Voet D., Voet Ju.G. Biochemistry
Подождите немного. Документ загружается.

(a)
(b)
erol digestion therefore depends on the surface area of the
interface, a quantity that is greatly increased by the churn-
ing peristaltic movements of the intestine combined with
the emulsifying action of bile salts. These are powerful di-
gestive detergents that, as we shall see in Section 25-6C, are
synthesized by the liver and secreted via the gallbladder
into the small intestine where lipid digestion and absorp-
tion mainly take place.
b. Pancreatic Lipase Requires Activation and
Has a Catalytic Triad
Pancreatic lipase (triacylglycerol lipase) catalyzes the
hydrolysis of triacylglycerols at their 1 and 3 positions to
form sequentially 1,2-diacylglycerols and 2-acylglycerols,
together with the Na
⫹
and K
⫹
salts of fatty acids (soaps).
These soaps, being amphipathic, aid in the lipid emulsifica-
tion process.
The enzymatic activity of pancreatic lipase greatly in-
creases when it contacts the lipid–water interface, a phe-
nomenon known as interfacial activation. Binding to the
lipid–water interface requires the presence of mixed mi-
celles of phosphatidylcholine (Fig. 12-4) and bile salts, as
well as the pancreatically produced protein named coli-
pase, which forms a 1:1 complex with lipase. This complex
aids in the adsorption of the enzyme to emulsified oil
droplets as well as stabilizes the enzyme in an active con-
formation. The X-ray structures, determined by Christian
Cambillau, of pancreatic lipase–colipase complexes, alone
and cocrystallized with mixed micelles of phosphatidyl-
choline and bile salts, have revealed the structural basis of
lipase activation as well as how colipase and micelles aid li-
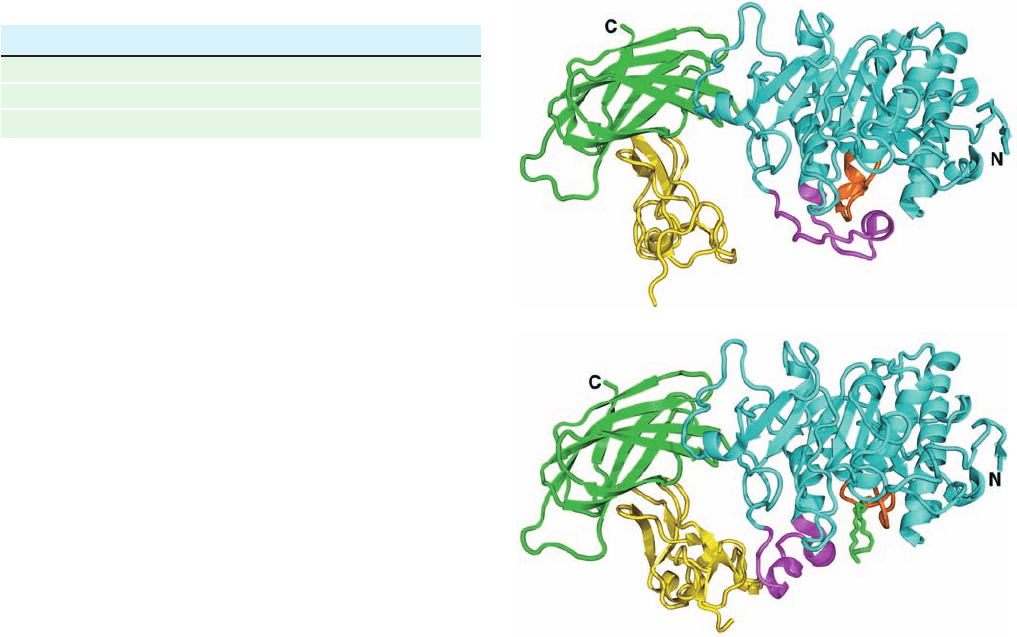
pase in binding to the lipid–water interface (Fig. 25-1).
The active site of the 449-residue pancreatic lipase,
which is contained in the enzyme’s N-terminal domain, has
a catalytic triad that closely resembles that in the serine
proteases (Section 15-3B; recall that ester hydrolysis is
mechanistically similar to peptide hydrolysis). In aqueous
solution (Fig. 25-1a), the lipase’s active site is covered by a
26-residue helical lid. However, in the presence of the
mixed micelles (Fig. 25-1b), the lid undergoes a complex
structural reorganization that exposes the active site;
causes a contacting 10-residue loop, the 5 loop, to change
conformation in a way that forms the active enzyme’s
oxyanion hole; and generates a hydrophobic surface about
the entrance to the active site. Indeed, the active site of the
mixed micelle–containing complex contains a long rod of
electron density that contacts the catalytic triad’s Ser
residue and appears to be a phosphatidylcholine molecule.
Colipase binds to the C-terminal domain of lipase such
that the hydrophobic tips of the three loops that comprise
much of this 90-residue protein extend from the complex on
the same face as lipase’s active site.A continuous hydropho-
bic plateau is thereby created that extends over a distance
of ⬎50 Å past the active site (bottom of Fig.25-1b) and that,
presumably, helps bind the complex to the lipid surface. In
the presence of the mixed micelles, colipase changes confor-
mation so as to form three hydrogen bonds to the opened
lid, thereby stabilizing it in this conformation.
The mixed micelles are not visible in the X-ray
structure. However, neutron diffraction studies, by Juan
Fontecilla-Camps, of crystals of a lipase–colipase–micelle
complex in which the lipase is in its active conformation
reveal that the activating micelle interacts, not with the
substrate site, but with the concave face of colipase and the
adjacent tip of the lipase’s C-terminal domain (left side of
Section 25-1. Lipid Digestion, Absorption, and Transport 941
Table 25-1 Energy Content of Food Constituents
Constituent ⌬H (kJ ⴢ g
⫺1
dry weight)
Carbohydrate 16
Fat 37
Protein 17
Source: Newsholme, E.A. and Leech, A.R., Biochemistry for the Medical
Sciences, p. 16, Wiley (1983).
