Wallace J.M., Hobbs P.V. Atmospheric Science. An Introductory Survey
Подождите немного. Документ загружается.


154 Atmospheric Chemistry
in 1774. It was named oxygen [from the Greek
oxus (acid) and genan (to beget)] by Lavoisier.
6
The
role of oxygen in the Earth system was discussed in
Section 2.4.
Oxygen occupies 20.946% by volume of dry air.
“Foul air” (now called nitrogen
8
) occupies 78.084%.
The next two most abundant gases in air are argon
(0.934%) and carbon dioxide
9
(0.03%). Together
these four gases account for 99.99% of the volume of
air. Many of the remaining minute amounts of the
many other gases in air (some of which are listed in
Table 5.1) are of prime importance in atmospheric
chemistry because of their reactivity.
The most common unit for expressing the quantity
of a gas in air is the fraction of the total volume of
air that the gas occupies. The volumes occupied by dif-
ferent gases at the same temperature and pressure are
proportional to the numbers of molecules of the
respective gases [see Eq. (3.6)]. Also, for a mixture of
ideal gases (such as air) the partial pressure exerted by
a gas is proportional to the mole fraction of the gas
in the mixture. For example, if CO
2
occupies 0.04%
of the volume of air, the fraction of the total number of
molecules in air that are CO
2
(i.e., the mole fraction of
CO
2
) is 0.04% and, if the total air pressure is 1 atm,
the partial pressure exerted by CO
2
is 0.04% of 1 atm.
Exercise 5.1 N
2
O occupies 310 ppbv of air, how
many N
2
O molecules are there in 1 m
3
of air at 1 atm
and 0 °C?
Solution: We need to calculate first the number of
molecules in 1 m
3
of any gas (or mixture of gases
such as air) at 1 atm and 0 °C (called Loschmidt’s
10
number).
From (3.8), p n
0
kT where n
0
is the number of
molecules in 1 m
3
. Substituting p 1 atm 1.013
10
5
Pa, T 273 K, and k 1.381 10
23
JK
1
molecule
1
into this expression yields Loschmidt’s
number
Because the volumes occupied by gases at the same
temperature and pressure are proportional to the
numbers of molecules of the gases,
The left side of this relation is equal to 310 ppbv
310 10
9
.Therefore, the number of N
2
O mole-
cules in 1 m
3
of air 310 10
9
n
0
(310 10
9
)
(2.687 10
25
) 8.33 10
18
. ■
Number of N
2
O molecules per m
3
Total number of molecules per m
3
(n
0
)
Volume occupied by N
2
O molecules
Volume occupied by air
2.687 10
25
molecules m
3
n
0
1.013 10
5
(1.381 10
23
) 273
6
Antoine-Laurent Lavoisier (1743–1794) French chemist. Father of modern chemistry. His early studies included the best means
for lighting large cities, analysis of gypsum, thunder, and the aurora. Confirmed that oxygen is absorbed by burning. Confirmed
Cavendish’s
7
conclusion that water is formed by the combustion of hydrogen and oxygen. Recognized some 30 elements and proposed
that compounds be named after the elements they contained. Beheaded in the French Terror. (“La république n’a pas besoin des
savants.”)
Lavoisier thought that all acids contain oxygen. It is now known that many acids do not contain oxygen (e.g., hydrochloric acid, HCl)
but they all contain hydrogen.
7
Henry Cavendish (1731–1810) English chemist and physicist. Perfected the technique of collecting gases above water. Discovered
“flammable air,” called oxygen by Lavoisier. Used a sensitive torsion balance to measure the gravitational constant (G), from which he
calculated the mass of the Earth. Terrified of women, with whom he communicated only by letter.
8
The Scottish chemist Daniel Rutherford (1749–1819) is attributed with the discovery of nitrogen (1772), which he called “phlogisti-
gated air.”
9
Carbon dioxide was discovered in 1750 by the Scottish physicist Joseph Black (1728–1799). Black is also known for his work on
melting and evaporation, which led to the concept of latent heats and specific heats.
10
Joseph Loschmidt (1821–1895) Czech physicist and chemist. Son of a poor Bohemian farmer. Moved to Vienna at age 20, where he
attended lectures in chemistry and physics at the Polytechnic Institute. Considered becoming a settler in the new state of Texas, but instead
started a company in Vienna to produce potassium nitrate. In 1856 became a high school teacher in Vienna. It was during this period that
he became the first person to use the kinetic theory of gases to obtain an estimate for the diameter of a molecule. Proposed the first struc-
tural chemical formulae for many molecules, including markings for double and triple carbon bonds. Appointed to a faculty position at the
University of Vienna in 1866. What is called “Avogadro’s number” in English textbooks is, in German-speaking countries, called
“Loschmidt’s number.”
P732951-Ch05.qxd 12/09/2005 09:05 PM Page 154

5.1 Composition of Tropospheric Air 155
Table 5.1 Some gases in dry tropospheric air at a pressure of 1 atm
Fraction of volume
Chemical of air occupied Residence time
Gas formula by the species
a
(or lifetime)
b
Major sources
Nitrogen N
2
78.084% 1.6 10
7
years Biological
Oxygen O
2
20.946% 3000–4000 years Biological
Argon Ar 0.934% — Radiogenic
Carbon dioxide CO
2
379 ppmv
c
3–4 years
d
Biological, oceanic,
combustion (concentration
increasing)
Neon Ne 18.18 ppmv — Volcanic (?)
