Voet D., Voet Ju.G. Biochemistry
Подождите немного. Документ загружается.

891
P
P
CH
2
OH
H
OH H
H NAc
H
UDP
H
O
HO
CH
2
OH
H
OH H
H NAc
H
H
O
HO
H
H
OH
HO
H
H
OH OH
H
O
O
–
P
H
HO
UDP–GlcNac Phosphatidylinositol (PI) GlcNac–PI
GlcNH
2
+
OO
OO
O
CC
R
2
R
1
CH
2
CH
2
CHO
H
H
OH
O
H
H
OH OH
H
O
O
–
P
H
HO
OO
OO
O
CC
R
2
R
1
CH
2
CH
2
CHO
1
P
1→4
P
CoA Acyl-CoA
P
4 3
P
1→4
P
1→41→61→2
P2
2
P
6,7
2
5
HOAc
Phosphatidyl
ethanolamine
O
O
O
OCH
2
CH
2
NH
2
P
O
O
O
P
–
–
P
1→41→6
6
1→2
O
O
O
O
P
CH
2
O
O
P
CH
2
H
2
N
–
O
–
98
Phosphatidyl
ethanolamine
lipid
remodeling
(a)
OCH
2
CH
2
NH
2
OCH
2
CH
2
NH
2
PI
C-TERMINAL PEPTIDE
P
1→41→6
6
1→2
O
O
O
OCH
2
CH
2
NH
2
P
OCH
2
O
O
O
PCH
2
+ H
2
NC-TERMINAL PEPTIDEHN
–
O
–
–
–
CTARGET PROTEIN
O
6
O
O
CH
2
O
O
PCH
2
NH
P
P
P
1→41→61→2
O
O
OCH
2
CH
2
NH
2
P
CTARGET PROTEIN
O
(b)
Dolichol phosphate
mannose
Glucosamine
Phosphalidylinositol (PI)
Acyl group
JWCL281_c23_871-900.qxd 6/8/10 9:43 AM Page 891
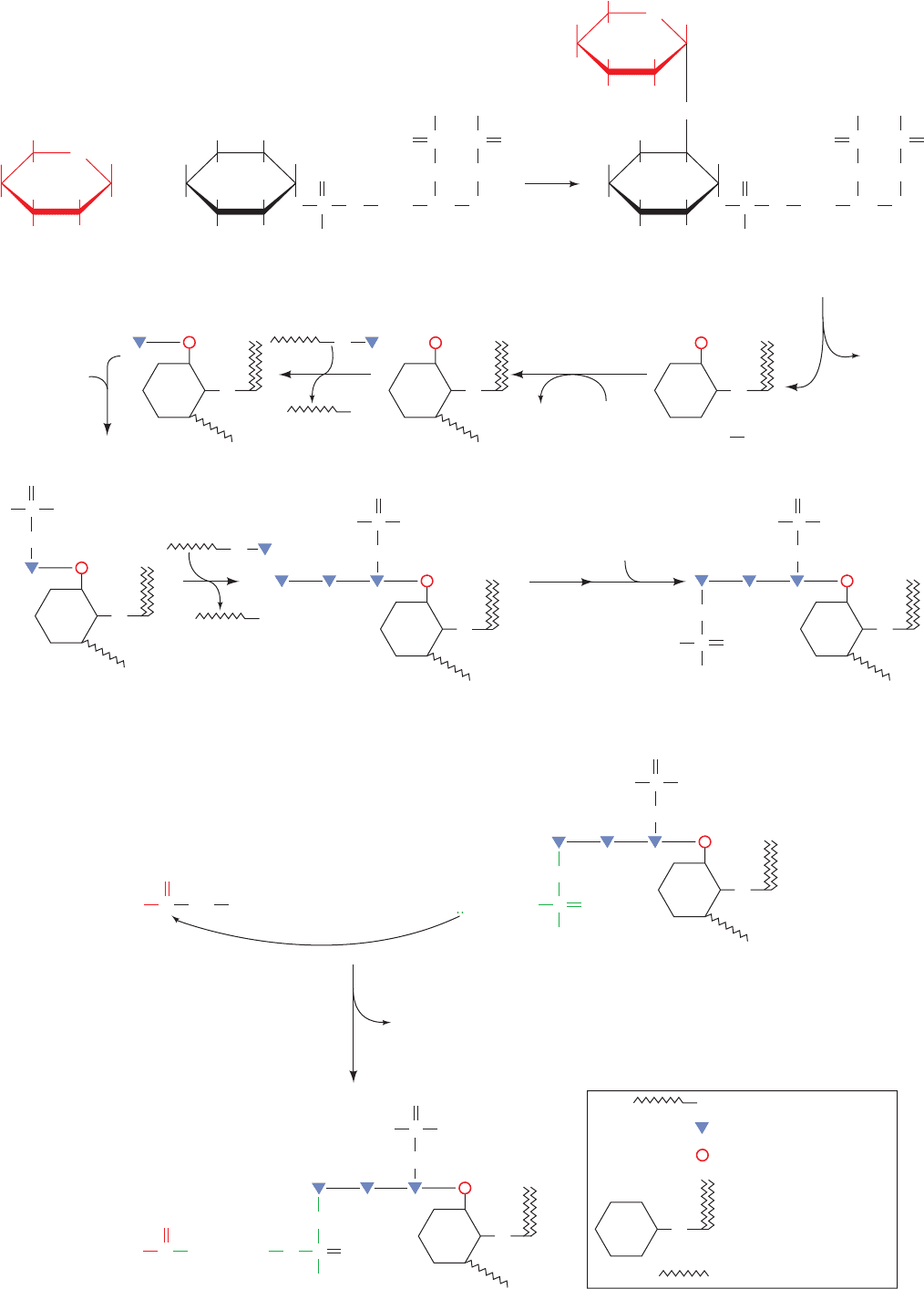
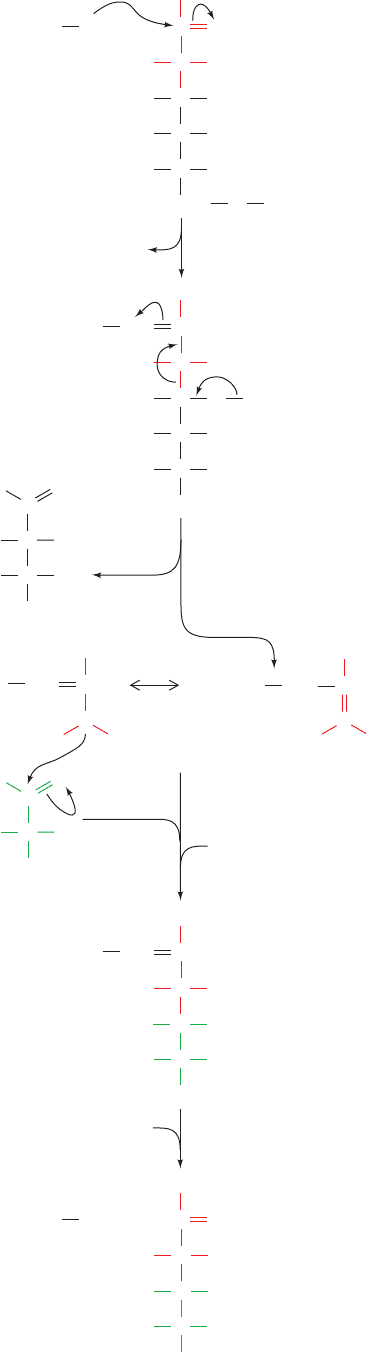
N-acetylglucosamine from UDP–N-acetylglucosamine
(UDP–GlcNAc) to the 6 hydroxyl of the inositol of phos-
phatidylinositol, followed by the removal of the acetyl
group. The mammalian pathway then continues with the
2-acylation of inositol, translocation to the luminal side of
the ER membrane, and the addition of mannose from
dolichol-P-mannose (Dol-P-Man; Fig. 23-16) and phos-
phoethanolamine from phosphatidylethanolamine (Table
12-2), as indicated in Fig. 23-25a. This core is modified with
a variety of additional sugar residues, depending on the
species and the protein to which it is attached.There is con-
siderable diversity in the fatty acid residues of GPI anchors
due to the extensive lipid remodeling that occurs during
anchor synthesis. Target proteins become anchored to the
membrane surface when the amino group of the GPI phos-
phoethanolamine nucleophilically attacks a specific amino
acyl group of the protein near its C-terminus, resulting in a
transamidation that releases a 20- to 30-residue hydropho-
bic C-terminal signal peptide (Fig. 23-25b). Since GPI
groups are appended to proteins on the luminal surface of
the RER, GPI-anchored proteins occur on the exterior
surface of the plasma membrane (Fig. 12-60). However,
they are distributed unevenly in the outer leaflet of the
plasma membrane because they prefer to associate with
sphingolipid–cholesterol rafts (Section 12-3Cb).
