Voet D., Voet Ju.G. Biochemistry
Подождите немного. Документ загружается.

unit; Section 11-1C). About 80 different kinds of naturally
occurring glycosidic linkages are known, most of which in-
volve mannose, N-acetylglucosamine, N-acetylmuramic
acid, glucose, galactose, fucose (6-deoxygalactose), N-acetyl-
neuraminic acid (sialic acid), and N-acetylgalactosamine
(Section 11-1C). Glycosidic linkages also occur to lipids
(e.g., glycosphingolipids; Section 12-1D) and proteins
(glycoproteins; Section 11-3C).
Glycosidic bond formation requires free energy input un-
der physiological conditions (G°¿ 16 kJ ⴢ mol
1
). This
free energy, as we have seen in the case of glycogen synthe-
sis (Section 18-2B), is acquired through the conversion of
monosaccharide units to nucleotide sugars.A nucleotide at a
sugar’s anomeric carbon atom is a good leaving group and
thereby facilitates formation of a glycosidic bond to a second
sugar unit via reactions catalyzed by glycosyltransferases
Section 23-3. Biosynthesis of Oligosaccharides and Glycoproteins 881
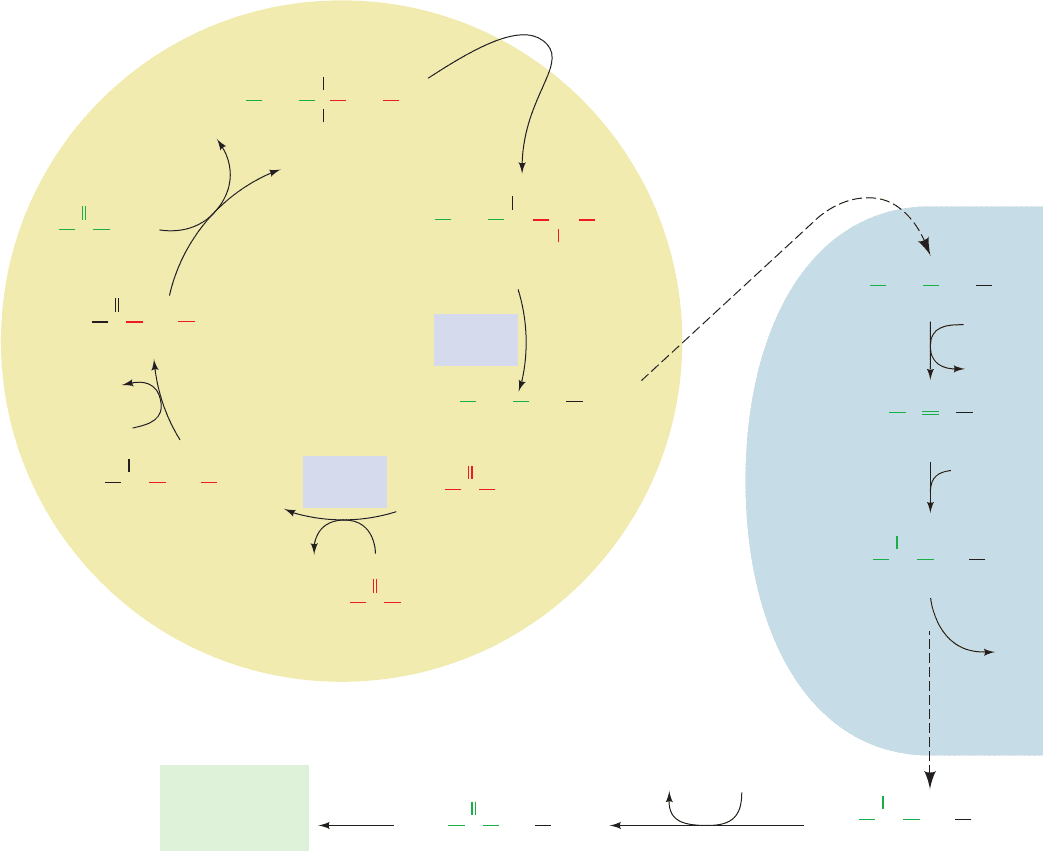
Figure 23-11 The glyoxylate cycle. The cycle results in the net
conversion of two acetyl-CoA to succinate in the glyoxysome,
which can be converted to malate in the mitochondrion for use
in gluconeogenesis. Isocitrate lyase and malate synthase, enzymes
unique to glyoxysomes (which occur only in plants), are boxed in
blue. (1) Glyoxysomal citrate synthase catalyzes the
condensation of oxaloacetate with acetyl-CoA to form citrate.
(2) Cytosolic aconitase catalyzes the conversion of citrate to
isocitrate. (3) Isocitrate lyase catalyzes the cleavage of isocitrate
to succinate and glyoxylate. (4) Malate synthase catalyzes the
COO
–
C
CH
2
–
OOC
COO
–
Citrate
OH
CH
2
COO
–
CH
CH
–
OOC
COO
–
Isocitrate
OH
CH
2
–
OOC
COO
–
CH
2
CH
2
Succinate
isocitrate
lyase
3
C
O
H COO
–
Glyoxylate
C
O
CH
3
SCoA
Acetyl-CoA
CoA
CoA
4
5
Malate
OH
CH
–
OOC
COO
–
CH
2
malate
dehydrogenase
malate
synthase
C
O
CH
2
–
OOC
COO
–
Oxaloacetate
citrate
synthase
C
O
CH
3
SCoA
Acetyl-CoA
–
OOC
COO
–
CH
2
CH
2
Succinate
–
OOC
COO
–
C
H
H
C
Fumarate
H
2
O
OH
CH
–
OOC
COO
–
Malate
CH
2
fumarase
succinate
dehydrogenase
OH
CH
–
OOC
COO
–
Malate
CH
2
C
O
CH
2
–
OOC
COO
–
Oxaloacetate
Gluconeogenesis
NADH+H
+
NAD
+
Glyoxysome MitochondrionCytosol
aconitase
1
6
citric
acid
cycle
FAD
FADH
2
7
8
NADH + H
+
NAD
+
+
2
condensation of glyoxylate with acetyl-CoA to form malate.
(5) Glyoxysomal malate dehydrogenase catalyzes the oxidation
of malate to oxaloacetate, completing the cycle. (6) Succinate is
transported to the mitochondrion, where it is converted to
malate via the citric acid cycle. (7) Malate is transported to the
cytosol, where malate dehydrogenase catalyzes its oxidation to
oxaloacetate, which can then be used in gluconeogenesis.
(8) Alternatively, malate can continue in the citric acid cycle,
making the glyoxylate cycle anaplerotic.
JWCL281_c23_871-900.qxd 3/24/10 11:33 AM Page 881
(Fig. 23-12).The nucleotides that participate in monosaccha-
ride transfers are UDP, GDP, and CMP; a given sugar is as-
sociated with only one of these nucleotides (Table 23-2).
