Wallace J.M., Hobbs P.V. Atmospheric Science. An Introductory Survey
Подождите немного. Документ загружается.


174 Atmospheric Chemistry
or
that is
For, N
0
N 2, N
0
1.00 10
11
m
3
and K 1.40
10
15
m
3
s
1
,
■
Improvements in visibility that frequently follow
periods of precipitation are due, in large part, to the
removal (i.e., scavenging) of particles by precipi-
tation. It is estimated that, on a global scale, precipi-
tation processes account for about 80–90% of the
mass of particles removed from the atmosphere.
Prior to the formation of precipitation, some of the
particles in the air serve as nuclei upon which cloud
particles (water and ice) can form (see Chapter 6).
As cloud particles grow, aerosols tend to be driven to
their surfaces by the diffusion field associated with
the flux of water vapor to the growing cloud particles
(called the diffusiophoretic force). Aerosol particles
less than 0.1
m are collected most efficiently by
diffusiophoresis. As precipitation particles fall
through the air, they collect aerosol particles greater
than 2
m in diameter with reasonable efficiency
by impaction. Aerosols are also removed by
impaction onto obstacles on the Earth’s surface
(such as newly washed automobiles).
The terminal fall speeds of particles 1
m diame-
ter are sufficiently large that gravitational settling (i.e.,
dry deposition) is important as a removal process. For
example, the fall speeds of particles 1 and 10
m in
diameter are 3 10
5
and 3 10
3
ms
1
,
respectively. It is estimated that 10–20% of the
t
2 1
(1.40 10
15
)10
11
7140 s 1.98 h
t
N
0
N
1
KN
0
1
N
0
1
N
Kt
mass of particles removed from the atmosphere is by
dry fallout.
5.4.5 Concentrations and Size
Distributions
One of the oldest and most convenient techniques
(which in various forms is still in widespread use) for
measuring the concentrations of particles in the air
is the Aitken
21
nucleus counter. In this instrument,
saturated air is expanded rapidly so that it becomes
supersaturated by several hundred percent with
respect to water (see Exercise 5.15). At these high
supersaturations, water condenses onto virtually all of
the particles in the air to form a cloud of small water
droplets. The concentration of droplets in the cloud
(which is close to the concentration of particles) can
be determined by allowing the droplets to settle out
onto a substrate, where they can be counted under a
microscope, or automatically by optical techniques.
The concentration of particles measured with an
Aitken nucleus counter is referred to as the Aitken
(or condensation) nucleus (CN) count.
Condensation nucleus counts near the Earth’s
surface vary widely from one location to another and
can also fluctuate by more than an order of mag-
nitude with time at any one site. Generally, they
range from average concentrations of 10
3
cm
3
over the oceans, to 10
4
cm
3
over rural land areas,
to 10
5
cm
3
or greater in urban polluted air. These
observations, together with the fact that CN counts
decline with increasing altitude, support the view that
land is an important source of atmospheric aerosol,
with human and industrial activities being particu-
larly prolific sources.
Atmospheric aerosol particles range in size from
10
4
m to tens of micrometers. The averages of
numerous measurements of particle number distribu-
tions in continental, marine, and urban polluted air are
shown in Fig. 5.9. The measurements are plotted in the
form of a number distribution in which the ordinate
[dNd(log D)] and the abscissa (D) are plotted on log-
arithmic scales, where dN is the number concentration
of particles with diameters between D and D dD.
22
21
John Aitken (1839–1919) Scottish physicist, although originally an apprentice marine engineer. In addition to his pioneering work on
atmospheric aerosol, he investigated cyclones, color, and color sensations.
22
Note that if the ordinate were linear in N (which it is not in Fig. 5.9), the concentration of particles within a diameter interval
d(log D) would be equal to the area under the curve in this diameter interval.
P732951-Ch05.qxd 12/09/2005 09:05 PM Page 174
5.4 Tropospheric Aerosols 175
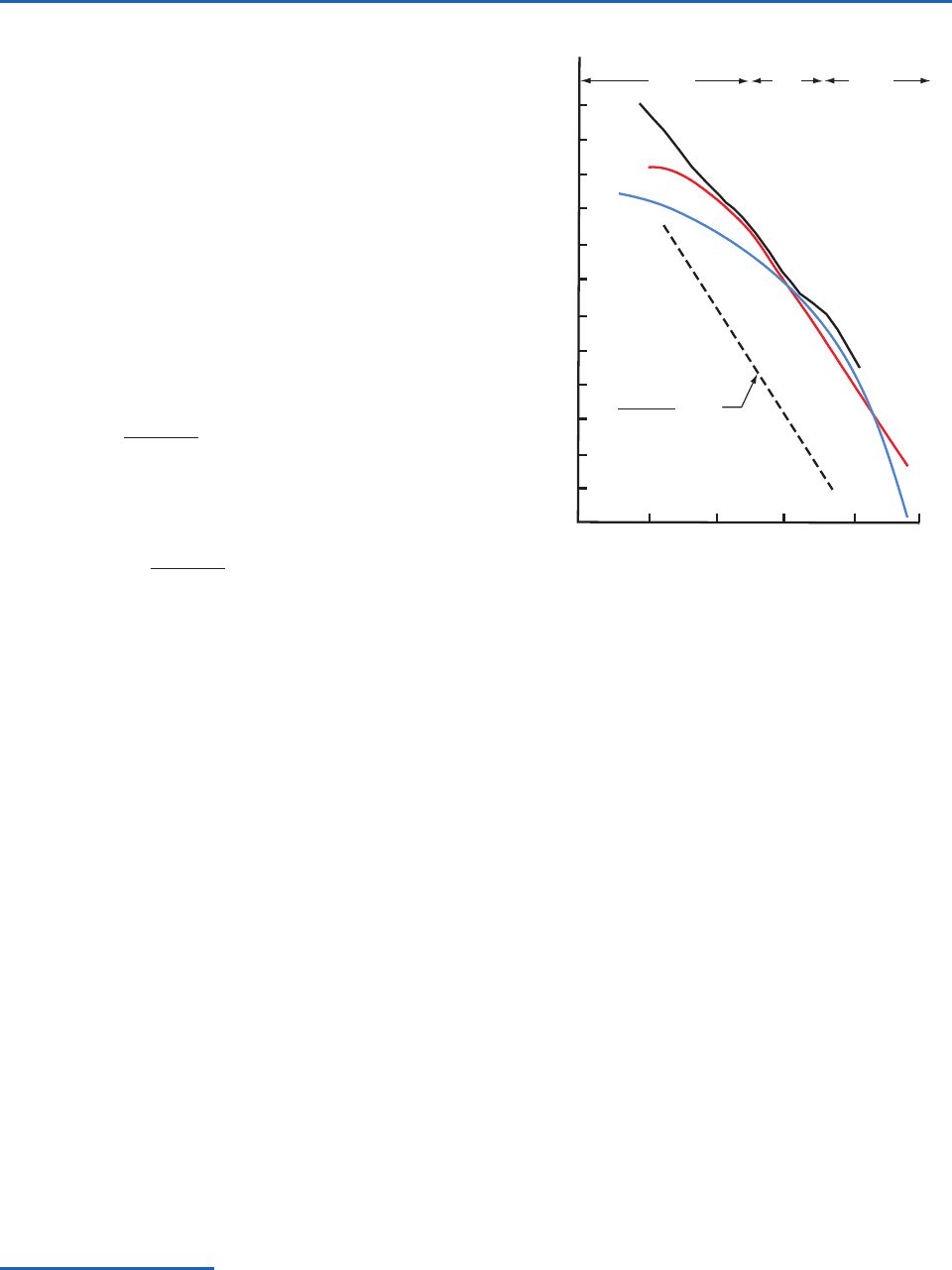
Several conclusions can be drawn from the results
shown in Fig. 5.9:
•The concentrations of particles fall off very
rapidly as they increase in size. Therefore, the
total number concentration (i.e., the Aitken or
CN count) is dominated by particles with
diameters 0.2
m, which are therefore
referred to as Aitken nuclei or condensation
nuclei.