The core GPI structure is evolutionarily conserved among
all eukaryotes, although there are differences between
species in its synthesis. For example, the cell surface of the
trypanosomes that cause African sleeping sickness (a debili-
tating and often fatal disease that afflicts millions of people in
sub-Saharan Africa) has a dense coating of variant surface
glycoprotein (VSG) that is GPI-anchored to its plasma mem-
brane. The VSG coating conceals the trypanosome’s plasma
membrane from the host’s immune system although it recog-
nizes and attacks the VSG itself.The parasite is nevertheless
able to evade the host’s immunological defenses because it
has a genetic repertoire of about a thousand immunologically
distinct VSGs.An individual trypanosome expresses only one
of its VSG genes and hence the host can mount an effective
immunological attack against the prevailing population of
VSGs, a process that takes around 1 week (Section 35-2A).
However, by switching VSG genes, a new population of
trypanosomes arises that replicates unchecked until the host
can mount a new immune response, a cycle that repeats until
the death of the host. The comparison of the GPI biosyn-
thetic pathway in trypanosomes with that in mammalian sys-
tems has revealed several differences in the pathway order.
For example, Steps 3 and 4 of Fig. 23-25a are reversed in try-
panosomes. This and other differences in the substrate speci-
ficities of the enzymes catalyzing this pathway have brought
to light several promising drug targets for the treatment of
African sleeping sickness.
4 THE PENTOSE PHOSPHATE PATHWAY
ATP is the cell’s “energy currency”;its exergonic hydrolysis is
coupled to many otherwise endergonic cell functions. Cells
have a second currency, reducing power. Many endergonic
reactions, notably the reductive biosynthesis of fatty acids
(Section 25-4) and cholesterol (Section 25-6A), as well as
photosynthesis (Section 24-3A), require NADPH in addition
to ATP. Despite their close chemical resemblance, NADPH
and NADH are not metabolically interchangeable (recall that
these coenzymes differ only by a phosphate group at the 2¿-
OH group of NADPH’s adenosine moiety; Fig. 13-2).
Whereas NADH participates in utilizing the free energy of
metabolite oxidation to synthesize ATP (oxidative phospho-
rylation), NADPH is involved in utilizing the free energy of
metabolite oxidation for otherwise endergonic reductive
biosynthesis. This differentiation is possible because the de-
hydrogenase enzymes involved in oxidative and reductive
metabolism exhibit a high degree of specificity toward their
respective coenzymes. Indeed, cells normally maintain their
[NAD
][NADH] ratio near 1000, which favors metabolite
oxidation, while keeping their [NADP
][NADPH] ratio
near 0.01, which favors metabolite reduction.
NADPH is generated by the oxidation of G6P via an al-
ternative pathway to glycolysis, the pentose phosphate path-
way [also called the hexose monophosphate (HMP) shunt
and the phosphogluconate pathway; Fig. 23-26]. The path-
way also produces ribose-5-phosphate (R5P), an essential
precursor in nucleotide biosynthesis (Sections 28-1, 28-2,
and 28-5). The first evidence of this pathway’s existence
was obtained in the 1930s by Otto Warburg, who discov-
ered NADP
through his studies on the oxidation of G6P
to 6-phosphogluconate. Further indications came from the
observation that tissues continue to respire in the presence
of high concentrations of fluoride ion, which, it will be re-
called, blocks glycolysis by inhibiting enolase (Section
17-2I). It was not until the 1950s, however, that the pentose
phosphate pathway was elucidated by Frank Dickens,
Bernard Horecker, Fritz Lipmann, and Efraim Racker.Tis-
sues most heavily involved in fatty acid and cholesterol
biosynthesis (liver, mammary gland, adipose tissue, and
adrenal cortex) are rich in pentose phosphate pathway
enzymes. Indeed, some 30% of the glucose oxidation in
liver occurs via the pentose phosphate pathway.
The overall reaction of the pentose phosphate pathway is
However, the pathway may be considered to have three
stages:
1. Oxidative reactions (Fig. 23-26, Reactions 1–3),
which yield NADPH and ribulose-5-phosphate (Ru5P).
2. Isomerization and epimerization reactions (Fig.
23-26, Reactions 4 and 5), which transform Ru5P either
to ribose-5-phosphate (R5P) or to xylulose-5-phosphate
(Xu5P).
3. A series of C¬C bond cleavage and formation reac-
tions (Fig. 23-26, Reactions 6–8) that convert two mole-
cules of Xu5P and one molecule of R5P to two molecules
3Ru5P Δ R5P 2Xu5P
6NADPH 6H
3CO
2
3Ru5P
3G6P 6NADP
3H
2
O
¡
6NADPH 6H
3CO
2
2F6P GAP
3G6P 6NADP
3H
2
O Δ
892 Chapter 23. Other Pathways of Carbohydrate Metabolism
JWCL281_c23_871-900.qxd 3/24/10 11:33 AM Page 892
of fructose-6-phosphate (F6P) and one of glyceraldehyde-
3-phosphate (GAP).
The reactions of Stages 2 and 3 are freely reversible so that
the products of the pathway vary with the needs of the cell.
R5P 2Xu5P Δ 2F6P GAP
For example, when R5P is required for nucleotide biosyn-
thesis, Stage 3 works in reverse, producing R5P from F6P
and GAP nonoxidatively. In this section, we discuss the
three stages of the pentose phosphate pathway and how
this pathway is controlled.We close by considering the con-
sequences of one of its abnormalities.
