Voet D., Voet Ju.G. Biochemistry
Подождите немного. Документ загружается.

associates with the M subunit BPheo b (Fig. 24-12a, left).
These various chromophores are closely associated with a
number of protein aromatic rings, which are therefore also
thought to participate in the electron-transfer process
described below. The Fe(II) is positioned between the
menaquinone and ubiquinone rings and is octahedrally lig-
anded by four His side chains and the two carboxyl oxygen
atoms of a Glu side chain. Curiously, the two symmetry re-
lated groups of chromophores are not functionally equiva-
lent; electrons, as we shall see, are almost exclusively trans-
ferred through the L subunit (the right sides of Figs. 24-11
and 24-12). This effect is generally attributed to subtle
structural and electronic differences between the L and M
subunits.
c. The Electronic States of Molecules Undergoing
Fast Reactions Can Be Monitored by EPR and
Laser Spectroscopy Techniques
The turnover time of a photosynthetic reaction cycle, as
we have seen, is only a few milliseconds. Its sequence of re-
actions can therefore only be traced by measurements that
can follow extremely rapid electronic changes in mole-
cules. Two techniques are well suited to this task:
1. Electron paramagnetic resonance (EPR) spectroscopy
[also called electron spin resonance (ESR) spectroscopy],
which detects the spins of unpaired electrons in a manner
analogous to the detection of nuclear spins in NMR spec-
troscopy. A molecular species with unpaired electrons,
such as an organic radical or a transition metal ion, has a
characteristic EPR spectrum because its unpaired elec-
trons interact with the magnetic fields generated by the
nuclei and the other electrons of the molecule. Paramag-
netic species as short lived as 10 ps can exhibit definitive
EPR spectra.
2. Optical spectroscopy using pulsed lasers. Laser flashes
as brief as 20 attoseconds (as; 1 as ⫽ 10
⫺18
s) have been
generated. By monitoring the bleaching (disappearance) of
certain absorption bands and the emergence of others,
laser spectroscopy can track the time course of a fast reac-
tion process.
Section 24-2. Light Reactions 911
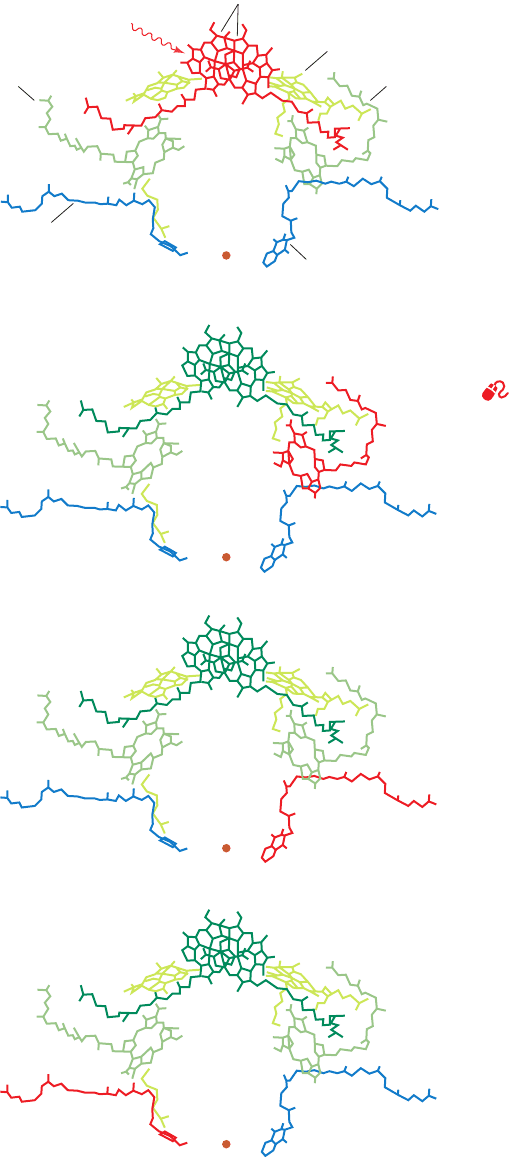
Fe(II)
0 s(a) Special pair
Menaquinone (Q
A
)
Ubiquinone (Q
B
)
BPheo b
Accessory BChl b
hν
3 × 10
–12
s(b)
200 × 10
–12
s(c)
100 × 10
–6
s(d)
BPheo b
Figure 24-12 Sequence of excitations in the bacterial RC of
Rps. viridis. The RC chromophores are shown in the same view
as in Fig. 12-26a, which resembles that in Fig. 24-11a. Note that
their rings, but not their aliphatic side chains, are arranged with
close to 2-fold symmetry. (a) At zero time, a photon is absorbed
by the “special pair” of BChl b molecules, thereby collectively
raising them to an excited state [in each step, the excited
molecule(s) is shown in red]. (b) Within 3 ps, an excited electron
has passed to the BPheo b of the L subunit (right arm of the
system) without becoming closely associated with the accessory
BChl b.The special pair is thereby left with a positive charge.
(c) Some 200 ps later, the excited electron has transferred to the
menaquinone (Q
A
, which is ubiquinone in Rb. sphaeroides).
(d) Within the next 100 s, the special pair has been reduced (via
an electron-transport chain discussed in the text), thereby
eliminating its positive charge, while the excited electron
migrates to the ubiquinone (Q
B
).After a second such electron
has been transferred to Q
B
, it picks up two protons from solution
and exchanges with the membrane-bound ubiquinone pool.
See Kinemage Exercise 8-2
JWCL281_c24_901-939.qxd 6/8/10 8:54 AM Page 911
d. Photon Absorption Rapidly Photooxidizes
the Special Pair
The sequence of photochemical events mediated by the
photosynthetic reaction center is diagrammed in Fig. 24-12:
(a) The primary photochemical event of bacterial photo-
synthesis is absorption of a photon by the special pair (P870
or P960 depending on whether it consists of BChl a or b;
here, for argument’s sake, we assume it to be P960). This
event is nearly instantaneous;it occupies the ⬃3-fs oscillation
time of a light wave. EPR measurements established that
P960 is, in fact, a pair of BChl b molecules and indicated that
the excited electron is delocalized over both of them.
(b) P960*, the excited state of P960, has but a fleeting
existence. Laser spectroscopy has demonstrated that
within ⬃3 ps after its formation, P960* has transferred an
electron to the BPheo b on the right in Fig. 24-12b to yield
P960
⫹
BPheo b
⫺
. In forming this radical pair, the trans-
ferred electron must pass near but seems not to reduce the
intervening BChl b (which is therefore termed an acces-
sory chlorophyll), although its position strongly suggests
that it has an important role in conveying electrons.
(c) By some 200 ps later, the electron has further mi-
grated to the menaquinone (or,in many species, the second
ubiquinone), designated Q
A
, to form the anionic semi-
quinone radical All these electron transfers, as dia-
grammed in Fig. 24-13, are to progressively lower energy
states, which makes this process all but irreversible.
Rapid removal of the excited electron from the vicinity of
P960
⫹
is an essential feature of the PbRC; this prevents
back reactions that would return the electron to P960
⫹
so
as to provide the time required for the wasteful internal
conversion of its excitation energy to heat. In fact, this se-
quence of electron transfers is so efficient that its overall
quantum yield (ratio of molecules reacted to photons ab-
sorbed) is virtually 100%. No man-made device has yet ap-
proached this level of efficiency.
e. Electrons Are Returned to the Photooxidized
Special Pair via an Electron-Transport Chain
The remainder of the photosynthetic electron-transport
process occurs on a much slower timescale.Within ⬃100 s
after its formation, , which occupies a hydrophobic
pocket in the protein, transfers its excited electron to the
more solvent-exposed ubiquinone, Q
B
, to form (Fig.
