Voet D., Voet Ju.G. Biochemistry
Подождите немного. Документ загружается.

any other movement—also produces heat. Nonshivering
thermogenesis through substrate cycling is discussed in
Section 17-4Fi.)
The mechanism of heat generation in brown fat involves
the regulated uncoupling of oxidative phosphorylation in
their mitochondria. These mitochondria contain the pro-
tein thermogenin [also called uncoupling protein (UCP)],
a transmembrane homodimer of 307-residue subunits that
acts as a channel to control the permeability of the inner
mitochondrial membrane to protons. In cold-adapted ani-
mals, thermogenin constitutes up to 15% of brown fat in-
ner mitochondrial membrane proteins.The flow of protons
through this channel protein is inhibited by physiological
concentrations of purine nucleotides (ADP, ATP, GDP,
GTP), but this inhibition can be overcome by free fatty
acids. The components of this system interact under hor-
monal control.
Thermogenesis in brown fat mitochondria is activated by
free fatty acids. These counteract the inhibitory effects of
purine nucleotides, thereby stimulating the flux through
the proton channel and uncoupling electron transport from
oxidative phosphorylation. The concentration of fatty acids
in brown adipose tissue is controlled by the adrenal hor-
mone norepinephrine (noradrenaline; Section 18-3E) with
cAMP acting as a second messenger (Section 18-3). Norep-
inephrine binds to the 
3
-adrenergic receptor, a G-protein
coupled receptor (GPCR) that, via an associated het-
erotrimeric G protein, stimulates adenylate cyclase to syn-
thesize cAMP (Fig.22-48), as described in Section 19-2.The
cAMP, in turn, activates protein kinase A (PKA), which ac-
tivates hormone-sensitive triacylglycerol lipase by phos-
phorylating it (Section 25-5). Finally, the activated lipase
hydrolyzes triacylglycerols to yield the free fatty acids that
open thermogenin’s proton channel. The transcription of
Section 22-3. Oxidative Phosphorylation 861
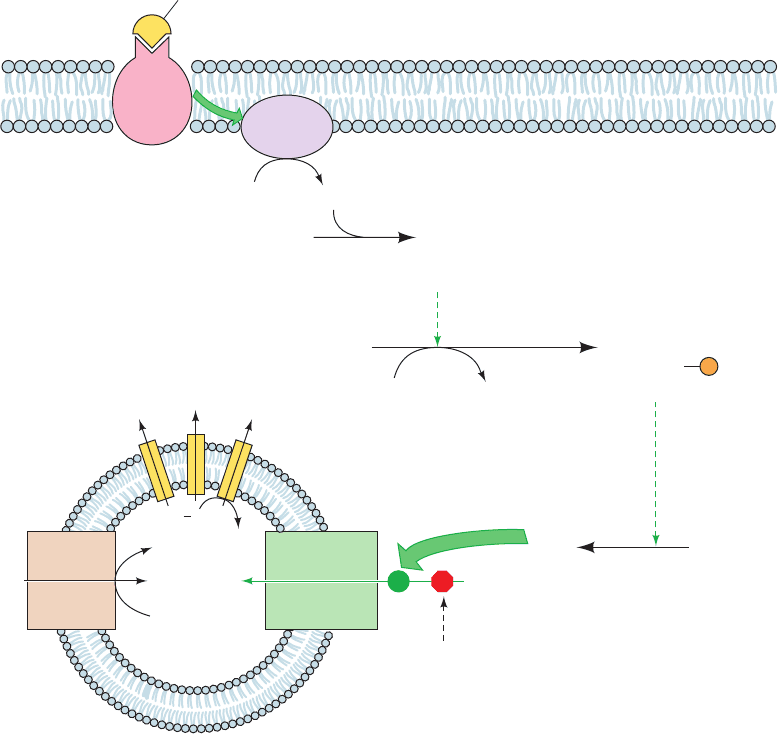
Figure 22-48 Mechanism of hormonally induced uncoupling
of oxidative phosphorylation in brown fat mitochondria.
(1) Norepinephrine binds to a 
3
-adrenergic receptor. (2) This
stimulates the associated heterotrimeric G protein to activate
adenylate cyclase (upper green arrow) to synthesize cAMP. (3)
cAMP binding activates protein kinase A (PKA). (4) PKA
ATP
Norepinephrine
1
2
cAMP
+ P
i
2C + R
2
(cAMP)
4
Cytosol
PKA
(inactive)
R
2
C
2
3
ATP
4
ADP
triacylglycerol
lipase
(active)
triacylglycerol
lipase
(inactive)
P
ATP
H
+
H
+
H
+
Thermogenin
proton
channel
H
+
opens
channel
6
Free
fatty
acids
5
Triacylglycerols
Mitochondrion
ATPase
ATP, ADP, GTP, GDP
block channel
ADP + P
i
H
2
O
2
O
2
1
PKA
(active)
Electron
transport
Adenylate
cyclase
H
+
+2H
+
F
1
F
O
β
3
-Adrenergic
receptor
phosphorylates hormone-sensitive triacylglycerol lipase, thereby
activating it. (5) Triacylglycerols are hydrolyzed, yielding free
fatty acids. (6) Free fatty acids overcome the purine nucleotide
block of thermogenin’s proton channel (lower green arrow),
allowing H
⫹
to enter the mitochondrion uncoupled from ATP
synthesis.
JWCL281_c22_823-870.qxd 3/19/10 11:00 AM Page 861

the gene encoding thermogenin is stimulated by the thy-
roid hormone triiodothyronine (T3; Section 19-1D).
b. Other Tissues Contain UCP Homologs
Although it originally seemed that only brown fat mito-
chondria contain an uncoupling protein, it is now apparent
that other tissues contain homologs of UCP1. Thus, UCP2
is expressed in many tissues including white adipose tissue,
whereas UCP3 occurs in both brown and white adipose tis-
sues as well as in muscle. These proteins may help regulate
metabolic rates, and variations in UCP levels or activity
might explain why some people seem to have a “fast” or
“slow” metabolism (Section 27-3E). UCPs are being stud-
ied as targets for treating obesity, since increasing the activ-
ity of UCPs could uncouple respiration from ATP synthe-
sis, thus permitting stored metabolic fuels (especially fat)
to be metabolized.The recent discovery that adult humans
have small depots of brown fat that are activated by cold
has made this an attractive weight loss strategy, although it
is possible that stimulating UCPs will cause a compensa-
tory increase in appetite.
Uncoupling proteins are not limited to animals. Some
plants express uncoupling proteins in response to cold
stress or to increase flower temperature, possibly to en-
hance the vaporization of scent to attract pollinators.
4 CONTROL OF ATP PRODUCTION
A typical adult woman requires some 1500 to 1800 kcal
(6300–7500 kJ) of metabolic energy per day. This corre-
sponds to the free energy of hydrolysis of over 200 mol of
ATP to ADP and P
i
. Yet the total amount of ATP present
in the body at any one time is ⬍0.1 mol; obviously, this
sparse supply of ATP must be continually recycled. As we
have seen, when carbohydrates serve as the energy supply
and aerobic conditions prevail, this recycling involves
glycogenolysis, glycolysis, the citric acid cycle, and oxida-
tive phosphorylation.
Of course the need for ATP is not constant. There is a
100-fold change in ATP utilization between sleep and vigor-
ous activity. The activities of the pathways that produce ATP
are under strict coordinated control so that ATP is never pro-
duced more rapidly than necessary. We have already dis-
cussed the control mechanisms of glycolysis, glycogenolysis,
and the citric acid cycle (Sections 17-4, 18-3, and 21-4). In
this section we discuss the mechanisms through which ox-
idative phosphorylation is controlled and observe how all
four systems are synchronized to produce ATP at precisely
the rate required at any particular moment.