Section 23-4. The Pentose Phosphate Pathway 893
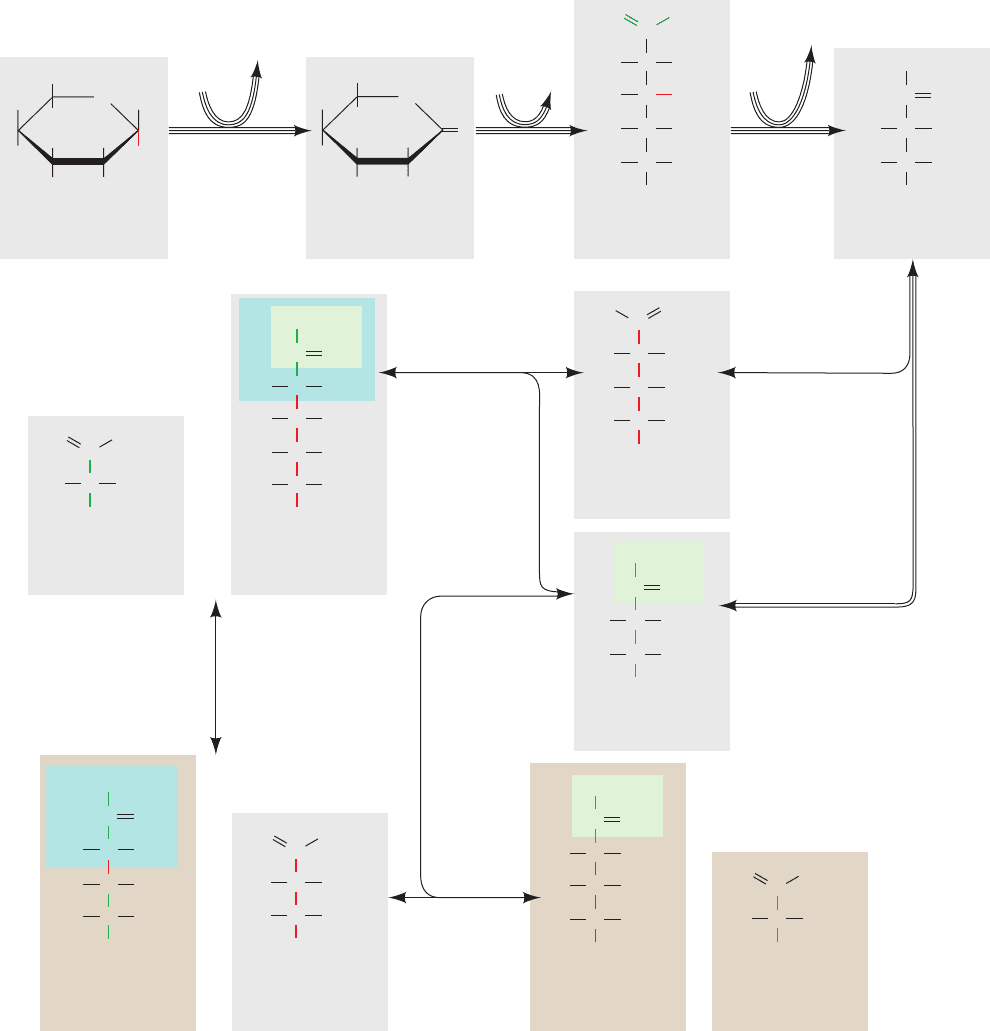
Figure 23-26 The pentose phosphate pathway. The number of
lines in an arrow represents the number of molecules reacting in
one turn of the pathway so as to convert three G6P to three CO
2
,
two F6P, and one GAP. For the sake of clarity, sugars from
Reaction 3 onward are shown in their linear forms.The carbon
CH
2
OH
OH
C
H
C
Glyceraldehyde-
3-phosphate
(GAP)
C
O
C
OH
OH
C
H
H
CH
2
OH
Ribulose-5-
phosphate (Ru5P)
NADP
+
6-phospho-
gluconate
dehydro-
genase
C
OH
OH
C
H
H
C
Ribose-5-
phosphate (R5P)
C
O
C
H
OH
C
C
H
2
OPO
3
2–
HO
H
Xylulose-5-
phosphate (Xu5P)
C
O
Fructose-6-
phosphate
(F6P)
C
OH
OH
C
H
H
CH
2
OPO
3
2–
CH
2
OPO
3
2–
C
H
HO
OH
C
H
C
Glyceraldehyde-
3-phosphate
(GAP)
Sedoheptulose-7-
phosphate (S7P)
C
OH
OH
C
H
H
C
H
HO
OH
C
H
OH
C
OH
OH
C
H
H
6-Phospho-
gluconate
O
–
O
C
H
HO
C
OH
H
C
6-phospho-
glucono-
lactonase
H
OH H
HOH
HO
H
O
H
OH H
HOH
HO
HOH
O
H
2
OH
+
NADP
H + CO
2
O
NADP
+
glucose-
6-phosphate
dehydrogenase
NADPH + H
+
CH
2
OPO
3
2–
CH
2
OPO
3
2–
CH
2
OPO
3
2–
CH
2
OPO
3
2–
H
6-Phosphoglucono-
-lactone
␦
Glucose-6-
phosphate (G6P)
C
OH
H
O H
OH
C
H
C
Erythrose-4-
phosphate (E4P)
C
O
Fructose-6-
phosphate (F6P)
OH
C
H
C
H
HO
OH
C
H
O H
+
+
OH
C
H
O H
1
2
3
4
ribulose-5-phosphate
epimerase
ribulose-5-phosphate
isomerase
transketolase
6
7
transaldolase
8
transketolase
CH
2
OH
CH
2
OH
CH
2
OH
C
O
CH
2
OPO
3
2–
CH
2
OPO
3
2–
CH
2
OPO
3
2–
CH
2
OPO
3
2–
CH
2
OPO
3
2–
5
+
skeleton of R5P and the atoms derived from it are drawn in red
and those from Xu5P are drawn in green.The C
2
units
transferred by transketolase are shaded in green and the C
3
units
transferred by transaldolase are shaded in blue. Double-headed
arrows indicate reversible reactions.
JWCL281_c23_871-900.qxd 3/24/10 11:33 AM Page 893
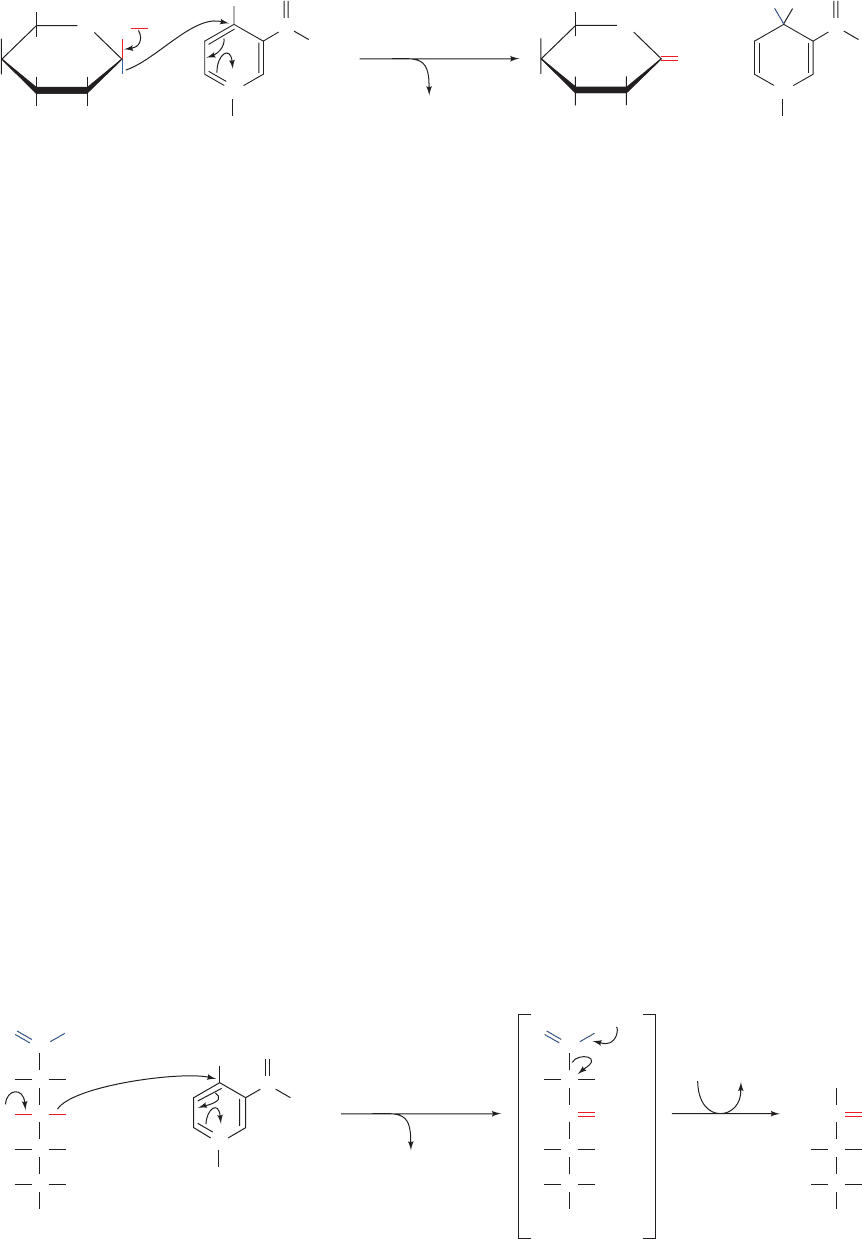
A. Oxidative Reactions of NADPH Production
Only the first three reactions of the pentose phosphate
pathway are involved in NADPH production.
1. Glucose-6-phosphate dehydrogenase (G6PD) cat-
alyzes net transfer of a hydride ion to NADP
from C1 of
G6P to form 6-phosphoglucono-␦-lactone (Fig. 23-27). G6P,
a cyclic hemiacetal with C1 in the aldehyde oxidation state,
is thereby oxidized to a cyclic ester (lactone).The enzyme is
specific for NADP
and is strongly inhibited by NADPH.
2. 6-Phosphogluconolactonase increases the rate of hy-
drolysis of 6-phosphoglucono--lactone to 6-phosphoglu-
conate (the nonenzymatic reaction occurs at a significant
rate), the substrate of the next oxidative enzyme in the
pathway.
3. 6-Phosphogluconate dehydrogenase catalyzes the ox-
idative decarboxylation of 6-phosphogluconate, a -hydroxy
acid, to Ru5P and CO
2
(Fig. 23-28). The reaction is similar
to that catalyzed by the citric acid cycle enzyme isocitrate
dehydrogenase (Section 21-3C).
