Voet D., Voet Ju.G. Biochemistry
Подождите немного. Документ загружается.

step and therefore “costs” the free energy of GTP hydroly-
sis. The liver lacks 3-ketoacyl-CoA transferase, which per-
mits it to supply ketone bodies to other tissues.
4 FATTY ACID BIOSYNTHESIS
Fatty acid biosynthesis occurs through condensation of C
2
units, the reverse of the -oxidation process. Through iso-
topic labeling techniques, David Rittenberg and Konrad
Bloch demonstrated, in 1945, that these condensation units
are derived from acetic acid.Acetyl-CoA was soon proven
to be a precursor of the condensation reaction, but its
mechanism remained obscure until the late 1950s when
Salih Wakil discovered a requirement for bicarbonate in
fatty acid biosynthesis and malonyl-CoA was shown to be
an intermediate. In this section, we discuss the reactions of
fatty acid biosynthesis.
A. Pathway Overview
The pathway of fatty acid synthesis differs from that of
fatty acid oxidation. This situation, as we saw in Section
18-1D, is typically the case of opposing biosynthetic and
degradative pathways because it permits them both to be
thermodynamically favorable and independently regulated
under similar physiological conditions. Figure 25-29 out-
lines fatty acid oxidation and synthesis with emphasis on
the differences between these pathways.Whereas fatty acid
oxidation occurs in the mitochondrion and utilizes fatty
acyl-CoA esters, fatty acid biosynthesis occurs in the
cytosol with, as Roy Vagelos discovered, the growing fatty
acids esterified to acyl-carrier protein (ACP; Fig. 25-30).
ACP, like CoA, contains a phosphopantetheine group that
forms thioesters with acyl groups.The phosphopantetheine
phosphoryl group is esterified to a Ser OH group of ACP,
whereas in CoA it is esterified to AMP. In animals, ACP is
part of a large multifunctional protein (Type I ACP; see
below), whereas in E. coli it is a 125-residue polypeptide
(Type II ACP). The phosphopantetheine group is trans-
ferred from CoA to apo-ACP to form the active holo-ACP
by phosphopantetheine transferase (alternatively, ACP
synthase).
The redox coenzymes of the animal fatty acid oxidative
and biosynthetic pathways differ (NAD
and FAD for ox-
idation; NADPH for biosynthesis) as does the stereochem-
istry of their intermediate steps, but their main difference is
the manner in which C
2
units are removed from or added
to the fatty acyl thioester chain. In the oxidative pathway,
-ketothiolase catalyzes the cleavage of the C
¬C
bond
of -ketoacyl-CoA so as to produce acetyl-CoA and a new
fatty acyl-CoA, which is shorter by a C
2
unit. The G°¿ of
this reaction is very close to zero so it can also function in
the reverse direction (ketone body formation). In the
biosynthetic pathway, the condensation reaction is coupled
to the hydrolysis of ATP, thereby driving the reaction to
completion. This process involves two steps: (1) the ATP-
dependent carboxylation of acetyl-CoA by acetyl-CoA
carboxylase (ACC) to form malonyl-CoA, and (2) the
Section 25-4. Fatty Acid Biosynthesis 961
CO
2
. Indeed, the breath of individuals with ketosis (also
called ketoacidosis), a potentially pathological condition in
which acetoacetate is produced faster than it can be metab-
olized (a symptom of diabetes; Section 27-4B), has the
characteristic sweet smell of acetone.
The liver releases acetoacetate and -hydroxybutyrate,
which are carried by the bloodstream to the peripheral tis-
sues for use as alternative fuels. There, these products are
converted to acetyl-CoA as is diagrammed in Fig. 25-27.
The proposed reaction mechanism of 3-ketoacyl-CoA
transferase (Fig. 25-28), which catalyzes this pathway’s sec-
ond step, involves the participation of an active site car-
boxyl group both in an enzyme–CoA thioester intermedi-
ate and in an unstable anhydride. Succinyl-CoA, which acts
as the CoA donor in this reaction, can also be converted to
succinate with the coupled synthesis of GTP in the succinyl-
CoA synthetase reaction of the citric acid cycle (Section
21-3Ea). The “activation” of acetoacetate bypasses this
Figure 25-28 Proposed mechanism of 3-ketoacyl-CoA
transferase involving an enzyme–CoA thioester intermediate.
O
C E
E
CH
2
CH
2
–
O
2
C
–
O
O
C E
–
O
C
O
SCo
A
–
SCoA
C
O
SCoA
Succinyl-CoA
CH
2
CH
2
–
O
2
CC
O
O C
O
Unstable anhydride
Enzyme–CoA
thioester intermediate
•
E
–
SCoACH
2
C
O
OCH
3
C
O
C
O
Unstable anhydride
•
CH
2
CH
2
–
O
2
CCO
2
–
CO
2
–
Succinate
ECoAS C
O
CH
3
CH
2
C
O
Acetoacetate
CH
3
CH
2
C
O
Acetoacetyl-CoA
JWCL281_c25_940-1018.qxd 4/20/10 1:59 PM Page 961

962 Chapter 25. Lipid Metabolism
the degradation of leucine; Section 26-3F). The ACC reac-
tion, like those of the other biotin-dependent carboxylases,
occurs in two steps, a CO
2
activation and a carboxylation:
E biotin
E biotin
–
O
2
C CH
2
C
O
SCoA
CH
3
C
O
SCoA
+
Biotinyl–enzyme
Carboxybiotinyl–enzyme
Malonyl-CoA
Acetyl-CoA
HCO
3
–
+ ATP
ADP
+ P
i
E biotin CO
2
–
exergonic decarboxylation of the malonyl group in the
condensation reaction catalyzed by fatty acid synthase.
These enzymes are discussed below.
B. Acetyl-CoA Carboxylase
ACC is a biotin-dependent enzyme that catalyzes the first
committed step of fatty acid biosynthesis and one of its rate-
controlling steps. It is a member of a family of biotin-
dependent carboxylases that, in humans, has only three
members besides ACC: propionyl-CoA carboxylase
(Section 25-2Ea), pyruvate carboxylase (Fig. 23-4), and
-methylcrotonyl-CoA carboxylase (which participates in
Figure 25-29 Comparison of fatty acid oxidation and fatty
acid biosynthesis. Differences occur in (1) cellular location,
(2) acyl group carrier, (3) electron acceptor/donor, (4) stereo-
Figure 25-30 The phosphopantetheine group in acyl-carrier protein (ACP) and in CoA.
