Voet D., Voet Ju.G. Biochemistry
Подождите немного. Документ загружается.

f. Plastocyanin Transports Electrons from
Cytochrome b
6
f to PSI
Electron transfer between cytochrome f, the terminal
electron carrier of the cytochrome b
6
f complex, and PSI is
mediated by plastocyanin (PC), a 99-residue, monomeric,
Cu-containing, peripheral membrane protein located on
the thylakoid luminal surface (Fig. 24-17). Thus PC is the
functional analog of cytochrome c, which transfers elec-
trons from Complex III to Complex IV in the mitochon-
drial electron-transport chain (Section 22-2C4).
PC’s redox center cycles between its Cu(I) and Cu(II)
oxidation states. The X-ray structure of PC from poplar
leaves, determined by Hans Freeman, shows that its single
Cu atom is coordinated with distorted tetrahedral geometry
by a Cys, a Met, and two His residues (Fig. 24-25). Cu(II)
complexes with four ligands normally adopt a square planar
coordination geometry, whereas those of Cu(I) are gener-
ally tetrahedral. Evidently, the strain of Cu(II)’s protein-
imposed tetrahedral coordination in PC promotes its
Section 24-2. Light Reactions 921
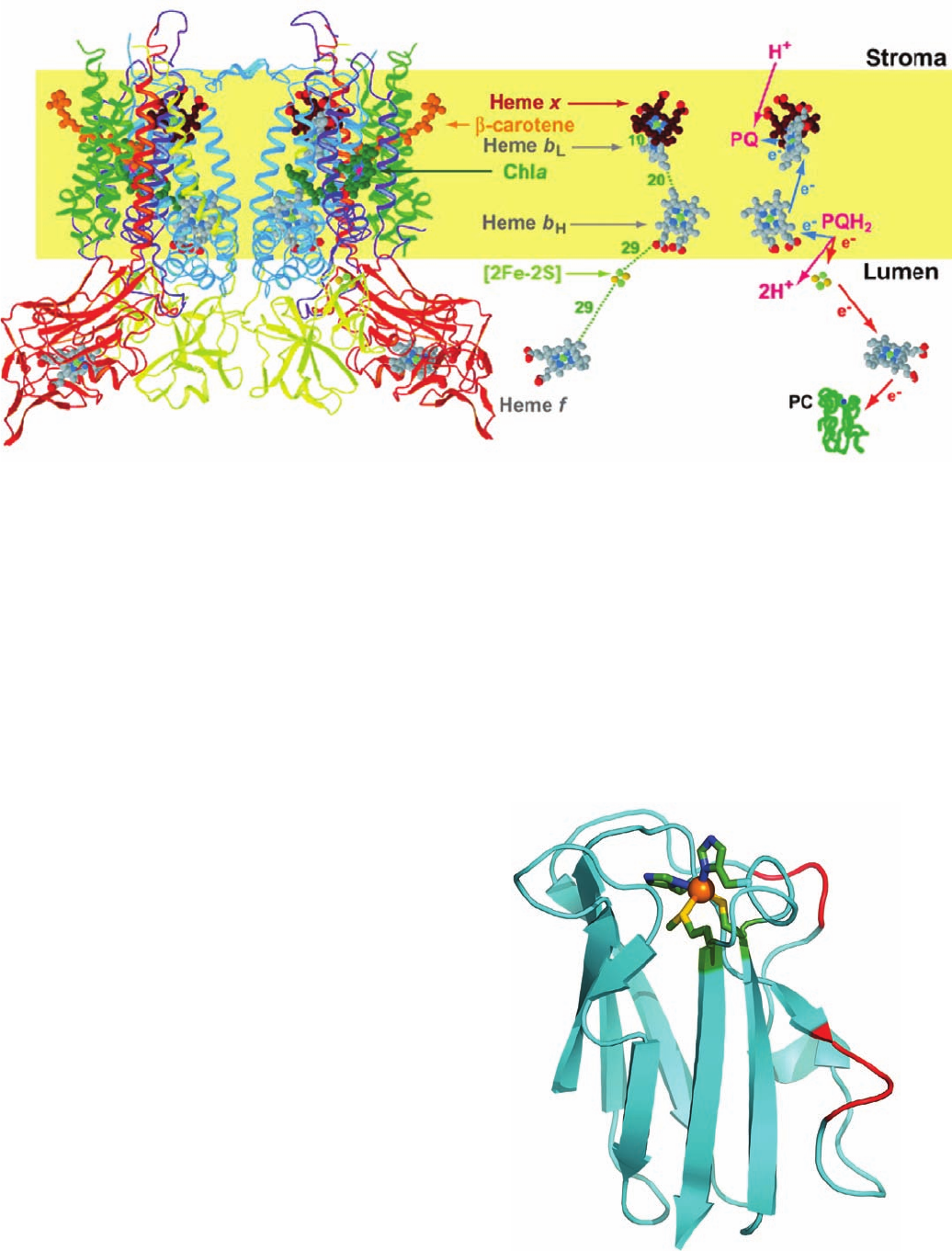
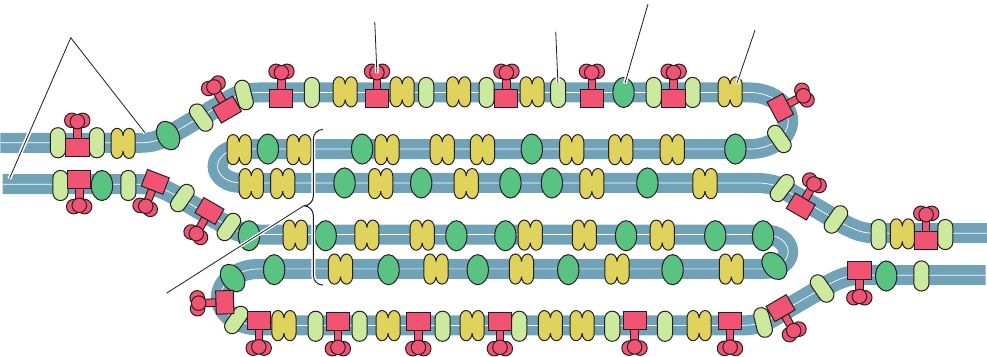
Figure 24-24 X-ray structure of the cytochrome b
6
f complex
from the thermophilic cyanobacterium Mastigocladus
laminosus. A ribbon diagram of the dimeric complex is drawn
on the left with cytochrome b
6
blue, subunit IV purple,
cytochrome f red, the iron–sulfur protein (ISP) yellow, and the
other subunits green.The inferred position of the lipid bilayer is
Figure 24-25 X-ray structure of plastocyanin (PC) from
poplar leaves. This 99-residue monomeric protein, a member of
the family of blue copper proteins (as is the globular domain of
Complex IV’s Subunit II, which binds the Cu
A
center; Section
22-2C5a), folds into a  sandwich. Its Cu atom (orange sphere),
which alternates between its Cu(I) and Cu(II) oxidation states, is
tetrahedrally coordinated by the side chains of His 37, Cys 84,
His 87, and Met 92, which are shown in stick form with their C, N,
and S atoms green, blue, and yellow. Seven conserved Asp and
Glu residues (red) form a negatively charged patch on the
surface of PC that has been implicated in electrostatically binding
to a positively charged patch on the surface of cytochrome f
formed by five Lys and Arg residues. [Based on an X-ray
structure by Mitchell Guss and Hans Freeman, University of
Sydney, Australia. PDBid 1PLC.]
indicated by a yellow band. Compare this figure to Fig. 22-23
(which is upside down relative to this figure).The paths of
electron and proton transfer through the complex and the
distances, in angstroms, between redox centers are shown on the
right. [Modified from a drawing by William A. Cramer and Janet
Smith, Purdue University. PDBid 1UM3.]