Formation of Ru5P completes the oxidative portion of
the pentose phosphate pathway. It generates two molecules
of NADPH for each molecule of G6P that enters the path-
way. The product Ru5P must subsequently be converted to
R5P or Xu5P for further use.
B. Isomerization and Epimerization of
Ribulose-5-Phosphate
Ru5P is converted to R5P by ribulose-5-phosphate iso-
merase (Fig. 23-26, Reaction 4) and to Xu5P by ribulose-
5-phosphate epimerase (Fig. 23-26, Reaction 5). These
isomerization and epimerization reactions, as discussed in
Section 16-2Db, are both thought to occur via enediolate
intermediates (Fig. 23-29).
R5P is an essential precursor in the biosynthesis of nu-
cleotides (Sections 28-1, 28-2, and 28-5). If, however, more
R5P is formed than the cell needs, the excess, along with
Xu5P, is converted to the glycolytic intermediates F6P and
GAP as described below.
C. Carbon–Carbon Bond Cleavage
and Formation Reactions
The conversion of three C
5
sugars to two C
6
sugars and one
C
3
sugar involves a remarkable “juggling act” catalyzed by
two enzymes, transaldolase and transketolase. As we dis-
cussed in Section 16-2E, enzymatic reactions that make or
break carbon–carbon bonds usually have mechanisms that
involve generation of a stabilized carbanion and its addi-
tion to an electrophilic center such as an aldehyde. This is
the dominant theme of both the transaldolase and the
transketolase reactions.
a. Transketolase Catalyzes the Transfer of C
2
Units
Transketolase, which has a thiamine pyrophosphate co-
factor (TPP; Section 17-3Ba), catalyzes the transfer of a C
2
unit from Xu5P to R5P, yielding GAP and sedoheptulose-
7-phosphate (S7P) (Fig. 23-26, Reaction 6). The reaction in-
volves the intermediate formation of a covalent adduct be-
tween Xu5P and TPP (Fig. 23-30).The X-ray structure of this
homodimeric enzyme shows that the TPP binds in a deep
cleft between the subunits such that residues from both
894 Chapter 23. Other Pathways of Carbohydrate Metabolism
Figure 23-27 The glucose-6-phosphate dehydrogenase reaction.
Figure 23-28 The phosphogluconate dehydrogenase reaction.
Oxidation of the OH group forms an easily decarboxylated
H
OH H
H OH
HO
H
O
O
6-Phosphoglucono-
δ-lactone
H
OH H
H OH
HO
H
O
H
H
O
G6P
CH
2
OPO
2
3
–
CH
2
OPO
2
3
–
glucose-6-phosphate
dehydrogenase
H
+
H
N
R
NADP
+
+
++
H
H
C
O
NH
2
N
R
NADPH
C
O
NH
2
6-phosphogluconate
dehydrogenase
C
C
C
OH
NADPH
+ H
+
O
–
O
O
CH
2
OPO
2
3
–
6-Phosphogluconate
H
H
C HHO
COH
NH
2
H
C
N
R
NADP
+
Ru5P
β-Keto acid
intermediate
OH
H
+
+
C
COH
O
–
CO
2
O
CH
2
OPO
2
3
–
H
H
+
C O
COH
H
COHH
CH
2
OH
C O
COH
H
COHH
CH
2
OPO
2
3
–
-keto acid (although the proposed intermediate has not been
isolated).
JWCL281_c23_871-900.qxd 3/24/10 11:33 AM Page 894
Section 23-4. The Pentose Phosphate Pathway 895
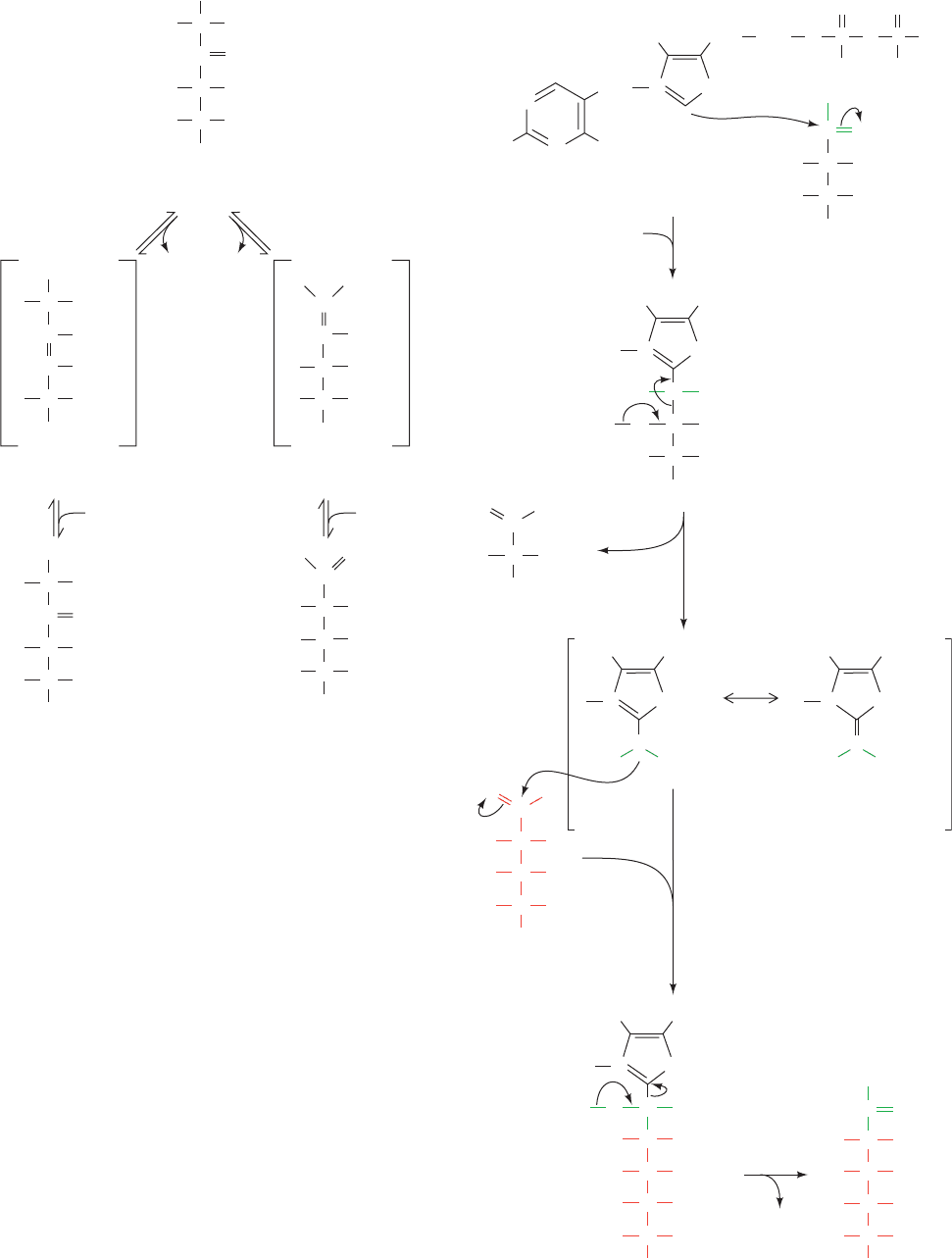
Figure 23-29 Ribulose-5-phosphate isomerase and
ribulose-5-phosphate epimerase. The reactions catalyzed
by both these enzymes involve enediolate intermediates.
In the isomerase reaction (right), a base on the enzyme
removes a proton from C1 of Ru5P to form a 1,2-enedio-
late and then adds a proton at C2 to form R5P. In the
epimerase reaction (left), a base on the enzyme removes
a C3 proton to form a 2,3-enediolate. A proton is then
added to the same carbon atom but with inversion of
configuration to yield Xu5P.
Figure 23-30 Mechanism of transketolase. Transketolase utilizes the coenzyme
thiamine pyrophosphate to stabilize the carbanion formed on cleavage of the
C2¬C3 bond of Xu5P. The reaction occurs as follows: (1) The TPP ylid attacks
the carbonyl group of Xu5P. (2) C2¬C3 bond cleavage yields GAP and enzyme-
bound 2-(1,2-dihydroxyethyl)-TPP, a resonance-stabilized carbanion. (3) The C2
carbanion attacks the aldehyde carbon of R5P forming an S7P–TPP adduct.
