Voet D., Voet Ju.G. Biochemistry
Подождите немного. Документ загружается.

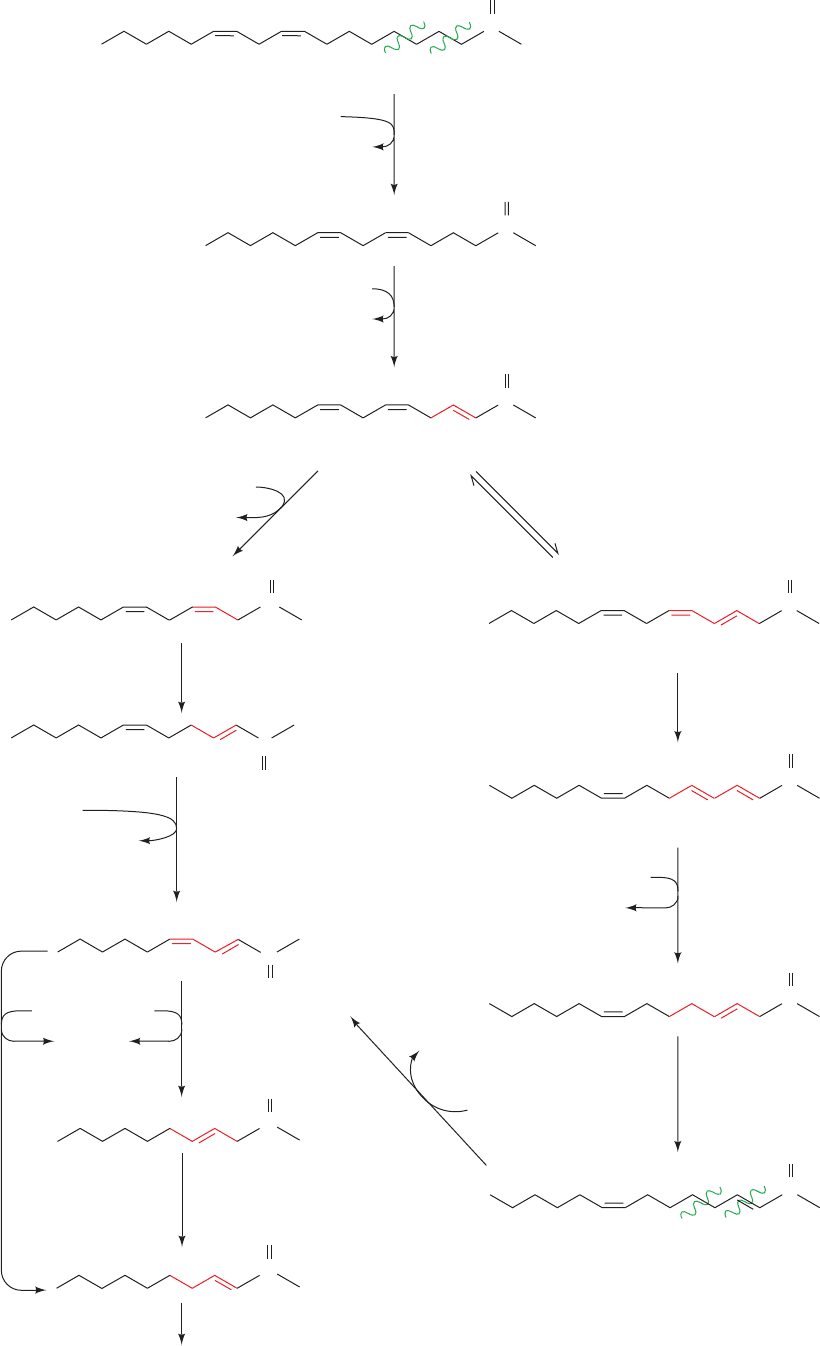
Section 25-2. Fatty Acid Oxidation 951
O
SCoA
2NAD
+
+ 2FAD + 2CoASH
2 rounds of β oxidation
γ
β
enoyl-CoA isomerase
3,5–2,4-dienoyl-CoA
isomerase
Problem 1:
,␥ double bond
Problem 3:
Isomerization
SCoA
C
O
one round of  oxidation
the first oxidation of
the next round
NAD
+
+ FAD + CoASH
NADH
+ FADH
2
+ Acetyl-CoA
3,2-enoyl-CoA
isomerase (mammalian)
Problem 2:
⌬
4
double bond
SCoA
C
O
C
O
SCoA
Continuation of  oxidation
2,4-dienoyl-CoA
reductase
(mammalian)
NADPH
+ H
+
NADP
+
2,4-dienoyl-
CoA reductase
(E. coli)
C
+
2
3
4
4
2
3
4
2
3
O
SCoA
C
C
O
SCoA
2,4-dienoyl-CoA
reductase
NADPH
+ H
+
NADP
+
3,2-enoyl-CoA
isomerase
2NADH + 2FADH
2
+ 2acetyl-CoA
FAD
FADH
2
acyl-CoA dehydrogenase
Linoleic acid
2,5,8-Trienoyl-CoA
O
SCoA
C
O
SCoA
C
8
12 9
5
O
SCoA
C
853
42
NAD
+
+ CoASH
2NADH
+ 2FADH
2
+ 2Acetyl-CoA
2NAD
+
+ 2FAD
+ 2CoA
NADH
+ acetyl-CoA
completion of
β-oxidation round
2 rounds
of -oxidation
3,5,8-Trienoyl-CoA
⌬
2
, ⌬
4
, ⌬
8
-Trienoyl-CoA
O
SCoA
C
853
42
O
SCoA
C
8
53
42
O
SCoA
C
8
8
3
2
3
2
3,2-enoyl-CoA
isomerase
JWCL281_c25_940-1018.qxd 6/18/10 7:27 AM Page 951
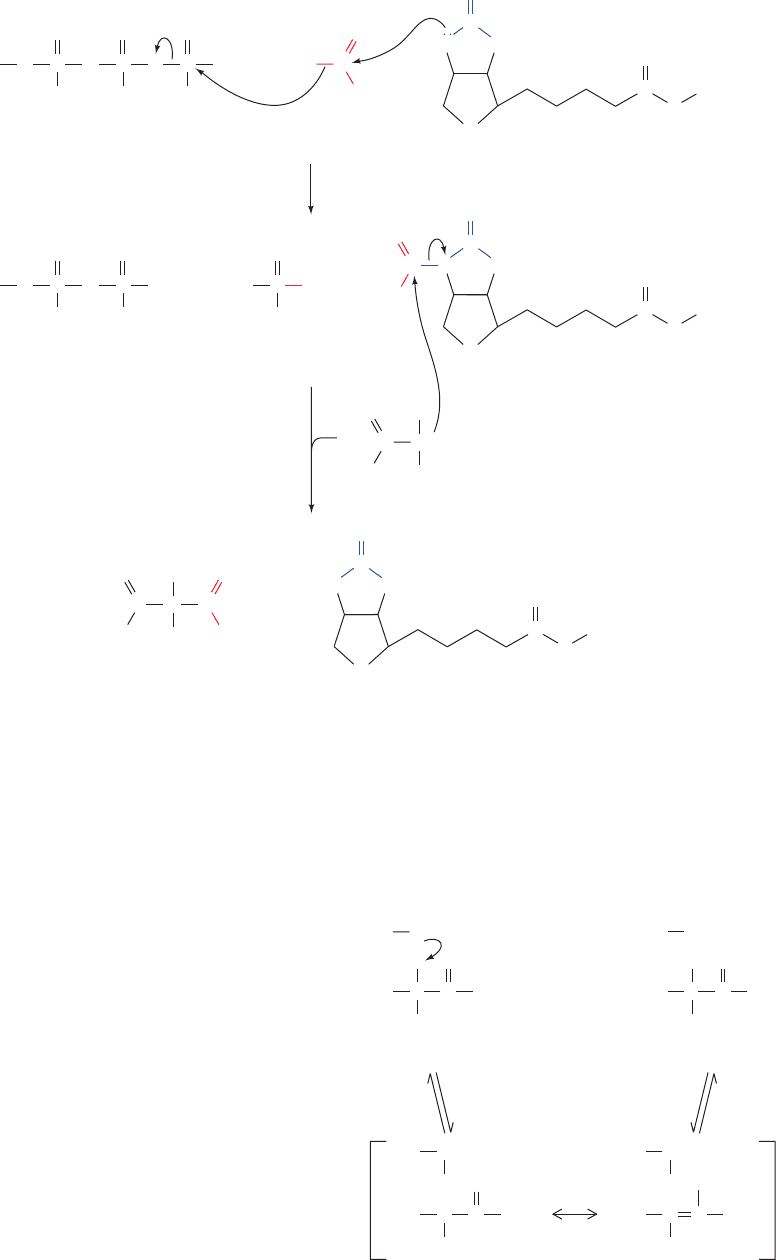
which is driven by the concomitant hydrolysis of ATP to
ADP and P
i
, activates the resulting carboxyl group for
transfer without further free energy input.