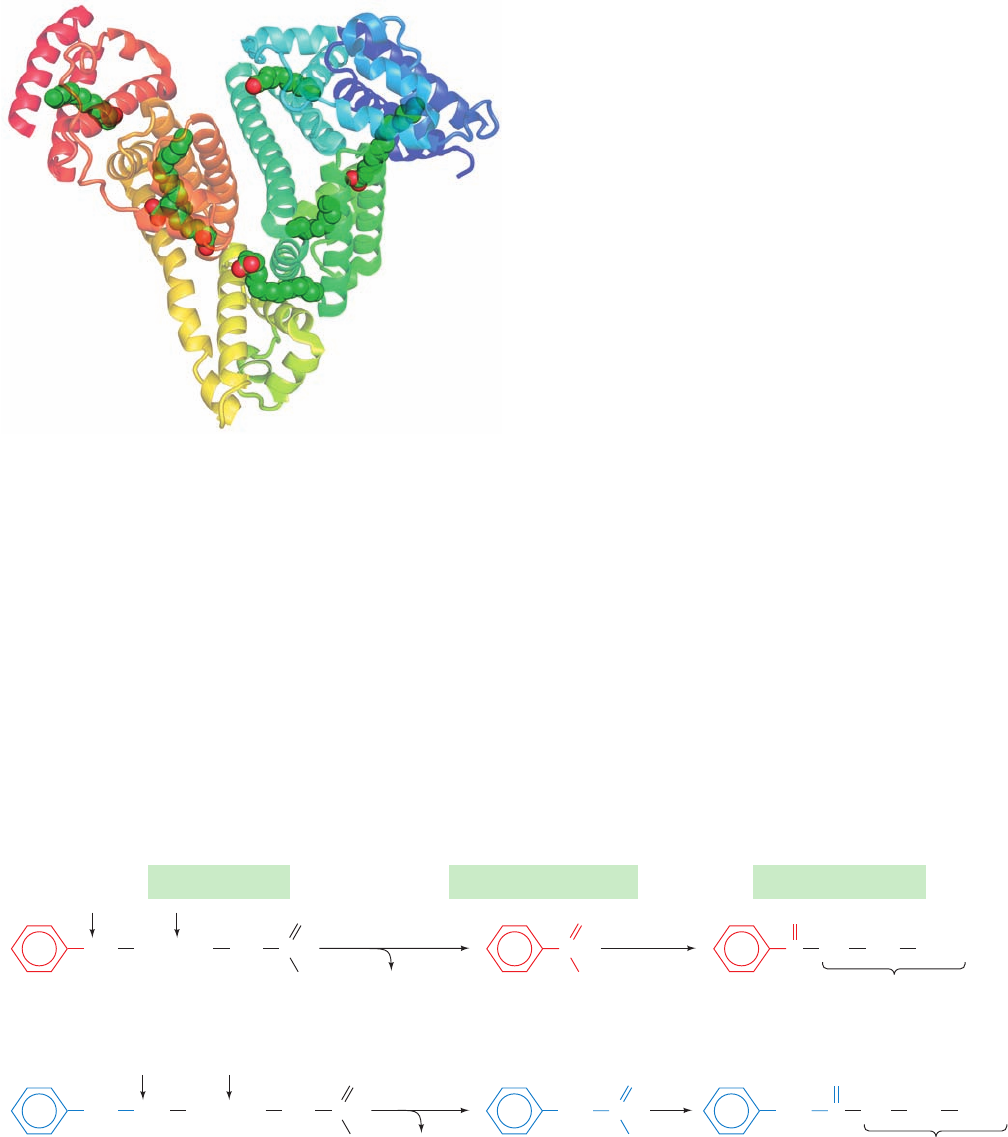
Figure 25-1 X-ray structures of pancreatic lipase in complex
with colipase. (a) In aqueous solution, and (b) cocrystallized with
mixed micelles of phosphatidylcholine and bile salts.The lipase is
drawn in ribbon form with its N-terminal domain (residues
1–336) cyan, its C-terminal domain (residues 337–449) green, the
lid (residues 237–262) magenta, and the 5 loop (residues 76–85)
orange. The colipase is yellow. A phosphatidylcholine molecule
that is bound in the lipase active site in Part b is shown in stick
form with C green, O red, and P orange.The micelles, which have
irregular structures (e.g., Fig. 2-9), are not visible. [Based on
X-ray structures by Christian Cambillau, LCCMB-CNRS,
Marseille, France. PDBids 1N8S and 1LPB.]
JWCL281_c25_940-1018.qxd 8/9/10 9:43 AM Page 941
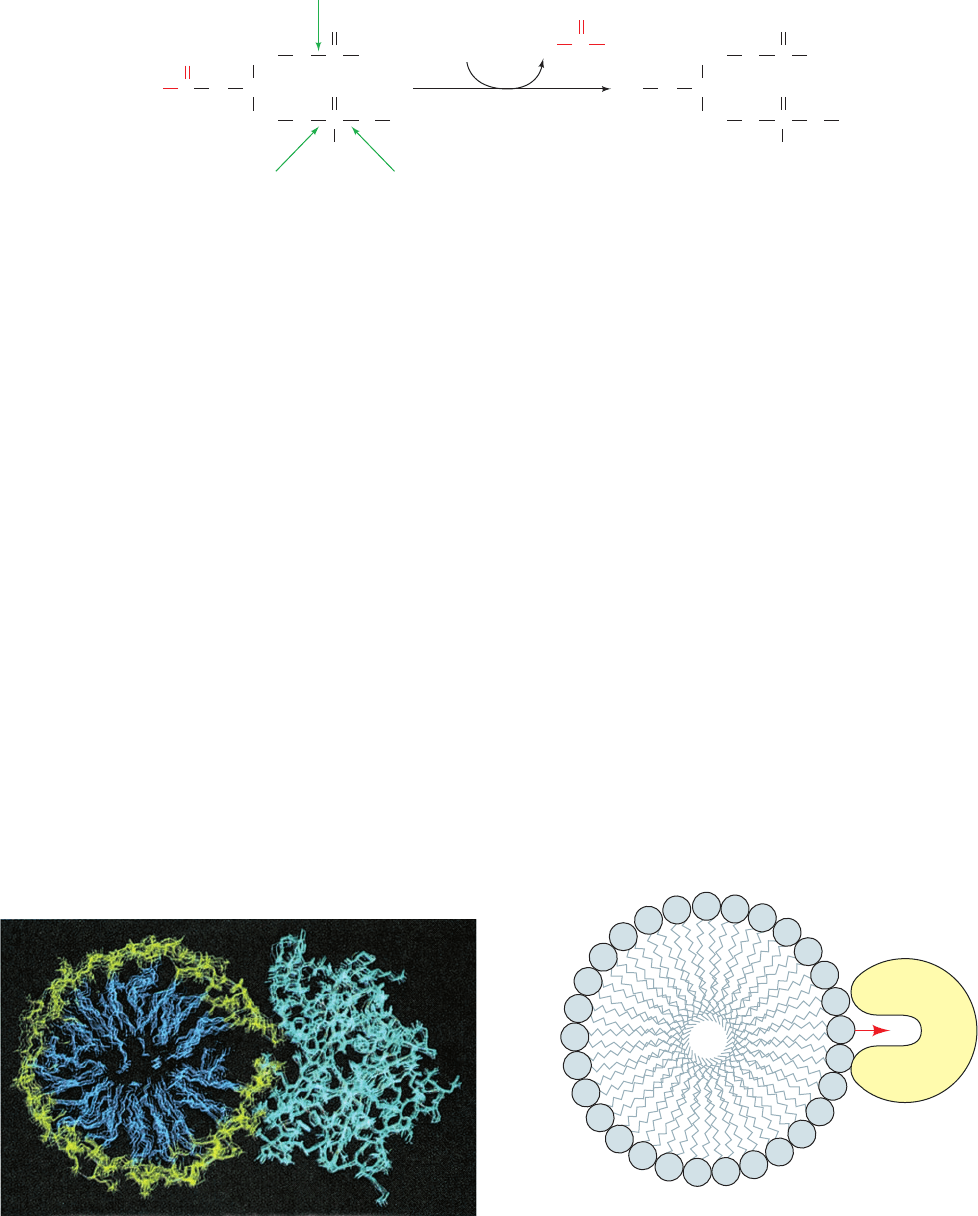
Fig. 25-1b).Apparently, micelle binding and substrate bind-
ing involve different regions of the lipase–colipase com-
plex. Hence, strictly speaking, lipase activation appears not
to be interfacial but, instead, occurs in the aqueous phase
and requires the binding of colipase and a micelle.
c. Pancreatic Phospholipase A
2
Has a
Modified Catalytic Triad
Phospholipids are degraded by pancreatic phospholipase
A
2
, which hydrolytically excises the fatty acid residue at C2
to yield the corresponding lysophospholipids (Fig. 25-2),
which are also powerful detergents. Indeed, the phospho-
lipid lecithin (phosphatidylcholine) is secreted in the bile,
presumably to aid in lipid digestion.
Phospholipase A
2
, as does triacylglycerol lipase, prefer-
entially catalyzes reactions at interfaces. However, as Paul
Sigler’s determinations of the X-ray structures of the
phospholipases A
2
from cobra venom and bee venom re-
vealed, its mechanism of interfacial activation differs from
that of triacylglycerol lipase in that it does not change its
conformation. Instead, phospholipase A
2
contains a hy-
drophobic channel that provides the substrate with direct
access from the phospholipid aggregate (micelle or mem-
brane) surface to the bound enzyme’s active site. Hence,
on leaving its micelle to bind to the enzyme, the substrate
need not become solvated and then desolvated (Fig. 25-3).
In contrast, soluble and dispersed phospholipids must first
surmount these significant kinetic barriers in order to bind
to the enzyme.
The catalytic mechanism of phospholipase A
2
also dif-
fers substantially from that of triacylglycerol lipase. Al-
though the phospholipase A
2
active site contains the His
and Asp components of a catalytic triad, an enzyme-bound
water molecule occupies the position expected for an ac-
tive site Ser. Moreover, the active site contains a bound
Ca
2
ion and does not form an acyl–enzyme intermediate.