(4) TPP is eliminated yielding S7P and the regenerated TPP–enzyme.
H
C
C
C
CH
2
OPO
2
3
–
C
2,3-Enediolate
intermediate
1,2-Enediolate
intermediate
ribulose-5-phosphate
isomerase
ribulose-5-phosphate
epimerase
Ru5P
O
–
OH
OH
OHH
H
H
C
C
C
CH
2
OPO
2
3
–
C
O
OH
OH
OHH
H
H
H
C
C
C
CH
2
OPO
2
3
–
C
Xu5P
O
OH
H
OHH
HO
H
H
C
C
C
CH
2
OPO
2
3
–
C
O
–
OH
OH
OHH
H
H
C
C
C
CH
2
OPO
2
3
–
C
OH
O
OH
OHH
H
H
H
+
H
+
H
+
H
+
R5P
NH
2
N
N
H
3
C
CH
2
N
H
3
C CH
2
CH
2
OP
+
•
E
+
–
Thiamine pyrophosphate (TPP)
ylid form
C
O
C
H
OH
C
HO
H
CH
2
OPO
3
2–
CH
2
OH
1
Xu5P
R
N
H
3
CR
•
E
S
C
CH
2
OH
HO
2-(1,2-Dihydroxyethyl)-
TPP
R
N
H
3
CR
+
•
E
S
C
CH
2
OH
HO
–
OH
C
H
C
HO
R5P
OH
C
H
OH
C
H
OH
C
H
OH
C
H
OH
C
H
H
C
HO
C
CH
OH
2
H
R
N
H
3
CR
+
•
E
S
OH
C
H
S7P
OH
C
H
OH
C
H
H
C
HO
C
O
CH
OH
2
TPP
O
C
H
OH
C
H
H
O
C
HO
CH
2
OH
R
N
H
3
CR
+
•
E
S
2
OH
C
H
C
HO
GAP
3
P
S
OO
–
O
–
O
–
OO
CH
2
OPO
3
2–
CH
2
OPO
3
2–
CH
2
OPO
3
2–
CH
O
2
PO
3
2–
CH
O
2
PO
3
2–
H
+
H
+
+
H
+
+
•
E
ylid
attack
bond
cleavage
4
ylid
elimination
C
2
unit
transfer
JWCL281_c23_871-900.qxd 3/24/10 11:33 AM Page 895
subunits participate in its binding, just as in pyruvate decar-
boxylase (another TPP-requiring enzyme; Figure 17-28). In
fact the structures are so similar that it is likely that they di-
verged from a common ancestor.
b. Transaldolase Catalyzes the Transfer of C
3
Units
Transaldolase catalyzes the transfer of a C
3
unit from
S7P to GAP, yielding erythrose-4-phosphate (E4P) and
F6P (Fig. 23-26, Reaction 7). The reaction occurs by aldol
cleavage, which begins with the formation of a Schiff base
between an ε-amino group of an essential enzyme Lys
residue and the carbonyl group of S7P (Fig. 23-31).Transal-
dolase and Class I aldolase (Section 17-2Da) share a com-
mon reaction mechanism and may also share a common
ancestor, despite their lack of significant sequence identity.
Both are barrel proteins (Section 8-3Bh), but while the
Schiff base–forming Lys is on 4 (the fourth strand from
the N-terminus) of transaldolase, it is on 6 of Class I al-
dolase. Superimposing the barrel structures of these two
enzymes while maintaining the alignment of the strands
bearing the Schiff base–forming Lys residues results in a
significantly better fit than doing so while maintaining the
alignment of their entire barrels. Moreover, five of the
pairs of matched active site residues in the former superpo-
sition are identical.This suggests that, during evolution, the
DNA sequence for two units was transferred from the
N-terminus to the C-terminus of the evolving Class I al-
dolase, moving the active site Lys from 6 to 4. Such a cir-
cular permutation of an / barrel’s structural elements
does not greatly change its structure.
c. A Second Transketolase Reaction Yields
GAP and a Second F6P Molecule
In a second transketolase reaction, a C
2
unit is trans-
ferred from a second molecule of Xu5P to E4P to form
GAP and another molecule of F6P (Fig. 23-26, Reaction 8).
The third phase of the pentose phosphate pathway thus
transforms two molecules of Xu5P and one of R5P to two
molecules of F6P and one molecule of GAP. These carbon
skeleton transformations (Fig. 23-26, Reactions 6–8) are
summarized in Fig. 23-32.
D. Control of the Pentose Phosphate Pathway
The principal products of the pentose phosphate pathway are
R5P and NADPH.The transaldolase and transketolase reac-
tions serve to convert excess R5P to glycolytic intermediates
896 Chapter 23. Other Pathways of Carbohydrate Metabolism
Figure 23-31 Mechanism of transaldolase. Transaldolase contains an
essential Lys residue that forms a Schiff base with S7P to facilitate an
aldol cleavage reaction.The reaction occurs as follows: (1) The ε-amino
group of an essential Lys residue forms a Schiff base with the carbonyl group
of S7P. (2) A Schiff base–stabilized C3 carbanion is formed in an aldol cleavage
reaction between C3 and C4 that eliminates E4P. (3) The enzyme-bound
resonance-stabilized carbanion adds to the carbonyl C atom of GAP, forming
F6P linked to the enzyme via a Schiff base. (4) The Schiff base hydrolyzes,
regenerating active enzyme and releasing F6P.
O
HC
PO
2
3
–
C
HO
OHC
H
OHC
C
H
CH
2
OH
H
CH
2
OH
O
NH
2
Lys(CH
2
)
4
OH
HC
C
HO
OC
H
OHC
C
H
CH
2
OPO
2
3
–
OH
H
CH
2
OH
NHLys(CH
2
)
4
+
H
C
OHC
C
H
CH
2
OPO
2
3
–
OH
H
H
O
C
C
CH
2
OH
NHLys(CH
2
)
4
+
H
C
C
HO
CH
2
OH
NHLys(CH
2
)
4
H
HO
1
2
S7P
E4P
1
2
3
4
5
6
7
–
C
C
CH
2
OPO
2
3
–
OH
H
H
O
3
H
+
O
HC
C
HO
OHC
H
C
OH
H
CH
2
OH
NH
2
Lys(CH
2
)
4
HC
C
HO
OHC
C
H
CH
2
OPO
2
3
–
OH
H
CH
2
OH
NHLys(CH
2
)
4
+
F6P
4
OH
+
CH
2
OPO
2
3
–
–
GAP
–
:
:
:
Schiff base
formation
aldol
cleavage
carbanion addition
to carbonyl
Schiff base
hydrolysis
JWCL281_c23_871-900.qxd 3/24/10 11:33 AM Page 896
when the metabolic need for NADPH exceeds that of
R5P in nucleotide biosynthesis. The resulting GAP and
F6P can be consumed through glycolysis and oxidative
phosphorylation or recycled by gluconeogenesis to form
G6P. In the latter case, 1 molecule of G6P can be converted,
via six cycles of the pentose phosphate pathway and gluco-
neogenesis, to 6 CO
2
molecules with the concomitant gener-
ation of 12 NADPH molecules. When the need for R5P
outstrips that for NADPH, F6P and GAP can be diverted
from the glycolytic pathway for use in the synthesis of R5P
by reversal of the transaldolase and transketolase reac-
tions. In fact, mass spectral analysis of the
13
C-labeled
carbons from [1,2-
13
C]glucose incorporated into RNA in
rapidly proliferating cancer cells has shown that more than
⬃70% of the de novo ribose synthesis arises through this
nonoxidative reversal of the pentose phosphate pathway
(rather than its forward direction).
Flux through the oxidative pentose phosphate pathway
and thus the rate of NADPH production is controlled by the
rate of the glucose-6-phosphate dehydrogenase reaction
(Fig. 23-26, Reaction 1). The activity of this enzyme, which
catalyzes the pentose phosphate pathway’s first committed
step (G 17.6 kJ ⴢ mol
1
in liver), is regulated by the
NADP
concentration (substrate availability). When the
cell consumes NADPH, the NADP
concentration rises,
increasing the rate of the glucose-6-phosphate dehydroge-
nase reaction, thereby stimulating NADPH regeneration.