Helium He 5.24 ppmv — Radiogenic
Methane
e
CH
4
1.7ppmv 9 years Biological, anthropogenic
Hydrogen H
2
0.56 ppmv 2 years Biological, anthropogenic
Nitrous oxide N
2
O 0.31 ppmv 150 years Biological, anthropogenic
Carbon monoxide CO 40–200 ppbv 60 days Photochemical, combustion,
anthropogenic
Ozone O
3
10–100 ppbv Days–weeks Photochemical
Nonmethane — 5–20 ppbv Variable Biological,
hydrocarbons (NMHC)
e
anthropogenic
Halocarbons — 3.8 ppbv Variable Mainly anthropogenic
Hydrogen peroxide H
2
O
2
0.1–10 ppbv 1 day Photochemical
Formaldehyde HCHO 0.1–1 ppbv 1.5h Photochemical
Nitrogen species NO
y
10 pptv–1 ppmv Variable Soils, anthropogenic, lightning
(NO NO
2
( NO
X
)
NO
3
N
2
O
5
HNO
3
PAN)
Ammonia NH
3
10 pptv–1 ppbv 2–10 days Biological
Sulfur dioxide SO
2
10 pptv–1 ppbv Days Photochemical, volcanic,
anthropogenic
Dimethyl sulfide (DMS) CH
3
SCH
3
10–100 pptv 0.7 days Biological, oceanic
Hydrogen sulfide H
2
S 5–500 pptv 1–5 days Biogenic, volcanic
Carbon disulfide CS
2
1–300 pptv 120 h Biological, anthropogenic
Hydroxyl radical
f
OH 0–0.4 pptv 1s Photochemical
Hydroperoxyl radical
f
HO
2
0–5 pptv — Photochemical
a
In addition to percentage by volume, the units used are parts per million by volume (ppmv), 10
6
; parts per billion by volume (ppbv), 10
9
; and parts per trillion
by volume (pptv), 10
12
.
b
See Box 5.1.
c
See Fig. 1.3.
d
This is the average time a CO
2
molecule is in the atmosphere before it is taken up by plants or dissolved in the ocean. However, the time required for atmospheric
CO
2
to adjust to a new equilibrium if its sources or sinks were changed is 50–200 years (see Sections 2.3 and 10.4.1).
e
Hydrocarbons other than CH
4
are referred to as nonmethane hydrocarbons (NMHC). They originate from fossil fuel combustion, biomass burning, forest vegetation, etc.
As is the case for CH
4
, the primary sink for most NMHC is oxidation by OH. Because NMHC are more reactive than CH
4
, their atmospheric residence times are much
shorter (hours to months). Also, unlike CH
4
, NMHC contribute significantly to O
3
formation in urban and regional smogs (see Section 5.5.2). For these reasons, it has
been traditional to separate CH
4
from NMHC. In addition to CH
4
and NMHC, a variety of organic compounds are important in tropospheric chemistry. These other
organics include volatile organic compounds (VOCs) such as carbonyls, organic sulfur compounds, and alcohols.
f
Radicals (sometimes called free radicals) are chemical species with an unpaired electron in their outer (valence) shell. Consequently, a radical has an odd number of total
electrons
g
(e.g., OH has 8 1 9 electrons). The unpaired electrons make radicals more reactive than nonradicals.
g
Important exceptions are atomic oxygen in its ground state [O(
3
P) in spectroscopic notation] and in its excited state [O(
1
D), or O* as we will indicate it], which,
despite the fact that each has 8 electrons, is very reactive.
P732951-Ch05.qxd 12/09/2005 09:05 PM Page 155
156 Atmospheric Chemistry
If the globally averaged concentration of a trace
constituent in the atmosphere does not change
significantly over a given time period, the rate
at which the constituent is injected into (and/or
produced within) the atmosphere must equal the
rate at which it is removed from the atmosphere.
Under such steady-state conditions, we can define
the residence time (or lifetime)
of a trace con-
stituent in the atmosphere as
(5.1)
where M is the amount of the constituent in the
atmosphere (in kg) and F is the rate of its removal
(in kg s
1
) from the atmosphere.
The following analogy may be helpful in under-
standing the concept of residence time. Suppose
a tank is full of water and is over-flowing at its
top due to water being pumped into the bottom
of the tank at a rate F.Then the rate of removal
of water from the tank is F. If we assume that the
M
F
water entering the bottom of the tank steadily
displaces the water above it by pushing it upward
without any mixing, the time spent by each small
element of water that enters the bottom of the
tank before it overflows at the top of the tank is
MF,where M is the volume of the tank in anal-
ogy with (5.1).
Although each atmospheric constituent can be
assigned a residence time in accordance with (5.1),
the residence times of individual molecules of
that constituent vary widely, especially if the
removal processes tend to be locally concen-
trated. Furthermore, residence time, defined in
