Voet D., Voet Ju.G. Biochemistry
Подождите немного. Документ загружается.

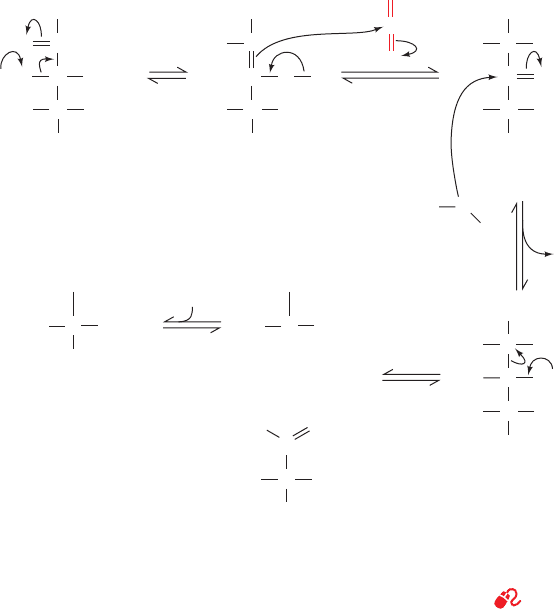
cleavage of the b-keto acid intermediate to yield an addi-
tional resonance-stabilized carboxylate group.
RuBP carboxylase activity requires a bound divalent
metal ion, physiologically Mg
2⫹
, which acts to stabilize the
developing negative charges during catalysis. The Mg
2⫹
is,
in part, bound to the enzyme by a catalytically essential
carbamate group that is generated by the reaction of a non-
substrate CO
2
with the ε-amino group of Lys 201 (R¬NH
2
⫹
CO
2
S R¬NH¬COO
⫺
⫹ H
⫹
).Although the in vitro acti-
vation reaction occurs spontaneously in the presence of
Mg
2⫹
and HCO
3
⫺
, it is blocked in vivo by the particularly
tight binding of RuBP to active sites lacking carbamate.
This inhibition is relieved, however,by the release of RuBP
in an ATP-driven process catalyzed by RuBP carboxylase
activase.
d. Calvin Cycle Products Are Converted to Starch,
Sucrose, and Cellulose
The overall stoichiometry of the Calvin cycle is
GAP, the primary product of photosynthesis, is used in a va-
riety of biosynthetic pathways, both inside and outside the
chloroplast. For example, fatty acids and amino acids are
synthesized from GAP as is described, respectively, in Sec-
tions 25-4 and 26-5. GAP can also be converted to fructose-
GAP ⫹ 9ADP ⫹ 8P
i
⫹ 6NADP
⫹
3CO
2
⫹ 9ATP ⫹ 6NADPH
¡
6-phosphate by the further action of Calvin cycle enzymes
and then to glucose-1-phosphate (G1P) by phosphoglucose
isomerase (Section 17-2B) and phosphoglucomutase
(Section 18-1B). G1P is the precursor of the higher carbohy-
drates characteristic of plants.
The polysaccharide ␣-amylose, a major component of
starch (Section 11-2D), is synthesized in the chloroplast
stroma as a temporary storage depot for glucose units. It is
also synthesized as a long-term storage molecule elsewhere
in the plant, including leaves, seeds, and roots. G1P is first
activated by its reaction with ATP to form ADP–glucose as
catalyzed by ADP–glucose pyrophosphorylase. Starch syn-
thase then transfers the glucose residue to a nonreducing
end of an ␣-amylose or amylopectin molecule, forming a
new glycosidic linkage (Fig. 24-35). The overall reaction is
driven by the exergonic hydrolysis of the PP
i
released in
the formation of ADP–glucose.A similar reaction sequence
occurs in glycogen synthesis, which uses UDP–glucose
(Section 18-2A). The ␣(1 S 6) branches of amylopectin
(Section 11-2D) are made by starch-branching enzyme,
which functions similarly to glycogen branching enzyme
(Section 18-2C).
Sucrose, a disaccharide of glucose and fructose (Section
11-2B), is the major transport sugar for delivering carbohy-
drates to nonphotosynthesizing cells and hence is the major
photosynthetic product of green leaves. Since sucrose is syn-
thesized in the cytosol, either glyceraldehyde-3-phosphate
Section 24-3. Dark Reactions 931
Figure 24-34 Probable mechanism of the carboxylation
reaction catalyzed by RuBP carboxylase. The reaction proceeds
via an enediolate intermediate that nucleophilically attacks CO
2
OH
C
CH
2
OPO
2
3
–
C
O
H
OH
CH
CH
2
OPO
2
3
–
RuBP
Enz-B:
O
C
CH
2
OPO
2
3
–
C
–
O
H
OH
CH
CH
2
OPO
2
3
–
O
C
O
O
C
CH
2
OPO
2
3
–
C
HO
OH
CH
CH
2
OPO
2
3
–
CO
–
2
Enediolate -Keto acid
O
–
C
CH
2
OPO
2
3
–
C
HO
OH
CH
CH
2
OPO
2
3
–
CO
–
2
OH
H
H
+
HO
C
CH
2
OPO
2
3
–
H
HO
CO
–
2
+
C
CH
2
OPO
2
3
–
HO CO
–
2
–
O
–
O
C
C
OH
H
CH
2
OPO
2
3
–
3PG
3PG
H
+
to form a -keto acid.This intermediate reacts with water to
yield two molecules of 3PG.
See the Animated Figures
JWCL281_c24_901-939.qxd 3/25/10 12:05 PM Page 931
or dihydroxyacetone phosphate is transported out of the
chloroplast by an antiporter that exchanges phosphate for a
triose phosphate. Two trioses combine to form fructose-6-
phosphate (F6P) and subsequently glucose-1-phosphate
(G1P), which is then activated by UTP to form UDP–
glucose. Next, sucrose-6-phosphate is produced in a reaction
catalyzed by sucrose-phosphate synthase. Finally, sucrose-6-
phosphate is hydrolyzed by sucrose-phosphate phosphatase
to yield sucrose (Fig. 24-36), which is then exported to other
plant tissues.
Cellulose, which consists of long chains of (1 S 4)-
linked glucose units and is the major polysaccharide of
plants, is also synthesized from UDP–glucose. Plant cell
walls consist of almost crystalline cables containing 36 par-
allel cellulose chains, each of 500 to 15,000 glucose units, all
embedded in an amorphous matrix of other polysaccha-
rides and lignin (Section 11-2C). Unlike starch in plants or
glycogen in mammals, cellulose is synthesized by multisub-
unit enzyme complexes in the plant plasma membrane and
extruded into the extracellular space.
B. Control of the Calvin Cycle
During the day, plants satisfy their energy needs via the
light and dark reactions of photosynthesis. At night, how-
ever, like other organisms, they must use their nutritional
reserves to generate their required ATP and NADPH
through glycolysis, oxidative phosphorylation, and the pen-
tose phosphate pathway. Since the stroma contains the en-
zymes of glycolysis and the pentose phosphate pathway as
well as those of the Calvin cycle, plants must have a light-
sensitive control mechanism to prevent the Calvin cycle
from consuming this catabolically produced ATP and
NADPH in a wasteful futile cycle.
As we saw in Section 17-4F,the control of flux in a meta-
bolic pathway occurs at enzymatic steps that are far from
equilibrium; that is, those that have a large negative value
of ⌬G. Inspection of Table 24-1 indicates that the four best
candidates for flux control in the Calvin cycle are the reac-
tions catalyzed by phosphoribulokinase, RuBP carboxylase,
FBPase, and SBPase (Reactions 1, 2, 7, and 10, Fig. 24-31).
In fact, the catalytic efficiencies of these four enzymes all
vary, in vivo, with the level of illumination.