24-12d). The nonheme Fe(II) is not reduced in this
process and, in fact, its removal only slightly affects the
electron transfer rate, so that the Fe(II) probably func-
tions to fine-tune the PbRC’s electronic character. Q
A
never becomes fully reduced; it shuttles between its oxi-
dized and semiquinone forms. Moreover, the lifetime of
is so short that it never becomes protonated. In con-
trast, once the PbRC again becomes excited, it transfers a
second electron to to form the fully reduced This
anionic quinol takes up two protons from the solution on
the cytoplasmic side of the plasma membrane to form
Q
B
H
2
.Thus Q
B
is a molecular transducer that converts two
Q
2⫺
B
.Q
B
⫺
ⴢ
Q
A
⫺
ⴢ
Q
B
⫺
ⴢ
Q
A
⫺
ⴢ
Q
A
⫺
ⴢ
light-driven one-electron excitations to a two-electron
chemical reduction.
The electrons taken up by Q
B
H
2
are eventually returned
to P960
⫹
via a complex electron-transport chain (Fig. 24-13).
The details of this process are more species dependent than
the preceding and are not so well understood. The avail-
able redox carriers include a membrane-bound pool of
ubiquinone molecules, cytochrome bc
1
, and cytochrome c
2
.
Cytochrome bc
1
is a transmembrane protein complex
composed of a [2Fe–2S] cluster–containing subunit; a heme
c-containing cytochrome c
1
; a cytochrome b that contains
two functionally inequivalent heme b’s, b
H
and b
L
(H and L
for high and low potential); and, in some species, a fourth
subunit. Note that cytochrome bc
1
is strikingly similar to
the proton-translocating Complex III of mitochondria
(Section 22-2C3a), which is also called cytochrome bc
1
.The
electron-transport pathway leads from Q
B
H
2
on the cyto-
plasmic side of the plasma membrane, through the
ubiquinone pool, with which Q
B
H
2
exchanges, to cy-
tochrome bc
1
, and then to cytochrome c
2
on the external
(periplasmic) side of the plasma membrane. The reduced
cytochrome c
2
, which, as its name implies, closely resembles
mitochondrial cytochrome c, diffuses along the external mem-
brane surface until it reacts with the membrane-spanning
PbRC to transfer an electron to P960
⫹
(the structures of
several c-type cytochromes, including that of cytochrome c
2
from Rs. rubrum, are diagrammed in Fig. 9-41). In Rps.
viridis, the four-heme c-type cytochrome bound to the
PbRC complex on the external side of the plasma mem-
brane (Fig. 12-26) is interposed between cytochrome c
2
and
P960
⫹
. Note that one of this c-type cytochrome’s hemes is
positioned to reduce the photooxidized special pair. The
PbRC is thereby prepared to absorb another photon.
f. Photosynthetic Electron Transport Drives the
Formation of a Proton Gradient
Since electron transport in PbRCs is a cyclic process (Fig.
24-13), it results in no net oxidation–reduction. Rather, it func-
tions to translocate the cytoplasmic protons acquired by Q
B
H
2
across the plasma membrane, thereby making the cell alkaline
relative to its environment. The mechanism of this process is
essentially identical to that of proton transport in mitochon-
drial Complex III (Section 22-3Be); that is, in addition to the
translocation of the two H
⫹
resulting from the two-electron
reduction of Q
B
to QH
2
, a Q cycle mediated by cytochrome
bc
1
translocates two H
⫹
for a total of four H
⫹
translocated per
two photons absorbed (Fig. 24-13a; also see Fig. 22-31). Syn-
thesis of ATP, a process known as photophosphorylation, is
driven by the dissipation of the resulting pH gradient in a man-
ner that closely resembles ATP synthesis in oxidative phospho-
rylation (Section 22-3C). We further discuss the mechanism of
photophosphorylation in Section 24-2D.
Photosynthetic bacteria use photophosphorylation-
generated ATP to drive their various endergonic processes.
However, unlike cyanobacteria and plants, which generate
their required reducing equivalents by the light-driven ox-
idation of H
2
O (see below), photosynthetic bacteria must
obtain their reducing equivalents from the environment.
912 Chapter 24. Photosynthesis
JWCL281_c24_901-939.qxd 3/25/10 12:04 PM Page 912
Various substances, such as H
2
S, S, S
2
O
3
2⫺
,H
2
, and many or-
ganic compounds, function in this capacity depending on
the bacterial species.
Modern photosynthetic bacteria are thought to resemble
the original photosynthetic organisms. These presumably
arose very early in the history of cellular life when environ-
mentally supplied sources of “high-energy” compounds
were dwindling but reducing agents were still plentiful (Sec-
tion 1-5Cb). During this era, photosynthetic bacteria were
no doubt the dominant form of life. However, their very
success eventually caused them to exhaust the available re-
ductive resources. The ancestors of modern cyanobacteria
adapted to this situation by evolving a photosynthetic sys-
tem with sufficient electromotive force to abstract electrons
from H
2
O. The gradual accumulation of the resulting toxic
waste product, O
2
, forced photosynthetic bacteria, which
cannot photosynthesize in the presence of O
2
(although
some species have evolved the ability to respire), into the
narrow ecological niches to which they are presently con-
fined (Section 1-1Ab).
C. Two-Center Electron Transport
See Guided Exploration 22: Two-center photosynthesis (Z-scheme)
overview
Plants and cyanobacteria use the reducing power
generated by the light-driven oxidation of H
2
O to produce
Section 24-2. Light Reactions 913
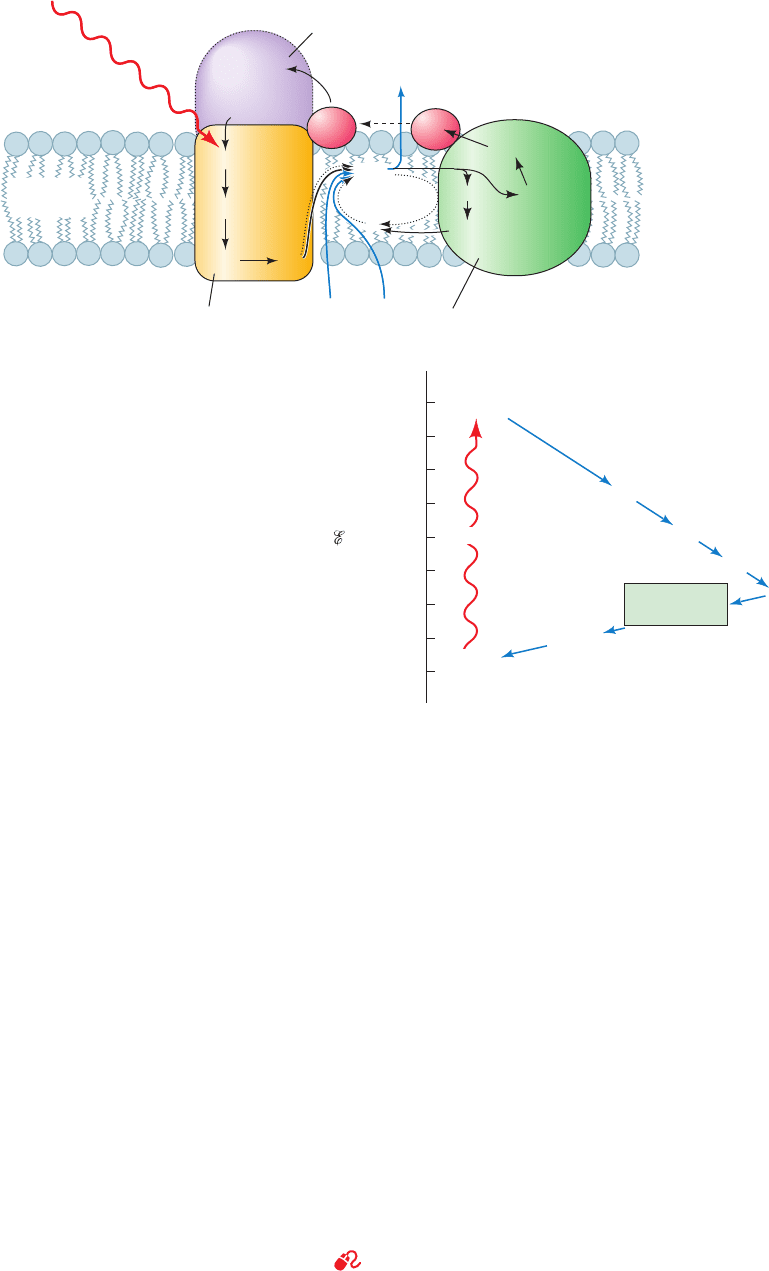
heme 4
heme 3
heme 2
heme 1
Four-heme c-type cytochrome
(not present in all species)
Exterior
(periplasm)
Cytoplasm
Plasma
membrane
hν
P870
Q
A
QH
2
2H
+
2H
+
Q
Q cycle
Q
B
BPheo a
Photosynthetic
reaction center
Cytochrome bc
1
4H
+
cyt
c
2
cyt
c
2
cyt b
L
[2Fe–2S]
cyt c
1
cyt b
H
(a)
+0.6
+0.4