A. Control of Oxidative Phosphorylation
In our discussion of the control of glycolysis, we saw that
most of the reactions in a metabolic pathway function close
to equilibrium. The few irreversible reactions constitute the
potential control points of the pathway and usually are cat-
alyzed by regulatory enzymes that are under allosteric con-
trol. In the case of oxidative phosphorylation, the pathway
from NADH to cytochrome c functions near equilibrium
(⌬G ⬇ 0):
for which
[22.2]
This pathway is therefore readily reversed by the addition
of ATP. In the cytochrome c oxidase reaction, however, the
terminal step of the electron-transport chain is irreversible
and is thus one of the important regulatory sites of the path-
way. Cytochrome c oxidase, in contrast to most regulatory
enzyme systems, appears to be controlled exclusively by
the availability of one of its substrates, reduced cytochrome
c (c
2⫹
). Since this substrate is in equilibrium with the rest of
the coupled oxidative phosphorylation system (Eq. [22.2]),
its concentration ultimately depends on the intramitochon-
drial [NADH]/[NAD
⫹
] ratio and the ATP mass action
ratio ([ATP]/[ADP][P
i
]). By rearranging Eq. [22.2], the ratio
of reduced to oxidized cytochrome c is expressed
[22.3]
Consequently, the higher the [NADH]/[NAD
⫹
] ratio and
the lower the ATP mass action ratio, the higher is the [c
2⫹
]
(reduced cytochrome c) and thus the higher is the cy-
tochrome c oxidase activity.
How is this system affected by changes in physical activ-
ity? In an individual at rest,ATP hydrolysis to ADP and P
i
is minimal and the ATP mass action ratio is high; the con-
centration of reduced cytochrome c is therefore low and
oxidative phosphorylation is minimal. Increased activity
results in hydrolysis of ATP to ADP and P
i
, thereby de-
creasing the ATP mass action ratio and increasing the con-
centration of reduced cytochrome c. This results in an in-
crease in the electron-transport rate and its coupled
phosphorylation. Such control of oxidative phosphoryla-
tion by the ATP mass action ratio is called acceptor control
because the rate of oxidative phosphorylation increases
with the concentration of ADP, the phosphoryl group
acceptor. In terms of a supply–demand system (Section
17-4D), acceptor control is understood as control by the
demand block.
The compartmentalization of the cell into mitochon-
dria, where ATP is synthesized, and cytoplasm, where ATP
is utilized, presents an interesting control problem: Is it the
ATP mass action ratio in the cytosol or in the mitochondr-
ial matrix that ultimately controls oxidative phosphoryla-
tion? Clearly the ATP mass action ratio that exerts direct
control must be that of the mitochondrial matrix where
ATP is synthesized. However, the inner mitochondrial
membrane, which is impermeable to adenine nucleotides
and P
i
, depends on specific transport systems to maintain
communication between the two compartments (Section
20-4C).This organization makes it possible for the transport
[c
2⫹
]
[c
3⫹
]
⫽ a
[NADH]
[NAD
⫹
]
b
1>
2
a
[ADP][P
i
]
[ATP]
bK
eq
K
eq
⫽ a
[NAD
⫹
]
[NADH]
b
1
Ⲑ
2
[c
2⫹
]
[c
3⫹
]
[ATP]
[ADP] [P
i
]
1
2
NAD
⫹
⫹ cytochrome c
2⫹
⫹ ATP
1
2
NADH ⫹ cytochrome c
3⫹
⫹ ADP ⫹ P
i
Δ
862 Chapter 22. Electron Transport and Oxidative Phosphorylation
JWCL281_c22_823-870.qxd 3/19/10 11:00 AM Page 862
of adenine nucleotides or P
i
to participate in the control of
oxidative phosphorylation.
Considerable research effort has been aimed at deter-
mining how oxidative phosphorylation is controlled in
terms of metabolic control analysis. For example, Hans
Westerhoff and Martin Kushmerick employed
31
P NMR to
measure the ATP/ADP ratios in human forearm muscle at
rest and during twitch contractions caused by external
electrical stimulation (the
31
P NMR spectrum of ATP is
shown in Fig. 16-15). Under conditions of low to moderate
ATP demand, the cytosolic mass action ratio as controlled
by the demand block of the system appears to be the major
control factor for mitochondrial oxidation. However, as
other laboratories have shown, as the demand for ATP in-
creases, the ADP–ATP translocator exerts greater control
until finally, when the demand for ATP is high, control
shifts to the supply block of the system, oxidative phospho-
rylation itself.
B. Coordinated Control of ATP Production
Glycolysis, the citric acid cycle, and oxidative phosphoryla-
tion constitute the major pathways for cellular ATP pro-
duction. Control of oxidative phosphorylation by the ATP
mass action ratio depends, of course, on an adequate sup-
ply of electrons to fuel the electron-transport chain. This
aspect of the system’s control is, in turn, dependent on the
[NADH]/[NAD
⫹
] ratio (Eq. [22.3]), which is maintained
high by the combined action of glycolysis and the citric acid
cycle in converting 10 molecules of NAD
⫹
to NADH per
molecule of glucose oxidized (Fig. 22-1). It is clear, there-
fore, that coordinated control is necessary for the three
processes. This is provided by the regulation of each of the
control points of glycolysis [hexokinase, phosphofructoki-
nase (PFK), and pyruvate kinase] and the citric acid cycle
(pyruvate dehydrogenase, citrate synthase, isocitrate dehy-
drogenase, and ␣-ketoglutarate dehydrogenase) by ade-
nine nucleotides or NADH or both as well as by certain
metabolites (Fig. 22-49).
a. Citrate Inhibits Glycolysis
The main control points of glycolysis and the citric acid cy-
cle are regulated by several effectors besides adenine nu-
cleotides or NADH (Fig. 22-49). This is an extremely com-
plex system with complex demands. Its many effectors, which
are involved in various aspects of metabolism, increase its
regulatory sensitivity. One particularly interesting regulatory
effect is the inhibition of PFK by citrate. When demand for
ATP decreases, [ATP] increases and [ADP] decreases. The
Section 22-4. Control of ATP Production 863
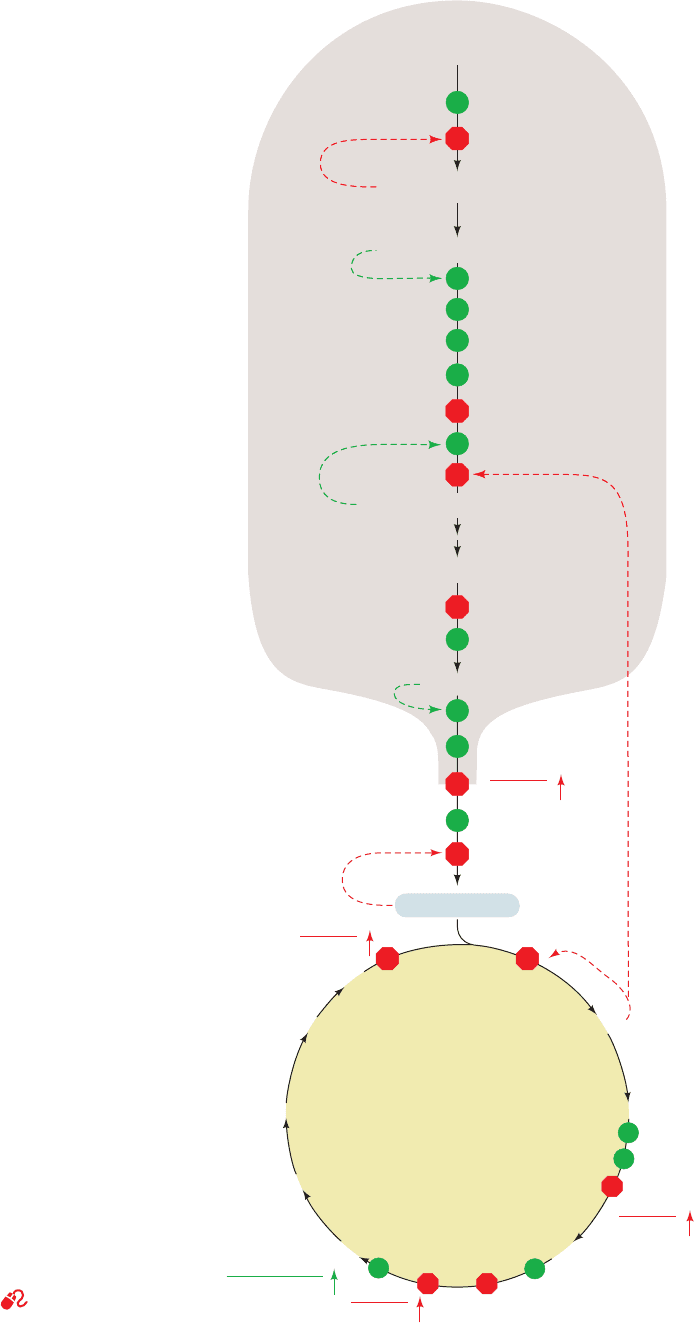
Figure 22-49 Schematic diagram depicting the coordinated
control of glycolysis and the citric acid cycle by ATP, ADP, AMP,
P
i
,Ca
2ⴙ
, and the [NADH]/[NAD
ⴙ
] ratio (the vertical arrows
indicate increases in this ratio). Here a green dot signifies
activation and a red octagon represents inhibition. [After
Newsholme, E.A. and Leech, A.R., Biochemistry for the Medical
Sciences, pp. 316 and 320, Wiley (1983).]