this manner, does not always give a representative
idea of how long it would take for the atmospheric
concentration of a species to react to an abrupt
change in the source. For example, CO
2
has a
residence time of only a few years in the atmos-
phere, but a much slower adjustment time (see
Section 2.3.2).
In the atmosphere, the very stable gas nitrogen
has a residence time of 10
7
years. In contrast,
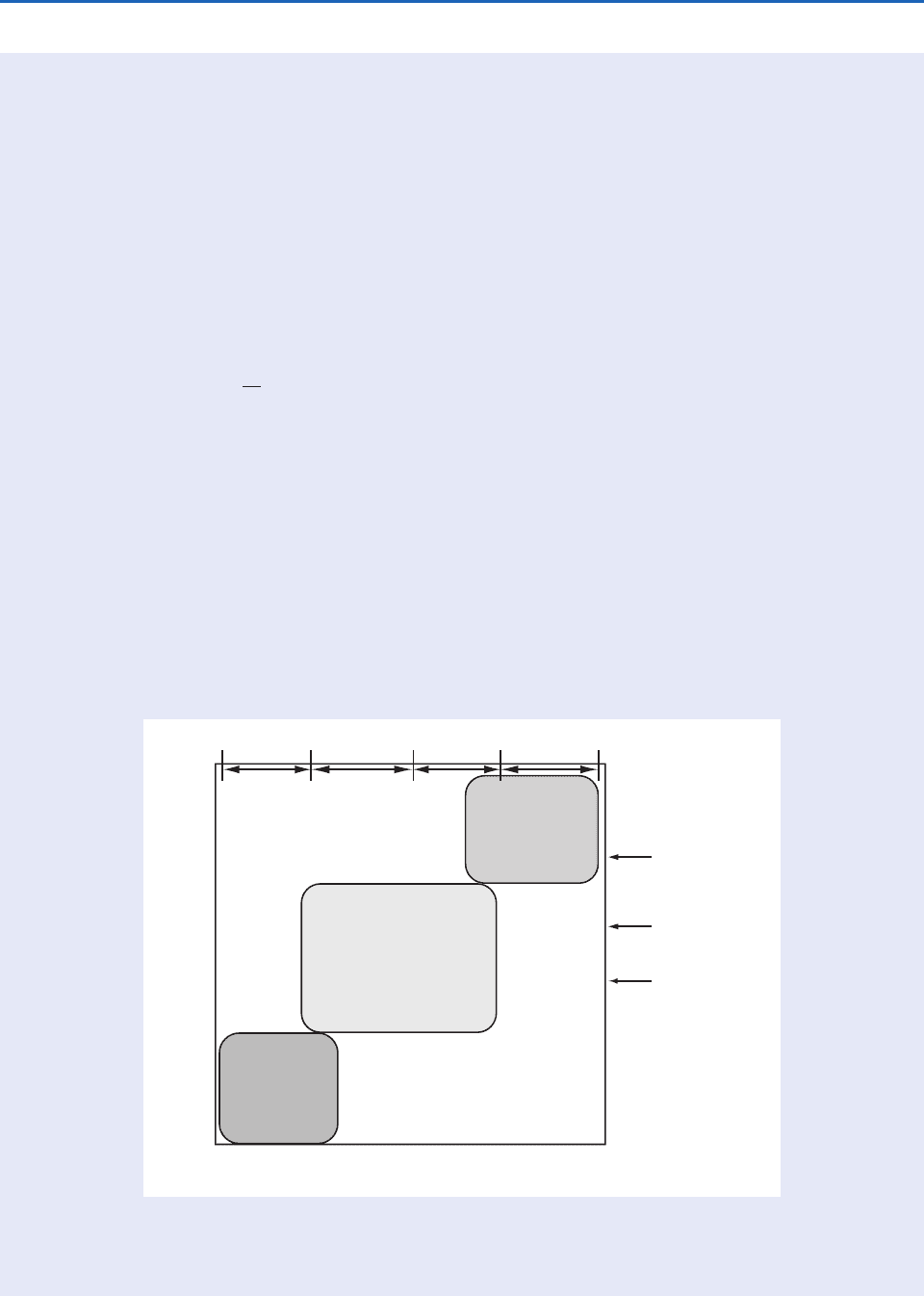
5.1 Residence Time and Spatial Scales of Variation of Chemicals in the Atmosphere
Micro-
scale
Urban or
local scale
Regional or
mesoscale
Synoptic or
global scale
Inter-hemispheric
mixing time
Intra-hemispheric
mixing time
Boundary layer
mixing time
Long-lived
species
Moderately long-
lived species
Short-lived
species
Residence time
100 yr
10 yr
1 yr
1 day
1 hr
100 s
1 s
Spatial scale of variability (m)
·CFC's
·N
2
O
·CH
4
·CH
3
CCl
3
·CH
3
Br
·CO
·Aerosols
·Trop O
3
·H
2
O
·SO
2
·H
2
O
2
·NO
x
·DMS
·C
3
H
6
·C
5
H
8
·CH
3
O
2
·HO
2
·NO
3
·OH
11010
2
10
3
10
4
10
5
10
6
10
7
Fig. 5.1 Spatial and temporal scales of variability for some atmospheric constituents. The temporal scale is represented
by residence time. [Adapted with permission from The Atmospheric Sciences Entering the Twenty-First Century, United States
National Academy Press, 1998, p. 137.]
Continued on next page
P732951-Ch05.qxd 12/09/2005 09:05 PM Page 156

5.2 Sources, Transport, and Sinks of Trace Gases 157
5.2 Sources, Transport, and
Sinks of Trace Gases
5.2.1 Sources
The major natural sources of gases in the troposphere
are biogenic, the solid Earth, the oceans, and in situ
formation. These sources are discussed, in turn, next.
a. Biogenic
As discussed in Sections 2.3 and 2.4, oxygen in the
Earth’s present atmosphere was liberated by biologi-
cal activity starting about 3.8 billion years ago, and
the Earth’s atmosphere is still strongly affected by
the biota. Of prime importance is the photosynthesis
reaction (2.5), which removes carbon from the atmos-
phere and stores it in organic matter and releases
oxygen to the atmosphere.
Exercise 5.2 If the photosynthesis reaction is repre-
sented by the reaction
11
(5.2)
where h
represents a photon. Is the carbon atom
reduced or oxidized by this reaction?
Solution: Because the oxidation number of each
oxygen atom in CO
2
is 2 and CO
2
has no net electric
charge, the oxidation number of the carbon atom in
CO
2
is 4. In CH
2
O, the oxidation numbers of the H
and O atoms are 1 and 2, respectively. Therefore,
the oxidation number of the carbon atom in CH
2
O
CO
2
(g) H
2
O(l) h
: CH
2
O(s) O
2
(g)
is 0. Therefore, the reaction decreases the oxidation
number of the carbon atom from 4 to 0 (i.e., the
carbon is reduced). ■
About 80% of the CH
4
in air derives from the decay
of recent organic materials (rather than fossil fuels)
through cud-chewing animals (cows, etc.), termites, rice
paddies, and wetlands.
Biological processes (often mediated by microbes)
convert N
2
into NH
3
(primarily via animal urine and
soils), N
2
O (through nitrate respiration by bacteria in
soils), and NO.
Regions of the ocean with high organic content
and biological productivity (e.g., upwelling regions,
coastal waters, and salt marshes) are a major source of
CS
2
and carbonyl sulfide (COS). Phytoplankton are
the major source of atmospheric DMS and dimethyl
disulfide (CH
3
SSCH
3
). DMS is oxidized to SO
2
and
then to sulfate aerosols. Microbial degradation of
dead organic matter releases H
2
S. The most abundant
halocarbon in the air, and the major natural source of
chlorine (Cl) in the stratosphere, is methyl chloride
(CH
3
Cl), which derives, in part, from biological
activity in seawater, wood molds, and biomass burn-
ing. Halogen compounds (e.g., chlorine and bromine
species) are also produced by biological activity in
the oceans.
Several thousand volatile organic compounds
(VOCs), emitted by plants and anthropogenic sources,
have been identified. In the United States, motor
vehicles are the primary source of VOCs, mainly in
the form of hydrocarbons produced by the incom-
plete combustion of fuel and from the vaporization
of fuel. The evaporation of solvents is the second
the very reactive hydroxyl radical (OH) has a
residence time in the atmosphere of only a second
or so. Of course, residence times may be deter-
mined by physical removal processes (e.g., scav-
enging by precipitation) as well as by chemical
processes.