+0.2
Q pool
Cytochrome c
2
0
Q
B
–0.2
–0.4
–0.6
BPheo b
–0.8
–1.0
P960*
hν
Q
A
Cytochrome
bc
1
complex
P960
o
′
(V)
(b)
Figure 24-13 Photosynthetic electron-transport system of
purple photosynthetic bacteria. (a) Schematic diagram indicating
the arrangement of the system components in the bacterial
plasma membrane and the flows of electrons (black arrows) and
protons (blue arrows) that photon (h) absorption promotes
through them.The system contains two protein complexes, the
RC and cytochrome bc
1
.Two electrons liberated from the special
pair, here P870 (as in Rb. sphaeroides), by the consecutive
absorption of two photons are taken up by ubiquinone (Q
B
)
together with two protons from the cytoplasm to yield ubiquinol
(QH
2
).The QH
2
is released from the RC and diffuses (dotted
arrows) through the membrane to cytochrome bc
1
, which, in a
two-electron reaction, oxidizes it to ubiquinone with the con-
comitant liberation of its two protons to the external medium.
One of the two electrons is passed, via the [2Fe–2S] cluster and
cytochrome c
1
, to cytochrome c
2
, a peripheral membrane protein
that then diffuses across the external surface of the membrane so
as to return the electron to P870 of the RC.The second electron
from QH
2
passes, via a Q cycle, through hemes b
L
and b
H
of
cytochrome bc
1
and then contributes to the reduction of a
molecule of ubiquinone (Q) with the concomitant uptake of two
more cytoplasmic protons (two rounds of a Q cycle are required
for the reduction of one molecule of Q to QH
2
; Fig. 22-31). The
resulting QH
2
diffuses back to cytochrome bc
1
.There it is again
oxidized, with the liberation of its two protons to the exterior
and the return of one of its two electrons, via cytochrome c
2
,to
P870, thereby completing the electrical circuit. Note that in every
turn of a Q cycle, half the electrons liberated by the oxidation of
QH
2
to Q are used to reduce Q to QH
2
, so that, after a large
number of turns, an electron that enters the Q cycle, on average,
passes through it twice before being returned to P870.Thus, the
net result of the absorption of two photons by the RC is the
translocation of four H
⫹
from the cytoplasm to the external
medium. (b) The approximate standard reduction potentials of
the photosynthetic electron-transport system’s various
components.The overall process is essentially irreversible
because electrons are transferred to progressively lower energy
states (more positive standard reduction potentials).
JWCL281_c24_901-939.qxd 6/8/10 8:54 AM Page 913
0.08
O
2
quantum yield
0.10
0.12
0.14
0.06
0.04
0.02
0
660 680 700 720
Wavelength (nm)
Absence
of yellow-
green light
Presence
of yellow-
green light
NADPH. The component half-reactions of this process, to-
gether with their standard reduction potentials, are
and
Hence, the overall four-electron reaction and its standard
redox potential is
This latter quantity corresponds (Eq. [16.5]) to a standard
free energy change of ⌬G°¿ ⫽ 438 kJ ⴢ mol
⫺1
, which Eq.
[24.1] indicates is the energy of one einstein of 223-nm
photons (UV light). Clearly, even if photosynthesis were
100% efficient, which it is not, it would require more than
one photon of visible light to generate a molecule of O
2
. In
fact, experimental measurements indicate that algae mini-
mally require 8 to 10 photons of visible light to produce
one molecule of O
2
. In the following subsections, we dis-
cuss how plants and cyanobacteria manage this multipho-
ton process.
a. Photosynthetic O
2
Production Requires
Two Sequential Photosystems
Two seminal observations led to the elucidation of the
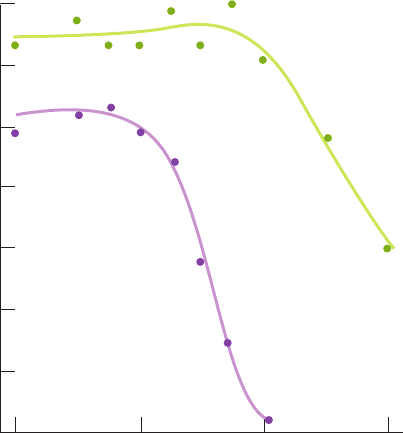
basic mechanism of photosynthesis in plants:
1. The quantum yield for O
2
evolution by Chlorella
pyrenoidosa varies little with the wavelength of the illumi-
nating light between 400 and 675 nm, but decreases precip-
itously above 680 nm (Fig. 24-14, lower curve). This phe-
nomenon, the “red drop,” was unexpected because Chl a
absorbs such far-red light (Fig. 24-5).
2. Shorter wavelength light, such as yellow-green
light, enhances the photosynthetic efficiency of 700-nm
light well in excess of the energy content of the shorter
wavelength light; that is, the rate of O
2
evolution by both
lights is greater than the sum of the rates for each light act-
ing alone (Fig. 24-14, upper curve). Moreover, this en-
hancement still occurs if the yellow-green light is
switched off several seconds before the red light is turned
on and vice versa.
These observations clearly indicate that two processes
are involved. They are explained by a mechanistic model,
the Z-scheme, which postulates that O
2
-producing photo-
synthesis occurs through the actions of two photosynthetic
RCs that are connected essentially in series (Fig. 24-15).
1. Photosystem I (PSI) generates a strong reductant
capable of reducing NADP
⫹
and, concomitantly, a weak
oxidant.
2. Photosystem II (PSII) generates a strong oxidant
capable of oxidizing H
2
O and, concomitantly, a weak re-
ductant.
e°¿ ⫽⫺1.135V
2NADP
⫹
⫹ 2H
2
O Δ 2NADPH ⫹ O
2
⫹ 2H
⫹
NADP
⫹
⫹ H
⫹
⫹ 2e
⫺
Δ NADPH
e°¿ ⫽⫺0.320V
O
2
⫹ 4e
⫺
⫹ 4H
⫹
Δ 2H
2
O
e°¿ ⫽⫹0.815V
The weak reductant reduces the weak oxidant, so that PSI
and PSII form a two-stage electron “energizer.” Both photo-
systems must therefore function for photosynthesis (electron
transfer from H
2
O to NADP
⫹
, forming O
2
and NADPH) to
occur.
The red drop is explained in terms of the Z-scheme by
the observation that PSII is only poorly activated by 680-
nm light. In the presence of only this far-red light, PSI is
activated but is unable to obtain more than a few of the
electrons it is capable of energizing. Yellow-green light,
however,efficiently stimulates PSII to supply these electrons.