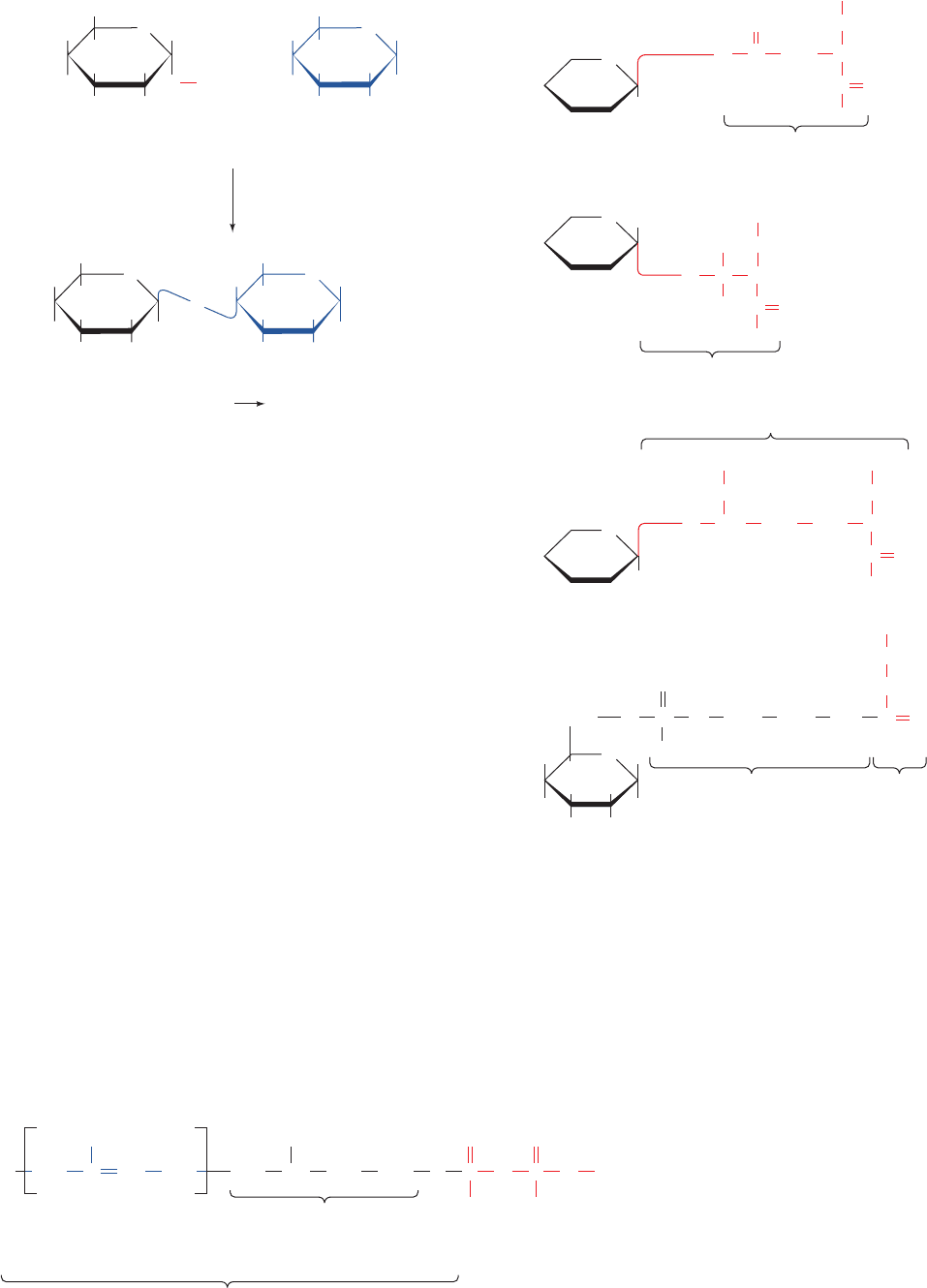
A. Lactose Synthesis
Several disaccharides are synthesized for future use as
metabolic fuels. In plants, the major fuel disaccharide is
sucrose (Section 11-2B), whose synthesis is discussed in
Section 24-3Ad. Typical of mammalian disaccharides is
lactose [-galactosyl-(1 S 4)-glucose; milk sugar], which is
synthesized in the mammary gland by lactose synthase
(Fig. 23-13). The donor sugar is UDP–galactose, which is
formed by epimerization of UDP–glucose (Section 17-5B).
The acceptor sugar is glucose.
Lactose synthase consists of two subunits:
1. Galactosyltransferase, the catalytic subunit, which
occurs in many tissues, where it catalyzes the reaction of
UDP–galactose and N-acetylglucosamine to yield N-acetyl-
lactosamine, a constituent of many complex oligosaccha-
rides (see, e.g., Fig. 23-20, Reaction 6).
2. ␣-Lactalbumin, a mammary gland protein with no
catalytic activity, which alters the specificity of galactosyl-
transferase such that it utilizes glucose as an acceptor,
rather than N-acetylglucosamine, to form lactose instead of
N-acetyllactosamine.
B. Glycoprotein Synthesis
Eukaryotic proteins destined for secretion, incorporation into
membranes, or localization inside membranous organelles
contain carbohydrates and are therefore classified as glyco-
proteins. Glycosylation and oligosaccharide processing play an
indispensable role in the sorting and the distribution of these
proteins to their proper cellular destinations. Their polypeptide
components are ribosomally synthesized and processed by
addition and modification of oligosaccharides.
The oligosaccharide portions of glycoproteins, as we
have seen in Sections 11-3C and 12-3Bc, are classified into
three groups:
1. N-Linked oligosaccharides, which are attached to
their polypeptide chain by a -N-glycosidic bond to the
side chain N of an Asn residue in the sequence Asn-X-Ser
or Asn-X-Thr, where X is any amino acid residue except
Pro (Fig. 23-14a).
2. O-Linked oligosaccharides, which are attached to
their polypeptide chain through an -O-glycosidic bond to
the side chain O of a Ser or Thr residue (Fig. 23-14b) or,
only in collagens (Section 8-2Bb), to that of a 5-hydroxyly-
sine (Hyl) residue (Fig. 23-14c).
3. Glycosylphosphatidylinositol (GPI) membrane an-
chors, which are attached to their polypeptide chain through
an amide bond between mannose-6-phosphoethanolamine
and the C-terminal carboxyl group (Fig. 23-14d).
We shall consider the synthesis of these three types of
oligosaccharides in turn.
a. N-Linked Glycoproteins Are Synthesized
in Four Stages
N-Linked glycoproteins are formed in the endoplasmic
reticulum and further processed in the Golgi apparatus.
Synthesis of their carbohydrate moieties occurs in four
stages:
1. Synthesis of a lipid-linked oligosaccharide precursor.
2. Transfer of this precursor to the side chain N of an
Asn residue on a growing polypeptide.
3. Removal of some of the precursor’s sugar units.
4. Addition of sugar residues to the remaining core
oligosaccharide.
882 Chapter 23. Other Pathways of Carbohydrate Metabolism
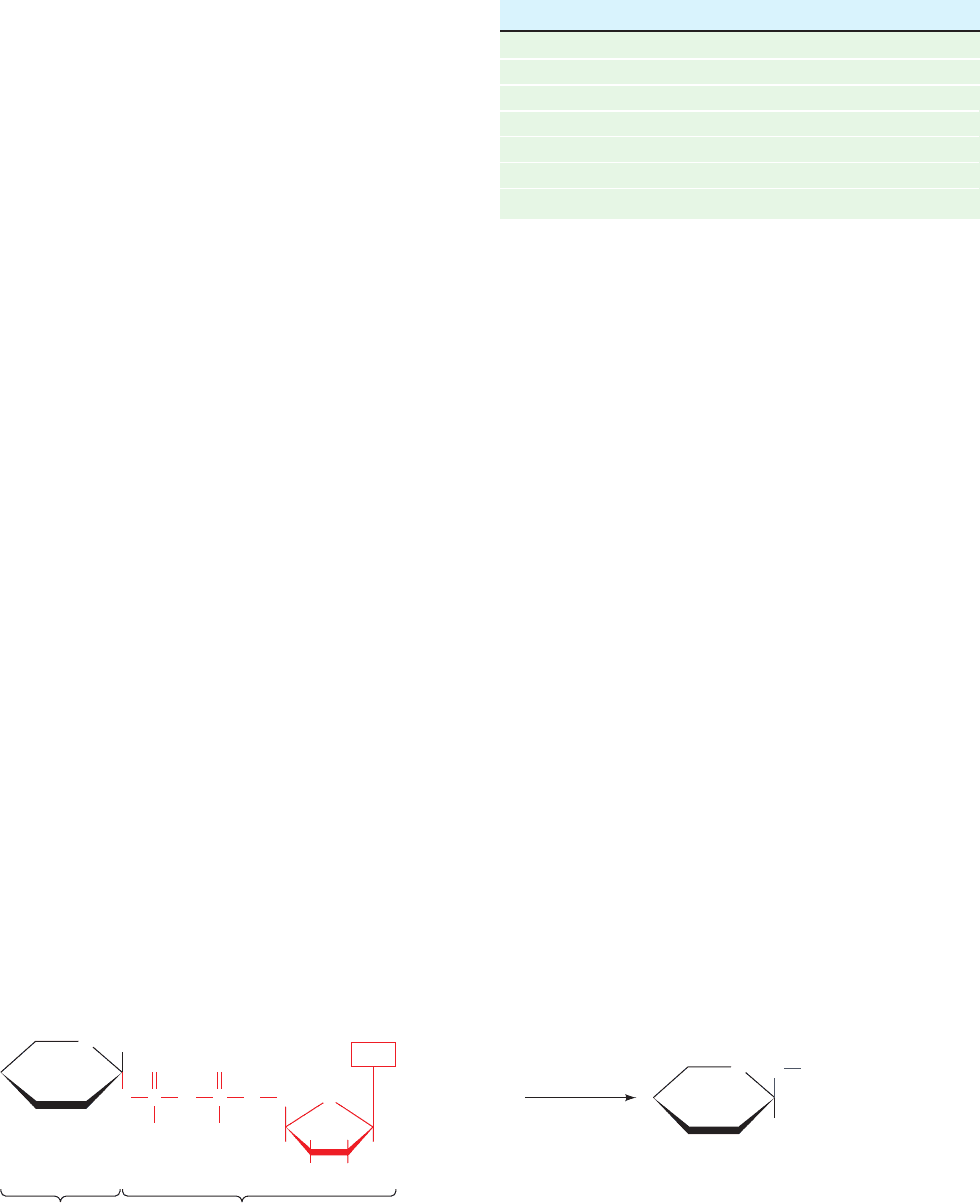
Figure 23-12 Role of nucleotide sugars. These compounds are the glycosyl
donors in oligosaccharide biosynthesis catalyzed by glycosyltransferases.