JWCL281_c24_901-939.qxd 6/8/10 8:54 AM Page 921
reduction to Cu(I). This hypothesis accounts for PC’s high
standard reduction potential (0.370 V) compared to that of
the normal Cu(II)/Cu(I) half-reaction (0.158 V). This is an
example of how proteins modulate the reduction potentials
of their redox centers so as to match them to their function—
in the case of plastocyanin,the efficient transfer of electrons
from the cytochrome b
6
f complex to PSI.
g. PSI Resembles Both PSII and the PbRC
Cyanobacterial PSIs are trimers of protomers that each
consist of at least 11 different protein subunits coordinat-
ing ⬎100 cofactors.The X-ray structure of PSI from T. elon-
gatus (Fig. 24-26), determined at 2.5-Å resolution by Nor-
bert Krauss, Saenger, and Petra Fromme, reveals that each
of its 356-kD protomers contains nine transmembrane sub-
units (PsaA, PsbB, PsaF, PsaI–M, and PsaX) and three
stromal (cytoplasmic) subunits (PsaC–E), which collec-
tively bind 127 cofactors that comprise 30% of PSI’s mass.
The cofactors forming the PSI RC are all bound by the
homologous subunits PsaA (755 residues) and PsaB (740
residues), whose 11 transmembrane helices each are
arranged in a manner resembling those in the L and M sub-
units of the PbRC (Fig. 24-11) and the D1 and D2 subunits
of PSII (Fig. 24-19), thus supporting the hypothesis that all
RCs arose from a common ancestor. PsaA and PsaB, to-
gether with other transmembrane subunits, also bind the
cofactors of the core antenna system (see below).
Figure 24-27 indicates that PSI’s RC consists of six mol-
ecules of chlorophyll and two molecules of phylloquinone
(vitamin K
1
; note that it has the same phytyl side chain as
chlorophylls; Fig. 24-3),
all arranged in two pseudosymmetrically related branches,
followed by three [4Fe–4S] clusters. The primary electron
donor of this system, P700, consists of a Chl a¿ (Fig. 24-3)
and a Chl a (A1 and B1, respectively), whose rings are
O
O
CH
3
CH
CH
2
(CH
2
CH
2
CH
2
)
3
CH H
CH
2
CCH
3
CH
3
Phylloquinone
922 Chapter 24. Photosynthesis
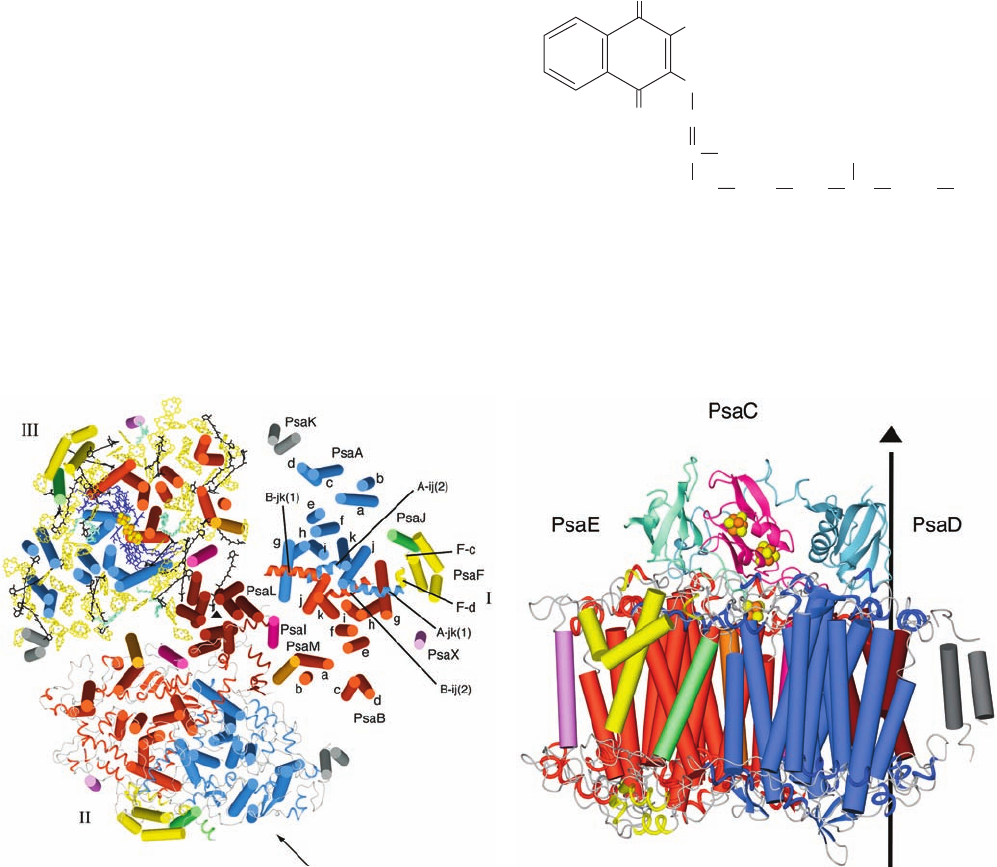
Figure 24-26 X-ray structure of PSI from T. elongatus.
(a) View of the trimer perpendicular to the membrane from its
stromal side. The stromal subunits have been removed for clarity.
PSI’s 3-fold axis of symmetry is represented by the small black
triangle. Different structural elements are shown for each of the
three protomers (I, II, and III). I shows the arrangement of
transmembrane helices (cylinders), which are differently colored
for each subunit.The transmembrane helices of both PsaA (blue)
and PsaB (red) are named a through k from their N- to
C-termini.The six helices in extramembranous loop regions are
drawn as spirals. II shows the transmembrane helices as cylinders
with the stromal and lumenal loop regions drawn in ribbon form.
III shows the transmembrane helices as cylinders together with
all cofactors.The RC Chl a’s and quinones, drawn in stick form,
are purple, the Fe and S atoms of the [4Fe–4S] clusters are drawn
as orange and yellow spheres, the antenna system Chl a’s (whose
side chains have been removed for clarity) are yellow, the
carotenoids are black, and the bound lipids are light green.
(b) One protomer as viewed parallel to the membrane along the
arrow in Part a with the stroma above. The transmembrane
subunits are colored as in Part a with the stromal subunits PsaC,
PsaD, and PsaE pink, cyan, and light green. The vertical line and
triangle mark the trimer’s 3-fold axis of symmetry. [Courtesy of
Wolfram Saenger, Freie Universität Berlin, Germany. PDBid
1JB0.]
(a)
(b)
JWCL281_c24_901-939.qxd 7/2/10 12:23 PM Page 922
parallel and whose Mg
2⫹
ions are separated by 6.3 Å.Thus
P700 resembles the special pair in the PbRC. However,
EPR studies indicate that ⬃80% of the unpaired electron
associated with photooxidized P700
⫹
resides on Chl a B1.
A1 is followed in the left branch of Fig. 24-27 by two more
Chl a rings, B2 and A3, and B1 is followed by A2 and B3 in
the right branch. One or both of the third pair of Chl a mol-
ecules, A3 and B3, probably form the spectroscopically
identified primary electron acceptor A
0
(right side of Fig.
24-18). The Mg
2⫹
ions of A3 and B3 are each axially lig-
anded by the S atom of a Met residue rather than by a His
side chain (thereby forming the only known biological ex-
amples of Mg
2⫹
¬S coordination). All of the residues in-
volved in Mg
2⫹
coordination and hydrogen bonding to
these second and third Chl a’s are strictly conserved in
PSI’s, from cyanobacteria to higher plants, thereby suggest-
ing that all of these interactions are important for fine-
tuning their redox potentials. Electrons are passed from A3
and B3 to the phylloquinones, Q
K
-A and Q
K
-B, which al-
most certainly correspond to the spectroscopically identi-
fied electron acceptor A
1
. Spectroscopically based kinetic
investigations indicate that, in contrast to the case for the
PbRC, electrons pass through both branches of the PSI
RC, although at different rates: 35 ⫻ 10
6
s
⫺1
for the branch
ending in Q
K
-B and 4.4 ⫻ 10
6
s
⫺1
for that ending in Q
K
-A.