The observation that the far-red and yellow-green lights
can be alternated indicates that both photosystems remain
activated for a time after the light is switched off.
The validity of the Z-scheme was established as follows.
The oxidation state of cytochrome f, a c-type cytochrome of
the electron-transport chain connecting PSI and PSII (see
below), can be spectroscopically monitored. Illumination
of algae with 680-nm (far-red) light results in the oxidation
of cytochrome f (Fig. 24-16). However, the additional
imposition of a 562-nm (yellow-green) light results in this
914 Chapter 24. Photosynthesis
Figure 24-14 Quantum yield for O
2
production by Chlorella
algae as a function of the wavelength of the incident light. The
experiment was conducted in the absence (lower curve) and the
presence (upper curve) of supplementary yellow-green light.The
upper curve has been corrected for the amount of O
2
production
stimulated by the supplementary light alone. Note that the lower
curve falls off precipitously above 680 nm (the red drop).
However, the supplementary light greatly increases the quantum
yield in the wavelength range above 680 nm (far-red) in which
the algae absorb light. [After Emerson, R., Chalmers, R., and
Cederstrand, C., Proc. Natl. Acad. Sci. 49, 137 (1957).]
JWCL281_c24_901-939.qxd 6/8/10 8:54 AM Page 914
protein’s partial re-reduction. In the presence of the herbi-
cide 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU),
which abolishes photosynthetic oxygen production, 680-
nm light still oxidizes cytochrome f but simultaneous 562-
nm light only oxidizes it further.The explanation for these
effects is that 680-nm light, which efficiently activates only
PSI, causes it to withdraw electrons from (oxidize) cy-
tochrome f. The 562-nm light also activates PSII, which
thereby transfers electrons to (reduces) cytochrome f.
DCMU blocks electron flow from PSII to cytochrome f
(Fig. 24-15), so an increased intensity of light, whatever its
wavelength, only serves to activate PSI further.
b. O
2
-Producing Photosynthesis Is Mediated by
Three Transmembrane Protein Complexes
Linked by Mobile Electron Carriers
The components of the Z-scheme, which mediate electron
transport from H
2
O to NADPH, are largely organized into
three thylakoid membrane-bound particles (Fig. 24-17): (1)
CH
3
CH
3
O
N
H
CN
3-(3,4-Dichlorophenyl)-1,1-dimethylurea
(DCMU)
Cl
Cl
PSII, (2) the cytochrome b
6
f complex, and (3) PSI. As in
oxidative phosphorylation, electrons are transferred be-
tween these complexes via mobile electron carriers. The
ubiquinone analog plastoquinone (Q), via its reduction to
plastoquinol (QH
2
),
O
O
H
CH
3
CH
2
CH C CH
2
n
Plastoquinone
H
3
C
H
3
C
H
2 [H•]
OH
OH
H
CH
3
CH C
H
3
C
H
3
C
Plastoquinol
CH
2
CH
2
n
H
Section 24-2. Light Reactions 915
Figure 24-15 The Z-scheme for photosynthesis in plants and
cyanobacteria. Two photosystems, PSI and PSII, function to drive
electrons from H
2
O to NADPH. The reduction potential increases
downward so that electron flow occurs spontaneously in this
direction.The herbicide DCMU (see text) blocks photosynthetic
electron transport from PSII to cytochrome f.
Figure 24-16 The oxidation state of cytochrome f in
Porphyridium cruentum algae as monitored by a weak beam of
420-nm (blue-violet) light. An increase in the transmitted light
signals the oxidation of cytochrome f. In the upper curve, strong
light at 680 nm (far-red) causes the oxidation of the cytochrome f
but the superposition of 562-nm (yellow-green) light causes its
partial re-reduction. In the lower curve, the presence of the
herbicide DCMU, which inhibits photosynthetic electron
transport, causes 562-nm light to further oxidize, rather than
reduce, the cytochrome f.
Strong
oxidant
Increasing
reduction
potential
2H
2
O
hν
e
–
Weak
reductant
O
2
+ 4H
+
cytochrome f
DCMU
Strong
reductant
NADP
+
+ H
+
NADPH
Weak
oxidant
hν
PSII
PSI
e
–
No
additions
Light transmitted at 420 nm
DCMU
added
On On Off Off
680 nm 562 nm 562 nm 680 nm
0 10203040
Time (s)
JWCL281_c24_901-939.qxd 3/25/10 12:04 PM Page 915
links PSII to the cytochrome b
6
f complex, which, in turn,
interacts with PSI through the mobile Cu-containing redox
protein plastocyanin (PC). In what follows, we trace the
electron pathway through this chloroplast system from
H
2
O to NADP
⫹
(Fig. 24-18).
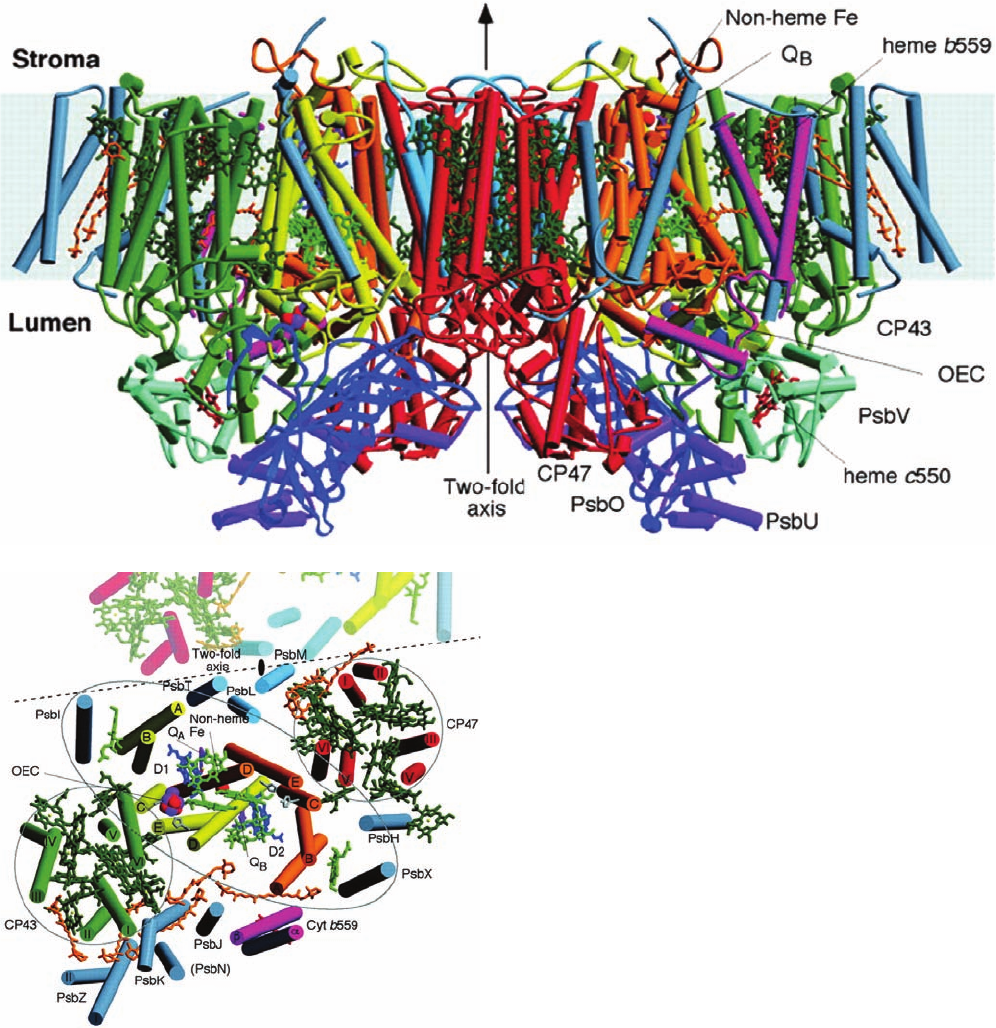
c. PSII Resembles the PbRC
PSII from the thermophilic cyanobacterium Ther-
mosynechococcus elongatus consists of 20 subunits, 14 of
which occupy the photosynthetic membrane. These trans-
membrane subunits include the reaction center proteins
D1 (PsbA) and D2 (PsbD), the chlorophyll-containing
inner-antenna subunits CP43 (PsbC) and CP47 (PsbB), and
cytochrome b
559
.The X-ray structure of this PSII (Fig. 24-19),
independently determined by James Barber and So Iwata
and by Wolfram Saenger, reveals that this ⬃340-kD protein
is a symmetric dimer, whose protomeric units each contain
35 transmembrane helices. Each protomer, which has
pseudo-2-fold symmetry, binds 36 Chl a’s, 2 pheophytin a’s
(Pheo a’s; Chl a with its Mg
2⫹
replaced by two protons),
one heme b, one heme c, 2 plastoquinones, one nonheme
Fe, 12 all-trans carotenoids presumed to be -carotene,
one ion, and one Mn
4
CaO
4
complex known as the
oxygen-evolving center [OEC; alternatively, the water-
oxidizing complex (WOC)]. In higher plants, the PSII pro-
tomer contains ⬃25 subunits and forms an ⬃1000-kD
transmembrane supercomplex with several antenna pro-
teins. The arrangement of the 5 transmembrane helices in
both D1 and D2 resembles that in the L and M subunits of
the PbRC (Fig. 24-11). Indeed, these two sets of subunits
have similar sequences, thereby indicating that they arose
from a common ancestor.