If a chemical species has a very short (or very
long) residence time in the atmosphere, significant
5.1 Continued
variations in the concentration of the species will
generally occur over very short (or very large)
spatial scales (Fig. 5.1). Species with short resi-
dence times will be present in high concentrations
close to localized sources and in low concentra-
tions far removed from their sources. In contrast,
chemical species with long residence times exhibit
more uniform concentrations.
11
When needed for clarity, the phase of a chemical species is indicated in parenthesis by g for gas, 1 for liquid, s for solid, and aq for
aqueous solution.
P732951-Ch05.qxd 12/09/2005 09:05 PM Page 157
158 Atmospheric Chemistry
largest source of VOCs worldwide. Some of the
more important VOCs are isoprene (C
5
H
8
), ethene
(C
2
H
4
), and monoterpenes. Isoprene accounts for
50% of the NMHC. The photooxidation of iso-
prene can produce compounds that have vapor
pressures low enough for them to condense onto
preexisting particles. This process could account for
5–20% of the annual secondary organic aerosol
from biogenic sources. Terpenes are a class of hydro-
carbons that evaporate from leaves. About 80% of
these emissions oxidize to organic aerosols in about
an hour. Emissions from vegetation are a significant
source of hydrocarbons, which can react photochem-
ically with NO and NO
2
to produce O
3
, thereby
playing a central role in atmospheric chemistry (see
Section 5.3.5).
Use of biological materials by humans results in the
emissions of many chemicals into the atmosphere, for
example, CO
2
,CO,NO
x
,N
2
O, NH
3
,SO
2
and hydro-
gen chloride—HCl (from the combustion of oil, gas,
coal, and wood), hydrocarbons (from automobiles,
refineries, paints, and solvents), H
2
S and DMS (from
paper mills, and oil refineries), carbonyl sulfide—
COS (from natural gas), and chloroform—CHCl
3
(from combustion of petroleum, bleaching of woods,
solvents).
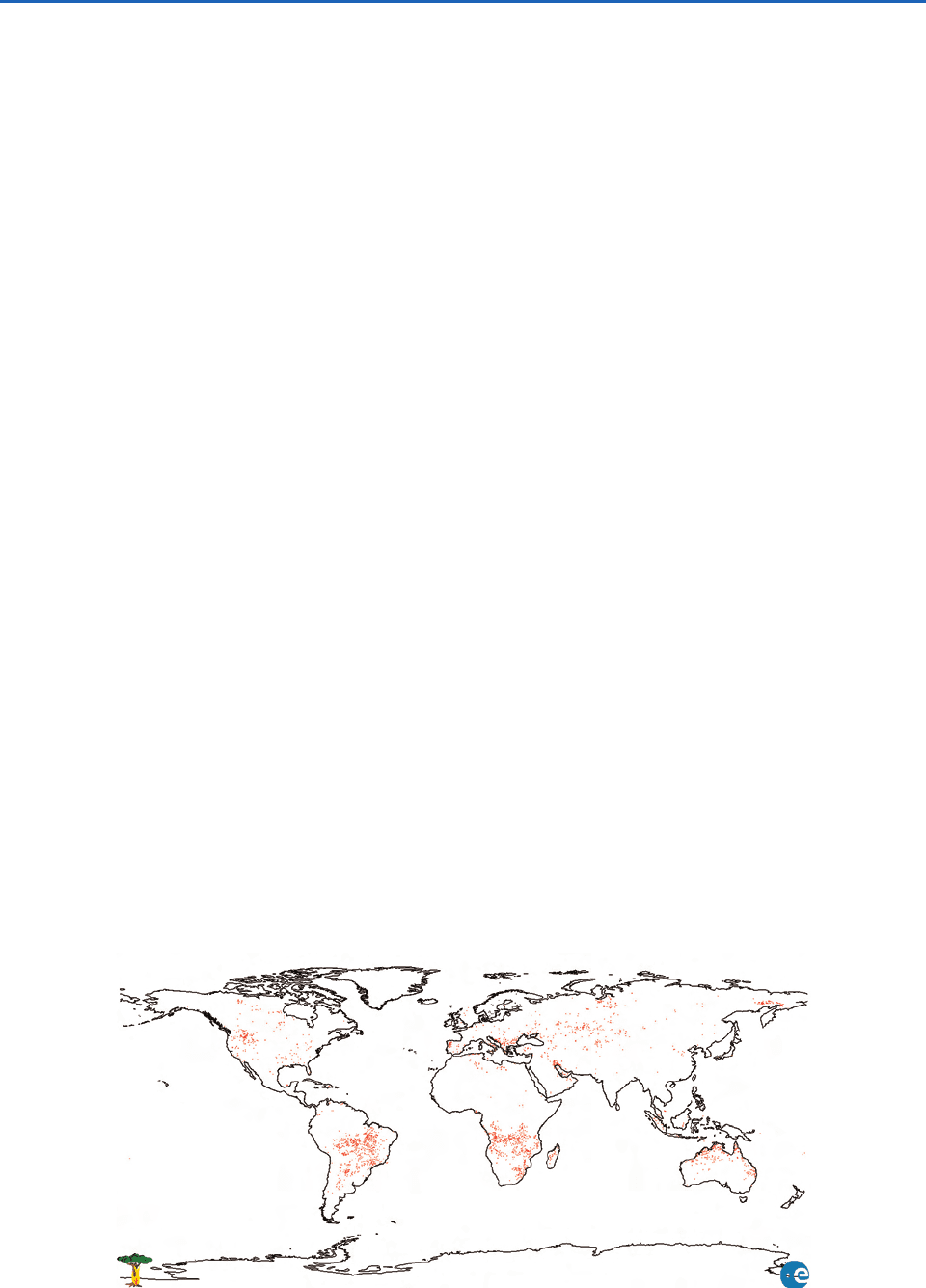
Figure 5.2 shows the global distribution of fires
during September 2000. September is in the biomass
burning season in South America and southern
Africa, and hence there are many fires in these two
locations. An area of vegetation equal to about half
the area of Europe is burned globally each year by
natural forest fires (many initiated by lightning) and
deliberate deforestation (e.g., in the Amazon Basin),
by refertilization of soils and grazing (e.g., in the
savannas of southern Africa), and by the use of wood
for heating and cooking (e.g., in Africa, India, and
southeast Asia). It has been estimated that on an
annual basis, biomass burning produces 38% of the
O
3
, 32% of the CO, 39% of the particulate carbon,
and more than 20% of the H
2
, NMHC, methyl chlo-
ride (CH
3
Cl), and NO
x
in the troposphere. Biomass
burning also produces 40% of the world’s annual
production of CO
2
, but this is largely offset by the
uptake of CO
2
by young vegetation that sprouts
quickly on burned areas.
