2
H H
2
S : H
2
O HS
P732951-Ch05.qxd 12/09/2005 09:05 PM Page 168
5.4 Tropospheric Aerosols 169
In the gas phase, SO
2
is oxidized by
(5.30a)
(5.30b)
(5.30c)
SO
2
is also oxidized to H
2
SO
4
in cloud water (see
Section 6.8.5).
The main sources of H
2
S are emissions from soils,
marshlands, oceans, and volcanoes. The only signifi-
cant sink for H
2
S is oxidation to SO
2
by (5.29).
Biological reactions in the oceans, involving
phytoplankton, emit several sulfur gases, of which
DMS has the largest emission rate. DMS is
removed from the atmosphere primarily by its
reaction with OH to produce SO
2
.The sulfur gas
with the largest concentration in the unpolluted
atmosphere is COS (0.5 ppbv). The major sources
of COS are biogenic and the oxidation of CS
2
by
OH; the source of CS
2
is also biogenic. Because
COS is very stable in the troposphere, it is eventu-
ally transported into the stratosphere where it is
the dominant source of sulfate particles during the
SO
3
H
2
O : H
2
SO
4
SO
2
O
2
: HO
2
SO
3
H SO
2
M : HOSO
2
M
Aitken
nuclei
Large
particles
Giant
particles
Cloud and precipitation
physics
Atmospheric electricity
Large
ions
Small
ions
Atmospheric
radiation
and optics
Air chemistry
(including
air pollution)
Particle diameter (
µ m)
10
–4
10
–3
10
–2
10
–1
10
0
10
1
10
2
10
3
Fig. 5.7 Size range of particles in the atmosphere and their
importance.
quiescent periods between large volcanic eruptions
(see Section 5.7.3).
5.4 Tropospheric Aerosols
Atmospheric aerosols are suspensions of small solid
andor liquid particles (excluding cloud particles) in
air that have negligible terminal fall speeds. Figure 5.7
shows the ranges of particle sizes that play a role in
the atmosphere.
Molecular aggregates that carry an electric charge
are called ions.The number density and type of
ions in the air determine the electrical conductiv-
ity of the air, which, in turn, affects the magnitude
of the fair weather atmospheric electric field. Ions
in the lower atmosphere are produced primarily
by cosmic rays, although very close to the Earth’s
surface ionization due to radioactive materials in
the Earth and atmosphere also plays a role. Ions
are removed by combining with ions of opposite
sign. Small ions, which are not much larger than
molecular size, have electrical mobilities (defined
as their velocity in a unit electric field) between
1 and 2 10
4
m s
1
for an electric field of
1Vm
1
at normal temperature and pressure
(NTP). Large ions have electrical mobilities in
the range from 3 10
8
to 8 10
7
m s
1
in a
field of 1 V m
1
at NTP. Concentrations of small
ions vary from about 40 to 1500 cm
3
at sea level,
whereas concentrations of large ions vary from
about 200 cm
3
in marine air to a maximum value
of about 8 10
5
cm
3
in some cities. It can be seen
from these numbers that the electrical conductiv-
ity of the air (which is proportional to the product
of the ion mobility and the ion concentration) is
generally dominated by small ions. However, when
the concentrations of large ions and uncharged
aerosols are large, as they are in cities, the concen-
tration of small ions tends to be low due to their
capture by both large ions and uncharged aerosols.
Consequently, the electrical conductivity of air is a
minimum (and the fair weather atmospheric elec-
tric field a maximum) when the concentration of
large ions, and similarly sized uncharged particles,
is a maximum. The observed decrease of at least
20% in the electrical conductivity of the air over
the north Atlantic Ocean during the 20th century
is attributed to a doubling in the concentration of
particles with diameters between 0.02 and 0.2
m,
probably due to pollution.
5.3 Charged Particles
P732951-Ch05.qxd 12/09/2005 09:05 PM Page 169
170 Atmospheric Chemistry
The effects of aerosols on the scattering and absorp-
tion of radiation have been discussed in Section 4.4.
The role that aerosols play in the formation of cloud
particles is discussed in Chapter 6. Here we are con-
cerned with tropospheric aerosols, in particular with
their sources, transport, sinks, properties, and with
their roles in tropospheric chemistry.
5.4.1 Sources
a. Biological
Solid and liquid particles are released into the atmos-
phere from plants and animals. These emissions,
which include seeds, pollen, spores, and fragments of
animals and plants, are usually 1–250
m in diameter.
