Voet D., Voet Ju.G. Biochemistry
Подождите немного. Документ загружается.

901
CHAPTER 24
Photosynthesis
1 Chloroplasts
2 Light Reactions
A. Absorption of Light
B. Electron Transport in Purple Photosynthetic Bacteria
C. Two-Center Electron Transport
D. Photophosphorylation
3 Dark Reactions
A. The Calvin Cycle
B. Control of the Calvin Cycle
C. Photorespiration and the C
4
Cycle
Life on Earth depends on the sun. Plants and cyanobacteria
chemically sequester light energy through photosynthesis, a
light-driven process in which CO
2
is “fixed” to yield carbo-
hydrates (CH
2
O).
This process, in which CO
2
is reduced and H
2
O is oxidized
to yield carbohydrates and O
2
, is essentially the reverse of
oxidative carbohydrate metabolism. Photosynthetically
produced carbohydrates therefore serve as an energy
source for the organism that produced them as well as for
nonphotosynthetic organisms that directly or indirectly
consume photosynthetic organisms. In fact, even modern
industry is highly dependent on the products of photosyn-
thesis because coal, oil, and gas (the so-called fossil fuels)
are thought to be the remains of ancient organisms. It is es-
timated that photosynthesis annually fixes ⬃10
11
tons of
carbon, which represents the storage of over 10
18
kJ of en-
ergy. Moreover, photosynthesis, over the eons, has pro-
duced the O
2
in Earth’s atmosphere (Section 1-5Cb).
The notion that plants obtain nourishment from such in-
substantial things as light and air took nearly two centuries
to develop. In 1648, the Flemish physician Jean Baptiste
van Helmont reported that growing a potted willow tree
from a shoot caused an insignificant change in the weight
of the soil in which the tree had been rooted.Although an-
other century was to pass before the law of conservation of
matter was formulated, van Helmont attributed the tree’s
weight gain to the water it had taken up. This idea was ex-
tended in 1727 by Stephen Hales, who proposed that plants
extract some of their matter from the air.
CO
2
⫹ H
2
O
¡
light
(CH
2
O) ⫹ O
2
The first indication that plants produce oxygen was
found by the English clergyman and pioneering chemist
Joseph Priestley, who reported:
Finding that candles burn very well in air in which plants had
grown a long time, and having some reason to think, that there
was something attending vegetation, which restored air that had
been injured by respiration, I thought it was possible that the
same process might also restore the air that had been injured by
the burning of candles.Accordingly, on the 17th of August,
1771, I put a sprig of mint into a quantity of air, in which a wax
candle had burned out, and found that, on the 27th of the same
month, another candle burned perfectly well in it.
Although Priestley later discovered oxygen, which he
named “dephlogisticated air,” it was Antoine Lavoisier
who elucidated its role in combustion and respiration. Nev-
ertheless, Priestley’s work inspired the Dutch physician Jan
Ingenhousz, who in 1779 demonstrated that the “purify-
ing” power of plants resides in the influence of sunlight on
their green parts. In 1782, the Swiss pastor Jean Senebier
showed that CO
2
, which he called “fixed air,” is taken up
during photosynthesis. His compatriot Nicolas-Théodore
de Saussure found, in 1804, that the combined weights of
the organic matter produced by plants and the oxygen they
evolve is greater than the weight of the CO
2
they consume.
He therefore concluded that water, the only other sub-
stance he added to his system, was also necessary for pho-
tosynthesis. The final ingredient in the overall photosyn-
thetic recipe was established in 1842 by the German
physiologist Robert Mayer, one of the formulators of the
first law of thermodynamics, who concluded that plants
convert light energy to chemical energy.
1 CHLOROPLASTS
The site of photosynthesis in eukaryotes (algae and higher
plants) is the chloroplast (Section 1-2Ag), a member of the
membranous subcellular organelles peculiar to plants
known as plastids.The first indication that chloroplasts have
a photosynthetic function was Theodor Englemann’s obser-
vation, in 1882, that small, motile, O
2
-seeking bacteria con-
gregate at the surface of the alga Spirogyra, overlying its sin-
gle chloroplast, but only while the chloroplast is illuminated.
Chloroplasts must therefore be the site of light-induced O
2
evolution, that is, photosynthesis. Chloroplasts, of which
there are 1 to 1000 per cell, vary considerably in size and
JWCL281_c24_901-939.qxd 3/25/10 12:04 PM Page 901
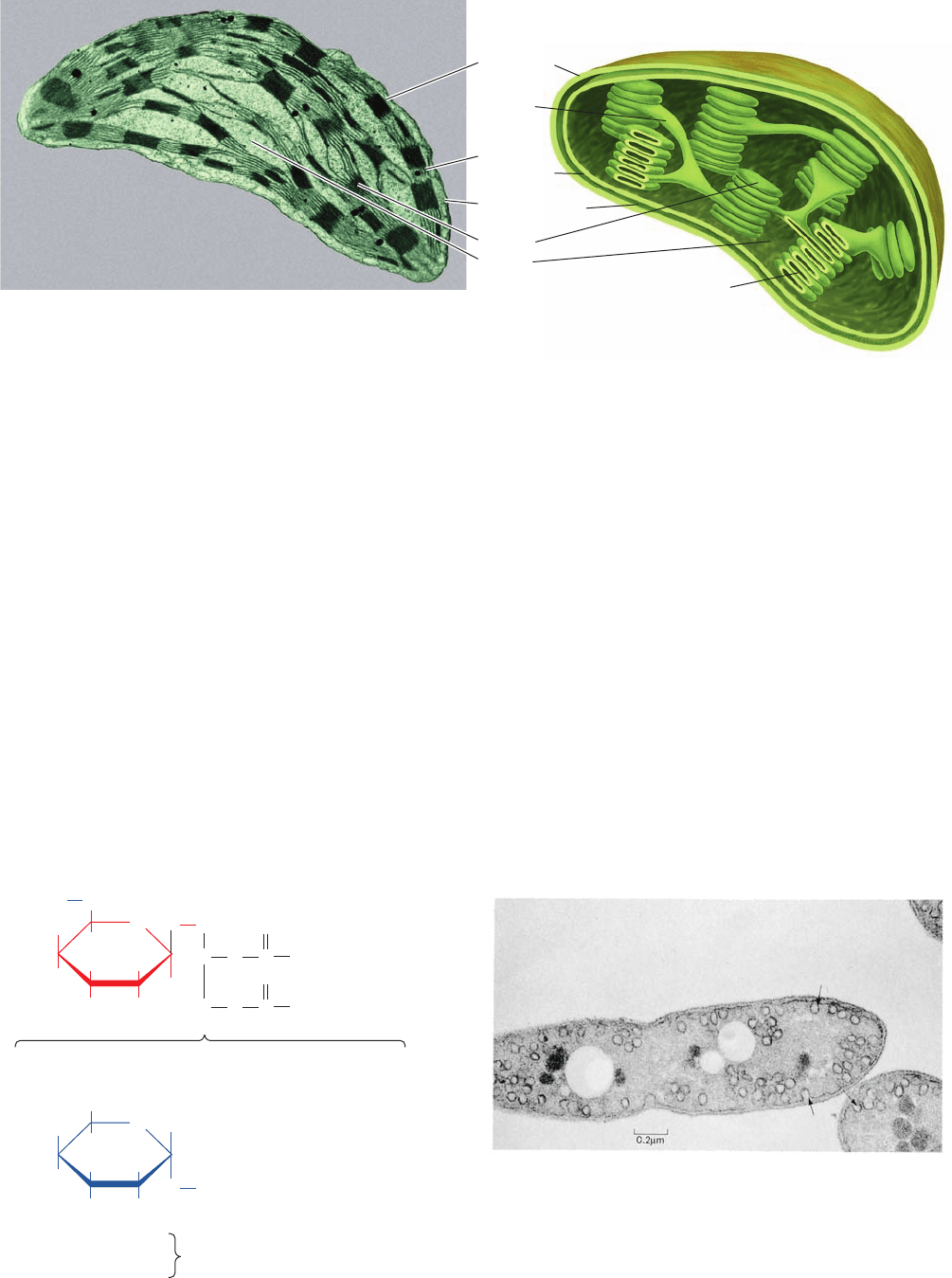
shape but are typically ⬃5-m-long ellipsoids. Like mito-
chondria, which they resemble in many ways, chloroplasts
have a highly permeable outer membrane and a nearly im-
permeable inner membrane separated by a narrow inter-
membrane space (Fig. 24-1). The inner membrane encloses
the stroma, a concentrated solution of enzymes much like
the mitochondrial matrix,that also contains the DNA, RNA,
and ribosomes involved in the synthesis of several chloro-
plast proteins. The stroma, in turn, surrounds a third mem-
branous compartment, the thylakoid (Greek: thylakos, a sac
or pouch). The thylakoid is probably a single highly folded
vesicle, although in most organisms it appears to consist of
stacks of disklike sacs named grana, which are intercon-
nected by unstacked stromal lamellae. A chloroplast usually
contains 10 to 100 grana. Thylakoid membranes arise from
invaginations in the inner membrane of developing chloro-
plasts and therefore resemble mitochondrial cristae.