FADH
2
H
2
O
NAD
+
NADH + H
+
CoA
Acetyl-CoA
CoA + CO
2
Malonyl-CoA
NADP
+
H
2
O
NADPH + H
+
NADP
+
NADPH + H
+
FAD
Fatty acyl-CoA (C
n +2
)
Fatty acyl-ACP (C
n +2
)
Fatty acyl-CoA (C
n
)
Fatty acyl-ACP (C
n
)
Enoyl-CoA
Enoyl-ACP
3-
L-Hydroxyacyl-CoA
3-
D-Hydroxyacyl-ACP
β-Ketoacyl-CoA
β-Ketoacyl-ACP
Biosynthesis
β
Oxidation
Occurs in mitochondrion Occurs in cytoplasm
CoA is acyl
group carrier
ACP is acyl
group carrier
FAD is electron
acceptor
NADPH is
electron donor
NAD
+
is electron
acceptor
NADPH is
electron donor
C
2
unit product
is acetyl-CoA
C
2
unit donor
is malonyl-CoA
L-β-Hydroxyacyl
group
D-β-Hydroxyacyl
group
1
2
3
4
3
5
chemistry of the hydration/dehydration reaction, and (5) the
form in which C
2
units are produced/donated. See the
Animated Figures
HS
Cysteamine
Phosphopantetheine prosthetic group of ACP
CH
2
CH
2
CH
2
CH
2
N C
O
H
N CH
2
CH
2
ACPO SerO
O
PC
O
C
OH CH
3
H
C
CH
3
O
–
H
HS
Cysteamine
Phosphopantetheine group of CoA
CH
2
CH
2
CH
2
CH
2
N C
O
H
N CH
2
PCH
2
O OO
O
PC
O
C
OH CH
3
H
C
CH
3
O
–
O
O
–
H
Adenine
O
HH
H
H
2–
O
3
PO OH
JWCL281_c25_940-1018.qxd 4/20/10 1:59 PM Page 962

E. coli ACC is a multienzyme complex in which these
steps are catalyzed by separate subunits: the homodimer
biotin carboxylase (BC; 456 residues) and the
2
2
heterotetramer carboxyltransferase (CT; 319 and 304
residues). In addition, the biotin is linked as a biocytin
residue (Fig. 23-3b) to the biotin carboxyl-carrier protein
(BCCP; 156 residues), which forms homodimers. In con-
trast, mammalian and avian ACCs contain both enzy-
matic activities as well as the biotin carboxyl carrier on a
single 2346-residue polypeptide chain in the order
BC–BCCP–CT (which differs from the order in pyruvate
carboxylase, which is BC–CT–BCCP; Section 23-1Ae).
The structure of an intact ACC has not been determined,
although the X-ray structure the E. coli BC subunit
closely resembles the BC domain of pyruvate carboxylase
(Fig. 23-5a). Interestingly, however,the CT domains of the
various biotin-dependent carboxylases differ greatly in
sequence and structure.
a. Acetyl-CoA Carboxylase Is Regulated by
Hormonally Controlled Reversible Phosphorylation
ACC is subject to hormonal regulation. Glucagon as well
as epinephrine and norepinephrine (adrenaline and nora-
drenaline; Section 18-3Ea) trigger the enzyme’s cAMP-
dependent increase in phosphorylation, which inactivates
the enzyme. Insulin, on the other hand, stimulates enzyme
dephosphorylation and thus its activation.
The mechanism by which cAMP causes an increase in
the phosphorylation state of ACC is interesting. ACC is
phosphorylated, in vitro, by two different kinases, the
cAMP-dependent protein kinase A (PKA; Section 18-
3Cb) at Ser 77 and AMP-dependent protein kinase
(AMPK; Sections 25-5a and 27-1) (which is cAMP inde-
pendent) at Ser 79, 1200, and 1215.Yet, when liver cells are
incubated with cAMP-elevating hormones in the presence
of
32
P-ATP, Ser 77 is found not to be labeled. Evidently, an
increase in [cAMP] results in a phosphorylation increase
at sites modified by AMPK rather than by PKA. How can
this be? It appears that, in vivo, the cAMP-dependent in-
crease in phosphorylation occurs not through the phos-
phorylation of new sites but, rather, through the inhibition
of dephosphorylation of previously phosphorylated posi-
tions. We have already seen such a mechanism in operation
in the control of glycogen metabolism,where the cAMP-de-
pendent phosphorylation of phosphoprotein phosphatase
inhibitor-1 causes the inhibition of dephosphorylation
(Section 18-3C). In the case of ACC, however, dephospho-
rylation is catalyzed by phosphoprotein phosphatase-2A,
which is not affected by phosphoprotein phosphatase in-
hibitor-1. The mechanism by which PKA causes the in-
crease in phosphorylation associated with AMPK activity
is, as yet, unknown.
b. Avian and Mammalian Acetyl-CoA Carboxylases
Undergo Enzyme Polymerization on Activation
Electron microscopy reveals that both avian and mam-
malian ACCs form long filaments of 20 to 40 protomers
(Fig. 25-31). This polymeric form of the enzyme is catalyti-
cally active but the protomer is not. The rate of fatty acid
biosynthesis is therefore controlled by the position of the
equilibrium between these forms:
Phosphorylation favors the inactive protomer while dephos-
phorylation favors the active polymer. Several metabolites
also affect the activity of acetyl-CoA carboxylase. Citrate
promotes the polymerization of ACC, whereas palmitoyl-
CoA and other fatty acyl-CoA’s promote its depolymeriza-
tion. Thus, cytosolic citrate, whose concentration increases
when the mitochondrial acetyl-CoA concentration builds up
(Section 25-4D), activates fatty acid biosynthesis and hence
is a feedforward activator, whereas palmitoyl-CoA, the
pathway product, is a feedback inhibitor.
c. Mammalian Acetyl-CoA Carboxylase
Has Two Major Isoforms
There are two major isoforms of mammalian ACC.ACC1
occurs in adipose tissue and ACC2 occurs in tissues that
oxidize but do not synthesize fatty acids, such as heart muscle.
Tissues that both synthesize and oxidize fatty acids, such as
liver,contain both isoforms, which are homologous although
the genes encoding them are located on different chromo-
somes. What is the function of ACC2? The product of the
ACC-catalyzed reaction, malonyl-CoA, strongly inhibits
Protomer(inactive) Δ polymer(active)
Section 25-4. Fatty Acid Biosynthesis 963
Figure 25-31 Association of acetyl-CoA carboxylase
protomers. An electron micrograph with an accompanying
interpretive drawing indicates that filaments of avian liver
acetyl-CoA carboxylase consist of linear chains of flat rectangular
protomers. [Courtesy of Malcolm Lane,The Johns Hopkins
University School of Medicine.]