Indeed, the PSI RC is most closely related to the RC of
green sulfur bacteria (a second class of photosynthetic bac-
teria), which is a true homodimer.
Up until this point, PSI’s RC resembles those of PSII
and purple photosynthetic bacteria. However, rather than
the reduced forms of either Q
K
-A or Q
K
-B dissociating
from PSI, both of these quinones directly pass their pho-
toexcited electron to a chain of three spectroscopically
identified [4Fe–4S] clusters designated F
X
,F
A
, and F
B
(right side of Fig. 24-18). F
X
, which lies on the pseudo-2-
fold axis relating PsaA and PsaB, is coordinated by two Cys
residues from each of these subunits. F
A
and F
B
are bound
to the stromal subunit PsaC, which structurally resembles
bacterial 2[4Fe–4S] ferredoxins (e.g., Fig. 22-16). Muta-
tional studies on the Cys residues of PsaC that coordinate
its two [4Fe–4S] clusters indicate that the cluster that lies
closer to F
X
is F
A
and the more distant cluster is F
B
(Fig. 24-
27). The observation that both branches of PSI’s electron-
transfer pathways are active, in contrast to only one active
branch in PSII and the PbRC, is rationalized by the fact
that the two quinones at the ends of each branch are func-
tionally equivalent in PSI but functionally different in PSII
and the PbRC.
PSI’s core antenna system consists of 90 Chl a molecules
and 22 carotenoids (Fig. 24-26a). The Mg
2⫹
ions of 79 of
these Chl a molecules are axially liganded by residues of
PsaA and PsaB (mostly His side chains or protein-bound
water molecules), whereas the remaining 11 are so lig-
anded by the smaller subunits PsaJ through M and PsaX.
The spatial distribution of these antenna Chl a’s resembles
that in the core antenna subunits CP43 and CP47 of PSII.
Indeed, the N-terminal domains of PsaA and PsaB are sim-
ilar in sequence to those of CP43 and CP47 and fold into
similar structures containing six transmembrane helices
each. The carotenoids, which are mostly -carotenes, are
deeply buried in the membrane, where they are in van der
Waals contact with Chl a rings. This permits efficient en-
ergy transfer from photoexcited carotenoids to Chl a as
well as protects PSI from photooxidative damage. PSI also
tightly binds four lipid molecules such that their fatty acyl
groups are embedded among the complex’s transmem-
brane helices. This strongly suggests that these lipids have
specific structural and/or functional roles rather than being
artifacts of preparation. Indeed, the head group of one of
them, a phospholipid, coordinates the Mg
2⫹
of an antenna
Chl a, an unprecedented interaction.
PSIs from higher plants are monomers rather than
trimers as are cyanobacterial PSIs. Nevertheless, the X-ray
structure of PSI from peas, determined by Nathan Nelson,
reveals that the positions and orientations of the chloro-
phylls in both species of PSIs are nearly identical, a re-
markable finding considering the ⬎1 billion years since
chloroplasts diverged from their cyanobacterial ancestors.
However, pea PSI has four antenna proteins not present in
cyanobacterial PSI that are arranged in a crescent-shaped
transmembrane belt around one side of its RC and which
collectively bind 56 chlorophyll molecules.
Section 24-2. Light Reactions 923
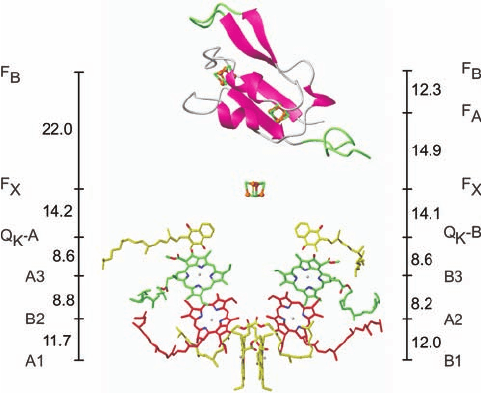
Figure 24-27 Cofactors of the PSI RC and PsaC. The
structure is viewed parallel to the membrane plane with the
stroma above. The Chl a and phylloquinone molecules are
arranged in two branches that are related by PSI’s 2-fold axis of
pseudosymmetry, which is vertical in this drawing. The Chl a’s are
labeled A or B to indicate that their Mg
2⫹
ions are liganded by
the side chains of PsaA or PsaB, respectively, and, from the
luminal side upward, by different colors and numbers, 1 to 3.
The phylloquinones are named Q
K
-A and Q
K
-B. PsaC is shown
in ribbon form with those portions resembling segments in
bacterial 2[4Fe–4S] ferredoxins pink and with insertions and
extensions green.The three [4Fe–4S] clusters are shown in
ball-and-stick form and labeled according to their spectroscopic
identities F
X
,F
A
, and F
B
.The center-to-center distances between
cofactors (vertical black lines) are given in angstroms. Compare
this figure with Figs. 24-20 and 24-12. [Courtesy of Wolfram
Saenger, Freie Universität Berlin, Germany. PDBid 1JB0.]
JWCL281_c24_901-939.qxd 7/2/10 12:24 PM Page 923
h. PSI-Activated Electrons May Reduce NADP
ⴙ
or
Motivate Proton Gradient Formation
Electrons ejected from F
B
in PSI may follow either of two
alternative pathways (Fig. 24-18):
1. Most electrons follow a noncyclic pathway by passing
to an ⬃100-residue, single [2Fe–2S]-containing, soluble
ferredoxin (Fd) that is located in the stroma.Reduced Fd, in
turn, reduces NADP
⫹
in a reaction mediated by the ⬃310-
residue, monomeric, FAD-containing ferredoxin–NADP
ⴙ
reductase (FNR, Fig. 24-28a), to yield the final product of
the chloroplast light reaction, NADPH. Two reduced Fd
molecules successively deliver one electron each to the
FAD of FNR, which thereby sequentially assumes the neu-
tral semiquinone and fully reduced states before transfer-
ring the two electrons and a proton to the NADP
⫹
via what
is formally a hydride ion transfer.The X-ray structure of the
complex between Fd and FNR from maize leaf (Fig.24-28b),
determined by Genji Kurisu, reveals that the shortest inter-
atomic approach between Fd’s [2Fe–2S] cluster and FNR’s
FAD is the 6.0 Å between an Fe atom and FAD atom C8a
(the methyl C closest to its ribitol residue; Fig. 16-8). This is
sufficiently close for direct electron transfer through space
between these prosthetic groups. The complex is stabilized
by five salt bridges, as similarly appears to be the case for the
interaction between cytochrome f and PC.
2. Some electrons are returned from PSI, via cy-
tochrome b
6
, to the plastoquinone pool, thereby traversing
a cyclic pathway that translocates protons across the thy-
lakoid membrane. A mechanism that has been proposed
for this process is that Fd transfers an electron to heme x
of cytochrome b
6
(Fig. 24-24) rather than to FNR. Since
heme x contacts heme b
L
at the periphery of cytochrome
b
6
f’s Q
i
site, an electron injected into heme x would be ex-
pected to reduce plastoquinone via a Q cycle-like mecha-
nism (Fig. 22-31). Note that the cyclic pathway is inde-
pendent of the action of PSII and hence does not result in
the evolution of O
2
.This accounts for the observation that
chloroplasts absorb more than eight photons per O
2
mole-
cule evolved.