Sigler therefore proposed that phospholipase A
2
catalyzes
the direct hydrolysis of phospholipid with a His–Asp “cat-
alytic dyad” activating an active site water molecule for
nucleophilic attack on the ester, and with the Ca
2
ion sta-
bilizing the oxyanion transition state. However, the subse-
quently determined X-ray structure, by Mahendra Jain and
942 Chapter 25. Lipid Metabolism
Figure 25-2 Catalytic action of phospholipase A
2
. Phospholipase
A
2
hydrolytically excises the C2 fatty acid residue from a
phospholipid to yield the corresponding lysophospholipid.The
Figure 25-3 Substrate binding to phospholipase A
2
. (a) A
hypothetical model of phospholipase A
2
in complex with a micelle
of lysophosphatidylethanolamine as shown in cross section.The
protein is drawn in cyan, the phospholipid head groups are yellow,
and their hydrocarbon tails are blue. The calculated atomic
CH
2
R
2
OO
O
–
PX
O
CH
2
H
2
OR
2
COH
CH
phospholipase C
phospholipase A
1
phospholipase A
2
phospholipase D
Phospholipid Lysophospholipid
OC
O
CHOH
CH
2
OO
O
–
P
X
O
R
1
O
1
2
3
C
O
CH
2
R
1
OC
O
O
bonds hydrolyzed by other types of phospholipases, which are
named according to their specificities, are also indicated.
Lipid micelle
(b)
Phospholipase A
2
motions of the assembly are indicated through a series of
superimposed images taken at 5-ps intervals. [Courtesy of
Raymond Salemme, E.I. du Pont de Nemours & Company.]
(
b) Schematic diagram of a productive interaction between
phospholipase A
2
and a phospholipid contained in a micelle.
(a)
JWCL281_c25_940-1018.qxd 4/20/10 1:58 PM Page 942
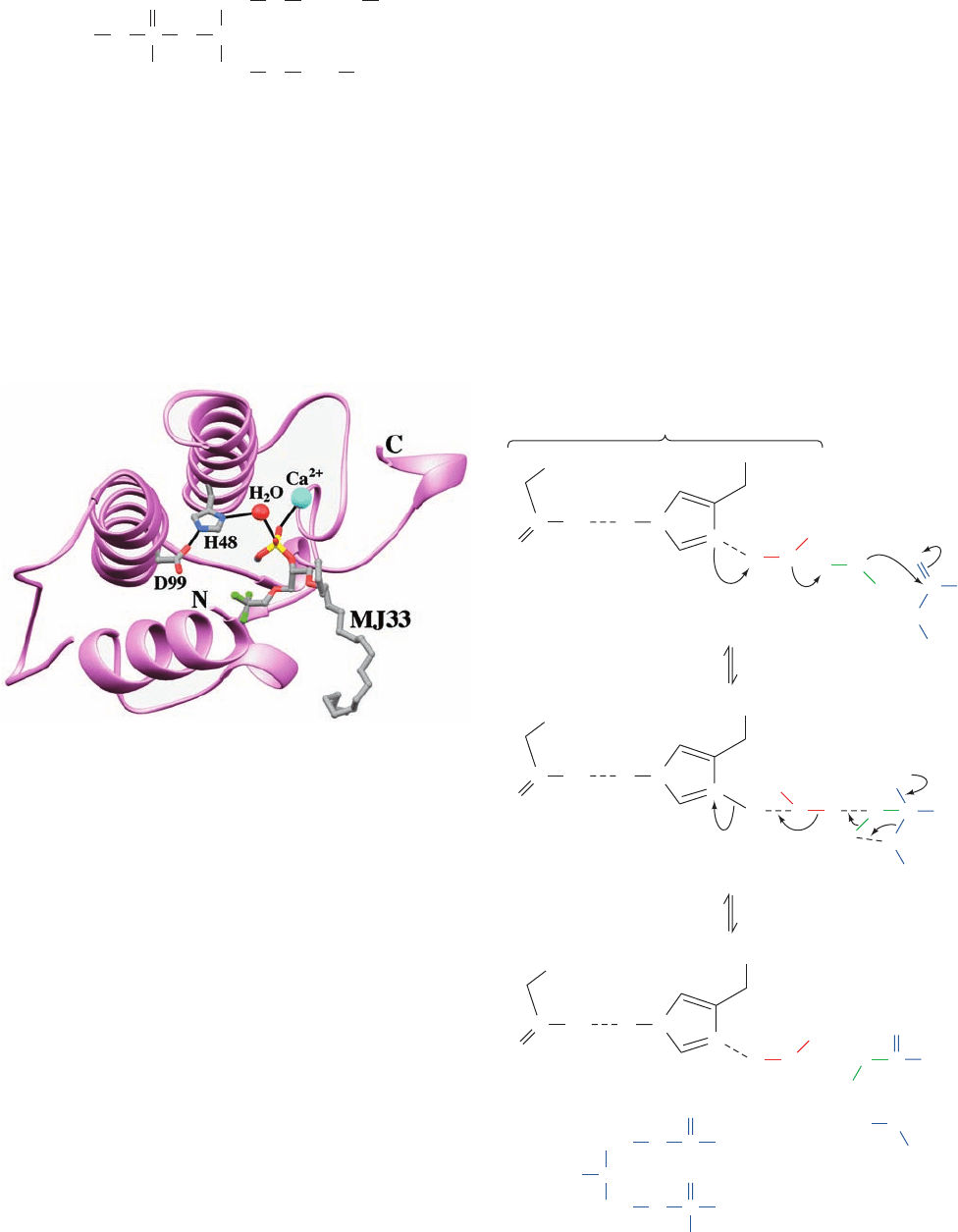
d. Bile Salts and Fatty Acid–Binding Protein
Facilitate the Intestinal Absorption of Lipids
The mixture of fatty acids and mono- and diacylglycerols
produced by lipid digestion is absorbed by the cells lining
the small intestine (the intestinal mucosa) in a process facil-
itated by bile salts.The micelles formed by the bile salts take
up the nonpolar lipid degradation products so as to permit
their transport across the unstirred aqueous boundary layer
at the intestinal wall. The importance of this process is
demonstrated in individuals with obstructed bile ducts:
They absorb little of their dietary lipids but, rather, elimi-
nate them in hydrolyzed form in their feces (steatorrhea).
Evidently, bile salts are not only an aid to lipid digestion but
are essential for the absorption of lipid digestion products.
Bile salts are likewise required for the efficient intestinal
absorption of the lipid-soluble vitamins A, D, E, and K.
Inside the intestinal cells, fatty acids form complexes
with intestinal fatty acid–binding protein (I-FABP), a cyto-
Section 25-1. Lipid Digestion, Absorption, and Transport 943
Brian Bahnson, of phospholipase A
2
in complex with the
tetrahedral intermediate mimic MJ33
suggests that a second, previously unobserved water mole-
cule, which is liganded by the Ca
2
ion, is the attacking nu-
cleophile (Fig. 25-4a). This has led to the formulation of a
reaction mechanism (Fig. 25-4b) in which the Asp–His–
water catalytic triad and the Ca
2
ion both activate the sec-
ond water molecule, with the Ca
2
ion also stabilizing the
resulting tetrahedral intermediate.
MJ33 [1-Hexadecyl-3-(trifluoroethyl)-sn-glycero-
2-phosphomethanol]
O O
O
O
–
PH
3
C
(CH
2
)
15
CH
3
CH
CH
2
O
CH
2
O CH
2
CF
3
Figure 25-4 Structure and mechanism of phospholipase A
2
.