As discussed in Section 5.3.5, ozone is produced in
the troposphere by photochemical reactions involv-
ing oxidation by OH of CO, CH
4
, and NMHC in
the presence of NO
x
. Because all of these precursors
are present in smoke from biomass burning, elevated
O
3
concentrations are produced in biomass smoke as
it disperses in the troposphere. Many of the emis-
sions from biomass burning are carcinogens, they
cause significant degradation to air quality on local
and regional scales, and they have global effects on
atmospheric chemistry and climate.
Biomass smoke can be dispersed over large dis-
tances in the atmosphere. For example, under appro-
priate wind conditions, biomass smoke from Africa is
dispersed across the south Atlantic Ocean and even
to Australia (Fig. 5.3). Indeed it is likely that even the
most remote regions of Earth are not immune from
pollution. Biomass smoke can also be lofted into the
middle and upper troposphere where it can become
a dominant source of HO
x
(where x 0, 1, or 2)
and NO
x
and result in the production of O
3
(see
Section 5.3.5).
Fig. 5.2 Global distribution of fires detected by satellite in September 2000. [Image courtesy of European Space Agency.]
P732951-Ch05.qxd 12/09/2005 09:05 PM Page 158

5.2 Sources, Transport, and Sinks of Trace Gases 159
b. Solid Earth
Various aspects of the role of the solid Earth in
the chemistry of the atmosphere have been discussed
in Chapter 2. Some additional information is given
here.
Volcanoes are the most important source of atmos-
pheric trace gases from the solid Earth. In addition to
ash and copious small particles, volcanoes emit H
2
O,
CO
2
,SO
2
,H
2
S, COS, HCl, hydrogen fluoride (HF),
hydrogen bromide (HBr), CH
4
,CH
3
Cl, H
2
,CO,and
heavy metals [e.g., mercury (Hg)]. Emissions from
violent volcanic eruptions can be blasted into the
stratosphere, where constituents with long residence
times can be dispersed around the globe.
14
Rocks are the major sources of He, Ar, and radon
(Rn) in the atmosphere. Helium is produced by the
radioactive decay of uranium-238 and thorium-232. It
does not significantly accumulate in the atmosphere
because it is so light that it escapes from the exos-
phere. Argon has accumulated in air over eons from
the radioactive decay of potassium-40 in rocks.
Radon-222 is a decay product of uranium in rocks; it
has a half-life of only 3.8 days.
Carbonate rocks, such as limestone (e.g., CaCO
3
),
contain about 20,000 times more carbon than the
atmosphere (see Table 2.3), but most of this is
sequestered. As described in Section 2.3.3 and 2.2.4,
carbonate rocks and marine sediments are involved
in a long-period cycle with atmospheric CO
2
.
c. Oceanic
As mentioned in Section 5.2.1(a), the oceans are a
huge reservoir of water-soluble gases (e.g., see the
major carbon reservoirs listed in Table 2.3). Thus, the
oceans may serve as either a sink or a source for
soluble gases.
The oceans are an atmospheric source for many
gases produced by biological activity, particularly
sulfur-containing gases.
Exercise 5.3 If the Henry’s
15
law constant for CO
2
in water is 3.40 10
2
mole liter
1
atm
1
, what is the
the solubility of CO
2
in water?
Solution: Henry’s law is
(5.3)
where C
is solubility of a gas in a liquid (in mole
liter
1
), k
H
is a temperature-dependent constant of
proportionality called the Henry’s law constant,
and p
is the partial pressure (in atm) of the gas
C
k
H
p
September–October
Dobson units
Longitude
Latitude
West East
–180 –120 –60 0 60 120 180
–50
–25
0
25
50
15 20 25 30 35 40 45 50
Fig. 5.3 Satellite measurements of tropospheric ozone in
September and October for the period 1979–1989. The high
column amounts of ozone (indicated by high Dobson
12
units
13
) over tropical and southern Africa are due to smoke
from biomass burning. [Excerpted with permission from
Fishman et al., Science 252, p. 1694. Copyright 1991 AAAS.]
12
G. M. B. Dobson (1889–1976) English physicist and meteorologist. Made the first measurements of the variation of wind with height
using pilot balloons (1913). In 1922 he discovered the presence of a warm layer of air at 50 km, which he correctly attributed to the
absorption of UV radiation by O
3
. Built a UV solar spectrograph for measuring the atmospheric O
3
column. Also obtained first measure-
ments of water vapor in the stratosphere.
13
One Dobson unit (DU) is the thickness, in hundredths of a millimeter, that the total O
3
column would occupy at 0 °C and 1 atm. The
Earth’s total atmospheric O
3
column is 300 DU (i.e., if all the O
3
in the atmosphere were brought to 0 °C and 1 atm, it would form a
layer just 3 mm deep).
14
The violent eruption in 1883 of the Indonesian volcano of Krakatau caused remarkable sunsets and lowered global temperatures
at the Earth’s surface by 0.5 °C in the year following the eruption. The largest volcanic eruption in the 20th century, in terms of its
atmospheric effects, was Pinatubo in the Philippines in 1991. The emissions from this eruption produced a global average cooling of
0.5 °C for 2 years and lowered ozone concentrations in the stratosphere. (See Sections 5.7.3 and 10.2.3.)
15
William Henry (1774–1836) English physician and chemist. First scientific paper was a refutation of a claim that carbon is not an
element. Together with his friend John Dalton, his experiments on the dissolution of gases were crucial in the development of the atomic
theory of matter. Committed suicide due to pain (from a childhood injury) and sleep deprivation.
P732951-Ch05.qxd 12/09/2005 09:05 PM Page 159

160 Atmospheric Chemistry
above the surface of the liquid. The partial pressure
of CO
2
in air is 3.79 10
4
atm (see Table 5.1).