400Å
JWCL281_c25_940-1018.qxd 6/8/10 8:59 AM Page 963
C
O
CH
3
SCoA
+ H SACP
Acetyl-CoA
S
E
C
O
CH
3
SACP
Acetyl-ACP
H
SCoA
H
SACPH
C
O
CH
3
S
E
S
E
H+
CO
2
C
O
CH
3
SACP
Acetoacetyl-ACP
C
O
CH
2
H
+
+ NADPH
H
+
NADP
+
C
CH
3
SACP
C
CH
2
OOH
H
D-
β
-Hydroxybutyryl-ACP
α,β
α,β
-trans-Butenoyl-ACP
H
+
+ NADPH
NADP
+
CH
3
SACP
C
O
Butyryl-ACP
CH
2
CH
2
CH
3
CH
2
SACP
C
O
Palmitoyl-ACP
(CH
2
)
13
H
2
O
SACP
C
O
Palmitate
H
O
–
palmitoyl thioesterase (TE)
enoyl-ACP reductase (ER)
β-ketoacyl-ACP reductase (KR)
β-ketoacyl-ACP synthase (KS)
malonyl/acetyl-CoA-ACP
transacylase (MAT)
+
C
O
CH
2
SCoA
+ H SACP
Malonyl-CoA
C
O
CH
2
SACP
Malonyl-ACP
malonyl/acetyl-CoA-ACP
transacylase (MAT)
CO
2
–
CO
2
–
1a
2a
2b
3
H
2
O
C
CH
3
SACP
C
C
OH
H
β-hydroxyacyl-ACP dehydrase (DH)
4
5
6
1b
SCoA
CH
3
CH
2
(CH
2
)
13
H
after 7 reaction cycles
recycle Reactions 2a–5
six more times
964 Chapter 25. Lipid Metabolism
the mitochondrial import of fatty acyl-CoA for fatty acid
oxidation, the major control point for this process. Thus it
appears that ACC2 has a regulatory function (Section 25-5).
Prokaryotic acetyl-CoA carboxylases are not subject to
any of these controls.This is because fatty acids in these or-
ganisms are not stored as fats but function largely as phos-
pholipid precursors.The E. coli enzyme is instead regulated
by guanine nucleotides so that fatty acids are synthesized
in response to the cell’s growth requirements.
C. Fatty Acid Synthase
The synthesis of fatty acids from acetyl-CoA and malonyl-
CoA involves seven enzymatic reactions that yield mainly
palmitic acid (see below). These reactions were first studied
in cell-free extracts of E. coli, in which they are catalyzed by
independent enzymes together with ACP.These proteins are
collectively known as type II fatty acid synthase (FAS-II).
FAS-II also occurs in chloroplasts, the only site of fatty acid
synthesis in plants, and in vertebrate mitochondria, whose
components are all nuclear encoded and closely resemble
their bacterial counterparts. In fungi,however,fatty acids are
synthesized by a 2600-kD ␣
6

6
multifunctional enzyme,
whereas in animals they are synthesized by a multifunctional
enzyme consisting of two identical ⬃275-kD polypeptide
chains, each containing all seven activities plus ACP and
known as type I fatty acid synthase (FAS-I). Most of the do-
mains catalyzing these reactions in these so-called megasyn-
thases are homologous to the corresponding proteins that
constitute FAS-II, which indicates that they arose through
the joining of what were previously independent proteins.
The reactions catalyzed by FAS-I to synthesize palmi-
tate are diagrammed in Fig. 25-32. The long flexible phos-
phopantetheine chain of ACP (Fig. 25-30) functions to
transport the substrate between the protein’s various enzy-
matic domains:
1a. The transfer of the acetyl group from acetyl-CoA to
ACP to yield acetyl-ACP as catalyzed by malonyl/acetyl-
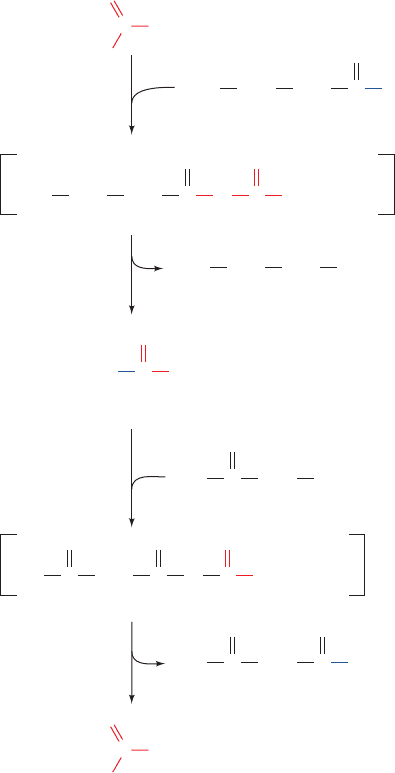
CoA-ACP transacylase (MAT).
Figure 25-32 Reaction cycle for the biosynthesis
of fatty acids. The biosynthesis of palmitate requires
seven cycles of C
2
elongation followed by a final
hydrolysis step.