Table 23-2 Sugar Nucleotides and Their Corresponding
Monosaccharides in Glycosyltransferase
Reactions
UDP GDP CMP
N-Acetylgalactosamine Fucose Sialic acid
N-Acetylglucosamine Mannose
N-Acetylmuramic acid
Galactose
Glucose
Glucuronic acid
Xylose
glycosyl-
transferase
O
O
P
CH
2
O
HH
H
H
ROH
OH OH
1
Base
O
Donor
sugar
H
1
O
O
H
R
Nucleotide sugar
Oligosaccharide
+
+
O
–
O O
O
P
O
–
Nucleoside
diphosphate
Acceptor
sugar
Nucleoside
diphosphate
JWCL281_c23_871-900.qxd 3/24/10 11:33 AM Page 882
We shall discuss these stages in order.
b. N-Linked Oligosaccharides Are Constructed on
Dolichol Carriers
N-Linked oligosaccharides are initially synthesized as
lipid-linked precursors. The lipid component in this process
is dolichol, a long-chain polyisoprenol of 14 to 24 isoprene
units (17–21 units in animals and 14–24 units in fungi and
plants; isoprene units are C
5
units with the carbon skeleton
of isoprene; Section 25-6A), which is linked to the oligosac-
charide precursor via a pyrophosphate bridge (Fig. 23-15).
Dolichol apparently anchors the growing oligosaccharide to
the endoplasmic reticulum membrane. Involvement of lipid-
linked oligosaccharides in N-linked glycoprotein synthesis
was first demonstrated in 1972 by Armando Parodi and Luis
Leloir,who showed that,when a lipid-linked oligosaccharide
containing [
14
C]glucose is incubated with rat liver micro-
somes (vesicular fragments of isolated endoplasmic reticu-
lum), the radioactivity becomes associated with protein.
c. N-Linked Glycoproteins Have a Common
Oligosaccharide Core
The pathway of dolichol-PP-oligosaccharide synthesis
involves stepwise addition of monosaccharide units to the
Section 23-3. Biosynthesis of Oligosaccharides and Glycoproteins 883
H
OH
CH
2
OH
H
H
HO
OH
H
H OH
O
Glucose
H
OH
CH
2
OH
H
H
HO
O
H
H OH
O
UDP–galactose
+
UDP
lactose synthase
H
OH
CH
2
OH
H
H
OH
H
H OH
O
Lactose
H
OH
CH
2
OH
H
H
HO
H
H OH
O
O
+ UDP
4)-glucose]
[-galactosyl-(1
Figure 23-13 Lactose synthase. This enzyme catalyzes the
formation of lactose from UDP–galactose and glucose.
Figure 23-15 Dolichol
pyrophosphate glycoside. The
carbohydrate precursors of N-
linked glycosides are synthesized
as dolichol pyrophosphate
glycosides. Dolichols are long-
chain polyisoprenols (n 14–24)
in which the -isoprene unit is
saturated.
Figure 23-14 Types of saccharide–polypeptide linkages in
glycoproteins. (a) An N-linked glycosidic bond to an Asn residue
in the sequence Asn-X-Ser/Thr. (b) An O-linked glycosidic bond
to a Ser (or Thr) residue. (c) An O-linked glycosidic bond to
a 5-hydroxylysine residue in collagen. (d) An amide bond
between the C-terminal amino acid of a protein and the
phosphoethanolamine bridge to the 6 position of mannose in the
glycophosphatidylinositol (GPI) anchor.The X group (green)
denotes the rest of the GPI anchor (Fig. 12-30).
Asn
Ser (Thr)
Phosphoethanolamine C-terminal
residue
H
O
H
O
N
H
NH
C
O C
H
H
CH
O
C
CH
2
(CH
3
)
CH
NH
CO
O
O
O
(a)
(b)
(c)
CH
2
CH
2
CH
2
NH
H
OP O
O
O
–
O
(d)
H
HO
OHHO
H
H
H
O
X
C
CHR
NH
Mannose
5-Hydroxylysine
H
O CH
CH
2
NH
3
C
CH
NH
O
CH
2
CH
2
+
O
CH
2
CH
3
CH
3
PO
CH
2
CHCH
2
CH
2
CHCH CH
2
O carbohydrate
O
–
O
P
O
O
–
n
Isoprene unit
Dolichol
Saturated
-isoprene
unit
JWCL281_c23_871-900.qxd 6/8/10 9:43 AM Page 883
884 Chapter 23. Other Pathways of Carbohydrate Metabolism
growing glycolipid by specific glycosyltransferases to form a
common “core” structure. Each monosaccharide unit is
added by a unique glycosyltransferase (Fig. 23-16). For ex-
ample, in Reaction 2 of Fig. 23-16, five mannosyl units are
added through the action of five different mannosyltrans-
ferases, each with a different oligosaccharide-acceptor
specificity. The oligosaccharide core, the product of Reac-
tion 9 in Fig. 23-16, has the composition (N-acetylglu-
cosamine)
2
(mannose)
9
(glucose)
3
.
Although nucleotide sugars are the most common
monosaccharide donors in glycosyltransferase reactions,
several mannosyl and glucosyl residues are transferred to
the growing dolichol-PP-oligosaccharide from their cor-
responding dolichol-P derivatives. This requirement for
dolichol-P-mannose was discovered by Stuart Kornfeld,
who found that mutant mouse lymphoma cells (lym-
phoma is a type of cancer) that are unable to synthesize
normal lipid-linked oligosaccharides formed a defective,
smaller glycolipid. These cells contain all the requisite
glycosyltransferases but are unable to synthesize
dolichol-P-mannose (Reaction 4 in Fig. 23-16 is blocked).
When this substance is supplied to the mutant cells, man-
nosyl units are added to the defective dolichol-PP-
oligosaccharide.