2. Stereospecific transfer of the activated carboxyl
group from carboxybiotin to propionyl-CoA to form (S)-
methylmalonyl-CoA. This step occurs via nucleophilic
attack on carboxybiotin by a carbanion at C2 of propionyl-
CoA (see below).
These two reaction steps occur at different catalytic sites
on propionyl-CoA carboxylase. It therefore appears that
the biotinyllysine linkage attaching the biotin ring to the
enzyme forms a flexible tether that permits the efficient
transfer of the biotin ring between these two active sites as
occurs in pyruvate carboxylase (Section 23-1Ae).
Formation of the C2 carbanion in the second stage of
the propionyl-CoA carboxylase reaction involves removal
of a proton to a thioester. This proton is relatively acidic
since, as we have seen in Section 25-2Cd, the negative
charge on a carbanion to a thioester can be delocalized
into the thioester’s carbonyl group. This explains the rela-
tively convoluted path taken in the conversion of propionyl-
CoA to succinyl-CoA (Fig. 25-18). It would seem simpler, at
least on paper, for this process to occur in one step, with car-
boxylation occurring on C3 of propionyl-CoA so as to form
succinyl-CoA directly. Yet, the C3 carbanion required for
952 Chapter 25. Lipid Metabolism
2,4-dienoyl-CoA reductase reduces the
4
double bond.The
E. coli reductase produces trans-2-enoyl-CoA, a normal
substrate of oxidation. The mammalian reductase, how-
ever, yields trans-3-enoyl-CoA, which, to proceed along
the -oxidation pathway, must first be isomerized to trans-
2-enoyl-CoA by 3,2-enoyl-CoA isomerase.
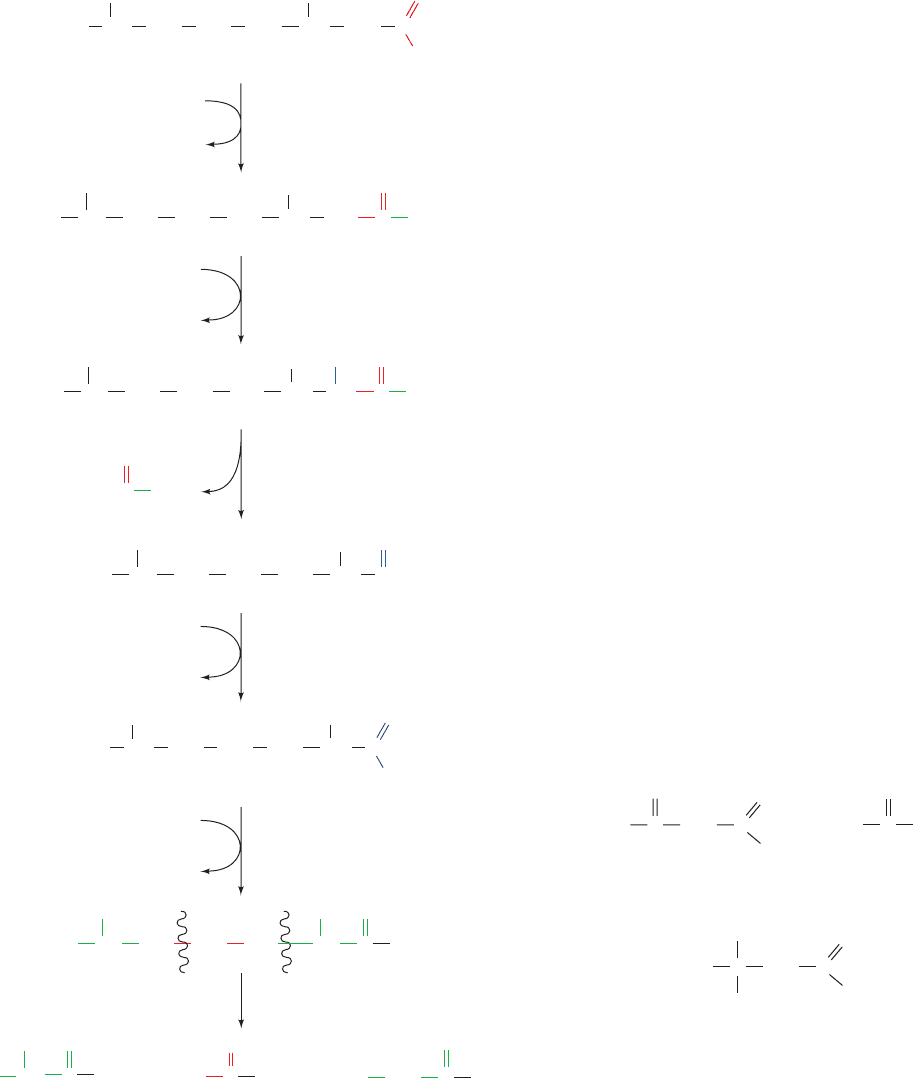
Problem 3: The Unanticipated Isomerization
of 2,5-Enoyl-CoA by 3,2-Enoyl-CoA Isomerase
Mammalian 3,2-enoyl-CoA isomerase catalyzes a re-
versible reaction that interconverts
2
and
3
double
bonds. A carbonyl group is stabilized by being conju-
gated to a
2
double bond. However, the presence of a
5
double bond (originating from an unsaturated fatty acid
with a double bond at an odd-numbered C atom such as
the
9
double bond of linoleic acid) is likewise stabilized
by being conjugated with a
3
double bond (right-hand
pathway of Fig. 25-17). If a 2,5-enoyl-CoA is converted by
3,2-enoyl-CoA isomerase to 3,5-enoyl-CoA, which occurs
up to 20% of the time, another enzyme is necessary to
continue the oxidation: 3,5–2,4-Dienoyl-CoA isomerase
isomerizes the 3,5-diene to a 2,4-diene, which is then re-
duced by 2,4-dienoyl-CoA reductase and isomerized by
3,2-enoyl-CoA isomerase as in Problem 2 above.After two
more rounds of oxidation, the cis-
4
double bond origi-
nating from the cis-
12
double bond of linoleic acid is also
dealt with as in Problem 2.