See the Animated Figures
JWCL281_c25_940-1018.qxd 8/9/10 9:43 AM Page 964
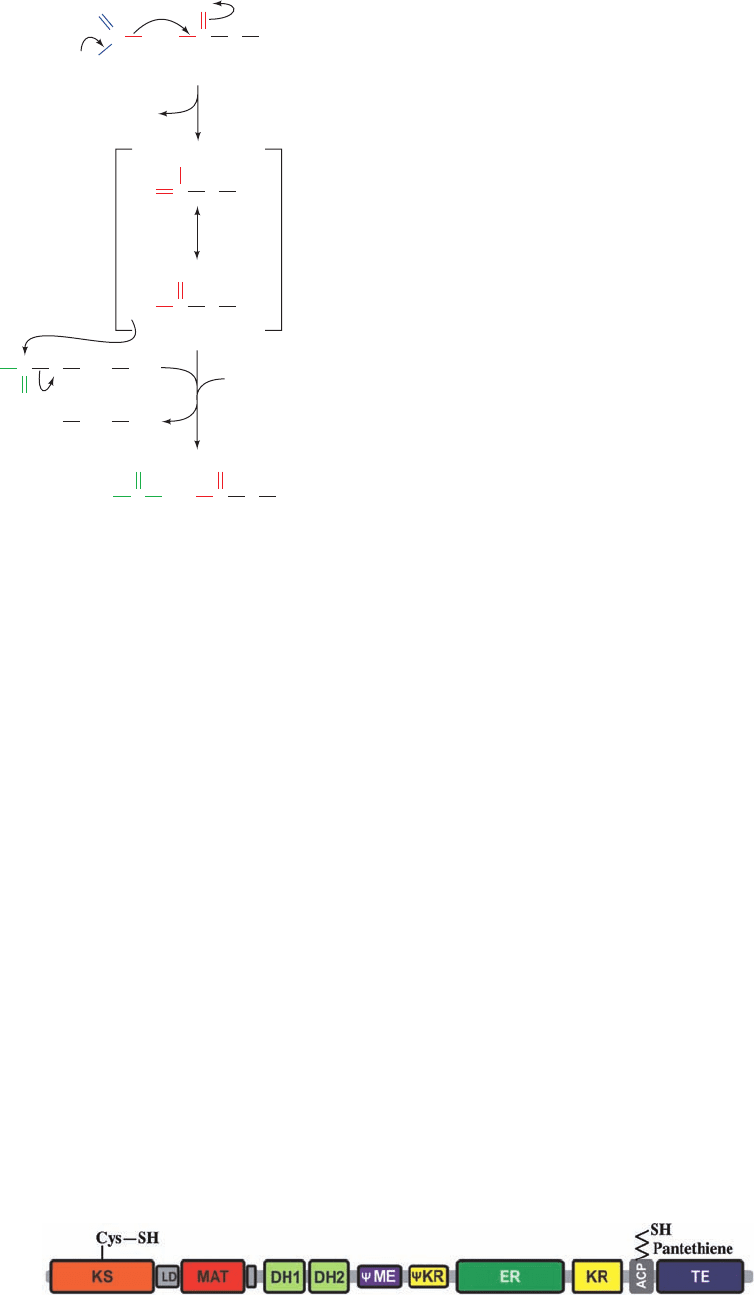
2a. The loading of -ketoacyl-ACP synthase (KS; also
known as condensing enzyme) by the transfer of the acetyl
group from ACP to a KS Cys residue, thus maintaining the
acetyl group’s thioester linkage.
1b. The formation of malonyl-ACP in a reaction analo-
gous to that of Reaction 1a, which in animals is catalyzed
by the same enzyme, MAT.
2b. The coupling of the acetyl group to the C
of the
malonyl group on the ACP with the malonyl group’s ac-
companying decarboxylation so as to form acetoacetyl-
ACP and free the KS active site Cys-SH group (Fig. 25-33).
Consequently, the CO
2
taken up in the acetyl-CoA carboxy-
lase reaction (Section 25-4B) does not appear in the product
fatty acid. Rather, the decarboxylation functions to drive
carbon–carbon bond formation in the condensation reac-
tion, which through the acetyl-CoA carboxylase reaction, is
coupled to ATP hydrolysis.
3–5. The reduction, dehydration, and further reduc-
tion of acetoacetyl-ACP so as to form butyryl-ACP as se-
quentially catalyzed by -ketoacyl-ACP reductase (KR),
-hydroxyacyl-ACP dehydrase (DH), and enoyl-ACP re-
ductase (ER). The coenzyme in both reductive steps is
NADPH, whereas in oxidation, the analogs of Reactions
3 and 5, respectively, use NAD
and FAD (Fig. 25-29).
Moreover, Reaction 3 produces and Reaction 4 requires a
D--hydroxyacyl group, whereas the analogous reactions in
oxidation involve the corresponding
L isomer.
2a to 5 Repeat. The butyryl group from the butyryl-
ACP is transferred to the Cys-SH of KS. Thus the acetyl
group with which the system was initially loaded has been
elongated by a C
2
unit. The ACP is “reloaded” with a mal-
onyl group (Step 1b), and another cycle of C
2
elongation
occurs. This process occurs altogether seven times to yield
palmitoyl-ACP.
6. The palmitoyl-ACP thioester bond is hydrolyzed by
palmitoyl thioesterase (TE), yielding palmitate, the normal
product of the fatty acid synthase pathway, and regenerat-
ing the enzyme for a new round of synthesis.
The stoichiometry of palmitate synthesis therefore is
Since the 7 malonyl-CoA are derived from acetyl-CoA as
follows:
the overall stoichiometry for palmitate biosynthesis is
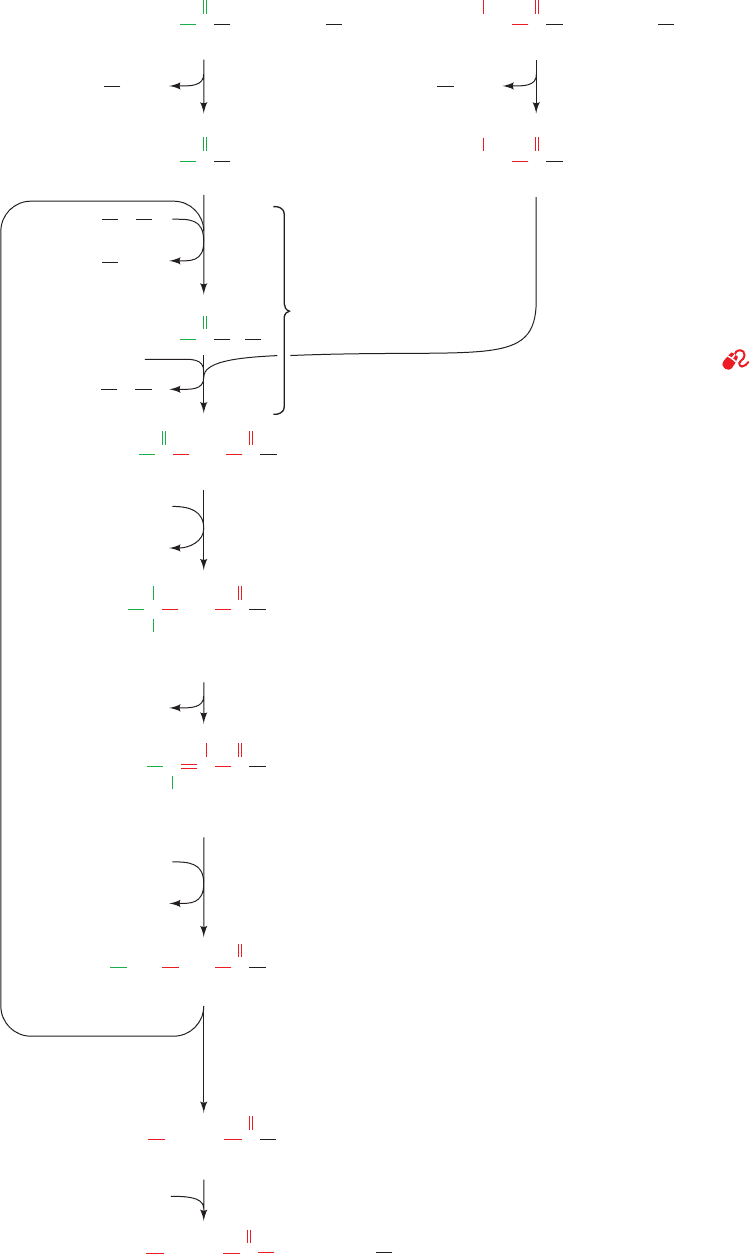
a. Animal FAS-I Is a Flexible X-Shaped Dimer
Most but not all of the enzymatic activities of animal
FAS-I remain functional when this dimeric enzyme is dis-
sociated into monomers. Moreover, fragments resulting
from the limited proteolysis of animal FAS-I exhibit many
of the enzymatic activities of the intact protein. Appar-
ently, contiguous stretches of its polypeptide chain fold to