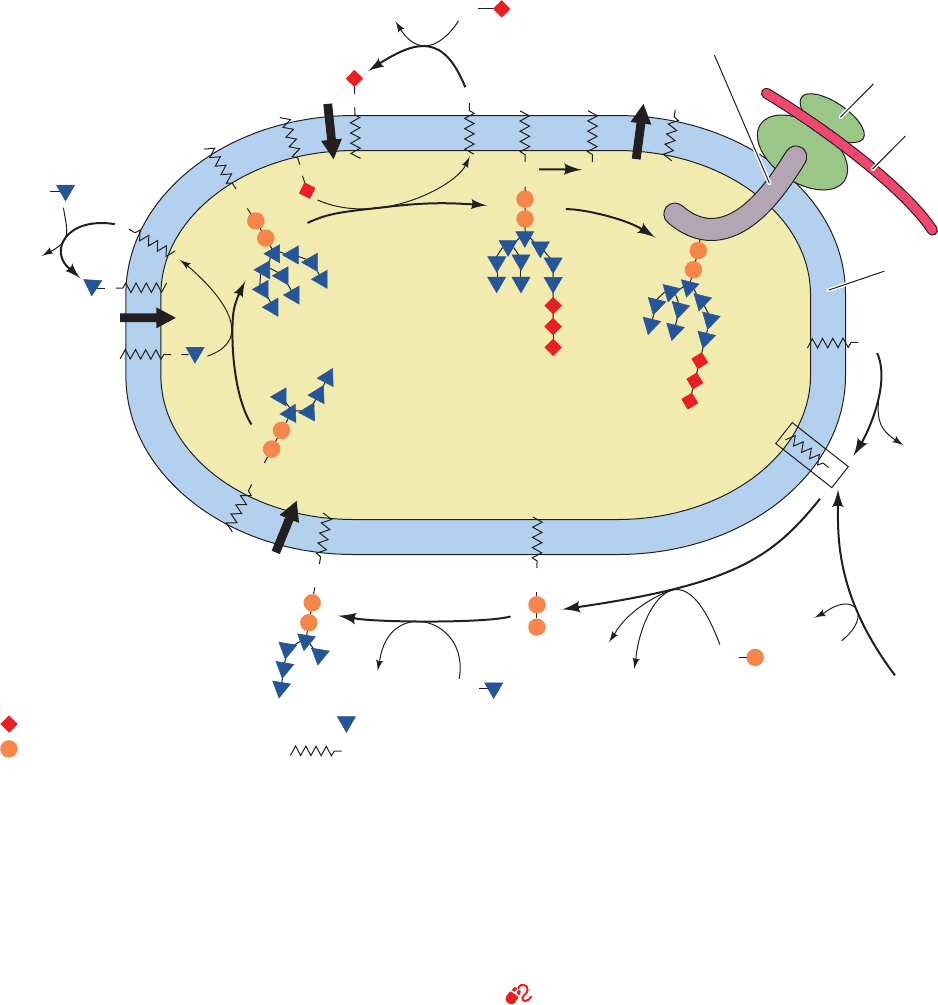
Figure 23-16 Pathway of dolichol-PP-oligosaccharide synthesis.
(1) Addition of N-acetylglucosamine-1-P and a second
N-acetylglucosamine to dolichol-P. (2) Addition of five mannosyl
residues from GDP–mannose in reactions catalyzed by five
different mannosyltransferases. (3) Membrane translocation of
dolichol-PP-(N-acetylglucosamine)
2
(mannose)
5
to the lumen of
the endoplasmic reticulum (ER). (4) Cytosolic synthesis of
dolichol-P-mannose from GDP–mannose and dolichol-P.
(5) Membrane translocation of dolichol-P-mannose to the lumen
of the ER. (6) Addition of four mannosyl residues from
dolichol-P-mannose in reactions catalyzed by four different
mannosyltransferases. (7) Cytosolic synthesis of dolichol-P-
P
PP
PP
PP
PP
PP
PP
PP
PP
P
P
P
P
P
P
i
12
13
1
2
3
2 UDP
Dolichol
5 GDP
5 GDP
1 UMP
1 UDP
CDP
CTP
4 GDP
3 UDP3 UDP
4 GDP
P
4
8
6
5
9
10
7
11
Lumen
Cytosol
Endoplasmic
reticulum
membrane
Endoplasmic Reticulum
mRNA
Ribosome
Nascent
polypeptide
= N-Acetylglucosamine
= Mannose
= Glucose
= Dolichol phosphate
P
-
A
s
n
-
X
-
S
e
r
/
T
h
r
-
glucose from UDP–glucose and dolichol-P. (8) Membrane
translocation of dolichol-P-glucose to the lumen of the ER.
(9) Addition of three glucosyl residues from dolichol-P-glucose.
(10) Transfer of the oligosaccharide from dolichol-PP to the
polypeptide chain at an Asn residue in the sequence Asn-X-
Ser/Thr, releasing dolichol-PP. (11) Translocation of dolichol-PP
to the cytoplasmic surface of the ER membrane. (12) Hydrolysis
of dolichol-PP to dolichol-P. (13) Dolichol-P can also be formed
by phosphorylation of dolichol by CTP. [Modified from Abeijon,
C. and Hirschberg, C.B., Trends Biochem. Sci. 17, 34 (1992).]
See the Animated Figures
JWCL281_c23_871-900.qxd 3/24/10 11:33 AM Page 884
d. Dolichol-PP-Oligosaccharide Synthesis Involves
Topological Changes of the Intermediates
Reactions 1, 2, 4, and 7 of Fig. 23-16 all occur on the cyto-
plasmic side of the endoplasmic reticulum (ER) membrane.
This was determined by using “right-side-out” rough ER
vesicles and showing that various membrane-impermeant
reagents can disrupt one or another of these reactions. Re-
actions 6, 9, and 10 occur in the lumen of the ER as judged
by the inability of concanavalin A, a lectin (carbohydrate-
binding protein), to bind to the products of these reactions
until the membrane is permeabilized. The (mannose)
5
(N-acetylglucosamine)
2
-PP-dolichol product of Reaction
2, the dolichol-P-mannose product of Reaction 4, and the
dolichol-P-glucose product of Reaction 7 must therefore
be translocated across the ER membrane (Reactions 3, 5,
and 8) such that they extend from its luminal surface in
order for the synthesis of N-linked oligosaccharides to
continue.The translocations are mediated by specific ATP-
independent flippases.
e. N-Linked Oligosaccharides Are Cotranslationally
Added to Proteins
Vesicular stomatitis virus (VSV), which infects cattle, pro-
ducing influenza-like symptoms, provides an excellent model
system for studying N-linked glycoprotein processing. The
VSV coat consists of host-cell membrane in which a single
viral glycoprotein, the VSV G-protein (not to be confused
with the GTPases involved in signal transduction; Chap-
ter 19), is embedded. Since a viral infection almost totally
usurps an infected cell’s protein synthesizing machinery, a
VSV-infected cell’s Golgi apparatus, which normally con-
tains hundreds of different types of glycoproteins, contains
virtually no other glycoprotein but G-protein. Consequently,
the maturation of the G-protein is relatively easy to follow.
Of the Asn-X-Ser/Thr sites in mature eukaryotic pro-
teins, 70 to 90% are N-glycosylated. Studies of VSV-
infected cells indicate that the transfer of the lipid-linked
oligosaccharide to a polypeptide chain occurs while the
polypeptide chain is still being synthesized. Structural pre-
dictions (Section 9-3A), together with glycosylation studies
of model polypeptides, suggest that the amino acid sequences
flanking known N-glycosylation sites occur at turns or
loops in which Asn’s backbone N¬H group is hydrogen
bonded to the Ser/Thr hydroxyl O atom (Fig. 23-17a). This
explains why Pro cannot occupy the X position; it would
prevent Asn-X-Ser/Thr from assuming the putative re-
quired hydrogen bonded conformation.
VSV G-protein is N-glycosylated by oligosaccharyltrans-
ferase (OST), a membrane-bound, ⬃300-kD, 8-subunit
enzyme that recognizes the amino acid sequence Asn-X-
Ser/Thr (Fig. 23-16, Reaction 10). Ernst Bause has proposed
a catalytic mechanism for OST in which an enzyme base
Section 23-3. Biosynthesis of Oligosaccharides and Glycoproteins 885
(b)
O
NH
CH
2
H
N
O
C
H
2
B
B
–
Enzyme
Enzyme
Dol-PP–OH
Inactive, doubly
labeled, enzyme
Epoxyethyl-Gly
O
O
NH
NH
O
O
H
2
C
H
H
N
H
N
CH
R
O
HN
S
a
c
O
NH
O
O
H
2
C
H
H
N
CH
R
O
HN
Asn
Dol-PP
..