See the Animated
Figures
Glycolysis
Glucose
Glucose-6-phosphate
Fructose-6-phosphate
Fructose-1,6-bisphosphate
PEP
Pyruvate
hexokinase
phosphoglucose isomerase
phosphofructokinase
pyruvate kinase
P
i
P
AMP
NH
4
+
ATP
ADP
ATP
ADP
Ca
2+
NADH
NAD
+
]
]
Acetyl-CoA
citrate synthase
Citrate
Isocitrate
α-KetoglutarateSuccinyl-CoA
Fumarate
Malate
Oxaloacetate
Succinate
Ca
2+
ADP
Ca
2+
ATP
CoASH
succinyl-CoA
]
]
isocitrate
dehydrogenase
α-ketoglutarate
dehydrogenase
Citric acid
cycle
i
NADH
NAD
+
]
]
NADH
NAD
+
]
]
NADH
NAD
+
]
]
pyruvate dehydrogenase
JWCL281_c22_823-870.qxd 6/8/10 9:19 AM Page 863
citric acid cycle slows down at its isocitrate dehydrogenase
(activated by ADP) and ␣-ketoglutarate dehydrogenase (in-
hibited by ATP) steps, thereby causing the citrate concentra-
tion to build up. Citrate can leave the mitochondrion via a
specific transport system and, once in the cytosol, acts to re-
strain further carbohydrate breakdown by inhibiting PFK.
b. Fatty Acid Oxidation Inhibits Glycolysis
As we shall see in Section 25-2, the oxidation of fatty
acids is an aerobic process that produces acetyl-CoA, which
enters the citric acid cycle, thereby increasing both the mi-
tochondrial and cytoplasmic concentrations of citrate. The
increased [acetyl-CoA] inhibits the pyruvate dehydroge-
nase complex, whereas the increased [citrate] inhibits
phosphofructokinase, leading to a buildup of glucose-6-
phosphate, which inhibits hexokinase (Fig. 22-49). This in-
hibition of glycolysis by fatty acid oxidation is called the
glucose–fatty acid cycle or Randle cycle (after its discov-
erer, Philip Randle), although it is not, in fact, a cycle. The
Randle cycle allows fatty acids to be utilized as the major
fuel for oxidative metabolism in heart muscle, while con-
serving glucose for organs such as the brain,which require it.
C. Physiological Implications of Aerobic versus
Anaerobic Metabolism
In 1861, Louis Pasteur observed that when yeast are exposed
to aerobic conditions, their glucose consumption and ethanol
production drop precipitously (the Pasteur effect; alcoholic
fermentation in yeast to produce ATP, CO
2
, and ethanol are
discussed in Section 17-3B). An analogous effect is ob-
served in mammalian muscle; the concentration of lactic
acid, the anaerobic product of muscle glycolysis, drops dra-
matically when cells switch to aerobic metabolism.
a. Hypoxia Causes an Increase in Glycolysis
In the presence of sufficient oxygen, oxidative phospho-
rylation supplies most of the body’s ATP needs. However,
during hypoxia (when oxygen is limiting), glycolysis must be
stimulated (with its inherent increased rate of glucose con-
sumption; the reverse of the Pasteur effect) to supply the
necessary ATP. F2,6P, the most potent activator of PFK-1,
also participates in this process. The concentration of F2,6P,
as we have seen (Section 18-3Fc), is regulated by the bifunc-
tional enzyme PFK-2/FBPase-2. In its heart isozyme, the
PFK-2 activity is stimulated by phosphorylation at its Ser
466. Among the enzymes that do so is AMP-activated pro-
tein kinase (AMPK; Sections 25-4Ba, 25-5, and 27-1). When
oxygen deficiency prevents oxidative phosphorylation from
providing sufficient ATP for heart function, as occurs in is-
chemia (insufficient blood flow), the resulting increased
[AMP] activates AMPK. The consequent phosphorylation
and hence activation of PFK-2 results in an increase of
[F2,6P], thereby activating PFK-1 and thus glycolysis.
b. Aerobic ATP Production Is Far More Efficient
than Anaerobic ATP Production
One reason for the decrease in glucose consumption on
switching from anaerobic to aerobic metabolism is clear
from an examination of the stoichiometries of anaerobic
and aerobic breakdown of glucose (C
6
H
12
O
6
).
Anaerobic glycolysis:
Aerobic metabolism of glucose:
(2.5 ATP for each of the 10 NADH generated per glucose
oxidized, 1.5 ATP for each of the 2 FADH
2
generated,
2 ATP produced in glycolysis, and 2GTP 34 2ATP pro-
duced in the citric acid cycle.) Thus aerobic metabolism is
16 times more efficient than anaerobic glycolysis in produc-
ing ATP. The switch to aerobic metabolism therefore rap-
idly increases the ATP mass action ratio. As the ATP mass
action ratio increases, the rate of electron transport
decreases, which has the effect of increasing the [NADH]/
[NAD
⫹
] ratio. The increases in [ATP] and [NADH] inhibit
their target enzymes in the citric acid cycle and in the gly-
colytic pathway. The activity of PFK, which is citrate- and
adenine nucleotide-regulated and one of the rate-controlling
enzymes of glycolysis, decreases manyfold on switching
from anaerobic to aerobic metabolism.This accounts for the
dramatic decrease in glycolysis.
c. Anaerobic Glycolysis Has Advantages as
Well as Limitations
Animals can sustain anaerobic glycolysis for only short
periods of time.This is because PFK, which cannot function
effectively much below pH 7, is inhibited by the acidifica-
tion arising from lactic acid production. Despite this limita-
tion and the low efficiency of glycolytic ATP production, the
enzymes of glycolysis are present in such great concentra-
tions that when they are not inhibited, ATP can be produced
much more rapidly than through oxidative phosphorylation.