Therefore,
■
d. In situ formation
In situ formation, which refers to the formation of
chemical species by chemical reactions in the atmos-
phere, is a major source of many important atmos-
pheric trace constituents. Most such gaseous reactions
are initiated by photolysis involving radicals and occur
by uni-, bi- and termolecular reactions.
In situ chemical reactions can be classified as homo-
geneous or heterogeneous.A homogeneous reaction is
one in which all of the reactants are in the same phase.
For example, the reaction
(5.4)
which is a major source of the nitrate radical (NO
3
)
in the atmosphere, is a homogeneous gas-phase
reaction.A heterogeneous reaction is one involving
reactants in two or more phases. The mixing of
an inorganic aerosol [e.g., sulfuric acid (H
2
SO
4
) or
nitric acid (HNO
3
)] with organic compounds (e.g.,
aldehydes), which can appreciably increase the rate
of aerosol growth, is an example of a heterogeneous
reaction.
Trace gases emitted from the biosphere, solid
Earth, and oceans are generally in a reduced (low)
oxidation state (e.g., hydrocarbons, ammonia, hydro-
gen sulfide), but they are oxidized (i.e., raised to
a higher oxidation state) by in situ reactions in the
atmosphere.
e. Anthropogenic sources
We will discuss anthropogenic (i.e., human) sources of
gases and particles in Sections 5.5 and 5.6. However,
it is important to note here that anthropogenic
sources play significant roles in the budgets of many
important trace gases in the atmosphere (Table 5.2).
As a result of increasing populations, anthropogenic
emissions of a number of important trace gases have
increased significantly over the past century. As a
consequence, the extent of human influences on the
NO
2
(g) O
3
(g) : NO
3
(g) O
2
(g)
1.29 10
5
mol liter
1
(3.40 10
2
mol liter
1
)(3.79 10
4
atm)
C
k
H
p
atmosphere is one of the main themes of current
research in atmospheric chemistry.
5.2.2 Transport
In the atmospheric boundary layer (ABL) the atmos-
phere interacts directly with the Earth’s surface
through turbulent mixing. Consequently, during the
day over land, chemicals in the ABL are generally
well mixed up to a height of 1–2 km. The dilution
of chemical compounds by turbulent mixing is less
efficient at night when the ABL depth is usually a
few hundred meters or less. Over the oceans, the
diurnal cycle is much less apparent.
If a chemical that originates from the Earth’s surface
is not returned to the surface or transformed by in situ
reactions in the ABL, it will eventually pass into the
free troposphere. Once in the free troposphere, chemi-
cals with long residence times are carried along with
the global circulation pattern. For example, in midlati-
tudes, where the winds are generally from west to east
and have speeds of 10–30 m s
1
,a chemical injected
into the atmosphere from a “point source,” such as a
volcano, will become distributed fairly uniformly longi-
tudinally around the latitude belt within a few weeks.
Since the transport of tropospheric air across the trop-
ics is relatively restricted, so is the transport of chemi-
cals. It follows that the chemistry of the troposphere in
the northern hemisphere is more strongly affected by
emissions from the use of fossil fuels than the chem-
istry of the southern hemisphere; the latter reflects
more the effects of emissions from the oceans and
from biomass burning. Transport is also restricted
between the free troposphere and the stratosphere;
most of the upward transport is in the tropics, and most
of the downward transport is in higher latitudes. Never-
theless, as shown in Section 5.7.2, certain long-lived
chemicals of anthropogenic origin can accumulate in
the stratosphere, where they can have major effects.
Satellite observations provide strong evidence for
the transport of tropospheric gases and particles. For
example, satellite observations reveal large plumes
of particles off the east coasts of the United States
and Asia, enormous dust plumes carried westward
from the Sahara Desert over the Atlantic Ocean, and
large smoke plumes from regions of biomass burn-
ing. During the winter monsoon (December through
April), a plume of pollutants extends from the south-
west coast of India over the Indian Ocean. In spring
and summer, dust and pollutants are transported
from sources in Asia across the north Pacific Ocean.
P732951-Ch05.qxd 12/09/2005 09:05 PM Page 160

Table 5.2 Estimates of natural and anthropogenic sources of a number of atmospheric trace gases in 2000
a
SO
2
NH
3
N
2
OCH
4
CO NO
x
NMHC
Sources [Tg(S) year
1
][Tg(N) year
1
][Tg(N) year
1
][Tg(CH
4
) year
1
][Tg(CO) year
1
][Tg(N) year
1
][Tg(C) year
1
]
Natural
Vegetation 5.1 100 (60–160) 400 (230–1150)
Wetlands 115 (55–150)
Wild animals 2.5
Termites 20 (10–50)
Oceans 25
b
7.03 (1–5) 10 (5–50) 50 (20–200) 50 (20–150)
Soils 6 (3.3–9.7) 7 (5–12)
Lightning 5 (2–20)
Volcanoes 10 (7–10)
Other 7.5
c
15 (10–40) 1.5 (0–5.7)
d
Total natural 42.5 14.6 9 (4–15) 160 (80–290) 150 (80–360) 13.5 (7–38) 450 (250–1300)
Anthropogenic
Natural gas 40 (25–50)
Coal mines 30 (15–45)
Fossil fuels related
Petroleum industry
75
e
15 (5–30)
Coal combustion ? (1–30)
Energy use 500 (300–900) 22 (20–24) 70 (60–100)
Aircraft 0.5 (0.2–1)
Enteric fermentation 85 (65–100)
Rice paddies 60 (20–100)
Biospheric carbon
Biomass burning
3
2 0.5 (0.2–1.0) 40 (20–80) 500 (400–700) 8 (3–13) 40 (30–90)
Landfills 40 (20–70)
Animal waste 22 25 (20–30)
Domestic sewage 25 (15–80)
Fertilizer 6.4
Cultivated soils 3.5 (1.8–5.3)
Cattle and feedlots 0.4 (0.2–0.5)
Industrial sources 1.3 (0.7–1.8)
Total anthropogenic 78 30.4 5.7 (3–9) 360 (206–615) 1000 (700–1600) 30.5 (23–38) 110 (90–190)
a
The last four listed species are ozone precursors. Numbers in parentheses are possible ranges of values. Estimates are based on several authoritative sources.
b
Via oxidation of DMS.
c
Oxidation of H
2
S [7 Tg(S) year
1
] and CS
2
[0.5 Tg(S) year
1
].
d
Oxidation of NH
3
[0.9 Tg(N) year
1
] and breakdown of N
2
O [0.6 Tg(N) year
1
] produced in the stratosphere and transported to the troposphere.
e
Including other industrial sources.