form a series of autonomous domains, each with a specific
but different catalytic activity. Several other enzymes, for
example, mammalian acetyl-CoA carboxylase (Section
25-4B), exhibit similar multifunctionality but none has as
many separate catalytic activities as does animal FAS-I. The
order of the domains along the animal FAS-I polypeptide
chain is indicated in Fig. 25-34. Three of these domains
besides ACP lack enzymatic activity: a linker domain
Palmitate 14NADP
8CoA 6H
2
O 7ADP 7P
i
8 Acetyl-CoA 14NADPH 7ATP
¡
7 malonyl-CoA 7ADP 7P
i
7H
7 Acetyl-CoA 7CO
2
7ATP
¡
Palmitate 7CO
2
14NADP
8CoA 6H
2
O
Acetyl-CoA 7 malonyl-CoA 14NADPH 7H
¡
Section 25-4. Fatty Acid Biosynthesis 965
CO
2
CH
2
C
O
S ACP
Malonyl-ACP
Acetoacetyl-CoA
H
+
C
–
O
–
O
H
3
CC
O
S Cys KS
HS Cys KS
CH
2
C
O
–
S ACP
CH
2
C
O
S ACP
CH
2
C
O
H
3
CC
O
S ACP
Figure 25-33 The mechanism of carbon–carbon bond forma-
tion in fatty acid biosynthesis. The condensation of an acetyl
group on the active site Cys of -ketoacyl-ACP synthase (KS)
with a malonyl group on the phosphopantetheine arm of ACP
forms a -ketoacyl-ACP.The reaction is driven by the exergonic
elimination of CO
2
from the malonyl group to generate a reso-
nance-stabilized acetyl-ACP carbanion intermediate that func-
tions as a good nucleophile.
Figure 25-34 Domain organization of porcine FAS-I at approximate sequence scale. [Modified
from a drawing by Timm Maier and Nenad Ban, ETH Zurich, Switzerland.]
JWCL281_c25_940-1018.qxd 6/8/10 8:59 AM Page 965
966 Chapter 25. Lipid Metabolism
(LD) that bridges the KS and MAT domains; a pseudo-
methyltransferase (ME) domain, so named because it is
homologous to the methyltransferase family; and a pseudo-
ketoreductase (KR) domain, so named because it is a
truncated form of the KR domain.
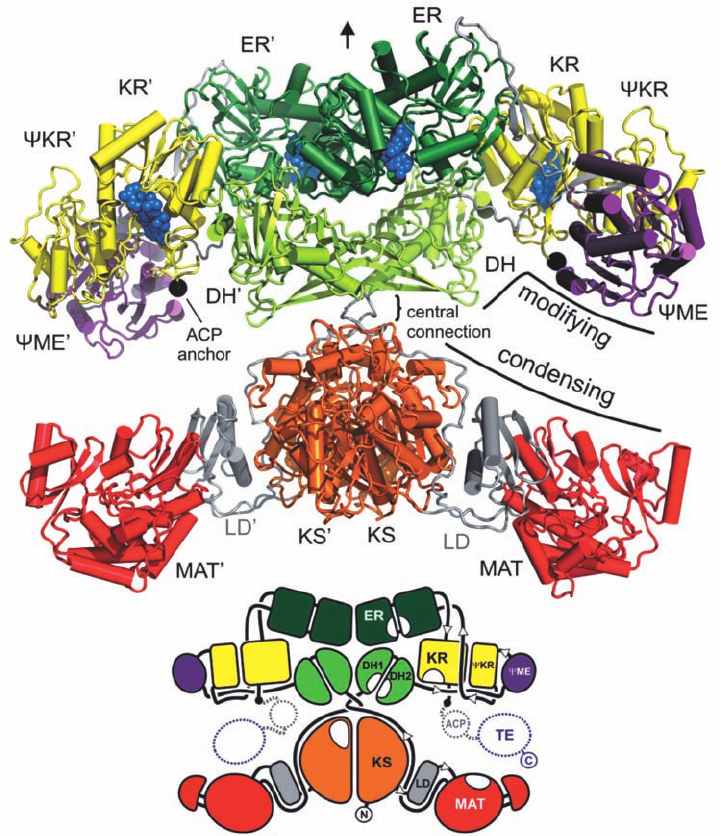
The X-ray structure of porcine FAS-I in complex with
NADP
, determined by Nenad Ban, reveals a pseudo-2-
fold symmetric, X-shaped dimer (Fig. 25-35). Its two sub-
units associate via an extensive interface involving over
150 residues per chain from the KS, ER, and DH domains.
However the upper and lower portions of the X are only
loosely connected.The lower portion of the X contains the
enzyme’s two condensing activities, MAT and KS (which
catalyze reactions 1 and 2 in Fig. 25-32), whereas the upper
portion of the X contains the enzyme’s -carbon modifying
activities, DH, KR,and ER (which catalyze reactions 3–5 in
Fig. 25-32). The C-terminal ACP and TE domains are flexi-
bly tethered to the enzyme and are therefore unobserved.
The KR–ACP linker consists of ⬃13 residues and can
therefore span a distance of up to ⬃40 Å, whereas the
ACP–TE linker has a length of ⬃25 residues and can there-
fore extend ⬃80 Å.