The different characteristics of aerobic and anaerobic
metabolism permit us to understand certain aspects of can-
cer cell metabolism and cardiovascular disease.
d. Cancer Cell Metabolism
As Warburg first noted in 1926, certain cancer cells pro-
duce more lactic acid under aerobic conditions than do
normal cells.This is because the glycolytic pathway in these
cells produces pyruvate more rapidly than the citric acid
cycle can accommodate. How can this happen given the in-
terlocking controls on the system? One explanation is that
these controls have broken down in cancer cells.Another is
that their ATP utilization occurs at rates too rapid to be re-
plenished by oxidative phosphorylation. This would alter
the ratios of adenine nucleotides so as to relieve the inhibi-
tion of PFK-1. In addition, many cancer cell lines have a
much larger [F2,6P] than do normal cells. These cells con-
tain an inducible isozyme of PFK-2/FBPase-2 that has an
AMPK-phosphorylatable site for activating PFK-2. Conse-
quently, an [AMP] increase in these cells results in an in-
crease in their [F2,6P], which further activates PFK-1 and
6CO
2
⫹ 38H
2
O ⫹ 32ATP
C
6
H
12
O
6
⫹ 32ADP ⫹ 32P
i
⫹ 6O
2
¡
2 lactate ⫹ 2H
⫹
⫹ 2H
2
O ⫹ 2ATP
C
6
H
12
O
6
⫹ 2ADP ⫹ 2P
i
¡
864 Chapter 22. Electron Transport and Oxidative Phosphorylation
JWCL281_c22_823-870.qxd 3/19/10 11:00 AM Page 864
Section 22-4. Control of ATP Production 865
glycolysis. Efforts to understand the metabolic differences
between cancer cells and normal cells may eventually lead
to a treatment of certain forms of this devastating disease.
e. Cardiovascular Disease
Oxygen deprivation of certain tissues resulting from
cardiovascular disease is of major medical concern. For ex-
ample, two of the most common causes of human death,
myocardial infarction (heart attack) and stroke, are caused
by interruption of the blood (O
2
) supply to a portion of the
heart or the brain, respectively. It seems obvious why this
should result in a cessation of cellular activity, but why does
it cause cell death?
In the absence of O
2
, a cell, which must then rely only on
glycolysis for ATP production, rapidly depletes its stores of
phosphocreatine (a source of rapid ATP production; Sec-
tion 16-4Cd) and glycogen. As the rate of ATP production
falls below the level required by membrane ion pumps for
the maintenance of proper intracellular ionic concentra-
tions, the osmotic balance of the system is disrupted, so that
the cell and its membrane-enveloped organelles begin to
swell.The resulting overstretched membranes become per-
meable, thereby leaking their enclosed contents. [In fact, a
useful diagnostic criterion for myocardial infarction is the
presence in the blood of heart-specific enzymes, such as the
H-type isozyme of lactate dehydrogenase (vs the M-type
isozyme, which predominates in skeletal muscle; Section
17-3A), which leak out of necrotic (dead) heart tissue.]
Moreover, the decreased intracellular pH that accompa-
nies anaerobic glycolysis (because of lactic acid produc-
tion; Section 17-3A) permits the released lysosomal en-
zymes (which are active only at acidic pH’s) to degrade the
cell contents. Thus, the cessation of metabolic activity re-
sults in irreversible cell damage. Rapidly respiring tissues,
such as those of heart and brain, are particularly suscepti-
ble to such damage.
f. IF
1
Inhibits F
1
F
0
–ATPase during Hypoxia
Under hypoxic conditions, the proton-motive force
across the inner mitochondrial membrane is reduced to the
point that F
1
F
0
–ATPase would switch from the synthesis of
ATP to its hydrolysis, resulting in a catastrophic loss of
ATP. This is prevented through the interaction of the
F
1
F
0
–ATPase with an 84-residue regulatory protein named
IF
1
. Under normal physiological conditions, IF
1
forms inac-
tive tetramers and higher order oligomers. However, when
the pH drops below 6.5, which occurs under anaerobic con-
ditions due to lactic acid production, IF
1
forms dimers in
which its almost entirely ␣ helical subunits associate via an
antiparallel coiled coil involving its residues 48 to 84. The
X-ray structure of F
1
in complex with AMPPNP and IF
1
,
determined by Leslie and Walker, reveals that each N-
terminal segment of the IF
1
dimer has bound to the
␣
DP
–
DP
interface (Fig. 22-38b) of a separate F
1
. This traps
AMPPNP and presumably ATP in the 
DP
binding site,
which would prevent it from hydrolyzing ATP (which,
since AMPPNP rather than ADP is bound to 
DP
, suggests
that this structure is that of a prehydrolysis step in the cat-
alytic reaction). When oxygen becomes available, the cell
re-energizes and its pH increases, thereby causing IF
1
to
dissociate from F
1
F
0
, which then commences synthesizing
ATP.
g. Partial Oxygen Reduction Produces Reactive
Oxygen Species (ROS)
Although the four-electron reduction of O
2
by cy-
tochrome c oxidase normally goes to completion, the en-
zyme infrequently releases partially reduced reactive oxygen
species (ROS) that readily react with a variety of cellular
components. The best known ROS is the superoxide
radical, . It is also produced by the occasional leakage
of electrons from Complexes I and III:
Its production is enhanced under hypoxic conditions.
Superoxide radical is a precursor of other reactive
species. Protonation of yields HO
2
ⴢ, a much stronger
oxidant than . The most potent oxygen species in bio-
logical systems is probably the hydroxyl radical, which
forms from the relatively harmless hydrogen peroxide
(H
2
O
2
):
The hydroxyl radical also forms through the reaction of su-
peroxide with H
2
O
2
:
ROS readily extract electrons from other molecules, con-
verting them to free radicals and thereby initiating a chain
reaction.
The random nature of ROS attacks makes it difficult to
characterize their reaction products, but all classes of bio-
logical molecules are susceptible to oxidative damage
caused by free radicals. The oxidation of polyunsaturated
lipids in cells may disrupt the structures of membranes, and
oxidative damage to DNA may result in point mutations.
Enzyme function may also be compromised through radi-
cal reactions with amino acid side chains. Because the mi-
tochondrion is the site of the bulk of the cell’s oxidative
metabolism, its lipids, DNA, and proteins bear the brunt of
free radical–related damage.
Several degenerative diseases, including Parkinson’s,
Alzheimer’s, and Huntington’s diseases, are associated
with oxidative damage to mitochondria. Such observations
have led to the free-radical theory of aging, which holds
that free-radical reactions arising during the course of nor-
mal oxidative metabolism are at least partially responsible
for the aging process. In fact, individuals with congenital
defects in their mitochondrial DNA suffer from a variety
of symptoms typical of old age, including neuromotor
difficulties, deafness, and dementia. These genetic defects
may increase the susceptibility of mitochondria to ROS-
generated damage.
h. Cells Are Equipped with Antioxidant Mechanisms
Antioxidants eliminate oxidative free radicals such as
and ⴢOH. In 1969, Irwin Fridovich discovered that the
O
2
⫺
ⴢ
O
2
⫺
ⴢ ⫹ H
2
O
2
¡
O
2
⫹ OH
⫺
⫹ ⴢOH
H
2
O
2
⫹ Fe
2⫹
¡
ⴢOH ⫹ OH
⫺
⫹ Fe
3⫹
O
2
⫺
ⴢ
O
2
⫺
ⴢ
O
2
⫹ e
⫺
¡
O
2
⫺
ⴢ
O
2
⫺
ⴢ
JWCL281_c22_823-870.qxd 7/5/10 11:43 AM Page 865

enzyme superoxide dismutase (SOD), which is present in
nearly all cells, catalyzes the conversion of to H
2
O
2
:
Mitochondrial and bacterial SOD are both Mn
2⫹
-containing
tetramers; eukaryotic cytosolic SOD is a dimer that contains
both Cu
2⫹
and Zn
2⫹
ions.Although the rate of nonenzymatic
superoxide breakdown is ⬃2 ⫻ 10
5
M
⫺1
ⴢ s
⫺1
, that of the
Cu,Zn-SOD–catalyzed reaction is ⬃2 ⫻ 10
9
M
⫺1
ⴢ s
⫺1
, close
to the diffusion-controlled limit (Section 14-2Bb). This is
apparently accomplished by electrostatic guidance of the
negatively charged superoxide substrate into the enzyme’s
active site (Fig. 14-10).