E. Oxidation of Odd-Chain Fatty Acids
Most fatty acids have even numbers of carbon atoms and
are therefore completely converted to acetyl-CoA. Some
plants and marine organisms, however, synthesize fatty
acids with an odd number of carbon atoms. The final
round of  oxidation of these fatty acids forms propionyl-
CoA, which, as we shall see, is converted to succinyl-CoA
for entry into the citric acid cycle. Propionate or propionyl-
CoA is also produced by oxidation of the amino acids
isoleucine, valine, and methionine (Section 26-3E). Fur-
thermore, ruminant animals such as cattle derive most of
their caloric intake from the acetate and propionate pro-
duced in their rumen (stomach) by bacterial fermentation
of carbohydrates. These products are absorbed by the ani-
mal and metabolized after conversion to the correspon-
ding acyl-CoA.
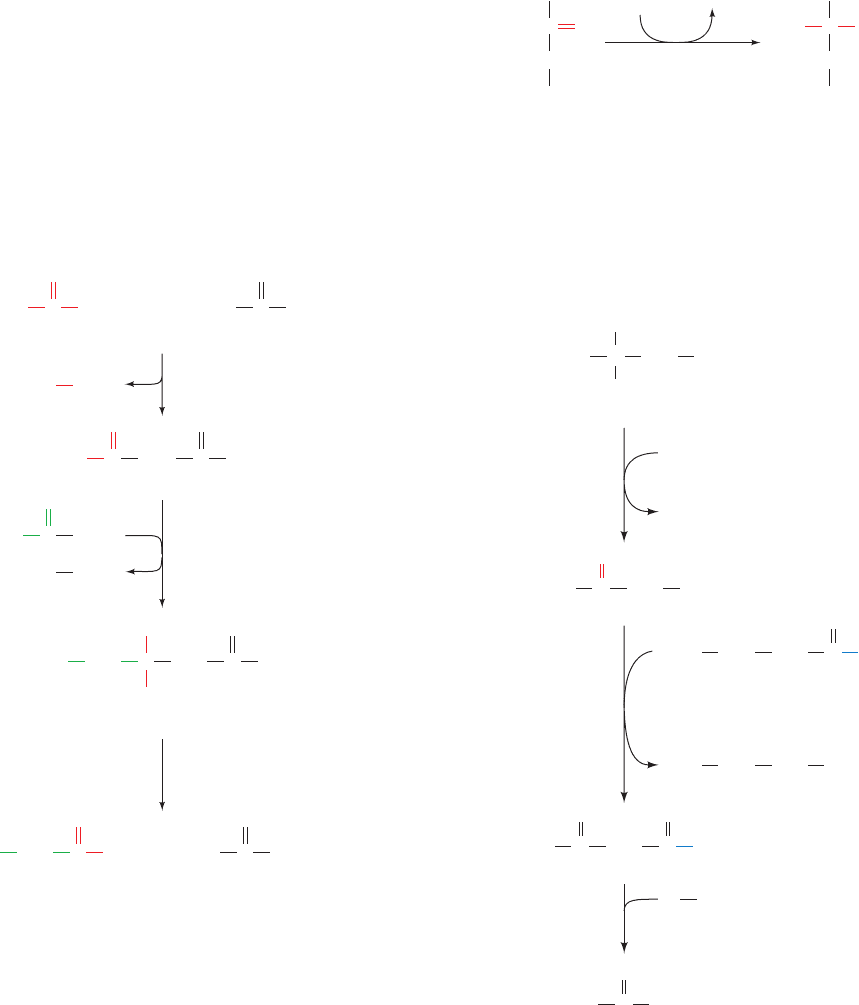
a. Propionyl-CoA Carboxylase Has
a Biotin Prosthetic Group
The conversion of propionyl-CoA to succinyl-CoA in-
volves three enzymes (Fig. 25-18). The first reaction is that
of propionyl-CoA carboxylase, a biotin-dependent enzyme
(Section 23-1Ab) with subunit composition
6
6
.The reac-
tion, which resembles that catalyzed by the homologous
biotin-containing enzyme pyruvate decarboxylase (Section
23-1Ac), occurs in two steps (Fig. 25-19):
1. Carboxylation of biotin at N1¿ by bicarbonate ion as
in the pyruvate carboxylase reaction (Fig. 23-4). This step,
Figure 25-18 Conversion of propionyl-CoA to succinyl-CoA.
CH
2
CH
3
SCoA
propionyl-CoA carboxylase
methylmalonyl-CoA racemase
ATP
+ CO
2
ADP + P
i
O
C
SCoA
OH
CC
CH
3
Propionyl-CoA
(S)-Methylmalonyl-CoA
–
O
2
C
–
O
2
C
methylmalonyl-CoA mutase
SCoA
OH
CC
CO
2
–
(R)-Methylmalonyl-CoA
Succinyl-CoA
CH
3
SCoA
O
C
CH
2
CH
2
JWCL281_c25_940-1018.qxd 4/20/10 1:58 PM Page 952
–
Resonance-stabilized carbanion
intermediate
(R)-Methylmalonyl-CoA
O
C SCoA
O
C
–
O
2
C
CH
3
BEnz
H
C SCoA
O
–
C
–
O
2
C
CH
3
CH
3
BEnz
H
C CoA
O
C
–
O
2
C
H
3
C
BEnz
H
(S)-Methylmalonyl-CoA
O
C CoA
OH
C
–
O
2
C
BEnz
such a carboxylation has a high free energy of formation.
Nature has instead chosen a more facile, albeit less direct
route, which carboxylates propionyl-CoA at a more reactive
position and then rearranges the C
4
skeleton to form the de-
sired product.
b. Methylmalonyl-CoA Mutase Contains
a Coenzyme B
12
Prosthetic Group
Methylmalonyl-CoA mutase, which catalyzes the third
reaction of the propionyl-CoA to succinyl-CoA conversion
(Fig. 25-18), is specific for (R)-methylmalonyl-CoA even
though propionyl-CoA carboxylase stereospecifically syn-
thesizes (S)-methylmalonyl-CoA. This diversion is recti-
fied by methylmalonyl-CoA racemase, which interconverts
the (R) and (S) configurations of methylmalonyl-CoA,
presumably by promoting the reversible dissociation of its
acidic -H via formation of a resonance-stabilized carban-
ion intermediate:
Section 25-2. Fatty Acid Oxidation 953
Figure 25-19 The propionyl-CoA carboxylase reaction. (1)
The carboxylation of biotin with the concomitant hydrolysis of
ATP is followed by (2) the carboxylation of a propionyl-CoA
O
A
denosine O
O
O
–
P
O
O
–
P
O
O
–
O
O
–
P
O HO C
O
O
–
O
–
O
–
O
++
ATP
O
A
denosine O
O
O
–
P
O
O
–
P
O
O
–
P
O
––
O OH++
ADP
O
C
O
C
N
H
S
Biotinyl–enzymeHCO
3
–
1
2
P
i
HN
NH
E
O
C
O
C
N
H
S
Carboxybiotinyl–enzyme
NC
O
CoAS
C
–
H
CH
3
CH
3
C
O
CoAS
C
NH
E
H
C C
+
(S)-Methylmalonyl-CoA
O
C
O
C
N
H
S
Biotinyl–enzyme
HN
NH
E
carbanion by its attack on carboxybiotin. Each reaction step
probably involves the intermediate formation of CO
2
as occurs
in the pyruvate carboxylase reaction (Fig. 23-4).