S
a
c
(a)
O
O
NH
NH
O
O
H
2
C
H
H
H
H
N
H
N
CH
R
O
O
O
HN
B
B
–
N-linked
glycoprotein
Enzyme
Enzyme
ⴙ
Asn
Dol-PP
Dol-PP–OH
CH
3
Thr
..
S
a
c
O
NH
NH
O
O
H
2
C
H
H
N
H
N
CH
R
O
HN
CH
3
S
a
c
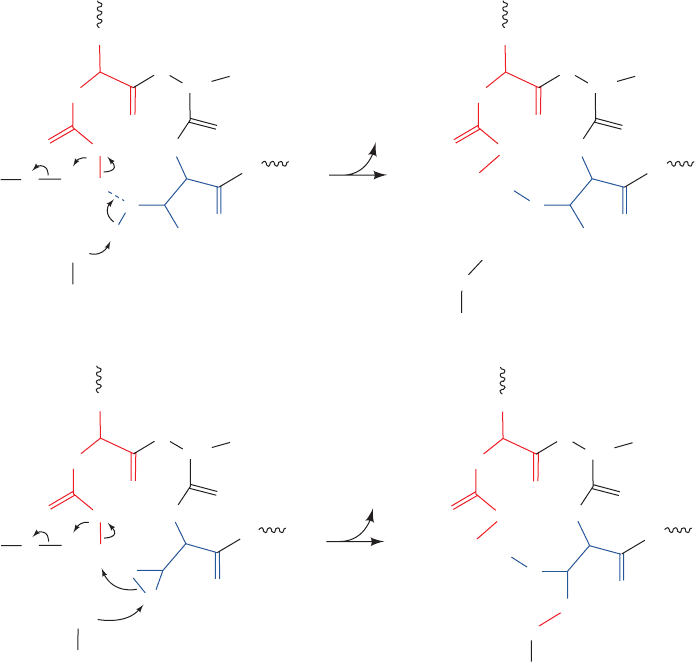
Figure 23-17 The
oligosaccharyltransferase
(OST) reaction. (a) The
Asn-X-Thr component of a
hexapeptide model substrate
forms a ring that is closed by
a hydrogen bond from the
Asn amide group to the Thr
hydroxyl group. A base on the
enzyme facilitates the
nucleophilic displacement of
dolichol pyrophosphate from
the oligosaccharide (Sac) by
the amide nitrogen. (b) The
inactivation of the OST by
reacting it with a hexapeptide
containing Asn-Gly-
epoxyethylGly in the presence
of dolichol-PP-oligosaccharide.
This chemically labels the
base with the oligopeptide to
which the oligosaccharide has
become covalently linked.
JWCL281_c23_871-900.qxd 6/8/10 9:43 AM Page 885
abstracts a proton from the Ser/Thr hydroxyl group, which in
turn abstracts a proton from the Asn NH
2
group, thereby
promoting its nucleophilic attack on the oligosaccharide
(Sac), which then displaces the dolichol pyrophosphate (Fig.
23-17a). This mechanism is supported by the observation
that reacting the OST with dolichol-PP-oligosaccharide and
a hexapeptide model substrate containing the sequence
Asn-X-epoxyethylGly (rather than Asn-X-Ser/Thr) irre-
versibly inactivates the enzyme by covalently linking it to
the now glycosylated hexapeptide (Fig. 23-17b).
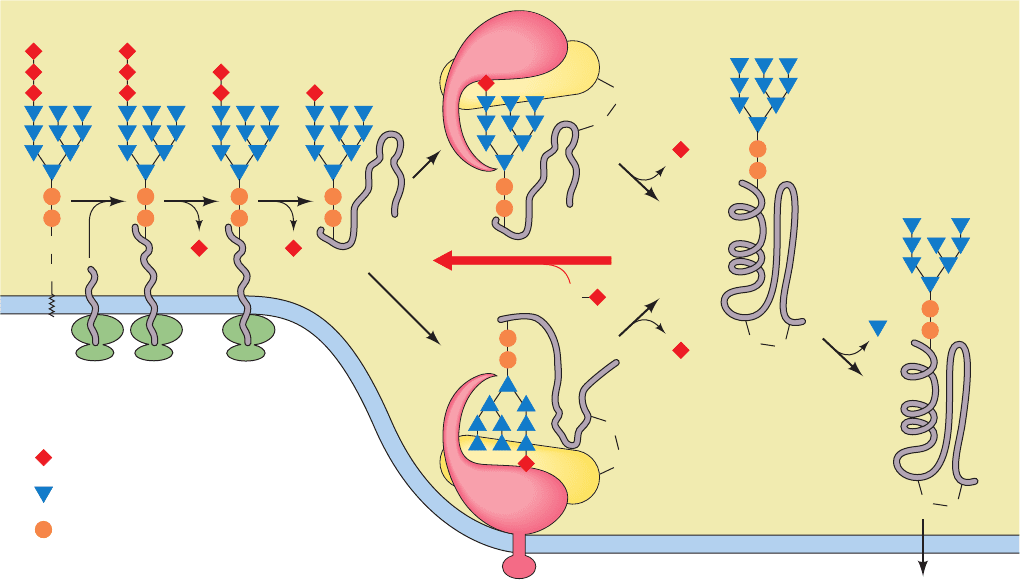
f. The Calnexin/Calreticulin Cycle Facilitates
Glycoprotein Folding
The processing of an N-linked core oligosaccharide
begins in the endoplasmic reticulum by the enzymatic trim-
ming (removal) of its three glucose residues (Fig. 23-18, Re-
actions 2 and 3) and one of its mannose residues (Fig. 23-18,
Reaction 4) before the protein has folded to its native
conformation. This is not a straightforward process, how-
ever, because UDP–glucose:glycoprotein glucosyltrans-
ferase (GT), a 1513-residue soluble protein, reglucosylates
the oligosaccharides of partially folded glycoproteins, a reac-
tion that reverses the removal of the last of the three glucose
residues by glucosidase II (Fig. 23-18, Reaction 3).This futile
cycle (most glycoproteins undergo reglucosylation at least
once) is part of a chaperone-mediated glycoprotein folding
process called the calnexin/calreticulin cycle. Calnexin
(CNX; ⬃570 residues), which is membrane bound, and cal-
reticulin (CRT; ⬃400 residues), its soluble homolog, are ER-
resident lectins that bind partially folded glycoproteins bear-
ing a monoglucosylated oligosaccharide in a way that
protects the glycoprotein from degradation and premature
transfer to the Golgi apparatus. If the glycoprotein is re-
leased and deglucosylated before it has correctly folded, GT,
which recognizes only non-native glycoproteins, reglucosy-
lates it so that the CNX/CRT cycle can repeat. CNX and
CRT both also bind ERp57, a 481-residue thiol oxidoreduc-
tase homologous to protein disulfide isomerase (PDI; Sec-
tion 9-2A). While the partially folded glycoprotein is bound
to the complex, ERp57 catalyzes disulfide interchange reac-
tions to facilitate the formation of the correctly paired disul-
fide bonds. The CNX/ ERp57 and CRT/ERp57 complexes
are therefore responsible for the correct folding and disul-
fide bond formation of the glycoproteins in the ER.The im-
portance of this process is demonstrated by the observation
that knockout mice lacking the gene for CRT die in utero.