H
2
O
2
is degraded to water and oxygen by enzymes such
as catalase, which catalyzes the reaction
and glutathione peroxidase, which uses glutathione (GSH;
Section 21-2Ba) as a reducing agent:
2GSH ⫹ H
2
O
2
¡
GSSG ⫹ 2H
2
O
2H
2
O
2
¡
2H
2
O ⫹ O
2
2O
2
⫺
ⴢ ⫹ 2H
⫹
¡
H
2
O
2
⫹ O
2
O
2
⫺
ⴢ
The latter enzyme also catalyzes the breakdown of organic
hydroperoxides. Some types of glutathione peroxidase re-
quire Se for activity, which is one reason why Se appears to
have antioxidant activity.
Other potential antioxidants are plant-derived com-
pounds such as ascorbic acid (vitamin C; Section 11-1Cb)
and vitamin E, a group of compounds whose most promi-
nent member is ␣-tocopherol.
These compounds may help protect plants from oxidative
damage during photosynthesis, a process in which H
2
O is ox-
idized to O
2
(Section 24-2). However, clinical trials indicate
that their use does not contribute to longevity in humans.
␣-Tocopherol (vitamin E)
HO
H
3
O
()
866 Chapter 22. Electron Transport and Oxidative Phosphorylation
1 The Mitochondrion Oxidative phosphorylation is the
process through which the NADH and FADH
2
produced by
nutrient oxidation are oxidized with the concomitant forma-
tion of ATP. The process takes place in the mitochondrion, an
ellipsoidal organelle that is bounded by a permeable outer
membrane and contains an impermeable and highly invagi-
nated inner membrane that encloses the matrix. Enzymes of
oxidative phosphorylation are embedded in the inner mito-
chondrial membrane. P
i
is imported into the mitochondrion by
a specific transport protein. Ca
2⫹
import and Ca
2⫹
export pro-
teins operate to maintain a constant cytosolic [Ca
2⫹
]. NADH’s
electrons are imported into the mitochondrion by shuttle sys-
tems such as the glycerophosphate shuttle and the malate–
aspartate shuttle.
2 Electron Transport The standard free energy change
for the oxidation of NADH by O
2
is ⌬G°¿ ⫽ –218 kJ ⴢ mol
⫺1
,
whereas that for the synthesis of ATP from ADP and P
i
is
⌬G°¿ ⫽ 30.5 kJ ⴢ mol
⫺1
. Consequently, the molar free energy of
oxidation of NADH by O
2
is sufficient to power the synthesis
of several moles of ATP under standard conditions. The elec-
trons generated by oxidation of NADH and FADH
2
pass
through four protein complexes, the electron-transport chain,
with the coupled synthesis of ATP. Complexes I, III, and IV
participate in the oxidation of NADH, producing ⬃2.5 ATPs
per NADH, whereas FADH
2
oxidation, which involves Com-
plexes II, III, and IV, produces only ⬃1.5 ATPs per FADH
2
.
Thus, the ratio of moles of ATP produced per mole of coenzyme
oxidized by O
2
, the P/O ratio, is ⬃2.5 for NADH oxidation and
⬃1.5 for FADH
2
oxidation. The route taken by electrons
through the electron-transport chain was elucidated, in part,
through the use of electron-transport inhibitors. Rotenone and
amytal inhibit Complex I, antimycin inhibits Complex III, and
CN
⫺
inhibits Complex IV.Also involved were measurements of
the reduction potentials of the electron-carrying prosthetic
groups contained in the electron-transport complexes.
Complex I contains FMN and nine iron–sulfur clusters in a
45-subunit (in mammals) transmembrane protein complex.
This L-shaped complex passes electrons from NADH to CoQ,
a nonpolar small molecule that diffuses freely within the mem-
brane. Complex II, which is also the citric acid cycle enzyme
succinate dehydrogenase, also passes electrons to CoQ, in this
case from succinate through FAD and three iron–sulfur clus-
ters.The X-ray structure of Complex II indicates that its redox
cofactors are arranged in a linear chain. CoQH
2
passes elec-
trons to Complex III (cytochrome bc
1
), a homodimeric com-
plex whose protomers each contain two b-type hemes bound
to a cytochrome b subunit, a Rieske iron–sulfur protein (ISP),
and a cytochrome c
1
.An electron from cytochrome c
1
of Com-
plex III is passed to the Cu
A
center of Complex IV (cy-
tochrome c oxidase) via the peripheral membrane protein cy-
tochrome c. This electron is then passed to cytochrome a,
which, in turn, passes it to a binuclear center composed of
heme a
3
and Cu
B
, which reduces O
2
to H
2
O. This process oc-
curs in four 1-electron steps that pump four protons from the
mitochondrial matrix/bacterial cytoplasm to the intermem-
brane space/periplasm. Complexes I, III, and IV form super-
complexes that increase the efficiency of electron transport.
3 Oxidative Phosphorylation The mechanism by which
the free energy released by the electron-transport chain is
stored and utilized in ATP synthesis is described by the
chemiosmotic hypothesis. This hypothesis states that the free
energy released by electron transport is conserved by the gen-
eration of an electrochemical proton gradient across the inner
mitochondrial membrane (bacterial cell membrane; outside
positive and acidic), which is harnessed to synthesize ATP. The
proton gradient is created and maintained by the obligatory
outward translocation of H
⫹
across the inner mitochondrial
membrane as electrons travel through Complexes I,III, and IV.
Complex III pumps protons via a redox loop mechanism
called the Q cycle, a bifurcated double cycle in which one
CHAPTER SUMMARY
JWCL281_c22_823-870.qxd 7/2/10 11:14 AM Page 866

References 867
molecule of CoQH
2
is oxidized to CoQ and then is rereduced to
CoQH
2
by a second molecule of CoQH
2
in a process that col-
lectively transfers four protons from the inside to the outside
while oxidizing one molecule of CoQH
2
to CoQ. Electrons are
transferred between the two CoQ’s, which are bound at differ-
ent sites, Q
o
and Q
i
, as well as between the CoQH
2
bound at Q
o
and cytochrome c
1
via the ISP, which undergoes a conforma-
tional change in doing so. Complex IV contains no (H
⫹
⫹ e
⫺
)
carriers such as CoQH
2
and hence translocates proteins via a
proton pump mechanism. Bacteriorhodopsin, the best charac-
terized proton pump, translocates protons in a light driven
process. This involves a trans to cis isomerization of bacterio-
rhodopsin’s retinal prosthetic group on absorbing a photon,
followed by the translocation of a proton through the hy-
drophilic central channel of this transmembrane protein via a
process that involves conformational and pK changes of the
polar groups lining the channel as the retinal relaxes to its
ground state. Complex IV is thought to pump protons via a sim-
ilar mechanism that is driven by the changes in the redox state
of its heme a
3
–Cu
B
binuclear center as it reduces O
2
to H
2
O.
The energy stored in the electrochemical proton gradient is
utilized by proton-translocating ATP synthase (Complex V,
F
1
F
0
–ATPase) in the synthesis of ATP via the binding change
mechanism, by coupling this process to the exergonic transport
of H
⫹
back to the inside. Mitochondrial proton-translocating
ATP synthase consists of two oligomeric components: F
1
(␣
3

3
␥␦ε), a peripheral membrane protein that appears as “lol-
lipops” in electron micrographs of the inner mitochondrial
membrane, and F
0
(ab
2
c
12
in E. coli), an integral membrane
protein that contains the proton channel. The conformational
changes that promote the synthesis of ATP from ADP ⫹ P
i
arise through the demonstrated rotation of the ␥ subunit rela-
tive to the catalytic ␣
3

3
assembly that contains the enzyme’s
three active sites. The ␥ subunit is attached to a ring of c sub-
units in F
0
, whose rotation is driven by the passage of protons
between it and the a subunit.
Compounds such as 2,4-dinitrophenol are uncouplers of
oxidative phosphorylation because they carry H
⫹
across the
mitochondrial membrane, thereby dissipating the proton gra-
dient and allowing electron transport to continue without con-
comitant ATP synthesis. Brown fat mitochondria contain a
regulated uncoupling system that, under hormonal control,
generates heat instead of ATP.