Bacteria, algae, protozoa, fungi, and viruses are gen-
erally 1
m in diameter. Some characteristic con-
centrations are: maximum values of grassy pollens
200 m
3
; fungal spores (in water) 100–400 m
3
;
bacteria over remote oceans 0.5 m
3
; bacteria in
New York City 80–800 m
3
; and bacteria over
sewage treatment plants 10
4
m
3
. Microorganisms
live on skin: when you change your clothes, you can
propel 10
4
bacteria per minute into the air, with
diameters from 1 to 5
m.
The oceans are one of the most important sources
of atmospheric aerosols [1000–5000 Tg per year,
although this includes giant particles (2–20
m
diameter) that are not transported very far]. Just
above the ocean surface in the remote marine atmos-
phere, sea salt generally dominates the mass of both
supermicrometer and submicrometer particles.
The major mechanism for ejecting ocean materials
into the air is bubble bursting (some materials enter
the air in drops torn from windblown spray and foam,
but because these drops are relatively large, their
residence times in the air are very short). Aerosols
composed of sea salt originate from droplets ejected
into the air when air bubbles burst at the ocean
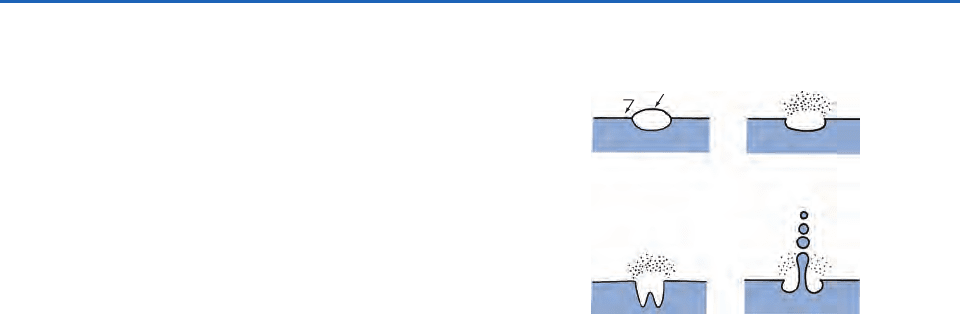
surface (Fig. 5.8). Many small droplets are produced
when the upper portion of an air bubble film bursts;
these are called film droplets (Fig. 5.8b). Bubbles
2 mm in diameter each eject 100–200 film
droplets into the air. After evaporation, the film
droplets leave behind sea-salt particles with diame-
ters less than 0.3
m. From one to five larger drops
break away from each jet that forms when a bubble
bursts (Fig. 5.8d), and these jet drops are thrown
about 15 cm up into the air. Some of these drops
subsequently evaporate and leave behind sea-salt
particles with diameters 2
m. The average rate
of production of sea-salt particles over the oceans
is 100 cm
2
s
1
.
Hygroscopic salts [NaCl (85%), KCl, CaSO
4
,
(NH
4
)
2
SO
4
] account for 3.5% of the mass of sea-
water. These materials are injected into the atmos-
phere by bubble bursting over the oceans. In
addition, organic compounds and bacteria in the sur-
face layers of the ocean are transported to the air by
bubble bursting.
Dry sea-salt particles will not form solution droplets
until the relative humidity exceeds 75%. Ambient
gases (e.g., SO
2
and CO
2
) are taken up by these
droplets, which changes the ionic composition of
the droplets. For example, the reaction of OH(g)
with sea-salt particles generates OH
(aq) in the
droplets, which leads to an increase in the production
of (aq) by aqueous-phase reactions and a
reduction in the concentration of Cl
(aq). Conse-
quently, the ratio of Cl to Na in sea-salt particles
collected from the atmosphere is generally much less
than in seawater itself. The excess of (aq) over
that of bulk seawater is referred to as non-sea-salt
sulfate (nss).