932 Chapter 24. Photosynthesis
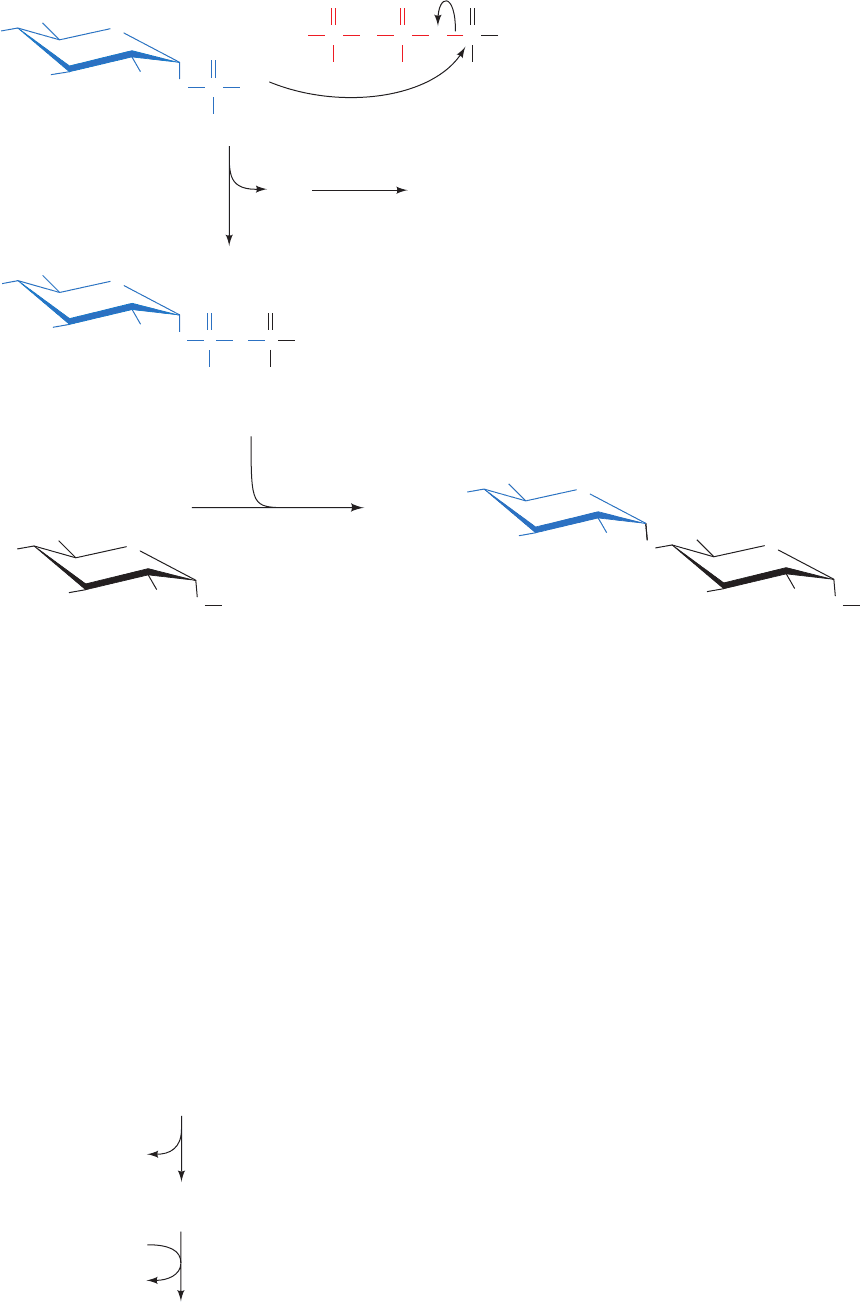
Figure 24-35 Starch synthesis. ADP–glucose
is formed from G1P and ATP in a
phosphoanhydride exchange reaction (1).The
PP
i
product is rapidly hydrolyzed.ADP–glucose
is the substrate for starch synthase (2), which
adds the glucose residue to a nonreducing end
of an existing polysaccharide, releasing ADP.
Figure 24-36 The synthesis of sucrose.
O
OH
HO
HO
CH
2
OH
O
O
OH
HO
CH
2
OH
O
Adenosine
Adenosine
ADP
ATP
+
α
-Amylose (n residues)
α
-Amylose (n + 1 residues)
starch synthase
O
PO
–
O
O
P
–
O
O
␥␣
O
P
–
O
–
O
ADP – glucose
PP
i
2 P
i
inorganic
pyrophos-
phatase
ADP–glucose
pyrophophorylase
O
P
O
–
O
–
O
O
P
G1P
1
2
O
OH
HO
HO
CH
2
OH
O
P
O
–
O
–
O
O
OH
HO
HO
CH
2
OH
O
O
OH
HO
HO
CH
2
OH
O
...
O
...
UDP–glucose ⫹ F6P
Sucrose-6-phosphate
sucrose-phosphate
synthase
UDP
sucrose-phosphate
phosphatase
H
2
O
P
i
Sucrose
JWCL281_c24_901-939.qxd 3/25/10 12:05 PM Page 932
The activity of RuBP carboxylase responds to three
light-dependent factors:
1. It varies with pH. On illumination, the pH of the
stroma increases from around 7.0 to about 8.0 as protons
are pumped from the stroma into the thylakoid lumen.
RuBP carboxylase has a sharp pH optimum near pH 8.0.
2. It is stimulated by Mg
2⫹
. Recall that the light-induced
influx of protons to the thylakoid lumen is accompanied by
the efflux of Mg
2⫹
to the stroma (Section 24-2Db).
3. It is strongly inhibited by its transition state analog
2-carboxyarabinitol-1-phosphate (CA1P; Section 24-3Ac),
which many plants synthesize only in the dark. RuBP car-
boxylase activase (Section 24-3Ac) also facilitates the re-
lease of the tight-binding CA1P from RuBP carboxylase.
FBPase and SBPase are also activated by increased pH
and Mg
2⫹
,and by NADPH as well.The action of these factors
is complemented by a second regulatory system that re-
sponds to the redox potential of the stroma. Thioredoxin
(Trx), an ⬃105-residue protein that occurs in many types of
cells, contains a redox-active disulfide group. Reduced Trx ac-
tivates five Calvin cycle enzymes by disulfide interchange re-
actions (Fig. 24-37): phosphoribulokinase, glyceraldehyde-3-
phosphate dehydrogenase, FBPase, SBPase, and RuBP
carboxylase activase.This explains why these enzymes are ac-
tivated by reduced disulfide reagents such as dithiothreitol.
The redox level of Trx is maintained by ferredoxin–thiore-
doxin reductase (FTR), which contains a redox-active disul-
fide that is closely associated with a [4Fe–4S] cluster through
which the protein directly responds to the redox state of sol-
uble ferredoxin (Fd) in the stroma. This, as we have seen
(Section 24-2Ch), varies with the illumination level.