(a) The X-ray structure of the 124-residue monomeric porcine
phospholipase A
2
(lavender) in complex with the tetrahedral
intermediate mimic MJ33.The enzyme’s active site contains a
catalytic triad similar to those of the serine proteases (Fig. 15-20)
with a water molecule replacing the catalytic Ser.The His 48 and
Asp 99 side chains of the catalytic triad together with MJ33 are
drawn in stick form colored according to atom type (C gray, N
blue, O red, F green, and P yellow). The water molecule of the
catalytic triad and the catalytically important Ca
2
ion are
represented by red and cyan spheres. Catalytically important
hydrogen bonds and Ca
2
liganding interactions are represented
by thin black lines.The tetrahedral phosphoryl group of MJ33
presumably occupies the site of the unobserved H
2
O. Residues
65 to 74 of the protein have been deleted for clarity. [Based on
an X-ray structure by Mahendra Jain and Brian Bahnson,
University of Delaware. PDBid 1FXF.] (b) The catalytic
mechanism of phospholipase A
2
. (1) The catalytic triad activates
a second water molecule to attack the scissile carbonyl carbon
with Ca
2
coordinating the activated water molecule as well as
electrostatically stabilizing the resulting tetrahedral intermediate
(rather than doing so via nucleophilic catalysis as occurs in the
serine proteases; Fig. 15-23). (2) The tetrahedral intermediate
decomposes to yield products. [After Berg, O.G., Gelb, M.H.,
Tsai, M.-D., and Jain, M.K., Chem. Rev. 101, 2638 (2001).]
N
N
H
O
H
His
Catalytic triad
H
R
2
C
O
O
O
H
H
1
R
1
Ca
2+
C
O
(b)
O
–
Asp
2
...
...
N
N
H
O
H
His
Tetrahedral
intermediate
H R
2
C
O
O
O
H
H
RCH
2
CHR
1
=
CO
O
OXCH
2
CO
O
R
1
Ca
2+
C
O
O
–
O
–
Asp
...
N
N
+
H
O
H
His
H
R
2
C
O
O
H
H
R
1
Ca
2+
C
O
O
–
O
–
Asp
...
+
(a)
JWCL281_c25_940-1018.qxd 6/8/10 8:59 AM Page 943
cles called chylomicrons. These, in turn, are released into
the bloodstream via the lymph system for delivery to the
tissues. Similarly, triacylglycerols synthesized by the liver
are packaged into very low density lipoproteins (VLDL)
and released directly into the blood. These lipoproteins,
whose origins, structures, and functions are discussed in
Section 12-5, maintain their otherwise insoluble lipid com-
ponents in aqueous solution.
The triacylglycerol components of chylomicrons and
VLDL are hydrolyzed to free fatty acids and glycerol in the
capillaries of adipose tissue and skeletal muscle by lipopro-
tein lipase (Section 12-5Ba). The resulting free fatty acids
are taken up by these tissues while the glycerol is trans-
ported to the liver or kidneys. There it is converted to the
glycolytic intermediate dihydroxyacetone phosphate by
the sequential actions of glycerol kinase and glycerol-3-
phosphate dehydrogenase (Fig. 25-6).
Mobilization of triacylglycerols stored in adipose tissue
involves their hydrolysis to glycerol and free fatty acids by
hormone-sensitive triacylglycerol lipase (or just hormone-
sensitive lipase). The free fatty acids are released into the
bloodstream, where they bind to serum albumin (or just al-
bumin), a soluble 585-residue monomeric protein that com-
prises about half of the blood serum protein. In the absence
of albumin, the maximum solubility of free fatty acids is
⬃10
6
M.Above this concentration, free fatty acids form mi-
celles that act as detergents to disrupt protein and mem-
brane structure and would therefore be toxic.However,the ef-
fective solubility of fatty acids in fatty acid–albumin complexes
is as much as 2 mM. Nevertheless, those rare individuals with
analbuminemia (severely depressed levels of albumin) suf-
fer no apparent adverse symptoms; evidently, their fatty
acids are transported in complex with other serum proteins.
The X-ray structure of human serum albumin in its com-
plexes with a variety of common fatty acids, determined by
Stephen Curry, reveals that each albumin molecule can bind
up to seven fatty acid molecules (Fig. 25-7). However, these
binding sites have different fatty acid–binding affinities so
that,under normal physiological conditions, albumin carries
between 0.1 and 2 fatty acid molecules per protein mole-
cule. Albumin also binds an extraordinarily broad range of
drugs and is thereby a major and usually unpredictable in-
fluence on their pharmacokinetics (Section 15-4Ba). In-
deed, the large amounts of fatty acids in the blood after
meals can significantly affect the pharmacokinetics of a
drug through competitive and/or cooperative interactions.
944 Chapter 25. Lipid Metabolism
plasmic protein, which serves to increase the effective sol-
ubility of these water-insoluble substances and also to
protect the cell from their detergent-like effects (recall
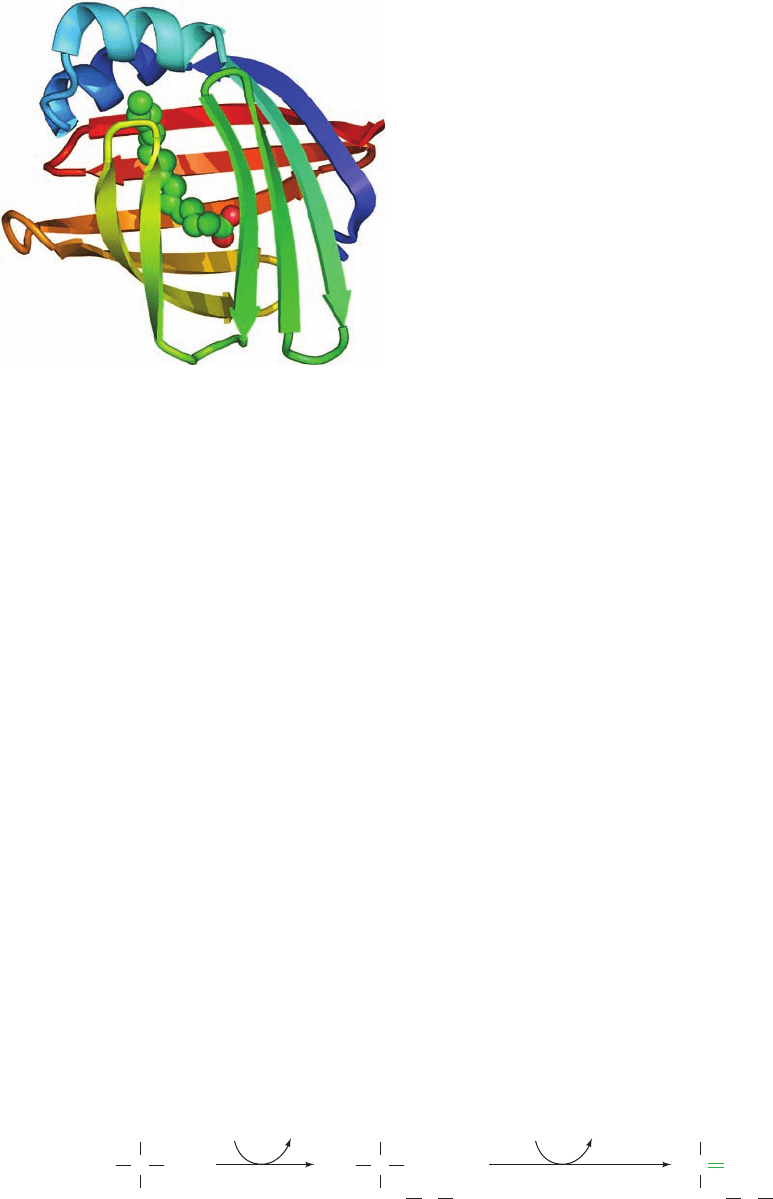
that soaps are fatty acid salts).The X-ray structures of rat
I-FABP, both alone and in complex with a single molecule
of palmitate, were determined by James Sacchettini. This
monomeric, 131-residue protein consists largely of 10 an-
tiparallel strands organized into a stack of two approxi-
mately orthogonal sheets (Fig. 25-5).The palmitate occu-
pies a gap between two of the strands such that it lies
between the sheets with an orientation that, over much of
its length, is more or less parallel to the gapped strands
(this structure has therefore been described as forming a
“-clam”). The palmitate’s carboxyl group interacts with
Arg 106, Gln 115, and two bound water molecules, whereas
the methylene chain is encased by the side chains of several
hydrophobic, mostly aromatic, residues.
e. Lipids Are Transported in Lipoprotein Complexes
The lipid digestion products absorbed by the intestinal
mucosa are converted by these tissues to triacylglycerols
(Section 25-4F) and then packaged into lipoprotein parti-
NAD
+
NADH + H
+
CH
2
OH
CH
2
PO
3
2
–
CHHO O
glycerol-3-
phosphate
dehydrogenase
glycerol
kinase
L-Glycerol-
3-phosphate
Dihydroxy-
acetone
phosphate
L-Glycerol
O
CH
2
OH
CH
2
PO
3
2–
C
O
HHO
CH
2
OH
CH
2
OH
C
ATP ADP
Figure 25-6 Conversion of glycerol to the glycolytic intermediate dihydroxyacetone phosphate.