The lipids of the thylakoid membrane have a distinctive
composition. They consist of only ⬃10% phospholipids; the
majority, ⬃80%, are uncharged mono- and digalactosyl
diacylglycerols, and the remaining ⬃10% are the sulfolipids
sulfoquinovosyl diacylglycerols (quinovose is 6-deoxyglucose):
O
O
O
O
C
C
R
1
R
2
O
H
O
OH
O
O
OH
H
H
HO
HC
H
CH
2
CH
2
H
2
C
H
X
X ⫽ OH
X ⫽ SO
⫺
3
* ⫽ C atom inverted
X ⫽
**
HOH
O
OH
H
H
HO
H
CH
2
OH
H
Galactosyl diacylglycerol
Digalactosyl diacylglycerol
Sulfoquinovosyl diacylglycerol
The acyl chains of these lipids have a high degree of unsat-
uration, which gives the thylakoid membrane a highly fluid
character.
Photosynthesis occurs in two distinct phases:
1. The light reactions, which use light energy to gener-
ate NADPH and ATP.
2. The dark reactions, actually light-independent reac-
tions, which use NADPH and ATP to drive the synthesis of
carbohydrate from CO
2
and H
2
O.
The light reactions occur in the thylakoid membrane and
involve processes that resemble mitochondrial electron
transport and oxidative phosphorylation (Sections 22-2
and 22-3). In photosynthetic prokaryotes, which lack
chloroplasts, the light reactions take place in the cell’s
plasma (inner) membrane or in highly invaginated struc-
tures derived from it called chromatophores (e.g., Fig. 24-2;
recall that chloroplasts evolved from cyanobacteria that as-
sumed a symbiotic relationship with a nonphotosynthetic
902 Chapter 24. Photosynthesis
Figure 24-2 Electron micrograph of a section through the
purple photosynthetic bacterium Rhodobacter sphaeroides. Its
plasma membrane invaginates to form externally connected
tubules known as chromatophores (arrows; seen here in circular
cross section) that are the sites of photosynthesis. [Courtesy of
Gerald A. Peters, Virginia Commonwealth University.]
Outer
membrane
Inner
membrane
Intermembrane
compartment
Thylakoid
compartment
(b)(a)
Granum
Stroma
Stromal
lamella
Figure 24-1 Chloroplast from corn. (a) An electron micrograph. (b) Schematic diagram.
[Electron micrograph courtesy of Lester Shumway, College of Eastern Utah.]
JWCL281_c24_901-939.qxd 6/16/10 11:54 AM Page 902

eukaryote; Section 1-2Ag). In eukaryotes, the dark reac-
tions occur in the stroma through a cyclic series of enzyme-
catalyzed reactions. In the following sections, we consider
the light and dark reactions in detail.
2 LIGHT REACTIONS
In the first decades of the twentieth century, it was gener-
ally assumed that light, as absorbed by photosynthetic pig-
ments, directly reduced CO
2
, which, in turn, combined with
water to form carbohydrate. In this view, CO
2
is the source
of the O
2
generated by photosynthesis. In 1931, however,
Cornelis van Niel showed that green photosynthetic bacte-
ria, anaerobes that use H
2
S in photosynthesis, generate
sulfur:
The chemical similarity between H
2
S and H
2
O led van Niel
to propose that the general photosynthetic reaction is
where H
2
A is H
2
O in green plants and cyanobacteria and
H
2
S in photosynthetic sulfur bacteria. This suggests that
photosynthesis is a two-stage process in which light energy
is harnessed to oxidize H
2
A (the light reactions):
and the resulting reducing agent [H] subsequently reduces
CO
2
(the dark reactions):
Thus, in aerobic photosynthesis, H
2
O, not CO
2
, is pho-
tolyzed (split by light).
The validity of van Niel’s hypothesis was established un-
equivocally by two experiments. In 1937, Robert Hill dis-
covered that when isolated chloroplasts that lack CO
2
are
illuminated in the presence of an artificial electron accep-
tor such as ferricyanide O
2
is evolved with
concomitant reduction of the acceptor [to ferrocyanide,
in our example]. This so-called Hill reaction
demonstrates that CO
2
does not participate directly in the
O
2
-producing reaction. It was discovered eventually that
the natural photosynthetic electron acceptor is NADP
⫹
(Fig. 13-2), whose reduction product, NADPH, is utilized in
the dark reactions to reduce CO
2
to carbohydrate (Section
24-3A). In 1941, when the oxygen isotope
18
O became
available, Samuel Ruben and Martin Kamen directly
demonstrated that the source of the O
2
formed in photo-
synthesis is H
2
O:
This section discusses the major aspects of the light reac-
tions.
H
2
18
O ⫹ CO
2
¡
light
(CH
2
O) ⫹
18
O
2
Fe(CN)
4⫺
6
,
[Fe(CN)
3⫺
6
],
4[H] ⫹ CO
2
¡
(CH
2
O) ⫹ H
2
O
2H
2
A
¡
light
2A ⫹ 4[H]
CO
2
⫹ 2H
2
A
¡
light
(CH
2
O) ⫹ 2A ⫹ H
2
O
CO
2
⫹ 2H
2
S
¡
light
(CH
2
O) ⫹ 2S ⫹ H
2
O
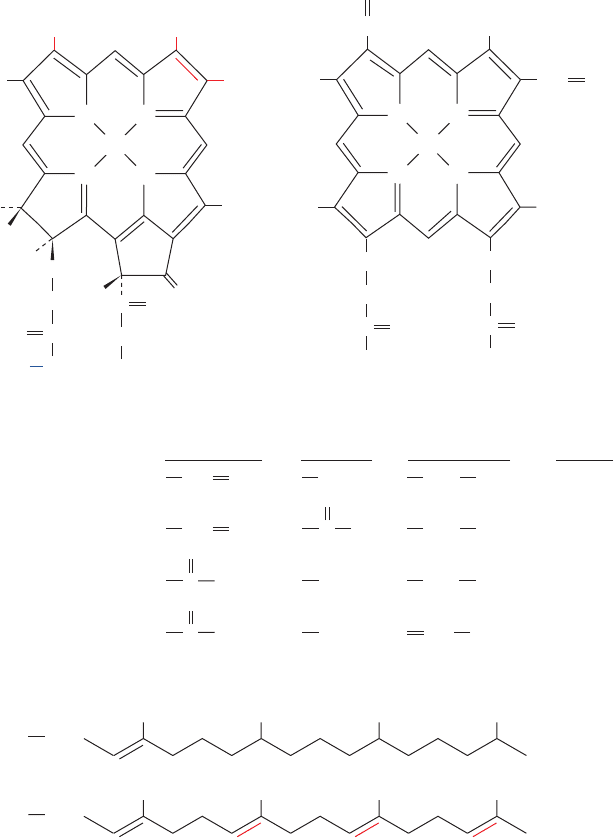
A. Absorption of Light
The principal photoreceptor in photosynthesis is chloro-
phyll. This cyclic tetrapyrrole, like the heme group of
globins and cytochromes (Sections 10-1A and 22-2C), is
derived biosynthetically from protoporphyrin IX. Chloro-
phyll, however, differs from heme in four major respects
(Fig. 24-3):
1. Its central metal ion is Mg
2⫹
rather than Fe(II) or
Fe(III).