Section 24-3. Dark Reactions 933
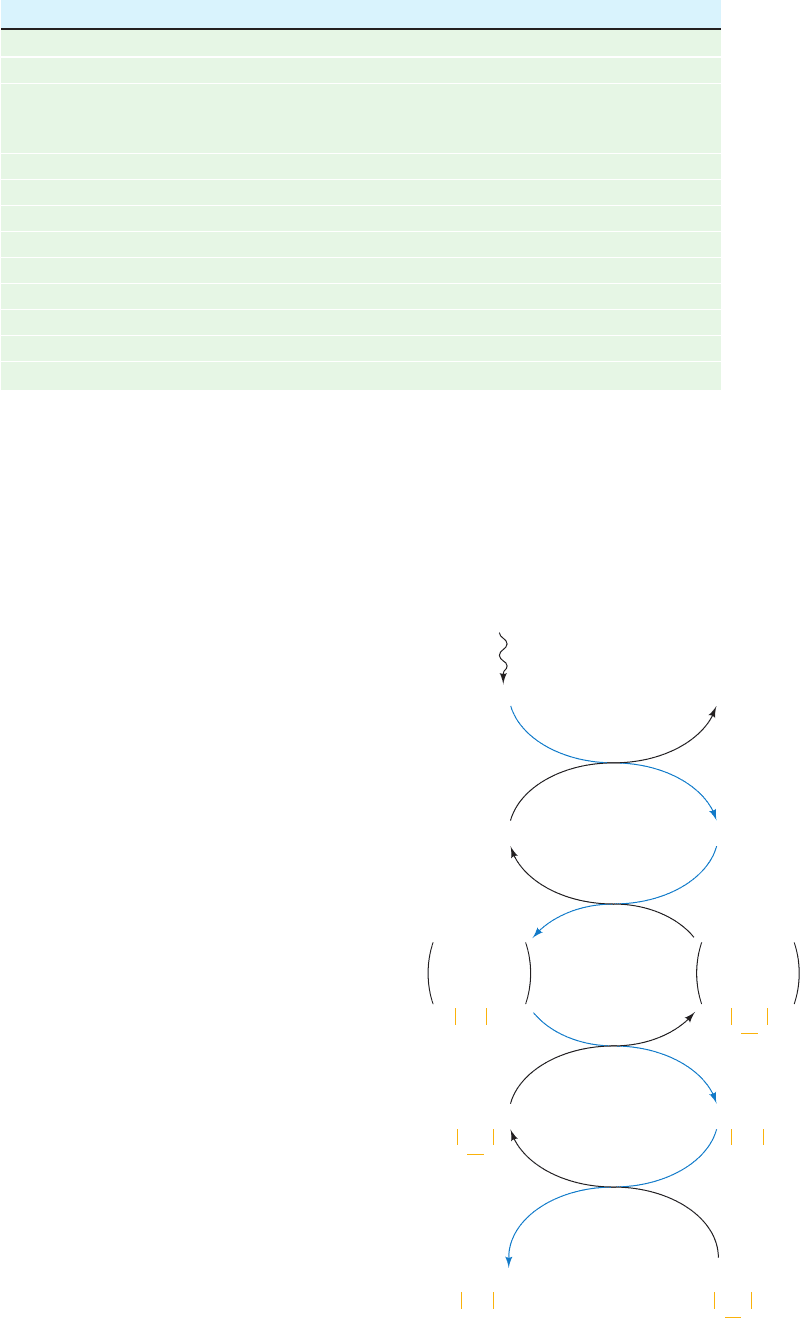
Figure 24-37 Light-activation mechanism of chloroplast enzymes.
Photoactivated PSI reduces soluble ferredoxin (Fd), which reduces
ferredoxin–thioredoxin reductase, which, in turn, reduces the disulfide
linkage of thioredoxin. Reduced thioredoxin reacts with its target enzymes
by disulfide interchange, thereby activating or deactivating these enzymes.
In the dark, these processses are quickly reversed by reaction with oxygen.
a
Refer to Fig. 24-31.
Source: Bassham, J.A. and Buchanan, B.B., in Govindjee (Ed.), Photosynthesis, Vol. II, p. 155, Academic Press
(1982).
Step
a
Enzyme ⌬G°¿ (kJ ⴢ mol
⫺1
) ⌬G (kJ ⴢ mol
⫺1
)
1 Phosphoribulokinase ⴚ21.8 ⴚ15.9
2 Ribulose bisphosphate carboxylase ⴚ35.1 ⴚ41.0
3 ⫹ 4 Phosphoglycerate kinase ⫹⫹18.0 ⫺6.7
glyceraldehyde-3-phosphate
dehydrogenase
5 Triose phosphate isomerase ⫺7.5 ⴚ0.8
6 Aldolase ⴚ21.8 ⴚ1.7
7 Fructose bisphosphatase ⴚ14.2 ⴚ27.2
8 Transketolase ⫹6.3 ⴚ3.8
9 Aldolase ⴚ23.4 ⴚ0.8
10 Sedoheptulose bisphosphatase ⴚ14.2 ⴚ29.7
11 Transketolase ⫹0.4 ⴚ5.9
12 Phosphopentose epimerase ⫹0.8 ⴚ0.4
13 Ribose phosphate isomerase ⫹2.1 ⴚ0.4
Table 24-1 Standard and Physiological Free Energy Changes for the Reactions
of the Calvin Cycle
Fd
ox
Fd
red
Thioredoxin Thioredoxin
SH
(Enzyme)
inactive
(Enzyme)
active
PSI
ox
PSI
red
PSI
red
hν
*
red ox
Ferredoxin–
thioredoxin
reductase
Ferredoxin–
thioredoxin
reductase
SH S S
SH SH
SH SH S S
SS
JWCL281_c24_901-939.qxd 3/25/10 12:05 PM Page 933
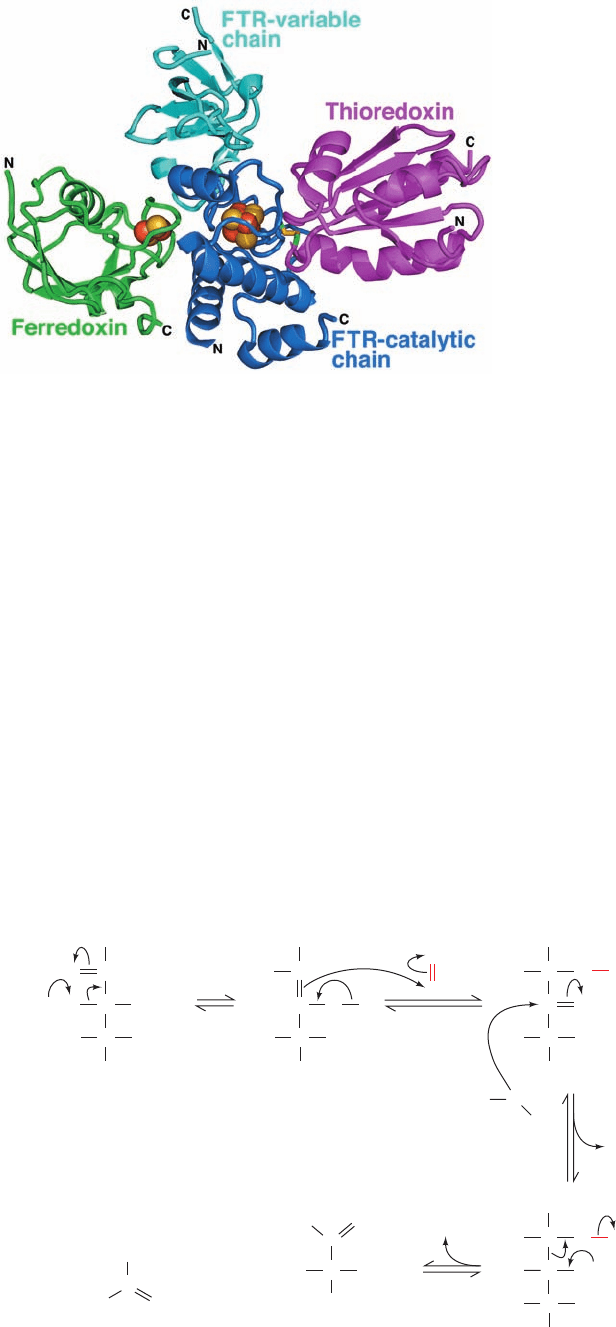
The X-ray structure of a Fd–FTR–Trx complex (Fig.
24-38), determined by Hans Eklund, reveals that redox-
active Cys residues of FTR and thioredoxin have formed
a disulfide bond. Moreover, the distance between this
disulfide bond and the [2Fe–2S] cluster of Fd is only ⬃20
Å as a consequence of the remarkably thin disklike cat-
alytic subunit of FTR, and that the [4Fe–4S] cluster of
FTR lies on a straight line between these two redox cen-
ters. This maximizes the efficiency of electron transfer
from the [2Fe–2S] cluster of Fd to the redox active disul-
fide of Trx.
Reduced Trx also deactivates the chloroplastic enzymes
phosphofructokinase (PFK), the main flux-generating en-
zyme of glycolysis (Section 17-4Fb), and glucose-6-phosphate
dehydrogenase, the first enzyme in the pentose phosphate
pathway (Section 23-4A), whose products, ATP and
NADPH, would otherwise be used by the Calvin cycle in a
futile cycle. Thus in plants, light stimulates the Calvin cycle
while deactivating glycolysis and the pentose phosphate
pathway, whereas darkness has the opposite effect (that is,
the so-called dark reactions do not occur in the dark). More-
over, chloroplast ATP synthase is activated by reduced Trx,
thus preventing it from uselessly hydrolyzing glycolytically
produced ATP in the dark. Indeed, the redox state of Trx
regulates a great variety of plant metabolic processes.