JWCL281_c25_940-1018.qxd 4/20/10 1:59 PM Page 953
954 Chapter 25. Lipid Metabolism
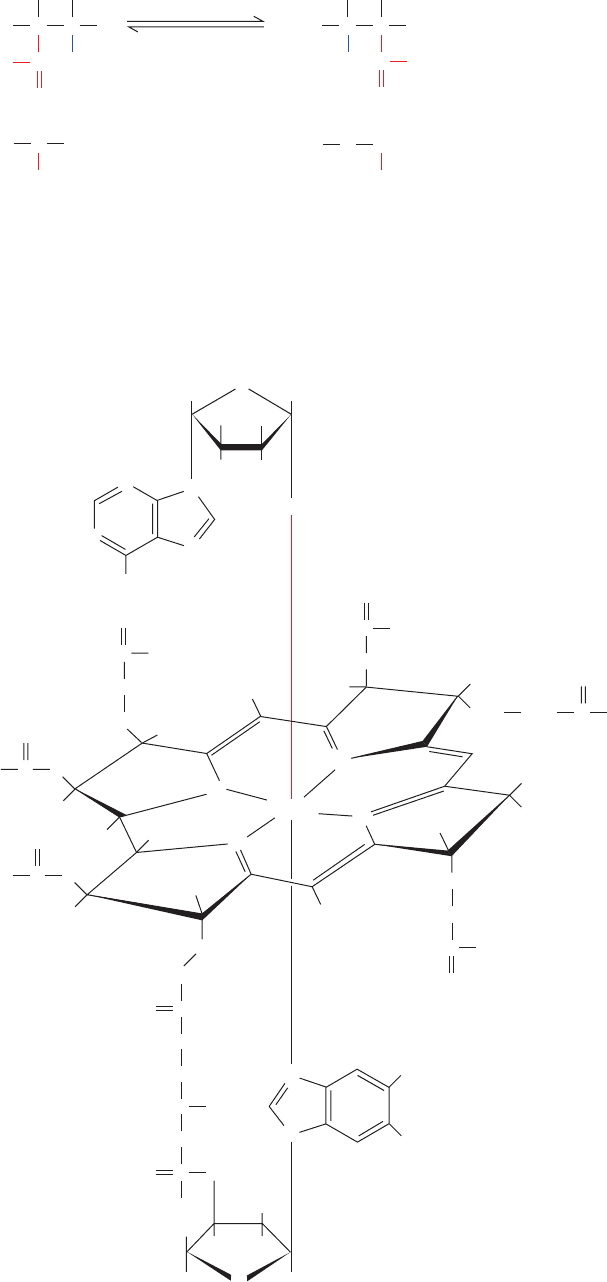
Methylmalonyl-CoA mutase, which catalyzes an un-
usual carbon skeleton rearrangement (Fig. 25-20), utilizes a
5-deoxyadenosylcobalamin (AdoCbl) prosthetic group
(also called coenzyme B
12
). Dorothy Hodgkin determined
the structure of this complex molecule (Fig. 25-21) in 1956,
a landmark achievement, through X-ray crystallographic
analysis combined with chemical degradation studies.
AdoCbl contains a hemelike corrin ring whose four pyr-
role N atoms each ligand a 6-coordinate Co ion. The fifth
Co ligand in the free coenzyme is an N atom of a 5,6-
dimethylbenzimidazole (DMB) nucleotide that is covalently
linked to the corrin D ring. The sixth ligand is a 5¿-
deoxyadenosyl group in which the deoxyribose C5¿ atom
forms a covalent C¬Co bond, one of only two known
carbon–metal bonds in biology (the other being a C¬Ni bond
in the bacterial enzyme carbon monoxide dehydrogenase).
In some enzymes, the sixth ligand instead is a CH
3
group
that likewise forms a C¬Co bond.
Figure 25-20 The rearrangement catalyzed by methylmalonyl-
CoA mutase.
H H
CC
CCC
H
C
C
O
–
O
2
C
H
CoAS
methylmalonyl-
CoA mutase
H
C
H
C
CCC
H
C
C
O
–
O
2
C
H
SCoA
Carbon
skeleton
Succinyl-CoA(R)-Methylmalonyl-CoA
O
OH OH
N
N
C
O
NH
2
CH
2
CH
2
H
N
N
N
N
N
Co(III)
CH
2
H H
H H
5
2
1
4
3
H
2
N
N
N
N
CH
3
CH
3
H
C
O
NH
2
CH
2
CH
2
C
O
NH
2
CH
2
CH
2
NH
2
C
O
C
O
NH
CH
2
CH
2
CH
2
CH
2
HC
CH
3
O
O
O
P
–
O
CH
2
CH
3
C
O
CH
3
CH
3
CH
3
CH
3
H
3
C
H
3
C
H
2
N
CH
2
C
O
H
2
N
H
3
C
H
H
H
B
C
D
A
O
H
H
H
H
5-Deoxyadenosylcobalamin (coenzyme B
12
)
HOH
2
C
OH
Figure 25-21 Structure of 5-deoxyadenosylcobalamin
(coenzyme B
12
).
JWCL281_c25_940-1018.qxd 4/20/10 1:59 PM Page 954
void of odd-chain fatty acids and low in the amino acid
residues that are degraded to propionyl-CoA (Ile, Val, and
Met; Section 26-3E).
c. The Methylmalonyl-CoA Mutase Reaction
Occurs via a Free Radical Mechanism
Methylmalonyl-CoA mutase from Propionibacterium
shermanii is an ␣ heterodimer whose catalytically active
728-residue ␣ subunit is 24% identical to its catalytically in-
active 638-residue  subunit. In contrast, the human en-
zyme is a homodimer whose subunits are 60% identical in
sequence to P.shermanii’s ␣ subunit. Hence P. shermanii’s 
subunit is thought to be an evolutionary fossil.
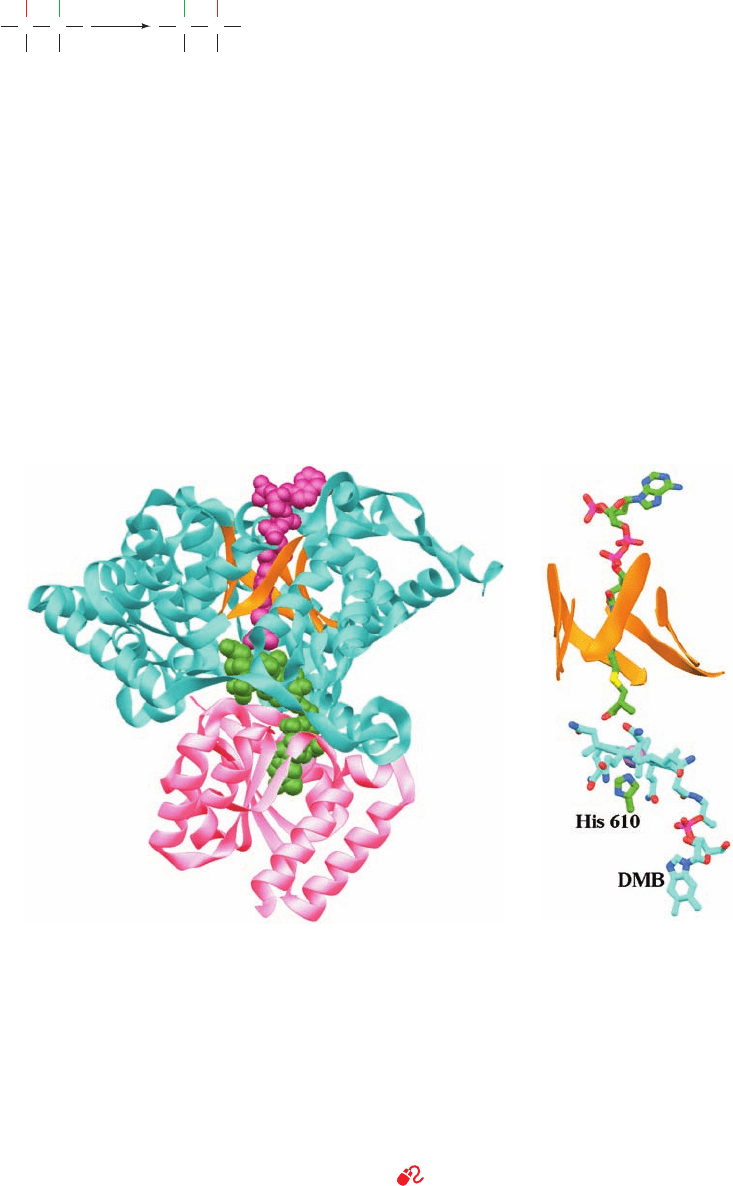
The X-ray structure of methylmalonyl-CoA mutase
from P. shermanii in complex with the substrate analog 2-
carboxypropyl-CoA (which lacks methylmalonyl-CoA’s
thioester oxygen atom) was determined by Philip Evans.