2. It has a cyclopentenone ring, Ring V, fused to pyrrole
Ring III.
3. Pyrrole Ring IV is partially reduced in chlorophyll a
(Chl a) and chlorophyll b (Chl b), the two major chloro-
phyll varieties in eukaryotes and cyanobacteria, whereas in
bacteriochlorophyll a (BChl a) and bacteriochlorophyll b
(BChl b), the principal chlorophylls of photosynthetic bac-
teria, Rings II and IV are partially reduced.
4. The propionyl side chain of Ring IV is esterified to a
tetraisoprenoid alcohol. In Chl a and b as well as in BChl b
it is phytol but in BChl a it is either phytol or geranylgeran-
iol, depending on the bacterial species.
In addition, Chl b has a formyl group in place of the
methyl substituent to atom C3 of Ring II of Chl a. Simi-
larly, BChl a and BChl b have different substituents to
atom C4.
a. Light and Matter Interact in Complex Ways
As photosynthesis is a light-driven process, it is worth-
while reviewing how light and matter interact. Electromag-
netic radiation is propagated as discrete quanta (photons)
whose energy E is given by Planck’s law:
[24.1]
where h is Planck’s constant (6.626 ⫻ 10
⫺34
J ⴢ s), c is the
speed of light (2.998 ⫻ 10
8
m ⴢ s
⫺1
in a vacuum), is the fre-
quency of the radiation, and is its wavelength (visible
light ranges in wavelength from 400 to 700 nm). Thus red
light with ⫽680 nm has an energy of 176 kJ ⴢ einstein
⫺1
(an einstein is a mole of photons).
Molecules, like atoms, have numerous electronic quan-
tum states of differing energies. Moreover, because mole-
cules contain more than one nucleus, each of their elec-
tronic states has an associated series of vibrational and
rotational substates that are closely spaced in energy (Fig.
24-4). Absorption of light by a molecule usually occurs
through the promotion of an electron from its ground
(lowest energy) state molecular orbital to one of higher
energy. However, a given molecule can only absorb pho-
tons of certain wavelengths because, as is required by the
law of conservation of energy, the energy difference be-
tween the two states must exactly match the energy of the
absorbed photon.
E ⫽ h⫽
hc
Section 24-2. Light Reactions 903
JWCL281_c24_901-939.qxd 6/8/10 8:53 AM Page 903
very rapidly, being complete in ⬍10
⫺11
s. Many molecules
relax in this manner to their ground states. Chlorophyll
molecules, however, usually relax only to their lowest ex-
cited states. Therefore, the photosynthetically applicable
excitation energy of a chlorophyll molecule that has ab-
sorbed a photon in its short-wavelength band, which corre-
sponds to its second excited state, is no different than if it
had absorbed a photon in its less energetic long-wavelength
band.
2. Fluorescence, in which an electronically excited mol-
ecule decays to its ground state by emitting a photon. Such
a process requires ⬃10
⫺8
s, so it occurs much more slowly
than internal conversion. Consequently, a fluorescently
emitted photon generally has a longer wavelength (lower
energy) than that initially absorbed. Fluorescence accounts
for the dissipation of only 3 to 6% of the light energy ab-
sorbed by living plants. However, chlorophyll in solution,
The various chlorophylls are highly conjugated mole-
cules (Fig. 24-3). It is just such molecules that strongly ab-
sorb visible light (the spectral band in which the solar radi-
ation reaching Earth’s surface is of peak intensity). In fact,
the peak molar extinction coefficients of the various
chlorophylls, ⬎10
5
M
⫺1
ⴢ cm
⫺1
, are among the highest
known for organic molecules (Fig. 24-5; absorbance spectra
are discussed in Section 5-3Ca). Yet the relatively small
chemical differences among the various chlorophylls
greatly affect their absorption spectra. These spectral dif-
ferences, as we shall see, are functionally significant.
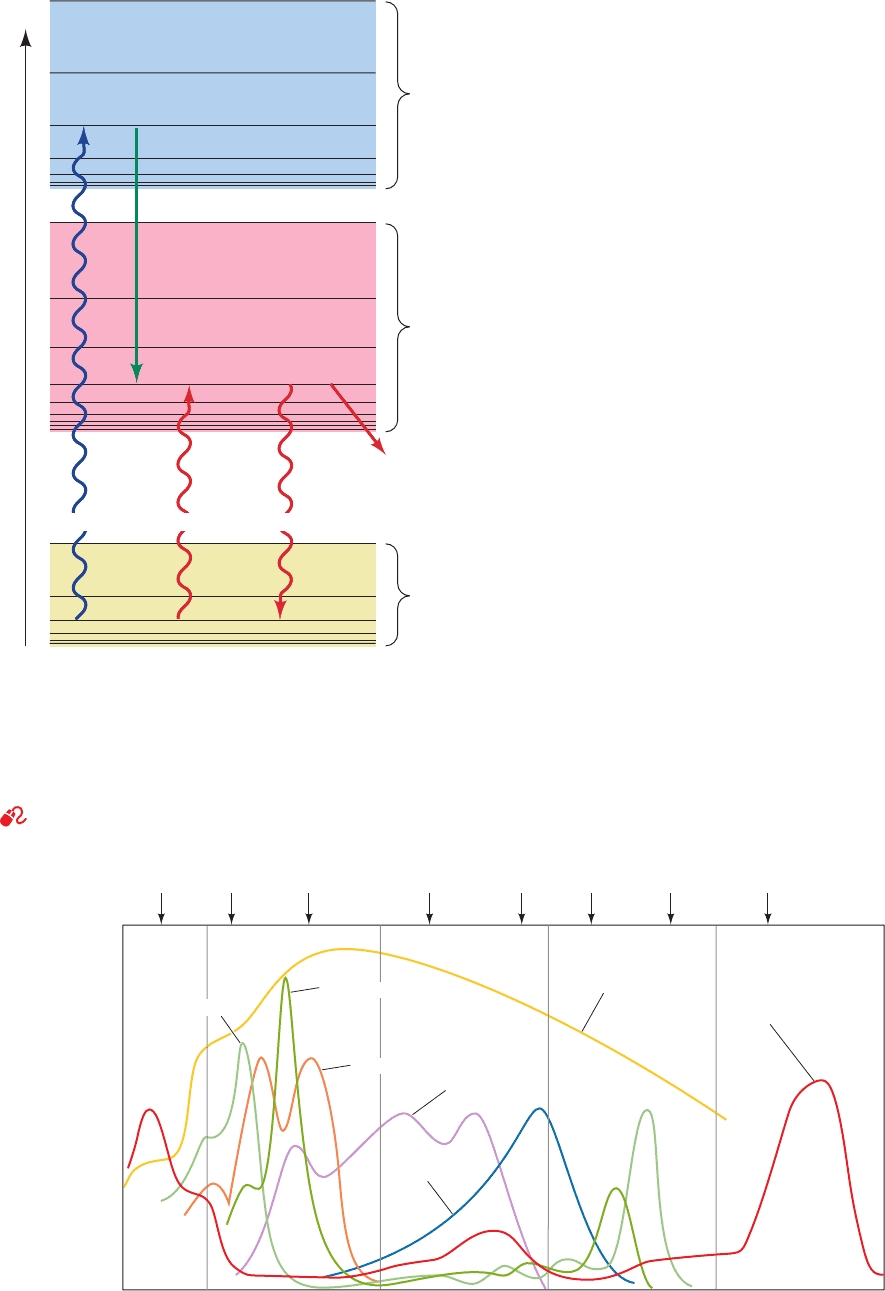
An electronically excited molecule can dissipate its ex-
citation energy in many ways. Those modes with the great-
est photosynthetic significance are as follows (Fig. 24-4):
1. Internal conversion, a common mode of decay in
which electronic energy is converted to the kinetic energy
of molecular motion, that is, to heat. This process occurs
904 Chapter 24. Photosynthesis
CH
3
N
CH
3
CH
2
CH
Fe
N
N N
C
CH
2
CH
2
CH
Iron–protoporphyrin IX
CH
2
H
3
C
H
3
C
H
3
C
CH
3
R
2
R
3
R
1
O
–
O
3
*
4
H
O
C
O
O
CH
3
H
V
IIIIV
I
II
R
1
Chlorophyll a
CH
2
CH
CH
2
CH
CH
3
C
O
CH
3
C
O
CH
3
C
O
H
CH
3
a
CH
3
a
CH
2
CH
3
CH
2
CH
3
CH
2
CH
3
a
CH CH
3
a
P
P
P or G
P
R
2
R
3
R
4
Chlorophyll b
Bacteriochlorophyll a
Bacteriochlorophyll b
a
No double bond between positions C3 and C4.