We have seen that ferredoxin reduces ferredoxin–
NADP
⫹
reductase (Section 24-2Ch) and FTR, as well as
supplying electrons to the cyclic pathway of chloroplast
photosynthesis (Section 24-2Ch). In addition, ferredoxin is
the reducing agent for three metabolically essential chloro-
plast enzymes: sulfite reductase (which reduces SO
3
2⫺
to
S
2
⫺
), nitrite reductase (which reduces NO
2
⫺
to ), and
glutamate synthase (which catalyzes the reaction of
␣-ketoglutarate and to form glutamate; Section 26-
5Aa).Thus Fd stands at the center of a complex web of en-
zymatic and regulatory processes.
C. Photorespiration and the C
4
Cycle
It has been known since the 1960s that illuminated plants con-
sume O
2
and evolve CO
2
in a pathway distinct from oxidative
phosphorylation. In fact, at low CO
2
and high O
2
levels, this
photorespiration process can outstrip photosynthetic CO
2
fixation. The basis of photorespiration was unexpected: O
2
competes with CO
2
as a substrate for RuBP carboxylase
(RuBP carboxylase is therefore also called RuBP carboxy-
lase–oxygenase or RuBisCO). In the oxygenase reaction, O
2
reacts with RuBisCO’s second substrate, RuBP, to form 3PG
and 2-phosphoglycolate (Fig. 24-39).The 2-phosphoglycolate
NH
⫹
4
NH
⫹
4
934 Chapter 24. Photosynthesis
Figure 24-38 X-ray structure of a Fd–FTR–Trx complex. The
subunits are drawn in ribbon form with Fd green, the catalytic
subunit of FTR blue, its variable subunit cyan, and Trx magenta.
The [2Fe–2S] cluster of Fd and the [4Fe–4S] cluster of FTR are
drawn in space-filling form with S yellow and Fe red-brown. The
two Cys side chains forming the disulfide bond between FTR
and Trx are shown in stick form with C green and S yellow.
[Based on an X-ray structure by Hans Eklund, Swedish Univer-
sity of Agricultural Sciences, Uppsala, Sweden. PDBid 2PVO.]
Figure 24-39 Probable mechanism of the
oxygenase reaction catalyzed by RuBP
carboxylase–oxygenase. Note the similarity
of this mechanism to that of the carboxylase
reaction catalyzed by the same enzyme
(Fig. 24-34).
OH
C
CH
2
OPO
2
3
–
C
O
H
OH
CH
CH
2
OPO
2
3
–
RuBP
Enz-B:
O
C
CH
2
OPO
2
3
–
C
–
O
H
OH
CH
CH
2
OPO
2
3
–
O
O
O
C
CH
2
OPO
2
3
–
C
HO
OH
CH
CH
2
OPO
2
3
–
OH
Enediolate
O
–
C
CH
2
OPO
2
3
–
C
HO
OH
CH
CH
2
OPO
2
3
–
OH
H
H
+
HO
C
CH
2
OPO
2
3
–
O
–
O
C
C
OH
H
CH
2
OPO
2
3
–
3PG
2-Phosphoglycolate
O
OHO
H
2
O
+
O
–
O
JWCL281_c24_901-939.qxd 3/25/10 12:05 PM Page 934
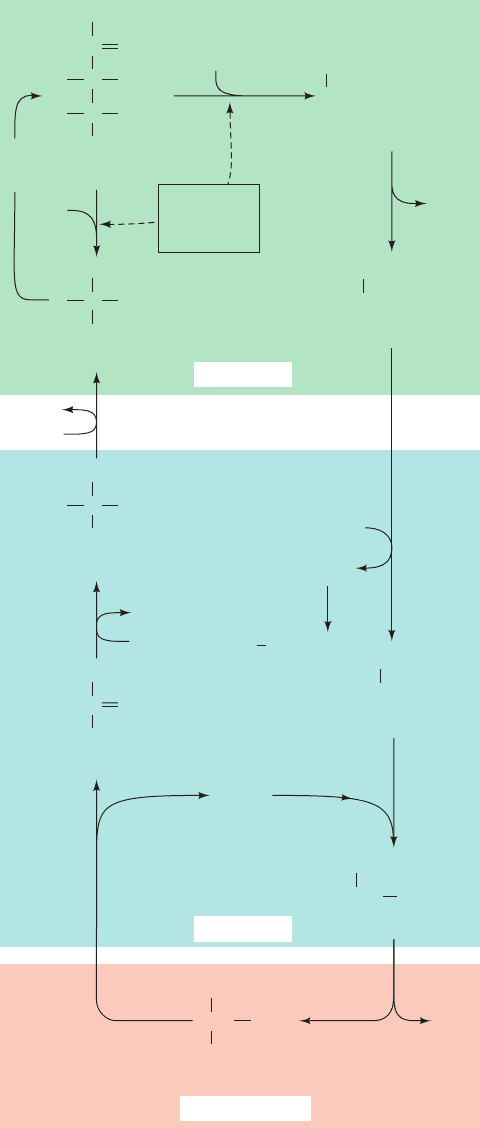
is hydrolyzed to glycolate by glycolate phosphatase and, as
described below, is partially oxidized to yield CO
2
by a se-
ries of enzymatic reactions that occur in the peroxisome
and the mitochondrion. Thus photorespiration is a seem-
ingly wasteful process that undoes some of the work of
photosynthesis. In the following subsections we discuss the
biochemical basis of photorespiration, its significance, and
how certain plants manage to evade its deleterious effects.
a. Photorespiration Dissipates ATP and NADPH
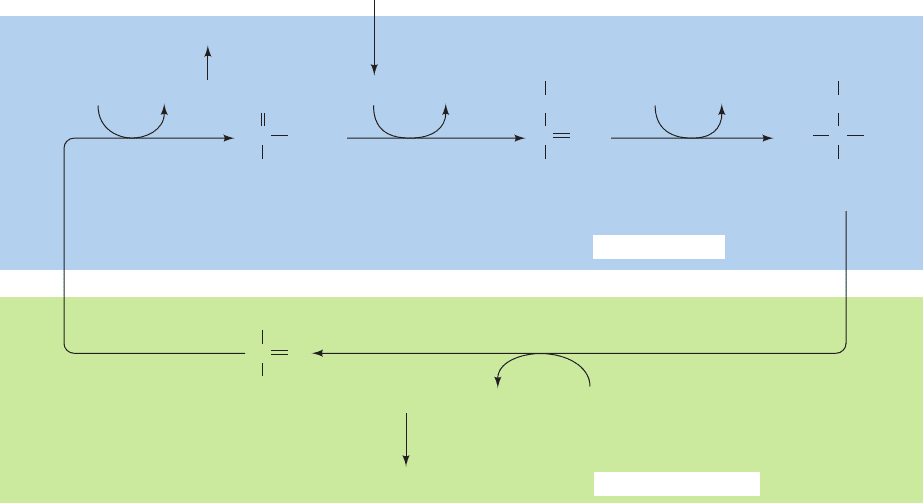
The photorespiration pathway is outlined in Fig. 24-40.
Glycolate is exported from the chloroplast to the peroxi-
some (also called the glyoxisome, Sections 1-2Ad and 23-2),
where it is oxidized by glycolate oxidase to glyoxylate and
H
2
O
2
. The H
2
O
2
, a powerful and potentially harmful oxi-
dizing agent, is disproportionated to H
2
O and O
2
in the
peroxisome by the heme-containing enzyme catalase.
Some of the glyoxylate is further oxidized by glycolate ox-
idase to oxalate. The remainder is converted to glycine in a
transamination reaction, as discussed in Section 26-1A,and
exported to the mitochondrion. There, two molecules of
glycine are converted to one molecule of serine and one of
CO
2
by a reaction described in Section 26-3B. This is the
origin of the CO
2
generated by photorespiration. The serine
is transported back to the peroxisome, where a transamina-
tion reaction converts it to hydroxypyruvate. This sub-
stance is reduced to glycerate and phosphorylated in the
cytosol to 3PG, which reenters the chloroplast, where it is
reconverted to RuBP in the Calvin cycle. The net result of
this complex photorespiration cycle is that some of the ATP
and NADPH generated by the light reactions is uselessly
dissipated.