4 Control of ATP Production Under aerobic conditions,
the rate of ATP synthesis by oxidative phosphorylation is reg-
ulated, in a phenomenon known as acceptor control, by the
ATP mass action ratio. ATP synthesis is tightly coupled to the
oxidation of NADH and FADH
2
by the electron-transport
chain. Glycolysis and the citric acid cycle are coordinately con-
trolled so as to produce NADH and FADH
2
only at a rate re-
quired to meet the system’s demand for ATP. IF
1
inhibits the
ATP hydrolysis by mitochondrial F
1
F
0
–ATPase that would
otherwise occur under hypoxic conditions. Incomplete and
side reactions of Complexes I, III, and IV produce damaging
reactive oxygen species (ROS) that are largely eliminated
through the actions of several cellular enzymes, most notably
superoxide dismutase.
Historical Overview
Ernster, L. and Schatz, G., Mitochondria: A historical review, J.
Cell Biol. 91, 227s–255s (1981).
Fruton, J.S., Molecules and Life,pp.262–396, Wiley–Interscience
(1972).
Krebs,H.,Otto Warburg.Cell Physiologist, Biochemist,and Eccentric,
Clarendon Press (1981). [A biography of one of the pioneers in
the biochemical study of respiration, by a distinguished student.]
Prebble, J., Peter Mitchell and the ox phos wars, Trends Biochem.
Sci. 27, 209–212 (2002).
Racker, E., A New Look at Mechanisms in Bioenergetics, Acade-
mic Press (1976). [A fascinating personal account by one of the
outstanding contributors to the field.]
General
Nicholls, D.G. and Ferguson, S.J., Bioenergetics (3rd ed.), Acade-
mic Press (2002). [An authoritative monograph devoted al-
most entirely to the mechanism of oxidative phosphorylation
and the techniques used to elucidate it.]
Schäfer,G. and Penefsky, H. (Eds.), Bioenergetics, Springer (2008).
Schultz, B.E. and Chan, S.I., Structures and proton-pumping
strategies of mitochondrial respiratory enzymes, Annu. Rev.
Biophys. Biomol. Struct. 30, 23–65 (2001).
Mitochondria
Goodsell, D.S., Mitochondrion, Biochem. Mol. Biol. Educ. 38,
134–140 (2010). [An illustrated guide to the mitochondrion.]
Frey,T.G. and Mannella, C.A.,The internal structure of mitochon-
dria, Trends Biochem. Sci. 23, 319–324 (2000).
Frey, T.G., Perkins, G.A., and Ellisman, M.H., Electron tomogra-
phy of membrane-bound cellular organelles, Annu. Rev. Bio-
phys. Biomol. Struct. 35, 199–224 (2006).
Logan, D.C., The mitochondrial compartment, J. Exp. Botany 57,
1225–1243 (2006).
Scheffler, I.E., Mitochondria (2nd ed.),Wiley-Liss (2008).
Electron Transport
Beinert, H., Holm, R.H., and Münck, E., Iron-sulfur clusters:
Nature’s modular, multipurpose structures, Science 277,
653–659 (1997).
Belevich, I. and Verkhovsky, M.I., Molecular mechanism of proton
translocation by cytochrome c oxidase, Antioxidants Redox
Signaling 10, 1–29 (2008). [A comprehensive review.]
Brandt, U., Energy converting NADH:quinone oxidoreductase
(Complex I), Annu. Rev. Biochem. 75, 69–92 (2006).
Collman, J.P., Rapta, M., Bröring, M., Raptova, L., Schwenninger,
R., Boitrel, B., Fu,L., and L’Her, M., Close structural analogues
of the cytochrome c oxidase Fe
a3
/Cu
B
center show clean
4e
⫺
electroreduction of O
2
to H
2
O at physiological pH, J. Am.
Chem. Soc. 121, 1387–1388 (1999).
Crofts,A.R.,The cytochrome bc
1
complex: Function in the context
of structure, Annu. Rev. Physiol. 66, 689–733 (2004).
Efremov, R.G., Baradaran, R., and Sazanov, L.A.,The architecture
of respiratory complex I, Nature 465, 441–445 (2010).
Hinkle, P.C., P/O ratios of mitochondrial oxidative phosporyla-
tion, Biochim. Biophys. Acta 1706, 1–11 (2005).
Hosler,J.P.,Ferguson-Miller, S.,and Mills, D.A., Energy transduction:
Proton transfer through the respiratory complexes, Annu. Rev.
REFERENCES
JWCL281_c22_823-870.qxd 7/20/10 6:20 PM Page 867
868 Chapter 22. Electron Transport and Oxidative Phosphorylation
Biochem. 75, 165–187 (2006). [A review that focuses on cy-
tochrome c oxidase.]
Huang, L., Sun, G., Cobessi, D., Wang, A.C., Shen, J.T., Tung, E.Y.,
Anderson,V.E., and Berry, E.A., 3-Nitropropionic acid is a sui-
cide inhibitor of mitochondrial respiration that, upon oxidation
by Complex II, forms a covalent adduct with a catalytic base
arginine in the active site of the enzyme, J. Biol. Chem. 281,
5965–5972 (2006). [X-ray structure of chicken Complex II.]
Hunte, C., Koepke, J., Lange, C., Rossmanith, T., and Michel, H.,
Structure at 2.3 Å resolution of the cytochrome bc
1
complex
from the yeast Saccharomyces cerevisiae co-crystallized with
an antibody Fv fragment, Structure 8, 669–684 (2000).
Iwata, S., Ostermeier, C., Ludwig, B., and Michel, H., Structure at
2.8 Å resolution of cytochrome c oxidase from Paracoccus den-
itrificans, Nature 376, 660–669 (1995).
Iwata, S., Lee, J.W., Okada, K., Lee, J.K., Iwata, M., Rasmussen, B.,
Link, T.A., Ramaswamy, S., and Jap, B.K., Complete structure
of the 11-subunit bovine mitochondrial cytochrome bc
1
com-
plex, Science 281, 64–71 (1998).
Johnson, D.C., Dean, D.R., Smith,A.D., and Johnson, M.K., Struc-
ture, function, and formation of biological iron–sulfur clusters,
Annu. Rev. Biochem. 74, 247–281 (2005).
Lenaz, G., Fato, R., Genova, M.L., Bergamini, C., Bianchi, C., and
Biondi,A., Mitochondrial complex I: Structural and functional
aspects, Biochim. Biophys. Acta 1757, 1406–1420 (2006).
Michel, H., Behr, J., Harrenga, A., and Kannt, A., Cytochrome c
oxidase: Structure and spectroscopy, Annu. Rev. Biophys.
Biomol. Struct. 27, 329–356 (1998).
Moser, C.C., Keske, J.M., Warncke, K., Farid, R.S., and Dutton,
L.S., Nature of biological electron transfer, Nature 355,
796–802 (1992).
Osyczka, A., Moser, C.C., and Dutton, P.L., Fixing the Q cycle,
Trends Biochem. Sci. 30, 176–182 (2005).
Radermacher, M., Ruiz, T., Clason, T., Benjamin, S., Brandt, U.,
and Zickerman, V., The three dimensional structure of com-
plex I from Yarrowia lipolytica: A highly dynamic enzyme, J.
Struct. Biol. 154, 269–279 (2006).
Sazanov, L.A. and Hinchliffe, P., Structure of the hydrophilic do-
main of respiratory Complex I from Thermus thermophilus,
Science 311, 1430–1436 (2006); and Sazanov, L.A., Respiratory
Complex I: Mechanistic and structural insights provided by the
crystal structure of the hydrophilic domain, Biochemistry 46,
2275–2288 (2007).