E. Glucose-6-Phosphate Dehydrogenase Deficiency
NADPH is required for several reductive processes in ad-
dition to biosynthesis. For example, erythrocyte mem-
brane integrity requires a plentiful supply of reduced glu-
tathione (GSH), a Cys-containing tripeptide (Sections
21-2Ba and 26-4C). A major function of GSH in the ery-
throcyte is to eliminate H
2
O
2
and organic hydroperoxides.
H
2
O
2
, a toxic product of various oxidative processes
(Section 22-4Cg), reacts with double bonds in the fatty
acid residues of the erythrocyte cell membrane to form or-
ganic hydroperoxides. These, in turn, react to cleave fatty
acid C¬C bonds, thereby damaging the membrane. In
erythrocytes, the unchecked buildup of peroxides results
in premature cell lysis. Peroxides are eliminated through
the action of glutathione peroxidase, one of the handful of
enzymes with a selenium cofactor, yielding glutathione
disulfide (GSSG).
Section 23-4. The Pentose Phosphate Pathway 897
2GSH R O O H+ GSSG ROH
glutathione
peroxidase
+ H
2
O+
Organic
hydroperoxide
GSH is subsequently regenerated by the NADPH reduc-
tion of GSSG catalyzed by glutathione reductase (Section
21-2Ba).
A steady supply of NADPH is therefore vital for erythro-
cyte integrity.
a. Primaquine Causes Hemolytic Anemia in
Glucose-6-Phosphate Dehydrogenase Mutants
A genetic defect, common in African, Asian, and
Mediterranean populations, results in severe hemolytic
anemia on infection or on the administration of certain
drugs including the antimalarial agent primaquine.
Similar effects, which go by the name of favism, occur when
individuals bearing this trait eat fava beans (broad beans,
Vicia faba), a staple Middle Eastern vegetable that con-
tains small quantities of toxic glycosides (the Greek
philosopher and mathematician Pythagoras, who lived in
the sixth century
BCE, forbade his followers from eating
fava beans, possibly because of their deleterious effects).
This trait has been traced to an altered gene for glucose-6-
phosphate dehydrogenase (G6PD). Under most condi-
tions, mutant erythrocytes have sufficient enzyme activity
for normal function. Agents such as primaquine and fava
beans, however, stimulate peroxide formation, thereby in-
creasing the demand for NADPH to a level that mutant
cells cannot meet.
The major reason for low enzymatic activity in affected
cells appears to be an accelerated rate of breakdown of the
mutant enzyme (protein degradation is discussed in Sec-
tion 32-6). This explains why patients with G6PD defi-
ciency react to primaquine with hemolytic anemia but re-
cover within a week despite continued primaquine
treatment. Mature erythrocytes lack a nucleus and protein
synthesizing machinery and therefore cannot synthesize
new enzyme molecules to replace degraded ones (they
likewise cannot synthesize new membrane components,
which is why they are so sensitive to membrane damage in
the first place). The initial primaquine treatments result in
NH CH CH
2
CH
3
H
3
CO
CH
2
NH
2
CH
2
N
Primaquine
GSSG NADPH H
+
2GSH NADP
+
glutathione
reductase
++ +
Figure 23-32 Summary of carbon skeleton rearrangements in
the pentose phosphate pathway. A series of carbon–carbon bond
formations and cleavages convert three C
5
sugars to two C
6
and
one C
3
sugar.The number to the left of each reaction is keyed to
the corresponding reaction in Fig. 23-26.
(6)
(7)
(8)
(Sum)
C
5
C
7
C
5
+
+
+
+
+
+
+
C
5
C
3
C
4
3 C
5
C
7
C
6
C
6
C
3
C
3
C
4
C
3
2 C
6
JWCL281_c23_871-900.qxd 3/24/10 11:33 AM Page 897

the lysis of old red blood cells whose defective G6PD has
been largely degraded. Lysis products stimulate the release
of young cells that contain more enzyme and are therefore
better able to cope with primaquine stress.
It is estimated that over 400 million people are deficient
in G6PD, which makes this condition the most common hu-
man enzymopathy. Indeed, ⬃400 G6PD variants have been
reported and at least 140 of them have been characterized
at the molecular level. G6PD is active in a dimer–tetramer
equilibrium. Many of the mutation sites in individuals with
the most severe G6PD deficiency are at the dimer inter-
face, shifting the equilibrium toward the inactive and un-
stable monomer.
Several G6PD variants occur with high incidence. For
example, the so-called type A
deficiency, which exhibits
⬃10% of the normal G6PD activity, has an incidence of
11% among African Americans. This variant is also the
most common form of G6PD deficiency in sub-Saharan
Africa. The variant “Mediterranean” is found throughout
the Mediterranean and Middle East regions, and occurs in
65% of Kurdish Jews, the population with the highest
known incidence of this trait.The high prevalence of defec-
tive G6PD in malarial areas of the world suggests that such
mutations confer resistance to the malarial parasite, Plas-
modium falciparum (as we likewise saw to be the case for
the sickle-cell trait; Section 7-3Ab). Indeed, two epidemio-
logical studies involving over 2000 African children with
A
G6PD deficiency indicate that this form is associated
with an ⬃50% reduction in the risk of severe malaria for
both female heterozygotes and male hemizygotes (G6PD
deficiency is an X-linked trait).
In vitro studies indicate that erythrocytes with G6PD
deficiency are less suitable hosts for plasmodia than are
normal cells. This is presumably because the parasite re-
quires the products of the pentose phosphate pathway
and/or because the erythrocyte is lysed before the parasite
has had a chance to mature.Thus, like the sickle-cell trait, a
defective G6PD confers a selective advantage on individuals
living where malaria is endemic.
898 Chapter 23. Other Pathways of Carbohydrate Metabolism
1 Gluconeogenesis Lactate, pyruvate, citric acid cycle in-
termediates, and many amino acids may be converted, by gluco-
neogenesis, to glucose via the formation of oxaloacetate. For
this to occur, the three irreversible steps of glycolysis must be
bypassed.The pyruvate kinase reaction is bypassed by convert-
ing pyruvate to oxaloacetate in an ATP-driven reaction cat-
alyzed by the biotinyl-containing enzyme pyruvate carboxylase.
The two phases of the pyruvate decarboxylase reaction are cat-
alyzed on different active sites of the homotetrameric enzyme,
which translocates its covalently linked carboxybiotinyl group
from its BC domain to the CT domain of a neighboring subunit.
The oxaloacetate is subsequently decarboxylated and phospho-
rylated by GTP to form PEP in a reaction catalyzed by PEPCK.
For this to happen in species in which PEPCK is a cytosolic en-
zyme, the oxaloacetate must be transported from the mitochon-
drion to the cytosol via its interim conversion to either malate
or aspartate. Conversion to malate concomitantly transports re-
ducing equivalents to the cytosol in the form of NADH.The two
other irreversible steps of glycolysis, the PFK reaction and the
hexokinase reaction, are bypassed by simply hydrolyzing their
products, FBP and G6P, by FBPase and glucose-6-phosphatase,
respectively. A glucose molecule may therefore be synthesized
from pyruvate at the expense of four ATPs more than are gen-
erated by the reverse process.
Glycolysis and gluconeogenesis are reciprocally regulated so
as to consume glucose when the demand for ATP is high and
synthesize it when the demand is low.The control points in these
processes are at pyruvate kinase/pyruvate carboxylase–PEPCK,
PFK/FBPase, and hexokinase/glucose-6-phosphatase. Regula-
tion of these enzymes is exerted largely through allosteric inter-
actions, cAMP-dependent enzyme modifications, and, for
PEPCK, gene expression. Muscle, which is incapable of gluco-
neogenesis, transfers much of the lactate it produces to the liver
via the blood for conversion to glucose and return to the muscle.
This Cori cycle shifts the metabolic burden of oxidative ATP
generation for gluconeogenesis from muscle to liver.
2 The Glyoxylate Cycle Animals cannot convert fatty
acids to glucose because they lack the enzymes necessary to
synthesize oxaloacetate from acetyl-CoA.Plants, however,can
do so via the glyoxylate cycle, a glyoxysomal process that con-
verts two molecules of acetyl-CoA to one molecule of succi-
nate via the intermediate formation of glyoxylate. Succinate is
converted to oxaloacetate for use in gluconeogensis or the cit-
ric acid cycle.