Figure 25-5 X-ray structure of rat intestinal fatty acid–binding
protein in complex with palmitate. The protein is drawn in ribbon
form colored in rainbow order from its N-terminus (blue) to its
C-terminus (red).The palmitate is shown in space-filling form
with C green and O red. [Based on an X-ray structure by James
Sacchettini,Albert Einstein College of Medicine. PDBid 2IFB.]
JWCL281_c25_940-1018.qxd 4/20/10 1:58 PM Page 944
labels to trace metabolic pathways, fed dogs fatty acids
labeled at their (last) carbon atom by a benzene ring
and isolated the phenyl-containing metabolic products
from their urine. Dogs fed labeled odd-chain fatty acids ex-
creted hippuric acid, the glycine amide of benzoic acid,
whereas those fed labeled even-chain fatty acids excreted
phenylaceturic acid, the glycine amide of phenylacetic acid
(Fig. 25-8). Knoop therefore deduced that the oxidation of
the carbon atom to the carboxyl group is involved in fatty
acid breakdown.Otherwise,the phenylacetic acid would be
further oxidized to benzoic acid. Knoop proposed that this
breakdown occurs by a mechanism known as oxidation
in which the fatty acid’s C
atom is oxidized. It was not un-
til after 1950, following the discovery of coenzyme A, that
the enzymes of fatty acid oxidation were isolated and their
reaction mechanisms elucidated. This work confirmed
Knoop’s hypothesis.
A. Fatty Acid Activation
Before fatty acids can be oxidized, they must be “primed”
for reaction in an ATP-dependent acylation reaction to
form fatty acyl-CoA.This activation process is catalyzed by
a family of at least three acyl-CoA synthetases (also called
thiokinases) that differ according to their chain-length
specificities. These enzymes, which are associated with
either the endoplasmic reticulum (ER) or the outer mito-
chondrial membrane, all catalyze the reaction
In the activation of
18
O-labeled palmitate by a long-
chain acyl-CoA synthetase, both the AMP and the acyl-
CoA products become
18
O labeled. This observation
fatty acyl-CoA AMP PP
i
Fatty acid CoA ATP Δ
2 FATTY ACID OXIDATION
The biochemical strategy of fatty acid oxidation was under-
stood long before the advent of biochemical techniques
involving enzyme purification or the use of radioactive
tracers. In 1904, Franz Knoop, in the first use of chemical
Section 25-2. Fatty Acid Oxidation 945
Figure 25-7 X-ray structure of human serum albumin in
complex with 7 molecules of palmitic acid. The protein is drawn
in semitransparent ribbon form colored in rainbow order from
its N-terminus (blue) to its C-terminus (red).The fatty acids are
shown in space-filling form with C green and O red. [Based on
an X-ray structure by Stephen Curry, Imperial College of Science,
Technology, and Medicine, London, U.K. PDBid 1E7H.]
Figure 25-8 Franz Knoop’s classic experiment indicating that
fatty acids are metabolically oxidized at their -carbon atom.
-Phenyl-labeled fatty acids containing an odd number of carbon
atoms are oxidized to the phenyl-labeled C
1
product, benzoic
acid, whereas those with an even number of carbon atoms are
oxidized to the phenyl-labeled C
2
product, phenylacetic acid.
CH
2
CH
2
NH
Glycine residue
Hippuric acidBenzoic acidOdd-chain fatty acid
Fatty acid fed
Breakdown product Excretion product
COOHCH
2
C
O
OH
C C
OO
OH
(CH
2
CH
2
)
n
(n + 1) C
2
CH
2
CH
2
CH
2
CH
2
CH
2
NH
Glycine residue
Phenylaceturic acidPhenylacetic acidEven-chain fatty acid
COOHCH
2
C
O
OH
C C
OO
OH
(CH
2
CH
2
)
n
(n + 1) C
2
These products are excreted as their respective glycine amides,
hippuric and phenylaceturic acids.The vertical arrows indicate
the deduced sites of carbon oxidation.The intermediate C
2
products are oxidized to CO
2
and H
2
O and were therefore not
isolated.
JWCL281_c25_940-1018.qxd 6/8/10 8:59 AM Page 945
indicates that the reaction has an acyladenylate mixed an-
hydride intermediate that is attacked by the sulfhydryl group
of CoA to form the thioester product (Fig. 25-9).The reac-
tion involves both the cleavage and the synthesis of bonds
with large negative free energies of hydrolysis so that the
free energy change associated with the overall reaction is
close to zero. The reaction is driven to completion in the
cell by the highly exergonic hydrolysis of the product py-
rophosphate (PP
i
) catalyzed by the ubiquitous inorganic
pyrophosphatase. Thus, as commonly occurs in metabolic
pathways, a reaction forming a “high-energy” bond through
the hydrolysis of one of ATP’s phosphoanhydride bonds is
driven to completion by the hydrolysis of its second such
bond.
B. Transport Across the Mitochondrial Membrane
Although fatty acids are activated for oxidation in the cy-
tosol, they are oxidized in the mitochondrion as Eugene
Kennedy and Albert Lehninger established in 1950. We
must therefore consider how fatty acyl-CoA is transported
across the inner mitochondrial membrane. A long-chain
fatty acyl-CoA cannot directly cross the inner mitochon-
drial membrane. Rather, its acyl portion is first transferred to
carnitine (Fig. 25-10), a compound that occurs in both plant
and animal tissues. This transesterification reaction has an
equilibrium constant close to 1, which indicates that the
O-acyl bond of acyl-carnitine has a free energy of hydrolysis
similar to that of the thioester. Carnitine palmitoyltrans-
ferases I and II, which can transfer a variety of acyl groups,
are located,respectively, on the external and internal surfaces
of the inner mitochondrial membrane. The translocation
process itself is mediated by a specific carrier protein that
transports acyl-carnitine into the mitochondrion while trans-
porting free carnitine in the opposite direction. Acyl-CoA
transport therefore occurs via four reactions (Fig. 25-11):
1. The acyl group of a cytosolic acyl-CoA is transferred
to carnitine, thereby releasing the CoA to its cytosolic pool.
2. The resulting acyl-carnitine is transported into the
mitochondrial matrix by the transport system.
3. The acyl group is transferred to a CoA molecule
from the mitochondrial pool.
4. The product carnitine is returned to the cytosol.
946 Chapter 25. Lipid Metabolism
–
OO
H
2
O
PP
i
2P
i
O
O
–
P
H SCoA
SCoA
+
O
O
O
–
P O
Adenosine
RO
*
O*
C
R
–
O
*
O*
C
O
O
O
–
P
Adenosine
O
O
O
–
P
Adenosine
inorganic
pyrophosphatase
ATP
AMPAcyl-CoA
Acyladenylate
mixed anhydride
Fatty acid
O
O
–
P
+
CR
O*
–
O*
(CH
3
)
3
N
+
C
O
SCo
A
CH
2
CH
R+
CH
2
COO
–
OH
Carnitine (4-trimethylamino-
3-hydroxybutyrate)
(CH
3
)
3
N
+
CH
2
CH CH
2
COO
–
Acyl-carnitine
C
O
R O
+ H SCoA
carnitine palmitoyltransferase
Figure 25-9 Mechanism of fatty acid activation catalyzed by
acyl-CoA synthetase. Experiments utilizing
18
O-labeled fatty
acids (*) demonstrate that the formation of acyl-CoA involves an
intermediate acyladenylate mixed anhydride.