Each of the two reaction chambers of porcine FAS-I is
lined by the full set of catalytic domains from one sub-
unit, all of which must be visited by the phosphopan-
tethiene arm of an ACP to carry out all the FAS reac-
Figure 25-35 X-ray structure of porcine FAS-I in complex
with NADP
. (a) The ⬃190-Å wide homodimer, as viewed
perpendicular to its pseudo-2-fold axis (vertical black arrow),
with its various domains colored as in Fig. 25-34 and its linkers
gray. The bound NADP
cofactors are drawn in space-filling
form in blue. The attachment sites of the disordered ACP/TE
(a)
(b)
domains are indicated by black dots.The domain names of the
second subunit have appended primes. (b) A corresponding
schematic diagram indicating how the various domains are
linked. [Courtesy of Timm Maier and Nenad Ban, ETH Zurich,
Switzerland. PDBid 2VZ9.]
JWCL281_c25_940-1018.qxd 6/8/10 8:59 AM Page 966
tions. The catalytic sites of these domains occur on both
the front and back faces of the protein and their distribu-
tion has no discernable relationship to the order of the
reactions in the catalytic cycle. In the structure shown in
Fig. 25-35a, the length of the KR–ACP linker would al-
low the ACP to reach all the catalytic sites in the same re-
action chamber but not those in the opposite reaction
chamber (e.g., the distance between the ACP attachment
site on one subunit and the MAT domain in the other is
⬃135 Å). Nevertheless, mutational studies by Stuart
Smith in which ACP on one subunit and MAT or KS on
the other subunit were inactivated yielded functional en-
zymes albeit with reduced activities, thus indicating that
an ACP domain can service both MAT domains and both
KS domains. The most plausible explanation for these
observations is that the upper portion of the X can rotate
180° with respect to the lower portion. This hypothesis is
supported by the observation that the quite tenuous
DH/KS contact that joins the top and bottom portions of
the X lacks perfect 2-fold symmetry in Fig. 25-35a, thus
indicating that this joint is flexible.
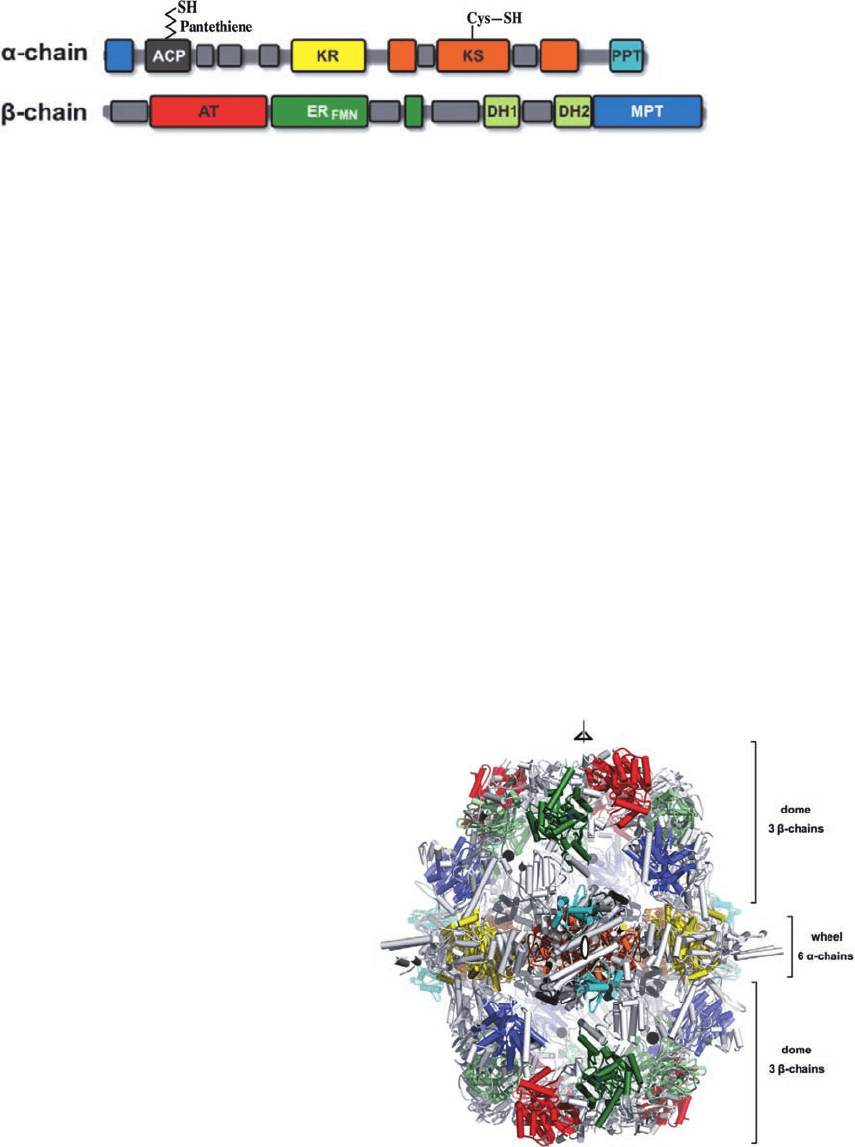
b. Fungal FAS-I Has a Barrel-Like Shape
The reactions catalyzed by
6
6
fungal FAS-I differ
from those mediated by animal FAS-I in several respects:
1. The bifunctional MAT activity of animal FAS-I
transfers both acetyl and malonyl groups from CoA to
ACP (Reactions 1a and 1b of Fig. 25-32). However, fungal
FAS-I employs a monofunctional acetyl transferase (AT)
activity to transfer the incoming acetyl group from CoA to
ACP and a malonyl/palmitoyl transferase (MPT) activity
to do so for malonyl groups.
2. Animal FAS-I synthesizes an ACP-linked palmitoyl
group, which its TE activity hydrolytically releases as
palmitate (Reaction 6 of Fig. 25-32). In contrast, fungal
FAS-I synthesizes ACP-linked palmitoyl (C
16
) and stearoyl
(C
18
) groups in a ratio of roughly 2:3, which its bifunctional
MPT activity then transfers to CoA to yield the pathway’s
final products, palmitoyl-CoA and stearoyl-CoA.
3. The ER activity in animal FAS-I uses NADPH to
directly reduce the C“C double bond in Reaction 5 of
Fig. 25-32. However, in fungal ER, NAPDH reduces FMN
to FMNH
2
, which in turn, reduces the double bond.
4. Fungal FAS-I has a phosphopantetheine transferase
(PPT) activity that attaches the phosphopantetheine group
to ACP.This activity is not part of animal FAS-I.