The oxidation of Br
(aq) and Cl
(aq) in solutions
of sea-salt particles can produce BrO
x
and ClO
x
species. Catalytic reactions involving BrO
x
and ClO
x
,
similar to those that occur in the stratosphere
(see Section 5.7.2), destroy O
3
.This mechanism has
SO
2
4
SO
2
4
Surface
of
water
Air
bubble
Film
droplets
(a) (b)
Jet
drops
(d)(c)
Fig. 5.8 Schematics to illustrate the manner in which film
droplets and jet drops are produced when an air bubble
bursts at the surface of water. Over the oceans some of
the droplets and drops evaporate to leave sea-salt particles
and other materials in the air. The time between (a) and (d)
is 2 ms. The film droplets are 5–30
m diameter before
evaporation. The size of the jet drops are 15% of the diam-
eter of the air bubble.
P732951-Ch05.qxd 12/09/2005 09:05 PM Page 170

5.4 Tropospheric Aerosols 171
been postulated to explain the depletion of O
3
,
from 40 to 0.5 ppbv, that occurs episodically over
periods of hours to days in the Arctic boundary layer
starting at polar sunrise and continuing through
April.
Smoke from forest fires is a major source of
atmospheric aerosols. Small smoke particles (prima-
rily organic compounds and elemental carbon) and
fly ash are injected directly into the air by forest fires.
Several million grams of particles can be released
by the burning of 1 hectare (10
4
m
2
). It is estimated
that about 54 Tg of particles (containing 6 Tg of
elemental carbon) are released into atmosphere each
year by biomass burning. The number distribution of
particles from forest fires peak at 0.1
m diameter,
which makes them efficient cloud condensation
nuclei. Some biogenic particles (e.g., bacteria from
vegetation) may nucleate ice in clouds.
b. Solid Earth
The transfer of particles to the atmosphere from the
Earth’s surface is caused by winds and atmospheric
turbulence. To initiate the motion of particles on the
Earth’s surface, surface wind speeds must exceed cer-
tain threshold values, which depend on the size of the
particle and the type of surface. The threshold values
are at least 0.2 m s
1
for particles 50–200
m in
diameter (smaller particles adhere better to the
surface) and for soils containing 50% clay or tilled
soils. To achieve a frictional speed of 0.2 m s
1
requires a wind speed of several meters per second
a few meters above ground level. A major source of
smaller (10–100
m diameter) particles is saltation,
in which larger sand grains become airborne, fly a
few meters, and then land on the ground, creating a
burst of dust particles.
On the global scale, semiarid regions and deserts
(which cover about one-third of the land surface) are
the main source of particles from the Earth’s surface.
They provide 2000 Tg per year of mineral particles.
Dust from these sources can be transported over
long distances (see Section 5.4.3).
Volcanoes inject gases and particles into the
atmosphere. The large particles have short residence
times, but the small particles (produced primarily by
gas-to-particle (g-to-p) conversion of SO
2
) can be
transported globally, particularly if they reach high
altitudes. Volcanic emissions play an important role
in stratospheric chemistry (see Section 5.7.3).
c. Anthropogenic
The global input of particles into the atmosphere
from anthropogenic activities is 20% (by mass) of
that from natural sources. The main anthropogenic
sources of aerosols are dust from roads, wind erosion
of tilled land, biomass burning, fuel combustion, and
industrial processes. For particles with diameters
5
m, direct emissions from anthropogenic sources
dominate over aerosols that form in the atmosphere
by g-to-p conversion (referred to as secondary parti-
cles) of anthropogenic gases. However, the reverse is
the case for most of the smaller particles, for which
g-to-p conversion is the over-whelming source of the
number concentration of anthropogenically derived
aerosols.
In 1997 the worldwide direct emission into the
atmosphere of particles 10
m diameter from
anthropogenic sources was estimated to be 350 Tg
per year (excluding g-to-p conversion). About 35%
of the number concentration of aerosols in the
atmosphere was sulfate, produced by the oxidation
of SO
2
emissions. Particle emissions worldwide
were dominated by fossil fuel combustion (prima-
rily coal) and biomass burning. These emissions are
projected to double by the year 2040, due largely
to anticipated increases in fossil fuel combustion,
with the greatest growth in emissions from China
and India.
During the 20th century, the emission of particles
into the atmosphere from anthropogenic sources was
a small fraction of the mass of particles from natural
sources. However, it is projected that by 2040 anthro-
pogenic sources of particles could be comparable to
those from natural processes.
d. In situ formation
In situ condensation of gases (i.e., g-to-p conversion)
is important in the atmosphere. Gases may condense
onto existing particles, thereby increasing the mass
(but not the number) of particles, or gases may
condense to form new particles. The former path is
favored when the surface area of existing particles
is high and the supersaturation of the gases is low.