Its AdoCbl cofactor is sandwiched between the ␣ subunit’s
two domains: a 559-residue N-terminal ␣/ barrel (TIM
barrel, the most common enzymatic motif; Section 8-3B)
and a 169-residue C-terminal ␣/ domain that resembles a
Rossmann fold (Section 8-3Bh). The structure of the ␣/
barrel contains several surprising features (Fig. 25-22):
1. The active sites of nearly all ␣/ barrel enzymes are
located at the C-terminal ends of the barrel’s  strands.
Section 25-2. Fatty Acid Oxidation 955
AdoCbl’s reactive C¬Co bond participates in two types
of enzyme-catalyzed reactions:
1. Rearrangements in which a hydrogen atom is di-
rectly transferred between two adjacent carbon atoms with
concomitant exchange of the second substituent, X:
where X may be a carbon atom with substituents, an oxy-
gen atom of an alcohol, or an amine.
2. Methyl group transfers between two molecules.
There are about a dozen known cobalamin-dependent
enzymes. However, only two occur in mammalian systems:
(1) methylmalonyl-CoA mutase, which catalyzes a carbon
skeleton rearrangement (the X group in the rearrangement
is ¬COSCoA; Fig. 25-20) and is the only B
12
-containing
enzyme that occurs in both eukaryotes and prokaryotes;
and (2) methionine synthase, a methyl transfer enzyme that
participates in methionine biosynthesis (Sections 26-3Ec
and 26-5B). Defects in methylmalonyl-CoA mutase result
in methylmalonic aciduria, a condition that is often fatal in
infancy due to acidosis (low blood pH) without a diet de-
X
C
2
H
C
1
X
C
2
H
C
1
Figure 25-22 X-ray structure of P. shermanii methylmalonyl-
CoA mutase in complex with 2-carboxypropyl-CoA and AdoCbl.
(a) The catalytically active ␣ subunit in which the N-terminal
domain is cyan with the  strands of its ␣/ barrel orange, and
the C-terminal domain is pink.The 2-carboxypropyl-CoA
(magenta) and AdoCbl (green) are drawn in space-filling form.
The 2-carboxypropyl-CoA passes through the center of the ␣/
barrel and is oriented such that the methylmalonyl group of
methylmalonyl-CoA would contact the corrin ring of the
AdoCbl, which is sandwiched between the enzyme’s N- and
C-terminal domains. (b) The arrangement of the AdoCbl and
2-carboxypropyl-CoA molecules which, together with the side
chain of His 610, are represented in stick form colored according
to atom type (AdoCbl and His C green, 2-carboxypropyl-CoA
C cyan, N blue, O red, P magenta, and S yellow). The corrin ring’s
Co atom is represented by a lavender sphere and the ␣/ barrel’s
 strands are represented by orange ribbons.The view is similar
to that in Part a. Note that the DMB group (bottom) has swung
away from the corrin ring (seen edgewise) to be replaced by the
side chain of His 610 from the C-terminal domain and that the
5¿-deoxyadenosyl group is unseen (due to disorder). [Based on
an X-ray structure by Philip Evans, MRC Laboratory of
Molecular Biology, Cambridge, U.K. PDBid 7REQ.]
See Interactive Exercise 24
(a)
(b)
JWCL281_c25_940-1018.qxd 10/19/10 9:42 AM Page 955
956 Chapter 25. Lipid Metabolism
However, in methylmalonyl-CoA mutase, the AdoCbl is
packed against the N-terminal ends of the barrel’s strands.
2. In free AdoCbl, the Co atom is axially liganded by
an N atom of its DMB group and by the adenosyl residue’s
5¿-CH
2
group (Fig. 25-21). However, in the enzyme, the
DMB has swung aside to bind in a separate pocket and
has been replaced by the side chain of His 610 from the C-
terminal domain. The adenosyl group is not visible in the
structure due to disorder and hence has probably also
swung aside.
3. In nearly all other / barrel–containing enzymes,
the center of the barrel is occluded by large, often branched,
hydrophobic side chains. However, in methylmalonyl-CoA
mutase, the 2-carboxypropyl-CoA’s pantetheine group
binds in a narrow tunnel through the center of the / bar-
rel so as to put the methylmalonyl group of an intact sub-
strate in close proximity to the unliganded face of the
cobalamin ring.This tunnel provides the only direct access
to the active site cavity, thereby protecting the reactive free
radical intermediates that are produced in the catalytic re-
action from side reactions (see below). The tunnel is lined
by small hydrophilic residues (Ser and Thr).
Methylmalonyl-CoA mutase’s substrate binding mode re-
sembles those of several other AdoCbl-containing en-
zymes of known structure, which are collectively unique
among / barrel–containing proteins.
The proposed methylmalonyl-CoA mutase reaction
mechanism (Fig. 25-23) begins with homolytic cleavage of
the cobalamin C¬Co(III) bond (the C and Co atoms each
acquire one of the electrons that formed the cleaved elec-
tron pair bond). The Co ion therefore fluctuates between
its Co(III) and Co(II) oxidation states [the two states are
spectroscopically distinguishable: Co(III) is red and dia-
magnetic (no unpaired electrons), whereas Co(II) is yellow
and paramagnetic (unpaired electrons)]. Note that a ho-
molytic cleavage reaction is unusual in biology; most other
biological bond cleavage reactions occur via heterolytic
cleavage (in which the electron pair forming the cleaved
bond is fully acquired by one of the separating atoms).