The cyclic electron flow presumably functions to in-
crease the amount of ATP produced relative to that of
924 Chapter 24. Photosynthesis
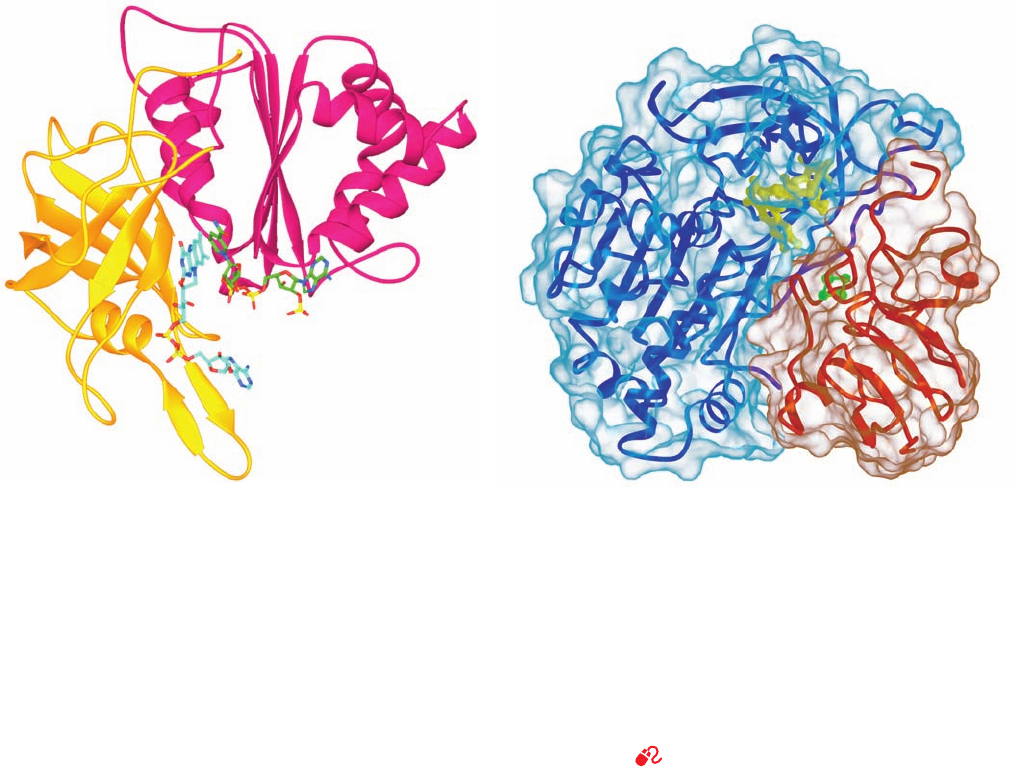
Figure 24-28 Ferredoxin–NADP
ⴙ
reductase. (a) The X-ray
structure of the Y308S mutant form of pea ferredoxin–NADP
⫹
reductase (FNR) in complex with FAD and NADP
⫹
.This
308-residue protein has two domains:The N-terminal domain
(gold), which forms the FAD binding site, folds into an
antiparallel  barrel, whereas the C-terminal domain (magenta),
which provides the NADP
⫹
binding site, forms a dinucleotide-
binding fold (Section 8-3Bi).The FAD and NADP
⫹
are shown in
stick form with NADP
⫹
C green, FAD C cyan, N blue, O red, and
P yellow. The flavin and nicotinamide rings are in opposition
with C4 of the nicotinamide ring and C5 of the flavin ring 3.0 Å
apart, an arrangement that is consistent with direct hydride
transfer as also occurs in glutathione reductase and dihydrolipoyl
dehydrogenase (Section 21-2Ba). However, in contrast to these
latter enzymes, whose bound flavin and nicotinamide rings are
parallel, those in FNR are inclined by ⬃30°, a heretofore
unobserved binding mode. [Based on an X-ray structure by
Andrew Karplus, Cornell University. PDBid 1QFY.] (b) The
X-ray structure of the complex between Fd (red) and FNR (blue)
from maize leaf with both proteins drawn in ribbon form
embedded in their transparent solvent-accessible surfaces.The
[2Fe–2S] cluster of Fd (green) and the FAD of FNR (yellow) are
drawn in ball-and-stick form.The Fd binds in a hollow between
FNR’s two domains (Part a) such that the line joining the two
Fe’s of the [2Fe–2S] cluster lies roughly in the plane of the flavin
ring. [Courtesy of Genji Kurisu, Osaka University, Osaka, Japan.
PDBid 1GAQ.]
See Interactive Exercise 22
(a) (b)
JWCL281_c24_901-939.qxd 10/19/10 9:36 AM Page 924
NADPH and thus permits the cell to adjust the relative
amounts of these two substances produced according to its
needs. However, the mechanism that apportions electrons
between the cyclic and noncyclic pathways is unknown.
i. PSI and PSII Occupy Different Parts of
the Thylakoid Membrane
Freeze-fracture electron microscopy (Section 12-3Ca)
revealed that the protein complexes of the thylakoid mem-
brane have characteristic distributions (Fig. 24-29):
1. PSI occurs mainly in the unstacked stroma lamellae,
in contact with the stroma, where it has access to NADP
⫹
.
2. PSII is located almost exclusively between the
closely stacked grana, out of direct contact with the stroma.
3. Cytochrome b
6
f is uniformly distributed throughout
the membrane.
The high mobilities of plastoquinone and plastocyanin, the
electron carriers that shuttle electrons between these com-
plexes, permits photosynthesis to proceed at a reasonable
rate.
What function is served by the segregation of PSI and
PSII, which are typically present in chloroplasts in equimo-
lar amounts? If these two photosystems were in close prox-
imity, the higher excitation energy of PSII (P680 vs P700)
would cause it to pass a large fraction of its absorbed pho-
tons to PSI via exciton transfer; that is, PSII would act as a
light-harvesting antenna for PSI (Fig. 24-7b). The separa-
tion of these particles by around 100 Å eliminates this
difficulty.
The physical separation of PSI and PSII also permits
the chloroplast to respond to changes in illumination. The
relative amounts of light absorbed by the two photosys-
tems vary with how the light-harvesting complexes
(LHCs) are distributed between the stacked and un-
stacked portions of the thylakoid membrane. Under high
illumination (normally direct sunlight, which contains a
high proportion of short-wavelength blue light), all else
being equal, PSII absorbs more light than PSI. PSI is then
unable to take up electrons as fast as PSII can supply
them, so the plastoquinone is predominantly in its reduced
state. The reduced plastoquinone activates a protein ki-
nase to phosphorylate specific Thr residues of the LHCs,
which, in response, migrate to the unstacked regions of the
thylakoid membrane, where they bind to PSI. A greater
fraction of the incident light is thereby funneled to PSI.
Under low illumination (normally shady light, which con-
tains a high proportion of long-wavelength red light), PSI
takes up electrons faster than PSII can provide them so
that plastoquinone predominantly assumes its oxidized
form. The LHCs are consequently dephosphorylated and
migrate to the stacked portions of the thylakoid mem-
brane, where they drive PSII. The chloroplast therefore
maintains the balance between its two photosystems by a
light-activated feedback mechanism.
D. Photophosphorylation
Chloroplasts generate ATP in much the same way as mito-
chondria, that is, by coupling the dissipation of a proton gra-
dient to the enzymatic synthesis of ATP (Section 22-3C).