•Those portions of the number distribution curves
that are straight lines in Fig. 5.9 can be
represented by an expression of the form
or, taking antilogs,
(5.31)
where C is a constant related to the
concentration of the particles and
is the
slope of the number distribution curve. The
value of
generally lies between 2 and 4.
Continental aerosol particles with diameters
larger than 0.2
m follow (5.31) quite closely
with
3. A size distribution with
3 is
called a Junge
23
distribution.
•The number distributions of particles shown in
Fig. 5.9 confirm CN measurements, which
indicate that the total concentrations of particles
are, on average, greatest in urban polluted air
and least in marine air.
•The concentrations of particles with diameters
2
m (giant particles) are, on average, rather
similar in continental, marine, and urban
polluted air.
Further insights into atmospheric particle size
distributions can be obtained by plotting particle
surface area distributions or particle volume distribu-
tions. In a surface area distribution the ordinate is
dSd(log D) and the abscissa is D ploted on a loga-
dN
d(log D)
CD
b
log
dN
d(log D)
const b log D
rithmic scale, where S is the total surface area of
particles with diameters between D and D dD.In
a volume distribution the ordinate is dVd(log D)
and the abscissa is D plotted on a logarithmic scale,
where V is the total volume of particles with diame-
ters between D and D dD.
If particle densities are independent of their diam-
eter, the mass concentration dM of particles with
diameters between D and D dD is proportional to
D
3
dN. If the number distribution follows (5.31) with
3, D
3
dN d(log D), therefore, dMd(log D)
has a fixed value. In this case, the particles in each
logarithmic increment of diameter contribute equally
to the total mass concentration of the aerosol. It fol-
lows from this result that, although particles with
diameters from 0.2 to 2
m (so-called large particles)
are present in much higher number concentrations
than giant particles, the large and giant particles in
23
Christian E. Junge (1912–1996) German meteorologist. Carried out pioneering studies of tropospheric and stratospheric aerosols
and trace gases. His book Atmospheric Chemistry and Radioactivity (Academic Press, 1963) initiated the modern era of atmospheric
chemistry research.
Diameter D (µ m)
10
–3
10
–2
10
–1
10
0
10
1
10
2
10
–3
10
2
10
1
10
0
10
–1
10
–2
10
–4
10
–5
10
–6
10
3
10
4
10
5
10
6
dN
∝ D
–3
d(log D)
dN/d(log D) (cm
–3
)
Aitken
nuclei
(or CN)
Large
particles
Giant
particles
Fig. 5.9 Number distributions of tropospheric particles
obtained from averaging many measurements in continental
(red), marine (blue), and urban polluted (black) air. Also
plotted is Eq. (5.31) with
3, with the line (dashed) dis-
placed from the other curves for the sake of clarity.
P732951-Ch05.qxd 12/09/2005 09:05 PM Page 175

176 Atmospheric Chemistry
continental air make similar contributions to the
total mass of the aerosol. However, despite their rela-
tively large number concentration, the CN contribute
only 10–20% to the total mass of the atmospheric
aerosol. This is because, as seen in Fig. 5.9, the CN do
not increase in concentration with decreasing size as
rapidly as indicated by (5.31).
Small fluctuations in the slope of a particle number
distribution about values of 2 and 3 (i.e.,
2
and 3, respectively) appear as local maxima and min-
ima in the surface and volume distribution plots,
respectively (see Exercise 5.16). Shown in Fig. 5.10
are particle surface area and particle volume distri-
bution plots for continental and urban polluted air
in Denver, Colorado. These curves show far more
structure, in the form of maxima and minima, than
the number distribution plots shown in Fig. 5.9. The
maxima and minima in the curves in Fig. 5.10 are
associated with sources and sinks, respectively, of the
aerosol. The prominent maxima in the surface and
volume distribution plots in the particle size range
0.2–2
m diameter is due primarily to the growth
of the CN by coagulation into this size range,
together with particles left behind when cloud
droplets evaporate. Since the sinks for particles
0.2–2
m diameter are weak, particles in this size
range tend to accumulate in the atmosphere: hence,
the peak in the particle surface area and volume dis-
tribution plots for particles between 0.2 and 2
m
(a)
(b)
Diameter D (µ
m)
600
400
200
0
10
–2
10
–1
10
0
10
1
10
2
dS/d(log D) (µ m
2
cm
–3
)
Diameter D (µ m)
80
60
40
20
0
10
–2
10
–1
10
0
10
1
10
2
dV/d(log D) (µ m
2
cm
–3
)
Fig. 5.10 (a) Typical particle surface area and (b) particle
volume distribution plots in urban-polluted air (black line)
and cleaner continental air (solid red line).
diameter is referred to as the accumulation mode.
Another peak, called the coarse particle mode, occurs
in the particle surface and volume distribution plots
for particles with D 1
m. This mode is attributable
to dusts and industrial processes, which produce fly
ash and other large particles, sea salt, and some bio-
logical materials.
Another common way of subdividing atmosphere
particles by size is to call particles with D 0.01
m
ultrafine, those with 0.01
m D 2.5
m fine par-
ticles, and those with D 2.5
m coarse particles.