Schäfer, E., Dencher, N.A., Vonck, J., and Parcej, D.N., Three
dimensional structure of the respiratory supercomplex
I
1
III
2
IV
1
from bovine heart mitochondria, Biochemistry 46,
12579–12585 (2007); and Vonck, J., and Schäfer, E., Supra-
molecular organization of protein complexes in the mitochon-
drial inner membrane, Biochim. Biophys. Acta 1793, 117–124
(2009).
Solmaz, S.R.N. and Hunte,C., Structure of Complex III with bound
cytochrome c in reduced state and definition of a minimal core
interface for electron transfer, J. Biol. Chem. 283, 17542–17549
(2008); and Lange, C. and Hunte, C., Crystal structure of the
yeast cytochrome bc
1
complex with its bound substrate cy-
tochrome c, Proc. Natl. Acad. Sci. 99, 2800–2805 (2002).
Sun, F., Huo, X., Zhai,Y.,Wang,A., Xu, J., Su, D., Bartlam, M., and
Rao, Z., Crystal structure of mitochondrial respiratory protein
Complex II, Cell 121, 1043–1057 (2005).
Tsukihara,T.,Aoyama, H.,Yamashita, E.,Tomizaki,T.,Yamaguchi,
H., Shinzawa-Itoh, K., Nakashima, R., Yaono, R., and
Yoshikawa, S., The whole structure of the 13-subunit oxidized
cytochrome c oxidase at 2.8 Å, Science 272, 1136–1144 (1996).
Xia, D.,Yu, C.-A., Kim, H., Xia, J.-Z., Kachurin,A.M.,Zhang,L.,Yu,
L., and Deisenhofer, J., Crystal structure of the cytochrome bc
1
complex from heart mitochondria, Science 277, 60–66 (1997).
Yankovskaya, V., Horsefield, R., Törnroth, S., Luna-Chavez, C.,
Miyoshi, H., Légar, C., Byrne, B., Cecchini, G., and Iwata, S.,
Architecture of succinate dehydrogenase and reactive oxygen
species generation, Science 299, 700–704 (2003).
Yoshikawa, S., et al., Redox-coupled crystal structural changes in
bovine heart cytochrome c oxidase, Science 280, 1723–1729
(1998); and Yoshikawa, S., Beef heart cytochrome c oxidase,
Curr. Opin. Struct. Biol. 7, 574–579 (1997).
Zhang, Z., Huang, L., Shulmeister, V.M., Chi, Y.-I., Kim, K.K.,
Huang, L.-W., Crofts, A.R., Berry, E.A., and Kim, S.H., Elec-
tron transfer by domain movement in cytochrome bc
1
, Nature
392, 677–684 (1998).
Bacteriorhodopsin
Heberle, J., Proton transfer reactions across bacteriorhodopsin
and along the membrane, Biochim. Biophys. Acta 1458,
135–147 (2000).
Kühlbrandt, W., Bacteriorhodopsin—the movie, Nature 406,
569–570 (2000).
Lanyi, J.K., Bacteriorhodopsin, Annu. Rev. Physiol. 66, 665–688
(2004).
Oxidative Phosphorylation
Abrahams, J.P., Leslie, A.G.W., Lutter, R., and Walker, J.E., Struc-
ture at 2.8 Å resolution of F
1
-ATPase from bovine heart mito-
chondria, Nature 370, 621–628 (1994).
Boyer, P.D., The binding change mechanism for ATP synthase—
some probabilities and possibilities, Biochim. Biophys. Acta
1140, 215–250 (1993).
Boyer, P.D., The ATP synthase—a splendid molecular machine,
Annu. Rev. Biochem. 66, 717–749 (1997).
Capaldi, R. and Aggeler, R., Mechanism of F
1
F
0
-type ATP syn-
thase, a biological rotary motor, Trends Biochem. Sci. 27,
154–160 (2002).
Dickson, V.K., Silvester, J.A., Fearnley, I.M., Leslie, A.G.W., and
Walker, J.E., On the structure of the stator of the mitochondrial
ATP synthase, EMBO J. 25, 2911–2918 (2006).
Gibbons, C., Montgomery, M.G., Leslie, A.G.W., and Walker, J.E.,
The structure of the central stalk in bovine F
1
-ATPase at 2.4 Å
resolution, Nature Struct. Biol. 7, 1055–1061 (2000).
Hausrath, A.C., Capaldi, R.A., and Matthews, B.M., The confor-
mation of the ε- and ␥-subunits of Escherichia coli F
1
ATPase,
J. Biol. Chem. 276, 47227–47232 (2001). [The X-ray structure of
the E. coli F
1
.]
Itoh, H., Takahashi, A., Adachi, K., Noji, H., Yasuda, R., Yoshida,
M., and Kinosita, K., Jr., Mechanically driven ATP synthesis by
F
1
-ATPase, Nature 427, 465–468 (2004).
Klingenberg,M., Mechanism and evolution of the uncoupling pro-
tein of brown adipose tissue, Trends Biochem. Sci. 15, 108–112
(1990).
Ma, J., Flynn, T.C., Cui, Q., Leslie, A.G.W., Walker, J.E., and
Karplus, M., A dynamic analysis of the rotation mechanism
for the conformational change in F
1
-ATPase, Structure 10,
921–931 (2002).
Meier, T., Polzer, P., Diederichs, K., Welte, W., and Dimroth, P.,
Structure of the rotor ring of F-type Na
⫹
-ATPase from Il-
yobacter tartaricus, Science 308, 659–662 (2005).
Mitchell, P., Vectorial chemistry and the molecular mechanics of
chemiosmotic coupling: Power transmission by proticity,
Biochem. Soc.Trans. 4, 398–430 (1976).
Nicholls, D.G. and Rial, E., Brown fat mitochondria, Trends
Biochem. Sci. 9, 489–491 (1984).
JWCL281_c22_823-870.qxd 7/5/10 10:27 AM Page 868

Problems 869
Noji, H. and Yoshida, M., The rotary engine in cell ATP synthase,
J. Biol. Chem. 276, 1665–1668 (2001).
Rastogi, V.K. and Girvin, M.E., Structural changes linked to pro-
ton translocation by subunit c of the ATP synthase,Nature 402,
262–268 (1999); and Girvin, M.E., Rastogi,V.K.,Abildgaard, F.,
Markley, J.L., and Fillingame, R.H., Solution structure of the
transmembrane H
⫹
-transporting subunit c of the ATP syn-
thase, Biochemistry 37, 8817–8824 (1998).
Rubinstein, J.L., Walker, J.E., and Henderson, R., Structure of the
mitochondrial ATP synthase by electron cryomicroscopy,
EMBO J. 22, 6182–6192 (2003).
Sambongi,Y., Iko,Y.,Tanabe, M., Omote, H., Iwamoto-Kihara,A.,
Ueda, I., Yanagida, T., Wada, Y., and Futai, M., Mechanical ro-
tation of the c subunit oligomer in ATP synthase (F
0
F
1
): Direct
observation, Science 286, 1722–1724 (1999).
Stock, D., Gibbons, C., Arechaga, I., Leslie, A.G.W., and Walker,
J.E., The rotary mechanism of ATP synthase, Curr. Opin.
Struct. Biol. 10, 672–679 (2000).
Verkhovsky, M.I., Jasaitis, A., Verkhovskaya, M.L., Morgan, J.E.,
and Wikström, M., Proton translocation by cytochrome c oxi-
dase, Nature 400, 480–483 (1999).
von Ballamoos, C., Wiedenmann, A., and Dimroth, P., Essentials
for ATP synthesis by F
1
F
0
ATP synthases, Annu. Rev. Biochem.
78, 649–672 (2009); and von Ballamoos, C., Cook, G.M., and
Dimroth, P., Unique rotary ATP synthase and its biological di-
versity, Annu. Rev. Biophys. 37, 43–64 (2008).
Wilkens, S., Rotary molecular motors, Adv. Prot. Chem. 71,
345–382 (2005).
Yasuda, R., Noji, H., Kinosita, K., Jr., and Yoshida, M., F
1
-ATPase
is a highly efficient molecular motor that rotates with discrete
120° steps, Cell 93, 1117–1124 (1998).