The distribution of the various enzymatic activities along
the and chains of fungal FAS-I (Fig. 25-36) bears no re-
semblance to that of animal FAS-I (Fig. 25-34). Note that
the MPT activity is shared by both the and chains.
The X-ray structure of FAS-I from the thermophilic
fungus Thermomyces lanuginosus (Fig. 25-37), determined
by Ban, reveals that it forms a hollow barrel-shaped protein
with D
3
symmetry (the rotational symmetry of a trigonal
prism; Fig. 8-65b). The six chains form a D
3
-symmetric
wheel that is capped on each side by a 3-fold symmetric
dome that is predominantly formed by the chains. The
central wheel splits the hollow interior of the barrel into
two identical reaction chambers, each of which has several
openings in its side walls through which small molecules
can enter. The active sites of all the enzymatic activities
face the interiors of the reaction chambers. Clearly, animal
and fungal FAS-I’s have divergently evolved from FAS-II.
Section 25-4. Fatty Acid Biosynthesis 967
Figure 25-36 Domain organization of fungal FAS-I at approximate sequence scale. [Modified
from a drawing by Simon Jenni and Nanad Ban, ETH Zurich, Switzerland.]
Figure 25-37 X-ray structure of T. lanuginosus FAS. The
⬃270-Å-high by ⬃250-Å-diameter
6
6
heterododecamer is
viewed along a 2-fold axis (ellipsoid) and perpendicular to its
3-fold axis (triangle) with its various domains colored as in Fig.
25-36 and its linker domains gray. The attachment sites of the
disordered ACP domains are indicated by black dots. [Courtesy
of Simon Jenni and Nenad Ban, ETH Zurich, Switzerland.
PDBids 2UV9 and 2UVA.]
JWCL281_c25_940-1018.qxd 6/8/10 8:59 AM Page 967
968 Chapter 25. Lipid Metabolism
The six ACP domains, which are N-terminally anchored
to the chamber walls and C-terminally anchored to the
middle of the central wheel, are disordered in the X-ray
structure of T. lanuginosus FAS-I. However, they are visi-
ble in the otherwise closely similar X-ray structure of yeast
FAS-I, which was independently determined by Ban and
by Thomas Steitz. Structural considerations suggest that
each doubly tethered ACP domain can swing to visit the re-
quired six catalytic centers, which in the case of the ACP
tethered to subchamber 1 are KR from 1, KS from 2,
MPT and DH from 1, and AT and ER from 2, where
subchamber 2 is on the clockwise side of subchamber 1 as
viewed from the top of the dome.
The PPT domains are located on the outside of the bar-
rel where they cannot interact with the ACP domains. This
suggests that they attach the phosphopantetheine groups
to the ACPs before the barrel has fully assembled.
c. Fatty Acid Synthase Inhibitors
Are Drug Candidates
In well-nourished individuals, fatty acid synthesis pro-
ceeds at a low rate. However, certain tissues, particularly
cancers, express high levels of FAS-I and produce fatty
acids at a high rate. Consequently, inhibitors of animal
FAS-I are being investigated as possible anticancer agents.
Moreover,the differences between the enzymatic activities
of the various types of FAS’s, particularly their ER activi-
ties, makes FAS-II and fungal FAS-I targets for the devel-
opment of novel antibiotics.
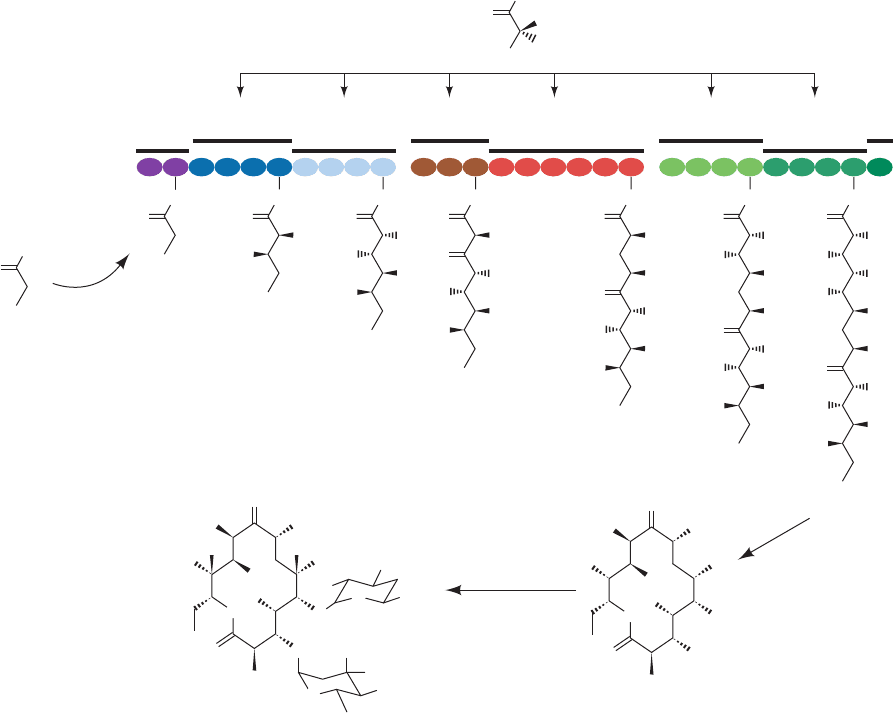
d. Variations on a Theme: Polyketide Biosynthesis
Polyketides are a family of 10,000 diverse and struc-
turally complex natural products, many of which have anti-
bacterial, antifungal, antitumor, and immunosuppressive
properties, that are synthesized by bacteria, fungi, plants,
and certain marine animals. They are made by the modular
condensation of acyl-CoA monomers such as acetyl-CoA
and propionyl-CoA with malonyl-CoA and methylmalonyl-
CoA extender units whose decarboxylation drives the
condensation reaction. The name polyketide comes from
the fact that the primary condensation products have
-keto functional groups. Palmitate is an example of a
polyketide since it is formed by the condensation of one
acetyl-CoA primer and seven malonyl-CoA extender
units. Following each condensation reaction, the new
-keto group may be reduced, dehydrated, and reduced
again as with fatty acids, or may undergo only partial
modification.