5.4.6 Residence Times
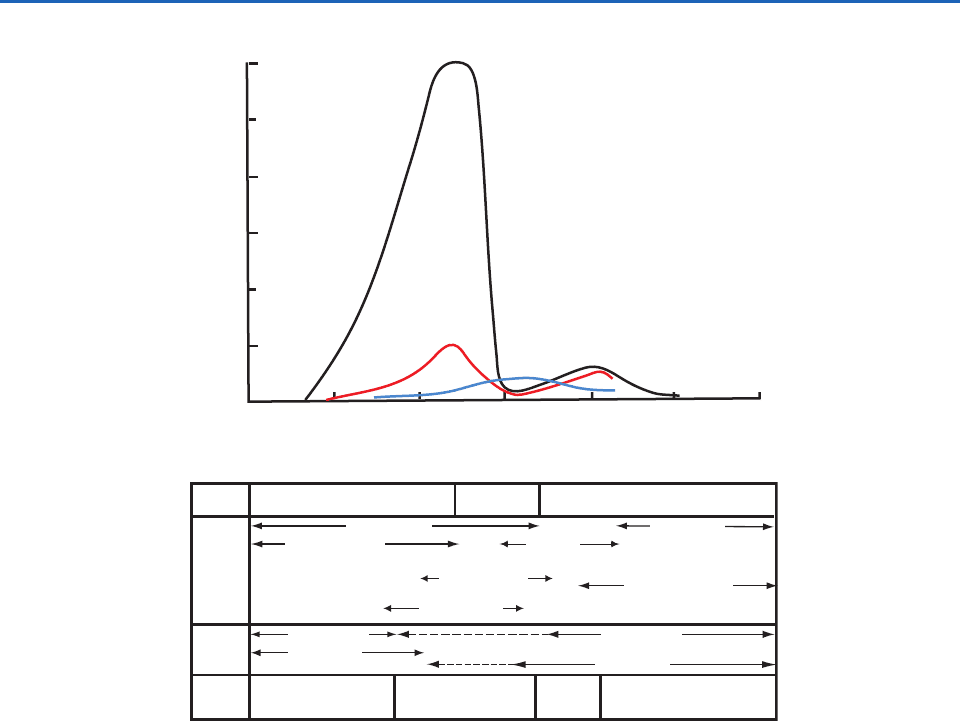
Figure 5.11 shows estimates of the residence times of
particles in the atmosphere as a function of their size.
Particles with diameter 0.01
m have residence
times 1 day; the major removal mechanisms for
particles in this size range are diffusion to cloud
particles and coagulation. Particles 20
m diameter
also have residence times 1 day, but they are
removed by sedimentation, impaction onto surfaces,
and precipitation scavenging. In contrast, particles
with diameters 0.2–2
m have strong sources (from
the coagulation of Aitken nuclei and particles left
behind by the evaporation of cloud droplets) but
weak sinks. Consequently, these particles have rela-
tively long residence times, reaching several hundred
days in the upper troposphere, but precipitation
scavenging and impaction reduce these residence
times to a few tens of days in the middle and lower
troposphere. It is for this reason that the accumula-
tion mode is in the size range of these particles (e.g.,
see Fig. 5.10).
5.5 Air Pollution
In urban and industrialized locations, anthropogenic
emissions can become so large that the concentra-
tions of various undesirable chemical species (pollu-
tants) cause significant deterioration in air quality
and visibility and can pose threats to human health.
Severe air pollution episodes, and associated visibility
reduction, occur when the rates of emissions or forma-
tion of pollutants greatly exceed the rates at which
the pollutants are dispersed by winds and vertical
transport or are removed from the atmosphere by
chemical reactions or deposition. Severe air pollution
episodes tend to occur in association with extended
intervals of light winds and strong static stability (see
Section 3.6 and Box 3.4).
P732951-Ch05.qxd 12/09/2005 09:05 PM Page 176
5.5 Air Pollution 177
5.5.1 Some Sources of Pollutants
Combustion (in power plants, smelters, automobiles,
and of wood, vegetation, etc.) is the largest source of
air pollutants. On a global scale, fossil-fuel combus-
tion is the major source of CO, CO
2
,NO
x
, and SO
2
.
Many other pollutants are released into the air by
combustion. For example, about 15% of the total
emissions of hydrocarbons are from anthropogenic
sources, most notably the burning of hydrocarbon
compounds (oil, natural gas, coal, and wood). Ideal
(or complete) combustion (or oxidation) of a hydro-
carbon fuel yields only CO
2
and H
2
O. However, for a
given quantity of fuel, a precise amount of oxygen is
required for complete combustion, and this ideal
combination of fuel and oxygen is rarely achieved.
Exercise 5.7 Determine the ratio of the mass of
dry air to the mass of isooctane (C
8
H
18
)—called the
air–fuel ratio—for ideal combustion.
Solution: The balanced chemical equation for the
ideal or complete combustion of C
8
H
18
can be writ-
ten as
Therefore, for complete combustion, 1 mol of C
8
H
18
reacts with 12.5 mol of O
2
or, because the molecular
weights of C
8
H
18
and O
2
are 114 and 32, respectively,
114 g of C
8
H
18
reacts with 12.5 32 or 400 g of O
2
.
We now need to calculate what mass of air contains
400 g of oxygen. The amount of oxygen in air by
volume (or by number of molecules) is 20.95% (see
Table 5.1). Because the apparent molecular weight of
dry air is 28.97, the amount of oxygen in air by mass
is 20.95 3228.97 23%. Therefore, the mass of
air containing 400 g of oxygen is 4000.23 1700 g.
Hence, for complete combustion, 114 g of C
8
H
18
reacts with 1700 g of air. Therefore, the air–fuel
ratio for ideal combustion is 1700114 15. ■
C
8
H
18
12.5O
2
: 8CO
2
9H
2
O
600
500
400
300
200
100
0
10
–3
10
–2
10
–1
10
0
10
1
10
2
10
3
Diameter D (µ m)
dS/d(log D) (µ m
2
cm
–3
)
Desig-
nation
Aitken nuclei
Large
particles
Giant particles
Sources
Sinks
Resi-
dence
time
Combustion
Windblown
dusts
Gas-to-particle
conversions
Fly ash,
sea-salt, pollens
Coagulation of
Aitken nuclei
Giant particles
from
industries
Cloud droplet
evaporation
Coagulation
Capture by
cloud particles
Precipitation
scavenging
Dry fallout
Less than an hour
in polluted air
or in clouds
Days to
weeks
Hours to
days
Minutes to hours
Fig. 5.11 Schematic curves of particle surface area distributions for urban polluted air (black line), continental air (red line),
and marine air (blue line). Shown below the curves are the principal sources and sinks of atmospheric particles and estimates of
their mean residence times in the troposphere. [Adapted from Atmos. Environ. 9, W. G. N. Slinn, Atmospheric aerosol particles in
surface-level air, 763, copyright (1975), with permission from Elsevier.]