The X-ray structure of the luminal domain of calnexin
(residues 61–458), determined by Miroslaw Cygler, reveals
a most unusual structure (Fig. 23-19): a compact globular
domain (residues 61–262 and 415–458) from which extends
886 Chapter 23. Other Pathways of Carbohydrate Metabolism
Figure 23-18 The calnexin/calreticulin cycle for glycoprotein
folding in the endoplasmic reticulum. The reactions are catalyzed
by: (1) oligosaccharyltransferase (OST); (2) -glucosidase I; (3)
-glucosidase II, UDP–glucose:glycoprotein glucosyltransferase
P
S
S
= Mannose
= N-Acetylglucosamine
= Glucose
2
3
4
3
31
S
P
UDP
GT
S
S
S
ERp57
ERp57
CNX
S
S
Transfer via vesicles
to the cis Golgi network
CRT
(GT), calreticulin (CRT), calnexin (CNX), and the thiol
oxidoreductase ERp57; and (4) ER -1,2-mannosidase. [After
Helenius,A. and Aebi, M., Science 291, 2367 (2001).]
JWCL281_c23_871-900.qxd 6/8/10 9:43 AM Page 886
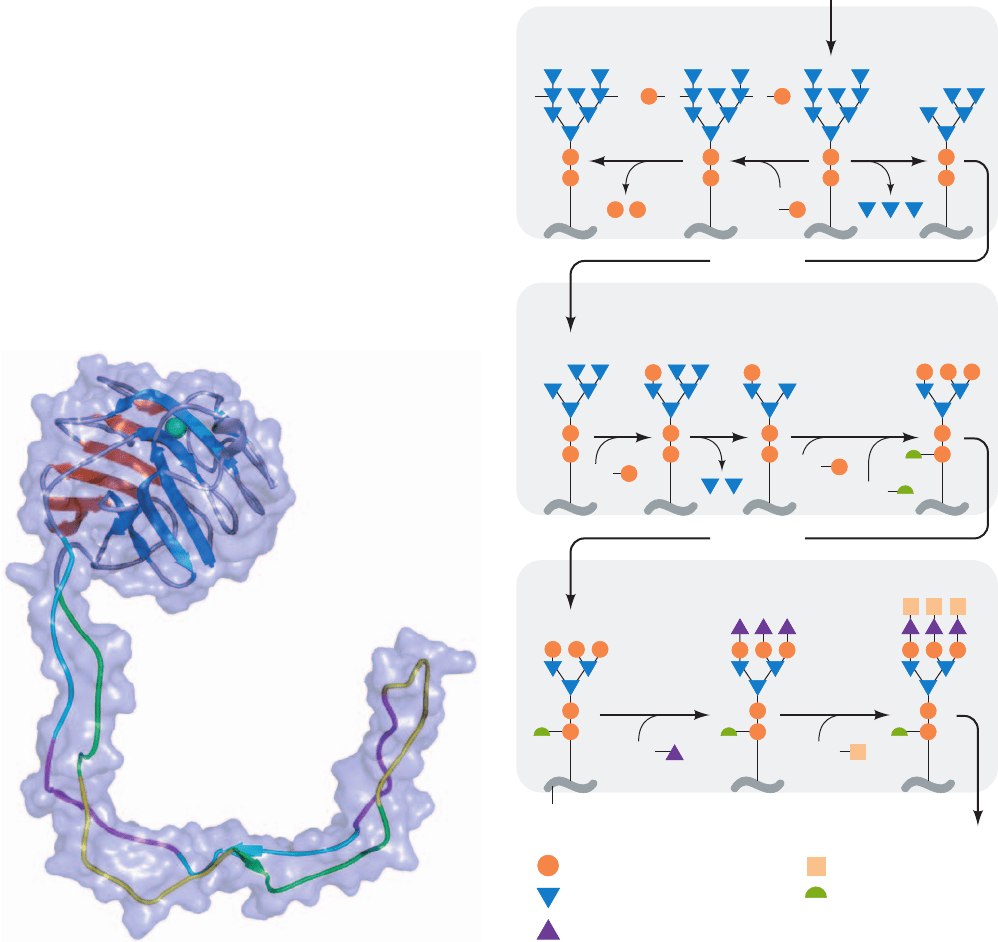
a 145-Å-long arm (residues 270–414).The globular domain
forms a sandwich of a 6-stranded and a 7-stranded antipar-
allel sheet that binds a Ca
2
ion and which resembles
legume lectins such as concanavalin A (Fig. 8-40). This do-
main binds glucose on its concave (blue) surface, which is
lined by hydrogen bonding groups that model building
suggests binds the (glucose)
1
(mannose)
3
portion of cal-
nexin’s natural (glucose)
1
(mannose)
9
substrate. The long
arm, which consists of an extended hairpin, is known as the
P domain because it has four copies each of two different
Pro-rich motifs arranged in the sequence 11112222, with
each ⬃18-residue motif 1 in antiparallel association with
an ⬃14-residue motif 2 on the opposite strand of the hair-
pin. Each of these motif pairs has a similar structure, with
its conserved residues maintaining identical interactions in
each pair.The P domain has been shown to form the bind-
ing site for ERp57 in both calnexin and calreticulin.
g. Glycoprotein Processing is Completed
in the Golgi Apparatus
Once a glycoprotein has folded to its native conforma-
tion and ER -1,2-mannosidase has removed one of its
mannosyl residues (Fig. 23-18, Step 4), the glycoprotein is
transported, in membranous vesicles, to the Golgi appara-
tus, where it is further processed (Fig. 23-20).The Golgi ap-
paratus (Fig. 12-58), as we discussed in Section 12-4C, con-
sists of, from opposite the ER outward, the cis Golgi
Section 23-3. Biosynthesis of Oligosaccharides and Glycoproteins 887
Figure 23-19 X-ray structure of the luminal portion of canine
calnexin. The protein is drawn in ribbon form embedded in its
semitransparent molecular surface. The 6- and 7-stranded
antiparallel sheets of its globular domain are colored orange
and blue, with its remaining portions gray and its bound Ca
2
ion
represented by a blue-green sphere. In the P domain, motifs 1 are
alternately colored green and yellow and motifs 2 are alternately
colored magenta and cyan. [Based on an X-ray structure by
Miroslaw Cygler, Biotechnology Research Institute, NRC,
Montreal, Quebec, Canada. PDBid 1JHN.]
Figure 23-20 Oligosaccharide processing of VSV G-protein in
the Golgi network. The reactions are catalyzed by: (1) Golgi
-mannosidase I, (2) N-acetylglucosaminyltransferase I, (3)
Golgi -mannosidase II, (4) N-acetylglucosaminyltransferase II,
(5) fucosyltransferase, (6) galactosyltransferase, and
(7) sialyltransferase. Lysosomal proteins are modified by:
(I) N-acetylglucosaminyl phosphotransferase and
(II) N-acetylglucosamine-1-phosphodiester
-N-acetylglucosaminidase. [Modified from Kornfeld, R. and
Kornfeld, S., Annu. Rev. Biochem. 54, 640 (1985).]
II 1I
P P P P
UDP
Via vesicles from the
Endoplasmic Reticulum
Exit
cis Golgi
2
UDP
2UDP
GDP
3 45
Via vesicles
medial Golgi
3CMP
7
Via vesicles
trans Golgi
Polypeptide
3UDP
6
= Galactose
= N-Acetylglucosamine
= Mannose
= Sialic acid
= L-Fucose
JWCL281_c23_871-900.qxd 3/24/10 11:33 AM Page 887
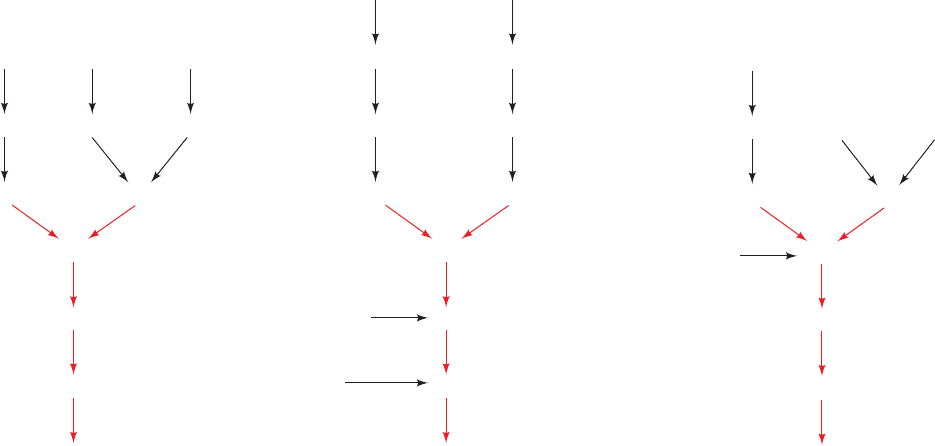
network, through which glycoproteins enter the Golgi ap-
paratus; a stack of at least three different types of sacs,
the cis, medial, and trans cisternae; and the trans Golgi net-
work, through which proteins exit the Golgi apparatus.