This was clearly demonstrated by the imposition of an arti-
ficially produced pH gradient across the thylakoid mem-
brane. Chloroplasts were soaked, in the dark, for several
hours in a succinic acid solution at pH 4 so as to bring the
thylakoid lumen to this pH (the thylakoid membrane is
permeable to unionized succinic acid). The abrupt transfer
of these chloroplasts to an ADP ⫹ P
i
–containing buffer at
pH 8 resulted in an impressive burst of ATP synthesis:
About 100 ATPs were synthesized per molecule of
cytochrome f present. Moreover, the amount of ATP
Section 24-2. Light Reactions 925
Figure 24-29 Segregation of PSI and PSII. The distribution of
photosynthetic protein complexes between the stacked (grana)
and the unstacked (stroma exposed) regions of the thylakoid
ATP synthase
Unstacked membranes
(stromal lamellae)
Stacked
membranes
(grana)
PSI complex
PSII complex
Cytochrome b
6
f
membrane is shown. [After Anderson, J.M. and Anderson, B.,
Trends Biochem. Sci. 7, 291 (1982).]
JWCL281_c24_901-939.qxd 3/25/10 12:05 PM Page 925
synthesized was unaffected by the presence of electron-
transport inhibitors such as DCMU. This, together with the
observations that photophosphorylation requires an intact
thylakoid membrane and that proton translocators such as
2,4-dinitrophenol (Section 22-3D) uncouple photophos-
phorylation from light-driven electron transport, provides
convincing evidence favoring the chemiosmotic hypothesis
(Section 22-3A).
a. Chloroplast Proton-Translocating ATP Synthase
Resembles That of Mitochondria
Electron micrographs of thylakoid membrane stromal
surfaces and bacterial plasma membrane inner surfaces re-
veal lollipop-shaped structures (Fig. 24-30). These closely
resemble the F
1
units of the proton-translocating ATP syn-
thase studding the matrix surfaces of inner mitochondrial
membranes (Fig. 22-36a). In fact, the chloroplast ATP syn-
thase, which is termed the CF
1
CF
0
complex (C for chloro-
plast), has remarkably similar properties to the mitochon-
drial F
1
F
0
complex (Section 22-3C). For example,
1. Both F
0
and CF
0
units are hydrophobic transmem-
brane proteins that contain a proton-translocating channel.
2. Both F
1
and CF
1
are hydrophilic peripheral mem-
brane proteins of subunit composition ␣
3

3
␥␦ε, of which 
is a reversible ATPase.
3. Both ATP synthases are inhibited by oligomycin.
4. Chloroplast ATP synthase translocates protons out
of the thylakoid lumen into the stroma (Fig. 24-17), and
mitochondrial ATP synthase conducts them into the matrix
space (the mitochondrial equivalent of the stroma) from
the intermembrane space (Section 22-3A).
Clearly, proton-translocating ATP synthases must have
evolved very early in the history of cellular life. Chloroplast
ATP synthase is located in the unstacked portions of the
thylakoid membrane, in contact with the stroma, where
there is room for the bulky CF
1
globule and access to ADP
(Fig. 24-29).
b. Photosynthesis with Noncyclic Electron
Transport Produces Around 1.25 ATP
Equivalents per Absorbed Photon
At saturating light intensities, chloroplasts generate
proton gradients of ⬃3.5 pH units across their thylakoid
membranes. This, as we have seen (Figs. 24-17 and 24-18),
arises from two sources:
1. The evolution of a molecule of O
2
from two H
2
O
molecules releases four protons into the thylakoid lumen.
These protons should be considered as being supplied from
the stroma by the protons and H atoms taken up in the syn-
thesis of NADPH.
2. The transport of the liberated four electrons through
the cytochrome b
6
f complex occurs with the translocation
of what is estimated to be eight protons from the stroma to
the thylakoid lumen.
Altogether ⬃12 protons are translocated per molecule of O
2
produced by noncyclic electron transport.
The thylakoid membrane, in contrast to the inner mito-
chondrial membrane, is permeable to ions such as Mg
2⫹
and Cl
⫺
. Translocation of protons and electrons across the
thylakoid membrane is consequently accompanied by the
passage of these ions so as to maintain electrical neutrality
(Mg
2⫹
out and Cl
⫺
in). This all but eliminates the mem-
brane potential, ⌬⌿ (Eq. [22.1]). The electrochemical gradi-
ent in chloroplasts is therefore almost entirely a result of the
pH gradient.
Chloroplast ATP synthase, according to most estimates,
produces one ATP for every three protons it transports out
of the thylakoid lumen. Noncyclic electron transport in
chloroplasts therefore results in the production of
⬃12/3 ⫽ 4 molecules of ATP per molecule of O
2
evolved
(although this quantity is subject to revision) or around
half an ATP per photon absorbed. Cyclic electron trans-
port is a more productive ATP generator since it yields
two-thirds of an ATP (two protons transported) per ab-
sorbed photon.The noncyclic process, of course, also yields
NADPH, each molecule of which has the free energy to
produce three ATPs (Section 22-2A; although this does not
normally occur), for a total of six more ATP equivalents
per O
2
produced. Consequently, the energetic efficiency of
the noncyclic process is 4兾8 ⫹ 6兾8 ⫽ 1.25 ATP equivalents
per absorbed photon.
3 DARK REACTIONS
In the previous section we saw how light energy is har-
nessed to generate ATP and NADPH. In this section we
discuss how these products are used to synthesize carbohy-
drates and other substances from CO
2
.
926 Chapter 24. Photosynthesis
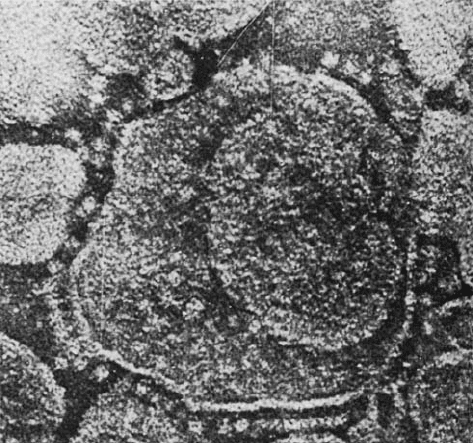
Figure 24-30 Electron micrograph of thylakoids. The CF
1
“lollipops” of their ATP synthases project from their stromal
surfaces. Compare this with Fig. 22-36a. [Courtesy of Peter
Hinkle, Cornell University.]
JWCL281_c24_901-939.qxd 3/25/10 12:05 PM Page 926

A. The Calvin Cycle
The metabolic pathway by which plants incorporate CO
2
into carbohydrates was elucidated between 1946 and 1953
by Melvin Calvin, James Bassham, and Andrew Benson.
They did so by tracing the metabolic fate of the radioactive
label from
14
CO
2
as it passed through a series of photosyn-
thetic intermediates. The basic experimental strategy they
used was to expose growing cultures of algae, such as
Chlorella, to
14
CO
2
for varying times and under differing il-
lumination conditions and then to drop the cells into boil-
ing alcohol so as to disrupt them while preserving their la-
beling pattern.The radioactive products were subsequently
separated and identified (an often difficult task) through
the use of the then recently developed technique of two-
dimensional paper chromatography (Section 6-3Dc) coupled
with autoradiography.The overall pathway, diagrammed in
Fig. 24-31, is known as the Calvin cycle or the reductive
pentose phosphate cycle.
Some of Calvin’s earliest experiments indicated that al-
gae exposed to
14
CO
2
for a minute or more had synthesized
a complex mixture of labeled metabolic products, including
sugars and amino acids. By inactivating the algae within 5 s
of their exposure to
14
CO
2
, however, it was shown that the
first stable radioactively labeled compound formed is 3-
phosphoglycerate (3PG), which is initially labeled only in its
carboxyl group. This result immediately suggested, in anal-
ogy with most biochemical experience, that the 3PG was
formed by the carboxylation of a C
2
compound. Yet the
failure to find any such precursor eventually forced this hy-
pothesis to be abandoned. The actual carboxylation reac-
tion was discovered through an experiment in which illu-
minated algae had been exposed to
14
CO
2
for ⬃10 min so
that the levels of their labeled photosynthetic intermedi-
ates had reached a steady state. The CO
2
was then with-
drawn. As expected, the carboxylation product, 3PG, de-
creased in concentration (Fig. 24-32) because it was
depleted by reactions farther along the pathway. The con-
centration of ribulose-5-phosphate (Ru5P),
however, simultaneously increased. Evidently, Ru5P is the
Calvin cycle’s carboxylation substrate. If so, the resulting
C
6
carboxylation product must split into two C
3
com-
pounds, one of which is 3PG (Fig. 24-31, Reaction 2). A
consideration of the oxidation states of Ru5P and CO
2
in-
dicates that, in fact, both C
3
compounds must be 3PG and
that the carboxylation reaction requires no external redox
source.