P732951-Ch05.qxd 12/09/2005 09:05 PM Page 161

162 Atmospheric Chemistry
5.2.3 Sinks
The final stage in the life history of a chemical in the
atmosphere is its removal. Sinks include transforma-
tions into other chemical species and gas-to-particle
(g-to-p) conversion, which can involve both chemical
and physical processes. The other important removal
process for both gases and aerosols is deposition
onto the Earth’s surface and vegetation. Deposition
is of two types: wet and dry. Wet deposition, which
involves the scavenging of gases and particles in the
air by clouds and precipitation, is one of the major
mechanisms by which the atmosphere is cleansed.
Dry deposition involves the direct collection of gases
and particles in the air by vegetation and the Earth’s
solid and liquid surfaces. Dry deposition is a much
slower process than wet deposition, but it is continu-
ous rather than episodic.
The oceans are important sinks for many trace
gases. The flux of a gas to the ocean depends on how
undersaturated the ocean is with respect to the gas
(see Section 5.2.1c). If the surface layers of the ocean
are supersaturated with a gas, then the flux is from
the ocean to the atmosphere (e.g., the estimated
global flux of DMS from the ocean to the atmos-
phere is 25 Tg of sulfur per year).
Exercise 5.4 If SO
2
were confined to a layer of
the atmosphere extending from the surface of the
Earth to a height of 5 km and the average deposition
velocity of SO
2
onto the ground were 0.800 cm s
1
,
how long would it take for all of the SO
2
to be
deposited on the ground if all sources of SO
2
were
suddenly switched off?
Solution: The deposition velocity of a gas onto a
surface is defined by
(5.5)
Since the units of flux are kg m
2
s
1
and the units of
concentration are kg m
3
, it follows from (5.5) that
the units of deposition velocity are m s
1
, which are
the usual units of velocity. Hence, we can consider
velocity
deposition
flux of the gas to a surface
mean concentration of the gas
just above the surface
the deposition velocity of a gas to be the counterpart
of the terminal fall speed of a particle.
Therefore, the time required to remove all of the SO
2
in a vertical column of height 5 km will be the time
taken for a molecule of SO
2
to move through a vertical
distance of 5 km toward the Earth’s surface, which is
.
The residence time of 7.23 days for SO
2
derived
here is an approximate upper limit since it neglects
other removal mechanisms, such as in situ chemical
reactions. ■
5.3 Some Important
Tropospheric Trace Gases
Prior to the 1970s, photochemical reactions, and the oxi-
dation of most trace gases, were thought to take place
primarily in the stratosphere where the intensity of UV
radiation is much greater than in the troposphere.
16
However, in the 1960–1970s it was recognized that the
very reactive hydroxyl radical OH can be produced by
photochemistry in the troposphere. At about the same
time, studies of photochemical smogs (such as those
that occur in Los Angeles) began to reveal the roles of
OH, nitrogen oxides, and hydrocarbons in the forma-
tion of O
3
and other pollutants (see Section 5.5.2b).
This section considers some of the trace gases
that play important roles in tropospheric chemistry,
including those mentioned earlier. Our main concern
in this section is with the nonurban troposphere.
Chemical reactions in heavily polluted air, which
can produce smogs, are considered in Section 5.5.
Stratospheric chemistry is discussed in Section 5.7.
5.3.1 The Hydroxyl Radical
Because of its high reactivity with both inorganic and
organic compounds, OH is one of the most important
chemical species in the atmosphere, even though it is
present in the troposphere in globally and diurnally
averaged concentrations of just a few tenths of a pptv
(10
12
OH molecules m
3
, or about 3 OH molecules
per 10
14
molecules in the air). Reaction with OH
5 km
0.800 10
5
km s
1
6.25 10
5
s 7.23 days
16
Photochemical reactions involve photons (represented by h
). To trigger a chemical reaction, the energy of the photons must exceed
a critical value. Because the energy of a photon is inversely proportional to the wavelength of the electromagnetic radiation, this means
that the wavelength of the radiation must be below a critical value if it is to trigger a specified photochemical reaction. In general, this
critical value places the effective radiation for photochemical reactions in the UV region.
P732951-Ch05.qxd 12/09/2005 09:05 PM Page 162
5.3 Some Important Tropospheric Trace Gases 163
is the major sink for most atmospheric trace gases.
Because it is so reactive, the average lifetime of an
OH molecule in the atmosphere is only 1s.