P732951-Ch05.qxd 12/09/2005 09:05 PM Page 177

178 Atmospheric Chemistry
Since 1981, gasoline-powered internal combustion
engines in the United States have used an oxygen
sensor in the exhaust system and a computer-
controlled fuel flow that keeps the air–fuel ratio
to within a few percent of that needed for ideal
combustion. This system, together with a catalytic
converter that reduces the emissions of particularly
undesirable substances, makes the emissions from
modern automobiles quite small. Older automobiles
tended to run with a fuel-rich mixture (i.e., a mixture
that contains less than the required amount of air for
ideal combustion) and these emit considerable pollu-
tants. For example, suppose the 12.5 mol of O
2
in
Exercise 5.7 is reduced to 11.25 mol. Then, 9 mol of
H
2
O is produced together with 5.5 mol of CO
2
and
2.5 mol of CO. In this case, more than one-third of
the carbon in the emissions is the highly poisonous
gas CO. Gas mileage is reduced because the fuel is
not completely burned. However, because the engine
is pumping less air, peak power can actually increase.
Even modern automobiles operate in this “fuel-rich
mode” for almost 1 min after the engine is started,
and for several seconds each time a strong accelera-
tion is required. Therefore, despite the fact that in
most countries automobiles account for only a small
percentage of the total fuel burned, they produce a
large fraction of the CO.
If an automobile has a broken catalyst or does
not have a catalyst, it will emit significant amounts
of unburned (and partially burned) hydrocarbons
(HC), many of which are toxic and carcinogenic.
Measurements of motor vehicle exhausts on high-
ways show that half of the emissions of CO and HC
come from less than 10% of the vehicles, namely
from those that are old or poorly maintained.
Hence the need for regular emission tests of older
automobiles.
The high temperatures associated with combustion
permit oxidation of atmospheric molecular nitrogen
to NO (referred to as thermal NO). At temperatures
below 4500 K, the reactions are
(5.32a)
(5.32b)
(5.32c)
Net: (5.33)
Because of the strong temperature dependence of
reactions (5.32a) and (5.32b), thermal formation of
O
2
N
2
M N 2NO M
2N 2O
2
N 2NO O
2
2O 2N
2
N 2NO 2N
O
2
M N 2O M
NO is highly temperature dependent. Under equi-
librium conditions, these reactions produce maxi-
mum NO concentrations at 3500 K (although
equilibrium is not attained in engines). As the
combustion gases cool rapidly to ambient tempera-
tures, the rates of the reverse reactions are reduced
drastically so that the NO concentration is “frozen
in” at the high temperature. An additional strong
source of NO during combustion is the oxidation
of nitrogen-containing compounds in the fuel
(fuel NO).
As shown in Section 5.5.2, NO
x
emissions from
automobiles play a key role in the formation of pho-
tochemical smog. However, over large geographic
areas, power stations and industries are generally
larger sources of NO
x
than automobiles.
Exercise 5.8 The balanced chemical equation for
the ideal combustion of a general hydrocarbon fuel
C
x
H
y
can be written as
(5.34)
If 1 mol of C
x
H
y
is completely burned, show that
mol of (unreacting) nitrogen will be con-
tained in the emissions. Hence, derive an expression
for the total number of moles of gases in the emis-
sions in terms of x and y.
Solution: From the percentages of O
2
and N
2
in air
by volume (namely 20.9 and 78) we have for air
(5.35)
Therefore, from (5.35), 1 mol of O
2
is associated with
3.73 mol of N
2
in air. From (5.34), mol of
N
2
will be associated with 1 mol of C
x
H
y
and
mol of O
2
burned. Therefore, the total
number of moles of gases in the emissions produced
by the combustion of 1 mol of C
x
H
y
is, from (5.34),
■
Combustion of fuels, particularly coal, dominates
the emissions of sulfur oxides, which are mainly SO
2
.
Heavy-metal smelters (e.g., Ni, Cu, Zn, Pb, and Ag)
can be large local sources of SO
2
.
There are also lower temperature sources of air
pollutants, e.g., leakages of hydrocarbons from natural
x
y
2
3.7(x
y
4
).
3.7(x
y
4
)
3.7(x
y
4
)
number of moles of O
2
number of moles of N
2
20.9
78
1
3.73
3.7(x
y
4
)
C
x
H
y
x
y
4
O
2
: xCO
2
y
2
H
2
O
P732951-Ch05.qxd 12/09/2005 09:05 PM Page 178

5.5 Air Pollution 179
gas lines, organics from the evaporation of solvents,
and nitrogen compounds from fertilizers.
5.5.2 Smogs
The term smog derives from smoke and fog; it was
originally coined to refer to heavily polluted air that
can form in cities (generally in winter under calm,
stable and moist conditions) due to the emissions of
sulfur dioxide and aerosols from the burning of fossil
fuels (primarily coal and oil). The term is now applied
to all forms of severe air pollution, particularly in
urban areas, that restrict visibility.
a. London (or classical) smog
Prior to the introduction of air pollution abatement
laws in the latter part of the 20th century, many large
cities in Europe and North America regularly suf-
fered from severe smogs. The London smogs were
sufficiently notorious that such pollution became
known as London smog.
24
In the London (or classi-
cal) type of smog, particles swell in size under high
relative humidity and some of the particles serve as
nuclei on which fog droplets form. Sulfur dioxide gas
dissolves in the fog droplets where it is oxidized to
form sulfuric acid (see Section 6.8.5).
In December 1952, cold air moved from the
English Channel and settled over London, producing
a pollution-trapping inversion fog. Over the next
5 days London experienced its worst air pollution
episode. The smog was so thick that people had to
grope their way along the streets, buses crawled
along at a walking pace led by pedestrians with flash-
lights, and indoor events were canceled because the
stage could not be seen.
25
By the time the smog
had lifted, 4000 people had died of respiratory prob-
lems, and the smog was implicated in an additional
8000 deaths in the months that followed. In the Great
Smog, as it was called, SO
2
reached peak mixing ratios
of 0.7 ppmv (compared to typical annual mean mix-
ing ratios of 0.1 ppmv in polluted cities with large
coal usage), and the peak particle concentrations
were 1.7 mg m
3
(compared to 0.1 mg m
3
under
more typical urban-polluted conditions). Interest-
ingly, there is no direct evidence that even these high
concentrations of pollutants would, in themselves,
have caused fatalities (see Box 5.4).
After the Great Smog, laws were passed in Britain
and elsewhere banning the use of coal on open fires
for domestic heating and the emissions of black
smoke, and requiring industries to switch to cleaner-
burning fuels. Nevertheless, pollution is still a serious
problem in many cities in Europe and the United
States. Also, many large cities, particularly in devel-
oping countries (e.g., China, India), still suffer from
London-type smogs due to the burning of coal and
wood and to the lack of strict air pollution controls.