Yoshida, M., Muneyuki, E., and Hisabori, T., ATP synthase—a
marvelous rotary engine of the cell, Nature Rev. Mol. Cell Biol.
2, 669–677 (2001); and Noji,H.and Yoshida, M.,The rotary ma-
chine in the cell ATP synthase, J. Biol. Chem. 276, 1665–1668
(2001).
Control of ATP Production
Brown, G.C., Control of respiration and ATP synthesis in mam-
malian mitochondria and cells, Biochem. J. 284, 1–13 (1992).
Cabezón, E., Montgomery, M.G., Leslie,A.G.W., and Walker, J.E.,
The structure of bovine F
1
-ATPase in complex with its regula-
tory protein IF
1
, Nature Struct. Biol. 10, 744–750 (2003).
Celi, F.S., Brown adipose tissue—when it pays to be inefficient,
New Engl. J. Med. 360 1553–1556 (2009).
Chesney J., Mitchell, R., Benigni, F., Bacher, M., Spiegel, L., Al-
Abed, Y., Han, J.H., Metz, C., and Bucala, R., An inducible
gene product for 6-phosphofructo-2-kinase with an AU-rich
instability element: role in tumor cell glycolysis and the
Warburg effect, Proc. Natl. Acad. Sci. 96, 3047–3052 (2000).
Harris, D.A. and Das,A.M., Control of mitochondrial ATP synthe-
sis in the heart, Biochem. J. 280, 561–573 (1991).
Jeneson, J.A.L., Westerhoff, H.V., and Kushmerick, M.J., A meta-
bolic control analysis of kinetic controls in ATP free energy
metabolism in contracting skeletal muscle, Am. J. Physiol. Cell
Physiol. 279, C813–C832 (2000).
Marsin, A-S., Bertrand, L., Rider, M.H., Deprez, J., Beauloye, C.,
Vincent, M.F., Van den Berghe, G., Carling, D., and Hue, L.,
Phosphorylation and activation of heart PFK-2 by AMPK has
a role in the stimulation of glycolysis during ischaemia, Curr.
Biol. 10, 1247–1255 (2000).
Marsin,A-S., Bouzin, C., Bertrand, L., and Hue, L.,The stimulation
of glycolysis by hypoxia in activated monocytes is mediated by
AMP-activated protein kinase and inducible 6-phosphofructo-
3-kinase, J. Biol. Chem. 277, 30778–30783 (2002).
Randle, P. J., Regulatory interactions between lipids and carbohy-
drates:The glucose fatty acid cycle after 35 years.Diabetes/Metab.
Rev. 14, 263–283 (1998).
Ricquier, D. and Bouillaud, F.,The mitochondrial uncoupling pro-
tein: Structural and genetic studies, Prog. Nucleic Acid Res.
Mol. Biol. 56, 83–108 (1997).
1. Rank the following redox-active coenzymes and prosthetic
groups of the electron-transport chain in order of increasing affin-
ity for electrons: cytochrome a, CoQ, FAD, cytochrome c, NAD
⫹
.
2. Why is the oxidation of succinate to fumarate only associ-
ated with the production of two ATPs during oxidative phospho-
rylation, whereas the oxidation of malate to oxaloacetate is asso-
ciated with the production of three ATPs?
3. What is the thermodynamic efficiency of oxidizing FADH
2
so
as to synthesize two ATPs under standard biochemical conditions?
4. Sublethal cyanide poisoning may be reversed by the admin-
istration of nitrites. These substances oxidize hemoglobin, which
has a relatively low affinity for CN
⫺
, to methemoglobin, which has
a relatively high affinity for CN
⫺
.Why is this treatment effective?
5. Match the compound with its behavior: (1) rotenone,
(2) dinitrophenol, and (3) antimycin. (a) Inhibits oxidative phos-
phorylation when the substrate is pyruvate but not when the sub-
strate is succinate. (b) Inhibits oxidative phosphorylation when
the substrate is either pyruvate or succinate. (c) Allows pyruvate
to be oxidized by mitochondria even in the absence of ADP.
6. Nigericin is an ionophore (Section 20-2C) that exchanges
K
⫹
for H
⫹
across membranes. Explain how the treatment of func-
tioning mitochondria with nigericin uncouples electron transport
from oxidative phosphorylation. Does valinomycin, an ionophore
that transports K
⫹
but not H
⫹
, do the same? Explain.
7. Why is it possible for electrons in an electron-transfer com-
plex to flow from a redox center to one with a lesser value of e°¿?
8. How do the P/O ratios for NADH differ in ATP synthases
that contain 10 and 15 c subunits?
9. The difference in pH between the internal and external
surfaces of the inner mitochondrial membrane is 1.4 pH units
(external side acidic). If the membrane potential is 0.06 V (inside
negative), what is the free energy released on transporting 1 mol
of protons back across the membrane? How many protons must
be transported to provide enough free energy for the synthesis of
1 mol of ATP (assume standard biochemical conditions)?
*10. (a) A simplistic interpretation of the Q cycle would pre-
dict that the proton pumping efficiency of cytochrome bc
1
would
be reduced by no more than 50% in the presence of saturating
amounts of antimycin. Explain. (b) Indicate why cytochrome bc
1
is
nearly 100% inhibited by antimycin.
PROBLEMS
JWCL281_c22_823-870.qxd 3/19/10 11:00 AM Page 869
870 Chapter 22. Electron Transport and Oxidative Phosphorylation
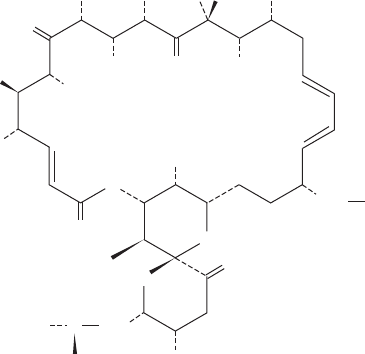
11. The antibiotic oligomycin B
binds to the F
0
subunit of the mitochondrial F
1
F
0
–ATPase and
thereby prevents it from synthesizing ATP [note that oligomycin-
sensitivity conferral protein (OSCP), the mitochondrial counter-
part of the E. coli ␦ subunit (Fig. 22-41), does not bind oligomycin
H
3
C
H
3
C
H
3
C
H
3
C
CH
3
CH
3
CH
3
CH
3
CH
3
CH
2
CH
3
CH
2
CH
3
C
HO
O
HO
OH
O
O
O
OH
O
O
O
OH
Oligomycin B
B.] Explain why: (a) Submitochondrial particles from which F
1
has
been removed are permeable to protons. (b) Addition of
oligomycin B to F
1
-depleted submitochondrial particles decreases
this permeability severalfold.
12. Oligomycin B (see Problem 11) and cyanide both inhibit
oxidative phosphorylation when the substrate is either pyruvate
or succinate. Dinitrophenol can be used to distinguish between
these inhibitors. Explain.
13. The E. coli F
1
F
0
–ATPase cannot synthesize ATP when
Met 23 of its ␥ subunit is mutated to Lys.Yet the F
1
component of
this complex still exhibits rotation of its ␥ subunit relative to its
␣
3

3
spheroid when it is supplied with ATP. Suggest a reason for
these effects.
14. For the oxidation of a given amount of glucose, does non-
shivering thermogenesis by brown fat or shivering thermogenesis
by muscle produce more heat?
15. What is the advantage of hormones activating a lipase to
stimulate nonshivering thermogenesis in brown fat rather than ac-
tivating UCP1 directly?
16. How does atractyloside affect mitochondrial respiration?
(Hint: See Section 20-4C.)
17. Certain unscrupulous operators offer, for a fee, to freeze
recently deceased individuals in liquid nitrogen until medical sci-
ence can cure the disease from which they died. What is the bio-
chemical fallacy of this procedure?
JWCL281_c22_823-870.qxd 3/19/10 11:00 AM Page 870