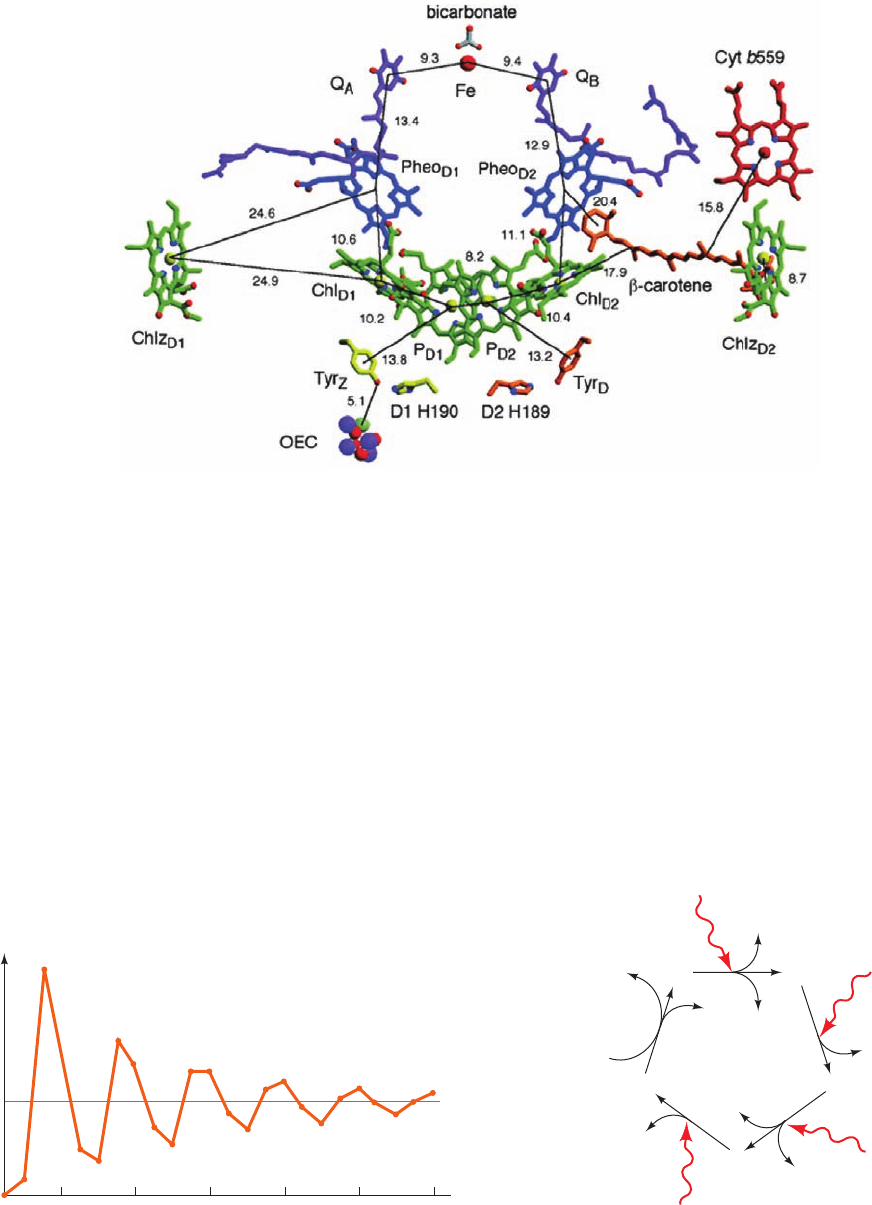
The cofactors of PSII’s RC (Fig. 24-20) are organized
similarly to those of the bacterial system (Fig. 24-12): They
have essentially the same components (with Chl a, Pheo a,
and plastoquinone replacing BChl b, BPheo b, and
menaquinone, respectively) and are symmetrically organ-
ized along the complex’s pseudo-2-fold axis. The two Chl a
rings labeled P
D1
and P
D2
in Fig. 24-20 are positioned anal-
ogously to the BChl b’s of P960’s “special pair” and are
therefore presumed to form PSII’s primary electron donor,
P680 (named after the wavelength at which its absorbance
HCO
⫺
3
916 Chapter 24. Photosynthesis
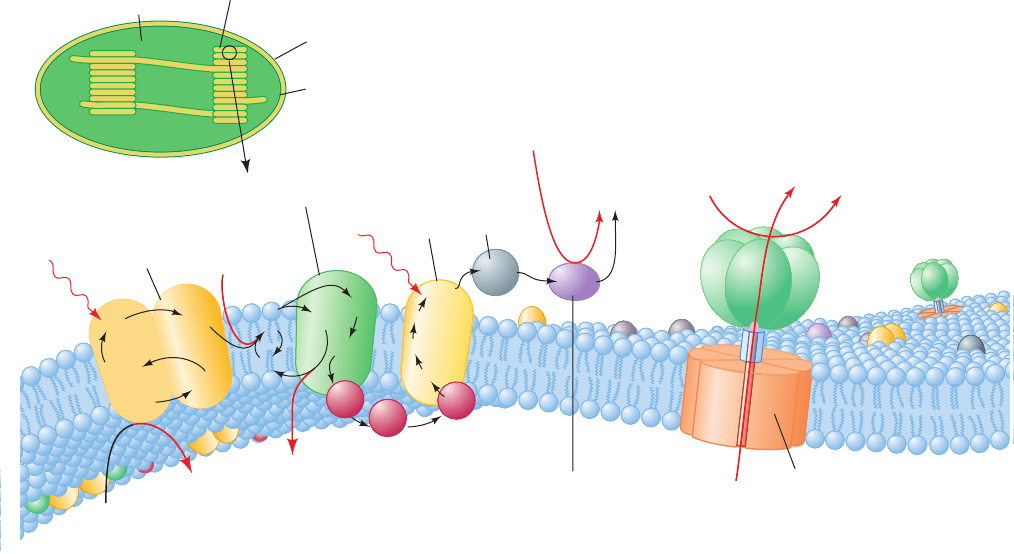
Figure 24-17 Schematic representation of the thylakoid
membrane showing the components of its electron-transport
chain. The system consists of three protein complexes: PSII, the
cytochrome b
6
f complex, and PSI, which are electrically
“connected” by the diffusion of the electron carriers plastoquinol
(Q) and plastocyanin (PC). Light-driven transport of electrons
(black arrows) from H
2
O to NADP
⫹
forming NADPH motivates
the transport of protons (red arrows) into the thylakoid space
(Fd is ferredoxin).Additional protons are split off from water by
Proton
translocating
ATP synthase
Fd–NADP
+
reductase
8H
+
PSII
complex
Cytochrome b
6
f
PSI
complex
Fd
Outer membrane
Inner membrane
Thylakoid
Chloroplast
Stroma
4H
+
+ O
2
8H
+
hν
2H
+
+ 2NADP
+
2NADPH
ADP + P
i
ATP
Stroma
3H
+
2H
2
O
3H
+
Thylakoid
lumen
Q
B
2QH
2
Cyt b
6
FeS
FeS
Cyt f
P700
A
0
A
1
PC
PC
PC
P680
OEC
Pheo
Q
A
Q
cycle
FAD
2Q
CF
0
CF
1
FeS
hν
the oxygen-evolving complex (OEC), yielding O
2
.The resulting
proton gradient powers the synthesis of ATP by the CF
1
CF
0
proton-translocating ATP synthase [CF
1
and CF
0
are chloroplast
(C) analogs of mitochondrial F
1
and F
0
].The membrane also
contains light-harvesting complexes whose component
chlorophylls and other chromophores transfer their excitations
to PSI and PSII. [After Ort, D.R. and Good, N.E., Trends
Biochem. Sci. 13, 469 (1988).]