P =
G =
Phytyl side chain
Geranylgeranyl side chain
CH
2
CH
2
C
CH
2
CH
2
O
–
O
N N
N N
Mg
Chlorophyll
C
CH
2
CH
2
O
O
R
4
H
3
C
H
Figure 24-3 Chlorophyll structures. The
molecular formulas of chlorophylls a and b
and bacteriochlorophylls a and b are
compared to that of iron protoporphyrin IX
(heme).The starred atom has the opposite
stereochemistry in chlorophyll aⴕ (Chl aⴕ).
The isoprenoid phytyl and geranylgeranyl
tails presumably increase the chlorophylls’
solubility in nonpolar media.
JWCL281_c24_901-939.qxd 3/25/10 12:04 PM Page 904
Section 24-2. Light Reactions 905
where of course the photosynthetic uptake of this energy
cannot occur, has an intense red fluorescence.
3. Exciton transfer (also known as resonance energy
transfer), in which an excited molecule directly transfers its
excitation energy to nearby unexcited molecules with simi-
lar electronic properties (the basis of FRET; Section 9-1Cd).
This process occurs through interactions between the mo-
lecular orbitals of the participating molecules in a manner
analogous to the interactions between mechanically coupled
pendulums of similar frequencies. An exciton (excitation)
may be serially transferred between members of a group of
molecules or, if their electronic coupling is strong enough,
the entire group may act as a single excited “supermolecule.”
We shall see that exciton transfer is of particular importance
in funneling light energy to photosynthetic reaction centers.
4. Photooxidation, in which a light-excited donor mole-
cule is oxidized by transferring an electron to an acceptor
molecule, which is thereby reduced. This process occurs be-
cause the transferred electron is less tightly bound to the
donor in its excited state than it is in the ground state. In
photosynthesis, excited chlorophyll (Chl*) is such a donor.
The energy of the absorbed photon is thereby chemically
transferred to the photosynthetic reaction system. Photooxi-
dized chlorophyll,Chl
⫹
,a cationic free radical, eventually re-
turns to its ground state by oxidizing some other molecule.
b. Light Absorbed by Antenna Chlorophylls Is
Transferred to Photosynthetic Reaction Centers
The primary reactions of photosynthesis, as is explained
in Sections 24-2B and 24-2C, take place at photosynthetic
reaction centers (RCs). Yet photosynthetic organelles con-
tain far more chlorophyll molecules than RCs. This was
Figure 24-4 Energy diagram indicating the electronic states of
chlorophyll and their most important modes of interconversion.
The thin black lines denote different vibrational and rotational
substates of each electronic state. The wiggly arrows represent
the absorption of photons or their fluorescent emission. Excitation
energy may also be dissipated in radiationless processes such as
internal conversion (heat production) or chemical reactions.
See the Animated Figures
Figure 24-5 Absorption spectra of various photosynthetic
pigments. The chlorophylls each have two absorption bands, one
in the red and one in the blue. Phycoerythrin absorbs blue and
Second
excited
state
First
excited
state
Ground
state
Absorption
of blue
light
Absorption
of red
light
Fluor-
escence
Internal conversion
(radiationless)
Energy
Photooxidation
(chemical
reactions)
hν hν hν
400 500 600 700 800
Wavelength (nm)
Phycocyanin
Phycoerythrin
Solar spectrum
Bacteriochlorophyll a
Chlorophyll a
Absorbance
Chlorophyll b
Carotenoids
UV Violet Blue Green Yellow Orange Red IR
green light, whereas phycocyanin absorbs yellow light.Together,
these pigments absorb most of the visible light in the solar
spectrum. [After a drawing by Govindjee, University of Illinois.]