3 Biosynthesis of Oligosaccharides and Glycoproteins
Glycosidic bonds are formed by transfer of the monosaccharide
unit of a sugar nucleotide to a second sugar unit. Such reactions
occur in the synthesis of disaccharides such as lactose and in the
synthesis of the carbohydrate components of glycoproteins. In
N-linked glycoproteins, the carbohydrate component is attached
to the protein via an N-glycosidic bond to an Asn residue in the
sequence Asn-X-Ser/Thr. In O-linked glycoproteins, the carbo-
hydrate attachment is an O-glycosidic bond to Ser or Thr or, in
collagens, to 5-hydroxylysine. In GPI-anchored proteins a glyco-
sylphosphatidylinositol group is linked to the protein through an
intermediary phosphoethanolamine bridge, which forms an
amide bond to the protein’s C-terminal amino acid residue.
Synthesis of N-linked oligosaccharides begins in the endo-
plasmic reticulum with the multistep formation of a lipid-linked
precursor consisting of dolichol pyrophosphate bonded to a
common 14-residue core oligosaccharide. The carbohydrate is
then transferred to an Asn residue of a growing polypeptide
chain.The correct folding of the immature N-linked glycoprotein
is assisted via the calnexin/calreticulin cycle and it is subse-
quently transferred, via a membranous vesicle, to the cis Golgi
network of the Golgi apparatus. Processing is completed by the
trimming of mannose residues followed by attachment of a vari-
ety of other monosaccharides as catalyzed by specific enzymes in
the cis, medial, and trans Golgi cisternae. Completed N-linked
glycoproteins are sorted in the trans Golgi network according to
the identities of their carbohydrate components for transport,via
membranous vesicles, to their final cellular destinations. Three
CHAPTER SUMMARY
JWCL281_c23_871-900.qxd 6/8/10 9:43 AM Page 898

major types of N-linked oligosaccharides have been identified,
high mannose, complex,and hybrid oligosaccharides,all of which
contain a common pentasaccharide core. Studies of glycoprotein
formation have been facilitated by the use of antibiotics, such as
tunicamycin and bacitracin, which inhibit specific enzymes in-
volved in the synthesis of these oligosaccharides.
O-Linked oligosaccharides are synthesized in the Golgi ap-
paratus by sequential attachments of specific monosaccharide
units to certain Ser or Thr residues. Carbohydrate components
of glycoproteins are thought to act as recognition markers for
the transport of glycoproteins to their proper cellular destina-
tions and for cell–cell and antibody recognition. The GPI mem-
brane anchor is appended to proteins on the luminal surface of
the endoplasmic reticulum, thereby targeting GPI-anchored
proteins to the external surface of the plasma membrane.
4 The Pentose Phosphate Pathway The cell uses NAD
⫹
in oxidative reactions and employs NADPH in reductive
biosynthesis. NADPH is synthesized by the pentose phos-
phate pathway, an alternate mode of glucose oxidation. This
pathway also synthesizes R5P for use in nucleotide biosynthe-
sis.The first three reactions of the pentose phosphate pathway
involve oxidation of G6P to Ru5P with release of CO
2
and for-
mation of two NADPH molecules. This is followed by reac-
tions that either isomerize Ru5P to R5P or epimerize it to
Xu5P. Each molecule of R5P not required for nucleotide
biosynthesis, together with two Xu5P, is converted to two
molecules of F6P and one molecule of GAP via the sequential
actions of transketolase, transaldolase, and, again, transketo-
lase. The products of the pentose phosphate pathway depend
on the needs of the cell. The F6P and GAP may be metabo-
lized through glycolysis and the citric acid cycle or recycled via
gluconeogenesis. If NADPH is in excess, the latter portion of
the pentose phosphate pathway may be reversed to synthesize
R5P from glycolytic intermediates. The pentose phosphate
pathway is controlled at its first committed step, the glucose-6-
phosphate dehydrogenase reaction, by the NADP
⫹
concentra-
tion. A genetic deficiency in glucose-6-phosphate dehydroge-
nase leads to hemolytic anemia on administration of the
antimalarial drug primaquine. This X-linked deficiency, which
results from the accelerated degradation of the mutant en-
zyme, provides resistance against severe malaria to female
heterozygotes and male hemizygotes for this sex-linked trait.
References 899
Gluconeogenesis
Croniger, C.M., Olswang, Y., Reshef, L., Kalhan, S.C., Tilghman,
S.M., and Hanson, R.W., Phosphoenolpyruvate carboxykinase
revisited. Insights into its metabolic role, Biochem. Mol. Biol.
Educ. 30, 14–20 (2002);and Croniger, C.M.,Chakravarty, K., Ol-
swang, Y., Cassuto, H., Reshef, L., and Hanson, R.W., Phospho-
enolpyruvate carboxykinase revisited. II. Control of PEPCK-C
gene expression, Biochem. Mol. Biol. Educ. 30, 353–362 (2002).
Knowles, J.R., The mechanism of biotin-dependent enzymes,
Annu. Rev. Biochem. 58, 195–221 (1989).
Matte, A., Tari, L.W., Goldie, H., and Delbaere,T.J., Structure and
mechanism of phosphoenolpyruvate carboxykinase, J. Biol.
Chem. 272, 8105–8108 (1997).
Pilkis, S.J., Mahgrabi, M.R., and Claus, T.H., Hormonal regulation
of hepatic gluconeogenesis and glycolysis, Annu. Rev.
Biochem. 57, 755–783 (1988).
Rothman, D.L., Magnusson, I., Katz, L.D., Shulman, R.G., and
Shulman, G.I., Quantitation of hepatic gluconeogenesis in fast-
ing humans with
13
C NMR, Science 254, 573–576 (1991).
St. Maurice, M., Reinhardt, L., Surinya, K.H., Attwood, P.V.,
Wallace, J.C., Cleland, W.W., and Rayment, I., Domain archi-
tecture of pyruvate carboxylase, a biotin-dependent multifunc-
tional enzyme, Science 317, 1076–1079 (2007).
Van Schaftingen, E., and Gerin, I.,The glucose-6-phosphatase sys-
tem, Biochem. J. 362, 513–532 (2002).
Yang, J.,Kalhan,S. C.,and Hanson,R.W.,What is the metabolic role
of phosphoenolpyruvate carboxykinase? and Yang, J., Rashef,
L., Cassuto, H., Aleman, G., and Hanson, R. W., Aspects of con-
trol of phosphoenolpyruvate carboxykinase gene transcription,
J. Biol. Chem. 284, 27025–27029 and 27031–27035 (2009).
The Glyoxylate Cycle
Eastmond, P.J. and Graham, I.A., Re-examining the role of the
glyoxylate cycle in oilseeds, Trends Plant Sci. 6, 72–77 (2001).
Oligosaccharide Biosynthesis
Abeijon, C. and Hirschberg, C.B., Topography of glycosylation
reactions in the endoplasmic reticulum, Trends Biochem. Sci.
17, 32–36 (1992).
Aebi, C., Bernasconi, R., Clerc, S., and Molinari, M., N-glycan
structures: recognition and processing in the ER, Trends
Biochem. Sci. 35, 74–82 (2010).
Bause, E., Wesemann, M., Bartoschek, A., and Breuer, W.,
Epoxyethylglycyl peptides as inhibitors of oligosaccharyltrans-
ferase: double-labeling of the active site, Biochem. J. 322,
95–102 (1997).
Burda, P. and Aebi, M.,The dolichol pathway of N-linked glycosy-
lation, Biochim. Biophys. Acta 1426, 239–257 (1999).
Elbein, A.D., Inhibitors of the biosynthesis and processing of N
linked oligosaccharide chains, Annu. Rev. Biochem. 56, 497–534
(1987).
Englund, P.T., The structure and biosynthesis of glycosyl phos-
phatidylinositol protein anchors, Annu. Rev. Biochem. 62,
65–100 (1993).
Ferguson, M.A.J., Brimacombe, J.S., Brown, J.R., Crossman, A.,
Dix,A., Field,R.A., Güther, M.L.S., Milne, K.G., Sharma, D.K.,
and Smith, T.K., The GPI biosynthetic pathway as a therapeu-
tic target for African sleeping sickness, Biochim. Biophys. Acta
1455, 327–340 (1999).