JWCL281_c24_901-939.qxd 6/8/10 8:54 AM Page 916
maximally decreases on photooxidation). The electron
ejected from P680 follows a similar asymmetric course as
that in the PbRC even though the two systems operate
over different ranges of reduction potential (compare Figs.
24-13b and 24-18). As indicated in the central part of Fig.
24-18, the electron is transferred to a molecule of Pheo a
(Pheo
D1
in Fig. 24-20), probably via a Chl a molecule (Chl
D1
),
and then to a bound plastoquinone (Q
A
).The electron is sub-
sequently transferred to a second plastoquinone molecule,
Q
B
, which after it receives a second electron in a like manner,
takes up two protons at the stromal (cytosolic in cyanobacte-
ria) surface of the thylakoid membrane.The resulting plasto-
quinol, Q
B
H
2
, then exchanges with a membrane-bound pool
of plastoquinone molecules. DCMU as well as many other
commonly used herbicides compete with plastoquinone for
the Q
B
-binding site on PSII, which explains how they inhibit
photosynthesis.
Two “extra” Chl a molecules, Chlz
D1
and Chlz
D2
, lie on
the periphery of the RC,where they are postulated to func-
tion in the transfer of excitation from the antenna systems
to P680. Cytochrome b
559
, whose function is unclear, breaks
the pseudosymmetry of the PSII protomer as does the Mn
cluster, whose function we now discuss.
d. O
2
Is Generated in a Five-Stage Water-Splitting
Reaction Mediated by an Mn-Containing
Protein Complex
The oxidation by the OEC of two molecules of H
2
O to
form one molecule of O
2
requires four electrons. Since
transfer of a single electron from H
2
O to NADP
⫹
requires
two photochemical events, this accounts for the observed
minimum of 8 to 10 photons absorbed per molecule of O
2
produced.
Must the four electrons necessary to produce a given O
2
molecule be removed by a single photosystem or can they
be extracted by several different photosystems? Pierre
Joliet and Bessel Kok answered this question by analyzing
the rate at which dark-adapted chloroplasts produce O
2
Section 24-2. Light Reactions 917
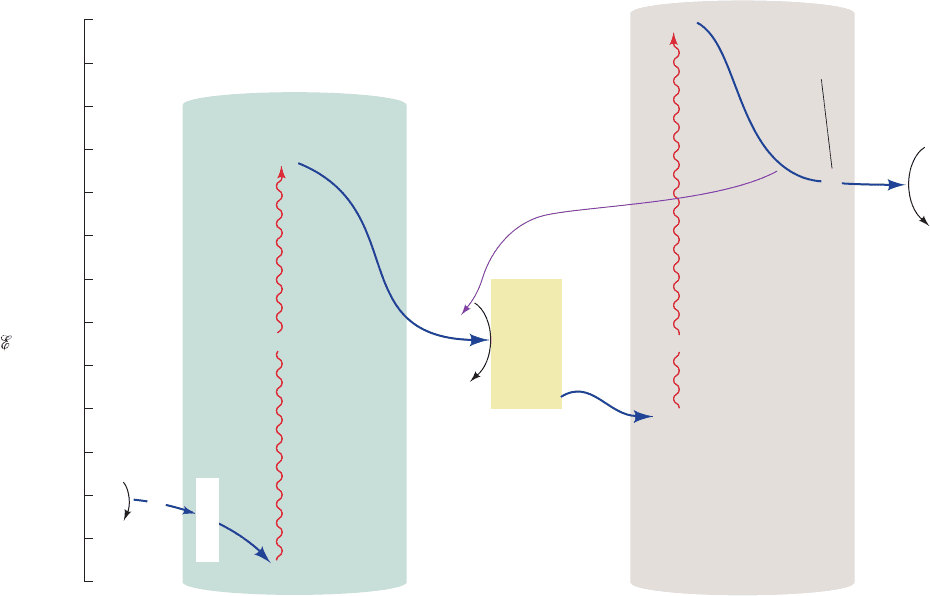
Figure 24-18 Detailed diagram of the Z-scheme of
photosynthesis. Electrons ejected from P680 by the absorption of
photons are replaced with electrons abstracted from H
2
O by an
Mn complex (OEC), thereby forming O
2
and four H
⫹
. Each
ejected electron is passed through a chain of electron carriers
to a pool of plastoquinone molecules (Q).The resulting
plastoquinol, in turn, reduces the cytochrome b
6
f particle (yellow
box) that transfers electrons with the concomitant translocation
of protons, via a Q cycle, into the thylakoid lumen. Cytochrome
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
hν
2H
2
O
O
2
+ 4H
+
e
–
(thylakoid)
complex
Mn
S
0
S
4
.....
P680
P680
cyt b
6
Q
[4Fe–4S]
cyt f
*
Chl a
Pheo a
Q
A
Q
B
Q
pool
PC
Cytochrome
b
6
f
Cyclic
pathway
8H
+
(stroma)
8H
+
(thylakoid)
P700
*
P700
Z
F
x
F
A
F
B
noncyclic
pathway
e
–
2NADP
+
+
2H
+
FNR
(stroma)
FQR
2NADPH
PSI
PSII
–1.0
–0.8
–1.2
–1.4
–0.6
–0.4
–0.2
0
A
0
A
1
Fd
•
•
OEC
hν
+0.6
+0.8
+1.0
+1.2
+0.4
+0.2
o
′
(V)
b
6
f then transfers the electrons to plastocyanin (PC).The
plastocyanin regenerates photooxidized P700.The electron
ejected from P700, through the intermediacy of a chain of
electron carriers (A
0
,A
1
,F
X
,F
A
,F
B
, and Fd), reduces NADP
⫹
to
NADPH in noncyclic electron transport.Alternatively, the
electron may be returned to the cytochrome b
6
f complex in a
cyclic process that only translocates protons into the thylakoid
lumen.