The role of AdoCbl in the catalytic process is that of a re-
versible free radical generator. The C¬Co(III) bond is well
suited to this function because it is inherently weak (disso-
ciation energy 109 kJ mol
1
) and appears to be further
weakened through steric interactions with the enzyme. In-
deed, as Fig. 25-22 indicates, the Co atom in methylmalonyl-
CoA mutase has no sixth ligand and hence, as confirmed by
spectroscopic measurements, is in its Co(II) state. The His
N¬Co bond is extremely long (2.5 Å vs 1.9–2.0 Å in vari-
ous other B
12
-containing structures). It is proposed that
this strained and hence weakened bond stabilizes the
Co(II) state with respect to the Co(III) state, thus favoring
the formation of the adenosyl radical and facilitating the
homolytic cleavage through which the catalyzed reaction
occurs (Fig. 23-23). The adenosyl radical presumably ab-
stracts a hydrogen atom from the substrate, thereby facili-
tating the rearrangement reaction through the intermedi-
ate formation of a cyclopropyloxy radical.
d. Succinyl-CoA Cannot Be Directly Consumed
by the Citric Acid Cycle
Methylmalonyl-CoA mutase catalyzes the conversion
of a metabolite to a C
4
citric acid cycle intermediate, not
acetyl-CoA. The route of succinyl-CoA oxidation is there-
fore not as simple as it may first appear. The citric acid
cycle regenerates all of its C
4
intermediates so that these
compounds are really catalysts, not substrates. Conse-
quently, succinyl-CoA cannot undergo net degradation by
citric acid cycle enzymes alone. Rather, in order for a
metabolite to undergo net oxidation by the citric acid cycle,
it must first be converted either to pyruvate or directly to
acetyl-CoA. Net degradation of succinyl-CoA begins with
its conversion, via the citric acid cycle, to malate. At high
concentrations, malate is transported, by a specific trans-
port protein, to the cytosol, where it may be oxidatively de-
carboxylated to pyruvate and CO
2
by malic enzyme
(malate dehydrogenase, decarboxylating):
(We previously encountered this enzyme in the C
4
cycle of
photosynthesis; Fig. 24-41.) Pyruvate is then completely ox-
idized via pyruvate dehydrogenase and the citric acid cycle.
e. Pernicious Anemia Results from
Vitamin B
12
Deficiency
The existence of vitamin B
12
came to light in 1926 when
George Minot and William Murphy discovered that perni-
cious anemia, a rare but often fatal disease of the elderly
characterized by decreased numbers of red blood cells,
low hemoglobin levels (for reasons explained in Section
26-4D), and progressive neurological deterioration (caused
by the accumulation of odd-chain fatty acid residues in
neuronal membranes), can be treated by the daily con-
sumption of large amounts of raw liver (a treatment that
some patients considered worse than the disease). It was
not until 1948, however, after a bacterial assay for antiper-
nicious anemia factor had been developed, that vitamin B
12
was isolated.
Vitamin B
12
is synthesized by neither plants nor animals
but only by a few species of bacteria. Herbivores obtain
their vitamin B
12
from the bacteria that inhabit their gut (in
fact, some animals, such as rabbits, must periodically eat
some of their feces to obtain sufficient amounts of this es-
sential substance). Humans, however, obtain almost all
their vitamin B
12
directly from their diet, particularly from
meat. The vitamin is specifically bound in the intestine by
the glycoprotein intrinsic factor that is secreted by the
stomach.This complex is absorbed by a specific receptor in
the intestinal mucosa, where the complex is dissociated and
the liberated vitamin B
12
transported to the bloodstream.
CO
–
2
C
C
CH
2
O
OO
–
CO
–
2
C
C
CH
2
H
OO
–
CO
2
CH
3
CO
CO
–
2
PyruvateMalate
NADPHNADP
+
H
+
+
HO
JWCL281_c25_940-1018.qxd 4/20/10 1:59 PM Page 956
cells that produce it. The normal human requirement for
cobalamin is very small, ⬃3 g ⴢ day
⫺1
, and the liver stores
a 3- to 5-year supply of this vitamin. This accounts for the
insidious onset of pernicious anemia and the fact that true
dietary deficiency of vitamin B
12
, even among strict vege-
tarians, is extremely rare.
Section 25-2. Fatty Acid Oxidation 957
There it is bound by at least three different plasma globu-
lins, called transcobalamins, which facilitate its uptake by
the tissues.
Pernicious anemia is not usually a dietary deficiency dis-
ease but, rather, results from insufficient secretion of in-
trinsic factor, often due to an autoimmune attack against the
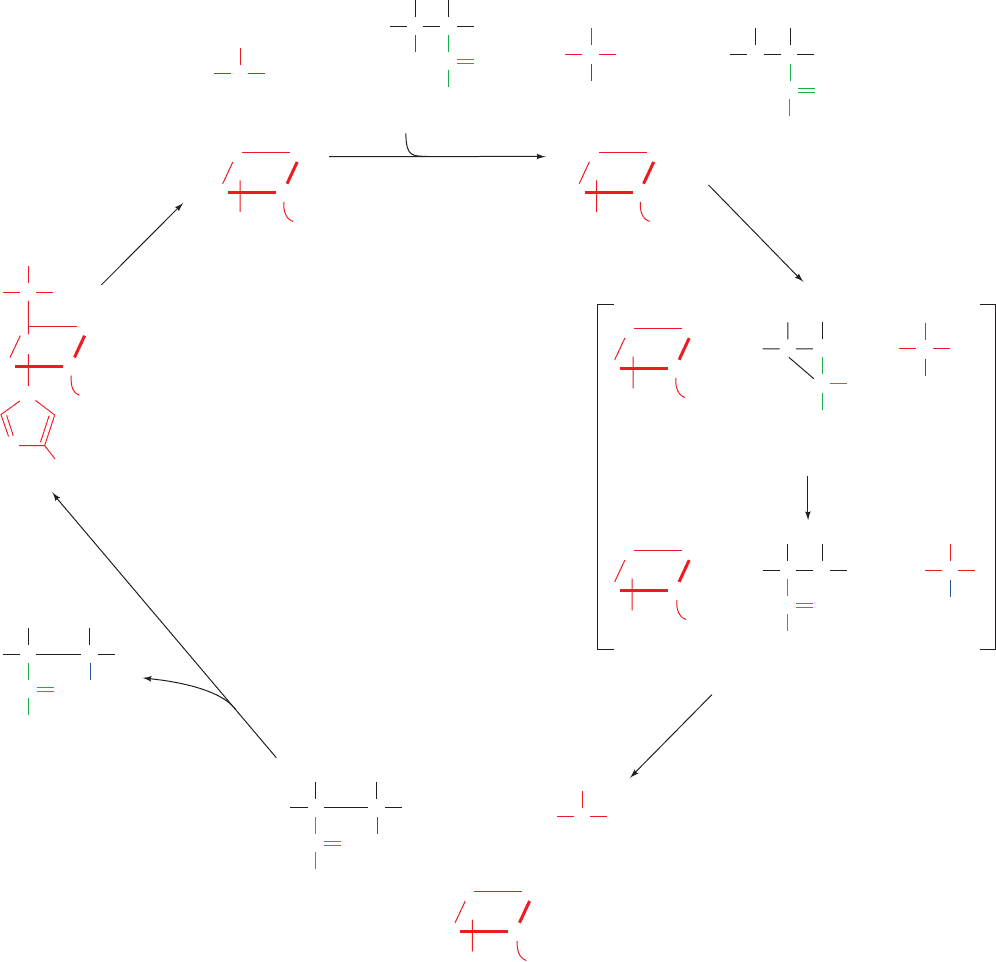
Figure 25-23 Proposed mechanism of methylmalonyl-CoA
mutase. (1) The homolytic cleavage of the C¬Co(III) bond
yielding a 5¿-deoxyadenosyl radical and cobalamin in its Co(II)
oxidation state. (2) Abstraction of a hydrogen atom from the
methylmalonyl-CoA by the 5¿-deoxyadenosyl radical, thereby
generating a methylmalonyl-CoA radical. (3) Carbon skeleton
H
H
HHC
CO
2
–
C
C
SCoA
O
H
H
HHC
CO
2
–
C
C
SCoA
O
SCoA
O
Ado
C
HH
H
+ HCC
C
H
H
CO
2
–
(R)-Methylmalonyl-CoA
2
N
N
N
N
Co(II)
N
N
N
N
Co(II)
DMB DMBHis 610
His 610
+
+
•
•
Ado
C
HH
1
H
H
HHC
CO
2
–
C
C
SCoA
O
DMB
His 610
N
N
N
N
Co(II)
•
Ado
C
HH
+
+
5
O
C
DMBHis 610
N
N
N
N
Co(II)
N
N
N
N
His 610
Co(II)
DMB
++
+
H
HHC
CO
2
–
C
C
SCoA
O
•
•
Ado
C
HH
H
+
H
HC
CO
2
–
CH
SCoA
Ado
C
HH
H
Cyclopropyloxy
radical
Hypothetical intermediates
Succinyl-CoA
rearrangement
3
4
N
N
N
C
N
N
N
Co(III)
DMB
Ado
HH
His 610
rearrangement to form a succinyl-CoA radical via a proposed
cyclopropyloxy radical intermediate. (4) Abstraction of a
hydrogen atom from 5¿-deoxyadenosine by the succinyl-CoA
radical to form succinyl-CoA and regenerate the 5¿-deoxyadenosyl
radical. (5) Release of succinyl-CoA and reformation of the
coenzyme.