Although photorespiration has no known metabolic
function, the RuBisCOs from the great variety of photo-
synthetic organisms so far tested all exhibit oxygenase ac-
tivity. Yet, over the eons, the forces of evolution must have
optimized the function of this important enzyme. It is
thought that photosynthesis evolved at a time when
Earth’s atmosphere contained large quantities of CO
2
and
very little O
2
, so that photorespiration was of no conse-
quence. It has therefore been suggested that the RuBisCO
reaction has an obligate intermediate that is inherently au-
tooxidizable. Another possibility is that photorespiration
protects the photosynthetic apparatus from photooxida-
tive damage when insufficient CO
2
is available to other-
wise dissipate its absorbed light energy. This hypothesis is
supported by the observation that when chloroplasts or
leaf cells are brightly illuminated in the absence of both
CO
2
and O
2
, their photosynthetic capacity is rapidly and ir-
reversibly lost.
b. Photorespiration Limits the Growth
Rates of Plants
The steady-state CO
2
concentration attained when a
photosynthetic organism is illuminated in a sealed system
is named its CO
2
compensation point. For healthy plants,
this is the CO
2
concentration at which the rates of photo-
synthesis and photorespiration are equal. For many species
it is ⬃40 to 70 ppm (parts per million) CO
2
(the normal
Section 24-3. Dark Reactions 935
Figure 24-40 Photorespiration. This pathway metabolizes the
phosphoglycolate produced by the RuBP carboxylase-catalyzed
oxidation of RuBP. The reactions occur, as indicated, in the
chloroplast, the peroxisome, the mitochondrion, and the cytosol.
Note that two glycines are required to form serine ⫹ CO
2
(Section 26-3B).
C
O
C
OH
OH
C
H
H
CH
O
2
CH
O
2
PO
C
OH
H
CO
2
CH
O
2
3PG
CO
2
Calvin
cycle
O
2
RuBP
carboxylase–
oxygenase
+ 3PG
2-Phosphoglycolate
phospho-
glycolate
phosphatase
P
i
Glycolate
Peroxisome
ADP
ATP
glycerate kinase
–
C
OH
H
CO
2
CH
OH
2
–
NAD
NADH
+
hydroxy-
pyruvate
reductase
Glycerate
C
CO
2
CH
OH
2
–
Hydroxypyruvate
O
O
2
H
2
O
2
Glyoxylate
Glycine
catalase
H
2
O+O
2
NH
3
()
Transamination
CO
2
CH
OH
2
–
Serine
CH NH
3
+
CO
2
Mitochondrion
RuBP
2
1
3
2–
PO
3
2–
PO
3
2–
glycolate
oxidase
CO
CH
O
2
PO
3
2–
2
CO
CH
OH
2
2
–
CO
CHO
2
–
CO
CH
NH
2
2
–
3
+
–
Cytosol
Chloroplast
JWCL281_c24_901-939.qxd 6/8/10 8:54 AM Page 935
atmospheric concentration of CO
2
is 330 ppm), so their
photosynthetic CO
2
fixation usually dominates their pho-
torespiratory CO
2
release. However, the CO
2
compensa-
tion point increases with temperature because the oxyge-
nase activity of RuBisCO increases more rapidly with
temperature than does its carboxylase activity. Thus, on a
hot bright day, when photosynthesis has depleted the level of
CO
2
at the chloroplast and raised that of O
2
, the rate of pho-
torespiration may approach that of photosynthesis. This
phenomenon is, in fact, a major limiting factor in the growth
of many plants. Indeed, plants possessing a RuBisCO with
significantly less oxygenase activity would not only have
increased photosynthetic efficiency but would need less
water because they could spend less time with their stomata
(the pores leading to their internal leaf spaces) open ac-
quiring CO
2
and would have a reduced need for fertilizer
because they would require less RuBisCO. The control of
photorespiration is therefore an important unsolved agri-
cultural problem that is presently being attacked through
genetic engineering studies (Section 5-5).
c. C
4
Plants Concentrate CO
2
Certain species of plants, such as sugarcane, corn, and
most important weeds, have a metabolic cycle that concen-
trates CO
2
in their photosynthetic cells, thereby almost to-
tally preventing photorespiration (their CO
2
compensation
points are in the range 2 to 5 ppm). The leaves of plants that
have this so-called C
4
cycle have a characteristic anatomy.
Their fine veins are concentrically surrounded by a single
layer of so-called bundle-sheath cells, which in turn are sur-
rounded by mesophyll cells.
The C
4
cycle (Fig. 24-41) was elucidated in the 1960s by
Marshall Hatch and Roger Slack. It begins with the uptake
of atmospheric CO
2
by the mesophyll cells, which, lacking
RuBisCO in their chloroplasts, do so by condensing it as
HCO
3
⫺
with phosphoenolpyruvate (PEP) to yield oxaloac-
etate. The oxaloacetate is reduced by NADPH to malate,
which is exported to the bundle-sheath cells (the name C
4
refers to these four-carbon acids). There the malate is ox-
idatively decarboxylated by NADP
⫹
to form CO
2
, pyru-
vate, and NADPH. The CO
2
, which has been concentrated
by this process, enters the Calvin cycle. The pyruvate is re-
turned to the mesophyll cells, where it is phosphorylated to
again form PEP. The enzyme that mediates this reaction,
pyruvate-phosphate dikinase, has the unusual action of ac-
tivating a phosphate group through the hydrolysis of ATP
to AMP ⫹ PP
i
. This PP
i
is further hydrolyzed to two P
i
,
which is tantamount to the consumption of a second ATP.
CO
2
is thereby concentrated in the bundle-sheath cells at
the expense of two ATPs per CO
2
. The dark reactions of
photosynthesis in C
4
plants therefore consume a total of five
ATPs per CO
2
fixed versus the three ATPs required by the
Calvin cycle alone. The additional ATP is presumably gen-
erated through the cyclic flow of electrons in the light reac-
tions (Section 24-2Ch).
C
4
plants, which comprise ⬃5% of terrestrial plants, oc-
cur largely in unshaded areas of tropical regions because
they grow faster under hot and sunny conditions than other,
so-called C
3
plants (so named because they initially fix CO
2
in the form of three-carbon acids). In cooler climates, where
photorespiration is less of a burden, C
3
plants have the ad-
vantage because they require less energy to fix CO
2
.
936 Chapter 24. Photosynthesis
Figure 24-41 The C
4
pathway. CO
2
is concentrated in the mesophyll cells and transported to
the bundle-sheath cells for entry into the Calvin cycle.
C
OH
H
CO
2
Malate
–
CH
2
CO
2
C
Oxaloacetate
CH
2
CO
2
CO
2
O
C
Phosphoenol-
pyruvate (PEP)
CH
2
CO
2
OPO
3
–
–––
PEP carboxylase malate
dehydrogenase
pyruvate-
phosphate
dikinase
C
Pyruvate
CH
3
CO
2
O
–
P
i
ATP + PP
i
AMP +
2 P
i
P
i
HCO
3
–
CO
2
NADPH NADP
+
malic enzyme
NADPH NADP
+
+CO
2
Calvin cycle
3PG
Mesophyll cell
Bundle-sheath cell
Air
carbonic
anhydrase
2–
JWCL281_c24_901-939.qxd 3/25/10 12:05 PM Page 936

d. CAM Plants Store CO
2
through a Variant
of the C
4
Cycle
Desert-adapted plants known as succulents (e.g., cacti)
employ a variant of the C
4
cycle that separates CO
2
acquisi-
tion and the Calvin cycle in time rather than in space. If, as
most plants, they opened their stomata by day to acquire
CO
2
, they would simultaneously transpire (lose by evapora-
tion) what for them would be unacceptable amounts of wa-
ter. To minimize this loss, these succulents only absorb CO
2
at night when the temperature is relatively cool. They store
this CO
2
, in a process known as crassulacean acid
metabolism (CAM; so named because it was first discovered
in plants of the family Crassulaceae), by the synthesis of
malate through the reactions of the C
4
pathway (Fig. 24-41).