Even with stringent pollution controls, increasing
numbers of automobiles in large cities can produce
high concentrations of NO, which can then be con-
verted to NO
2
by
(5.36)
The NO
2
can then be involved in the production of
O
3
(see Section 5.5.2b). Because the magnitude of the
rate coefficient for (5.36) increases with decreasing
temperature, the production of NO
2
by (5.36) can
become significant in cities that have cold winters.
2NO O
2
: 2NO
2
24
In 1661 the English diarist John Evelyn noted the effects of industrial emissions (and, no doubt, domestic wood burning) on the
health of plants and people. In the 17
th
century there were days when a plume of smoke half a mile high and 20 miles wide emanated from
London. Evelyn suggested that industries be placed outside of towns and that they be equipped with tall chimneys to disperse the smoke.
What are the pros and cons of this suggestion?
25
Such events were experienced by one of the authors (PVH) of this book.
Leonardo da Vinci noted, alongside a sketch of the
lung, “dust causes damage.” Some 500 years later
evidence began to mount supporting this conclu-
sion. There appears to be an increasing risk of lung
cancer and mortality with increasing concentra-
tion of the total mass of particulate matter (PM)
in the air, even at concentrations below 0.1 mg m
3
.
However, because other pollutants, such as SO
2
5.4 Effects of Particulates on Human Health
Continued on next page
P732951-Ch05.qxd 12/09/2005 09:05 PM Page 179

180 Atmospheric Chemistry
b. Photochemical (or Los Angeles) smog
During the second half of the 20th century emissions
from automobiles became increasingly important as
a source of pollutants in many urban areas. When
subjected to sunlight and stagnant meteorological
conditions, the combination of chemical species in
such strongly polluted urban air can lead to photo-
chemical (or Los Angeles-type) smog.These smogs
are characterized by high concentrations of a large
variety of pollutants, such as nitrogen oxides, O
3
,CO,
hydrocarbons, aldehydes (and other materials that
are eye irritants), and sometimes sulfuric acid as
well. The chemical reactions that lead to photo-
chemical smog are complex and still not completely
understood. However, some of the major chemical
reactions thought to be involved are described here.
Photochemical smogs are due to the interactions
between a variety of organic pollutants (e.g., hydro-
carbons such as ethylene and butane) and oxides of
nitrogen. The reactions start with (5.15) and (5.16),
which form O
3
. However, the O
3
is then depleted by
the rapid reaction (5.17). As shown in Exercise 5.5,
if there were no other reactions (5.15)–(5.17) would
lead to a steady-state concentration of O
3
given by
(5.18). However, (5.18) predicts O
3
concentrations in
urban-polluted air of only 0.03 ppmv, whereas typi-
cal values are well above this concentration and can
reach 0.5 ppmv. Therefore, other chemical reactions
must be involved in photochemical smogs. Most
effective are reactions that oxidize NO to NO
2
with-
out consuming O
3
, since this would allow net O
3
production, leading to a buildup of ozone concentra-
tions during the day. The OH radical can initiate a
chain reaction that can act in this way by attacking
the hydrocarbon pollutants in urban air, e.g.,
(5.37)
or
(5.38)
or
(5.39)
The resulting radicals, CH
3
in (5.37), H in (5.38), and
CH
3
CO in (5.39), then become involved in reactions
that oxidize NO to NO
2
and regenerate OH. For
example, CH
3
from (5.37) can initiate the series of
reactions
(5.40a)
(5.40b)
(5.40c)
(5.40d)
The net results of (5.40), together with (5.37), are
(5.41)
CH
4
2O
2
2NO : H
2
O 2NO
2
HCHO
HO
2
NO : NO
2
OH
CH
3
O O
2
: HCHO HO
2
CH
3
O
2
NO : CH
3
O NO
2
CH
3
O
2
: CH
3
O
2
OH CH
3
CHO : H
2
O CH
3
CO
OH CO : H CO
2
OH CH
4
: H
2
O CH
3
and O
3
, generally increase with increasing concen-
trations of PM, it is difficult to separate the health
effects of the different pollutants. Weather also
plays a confounding role because of the effects of
relative humidity on particles and of temperature
on humans.
Most statistical studies of the effects of PM on
human health have utilized the total mass of PM
with diameters below 10
m (PM-10). However,
there are several reasons for suspecting that parti-
cles 1
m in diameter have a more deleterious
effect on human health than the larger particles
that dominate PM-10 measurements. Smaller par-
ticles can penetrate deeper into the small airways
of the lungs. There is also evidence that very small
5.4 Continued
particles (0.05
m diameter) have enhanced tox-
icity. A recent examination of lung tissue samples
preserved from the victims of the Great Smog of
London (see Section 5.5.2) has revealed high con-
centrations of small particles and metals such as
lead of the type that derive from coal combustion
and diesel engines. It might not be chance coin-
cidence that 1952 (the year of the Great Smog)
was also the year that London completed a change
from electric trams to diesel buses, which emit
many small toxic particles.
Despite the introduction of air pollution con-
trol strategies, small particles are still prevalent
in significant number concentrations in large
cities.