Glycoproteins traverse the Golgi stack, from the cis to the
medial to the trans cisternae, each of which, as shown by
James Rothman and Kornfeld, contains different sets of
glycoprotein processing enzymes. As this occurs, mannose
residues are trimmed from each oligosaccharide group and
N-acetylglucosamine, galactose, fucose, and/or sialic acid
residues are added to complete the processing of the glyco-
protein (Fig. 23-20; Reactions 1–7). The glycoproteins are
then sorted in the trans Golgi network for transport to
their respective cellular destinations via membranous vesi-
cles (Sections 12-4C and 12-4D).
There is enormous diversity among the different
oligosaccharides of N-linked glycoproteins, as is indicated,
for example, in Fig. 11-32c. Indeed, even glycoproteins with
a given polypeptide chain exhibit considerable microhetero-
geneity (Section 11-3C), presumably as a consequence of
incomplete glycosylation and lack of absolute specificity
on the part of glycosyltransferases and glycosylases.
The processing of all N-linked oligosaccharides is iden-
tical through Reaction 4 of Fig. 23-18, so that all of them
have a common (N-acetylglucosamine)
2
(mannose)
3
core
(five “noncore” mannose residues are subsequently trimmed
from VSV G-protein; Fig. 23-20, Reactions 1 and 3). The
diversity of the N-linked oligosaccharides therefore arises
through divergence from this sequence after Fig. 23-20,
Reaction 3. The resulting oligosaccharides are classified
into three groups:
1. High-mannose oligosaccharides (Fig. 23-21a), which
contain 2 to 9 mannose residues appended to the common
pentasaccharide core (red residues in Fig. 23-21).
2. Complex oligosaccharides (Fig. 23-21b), which con-
tain variable numbers of N-acetyllactosamine units as well
as sialic acid and/or fucose residues linked to the core.
3. Hybrid oligosaccharides (Fig. 23-21c), which contain
elements of both high-mannose and complex chains.
It is unclear how different types of oligosaccharides are
related to the functions and/or final cellular locations of
their glycoproteins. Lysosomal glycoproteins, however, ap-
pear to be of the high-mannose variety.
h. Inhibitors Have Aided the Study
of N-Linked Glycosylation
Elucidation of the events in the glycosylation process
has been greatly facilitated through the use of inhibitors
that block specific glycosylation enzymes. Two of the most
useful are the antibiotics tunicamycin (Fig. 23-22a), a hy-
drophobic analog of UDP–N-acetylglucosamine, and baci-
tracin (Fig. 23-23), a cyclic polypeptide. Both were discov-
ered because of their ability to inhibit bacterial cell wall
biosynthesis, a process that also involves the participation
of lipid-linked oligosaccharides. Tunicamycin blocks the
formation of dolichol-PP-oligosaccharides by inhibiting
the synthesis of dolichol-PP-N-acetylglucosamine from
dolichol-P and UDP–N-acetylglucosamine (Fig. 23-16, Re-
action 1). Tunicamycin resembles an adduct of these reac-
tants (Fig. 23-22b) and, in fact, binds to the enzyme with a
dissociation constant of 7 ⫻ 10
⫺9
M.
888 Chapter 23. Other Pathways of Carbohydrate Metabolism
Man Man Man
Man
Man
Man
Man
ManMan
Man Man
Man
α 1,2
α 1,2 α 1,3
α 1,3 α 1,6
α 1,6
α 1,2
α 2,3, or 6 α 2,3, or 6
β 1,4
β 1,4
β 1,2
β 1,4
β 1,4
β 1,2
β 1,4
β 1,4
α 1,6
α 1,6
α 1,3
α 1,3
α 1,3
α 1,6
α 1,6
β 1,4
α 1,2
SA SA
GalGal
GlcNAc GlcNAc
Man
Man Man
Man
Man
β 1,2
β 1,4
Gal
GlcNAc
β 1,4
β 1,4
GlcNAc
GlcNAc
GlcNAc
GlcNAc
Asn Asn
GlcNAc
GlcNAc
Asn
–
+
GlcNAc
β1,4
GlcNAc
–
+
Fuc
High mannose Complex Hybrid
(a) (b)
(c)
Figure 23-21 Types of N-linked oligosaccharides. Typical
primary structures of (a) high-mannose, (b) complex, and
(c) hybrid N-linked oligosaccharides.The pentasaccharide core
common to all N-linked oligosaccharides is indicated in red.
[After Kornfeld, R. and Kornfeld, S., Annu. Rev. Biochem. 54,
633 (1985).]
JWCL281_c23_871-900.qxd 7/2/10 12:03 PM Page 888
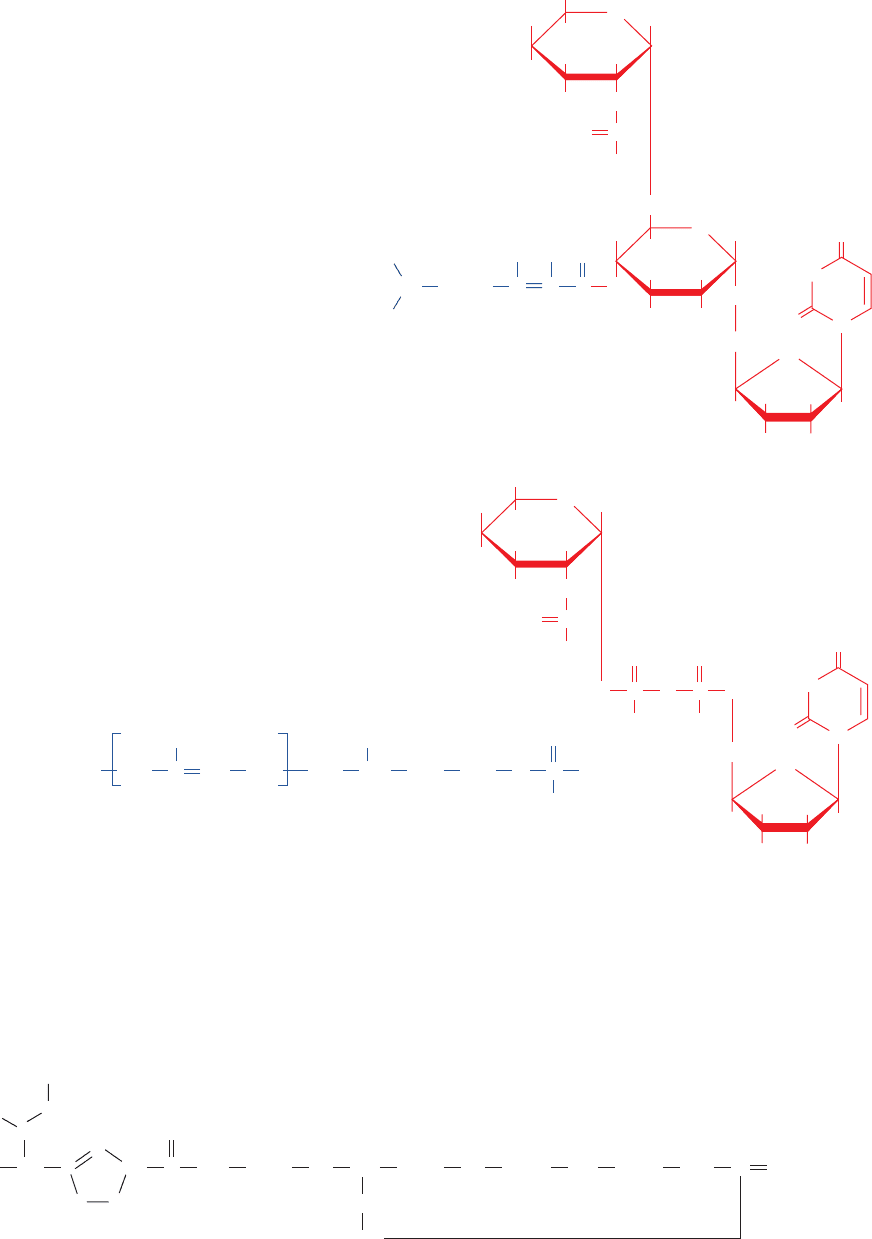
Bacitracin forms a complex with dolichol-PP that in-
hibits its dephosphorylation (Fig. 23-16, Reaction 12),
thereby preventing glycoprotein synthesis from lipid-
linked oligosaccharide precursors. Bacitracin is clinically
useful because it destroys bacterial cell walls but does not
affect animal cells because it cannot cross cell membranes
(bacterial cell wall biosynthesis is an extracellular
process).