The large amount of PEP necessary to store a day’s supply
of CO
2
is obtained by the breakdown of starch via glycolysis.
During the course of the day, this malate is broken down to
CO
2
, which enters the Calvin cycle, and pyruvate, which is
used to resynthesize starch.CAM plants are able, in this way,
to carry out photosynthesis with minimal water loss.
Chapter Summary 937
1 Chloroplasts Photosynthesis is the light-driven fixa-
tion of CO
2
to form carbohydrates and other biological mole-
cules. In plants, photosynthesis takes place in the chloroplast,
which consists of an inner and an outer membrane surround-
ing the stroma, a concentrated enzyme solution in which the
thylakoid membrane system is immersed. Photosynthesis oc-
curs in two stages, the so-called light reactions in which light
energy is harnessed to synthesize ATP and NADPH, and the
dark reactions in which these products are used to drive the
synthesis of carbohydrates from CO
2
and H
2
O. The thylakoid
membrane is the site of the photosynthetic light reactions,
whereas the dark reactions take place in the stroma.The coun-
terpart of the thylakoid in photosynthetic bacteria is a
specialized portion of the plasma membrane named the
chromatophore.
2 Light Reactions Chlorophyll is the principal photore-
ceptor of photosynthesis. Light is absorbed initially by light-
harvesting complexes (LHCs) that contain chlorophyll and ac-
cessory pigments such as carotenoids. The resulting excitation
then migrates via exciton transfer until it reaches the reaction
center chlorophyll, where it is trapped. LH2 from purple pho-
tosynthetic bacteria is a transmembrane protein that consists
of eight or nine rotationally related subunits that each bind
three BChl a molecules and one carotenoid. LH1, which is
similarly arranged but 16-fold symmetric, contains a central
hole that binds a photosynthetic reaction center (RC). Light
energy absorbed by LH2 is transmitted to LH1, which, in turn,
transmits it to the RC.
The purple photosynthetic bacterial RC (PbRC) is a pro-
tein that consists of three subunits and several redox-active
small molecules that are arranged as two pseudosymmetri-
cally related chains of electron carriers. The primary photon
absorbing species of the Rps. viridis bacterial reaction center is
a special pair of BChl b molecules known as P960. By rapid
measurement techniques it has been determined that the elec-
tron ejected by P960* passes by a third BChl b to a BPheo b
molecule located in only one of the two chains (the other
is apparently nonfunctional) and then sequentially to a
menaquinone (Q
A
) and a ubiquinone (Q
B
).The resulting is
subsequently further reduced in a second one-electron trans-
fer process and then takes up two protons from the cytosol to
form Q
B
H
2
. The electrons taken up by this species are re-
turned to P960 via a cytochrome bc
1
complex, cytochrome c
2
,
and, in some purple photosynthetic bacteria, a four-heme c-
type cytochrome associated with the photosynthetic reaction
center. This cyclic electron-transport process functions to
Q
B
⫺
ⴢ
translocate protons, via a Q cycle mediated by the cytochrome
bc
1
, from the cytoplasm to the outside of the cell.The resulting
proton gradient, in a process known as photophosphorylation,
drives the synthesis of ATP. Since bacterial photosynthesis
does not generate the reducing equivalents needed in many
biosynthetic processes, photosynthetic bacteria require an
outside source of reducing agents such as H
2
S.
In plants and cyanobacteria,the light reactions occur in two
reaction centers, those of PSI and PSII, which are electrically
“connected” in series.This enables the system to generate suf-
ficient electromotive force to form NADPH by oxidizing H
2
O
in a noncyclic pathway known as the Z-scheme. PSI and PSII
both contain core antenna systems and their RCs are evolu-
tionarily related to each other and to the PbRC. PSII contains
an Mn
4
CaO
4
complex that oxidizes two H
2
O molecules to four
H
⫹
and O
2
in four one-electron steps.The electrons are passed
singly, through a Tyr side chain named Z, to photooxidized
P680, the reaction center’s photon-absorbing species, a special
pair that consists of two Chl a molecules. The electron previ-
ously ejected from P680* passes through a series of carriers re-
sembling those of the PbRC to a pool of plastoquinone mole-
cules. The electrons then enter the cytochrome b
6
f complex,
which transports protons, via a Q cycle, from the stroma to the
thylakoid space. These electrons are transferred individually,
by a plastocyanin carrier, directly to PSI’s photooxidized
photon-absorbing pigment, P700, a pair of Chl a’s that resem-
bles the PbRC’s special pair.The electron that had been previ-
ously ejected from P700* migrates through both sides of a
bifurcated chain of Chl a molecules and then through a chain
of three [4Fe–4S] clusters to a soluble ferredoxin (Fd) that
contains a [2Fe–2S] cluster. The electron then reduces NADP
⫹
in a noncyclic process mediated by ferredoxin–NADP
⫹
reductase. Alternatively, it may be returned, presumably via
ferredoxin–plastoquinone reductase, to the plastoquinone
pool in a cyclic process that does not require electron input
from PSII and only translocates protons across the thylakoid
membrane. ATP is synthesized by the CF
1
CF
0
-ATP synthase,
which closely resembles the analogous mitochondrial com-
plex, in a reaction driven by the dissipation of the proton gra-
dient across the thylakoid membrane.
3 Dark Reactions CO
2
is fixed in the photosynthetic
dark reactions of plants and cyanobacteria by the reactions of
the Calvin cycle.The first stage of the Calvin cycle, in sum, me-
diates the reaction 3RuBP ⫹ 3CO
2
S 6GAP with the con-
sumption of 9 ATP and 6 NADPH generated by the light reac-
tions. The second stage reshuffles the atoms of five GAPs to
CHAPTER SUMMARY
JWCL281_c24_901-939.qxd 3/25/10 12:05 PM Page 937

reform the three RuBPs with which the cycle began, a process
that requires no further input of free energy or reduction
equivalents. The sixth GAP, the product of the Calvin cycle, is
used to synthesize carbohydrates, amino acids, and fatty acids.
The flux-controlling enzymes of the Calvin cycle are activated
in the light through variations in the pH and the Mg
2⫹
and
NADPH concentrations, and by the redox level of thiore-
doxin.The central enzyme of the Calvin cycle, RuBP carboxy-
lase, catalyzes both a carboxylase and an oxygenase reaction
with RuBP. The latter reaction is the first step in the photores-
piration cycle that liberates CO
2
.The rate of photorespiration
increases with temperature and decreases with CO
2
concen-
tration, so photorespiration constitutes a significant energetic
drain on most plants on hot bright days. Calvin cycle products
are converted to sucrose, starch, and cellulose, as well as fatty
acids and amino acids. C
4
plants, which are most common in
the tropics, have a system for concentrating CO
2
in their pho-
tosynthetic cells so as to minimize the effects of photorespira-
tion but at the cost of 2 ATP per CO
2
fixed. Certain desert
plants conserve water by absorbing CO
2
at night and releasing
it to the Calvin cycle by day.This crassulacean acid metabolism
(CAM) occurs through a process similar to the C
4
cycle.
938 Chapter 24. Photosynthesis
General
Blankenship, R.E., Molecular Mechanisms of Photosynthesis,
Blackwell Science (2002).
Buchanan, B.B., Gruissem, W., and Jones, R.L. (Eds.), Biochem-
istry and Molecular Biology of Plants, American Society of
Plant Physiologists (2000).
Hall, D.O. and Rao, K.K., Photosynthesis (6th ed.), Cambridge
(1999).
Heldt, H.-W., Plant Biochemistry, Elsevier (2005).
Lawlor,D.W., Photosynthesis (3rd ed.), BIOS Scientific Publishers
Ltd. (2001).
Nicholls, D.G. and Ferguson, S.J., Bioenergetics 3, Chapter 6,
Academic Press (2002).