P732951-Ch05.qxd 12/09/2005 09:05 PM Page 180
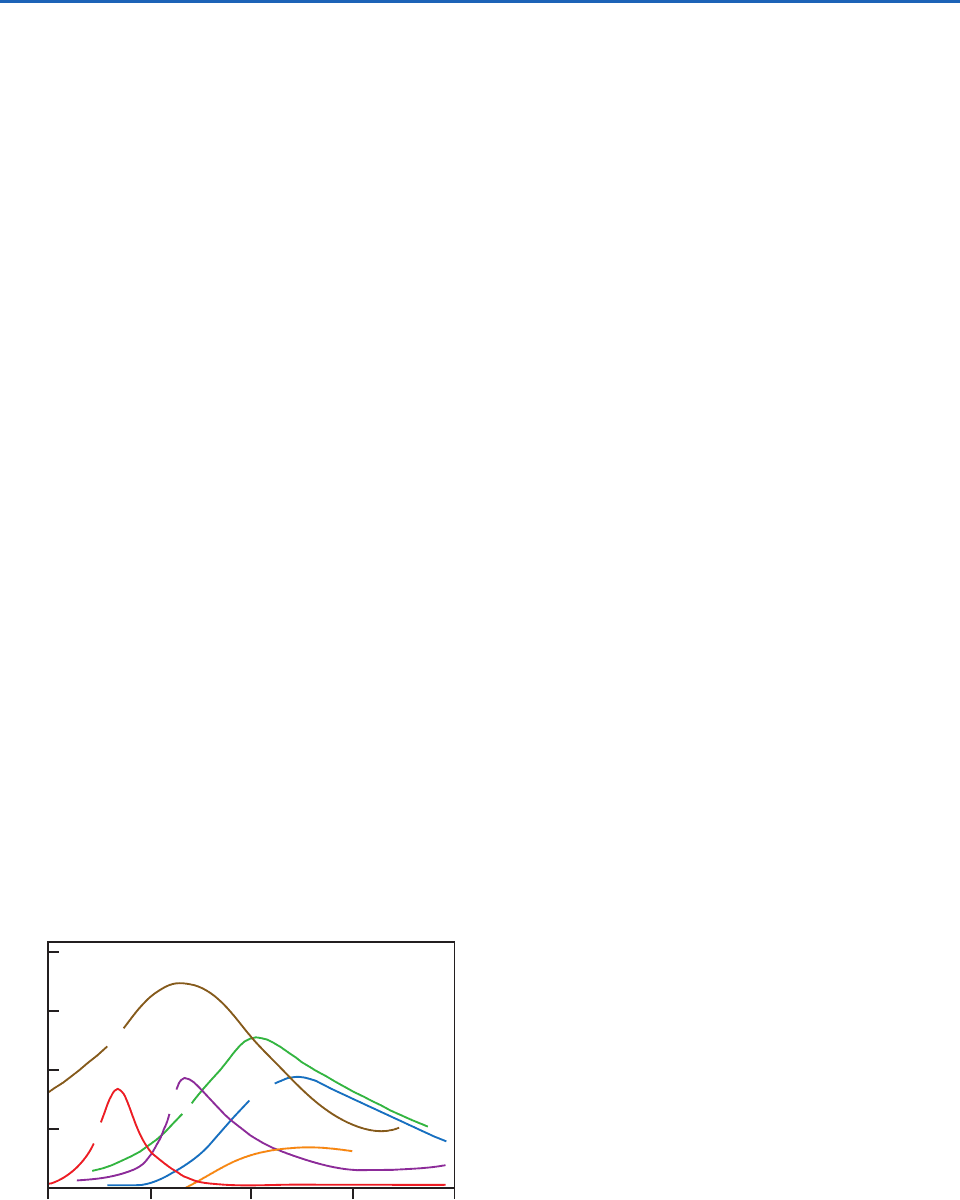
5.5 Air Pollution 181
Thus, in this case, the oxidation of CH
4
results in
the oxidation of NO to NO
2
without consuming O
3
.
Reaction (5.41) produces formaldehyde (HCHO),
which is an eye irritant and a source of HO
x
[see
(5.26) and (5.27)].
Similarly, the acetyl radical (CH
3
CO) from (5.39)
is involved in a series of reactions leading to the
methyl radical CH
3
and the peroxyacetyl radical
(CH
3
COO
2
). The methyl radical oxidizes NO by
reactions (5.40), and the peroxyacetyl radical reacts
with nitrogen dioxide
(5.42)
The chemical species on the right-hand side of (5.42)
is the vapor of a colorless and dangerously explosive
liquid called peroxyacetyl nitrate (PAN), which is
an important component of photochemical smogs
and another major eye irritant. Other alkenes oxidize
NO to NO
2
without consuming O
3
and regenerate
OH and can do so faster than the aforementioned
reactions.
Shown in Fig. 5.12 are typical variations through
the course of a day in the concentrations of some of
the major components of photochemical smogs in
Los Angeles. Ozone precursors (NO
x
and hydrocar-
bons) build up during the morning rush hour, and
aldehydes, O
3
, and PAN peak in the early afternoon.
The role of polycyclic aromatic hydrocarbons
(PAHs) in air pollution and public health was first
realized in the early 1940s following the discovery that
organic extracts of particles from polluted air (e.g.,
CH
3
COO
2
NO
2
: CH
3
COO
2
NO
2
benzo[a]pyrene) produce cancer in laboratory exper-
iments on animals. PAHs are emitted by diesel and
gasoline engines, coal-fired electric power-generating
plants, biomass burning, and cigarettes. They are pres-
ent in air as volatile particulates and gases. Reactions
initiated by OH in the day and NO
3
at night convert
gaseous PAHs to nitro-PAH derivatives, which are
responsible for 50% of the mutagenic activity of
respirable airborne particles in southern California.
5.5.3 Regional and Global Pollution
The effects of anthropogenic pollution now extend
to regional and global scales. Europe, Russia, the
northeastern United States, India, and large areas of
southeastern Asia are regularly covered by enormous
palls of polluted air that reduce visibility significantly,
produce acid deposition, soil and erode buildings
and other materials, and have deleterious effects on
human health, animals, and plants.
The fact that pollutants can be transported over
large distances is well illustrated by air pollution
episodes in the Arctic, known as arctic haze,which
can be as severe as those in cities. The pollutants
originate from fossil-fuel combustion, smelting, and
other industrial processes in northern Europe and
Russia. The pollutants are transported to the Arctic
by synoptic-scale flow patterns, primarily from
December to April. Because the arctic atmosphere
is generally stably stratified during this time of
the year, vertical mixing is limited; also, precipitation
is rare so that wet removal processes are weak.
Consequently, the pollutants can be transported over
large distances with relatively little dilution. A major
contributor to arctic haze is SO
2
, which is converted
to sulfate particles over the long transport distances.
Glacial records show that air pollution in the
Arctic has increased markedly since the 1950s, paral-
leling the increases in SO
2
and NO
x
emissions in
Europe. Interestingly, ice cores from Greenland show
unusually high lead concentrations from 500 B.C.
to 300 A.D. This is attributed to Greek and Roman
lead and silver mining and smelting activities, which
apparently polluted large regions of the northern
hemisphere. However, cumulative lead deposits in
the Greenland ice during these eight centuries were
only 15% of those caused by the widespread use
of lead additives in gasoline from 1930 to 1995.
Lead additives to gasoline were eliminated in the
United States in 1986, more than 60 years after their
introduction.
Time of day (hour)
Aldehydes
PAN
O
3
NO
2
NO
Hydrocarbons
Mixing ratio (ppbv)
4
812
16
20
200
400
Fig. 5.12 Typical variations during the course of a day of
some important pollutants in photochemical smogs in Los
Angeles. [Adapted from P. A. Leighton, Photochemistry of Air
Pollution, Academic Press, New York, 1961, p. 273, with
permission of Elsevier.]