JWCL281_c24_901-939.qxd 3/25/10 12:04 PM Page 917
when exposed to a series of short flashes. O
2
was evolved
with a peculiar oscillatory pattern (Fig. 24-21). There is vir-
tually no O
2
evolved by the first two flashes.The third flash
results in the maximal O
2
yield. Thereafter, the amount of
O
2
produced peaks with every fourth flash until the oscilla-
tions damp out to a steady state. This periodicity indicates
that each OEC cycles through five different states, S
0
through S
4
(Fig. 24-22). Each of the transitions between S
0
and S
4
is a photon-driven redox reaction; that from S
4
to S
0
results in the release of O
2
.Thus, each O
2
molecule must be
produced by a single photosystem. The observation that O
2
evolution peaks at the third rather than the fourth flash in-
dicates that the OEC’s resting state is predominantly S
1
rather than S
0
.The oscillations gradually damp out because
a small fraction of the RCs fail to be excited or become
doubly excited by a given flash of light, so that they eventu-
ally lose synchrony.The five reaction steps release a total of
four water-derived protons into the inner thylakoid space
918 Chapter 24. Photosynthesis
Figure 24-19 X-ray structure of PSII from T. elongatus.
(a) The PSII dimer is viewed from within the plane of the
membrane with the stroma above. Its transmembrane subunits
include D1 (yellow), D2 (orange), CP47 (red), CP43 (green), and
cytochrome b
559
(magenta). Other transmembrane subunits are
colored light blue and blue-gray. Its membrane-extrinsic proteins
are PsbO (dark blue), PsbU (purple), and PsbV (light green). The
various cofactors are drawn in stick form with the chlorophylls of
the D1/D2 reaction center light green, those of the antenna
complexes dark green, pheophytins dark blue, hemes red,
-carotenes orange, Q
A
and Q
B
purple, and the nonheme Fe
represented by a red sphere. The inferred position of the
membrane is indicated by the light blue band. (b) View of a PSII
protomer perpendicular to the membrane from the thylakoid
lumen showing only the transmembrane portions of the complex
and colored as in Part a.A portion of the other protomer in the
PSII dimer is shown in muted colors with the dashed line
indicating the region of monomer–monomer interactions and the
black ellipse indicating the position of the 2-fold axis.The
pseudo-2-fold axis, which is perpendicular to the membrane and
passes through the nonheme Fe, relates the transmembrane
helices of the D1/D2 heterodimer, CP43 and CP47, and PsbI and
PsbX as emphasized by the black lines encircling these subunits.
[Courtesy of James Barber and So Iwata, Imperial College
London, U.K. PDBid 1S5L.]
(a)
(b)
JWCL281_c24_901-939.qxd 6/8/10 8:54 AM Page 918
(lumen) in a stepwise manner (Fig. 24-22). These protons
contribute to the transmembrane proton gradient.
Since the OEC abstracts electrons from H
2
O, its five
states must have extraordinarily high reduction potentials
(recall from Table 22-1 that the O
2
/H
2
O half-reaction has a
standard reduction potential of 0.815 V). PSII must also
stabilize the highly reactive intermediates for extended pe-
riods (as much as minutes) in close proximity to water.
The OEC, which is located at the lumenal surface of the
D1 subunit (Fig. 24-20), is a Mn
4
CaO
4
or Mn
4
CaO
5
complex
in which the O atoms bridge neighboring Mn atoms. The
structure of the OEC remains elusive due to PSII’s rela-
tively poorly resolved X-ray structures and the observation
Section 24-2. Light Reactions 919
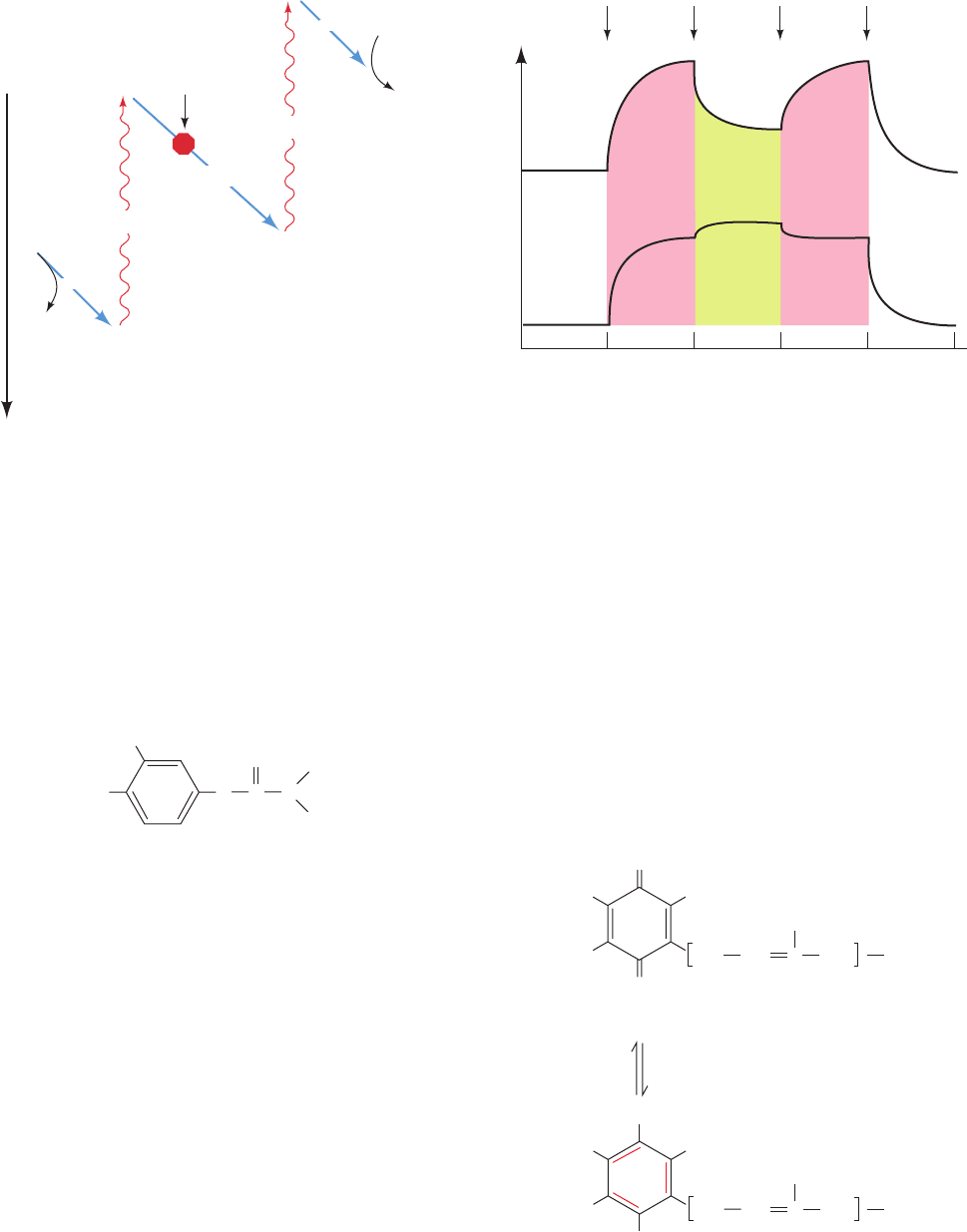
4 8 12 16 20 24
O
2
yield per flash
Flash number
H
+
H
+
2H
+
S
0
S
4
S
2
S
3
S
1
O
2
2H
2
O
e
–
e
–
e
–
e
–
hν
hν
hν
hν
Figure 24-20 The arrangement of electron-transfer cofactors
in PSII from T. elongatus. The complex is viewed along the
membrane plane with the thylakoid lumen below. The cofactors
are colored as in Fig. 24-19 but with Mg
2⫹
yellow, N blue, and O
red.The phytyl tails of the chlorophylls and pheophytins have
been removed for clarity. The side chain C atoms of Tyr
Z
(D1 Tyr
161) and D1 His 190 are yellow, and those of Tyr
D
(D2 Tyr 160)
Figure 24-21 The O
2
yield per flash in dark-adapted spinach
chloroplasts. Note that the yield peaks on the third flash and
then on every fourth flash thereafter until the curve eventually
damps out to its average value. [After Forbush, B., Kok, B., and
McGloin, M.P., Photochem. Photobiol. 14, 309 (1971).]