Hydroxyl radicals are produced when solar UV
radiation (with
0.32
m) decomposes O
3
into
molecular oxygen and energetically excited oxygen
atoms (O*)
(5.6a)
Most of the O* atoms produced by (5.6a) dissipate
their excess energy as heat and eventually recombine
with O
2
to form O
3
, which, together with (5.6a), is
a null cycle (i.e., it has no net chemical effect). How-
ever, a small fraction (1%) of the O* atoms reacts
with water vapor to form two hydroxyl radicals
(5.6b)
The net effect for those O* atoms produced by (5.6a)
and removed by (5.6b) is
(5.7)
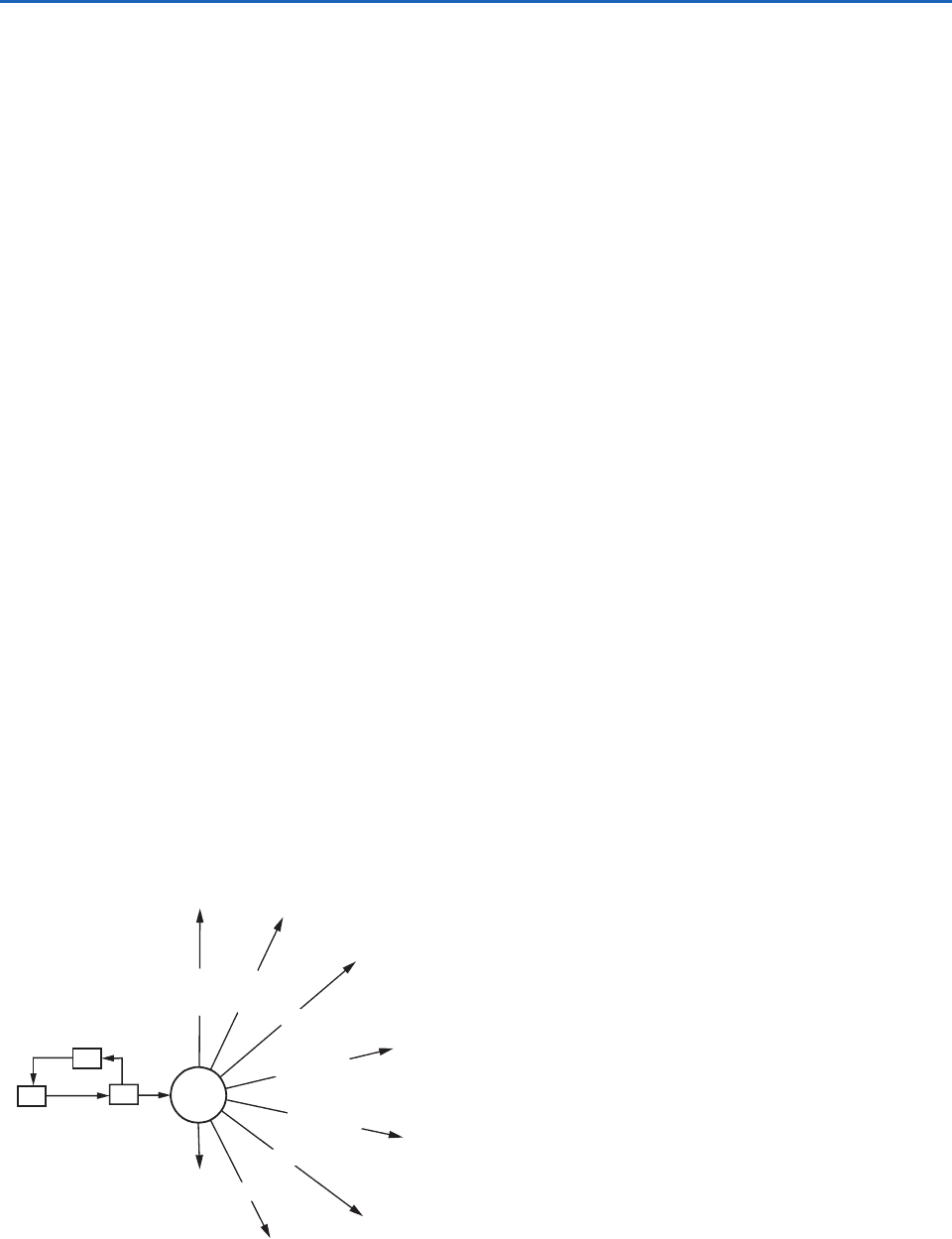
Once formed, the OH radical is a powerful oxidant
that reacts quickly with almost all trace gases contain-
ing H, C, N, O, and S and the halogens [except N
2
O
and the chlorofluorocarbons (CFC)]. For example,
OH reacts with CO to form CO
2
,NO
2
to form
HNO
3
,H
2
S to form SO
2
,SO
2
to form H
2
SO
4
, etc.
(Fig. 5.4). Because of its role in removing many pollu-
tants, OH has been called the atmosphere’s detergent.
O
3
H
2
O h
: O
2
2OH
O
*
H
2
O : 2OH
O
3
h
: O
2
O
*
The dominant sinks for OH in the global tropo-
sphere are the oxidation of CO and CH
4
. Over the
continents, reactions with NMHC can be strong local
sinks for OH. In forests, the dominant reactant with
OH is often isoprene (C
5
H
8
), which is emitted by
deciduous trees.
The production of O
3
through reaction (5.7) has
probably increased over the past two centuries due to
increases in anthropogenic sources of its precursors,
and also due to the increasing flux of solar UV radia-
tion into the atmospheric boundary layer in response
to the thinning of the stratospheric ozone layer.
However, sinks for OH have also increased due to
increases in the concentrations of CO and hydrocar-
bons. Consequently, it is not clear whether the concen-
tration of OH in the troposphere has changed
significantly over the past several decades in response
to human activity.
5.3.2 Some Reactive Nitrogen Compounds
a. Nitrogen oxides
The oxides of nitrogen, NO (nitric oxide), and NO
2
(nitrogen dioxide), which together are referred to
as NO
x
, play important roles in atmospheric chem-
istry. They are produced by fossil fuel combustion, bio-
mass burning, and from soils, lightning, NH
3
oxidation,
aircraft emissions, and transport from the strato-
sphere. NO
x
is emitted into the troposphere primarily
as NO, but during the day NO rapidly establishes an
equilibrium with NO
2
through the following null cycle.
(5.8a)
(5.8b)
where M represents an inert molecule that absorbs
excess molecular energies. Once NO is converted to
NO
2
,a number of reaction paths are available. At
night NO
x
is present only as NO
2
due to reaction
(5.8a). The principal sink for NO
x
in the daytime is
(5.9)
The nitric acid (HNO
3
) is removed in about 1 week
by dry and wet deposition. At night, NO
2
is oxidized
by O
3
to NO
3
, the NO
3
then reacts with NO
2
to
produce N
2
O
5
, and the N
2
O
5
reacts with water on
particles to produce HNO
3
.The resulting residence
time of NO
2
is 1 day.
NO
2
OH M : HNO
3
M
NO
2
O
2
M h
: NO O
3
M
NO O
3
: NO
2
O
2
M
H
2
O
( < 0.32 µ m)
CO
2
Carbon
monoxide
H
2
SO
4
Ammonia
NO and H
2
O
Sulfur
dioxide
SO
2
CO and H
2
O
Hydrocarbons
HNO
3
Hydrogen halides and water
Trichloroethane
Ozone
A halogen
monoxide
CO,
HCl and H
2
O
O
3
M,O
2
O
*
Hydroxyl
(OH)
radical
O
Hydrogen
sulfide
Nitrogen dioxide
Fig. 5.4 Illustration of the central role of the OH radical
in the oxidation of tropospheric trace gases. Little escapes
oxidation by OH. [Adapted from Global Tropospheric Chemistry,
United States National Academy Press, 1984, p. 79.]
P732951-Ch05.qxd 12/09/2005 09:05 PM Page 163