P732951-Ch05.qxd 12/09/2005 09:05 PM Page 181
182 Atmospheric Chemistry
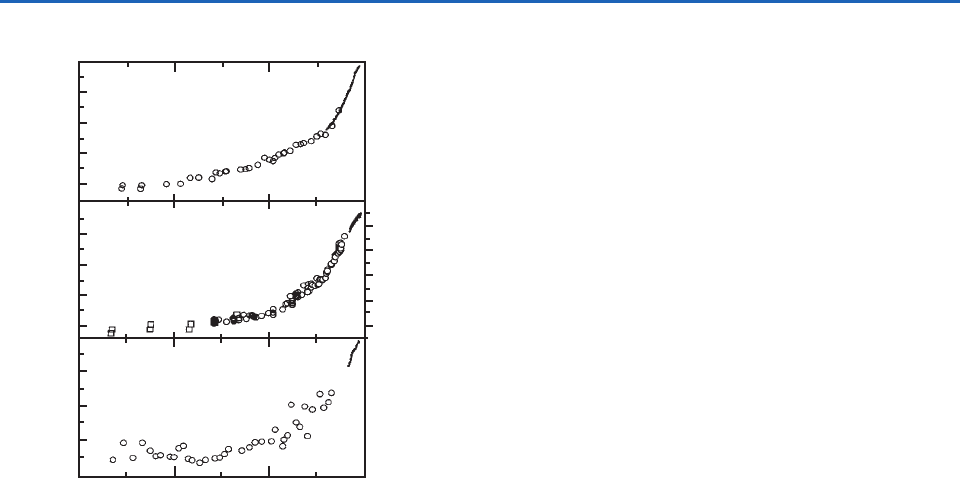
Anthropogenic influences are now apparent on a
global scale, as illustrated by the worldwide increase
in CO
2
concentrations since the industrial revolution
(Fig. 5.13a). Other trace gases (e.g., CH
4
and N
2
O)
also show increasing concentrations worldwide over
the past 150 years or so (Figs. 5.13b and 5.13c).
5.6 Tropospheric Chemical
Cycles
The reservoirs of chemical species in the Earth sys-
tem are the solid Earth, the hydrosphere (oceans and
fresh water), the cryosphere (ice and snow), the
biosphere, and the atmosphere. Chemical species can
be transferred between these reservoirs. Because
under steady-state conditions, a chemical species can-
not accumulate indefinitely in any of the reservoirs,
there must be continual cycling of species through
the various reservoirs. This is termed geochemical
cycling.
The geochemical cycling of carbon has been dis-
cussed in some detail in Chapter 2. Here we consider
the tropospheric portions of the geochemical cycles
of two other important chemical species, namely
nitrogen and sulfur. We will be concerned with rela-
tively rapid interchanges involving the atmosphere
and other reservoirs (generally the oceans and the
biosphere). Many aspects of global chemical cycles
are not well understood; therefore, in many cases, the
magnitudes of the sources and sinks given here are
only rough estimates. Also, the relative importance of
the various species is not determined exclusively by
the magnitude of the emissions: atmospheric resi-
dence times must also be taken into account.
5.6.1 The Nitrogen Cycle
Nitrogen gas (N
2
) constitutes more than 99.99% of
the nitrogen present in the atmosphere, and N
2
O (an
important greenhouse gas) makes up more than 99%
of the rest of the nitrogen. The other nitrogen species
in the atmosphere are therefore present in only trace
concentrations (see Table 5.1), but are nonetheless
of crucial importance in atmospheric chemistry. For
example, NH
3
is the only basic gas in the atmosphere.
Therefore, it is solely responsible for neutralizing
acids produced by the oxidation of SO
2
and NO
2
;the
ammonium salts of sulfuric and nitric acid so formed
become atmospheric aerosols. Nitric oxide (NO) and
NO
2
play important roles in both tropospheric and
stratospheric chemistry.
All of the nitrogen-containing gases in the air are
involved in biological nitrogen fixation and denitrifi-
cation. Fixation refers to the reduction and incorpo-
ration of nitrogen from the atmosphere into living
biomass. This transformation is accomplished by bac-
teria that are equipped with a special enzyme system
for this task; the usual product is NH
3
.The term fixed
nitrogen refers to nitrogen contained in chemical
compounds that can be used by plants and microor-
ganisms. Under aerobic (i.e., oxygen-rich) conditions,
other specialized bacteria can oxidize ammonia to
nitrite and then to nitrate; a process referred to as
nitrification. Most plants use nitrate taken through
their roots to satisfy their nitrogen needs. Some of the
nitrate undergoes bacterial reduction to N
2
and N
2
O
(termed denitrification), which returns fixed nitrogen
from the biosphere to the atmosphere. In this case,
nitrate acts as the oxidizing agent; therefore, denitri-
fication generally occurs under anaerobic conditions,
where oxygen is unavailable. Fixed nitrogen can also
be returned from plants to the atmosphere in the
CO
2
(ppbv)
N
2
O (ppbv)
CH
4
(ppbv)
a) Carbon dioxide (CO
2
)
b) Methane (CH
4
)
c) Nitrous oxide (N
2
O)
360
340
320
300
280
310
300
290
280
270
1800
1600
1400
1200
1000
800
1800
19001700 2000
Years A.D.
Fig. 5.13 Changes in the concentrations of (a) CO
2
,
(b) CH
4
, and (c) N
2
O over 300 years deduced from analy-
sis of Greenland and Antarctic ice cores. [Adapted from
D. J. Wuebbles et al., “Changes in the chemical composition
of the atmosphere and potential impacts,” in Brasseur, Prinn
and Pszenny, eds, Atmospheric Chemistry in a Changing World,
Springer, 2003, p. 12, Copyright 2003 Springer-Verlag.]