Figure 24-22 Schematic mechanism of O
2
generation in
chloroplasts. Four electrons are stripped, one at a time in light-
driven reactions (S
0
S S
4
), from two bound H
2
O molecules. In
the recovery step (S
4
S S
0
), which is light independent, O
2
is
released and two more H
2
O molecules are bound.Three of these
five steps release protons into the thylakoid lumen.
and D2 His 189 are orange. The OEC is drawn in space-filling form
with Mn purple, Ca
2⫹
cyan, and O red.The numbers indicate the
center-to-center distances, in angstroms, between the cofactors
spanned by the accompanying thin black lines. Compare this
figure to Fig. 24-12 (which is drawn upside down relative to this
figure). [Courtesy of James Barber and So Iwata, Imperial
College London, U.K. PDBid 1S5L.]
JWCL281_c24_901-939.qxd 6/16/10 12:04 PM Page 919
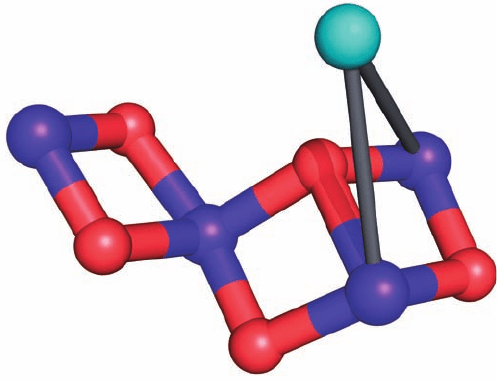
that the OEC decomposes when illuminated with X-rays at
the intensities used in X-ray structure determinations. How-
ever, the use of X-ray spectroscopy techniques of lower in-
tensity that are sensitive to bond lengths have led to the
formulation of several related models for the OEC that
are compatible with the X-ray structure of PSII. One of
these models is shown in Fig. 24-23.
The water-splitting reaction is driven by the excitation
of the PSII RC.A variety of evidence indicates that the Mn
ions in the OEC’s various S states (Fig. 24-22) cycle
through specific combinations of Mn(II), Mn(III), Mn(IV),
and Mn(V) while abstracting protons and electrons from
two H
2
O molecules to yield O
2
, which is released into the
thylakoid lumen. However, the mechanism whereby this
occurs, that is, the nature of the five S states, remains un-
known due to the lack of structural information concerning
these states.
The next link in the PSII electron transport chain is an
entity, originally named Z (Fig. 24-18), which relays elec-
trons from the OEC to P680.The existence of Z is signaled
by a transient EPR spectrum of illuminated chloroplasts
that parallels the S-state transitions. The change in this
spectrum on supplying deuterated tyrosine to cyanobacte-
ria indicates that Z
⫹
is a tyrosyl radical (TyrOⴢ; EPR spec-
tra reflect the nuclear spins of the atoms with which the un-
paired electrons interact). It has been identified as Tyr
Z
in
PSII (Fig. 24-20) due to its position between the Mn cluster
and P680’s chlorophyll P
D1
. Recall that a tyrosyl radical has
also been implicated in the reduction of O
2
to 2 H
2
O by
cytochrome c oxidase (Complex IV) in the respiratory
electron-transport chain (Section 22-2C5c).
e. Electron Transport through the Cytochrome
b
6
f Complex Generates a Proton Gradient
From the plastoquinone pool, electrons pass through
the cytochrome b
6
f complex. This integral membrane as-
sembly resembles cytochrome bc
1
, its purple bacterial
counterpart (Section 24-2Be), as well as Complex III of the
mitochondrial electron-transport chain (also called cy-
tochrome bc
1
; Section 22-2C3a). Electron flow through the
cytochrome b
6
f complex occurs through a Q cycle (Fig.
22-31) in which plastoquinone is the (H
⫹
⫹ e
⫺
) carrier.
Accordingly, two protons are translocated across the thy-
lakoid membrane for every electron transported. The four
electrons abstracted from 2 H
2
O by the OEC therefore
lead to the translocation of eight H
⫹
from the stroma to the
thylakoid lumen. Electron transport via the cytochrome b
6
f
complex generates much of the electrochemical proton gra-
dient that drives the synthesis of ATP in chloroplasts.
The X-ray structure of cytochrome b
6
f (Fig. 24-24) was
independently determined by Janet Smith and William
Cramer and by Jean-Luc Popot and Daniel Picot. Cy-
tochrome b
6
f is a dimer of ⬃109-kD protomers, each con-
taining four large subunits (18–32 kD) that have counter-
parts in cytochrome bc
1
: cytochrome b
6
, a homolog of the
N-terminal half of cytochrome b; subunit IV, a homolog of
the C-terminal half of cytochrome b; a Rieske iron–sulfur
protein (ISP), which is also present in cytochrome bc
1
; and
cytochrome f (f for feuille, French for leaf), a c-type cy-
tochrome that is a functional analog of cytochrome c
1
,al-
though the two are unrelated in structure or sequence. In
fact, cytochrome f is an elongated, two-domain protein that
is dominated by  sheets and hence has an entirely differ-
ent fold from those of other c-type cytochromes of known
structure. Cytochrome f’s single heme c is, nevertheless, co-
valently linked to the protein’s larger domain via the two
Cys residues in a Cys-X-Y-Cys-His sequence that is charac-
teristic of c-type cytochromes (Fig. 9-41) and whose His
residue forms one of the Fe ion’s two axial ligands (Fig.22-21).
Intriguingly, however, the second axial ligand is not a Met
S atom, as occurs in most c-type cytochromes, but instead is
the protein’s N-terminal amino group, a group that had
previously not been observed to be a heme ligand.
In addition, cytochrome b
6
f has four small hydrophobic
subunits that have no equivalents in cytochrome bc
1
. Each
protomer contains 13 transmembrane helices, four in cy-
tochrome b
6
, three in subunit IV, and one each in the re-
maining subunits. Cytochrome b
6
f binds cofactors that are
the equivalents of all of those in cytochrome bc
1
: heme f, a
c-type heme bound by cytochrome f; a [2Fe–2S] cluster
bound by the ISP; hemes b
H
and b
L
; a plastoquinone mole-
cule that occupies either the Q
i
site (the quinone-binding
site at which fully reduced quinone is regenerated during
the Q cycle; Section 22-3Be) or the Q
o
site. In addition,
cytochrome b
6
f binds several cofactors that have no coun-
terparts in cytochrome bc
1
: a Chl a, a -carotene, and, un-
expectedly, a novel heme named heme x (alternatively,
heme c
i
), which is covalently linked to the protein via a sin-
gle thioether bond to Cys 35 of cytochrome b
6
, and whose
only axial ligand is a water molecule (compare with hemes
a, b, and c; Fig. 22-21).
920 Chapter 24. Photosynthesis
Figure 24-23 A model of the OEC. This Mn
4
CaO
5
complex is
shown in ball-and-stick form with Mn ions purple, the Ca
2⫹
ion
cyan, and O red.The bonds between the Ca and Mn ions are
drawn in gray to indicate that the position of the Ca ion is rela-
tively poorly defined. Presumably, numerous protein side chains
and water molecules ligand the Ca and Mn ions. Several related
models are also compatible with the structural data. [Based on a
model by Vittal Yachandra, Lawrence Berkeley National
Laboratory, Berkeley, California.]
JWCL281_c24_901-939.qxd 3/25/10 12:04 PM Page 920