Florman, H.M. and Wasserman, P.M., O-Linked oligosaccharides
of mouse egg ZP3 account for its sperm receptor activity, Cell
41, 313–324 (1985).
Helenius, A. and Aebi, M., Intracellular functions of N-linked
glycans, Science 291, 2364–2369 (2001).
Helenius, A., Trombetta, E.S., Hebert, J.N., and Simons, J.F.,
Calnexin, calreticulin and the folding of glycoproteins, Trends
Cell Biol. 7, 193–200 (1997).
Hirschberg, C.B. and Snider, M.D.,Topography of glycosylation in
the rough endoplasmic reticulum and the Golgi apparatus,
Annu. Rev. Biochem. 56, 63–87 (1987).
Kornfeld, R. and Kornfeld, S., Assembly of asparagine-linked
oligosaccharides, Annu. Rev. Biochem. 54, 631–664 (1985).
Lairson, L.L., Henrissat, B., Davies, G.J., and Withers, S.G., Glyco-
syltransferases: Structures, functions, and mechanisms, Annu.
Rev. Biochem. 77,
521–555 (2008).
Maeda, Y., Watanabe, R., Harris, C.L., Hong, Y., Ohishi, K.,
Kinoshita, K., and Kinoshita, T., PIG-M transfers the first
REFERENCES
JWCL281_c23_871-900.qxd 7/2/10 12:04 PM Page 899

mannose to glycosylphosphatidylinositol on the lumenal side
of the ER, EMBO J. 20, 250–261 (2001).
Parodi, A.J., Role of N-oligosaccharide endoplasmic reticulum
processing reactions in glycoprotein folding and degradation,
Biochem. J. 348, 1–13 (2000); and Protein glucosylation and its
role in protein folding, Annu. Rev. Biochem. 69, 69–93 (2000).
Sanyal, S., Frank, C.G., and Menon, A.K., Distinct flippases
translocate glycerophospholipids and oligosaccharide diphos-
phate dolichols across the endoplasmic reticulum, Biochem-
istry 47, 7937–7946 (2008).
Schachter, H., Enzymes associated with glycosylation, Curr. Opin.
Struct. Biol. 1, 755–765 (1991).
Schrag,J.D., Bereron,J.J.M., Li,Y., Borisova, S., Hahn, M.,Thomas,
D.Y., and Cygler, M., The structure of calnexin, an ER chaper-
one involved in quality control of protein folding, Mol. Cell 8,
633–644 (2001).
Shaper, J.H. and Shaper, N.L., Enzymes associated with glycosyla-
tion, Curr. Opin. Struct. Biol. 2, 701–709 (1992).
Tartakoff, A.M. and Singh, N., How to make a glycoinositol phos-
pholipid anchor, Trends Biochem. Sci. 17, 470–473 (1992).
Taylor, M.E. and Drickamer, K., Introduction to Glycobiology
(2nd ed.), Oxford University Press (2006).
von Figura, K. and Hasilik, A., Lysosomal enzymes and their re-
ceptors, Annu. Rev. Biochem. 55, 167–193 (1986).
The Pentose Phosphate Pathway
Adams, M.J., Ellis, G.H., Gover, S., Naylor, C.E., and Phillips, C.,
Crystallographic study of coenzyme, coenzyme analogue and
substrate binding in 6-phosphogluconate dehydrogenase: Im-
plications for NADP specificity and enzyme mechanism, Struc-
ture 2, 651–668 (1994).
Au, S.W.N., Gover, S., Lam, V.M.S., and Adams, M.J., Human
glucose-6-phosphate dehydrogenase: The crystal structure re-
veals a structural NADP
⫹
molecule and provides insights into
enzyme deficiency, Structure 8, 293–303 (2000).
Beutler, E., The molecular biology of G6PD variants and other
red cell enzyme defects, Annu. Rev. Med. 43, 47–59 (1992).
Cappellini, M.D. and Fiorelli, G., Glucose-6-phosphate dehydro-
genase deficiency, Lancet 375, 64–74 (2008).
Jia, J., Huang, W., Schörken, U., Sahm, H., Sprenger, G.A.,
Lindqvist, Y., and Schneider, G., Crystal structure of transal-
dolase B from Escherichia coli suggests a circular permutation
of the ␣Ⲑ barrel within the class I aldolase family, Structure 4,
715–724 (1996).
Lindqvist, Y. and Schneider, G., Thiamin diphosphate dependent
enzymes: transketolase, pyruvate oxidase and pyruvate decar-
boxylase, Curr. Opin. Struct. Biol. 3, 896–901 (1993); and
Muller, Y.A., Lindqvist, Y., Furey, W., Schulz, G.E., Jordan, F.,
and Schneider, G., A thiamin diphosphate binding fold re-
vealed by comparison of the crystal structures of transketolase,
pyruvate oxidase and pyruvate decarboxylase, Structure 1,
95–103 (1993).
Luzzato, L., Mehta, A., and Vulliamy, T., Glucose-6-phosphate
dehydrogenase deficiency, Chap. 138 in Valle, D. (Ed.), The
Online Metabolic & Molecular Bases of Inherited Disease
http://www.ommbid.com/
Ruwende, C., et al., Natural selection of hemi- and heterozygotes
for G6PD deficiency in Africa by resistance to severe malaria,
Nature 376, 246–249 (1995).
Wood, T., The Pentose Phosphate Pathway, Academic Press
(1985).
900 Chapter 23. Other Pathways of Carbohydrate Metabolism
1. Compare the relative energetic efficiencies, in ATPs per
mole of glucose oxidized, of glucose oxidation via glycolysis ⫹ the
citric acid cycle versus glucose oxidation via the pentose phos-
phate pathway ⫹ gluconeogenesis. Assume that NADH and
NADPH are each energetically equivalent to 2.5 ATP.
2. Although animals cannot synthesize glucose from acetyl-
CoA, if a rat is fed
14
C-labeled acetate, some of the label will ap-
pear in the glycogen extracted from its muscles. Explain.
3. Substances that inhibit specific trimming steps in the pro-
cessing of N-linked glycoproteins have been useful tools in eluci-
dating the pathway of this process. Explain.
4. Through clever genetic engineering you have developed an
unregulatable enzyme that can interchangeably use NAD
⫹
or
NADP
⫹
in a redox reaction.What would be the physiological con-
sequence(s) on an organism of having such an enzyme?
5. What is the free energy change of the reaction
under physiological conditions? Assume that ⌬G°¿ ⫽ 0 for this re-
action and that T ⫽ 37°C.
6. If G6P is
14
C-labeled at its C2 position, what is the distribu-
tion of the radioactive label in the products of the pentose phos-
phate pathway after one turnover of the pathway? What is the dis-
tribution of the label after passage of these products through
gluconeogenesis followed by a second round of the pentose phos-
phate pathway?
NADH ⫹ NADP
⫹
Δ NAD
⫹
⫹ NADPH
7. After feeding rapidly growing and proliferating cells [1,2-
13
C]glucose and isolating the RNA, you find that both the C1 and
C2 atoms of the ribosyl units are labeled. Show, using chemical
structures and the appropriate enzymes, how the pentose phos-
phate pathway can yield this distribution of the label.
8. The relative metabolic activities in an organism of glycoly-
sis ⫹ the citric acid cycle versus the pentose phosphate path-
way ⫹ gluconeogenesis can be measured by comparing the rates
of
14
CO
2
generation on administration of glucose labeled with
14
C at C1 with that of glucose labeled at C6. Explain.
9. (a) Describe the lengths of the products of the transketo-
lase reaction when the two substrates are both five-carbon sugars.
(b) Describe the products of the reaction when the substrates are
a five-carbon aldose and a six-carbon ketose. Does it matter which
of the substrates binds to the enzyme first?
10. In light of the finding that an otherwise benign or even ad-
vantageous mutation leads to abnormal primaquine sensitivity
combined with the fact that human beings have enormous genetic
complexity, comment on the possibility of developing drugs that
exhibit no atypical side effects in any individual.
11. Glucose-6-phosphatase is located inside the endoplasmic
reticulum. Describe the probable symptoms of a defect in G6P
transport across the endoplasmic reticulum membrane.
PROBLEMS
JWCL281_c23_871-900.qxd 6/16/10 11:45 AM Page 900