Figure 25-10 Acylation of carnitine catalyzed by carnitine
palmitoyltransferase.
Figure 25-11 Transport of fatty acids into the mitochondrion.
Cytosol Matrix
Inner
mitochondrial
membrane
Carnitine
carrier protein
carnitine
palmitoyl
transferase I
carnitine
palmitoyl
transferase II
Carnitine Carnitine
Carnitine
4
2
SCoA
CR
O
SCo
A
CR
O
SCoAHSCoAH
C
O
R CarnitineC
O
R
13
JWCL281_c25_940-1018.qxd 6/8/10 8:59 AM Page 946
2. Hydration of the double bond by enoyl-CoA hydratase
(EH) to form a 3-
L-hydroxyacyl-CoA.
3. NAD
⫹
-dependent dehydrogenation of this -
hydroxyacyl-CoA by 3-
L-hydroxyacyl-CoA dehydroge-
nase (HAD) to form the corresponding -ketoacyl-CoA.
4. C
␣
¬C

cleavage in a thiolysis reaction with CoA as
catalyzed by -ketoacyl-CoA thiolase (KT; also called just
thiolase) to form acetyl-CoA and a new acyl-CoA contain-
ing two less C atoms than the original one.
The first three steps of this process chemically resemble the
citric acid cycle reactions that convert succinate to oxalo-
acetate (Sections 21-3F–H):
CH
2
CH
2
CO
–
2
CO
–
2
C
C
CO
–
2
CO
–
2
Succinate
FAD FADH
2
succinate
dehydrogenase
H
H
C
CH
2
CO
–
2
CO
–
2
HO H
C
CH
2
CO
–
2
CO
–
2
O
Fumarate
H
2
O
fumarase
L-Malate
malate
dehydrogenase
NAD
+
NADH
+ H
+
Oxaloacetate
The cell thereby maintains separate cytosolic and mito-
chondrial pools of CoA. The mitochondrial pool functions
in the oxidative degradation of pyruvate (Section 21-2A)
and certain amino acids (Sections 26-3E–G) as well as fatty
acids, whereas the cytosolic pool supplies fatty acid biosyn-
thesis (Section 25-4). The cell similarly maintains separate
cytosolic and mitochondrial pools of ATP and NAD
⫹
.
C.  Oxidation
Fatty acids are dismembered through the  oxidation of fatty
acyl-CoA, a process that occurs in four reactions (Fig. 25-12):
1. Formation of a trans-␣, double bond through dehydro-
genation by the flavoenzyme acyl-CoA dehydrogenase (AD).
Section 25-2. Fatty Acid Oxidation 947
Figure 25-12 The -oxidation pathway of fatty acyl-CoA.
See the Animated Figures
C
O
SCoA
CH
3
C
β
H
Fatty acyl-CoA
C
α
H
H H
FADH
2
FAD
ETF
ox
ETF
red
ETF:ubiquinone
oxidoreductase
ox
ETF:ubiquinone
oxidoreductase
red
Q
QH
2
Mitochondrial
electron-
transport
chain
H
2
O
1
C
O
SCoA
CH
3
C
trans-
Δ
2
-Enoyl-CoA
C
H
H
H
2
O
enoyl-CoA hydratase (EH)
C
O
SCoA
C
OH
3-L-Hydroxyacyl-CoA
H
CH
2
acyl-CoA
dehydrogenase (AD)
NADH
NAD
+
3-L-hydroxyacyl-CoA
dehydrogenase (HAD)
+ H
+
C
O
SCoA
C
β
-Ketoacyl-CoA
CH
2
O
CoASH
-ketoacyl-CoA thiolase (KT)β
(CH
2
)
n
(CH
2
)
n
(CH
2
)
n
(CH
2
)
n
(CH
2
)
n
SCoA
CH
3
C
Fatty acyl-CoA
(2 C atoms shorter)
O
SCoA
CH
3
C
Acetyl-CoA
O
+
2
O
2
2ADP
+ 2P
i
2ATP
15678
2
3
4
CH
3
CH
3
JWCL281_c25_940-1018.qxd 8/9/10 9:43 AM Page 947
Mitochondria contain four acyl-CoA dehydrogenases,
with specificities for short- (C
4
to C
6
), medium- (C
6
to C
10
),
long- (between medium and very long), and very long-
chain (C
12
to C
18
) fatty acyl-CoAs. The reaction catalyzed
by these enzymes is thought to involve removal of a proton
at C
␣
and transfer of a hydride ion equivalent from C

to
FAD (Fig. 25-12, Reaction 1). The X-ray structure of the
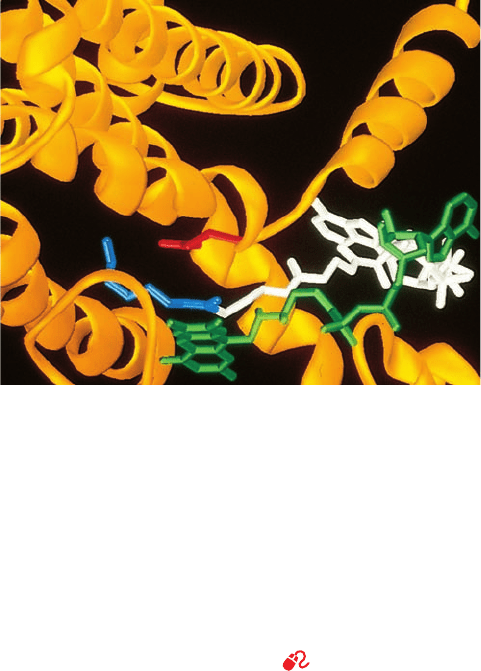
medium-chain acyl-CoA dehydrogenase (MCAD) in com-
plex with octanoyl-CoA, determined by Jung-Ja Kim,
clearly shows how the enzyme orients the enzyme’s base
(Glu 376), the substrate C
␣
¬C

bond, and the FAD pros-
thetic group for reaction (Fig. 25-13).
a. Acyl-CoA Dehydrogenase Is Reoxidized
via the Electron-Transport Chain
The FADH
2
resulting from the oxidation of the fatty
acyl-CoA substrate is reoxidized by the mitochondrial
electron-transport chain through the intermediacy of a se-
ries of electron-transfer reactions. Electron-transfer flavo-
protein (ETF) transfers two electrons from FADH
2
to the
flavoiron–sulfur protein ETF:ubiquinone oxidoreductase,
which in turn transfers two electrons to the mitochondrial
electron-transport chain by reducing coenzyme Q (CoQ;
Fig. 25-12, Reactions 5–8). Reduction of O
2
to H
2
O by the
electron-transport chain beginning at the CoQ stage re-
sults in the synthesis of 1.5 ATPs per two electrons trans-
ferred (Section 22-2Bc).
b. Acyl-CoA Dehydrogenase Deficiency
Has Fatal Consequences
The unexpected death of an apparently healthy infant,
often overnight, has been, for lack of any real explanation,
termed sudden infant death syndrome (SIDS). MCAD has
been shown to be deficient in up to 10% of these infants,
making this genetic disease more prevalent than phenylke-
tonuria (PKU) (Section 26-3Hd), a genetic defect in
phenylalanine degradation for which babies born in the
United States are routinely tested. Glucose is the principal
energy metabolism substrate just after eating,but when the
glucose level later decreases, the rate of fatty acid oxida-
tion must correspondingly increase. The sudden death of
infants lacking MCAD may be caused by the imbalance
between glucose and fatty acid oxidation.
Lys 304, which becomes Glu in the most prevalent mu-
tation among individuals with MCAD deficiency, is ⬃20 Å
distant from the enzyme’s active site and hence cannot par-
ticipate in binding substrate or FAD. However, since the
side chains of Asp 300 and Asp 346 lie within 6 Å of Glu
304, near a subunit–subunit interface, it seems likely that
the high concentration of negative charges resulting from
the Lys 304 S Glu mutation structurally destabilizes the
enzyme.