While the search for the carboxylation substrate was go-
ing on, several other photosynthetic intermediates had
CH
2
OH
Ribulose-5-phosphate (Ru5P)
CO
HCOH
HCOH
CH
2
OPO
2
3
–
been identified and, through chemical degradation studies,
their labeling patterns had been elucidated. For example,
the hexose fructose-1,6-bisphosphate (FBP) is initially la-
beled only at its C3 and C4 positions (Fig. 24-31) but later
becomes labeled to a lesser degree at its other atoms. Sim-
ilarly, a series of tetrose, pentose, hexose, and heptose phos-
phates were isolated that had the identities and initial la-
beling patterns indicated in Fig. 24-31. A consideration of
the flow of the labeled atoms through these various inter-
mediates led, in what was a milestone of metabolic bio-
chemistry, to the deduction of the Calvin cycle as is dia-
grammed in Fig. 24-31. The existence of many of its
postulated reactions was eventually confirmed by in vitro
studies using purified enzymes.
a. The Calvin Cycle Generates GAP from
CO
2
via a Two-Stage Process
The Calvin cycle may be considered to have two stages:
Stage 1 The production phase (top line of Fig. 24-31), in
which three molecules of Ru5P react with three molecules
of CO
2
to yield six molecules of glyceraldehyde-3-phosphate
(GAP) at the expense of nine ATP and six NADPH mole-
cules. The cyclic nature of the pathway makes this process
equivalent to the synthesis of one GAP from three CO
2
molecules. Indeed, at this point, one GAP can be bled off
from the cycle for use in biosynthesis (see Stage 2).
Stage 2 The recovery phase (bottom lines of Fig. 24-31),
in which the carbon atoms of the remaining five GAPs are
shuffled in a remarkable series of reactions, similar to those
of the pentose phosphate pathway (Section 23-4), to re-
form the three Ru5Ps with which the cycle began. Indeed,
the elucidation of the pentose phosphate pathway at about
the same time that the Calvin cycle was being worked out
provided much of the biochemical evidence in support of
the Calvin cycle. This stage can be conceptually decom-
posed into four sets of reactions (with the numbers keyed
to the corresponding reactions in Fig. 24-31):
6. C
3
⫹ C
3
C
6
8. C
3
⫹ C
6
C
4
⫹ C
5
9. C
3
⫹ C
4
C
7
11. C
3
⫹ C
7
C
5
⫹ C
5
The overall stoichiometry for this process is therefore
Note that this stage of the Calvin cycle occurs without fur-
ther input of free energy (ATP) or reducing power
(NADPH).
b. Most Calvin Cycle Reactions Also Occur in
Other Metabolic Pathways
The types of reactions in the Calvin cycle are all familiar
(Section 23-4), with the exception of the carboxylation
reaction. This first stage of the Calvin cycle begins with
the phosphorylation of Ru5P by phosphoribulokinase to
form ribulose-1, 5-bisphosphate (RuBP). Following the
5C
3
¡
3C
5
¡
¡
¡
¡
Section 24-3. Dark Reactions 927
JWCL281_c24_901-939.qxd 3/25/10 12:05 PM Page 927
928 Chapter 24. Photosynthesis
ribulose
bisphos-
phate
carboxylase
phospho-
glycerate
kinase
triose phosphate
isomerase
aldolase
aldolase
Ribulose-
1,5-bis-
phosphate
(RuBP)
3-Phospho-
glycerate
(3PG)
1,3-Bisphospho-
glycerate
(BPG)
Glyceraldehyde-
3-phosphate
(GAP)
phospho-
ribulo-
kinase
ATP
fructose
bisphosphatase
phosphopentose
epimerase
transketolase
ADP
ATP ADP
CH
2
OPO
3
2–
CO
2
CH
2
OPO
3
2–
C
COHH
COHH
O
COHH
CH
2
OPO
3
2–
OPO
3
2–
O
CH
2
OPO
3
2–
C
+
HHO
CO
2
–
CO
2
–
Ribulose-5-
phosphate
(Ru5P)
CH
2
OH
CH
2
OPO
3
2–
C
C OHH
COHH
O
glyceraldehyde-
3-phosphate
dehydrogenase
NADPH NADP
+
+ P
i
CCHO
COHH
CH
2
OPO
3
2–
COHH
CH
2
OPO
3
2–
Ribulose-5-
phosphate
(Ru5P)
Dihydroxyacetone
phosphate
(DHAP)
CH
2
OH
CH
2
OPO
3
2–
C
C OHH
COHH
O
Fructose-1,6-
bisphosphate
(FBP)
P
i
Sedoheptulose-1,7-
bisphosphate (SBP)
Sedoheptulose-7-
phosphate (S7P)
CH
2
OPO
3
2–
CH
2
OPO
3
2–
C
C OHH
COHH
H
CO
HO
CH
2
OPO
3
2–
C
C OHH
C OHH
H
CO
HO
CH
2
OPO
3
2–
COHH
C
C OHH
C OHH
H
HO
CH
2
OPO
3
2–
COHH
Fructose-6-
phosphate
(F6P)
Xylulose-5-
phosphate
(Xu5P)
Ribose-5-
phosphate
(R5P)
CH
2
OPO
3
2–
C
C OHH
COHH
H
CH
2
OH
CO
CH
2
OH
CO
C OHH
C OHH
CH
2
OPO
3
2–
COHH
CHO
HO
CH
2
OPO
3
2–
C
COHH
H
CH
2
OH
CO
HO
CH
2
OH
CH
2
OPO
3
2–
CO
Products
Erythrose-4-
phosphate (E4P)
sedoheptulose
bisphosphatase
P
i
CHO
CH
2
OPO
3
2–
C OHH
COHH
ribose
phosphate
isomerase
1
2
5
6
12
9
7
8
34
10
13
11 transketolase
JWCL281_c24_901-939.qxd 3/25/10 12:05 PM Page 928
carboxylation step, which is discussed below, the resulting
3PG is converted first to 1,3-bisphosphoglycerate (BPG)
and then to GAP. This latter sequence is the reverse of
two consecutive glycolytic reactions (Sections 17-2G and
17-2F) except that the Calvin cycle reaction involves
NADPH rather than NADH.