JWCL281_c24_901-939.qxd 3/25/10 12:04 PM Page 905
906 Chapter 24. Photosynthesis
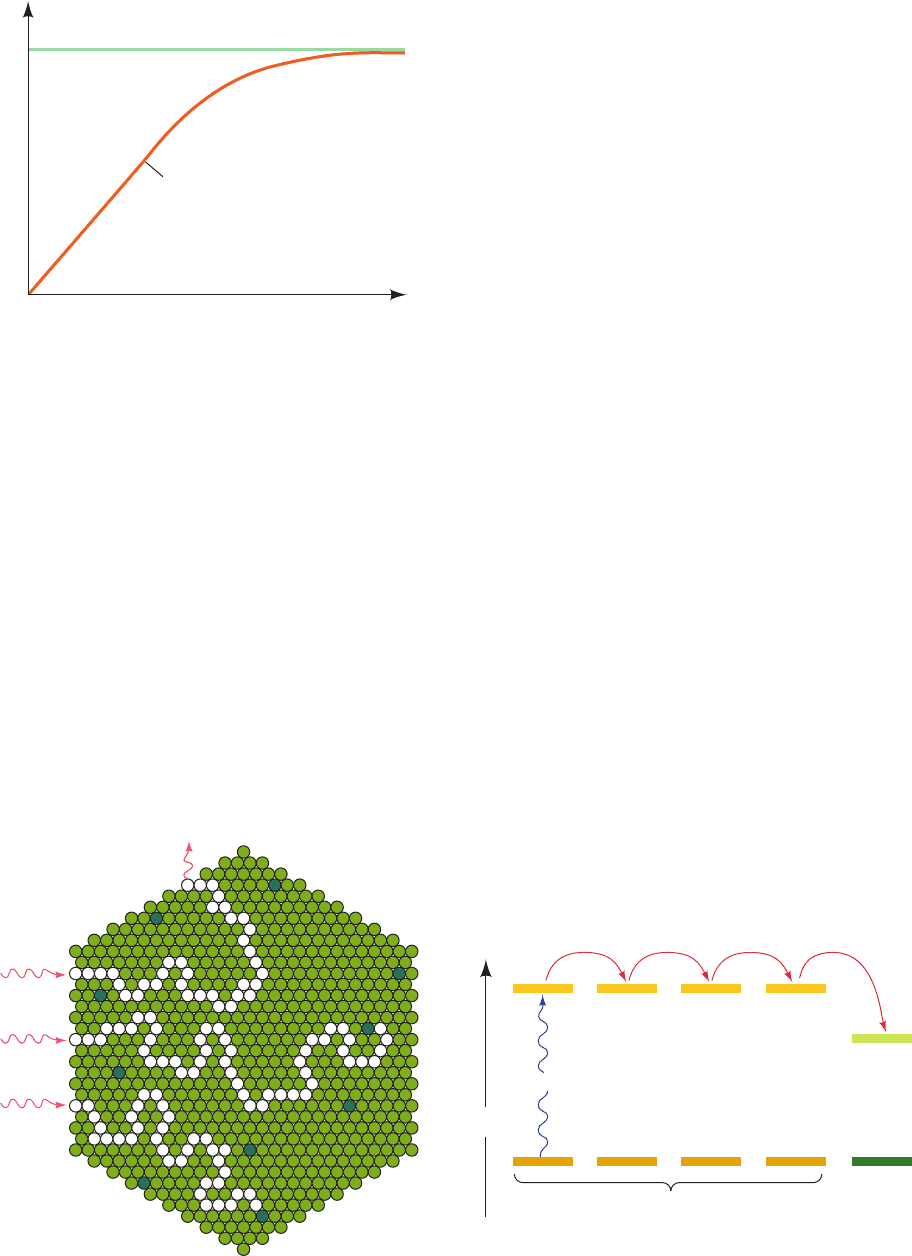
demonstrated in 1932 by Robert Emerson and William
Arnold in their studies of O
2
production by the green alga
Chlorella (a favorite experimental subject), which had
been exposed to repeated brief (10-s) flashes of light.The
amount of O
2
generated per flash was maximal when the
interval between flashes was at least 20 ms. Evidently, this
is the time required for a single turnover of the photosyn-
thetic reaction cycle. Emerson and Arnold then measured
the variation of O
2
yield with flash intensity when the flash
interval was the optimal 20 ms. With weak flashes, the O
2
increased linearly with flash intensity such that about one
molecule of O
2
was generated per eight photons absorbed
(Fig. 24-6). With increasing flash intensity the efficiency of
this process fell off, no doubt because the number of pho-
tons began to approach the number of photochemical
units.What was unanticipated, however, was that each flash
of saturating intensity produced only one molecule of O
2
per ⬃2400 molecules of chlorophyll present. Since at least
eight photons must be sequentially absorbed to liberate
one O
2
molecule (Section 24-2C), these results suggest that
the photosynthetic apparatus contains ⬃2400兾8 ⫽ 300
chlorophyll molecules per RC.
With such a great excess of chlorophyll molecules per
RC, it seems unlikely that all participate directly in photo-
chemical reactions. Rather,as subsequent experiments have
shown, most chlorophylls function to gather light; that is, they
act as light-harvesting antennas. These antenna chlorophylls
pass the energy of an absorbed photon, by exciton transfer,
from molecule to molecule until the excitation reaches an
RC (Fig. 24-7a).There, the excitation is trapped because RC
chlorophylls, although chemically identical to antenna
chlorophylls, have slightly lower excited state energies be-
cause of their different environments (Fig. 24-7b).
Transfer of energy from the antenna system to an RC
occurs in ⬍10
⫺10
s with an efficiency of ⬎90%.This high ef-
ficiency depends on the chlorophyll molecules having ap-
propriate spacings and relative orientations. Even in bright
sunlight, an RC intercepts only ⬃1 photon per second, a
metabolically insignificant rate, and hence, these light-
harvesting complexes (LHCs) serve an essential function.
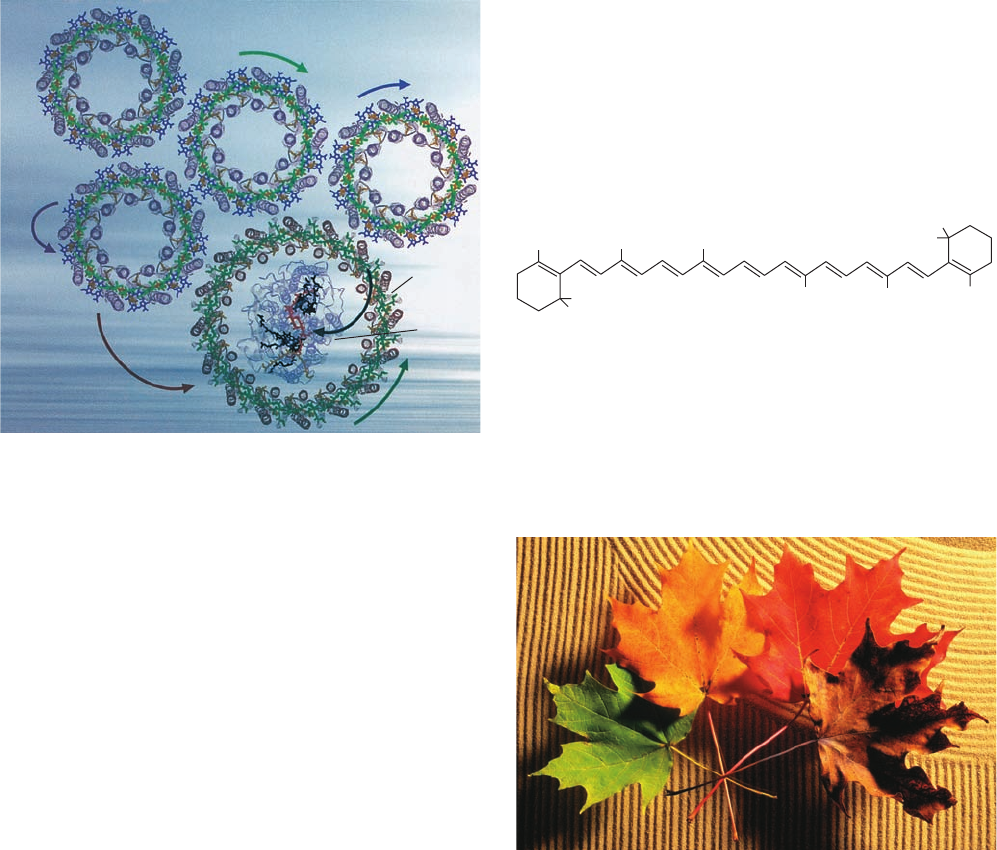
c. The LHCs of Purple Photosynthetic Bacteria
Contain Multiple Symmetrically Arranged
Light-Absorbing Molecules
Most purple photosynthetic bacteria, which are among
the simplest photosynthetic organisms, have two types of
LHCs, LH1 and LH2, that are transmembrane proteins but
have different spectral and biochemical properties. LH2,
which absorbs light at shorter wavelengths than LH1,
rapidly passes the energy from the photons it absorbs to
Maximum ~ 1 O
2
/2400 chlorophylls
slope ~ 8 quanta
per
O
2
molecule evolved
Intensity of each flash
O
2
Evolved
per flash
~
Figure 24-6 The amount of O
2
evolved by Chlorella algae
versus the intensity of light flashes. Flashes are separated by dark
intervals of ⬎20 ms.
Figure 24-7 Flow of energy through a photosynthetic antenna
complex. (a) The excitation resulting from photon absorption
randomly migrates by exciton transfer among the molecules of
the antenna complex (light green circles) until it is either trapped
Photon
Photon
Photon
Photon(a)
Energy
Excited states
Ground states
Antenna pigment molecules
Exciton transfer
Reaction
center
chlorophyll
hν
(b)
by an RC chlorophyll (dark green circles) or, less frequently,
fluorescently reemitted. (b) The excitation is trapped by the RC
chlorophyll because its lowest excited state has a lower energy
than those of the antenna pigment molecules.