If new particles are formed, they are generally
0.01
m diameter. The quantities of aerosols pro-
duced by g-to-p conversion exceed those from direct
emissions for anthropogenically derived aerosols and
are comparable to direct emission in the case of
naturally derived aerosols.
P732951-Ch05.qxd 12/09/2005 09:05 PM Page 171

172 Atmospheric Chemistry
Three major families of chemical species are
involved in g-to-p conversion: sulfur, nitrogen, and
organic and carbonaceous materials. Various sulfur
gases (e.g., H
2
S, CS
2
, COS, DMS) can be oxidized to
SO
2
.The SO
2
is then oxidized to sulfate ( ), the
dominant gas phase routes being reactions (5.30).
However, on a global scale, heterogeneous reactions
of SO
2
in cloud water dominate the conversion of
SO
2
to (see Section 6.8.9).
Over the oceans, sulfates derived from DMS con-
tribute to the growth of existing particles. Sulfates
are also produced in and around clouds, and nitric
acid can form from N
2
O
5
in cloud water.
Subsequent evaporation of cloud water releases
these sulfate and nitrate particles into the air (see
Section 6.8).
Organic and carbonaceous aerosols are produced
by g-to-p conversion from gases released from the
biosphere and from volatile compounds such as crude
oil that leak to the Earth’s surface. Carbonaceous
particles emitted directly into the atmosphere derive
mainly from biomass fires.
e. Summary
Table 5.3 summarizes estimates of the magnitudes of
the principal sources of direct emission of particles
into the atmosphere and in situ sources.
Anthropogenic activities emit large numbers of
particles into the atmosphere, both directly and
through g-to-p conversion (see Sections 5.4.1c and
5.4.1d). For particles 5
m diameter, human acti-
vities worldwide are estimated to produce 15%
of natural emissions, with industrial processes, fuel
combustion, and g-to-p conversion accounting for
80% of the anthropogenic emissions. However, in
urban areas, anthropogenic sources are much more
important. For particles 5
m diameter, human
activities produce 20% of natural emissions, with
g-to-p conversion accounting for 90% of the human
emissions.
5.4.2 Chemical Composition
Except for marine aerosols, the masses of which are
dominated by sodium chloride, sulfate is one of the
prime contributors to the mass of atmospheric
aerosols. The mass fractions of range from
22–45% for continental aerosols to 75% for
aerosols in the Arctic and Antarctic. Because the
sulfate content of the Earth’s crust is too low to
SO
2
4
SO
2
4
SO
2
4
explain the large percentages of sulfate in atmos-
pheric aerosols, most of the must derive from
g-to-p conversion of SO
2
.The sulfate is contained
mainly in submicrometer particles.
Ammonium is the main cation associated
with in continental aerosol. It is produced by
gaseous ammonia neutralizing sulfuric acid to pro-
duce ammonium sulfate [(NH
4
)
2
SO
4
]—see reaction
SO
2
4
(NH
4
)
SO
2
4
Table 5.3 Estimates (in Tg per year) for the year 2000 of
(a) direct particle emissions into the atmosphere and (b) in situ
production
(a) Direct emissions
Northern Southern
hemisphere hemisphere
Carbonaceous aerosols
Organic matter (0–2
m)
a
Biomass burning 28 26
Fossil fuel 28 0.4
Biogenic (1
m) — —
Black carbon (0–2
m)
Biomass burning 2.9 2.7
Fossil fuel 6.5 0.1
Aircraft 0.005 0.0004
Industrial dust, etc. (1
m)
Sea salt
1
m2331
1–16
m1,420 1,870
Total1,440 1,900
Mineral (soil) dust
1
m9017
1–2
m 240 50
2–20
m1,470 282
Total1,800 349
(b) In situ
Northern Southern
hemisphere hemisphere
Sulfates (as NH
4
HSO
4
) 145 55
Anthropogenic 106 15
Biogenic 25 32
Volcanic 14 7
Nitrate (as )
Anthropogenic 12.4 1.8
Natural 2.2 1.7
Organic compounds
Anthropogenic 0.15 0.45
Biogenic 8.2 7.4
a
Sizes refer to diameters. [Adapted from Intergovernmental Panel on Climate
Change, Climate Change 2001, Cambridge University Press, pp. 297 and 301, 2001.]