Chloroplasts
Bogorad, L. and Vasil, I.K. (Eds.), The Molecular Biology of Plas-
tids, Academic Press (1991).
Hoober, J.K., Chloroplasts, Plenum Press (1984).
Light Reactions
Amunts,A., Drory, O., and Nelson, N., The structure of plant pho-
tosystem I at 3.4 Å resolution, Nature 447, 58–63 (2007).
Barber, J., Photosystem II: a multisubunit membrane protein that
oxidises water, Curr. Opin. Struct. Biol. 12, 523–530 (2002).
Chitnis, P.R., Photosystem I: Function and physiology, Annu. Rev.
Plant Physiol. Plant Biol. 52, 593–626 (2001).
Deisenhofer, J., Epp, O., Sinning, I., and Michel, H., Crystallo-
graphic refinement at 2.3 Å resolution and refined model of
the photosynthetic reaction centre from Rhodopseudomonas
viridis, J. Mol. Biol. 246, 429–457 (1995).
Deisenhofer, J. and Michel, H., High-resolution structures of
photosynthetic reaction centers, Annu. Rev. Biophys. Bio-
phys. Chem. 20, 247–266 (1991); and Structures of bacterial
photosynthetic reaction centers, Annu. Rev. Cell Biol. 7, 1–23
(1991).
Deng, Z., Aliverti, A., Zanetti, G., Arakaki, A.K., Ottado, J.,
Orellano, E.G., Calcaterra, N.B., Ceccarelli, E.A., Carrillo, N.,
and Karplus, P.A., A productive NADP
⫹
binding mode of
ferredoxin–NADP
⫹
reductase revealed by protein engineer-
ing and crystallographic studies, Nature Struct. Biol. 6, 847–853
(1999); and Bruns, C.M. and Karplus, P.A., Refined crystal
structure of spinach ferredoxin reductase at 1.7 Å resolution:
Oxidized, reduced, and 2¿-phospho-5¿-AMP bound states, J.
Mol. Biol. 247, 125–145 (1995).
Diner,B.A. and Rappaport, F., Structure,dynamics, and energetics
of the primary photochemistry of photosystem II of oxygenic
photosynthesis, Annu. Rev. Plant Biol. 53, 551–580 (2002).
Eberhard, S., Finazzi, G., and Wollman, F.-A., The dynamics of
photosynthesis, Annu. Rev. Genet. 42, 463–515 (2008).
El-Kabbani, O., Chang,C.-H.,Tiede, D., Norris, J., and Schiffer, M.,
Comparison of reaction centers from Rhodobacter sphaeroides
and Rhodopseudomonas viridis: Overall architecture and
protein-pigment interactions, Biochemistry 30, 5361–5369
(1991).
Fromme, P. (Ed.), Photosynthetic Protein Complexes. A Structural
Approach, Wiley–Blackwell (2008).
Guskov,A., Kern, J., Gabdulkhakov,A., Broser, M., Zouni,A., and
Saenger, W., Cyanobacterial photosystem II at 2.9-Å resolu-
tion and the roles of quinones, lipids, channels, and chloride,
Nature Struct. Mol. Biol. 16, 334–342 (2009); Loll, B., Kern, J.,
Saenger,W., Zouni,A., and Biesiadka, J.,Towards complete co-
factor arrangement in the 3.0 Å resolution structure of photo-
system II, Nature 438, 1040–1044 (2005); and Ferreira, K.N.,
Iverson,T.M., Maghlaoui, K., Barber, J., and Iwata, S.,Architec-
ture of the photosynthetic oxygen-evolving center, Science
303, 1831–1838 (2004). [X-ray structures of PSII.]
Heathcote, P., Fyfe, P.K., and Jones, M.R., Reaction centers: The
structure and evolution of biological solar power, Trends
Biochem. Sci. 27, 79–87 (2002).
Jordan, P., Fromme, P., Witt, H.T., Klukas, O., Saenger, W., and
Krauss, N.,Three-dimensional structure of cyanobacterial pho-
tosystem I at 2.5 Å resolution, Nature 411, 909–917 (2001).
Koepke, J., Hu, X., Muenke, C., Schulen, K., and Michel, H., The
crystal structure of the light-harvesting complex II (B800–850)
from Rhodospirillum molischianum, Structure 4, 581–597
(1996); and McDermott, G., Prince, S.M., Freer, A.A.,
Horthornthwaite-Lawless, A.M., Papiz, M.Z., Cogdell, R.J.,
and Isaacs, N.W., Crystal structure of an integral membrane
light-harvesting complex from photosynthetic bacteria, Nature
374, 517–521 (1995). [The X-ray structures of LH2s.]
Kurisu, G., Kusunoki, M., Katoh, E., Yamazaki, T., Teshima, K.,
Onda,Y., Kimata-Ariga,Y., and Hase,T., Structure of the elec-
tron transfer complex between ferredoxin and ferredoxin-
NADP
⫹
reductase, Nature Struct. Biol. 8, 117–121 (2001).
Kurisu, G., Zhang, H., Smith, J.L., and Cramer, W.A., Structure of
the cytochrome b
6
f complex of oxygenic photosynthesis:
Tuning the cavity, Science 302, 1009–1014 (2003); Stroebel, D.,
Choquet,Y., Popot, J.-L., and Picot, D.,An atypical haem in the
cytochrome b
6
f complex, Nature 426, 413–418 (2003); and
Cramer, W.A., Zhang, H., Yan, J., Kurisu, G., and Smith, J.L.,
Transmembrane traffic in the cytochrome b
6
f complex, Annu.
Rev. Biochem. 75, 769–790 (2006).
Nelson, N. and Yocum, C.F., Structure and function of photosys-
tems I and II, Annu. Rev. Plant Biol. 57, 521–565 (2006).
REFERENCES
JWCL281_c24_901-939.qxd 7/20/10 7:29 PM Page 938

Renger, G. and Tenger,T., Photosystem II: The machinery of pho-
tosynthetic water splitting, Photosyn. Res. 98, 53–80 (2008).
Ruban,A.V., et al., Identification of a mechanism of photoprotec-
tive energy dissipation in higher plants, Nature 450, 575–578
(2007).
Standfuss, J., van Scheltinga, A.C.T., Lamborghini, M., and
Kühlbrandt, W., Mechanisms of photoprotection and nonpho-
tochemical quenching in pea light-harvesting complex at 2.5 Å
resolution, EMBO J. 24, 919–928 (2005). [The X-ray structure
of LHC-II.]
Yano, J., et al., Where water is oxidized to dioxygen: Structure of
the photosynthetic Mn
4
Ca cluster,Science 314, 821–825 (2006).
Dark Reactions
Black, C.C. and Osmond, C.B., Crassulacean acid metabolism
pnotosynthesis: ‘working the night shift,’ Photosynthesis Res.
76, 329–31 (2003).
Cushman, J.C. and Bohnert, H.J., Crassalacean acid metabolism:
Molecular genetics, Annu. Rev. Plant Physiol. Plant Mol. Biol.
50, 305–332 (1999).
Dai, S., Friemann, R., Glauser, D.A., Bourquin, F., Manieri, W.,
Schürmann, P., and Eklund, H., Structural snapshots along the
reaction pathway of ferredoxin–thioredoxin reductase, Nature
448, 92–96 (2007).
Hartman, F.C. and Harpel, M.R., Chemical and genetic probes of
the active site of
D-ribulose-1,5-bisphosphate carboxylase/
oxygenase: A retrospective based on the three-dimensional
structure, Adv.Enzymol.Relat.Areas Mol. Biol. 67, 1–75 (1993).
Hatch, M.D., C
4
photosynthesis: A unique blend of modified bio-
chemistry, anatomy, and ultrastructure, Biochim. Biophys.Acta
895, 81–106 (1987).
Portis,A.R.,Jr., Regulation of ribulose 1,5-bisphosphate carboxylase/
oxygenase activity, Annu. Rev. Plant Physiol. Plant Mol. Biol.
43, 415–437 (1992); and Rubisco activase, Biochim. Biophys.
Acta 1015, 15–28 (1990).