Section 23-3. Biosynthesis of Oligosaccharides and Glycoproteins 889
(a)
(b)
H
OH
HN
N
H
O
O
O
H
OH
H
H
N
H
CCC
OHH
CH (CH
2
)
n
H
3
C
H
H
3
C
O
H
OH
OH
H
H
O
CHOH
CH
2
H
Tunicamycin
n = 8,9,10, or 11
H
HN
N
O
O
H
OH
OH
H
H
O
OC
NH
H
H
OH
H
O
HO
HH
CH
3
CH
2
OH
O
O
–
O
P
O O
CH
2
P
O
O
–
O
O
–
OC
NH
H
H
OH
H
O
HO
HH
CH
3
CH
2
OH
CH
2
CCHCH
2
CH
2
CH
2
O P O
–
CHCH
2
CH
3
CH
3
+
Dolichol phosphate
UDP-N-Acetylglucosamine
n
Bacitracin
OCHis
(CH
2
)
4
AsnD-Asp
CH
CH
3
D-PheIleD-OrnIle
NH
CysIle
D-Glu LysLeuC
S
C
N
CHCHH
3
N
H
3
C
CH
2
CH
2
O
+
Figure 23-22 Chemical structure of tunicamycin. The
structure of (a) the glycosylation inhibitor tunicamycin is
compared to that of (b) dolichol-P UDP–N-
acetylglucosamine.
Figure 23-23 Chemical structure of bacitracin. Note that this
dodecapeptide has four
D-amino acid residues and two unusual
intrachain linkages.“Orn” represents the nonstandard amino
acid residue ornithine (Fig. 26-7).
JWCL281_c23_871-900.qxd 3/24/10 11:33 AM Page 889
i. O-Linked Oligosaccharides Are Post-
Translationally Formed
The study of the biosynthesis of mucin, an O-linked gly-
coprotein secreted by the submaxillary salivary gland, indi-
cates that O-linked oligosaccharides are synthesized in the
Golgi apparatus by serial addition of monosaccharide units
to a completed polypeptide chain (Fig.23-24).Synthesis starts
with the transfer of N-acetylgalactosamine (GalNAc) from
UDP–GalNAc to a Ser or Thr residue on the polypeptide by
GalNAc transferase. In contrast to N-linked oligosaccha-
rides, which are transferred to an Asn in a specific amino
acid sequence, the O-glycosylated Ser and Thr residues are
not members of any common sequence. Rather, it appears
that the location of glycosylation sites is specified only by
the secondary or tertiary structure of the polypeptide. Gly-
cosylation continues with stepwise addition of galactose,
sialic acid, N-acetylglucosamine, and/or fucose by the corre-
sponding glycosyltransferases.
j. Oligosaccharides on Glycoproteins Act as
Recognition Sites
Glycoproteins that are synthesized in the endoplasmic
reticulum and processed in the Golgi apparatus are targeted
for secretion, insertion into cell membranes, or incorpora-
tion into cellular organelles such as lysosomes. This suggests
that oligosaccharides serve as recognition markers for this
sorting process. For example, the study of I-cell disease (Sec-
tion 12-4Cg) demonstrated that in glycoprotein enzymes
destined for the lysosome, a mannose residue is converted to
mannose-6-phosphate (M6P) in the cis cisternae of the
Golgi. The process involves two enzymes (Fig. 23-20, Reac-
tions I and II), which are thought to recognize lysosomal
protein precursors by certain structural features on these
proteins rather than a specific amino acid sequence. In the
trans Golgi network, M6P-bearing glycoproteins are sorted
into lysosome-bound coated vesicles through their specific
binding to one of two M6P receptors, one of which is a 275-
kD membrane glycoprotein called the M6P/IGF-II receptor
(because it has been found that this M6P receptor and the
insulinlike growth factor II receptor are the same protein).
Individuals with I-cell disease lack the enzyme catalyzing
mannose phosphorylation (Fig. 23-20, Reaction I), resulting
in the secretion of the normally lysosome-resident enzymes.
ABO blood group antigens (Section 12-3E) are O-linked
glycoproteins.Their characteristic oligosaccharides are com-
ponents of both cell-surface lipids and of proteins that occur
in various secretions such as saliva. These oligosaccharides
form antibody recognition sites.
Glycoproteins are believed to mediate cell–cell recogni-
tion. For example, an O-linked oligosaccharide on a glyco-
protein that coats the mouse ovum surface (zona pellu-
cida) acts as the sperm receptor. Even when this
oligosaccharide is separated from its protein, it retains the
ability to bind mouse sperm.
k. GPI-Linked Proteins
Glycosylphosphatidylinositol (GPI) groups function to
anchor a wide variety of proteins to the exterior surface of
the eukaryotic plasma membrane, thus providing an alter-
native to transmembrane polypeptide domains (Section
12-3Bc; Fig. 12-30). This anchoring results from transami-
dation of a preformed GPI glycolipid within 1 min of the
synthesis and transfer of a target protein to the ER.
Biosynthesis of the GPI core structure (Fig. 23-25a) be-
gins on the cytoplasmic side of the ER with the transfer of
890 Chapter 23. Other Pathways of Carbohydrate Metabolism
Figure 23-24 Proposed synthesis pathway for the carbohydrate
moiety of an O-linked oligosaccharide chain of canine
submaxillary mucin. SA and Fuc represent sialic acid and fucose.
Figure 23-25 GPI anchors. (Opposite) (a) The pathway of
synthesis of the tetrasaccharide core of glycophosphatidylinositol
(GPI).The following enzymes and steps are involved: (1) UDP–
GlcNAc:PI 1 S 6 N-acetylglucosaminyltransferase complex,
(2) GlcNAc–PI de-N-acetylase, (3) inositol acyltransferase,
(4) Dol-P-Man:GlcN–PI/GlcN–(acyl)PI 1 S 4 mannosyltrans-
ferase (MT-I), (5) an ethanolamine phosphotransferase, (6) Dol-
P-Man:Man
1
GlcN–(acyl)PI 1 S 6 mannosyltransferase (MT-II),
(7) Dol-P–Man:Man
2
GlcN–(acyl)PI 1 S 2 mannosyltransferase
(MT-III), (8) lipid remodeling (replacement of the fatty acyl
groups on PI), and (9) transfer of phosphoethanolamine from
phosphatidylethanolamine to the 6-hydroxyl group of the
terminal mannose residue of the core tetrasaccharide by an
ethanolamine phosphotransferase. (b) Transamidation of the target
protein, resulting in a C-terminal amide link to the GPI anchor.
UDP
UDP
Ser
GalNAc
transferase
UDP
UDP
Ser
CMP
CMP
Ser
GDP
GDP
Ser
GalNAc
GalNAc
GalGalNAc
Gal
β 1,3
SA
GalGalNAc
SA
α 2,6
Fuc
Ser
GalGalNAc
SA
1,2
Fuc
JWCL281_c23_871-900.qxd 3/24/10 11:33 AM Page 890