NO
3
P732951-Ch05.qxd 12/09/2005 09:05 PM Page 172

5.4 Tropospheric Aerosols 173
(5.13). The ratio of the molar concentrations of
to ranges from 1 to 2, corresponding to an
aerosol composition intermediate between that for
NH
4
HSO
4
and (NH
4
)
2
SO
4
.The average mass frac-
tions of submicrometer non sea-salt sulfates plus
associated range from 16 to 54% over large
regions of the world.
In marine air the main contributors to the mass
of inorganic aerosols are the ions Na
,Cl
,Mg
2
,
,K
, and Ca
2
. Apart from , these com-
pounds are contained primarily in particles a few
micrometers in diameter because they originate from
sea salt derived from bubble bursting (see Fig. 5.8).
Sulfate mass concentrations peak for particles with
diameters 0.1–1
m. Particles in this size range are
effective in scattering light (and therefore reducing
visibility) and as cloud condensation nuclei.
Nitrate occurs in larger sized particles
than sulfate in marine air. Because seawater con-
tains negligible nitrate, the nitrate in these particles
must derive from the condensation of gaseous
HNO
3
, possibly by g-to-p conversion in the liquid
phase.
Nitrate is also common in continental aerosols,
where it extends over the diameter range 0.2–20
m.
It derives, in part, from the condensation of HNO
3
onto larger and more alkaline mineral particles.
Organic compounds form an appreciable fraction
of the mass of atmospheric aerosols. The most abun-
dant organics in urban aerosols are higher molecular
weight alkanes (C
x
H
2x 2
), 1000–4000 ng m
3
, and
alkenes (C
x
H
2x
), 2000 ng m
3
. Many of the parti-
cles in urban smog are by-products of photochemical
reactions involving hydrocarbons and nitrogen oxides,
which derive from combustion. In the United States,
carbonaceous materials can account for 50% or
more of the total dry aerosol mass.
Elemental carbon (commonly referred to as
“soot”), a common component of organic aerosols
in the atmosphere, is a strong absorber of solar
radiation. For example, in polluted air masses from
India, elemental carbon accounts for 10% of the
mass of submicrometer sized particles.
5.4.3 Transport
Aerosols are transported by the airflows they
encounter during the time they spend in the atmos-
phere. The transport can be over intercontinental,
even global, scales. Thus, Saharan dust is transported
to the Americas, and dust from the Gobi Desert
(NO
3
)
SO
2
4
SO
2
4
NH
4
SO
2
4
NH
4
can reach the west coast of North America. If the
aerosols are produced by g-to-p conversion, long-
range transport is likely because the time required
for g-to-p conversion and the relatively small sizes
of the particles produced by this process lead to long
residence times in the atmosphere. This is the case
for sulfates that derive from SO
2
blasted into the
stratosphere by large volcanic eruptions. It is also
the case for acidic aerosols such as sulfates and
nitrates, which contribute to acid rain.Thus, SO
2
emitted from power plants in the United Kingdom
can be deposited as sulfate far inland in continental
Europe.
5.4.4 Sinks
On average, particles are removed from the atmos-
phere at about the same rate as they enter it.
Small particles can be converted into larger parti-
cles by coagulation. Because the mobility of a parti-
cle decreases rapidly as it increases in size,
coagulation is essentially confined to particles less
than 0.2
m in diameter. Although coagulation
does not remove particles from the atmosphere, it
modifies their size spectra and shifts small particles
into size ranges where they can be removed by
other mechanisms.
Exercise 5.6 If the rate of decrease in the number
concentration N of a monodispersed (i.e., all parti-
cles of the same size) aerosol due to coagulation
is given by dNdt KN
2
,where K is a constant
(assume K 1.40 10
15
m
3
s
1
for 0.10-
m-
diameter particles at 20 °C and 1 atm), determine
the time required at 20 °C and 1 atm for coagulation
to decrease the concentration of a monodispersed
atmospheric aerosol with particles of a diameter of
0.100
m to one-half of its initial concentration of
1.00 10
11
m
3
.
Solution: Because dNdt KN
2
where N
0
and N are the number concentrations of
particles at time t 0 and time t, respectively.
Therefore,
1
N
N
N
0
Kt
N
N
0
dN
N
2
K
t
0
dt
P732951-Ch05.qxd 12/09/2005 09:05 PM Page 173