Saxena, I.M. and Brown, R.M., Jr., Cellulose biosynthesis: current
views and evolving concepts, Ann. Bot. 96, 9–21 (2005).
Schneider, G., Lindqvist,Y., and Branden, C.-I., RUBISCO: Struc-
ture and mechanism, Annu. Rev. Biophys. Biomol. Struct. 21,
119–143 (1992).
Schreuder, H.A., Knight, S., Curmi, P.M.G., Andersson, I., Cascio,
D., Sweet, R.M., Brändén, C.-I., and Eisenberg, D., Crystal
structure of activated tobacco rubisco complexed with the
reaction-intermediate analogue 2-carboxy-arabinitol 1,5-
bisphosphate, Protein Sci. 2, 1136–1146 (1993).
Schürmann, P., Redox signaling in the chloroplast: The ferredoxin/
thioredoxin system, Antioxidants Redox Signaling 5, 69–79
(2003).
Spreitzer, R.J. and Salvucci, M.E., Rubisco: structure, regulatory
interactions, and possibilities for a better enzyme, Annu. Rev.
Plant Biol. 53, 449–475 (2002).
Taylor, T.C. and Andersson, I., The structure of the complex be-
tween rubisco and its natural substrate ribulose 1,5-bisphos-
phate, J. Mol. Biol. 265, 432–444 (1997).
Problems 939
1. Why is chlorophyll green in color when it absorbs in the red
and the blue regions of the spectrum (Fig. 24-5)?
2. The “red tide” is a massive proliferation of certain algal
species that cause seawater to become visibly red. Describe the
spectral characteristics of the dominant photosynthetic pigments
in these algae.
3. H
2
18
O is added to a suspension of chloroplasts capable of
photosynthesis.Where does the label appear when the suspension
is exposed to light?
4. Indicate, where appropriate, the analogous components in
the photosynthetic electron-transport chains of purple photosyn-
thetic bacteria and chloroplasts.
5. Antimycin inhibits photosynthesis in chloroplasts. Indicate
its most likely site of action and explain your reasoning.
6. Calculate the energy efficiency of cyclic and noncyclic pho-
tosynthesis in chloroplasts using 680-nm light.What would this ef-
ficiency be with 500-nm light? Assume that ATP formation re-
quires 59 kJ ⴢ mol
⫺1
under physiological conditions.
*7. What is the minimum pH gradient required to synthesize
ATP from ADP ⫹ P
i
? Assume [ATP]/([ADP][P
i
]) ⫽ 10
3
,T ⫽ 25°C,
and that three protons must be translocated per ATP generated.
(See Table 16-3 for useful thermodynamic information.)
8. Indicate the average Calvin cycle labeling pattern in
ribulose-5-phosphate after two rounds of exposure to
14
CO
2
.
9. Chloroplasts are illuminated until the levels of their Calvin
cycle intermediates reach a steady state. The light is then turned
off. How do the levels of RuBP and 3PG vary after this time?
10. What is the energy efficiency of the Calvin cycle combined
with glycolysis and oxidative phosphorylation; that is, what per-
centage of the input energy can be metabolically recovered in syn-
thesizing starch from CO
2
using photosynthetically produced
NADPH and ATP rather than somehow directly storing these
“high-energy” intermediates? Assume that each NADPH is ener-
getically equivalent to three ATPs and that starch synthesis and
breakdown are energetically equivalent to glycogen synthesis and
breakdown.
11. Predict the effect of an uncoupler such as dinitrophenol
(Fig. 22-47) on production of (a) ATP and (b) NADPH in a
chloroplast.
12. Describe the effects of an increase in oxygen pressure on
the dark reactions of photosynthesis.
13. If a C
3
plant and a C
4
plant are placed together in a sealed
illuminated box with sufficient moisture, the C
4
plant thrives while
the C
3
plant sickens and eventually dies. Explain.
14. The leaves of some species of desert plants taste sour in
the early morning but, as the day wears on, they become tasteless
and then bitter. Explain.
PROBLEMS
JWCL281_c24_901-939.qxd 7/2/10 12:24 PM Page 939
940
CHAPTER 25
Lipid Metabolism
1 Lipid Digestion, Absorption, and Transport
2 Fatty Acid Oxidation
A. Fatty Acid Activation
B. Transport Across the Mitochondrial Membrane
C. Oxidation
D. Oxidation of Unsaturated Fatty Acids
E. Oxidation of Odd-Chain Fatty Acids
F. Peroxisomal Oxidation
G. Minor Pathways of Fatty Acid Oxidation
3 Ketone Bodies
4 Fatty Acid Biosynthesis
A. Pathway Overview
B. Acetyl-CoA Carboxylase
C. Fatty Acid Synthase
D. Transport of Mitochondrial Acetyl-CoA Into the Cytosol
E. Elongases and Desaturases
F. Synthesis of Triacylglycerols
5 Regulation of Fatty Acid Metabolism
6 Cholesterol Metabolism
A. Cholesterol Biosynthesis
B. Control of Cholesterol Biosynthesis and Transport
C. Cholesterol Utilization
7 Eicosanoid Metabolism: Prostaglandins,
Prostacyclins, Thromboxanes, Leukotrienes,
and Lipoxins
A. Background
B. The Cyclic Pathway of Eicosanoid Metabolism:
Prostaglandins, Prostacyclins, and Thromboxanes
C. The Linear Pathway of Eicosanoid Metabolism: Leukotrienes
and Lipoxins
8 Phospholipid and Glycolipid Metabolism
A. Glycerophospholipids
B. Sphingophospholipids
C. Sphingoglycolipids
Lipids play indispensable roles in cell structure and metab-
olism. For example, triacylglycerols are the major storage
form of metabolic energy in animals; cholesterol is a vital
component of cell membranes and a precursor of the
steroid hormones and bile salts; arachidonate, a C
20
unsat-
urated fatty acid, is the precursor of the prostaglandins,
prostacyclins, thromboxanes, leukotrienes, and lipoxins, po-
tent intercellular mediators that control a variety of com-
plex processes; and complex glycolipids and phospholipids
are major components of biological membranes. We dis-
cussed the structures of simple and complex lipids in Sec-
tion 12-1. In the first half of this chapter, we consider the
metabolism of fatty acids and triacylglycerols, including
their digestion, oxidation, and biosynthesis. We then con-
sider how cholesterol is synthesized and utilized, and how
prostaglandins, prostacyclins, thromboxanes, leukotrienes,
and lipoxins are synthesized.We end by studying how com-
plex glycolipids and phospholipids are synthesized from
their simpler lipid and carbohydrate components.
1 LIPID DIGESTION, ABSORPTION,
AND TRANSPORT
Triacylglycerols (also called fats or triglycerides) constitute
⬃90% of the dietary lipid and are the major form of meta-
bolic energy storage in humans. Triacylglycerols consist of
glycerol triesters of fatty acids such as palmitic and oleic
acids
(the names and structural formulas of some biologically
common fatty acids are listed in Table 12-1). Like glucose,
they are metabolically oxidized to CO
2
and H
2
O.Yet, since
most carbon atoms of triacylglycerols have lower oxidation
states than those of glucose, the oxidative metabolism of
fats yields over twice the energy of an equal weight of dry
carbohydrate or protein (Table 25-1). Moreover, fats, being
nonpolar, are stored in an anhydrous state, whereas glyco-
gen, the storage form of glucose, is polar and is conse-
quently stored in a hydrated form that contains about twice
its dry weight of water. Fats therefore provide up to six
times the metabolic energy of an equal weight of hydrated
glycogen.
a. Lipid Digestion Occurs at Lipid–Water Interfaces
Since triacylglycerols are water insoluble, whereas di-
gestive enzymes are water soluble, triacylglycerol digestion
takes place at lipid–water interfaces. The rate of triacylglyc-
H
2
C
1
HC
2
O
O
C
1
H
2
C
3
O
O
C
1
O
O
C
1
16
18
9
18
9
1-Palmitoyl-2,3-dioleoyl-glycerol
JWCL281_c25_940-1018.qxd 6/8/10 8:59 AM Page 940