JWCL281_c25_940-1018.qxd 8/9/10 9:43 AM Page 957
958 Chapter 25. Lipid Metabolism
through the action of peroxisomal catalase (Section
1-2Ad).
2. Peroxisomal enoyl-CoA hydratase and 3-
L-hydroxy-
acyl-CoA dehydrogenase are activities that occur on a sin-
gle polypeptide and therefore join the growing list of mul-
tifunctional enzymes. The reactions catalyzed are identical
to those of the mitochondrial system (Fig. 25-12).
3. Peroxisomal thiolase has a different chain-length
specificity than its mitochondrial counterpart. It is almost
inactive with acyl-CoAs of length C
8
or less so that fatty
acids are incompletely oxidized by peroxisomes.
Although peroxisomal oxidation is not dependent on
the transport of acyl groups into the peroxisome as their
carnitine esters, the peroxisome contains carnitine acyl-
transferases. Acyl-CoAs that have been chain-shortened
by peroxisomal oxidation are thereby converted to their
carnitine esters. These substances, for the most part, pas-
sively diffuse out of the peroxisome to the mitochondrion,
where they are oxidized further.
G. Minor Pathways of Fatty Acid Oxidation
Oxidation is blocked by an alkyl group at the C
of a fatty
acid, and thus at any odd-numbered carbon atom. One
such branched-chain fatty acid, a common dietary compo-
nent, is phytanic acid. This metabolic breakdown product
of chlorophyll’s phytyl side chain (Section 24-2A) is pres-
ent in dairy products, ruminant fats, and fish although, sur-
prisingly, chlorophyll itself is but a poor dietary source of
phytanic acid for humans.The oxidation of branched-chain
fatty acids such as phytanic acid is facilitated by oxidation
(Fig. 25-25). In this process, the fatty acid is converted to
its CoA thioester and its C
is hydroxylated by the Fe
2
-
containing phytanoyl-CoA hydroxylase. The resulting
F. Peroxisomal  Oxidation
In mammalian cells, the bulk of oxidation occurs in the
mitochondria, but peroxisomes (Fig. 25-24) also oxidize
fatty acids, particularly those with very long chains or
branched chains. Peroxisomal  oxidation in animals func-
tions to shorten very long chain fatty acids (22 C atoms) so
as to facilitate their degradation by the mitochondrial -
oxidation system. In yeast and plants, fatty acid oxidation
occurs exclusively in the peroxisomes and glyoxysomes
(specialized peroxisomes, Sections 23-2 and 1-2Ad).
The peroxisomal pathway results in the same chemical
changes to fatty acids as does the mitochondrial pathway,
although the enzymes in these two organelles are different.
The protein that transports very long-chain fatty acids into
the peroxisome, ALD protein (see below), does not have a
carnitine requirement. The very long-chain fatty acids that
enter this compartment are activated by a peroxisomal
very long-chain acyl-CoA synthetase to form their CoA es-
ters, and are oxidized directly. The shorter chain acyl prod-
ucts of this -oxidation process are then linked to carnitine
for transport into mitochondria for further oxidation.
a. Adrenoleukodystrophy Is Caused by
a Defect in ALD Protein
Adrenoleukodystrophy (ALD) is a rare X-linked inher-
ited disease that results in progressive brain damage and
adrenal gland failure. It causes very long-chain saturated
fatty acids to accumulate in the blood and destroy the insu-
lating myelin sheath surrounding the axons of many neu-
rons (Section 20-5Bc). Its varied neurological symptoms
present (become evident) between the ages of 4 and 10
years and are usually fatal within 1 to 10 years (except after
a successful bone marrow transplant). ALD is caused by
a defective ALD protein, an ABC transporter (Section
20-3E). Thus in ALD patients, lignoceric acid (24:0; recall
that the symbol n:m indicates a C
n
fatty acid with m double
bonds) is converted to lignoceroyl-CoA at only 13% of the
normal rate, although once formed, it undergoes oxida-
tion at the normal rate.
b. Peroxisomal Oxidation Differs in Detail
from Mitochondrial Oxidation
The -oxidation pathway in peroxisomes differs from
that in mitochondria as follows:
1. The first enzyme in the peroxisomal pathway, acyl-
CoA oxidase, catalyzes the reaction
This reaction involves participation of an FAD cofactor
but differs from its mitochondrial counterpart in that the
abstracted electrons are transferred directly to O
2
rather
than passing through the electron-transport chain with its
concomitant oxidative phosphorylation (Fig. 25-12). Perox-
isomal fatty acid oxidation is therefore less efficient than
the mitochondrial process by two ATPs for each C
2
cycle.
The H
2
O
2
produced is disproportionated to H
2
O and O
2
Fatty acyl-CoA O
2
¡
trans-¢
2
-enoyl-CoA H
2
O
2
Figure 25-24 Peroxisomes. These membrane-bounded
organelles perform a variety of metabolic functions, including
the oxidation of very long chain fatty acids. [© Donald Fawcett/
Visuals Unlimited.]
JWCL281_c25_940-1018.qxd 4/20/10 1:59 PM Page 958
CoA thioester is, in effect, oxidatively decarboxylated to
yield a new fatty acid with an unsubstituted C
. Further
degradation of the molecule can then continue via six
cycles of normal oxidation to yield three propionyl-
CoAs, three acetyl-CoAs, and one 2-methylpropionyl-CoA
(which is converted to succinyl-CoA).
A rare genetic defect, Refsum’s disease or phytanic acid
storage syndrome, results from the accumulation of this
metabolite throughout the body.The disease,which is char-
acterized by progressive neurological difficulties such as
tremors, unsteady gait, and poor night vision, results from a
defective phytanoyl-CoA hydroxylase. Its symptoms can
therefore be attenuated by a diet that restricts the intake of
phytanic acid–containing foods.
Medium- and long-chain fatty acids are converted to di-
carboxylic acids through oxidation (oxidation of the last
carbon atom). This process, which is catalyzed by enzymes
of the ER, involves hydroxylation of a fatty acid’s C
atom
by a cytochrome P450, a monooxygenase that utilizes
NADPH and O
2
(Section 15-4Bc).This CH
2
¬OH group is
then oxidized to a carboxyl group, converted to a CoA de-
rivative at either end, and oxidized via the -oxidation
pathway. Oxidation is probably of only minor signifi-
cance in fatty acid oxidation.