Deficiency of acyl-CoA dehydrogenase has also been
implicated in Jamaican vomiting sickness, whose victims
suffer violent vomiting followed by convulsions, coma, and
death. Severe hypoglycemia is observed in most cases. This
condition results from eating unripe ackee fruit, which con-
tains hypoglycin A, an unusual amino acid, which is metab-
olized to methylenecyclopropylacetyl-CoA (MCPA-CoA;
Fig. 25-14). MCPA-CoA, a substrate for acyl-CoA dehy-
drogenase, is thought to undergo the first step of the reac-
tion that this enzyme catalyzes, removal of a proton from
C
␣
, to form a reactive intermediate that covalently modi-
fies the enzyme’s FAD prosthetic group (Fig. 25-14). Since
a normal step in the enzyme’s reaction mechanism gener-
ates the reactive intermediate, MCPA-CoA is said to be a
mechanism-based inhibitor.
c. Long-Chain Enoyl-CoAs Are Converted to
Acetyl-CoA and a Shorter Acyl-CoA by
Mitochondrial Trifunctional Protein
The products of acyl-CoA dehydrogenases are 2-enoyl-
CoAs. Depending on their chain lengths their processing is
continued by one of three systems (Fig. 25-12): the short-
chain, medium-chain, or long-chain 2-enoyl-CoA hydratases
(EHs), hydroxyacyl-CoA dehydrogenases (HADs), and
-ketoacyl-CoA thiolases (KTs). The long-chain (LC) ver-
sions of these enzymes are contained on one ␣
4

4
octameric
protein, mitochondrial trifunctional protein, located in the
inner mitochondrial membrane. LCEH and LCHAD are
contained on the ␣ subunits while LCKT is located on the
 subunits. The protein is therefore a combination multi-
functional protein (more than one enzyme activity on a sin-
948 Chapter 25. Lipid Metabolism
Figure 25-13 Ribbon diagram of the active site region in a
subunit of medium-chain acyl-CoA dehydrogenase from pig liver
mitochondria in complex with octanoyl-CoA. The enzyme is a
tetramer of identical 385-residue subunits, each of which binds an
FAD prosthetic group (green) and its octanoyl-CoA substrate
(whose octanoyl and CoA moieties are blue and white) in largely
extended conformations.The octanoyl-CoA binds such that its
C
␣
¬C

bond is sandwiched between the carboxylate group of
Glu 376 (red) and the flavin ring (green), consistent with the
proposal that Glu 376 is the general base that abstracts the ␣
proton in the ␣, dehydrogenation reaction catalyzed by the
enzyme. [Based on an X-ray structure by Jung-Ja Kim, Medical
College of Wisconsin. PDBid 3MDE.]
See Interactive
Exercise 23
JWCL281_c25_940-1018.qxd 10/19/10 9:41 AM Page 948
gle polypeptide chain)–multienzyme complex (a complex
of polypeptides catalyzing more than one reaction). The
advantage of such a trifunctional enzyme is the ability to
channel the intermediates toward the final product. In-
deed, no long-chain hydroxyacyl-CoA or ketoacyl-CoA in-
termediates are released into solution by this system.
d. The Thiolase Reaction Occurs via
Claisen Ester Cleavage
The final stage of the fatty acid -oxidation process, the
thiolase reaction, forms acetyl-CoA and a new acyl-CoA
which is two carbon atoms shorter than the one that began
the cycle.This occurs in five reaction steps (Fig. 25-15):
1. An active site thiol is added to the substrate -keto
group.
2. Carbon–carbon bond cleavage forms an acetyl-CoA
carbanion intermediate that is stabilized by electron with-
drawal into this thioester’s carbonyl group. Such a reaction
is known as a Claisen ester cleavage (the reverse of a
Claisen condensation). The citric acid cycle enzyme citrate
synthase also catalyzes a reaction that involves a stabilized
acetyl-CoA carbanion intermediate (Section 21-3A).
3. The acetyl-CoA carbanion intermediate is proto-
nated by an enzyme acid group, yielding acetyl-CoA.
4 and 5. Finally, CoA displaces the enzyme thiol group
from the enzyme–thioester intermediate, yielding acyl-CoA.
The formation of an enzyme–thioester intermediate in-
volving an active site thiol group is based on the observa-
tion that incubation of the enzyme with [
14
C]acetyl-CoA
Section 25-2. Fatty Acid Oxidation 949
Figure 25-14 Metabolic conversions of hypoglycin A to yield a
product that inactivates acyl-CoA dehydrogenase. Spectral
Figure 25-15 Mechanism of action of -ketoacyl-CoA thiolase. An active site
Cys residue participates in the formation of an enzyme–thioester intermediate.
COO
–
–
–
H
+
H
2
CCH
2
CH
2
CCH
O
SCoACH
2
C
H
2
C
H
2
C
CH
2
+
NH
3
CH
2
CCH
H
C
metabolism
Possible reactive intermediate that reacts with the FAD of acyl-CoA dehydrogenase
Hypoglycin A Methylenecyclopropylacetyl-Co
A
(MCPA-CoA)
H
2
CCH
CH
2
CCH
O
SCoACCH CCH
O
SCoAC
acyl-CoA
dehydrogenase
changes suggest that the enzyme’s FAD prosthetic group has
been modified.
O
CH
2
C
SCoA
β
-Ketoacyl-CoA
O
C
R
S
–
BH
+
+
S
BH
+
O
O
–
C
SCoA
R
C
CH
2
C
SCoA
O
S
BH
+
O
R
C
+
–
CH
2
O
–
C
SCoA
CH
2
O
C
SCoA
S
B
O
R
C
H
3
C
Enzyme–thioester
intermediate
Acetyl-CoA
S
BH
+
O
–
R
C
SCoA
S
–
BH
+
SCoA
O
C
R
Acyl-CoA
+
1
2
3
4
5
CoAS H
JWCL281_c25_940-1018.qxd 4/20/10 1:58 PM Page 949
The resulting cis-, double bond–containing enoyl-CoA
is not a substrate for enoyl-CoA hydratase. Enoyl-CoA iso-
merase, however, mediates conversion of the cis-
3
double
bond to the more stable, ester-conjugated trans-
2
form:
Such compounds are normal substrates of enoyl-CoA
hydratase so that oxidation can then continue.
Problem 2: A
4
Double Bond Inhibits
Hydratase Action
The next difficulty arises on the left-hand pathway in
Fig. 25-17 in the fifth round of oxidation.The presence of
a double bond at an even-numbered carbon atom results in
the formation of 2,4-dienoyl-CoA, which is a poor substrate
for enoyl-CoA hydratase. However, NADPH-dependent
C
SCoA
O
H
H
Enz
HH
enoyl-CoA
isomerase
...
...
HH
B
O
C
SCoA
O
HH
Enz
B
O
950 Chapter 25. Lipid Metabolism
yields a specifically labeled enzyme Cys residue (the re-
verse of steps 4 and 5):
e. Fatty Acid Oxidation Is Highly Exergonic
The function of fatty acid oxidation is, of course, to gen-
erate metabolic energy. Each round of oxidation pro-
duces one NADH, one FADH
2
, and one acetyl-CoA. Oxi-
dation of acetyl-CoA via the citric acid cycle generates
additional FADH
2
and NADH, which are reoxidized
through oxidative phosphorylation to form ATP. Complete
oxidation of a fatty acid molecule is therefore a highly ex-
ergonic process, which yields numerous ATPs. For exam-
ple, oxidation of palmitoyl-CoA (which has a C
16
fatty acyl
group) involves seven rounds of oxidation, yielding 7
FADH
2
, 7 NADH, and 8 acetyl-CoA. Oxidation of the 8
acetyl-CoA, in turn, yields 8 GTP, 24 NADH, and 8
FADH
2
. Since oxidative phosphorylation of the 31 NADH
molecules yields 77.5 ATP and that of the 15 FADH
2
yields
22.5 ATPs, subtracting the 2 ATP equivalents required for
fatty acyl-CoA formation (Section 25-2A), the oxidation of
one palmitate molecule has a net yield of 106 ATP.