JWCL281_c24_901-939.qxd 3/25/10 12:04 PM Page 906

The ␣ and  subunits (56 and 45 residues, respectively)
both consist largely of single helices that are aligned nearly
perpendicularly to the plane of the membrane in which
they are embedded. The eight ␣ subunits pack side by side
to form a hollow cylinder of diameter ⬃31 Å (as measured
between helix axes). Each of the eight  subunits occupies
a position radially outward from an ␣ subunit to form a
concentric cylinder of diameter ⬃62 Å.Sixteen of the BChl
a molecules are packed between these rings of helices in an
arrangement resembling a 16-bladed turbine: Successive
nearly parallel BChl a ring systems are in partial van der
Waals contact (their Mg
2⫹
ions are ⬃9 Å apart) with their
planes perpendicular to the plane of the membrane. Their
Mg
2⫹
atoms are each singly axially liganded by His side
chains [much like the Fe(II) in deoxyhemoglobin] that al-
ternately extend from an ␣ and a  subunit around the
lower end of the cylinder.The remaining eight BChl a mol-
ecules, which are each singly axially liganded by a side
chain of Asp 6␣ near the upper end of the cylinder, are
arranged in an 8-fold symmetric ring between successive
 subunit helices and are oriented with the planes of their
ring systems tilted by ⬃35° relative to the plane of the mem-
brane. The eight lycopene molecules are sandwiched be-
tween the ␣ and  subunits and extend along much of their
lengths, thereby contacting both sets of BChl a molecules.
The LH2 from Rhodopseudomonas (Rps.) acidophila, an-
other purple photosynthetic bacterium, is an ␣
9

9
18-mer
but otherwise has a similar structure in its transmembrane
region to that of Rs. molischianum, even though their ␣ and
 subunits are only 26 and 31% identical.
Spectroscopic measurements indicate that an LH2’s
His-liganded and closely associated BChl a molecules max-
imally absorb radiation at a wavelength of 850 nm (and
hence are called B850) and are strongly coupled, that is,
they absorb radiation almost as a unit. The other, more
loosely associated BChl a molecules (B800) maximally ab-
sorb radiation at 800 nm, largely as individual molecules
(BChl a’s local environment in the protein alters its spec-
trum from that in solution; Fig. 24-5). When a B800 BChl a
absorbs a photon, the excitation is rapidly [in ⬃700 fem-
toseconds (fs); 1 fs ⫽ 10
⫺15
s] transferred to a lower energy
B850 BCl a (which may independently absorb a photon),
LH1, which, in turn, passes it to the RC. The X-ray struc-
ture of LH2 from the purple photosynthetic bacterium
Rhodospirillum (Rs.) molischianum (Fig. 24-8),determined
by Hartmut Michel, reveals that this protein is an 8-fold ro-
tationally symmetric ␣
8

8
16-mer that binds 24 bacteri-
ochlorophyll a (BChl a) molecules and 8 lycopene mole-
cules (a carotenoid; see below):
Lycopene
Section 24-2. Light Reactions 907
Figure 24-8 X-ray structure of LH2 from Rs. molischianum.
The ␣ subunits are purple and the  subunits are pink. The
bound chromophores are drawn in stick form with the BChl a’s
green and the lycopenes yellow. The phytyl tails of the BChl a’s
have been truncated for clarity. (a) View perpendicular to the
bacterial membrane from the cytoplasm.The polypeptide chains
are drawn in worm form. (b) View parallel to the membrane with
the cytoplasm above.The protein subunits are represented by
only their helices, which are shown as cylinders.The Mg
2⫹
ions
are represented by white spheres. [Courtesy of Juergen Koepke
and Hartmut Michel, Max-Planck-Institut für Biochemie,
Frankfurt, Germany. PDBid 1LGH.]
See Interactive
Exercise 20
(a)
(b)
JWCL281_c24_901-939.qxd 10/19/10 9:35 AM Page 907
which even more rapidly (in ⬃100 fs) exchanges the excita-
tion among the other B850 BChl a molecules. Hence, the
B850 system acts as a kind of energy storage ring that delo-
calizes the excitation over a large region. The carotenoid
molecules in this system absorb visible light (⬍800 nm) and
may also facilitate the transmission of excitation between
the rather distantly separated (19 Å between Mg atoms)
nearest-neighbor B850 and B800 BChl a molecules.
LH1, like LH2, has ␣ and  subunits of ⬃50 residues
each.The low (8.5-Å) resolution structure of LH1 from Rs.
rubrum, as determined by electron crystallography, reveals
that it resembles LH2 but with 16-fold rotational symme-
try, and forms a 116-Å-diameter cylinder with a 68-Å-
diameter hole down its center.This hole is of sufficient size
to contain an RC (see below), as electron microscopy stud-
ies indicate is, in fact, the case (Fig. 24-9). LH1’s BChl a
molecules absorb radiation at a longer wavelength than
those of LH2 and consequently, when these two assemblies
are in contact, excitation is rapidly [in 1–5 picoseconds (ps);
1 ps ⫽ 10
⫺12
s] transferred from LH2 to LH1 and then (in
20–40 ps) to LH1’s enclosed RC. Excitations may also be
rapidly exchanged between contacting LH2s. Thus, this an-
tenna system transfers virtually all of the radiation energy
it absorbs to the RC in far less than the few nanoseconds
(ns; 1 ns ⫽ 10
⫺9
s) over which these excitations would oth-
erwise decay. It should be noted that this complicated
arrangement of chromophores (light-absorbing molecules)
is among the simplest known; those of the light-harvesting
systems of plants are even more elaborate (see below).
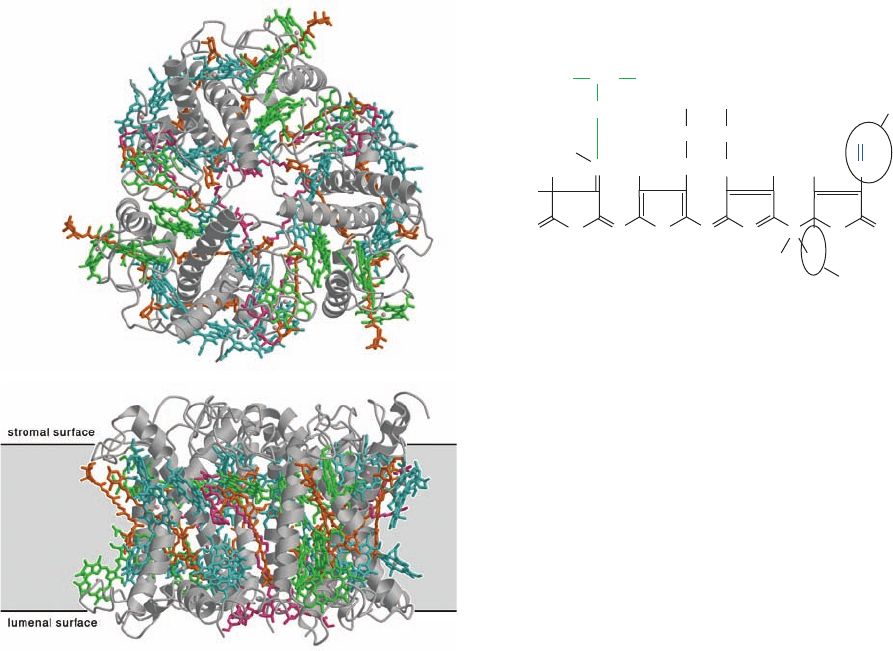
d. LHCs Contain Accessory Pigments
Most LHCs contain organized arrays of other light-
absorbing substances in addition to chlorophyll. These
accessory pigments function to fill in the absorption spectra
of the antenna complexes in spectral regions where chloro-
phylls do not absorb strongly (Fig. 24-5). Carotenoids,
which are C
40
, largely linear polyenes such as lycopene and
-carotene,
are components of all green plants and many photosyn-
thetic bacteria and are therefore the most common acces-
sory pigments.They are largely responsible for the brilliant
fall colors of deciduous trees as well as for the orange color
of carrots (after which carotenoids are named).