P732951-Ch05.qxd 12/09/2005 09:05 PM Page 182
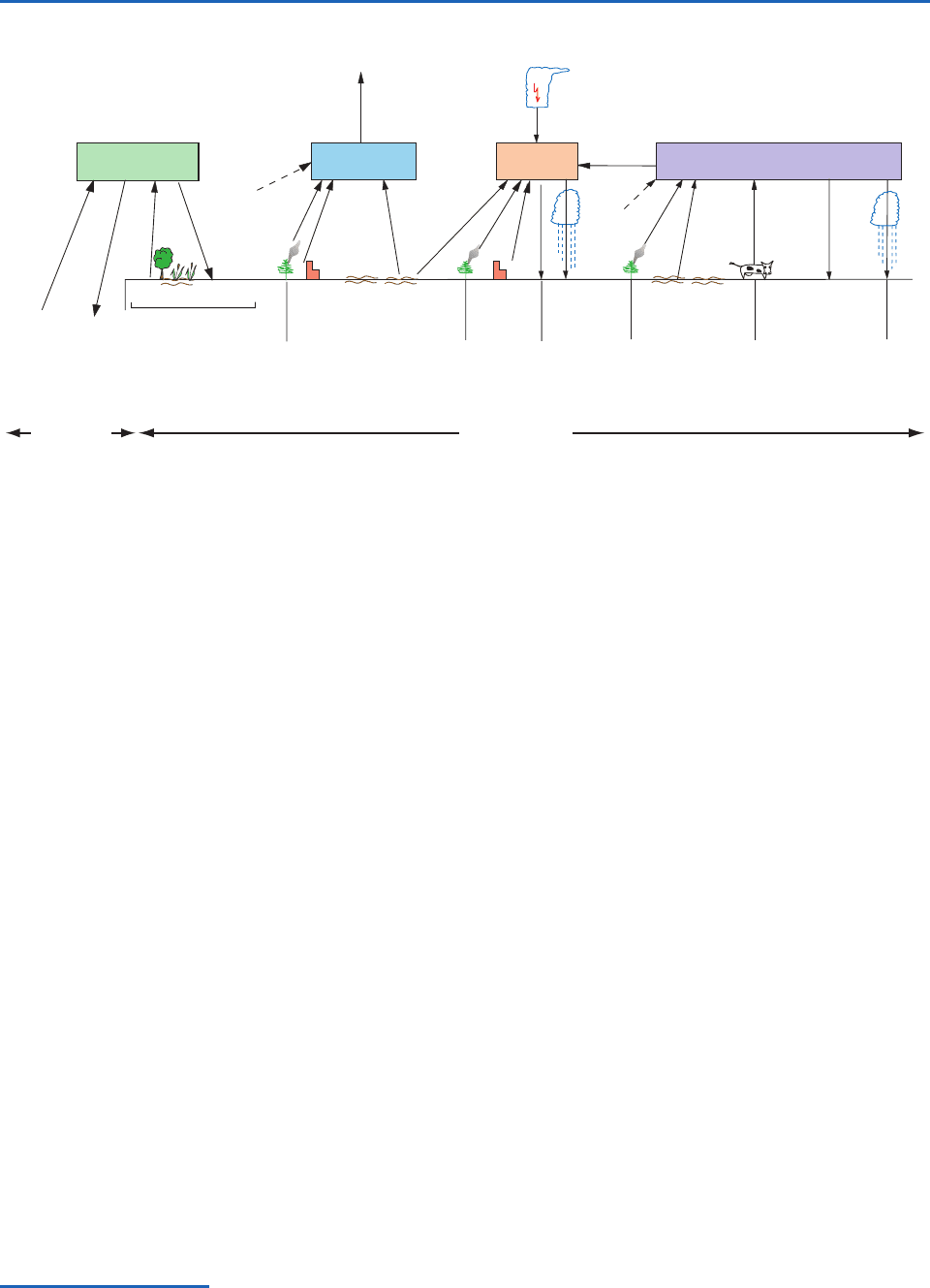
5.6 Tropospheric Chemical Cycles 183
form of N
2
O. Biomass burning returns fixed nitrogen
to the atmosphere as N
2
,NH
3
,N
2
O, and NO
x
.
The principal sources and sinks of nitrogen-
containing species in the atmosphere are shown in
Fig. 5.14. Assuming that fixation and denitrification
of N
2
are in approximate balance, the main atmos-
pheric sources of nitrogen-containing species are
biogenic emissions from the terrestrial and marine
biosphere (NH
3
,N
2
O, and NO
x
), decomposition of
proteins and urea from animals (NH
3
), biomass
burning and fossil fuel consumption (NO
x
,NH
3
,
and N
2
), and lightning (NO
x
). Nitrous oxide (N
2
O)
is by far the largest reservoir of nitrogen in the
atmosphere and, except for N
2
, it has a much longer
residence time than other nitrogen species.
The main sinks of nitrogen-containing species
are wet removal by precipitation (NH
3
and NO
x
as
dry deposition (NO
x
and NH
3
), and the chem-
ical breakdown of N
2
O in the stratosphere. Because
anthropogenic sources of NH
3
,N
2
O, and NO
x
(from
fossil fuel consumption, biomass burning, and agri-
cultural nitrate fertilization) are appreciable, human
NO
3
),
activities may be causing significant perturbations in
the budgets of these species in the atmosphere.
5.6.2 The Sulfur Cycle
Sulfur species enter the atmosphere primarily in a
chemically reduced state. The most important reduced
sulfur gases are H
2
S, DMS, COS, and CS
2
.Their main
natural sources are biogenic reactions in soils, marsh-
land, and plants. Biogenic reactions in the oceans,
due primarily to phytoplankton, are sources of DMS,
COS, and CS
2
.When reduced sulfur gases are
released into the oxygen-rich atmosphere, they are
oxidized to SO
2
and then over 65% of the SO
2
is oxi-
dized to (the remainder of the SO
2
is removed
by dry deposition). Estimates of the fluxes of these
natural emissions of sulfur gases and their transfor-
mations to SO
2
and are given in Fig. 5.15.
It can be seen from Fig. 5.15 that DMS dominates
the emissions of sulfur gases from the oceans.
26
Most
of the sulfides in the air are oxidized rapidly by the
OH radical; therefore, their residence times are only
SO
2
4
SO
2
4
NH
3
+ NH
+
[0.6 Tg(N)]
5
3
Biomass
burning
[OCEANS]
2
26 15
32
12
Soil emissions
including
fertilizer loss
140
N
2
[2 x 10
9
Tg(N)]
Biomass
burning
Biomass burning
and fossil fuel
consumption and
industrial
processes
Fixation
Denitrification
Dry
removal
of NO
x
9
4
140100
2
(
(
(
(
(
(
(
(
(
(
(
(
(
(
(
(
(
(
Fixation
Denitrification
100
[11 x 10
6
Tg(N)]
N
2
O
[1300 Tg(N)]
by bacteria in plants,
soils and water
6
Soil emissions
from nitrification,
denitrification
and fertilizers
Fossil
fuel
combustion
To the stratosphere
NO
x
[0.15 Tg(N)]
6 12 20
20
25
Wet
removal
of NO
x
Decomposition
of protein,
urea from
domestic (23)
and wild
animals (3)
[OCEANS]
7
Lightning
Dry
removal
of NO
3
and
NH
4
+
Wet
removal
of NH
3
and
NH
4
+
0.5
4
OCEANS
CONTINENTS
[9 x 10
9
Tg(N)]
Fig. 5.14 Principal sources and sinks of nitrogen-containing gases in the atmosphere. Numbers alongside the arrows are esti-
mates of average annual fluxes in Tg(N) per year; various degrees of uncertainty, some quite large, are associated with all of the
fluxes. Numbers in square brackets are total amounts of the species in the atmosphere. [Adapted from P. V. Hobbs, Introduction
to Atmospheric Chemistry, Camb. Univ. Press, 2000, p. 148. Reprinted with the permission of Cambridge University Press.]
26
An enormous amount of sulfate is ejected into the air from the oceans in the form of sea salt. However, because these are relatively
large particles, they are recycled quickly back to the ocean.Therefore, they do not have a significant effect on the global sulfur cycle.
P732951-Ch05.qxd 12/09/2005 09:05 PM Page 183