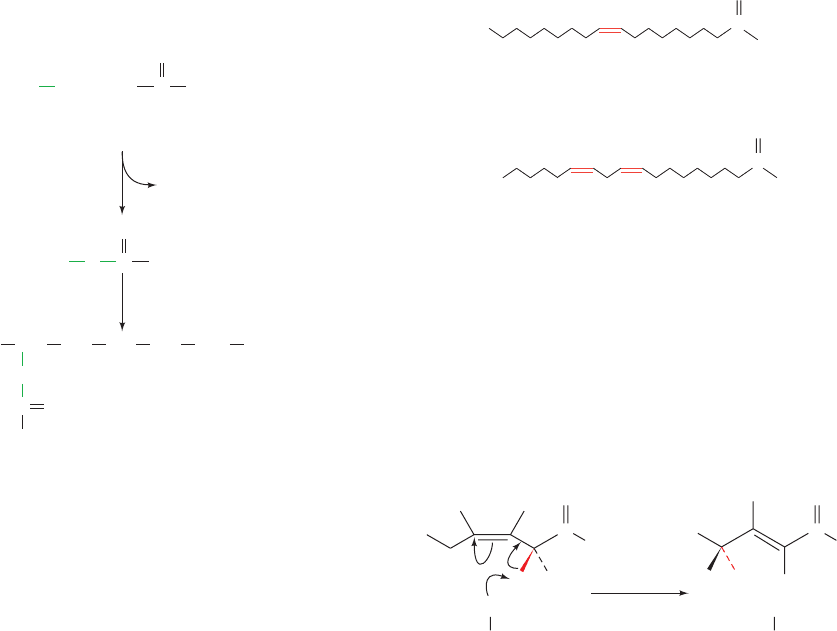
D. Oxidation of Unsaturated Fatty Acids
Almost all unsaturated fatty acids of biological origin (Sec-
tion 12-1A) contain only cis double bonds, which most often
begin between C9 and C10 (referred to as a ⌬
9
or 9-double
bond; Table 12-1). Additional double bonds, if any, occur at
three-carbon intervals and are therefore never conjugated.
Two examples of unsaturated fatty acids are oleic acid and
linoleic acid (Fig. 25-16). Note that one of the double bonds
in linoleic acid is at an odd-numbered carbon atom and the
other is at an even-numbered carbon atom. Double bonds
at these positions in fatty acids pose three problems for the
-oxidation pathway that are solved through the actions of
four additional enzymes (Fig. 25-17):
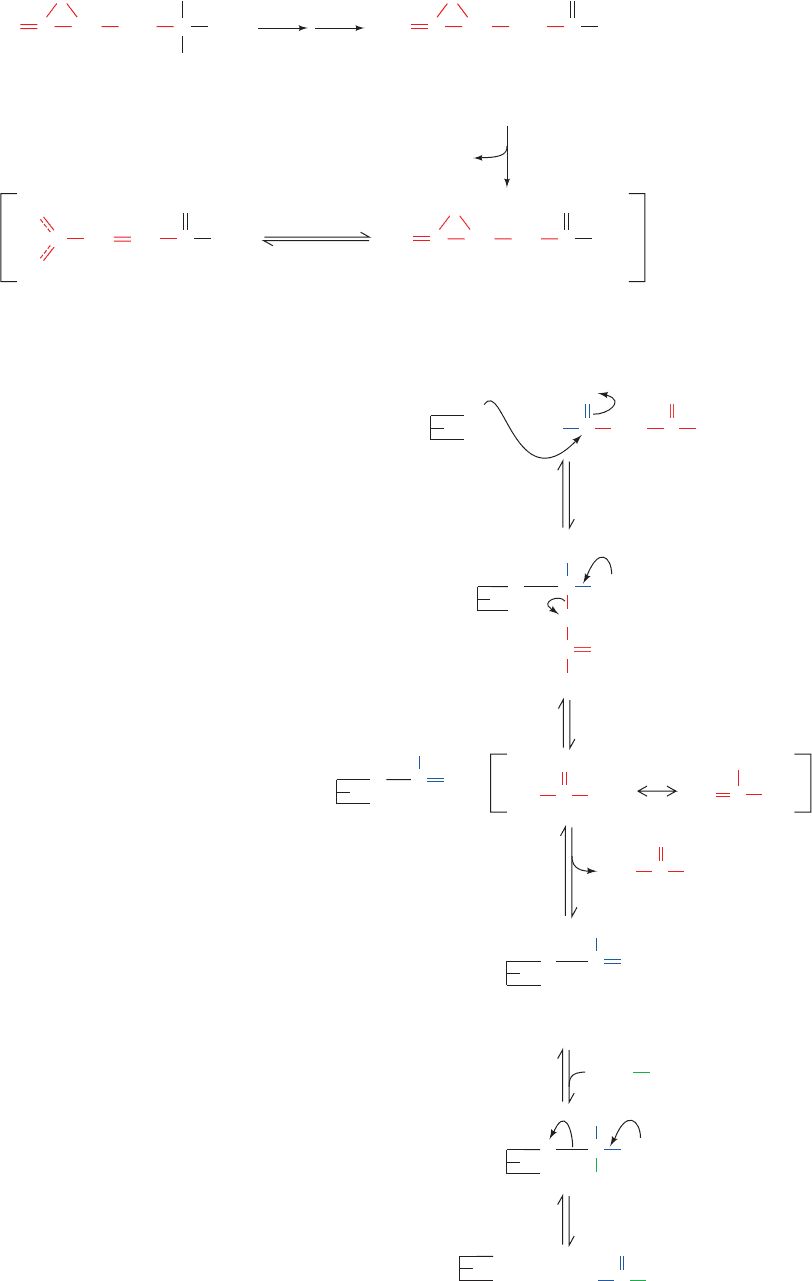
Problem 1: A ,␥ Double Bond
The first enzymatic difficulty occurs on the left-hand
pathway in Fig. 25-17 after the third round of oxidation:
Acetyl-CoAThiolase
C
*
SCoA
trypsin degradation
CoASH
+
O
CH
3
CH
3
SH
C
*
CH
3
O
S
E
E
C
*
O
S
Cys Ala Ser
Gly MetVal Lys
Figure 25-16 Structures of two common unsaturated fatty
acids. Most unsaturated fatty acids contain unconjugated cis
double bonds.
Figure 25-17 (Opposite) Problems in the oxidation of
unsaturated fatty acids and their solutions. Linoleic acid is used
as an example. The first problem, the presence of a , double
bond seen in the left-hand pathway, is solved by the bond’s
enoyl-CoA isomerase–catalyzed conversion to a trans-,
double bond.The second problem in the left-hand pathway, that
a 2,4-dienoyl-CoA is not a substrate for enoyl-CoA hydratase, is
eliminated by the NADPH-dependent reduction of the
4
bond
by 2,4-dienoyl-CoA reductase to yield the -oxidation substrate
trans-2-enoyl-CoA in E. coli but trans-3-enoyl-CoA in mammals.
Mammals therefore also have 3,2-enoyl-CoA isomerase, which
converts the trans-3-enoyl-CoA to trans-2-enoyl-CoA.The third
problem, the isomerization of 2,5-dienoyl-CoA (originating from
the oxidation of unsaturated fatty acids with double bonds at
odd-numbered C atoms) to 3,5-dienoyl-CoA by 3,2-enoyl-CoA
isomerase, is solved by 3,5–2,4-dienoyl-CoA isomerase, which
converts the 3,5-dienoyl-CoA to 2,4-dienoyl-CoA, a substrate
for 2,4-dienoyl-CoA reductase.
918
C
1
9
18
C
OH
O
112
Oleic acid
(9-cis-Octadecenoic acid)
Linoleic acid
(9,12-cis-Octadecadienoic acid)
OH
O
JWCL281_c25_940-1018.qxd 4/20/10 1:58 PM Page 950