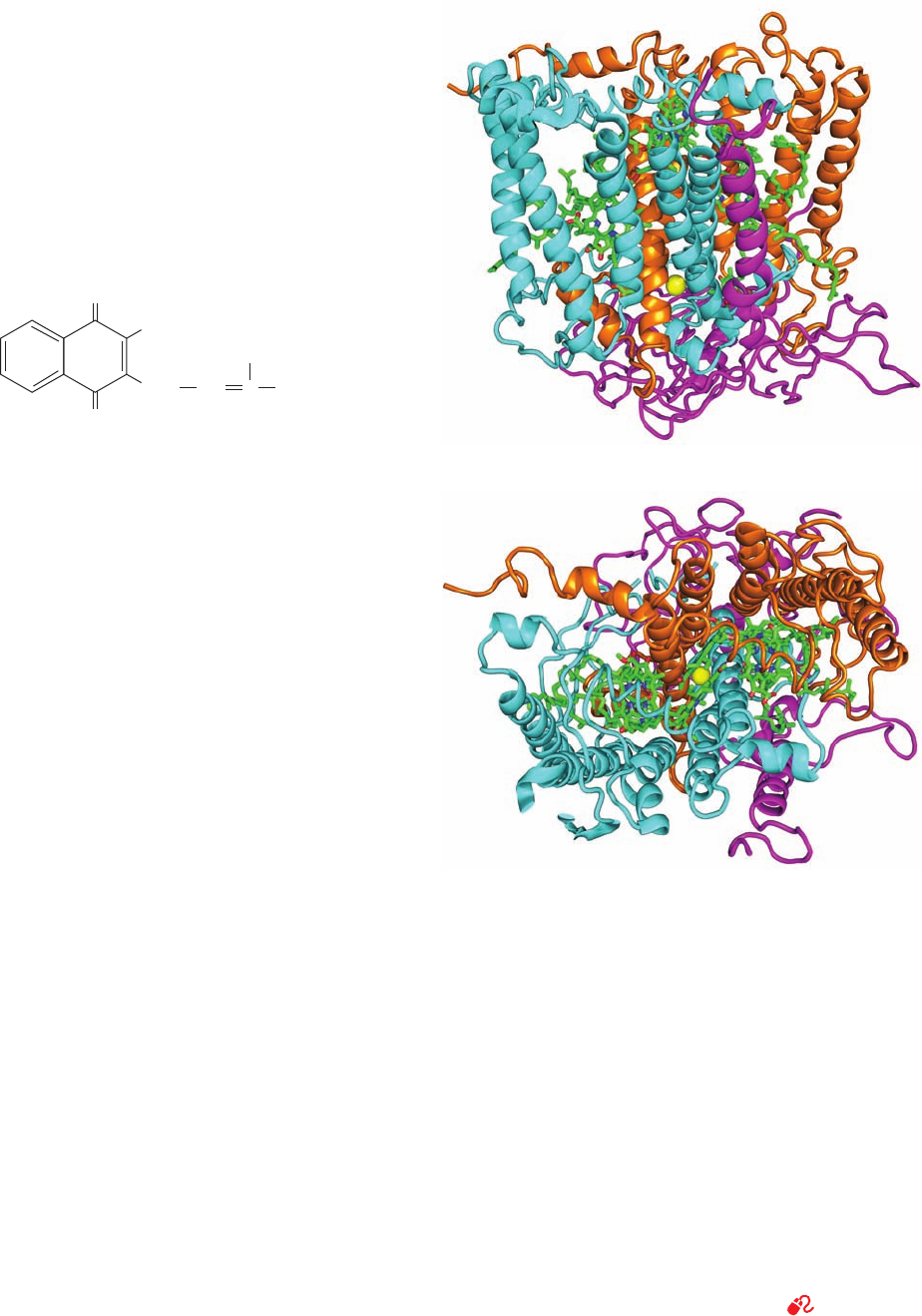
The light-harvesting protein LHC-II of green plants
comprises ⬃30% of the protein in chloroplast membranes,
which makes it the most abundant membrane protein in
nature. Each subunit of this highly conserved, 232-residue,
trimeric protein binds eight Chl a’s, six Chl b’s, and four
carotenoids (Fig. 24-10), thereby accounting for around
half the chlorophyll in the biosphere. The orientations of
the 42 chlorophylls in each LHC-II trimer evenly sample
nearly all directions in space, thus maximizing the effi-
ciency of light harvesting.
Carotenoids serve an additional function besides that of
light-gathering antennas: Through electronic interactions,
they prevent their associated light-excited chlorophyll mol-
ecules from transferring this excitation to O
2
, which would
otherwise yield highly destructive reactive oxygen species
(ROS; Section 22-4Cg). This is particularly important un-
der full sunlight,when the rate that light energy is absorbed
exceeds the rate that it can be used in photosynthesis.Then,
the excess energy is dissipated as heat through internal
CH
3
CH
3
CH
3
CH
3
CH
3
CH
3
CH
3
CH
3
H
3
C
H
3
C
-Carotene
908 Chapter 24. Photosynthesis
Figure 24-9 Model of the light-absorbing antenna system of
purple photosynthetic bacteria. Several LH2s associate with each
other and with LH1, which surrounds the photosynthetic reaction
center (RC).The BChl a’s of LH2 B850 and LH1 are green,
those of LH2 B800 are purple, and the light-absorbing pigments
of the RC (see below) are red and black. Light absorbed by the
Bchl a and lycopene molecules of an LH2 is rapidly transferred
(curved arrows), often via other contacting LH2s, to LH1, which,
in turn, transfers the excitation to its enclosed RC. [From
Bhattarchardee, Y., Nature 412, 474 (2001).]
LH2
LH2
LH2
LH2
LH1
RC
[Ross M. Horowitz/The Image Bank/Getty Images.]
JWCL281_c24_901-939.qxd 7/20/10 7:27 PM Page 908
(a)
(b)
conversion by carotenoids, thereby minimizing the irre-
versible damage to the photosynthetic system that would
otherwise occur. In fact, the acidification of the thylakoid
lumen resulting from high photosynthetic activity (see below)
induces a conformational change in LHC-II. This converts
it to a dissipative state by twisting one of its carotenoids
and hence changing its electronic properties.
Aquatic photosynthetic organisms, which are responsible
for nearly half of the photosynthesis on Earth, additionally
contain other types of accessory pigments. This is because
light outside the wavelengths 450 to 550 nm (blue and green
light) is absorbed almost completely by passage through
more than 10 m of water. In red algae and cyanobacteria,
Chl a is therefore replaced as an antenna pigment by a
series of linear tetrapyrroles, notably the red phycoeryth-
robilin and the blue phycocyanobilin:
The lowest excited states of these so-called bilins have
higher energies than those of the chlorophylls, thereby facil-
itating energy transfer to the RC. The bilins are covalently
linked via Cys S atoms to phycobiliproteins to form phyco-
erythrin and phycocyanin (spectra in Fig. 24-5). These, in
turn, are organized in high molecular mass particles called
phycobilisomes that are bound to the outer faces of photo-
synthetic membranes so as to funnel excitation energy to
RCs over long distances with ⬎90% efficiency.