The second stage of the Calvin cycle begins with the re-
verse of a familiar glycolytic reaction, the isomerization of
GAP to dihydroxyacetone phosphate (DHAP) by triose
phosphate isomerase (Section 17-2E). Following this,
DHAP is directed along two analogous paths (Fig. 24-31):
Reactions 6–8 or Reactions 9–11. Reactions 6 and 9 are
aldolase-catalyzed aldol condensations in which DHAP is
linked to an aldehyde (aldolase is specific for DHAP but
accepts a variety of aldehydes). Reaction 6 is also the re-
verse of a glycolytic reaction (Section 17-2D). Reactions 7
and 10 are phosphate hydrolysis reactions that are cat-
alyzed, respectively, by fructose bisphosphatase (FBPase,
which we previously encountered in our discussion of
glycolytic substrate cycles and gluconeogenesis; Sections
17-4Ff and 23-1Ah) and sedoheptulose bisphosphatase
(SBPase). The remaining Calvin cycle reactions are cat-
alyzed by enzymes that also participate in the pentose phos-
phate pathway. In Reactions 8 and 11, both catalyzed by
transketolase, a C
2
keto unit (shaded in green in Fig. 24-31)
is transferred from a ketose to GAP to form xylulose-
5-phosphate (Xu5P) and leave the aldoses erythrose-
4-phosphate (E4P) in Reaction 8 and ribose-5-phosphate
(R5P) in Reaction 11. The E4P produced by Reaction 8
feeds into Reaction 9. The Xu5Ps produced by Reactions 8
and 11 are converted to Ru5P by phosphopentose
epimerase in Reaction 12.The R5P from Reaction 11 is also
converted to Ru5P by ribose phosphate isomerase in Reac-
tion 13, thereby completing a turn of the Calvin cycle. Thus
only 3 of the 11 Calvin cycle enzymes, phosphoribulokinase,
the carboxylation enzyme ribulose bisphosphate carboxy-
lase, and SBPase, have no equivalents in animal tissues.
c. RuBP Carboxylase Catalyzes CO
2
Fixation
in an Exergonic Process
The enzyme that catalyzes CO
2
fixation, ribulose bis-
phosphate carboxylase (RuBP carboxylase), is arguably
the world’s most important enzyme, since nearly all life on
Earth ultimately depends on its action. This protein, pre-
sumably as a consequence of its particularly low catalytic
efficiency (k
cat
⫽ ⬃3 s
⫺1
), comprises up to 50% of leaf pro-
teins and is therefore the most abundant protein in the
biosphere (it is estimated to be synthesized at the rate of
⬃4 ⫻ 10
9
tons/year, which fixes ⬃10
11
tons of CO
2
/year; in
comparison crude oil is consumed at the rate of ⬃3 ⫻ 10
9
tons/year). RuBP carboxylase from higher plants and most
photosynthetic microorganisms consists of eight large (L)
subunits (477 residues in tobacco leaves) encoded by
chloroplast DNA and eight small (S) subunits (123
residues) specified by a nuclear gene (the RuBP carboxy-
lase from certain photosynthetic bacteria is an L
2
dimer
whose L subunit has 28% sequence identity with and is
structurally similar to that of the L
8
S
8
enzyme). X-ray stud-
ies by Carl-Ivar Brändén and by David Eisenberg demon-
strated that the L
8
S
8
enzyme has the D
4
symmetry of a square
prism (Fig. 24-33a,b). The L subunit contains the enzyme’s
catalytic site, as is demonstrated by its enzymatic activity in
the absence of the S subunit. It consists of two domains
(Fig. 24-33c): Residues 1 to 150 form a mixed five-stranded
 sheet and residues 151 to 477 fold into an ␣/ barrel (Fig.
8-19b,c) which, as do nearly all known ␣/ barrel enzymes
(Section 8-3Bh), contains the enzyme’s active site at the
mouth of the barrel near the C-terminus of its  strands.
The function of the S subunit is unknown; attempts to show
that it has a regulatory role, in analogy with other enzymes,
have been unsuccessful.
Section 24-3. Dark Reactions 929
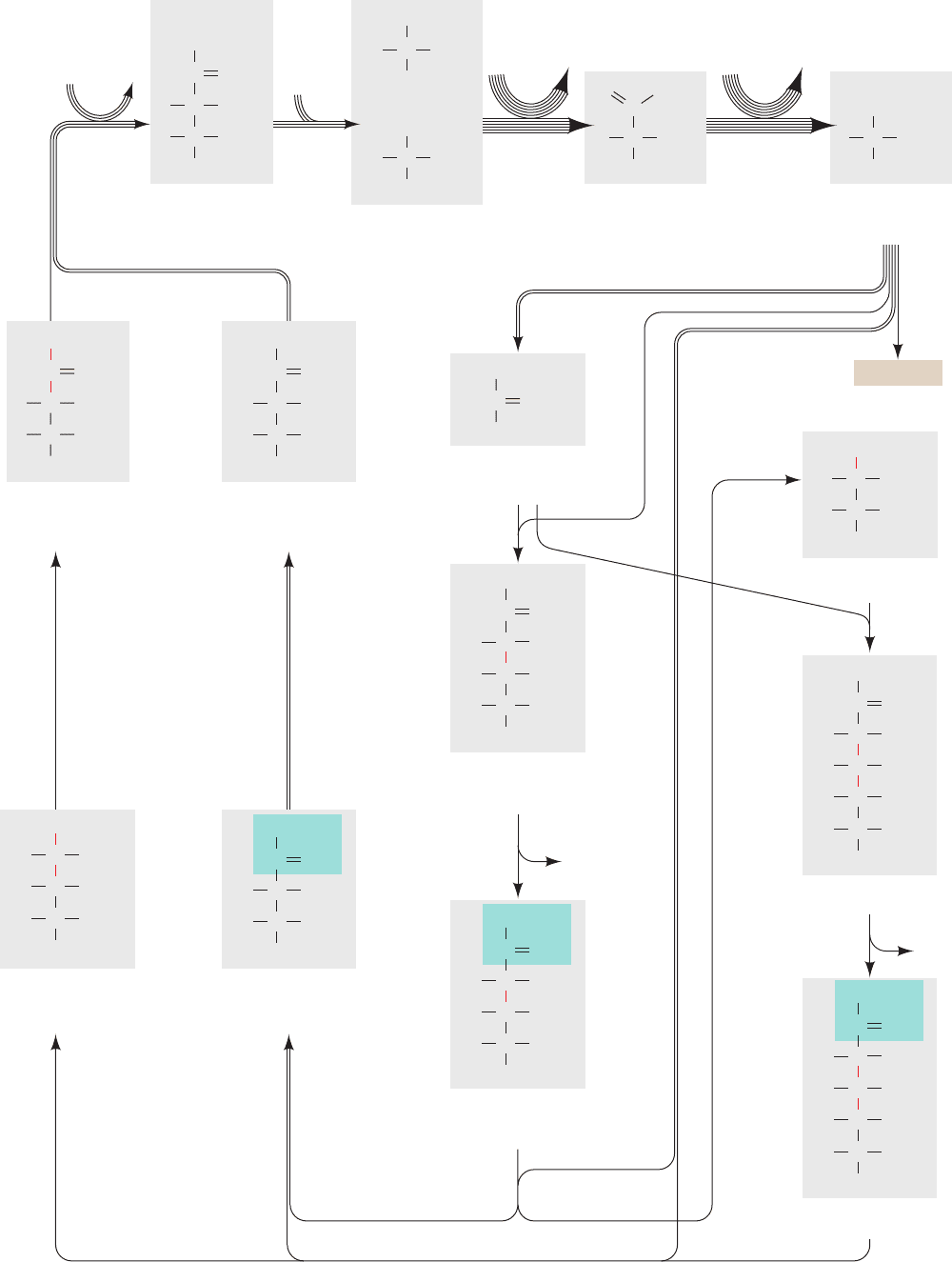
Figure 24-31 The Calvin cycle. (Opposite) The number of
lines in an arrow indicates the number of molecules reacting in
that step for a single turn of the cycle that converts three CO
2
molecules to one GAP molecule. For the sake of clarity, the
sugars are all shown in their linear forms, although the hexoses
and heptoses predominantly exist in their cyclic forms (Section
11-1B).The
14
C-labeling patterns generated in one turn of the
cycle through the use of
14
CO
2
are indicated in red. Note that two
of the Ru5Ps are labeled only at C3, whereas the third Ru5P is
equally labeled at C1, C2, and C3.