3 KETONE BODIES
Acetyl-CoA produced by oxidation of fatty acids in liver
mitochondria can be further oxidized via the citric acid
cycle as is discussed in Chapter 21.A significant fraction of
this acetyl-CoA has another fate, however. By a process
known as ketogenesis, which occurs primarily in liver mito-
chondria, acetyl-CoA is converted to acetoacetate or
D--
hydroxybutyrate. These compounds, which together with
acetone are somewhat inaccurately referred to as ketone
bodies,
serve as important metabolic fuels for many peripheral tis-
sues, particularly heart and skeletal muscle.The brain, under
normal circumstances, uses only glucose as its energy
source (fatty acids are unable to pass the blood–brain bar-
rier), but during starvation, ketone bodies become the
brain’s major fuel source (Section 27-4A). Ketone bodies
are water-soluble equivalents of fatty acids.
H
3
CCH
2
CC
O
–
O
O
H
3
C
H
3
C
CH
3
CH
2
C
C C
O
Acetone
D--Hydroxybutyrate
Acetoacetate
O
–
O
H
OH
Section 25-3. Ketone Bodies 959
Figure 25-25 Pathway of oxidation of fatty acids. Phytanic
acid, a degradation product of the phytyl side chain of
chlorophyll, is metabolized through oxidation to pristanic
acid followed by oxidation.
CH
3
CH CCH
2
SCoACH
2
CH
2 3
CH
3
CH
3
O
CH C SCoA
CH
3
O
CH
)
CH
3
CCH
2
SCoACH
2
CH
2
CH
2
CH
3
O
CH
(
PP
i
Phytanic acid
6 cycles of oxidation
2-hydroxyphytanoyl-CoA
lyase
phytanoly-CoA
hydroxylase
ATP
+ CoASH
AMP
+
NAD(P)
+
Phytanoyl-CoA
CH
3
CCH
2
SCoACH
2
CH
2
CH
CH
3
OH O
HC SCoA
O
CH
(
2-Hydroxyphytanoyl-CoA
Formyl-CoA
CH
3
CH
2
CH
2
CH
2
CH
CH
3
O
CH
(
Pristanal
Fe
2
-Ketoglutarate + O
2
succinate + CO
2
acyl-CoA synthase
ATP
+ CoA
++
AMP + PP
i
aldehyde dehydrogenase
NAD(P)H
C
CH
3
CH
2
CH
2
CH
2
CH
3
CH
3
CH CH
()
O
–
O
3
C
CH
3
CH
2
CH
2
CH
2
CH
2
CH
3
CH
3
CH CH
()
O
–
O
3
CH
3
CH
)
3
CH
3
CH
)
3
CH
3
CH
)
3
Pristanic acid
(
O
C
SCoA
Acetyl-CoA2-Methyl-
propionyl-CoA
CH
2
3CH
3
3CH
3
CH
3
SCo
A
O
C
Propionyl-CoA
JWCL281_c25_940-1018.qxd 6/8/10 8:59 AM Page 959
960 Chapter 25. Lipid Metabolism
The overall reaction catalyzed by HMG-CoA synthase
and HMG-CoA lyase is
One may well ask why this apparently simple hydrolysis re-
action occurs in such an indirect manner.The answer is un-
clear but may lie in the regulation of the process.
Acetoacetate may be reduced to
D--hydroxybutyrate
by -hydroxybutyrate dehydrogenase:
Note that this product is the stereoisomer of the
L--
hydroxyacyl-CoA that occurs in the -oxidation pathway.
Acetoacetate, being a -keto acid, also undergoes rela-
tively facile nonenzymatic decarboxylation to acetone and
H
+
+
NADH NAD
+
C O
CH
3
CH
2
CO
2
–
CO
2
–
C HHO
CH
3
CH
2
D--Hydroxybutyrate
-hydroxybutyrate
dehydrogenase
Acetoacetate
Acetoacetyl-CoA H
2
O
¡
acetoacetate CoA
Acetoacetate formation occurs in three reactions (Fig.
25-26):
1. Two molecules of acetyl-CoA are condensed to
acetoacetyl-CoA by thiolase (also called acetyl-CoA
acetyltransferase) working in the reverse direction from the
way it does in the final step of oxidation (Section 25-2Cd).
2. Condensation of the acetoacetyl-CoA with a third
acetyl-CoA by HMG-CoA synthase forms -hydroxy--
methylglutaryl-CoA (HMG-CoA). The mechanism of this
reaction resembles the reverse of the thiolase reaction (Fig.
25-15) in that an active site thiol group forms an
acyl–thioester intermediate.
3. Degradation of HMG-CoA to acetoacetate and
acetyl-CoA in a mixed aldol–Claisen ester cleavage is cat-
alyzed by HMG-CoA lyase. The mechanism of this reac-
tion is analogous to the reverse of the citrate synthase reac-
tion (Section 21-3A). (HMG-CoA is also a precursor in
cholesterol biosynthesis and hence may be diverted to this
purpose as is discussed in Section 25-6A.)
Figure 25-26 Ketogenesis: the enzymatic reactions forming
acetoacetate from acetyl-CoA. (1) Two molecules of acetyl-CoA
condense to form acetoacetyl-CoA in a thiolase-catalyzed
reaction. (2) A Claisen ester condensation of the acetoacetyl-CoA
with a third acetyl-CoA to form -hydroxy--methylglutaryl-CoA
(HMG-CoA) as catalyzed by HMG-CoA synthase. (3) The
degradation of HMG-CoA to acetoacetate and acetyl-CoA in a
mixed aldol–Claisen ester cleavage catalyzed by HMG-CoA
lyase.
Figure 25-27 The metabolic conversion of ketone bodies to
acetyl-CoA.
O
C SCoA
CH
3
+
CH
3
C
O
SCoA
Acetyl-CoA Acetyl-CoA
thiolase
(acetyl-CoA acetyltransferase)
1
CH
3
C
O
CH
2
O
C
SCoA
O
Acetoacetyl-CoA
OH
H
2
O CH
3
+
C
SCoA
H
SCoA
H
SCoA
2
C CCH
2
CH
2
CH
3
–
O
2
C
O
CCH
2
CH
3
–
O
2
C
SCoA
O
CCH
3
SCoA
O
hydroxymethylglutaryl-CoA synthase
(HMG-CoA synthase)
-Hydroxy--methylglutaryl-CoA (HMG-CoA)
3
hydroxymethylglutaryl-CoA lyase
(HMG-CoA lyase)
+
Acetoacetate Acetyl-CoA
C
H
CH
2
CH
3
OH
CO
2
–
D-
β
-Hydroxybutyrate
NAD
+
NADH
+ H
+
β-hydroxybutyrate dehydrogenase
C
CH
2
CH
3
CO
2
–
Acetoacetate
O
C
CH
2
CH
2
SCo
A
O
–
O
2
C
Succinyl-CoA
3-ketoacyl-CoA transferase
CH
2
CH
22
–
OC
Succinate
CO
2
–
C
CH
2
CH
3
SCoA
Acetoacetyl-CoA
O
C
O
SCoAH
thiolase
C
SCoA
Acetyl-CoA
O
2 CH
3
JWCL281_c25_940-1018.qxd 4/20/10 1:59 PM Page 960