B. Electron Transport in Purple
Photosynthetic Bacteria
Photosynthesis is a process in which electrons from excited
chlorophyll molecules are passed through a series of accep-
tors that convert electronic energy to chemical energy. Thus
two questions arise: (1) What is the mechanism of energy
transduction; and (2) how do photooxidized chlorophyll
molecules regain their lost electrons? We shall see that
photosynthetic bacteria solve these problems somewhat
differently from cyanobacteria and plants. We first discuss
these mechanisms in photosynthetic bacteria, where they
are simpler and better understood. Electron transport in
cyanobacteria and plants is the subject of Section 24-2C.
a. The Photosynthetic Reaction Center Is a
Transmembrane Protein Containing a Variety
of Chromophores
The first indication that chlorophyll undergoes direct
photooxidation during photosynthesis was obtained by
Louis Duysens in 1952. He observed that illumination of
membrane preparations from the purple photosynthetic
bacterium Rs. rubrum caused a slight (⬃2%) bleaching of
their absorbance at 870 nm, which returned to their origi-
nal levels in the dark. Duysens suggested that this bleach-
ing is caused by photooxidation of a bacteriochlorophyll
complex that he named P870 (P for pigment and 870 nm
for the position of the major long-wavelength absorption
band of BChl a; photosynthetic bacteria tend to inhabit
murky stagnant ponds, so that they require an infrared-
absorbing species of chlorophyll). The ability to detect the
presence of P870 eventually led to the purification and
characterization of the RC to which it is bound.
N
CH
3
C
H
3
C
H
S
H
C
H
N
CH
2
CH
2
CH
3
H
C
H
N
CH
2
H
C
HH
N
CH
3
CH
3
CH
2
CH
H
H
O
O
–
OOC
Cys
CH
2
COO
–
ethyl in
phycocyanobilin
dehydrogenated in
phycocyanobilin
Peptide-linked phycoerythrobilin and phycocyanobilin
Section 24-2. Light Reactions 909
Figure 24-10 X-ray structure of the homotrimeric protein
LHC-II from pea chloroplasts. The protein is drawn in ribbon
form (gray) viewed (a) perpendicular to the thylakoid membrane
from the stroma and along its 3-fold axis; and (b) parallel to the
membrane (gray band) with the stroma above. Its bound
carotenoids and chlorophylls are drawn in stick form with Chl a
cyan, Chl b green, carotenoids orange, and lipids magenta. Mg
2⫹
ions are represented by light pink spheres. Each subunit has
three transmembrane helices oriented with its N-terminus on the
stromal surface. Note the unusually high density of cofactors:
Nearly 40% of this protein’s nonhydrogen atoms comprise its
chlorophylls and carotenoids. [Courtesy of Werner Kühlbrandt,
Max Planck Institute of Biophysics, Frankfurt, Germany. PDBid
2BHW.]
JWCL281_c24_901-939.qxd 6/8/10 8:54 AM Page 909
(a)
(b)
RC particles from several species of purple photosyn-
thetic bacteria (PbRCs) have similar compositions. That
from Rps. viridis consists of three hydrophobic subunits: H
(258 residues), L (273 residues), and M (323 residues). The
L and M subunits of this membrane-spanning protein col-
lectively bind four molecules of BChl b (which maximally
absorbs light at 960 nm), two molecules of bacteriopheo-
phytin b (BPheo b; BChl b in which the Mg
2⫹
is replaced
by two protons), one nonheme/non-Fe–S Fe(II) ion, one
molecule of the redox coenzyme ubiquinone (Fig. 22-17b),
and one molecule of the related menaquinone
(vitamin K
2
, a substance required for proper blood clot-
ting; Section 35-1Ba). In many PbRCs, however, the BChl
b, BPheo b, and menaquinone are replaced by BChl a,
BPheo a, and a second ubiquinone, respectively.
The RC of Rps. viridis, whose X-ray structure was deter-
mined by Johann Deisenhofer, Robert Huber, and Hart-
mut Michel in 1984, was the first transmembrane protein to
be described in atomic detail (Fig. 12-26). The protein’s
transmembrane portion consists of 11 ␣ helices that form a
45-Å-long flattened cylinder with the expected hydrophobic
surface. A c-type cytochrome containing four hemes, which
is an integral constituent of the PbRC complex in only
some photosynthetic bacteria, binds to the PbRC on the
external side of the plasma membrane. In fact, the PbRC from
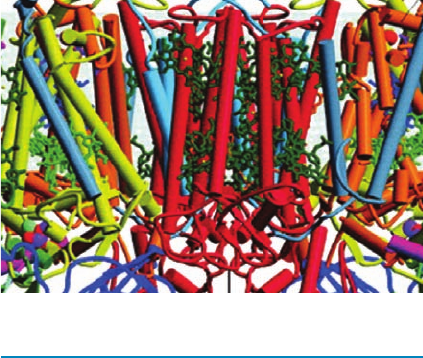
another bacterial species, Rhodobacter (Rb.) sphaeroides,
whose X-ray structure (Fig. 24-11) was independently deter-
mined by Marianne Schiffer and by Douglas Rees and
George Feher, is nearly identical to that of Rps. viridis but
lacks such a bound cytochrome.
b. Two BChl Molecules Form a “Special Pair”
The most striking aspect of the PbRC is that its chro-
mophoric prosthetic groups are arranged with nearly perfect
2-fold symmetry (Fig. 24-12a). This symmetry arises be-
cause the L and M subunits, with which these prosthetic
groups are exclusively associated, have homologous se-
quences and similar folds. Two of the BChl b molecules in
the Rps. viridis PbRC, the so-called special pair, are closely
associated; they are nearly parallel and have an Mg¬Mg
distance of ⬃7 Å. The special pair occupies a predomi-
nantly hydrophobic region of the protein and each of its
Mg
2⫹
ions has a His side chain as a fifth ligand. Each mem-
ber of the special pair is in contact with another His-
liganded BChl b molecule, which, in turn, is associated with a
BPheo b molecule.The menaquinone is in close association
with the L subunit BPheo b (Fig. 24-12a, right), whereas
the ubiquinone, which is but loosely bound to the protein,
O
O
CH
3
CH
3
(CH
2
CH C CH
2
)
8
H
Menaquinone
910 Chapter 24. Photosynthesis
Figure 24-11 A ribbon diagram of the photosynthetic reaction
center (RC) from Rb. sphaeroides. (a) The H, M, and L subunits,
as viewed from within the plane of the plasma membrane with
the cytoplasm below, are magenta, cyan, and orange, respectively.
The prosthetic groups are drawn in stick form with C green,
N blue, and O red.The Fe(II) atom is represented by a yellow
sphere. The 11 largely vertical helices that form the central
portion of the protein constitute its transmembrane region.
Compare this structure with that of the RC from Rps. viridis
(Fig. 12-26), whose H, M, and L subunits are 39, 50, and 59%
identical to those of Rb. sphaeroides. Note that the Rb.
sphaeroides protein lacks the four-heme c-type cytochrome
(green in Fig. 12-26) on its periplasmic surface and that the Q
A
prosthetic group, whose quinone ring lies to the right of the
Fe(II), is ubiquinone in Rb. sphaeroides but menaquinone in Rps.
viridis.(b) View from the extracellular side of the membrane.
Note how the transmembrane portions of the M and L subunits
are related by a pseudo-2-fold axis passing through the Fe(II) ion
and that the prosthetic groups are sandwiched between these
two subunits. [Based on an X-ray structure by Marianne Schiffer,
Argonne National Laboratory. PDBid 2RCR.]
See
Interactive Exercise 21 and Kinemage Exercise 8-2
JWCL281_c24_901-939.qxd 10/19/10 9:36 AM Page 910