See the Animated Figures
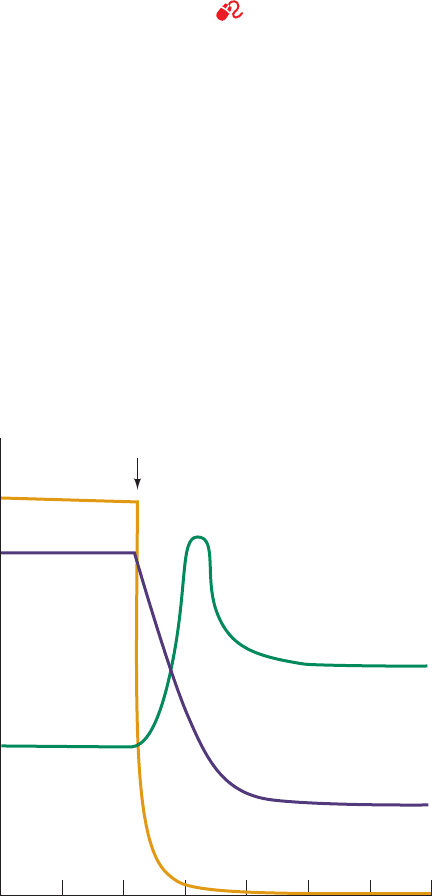
Figure 24-32 Algal 3PG and RuBP levels on removal of CO
2
.
The time course of the levels of 3PG (purple curve) and RuBP
(green curve) in steady-state
14
CO
2
-labeled, illuminated algae is
shown during a period in which the CO
2
(orange curve) is
abruptly withdrawn. In the absence of CO
2
, the 3PG
concentration rapidly decreases because it is taken up by the
reactions of the Calvin cycle but cannot be replenished by them.
Conversely, the RuBP concentration transiently increases as it is
synthesized from the residual pool of Calvin cycle intermediates
but, in the absence of CO
2
, cannot be used for their regeneration.
01234567
Reservoir size
Time (min)
CO
2
withdrawal
RuBP
3PG
CO
2
JWCL281_c24_901-939.qxd 6/16/10 12:04 PM Page 929
The accepted mechanism of RuBP carboxylase, which
was largely formulated by Calvin, is indicated in Fig. 24-34.
Abstraction of the C3 proton of RuBP, the reaction’s rate-
determining step, generates an enediolate that nucleophili-
cally attacks CO
2
(not HCO
3
⫺
). The resulting -keto acid is
rapidly attacked at its C3 position by H
2
O to yield an
adduct that splits, by a reaction similar to aldol cleavage, to
yield the two product 3PG molecules. The following evi-
dence favors this mechanism:
1. The C3 proton of enzyme-bound RuBP exchanges
with solvent, an observation compatible with the existence
of the enediolate intermediate.
2. The C2 and C3 oxygen atoms remain attached to
their respective C atoms, which eliminates mechanisms in-
volving a covalent adduct such as a Schiff base between
RuBP and the enzyme.
3. The trapping of the proposed -keto acid intermedi-
ate by borohydride reduction and the tight enzymatic bind-
ing of its analogs, such as 2-carboxyarabinitol-1-phosphate
(CA1P) and 2-carboxyarabinitol-1,5-bisphosphate (CABP),
provide strong evidence for the existence of this intermediate.
The driving force for the overall reaction, which is highly
exergonic (⌬G°¿ ⫽⫺35.1 kJ ⴢ mol
⫺1
), is provided by the
2-Carboxyarabinitol-
1-phosphate
(CA1P)
HCOH
HCOH
HO C CO
2
CH
2
OPO
2
3
–
CH
2
OH
–
2-Carboxyarabinitol-
1,5-bisphosphate
(CABP)
HCOH
HCOH
HO C CO
2
CH
2
OPO
2
3
–
CH
2
OPO
2
3
–
–
930 Chapter 24. Photosynthesis
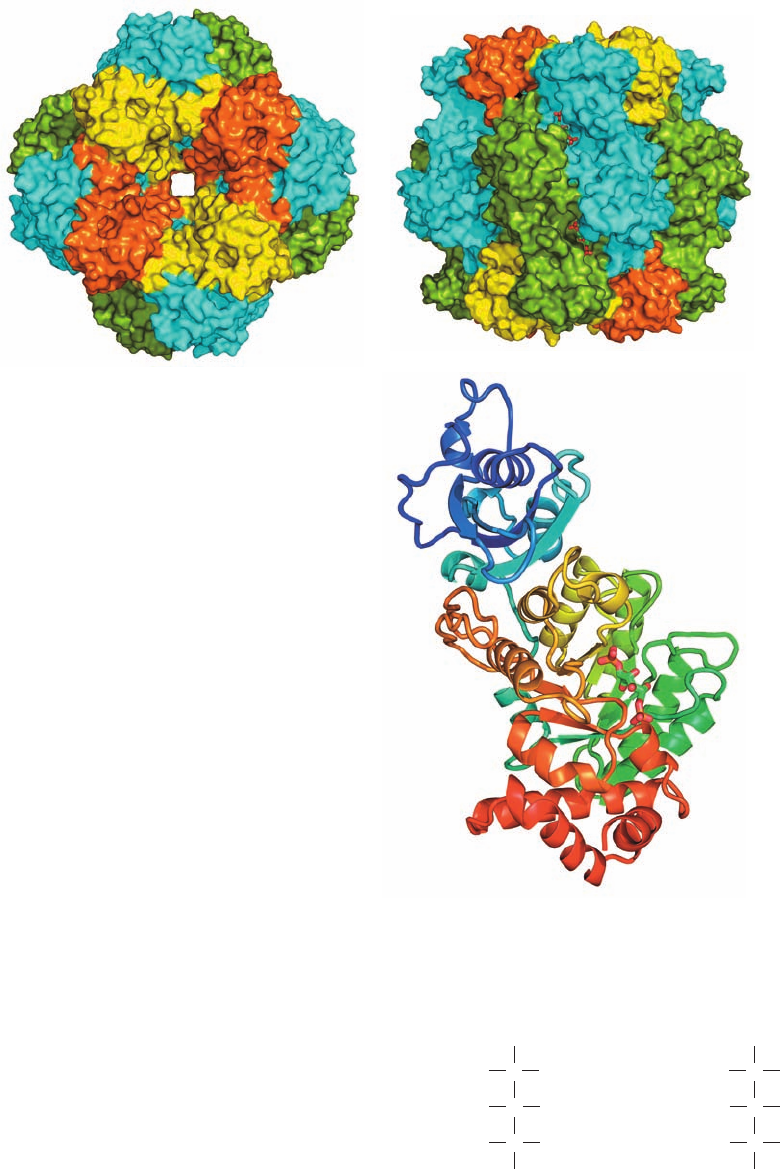
Figure 24-33 X-ray structure of tobacco RuBP carboxylase in
complex with the transition state inhibitor 2-carboxyarabinitol-
1,5-bisphosphate. The ⬃535-kD L
8
S
8
protein has D
4
symmetry
(the rotational symmetry of a square prism; Fig. 8-65b).
(a) Surface diagram viewed along the protein’s 4-fold axis, and
(b) along a 2-fold axis. Parts a and b are related by a 90° rotation
about the horizontal axis.The elongated L subunits can be
considered to associate as two interdigitated tetramers, with that
extending from the top in Part b cyan and that extending from
the bottom green.The S subunits, which form C
4
tetramers that
cap the top and bottom of the complex, are alternately yellow
and orange. The transition state inhibitor 2-carboxyarabinitol-
1,5-bisphosphate (CABP; see text), which is partially visible in
Part b, is drawn in stick form with C green, O red, and P orange.
(c) Ribbon diagram of an L subunit oriented as is the central
green subunit in Part b and colored in rainbow order from its
N-terminus (blue) to its C-terminus (red).The 2-carboxyarabinitol-
1,5-bisphosphate is bound in the mouth of the enzyme’s ␣/
barrel. [Based on an X-ray structure by David Eisenberg, UCLA.
PDBid 1RLC.]
(a)
(b)
(c)
JWCL281_c24_901-939.qxd 6/8/10 8:54 AM Page 930