Polyketides are synthesized by megasynthases. We
have already seen that animal FAS-I contains seven enzy-
matic activities as well as ACP. Another example of a
polyketide is 6-deoxyerythronolide B (6dEB), the parent
macrolactone of the antibiotic erythromycin A (Section
32-3G), which is synthesized in the soil bacterium Saccha-
ropolyspora erythraea from one propionyl-CoA primer
and six (S)-methylmalonyl-CoA extenders by deoxyery-
thronolide B synthase (DEBS; Fig. 25-38). DEBS is a
2000-kD,
2
2
2
complex of 3000-residue subunits
whose three homodimeric units each catalyze two elonga-
tion/modification cycles. Unlike FAS-I, which catalyzes
several cycles of elongation/modification with the same
active sites, DEBS catalyzes each elongation/modification
cycle on a different module, which permits the differences
in the modifications that occur at each cycle. Thus, DEBS,
which has 28 different active sites, functions much like an
assembly line. Module 4, as Fig. 25-38 indicates, is almost
identical in function to FAS-I, containing KS, AT, ACP,
KR, DH, and ER, and reducing its primary -ketone con-
densation product to a methylene group. However, it does
not contain TE because the elongation process is not
complete after this phase. Module 3 contains only ACP,
KS, and AT, the minimal set of activities for a module, and
passes its -ketone condensation product to module 4
without further modification. Modules 1, 2, 5, and 6 con-
tain only ACP,AT, KS, and KR, the sites necessary for the
condensation and ketone reduction steps, thereby gener-
ating hydroxy products. The overall organization of the
modules therefore creates a polyhydroxy product con-
taining one keto group and one methylene group in the
chain. The DEBS final product, 6dEB, is a lactone pro-
duced by the reaction of the terminal hydroxyl group with
the thioester anchoring the growing chain to the synthase.
The various polyketide synthases have different organiza-
tions of modules, and consequently synthesize a multi-
tude of different compounds.
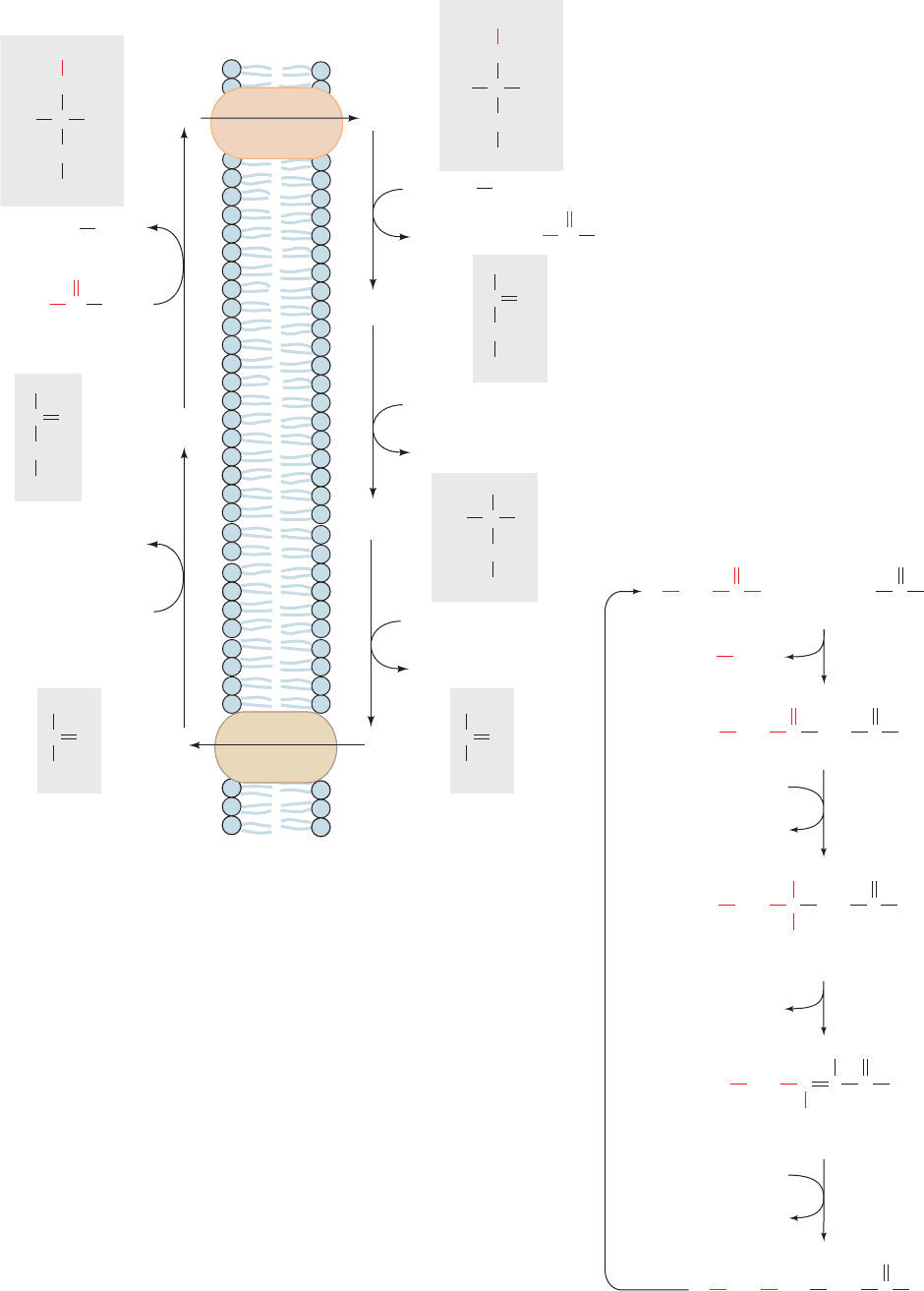
D. Transport of Mitochondrial Acetyl-CoA
Into the Cytosol
Acetyl-CoA is generated in the mitochondrion by the ox-
idative decarboxylation of pyruvate as catalyzed by pyru-
vate dehydrogenase (Section 21-2A) as well as by the oxi-
dation of fatty acids. When the need for ATP synthesis is
low, so that the oxidation of acetyl-CoA via the citric acid
cycle and oxidative phosphorylation is minimal, this mito-
chondrial acetyl-CoA may be stored for future use as fat.
Fatty acid biosynthesis occurs in the cytosol but the mito-
chondrial membrane is essentially impermeable to acetyl-
CoA. Acetyl-CoA enters the cytosol in the form of citrate via
the tricarboxylate transport system (Fig. 25-39). Cytosolic
ATP-citrate lyase then catalyzes the reaction
which resembles the reverse of the citrate synthase reac-
tion (Section 21-3A) except that ATP hydrolysis is
required to drive the intermediate synthesis of the “high-
energy” citryl-CoA, whose hydrolysis drives the citrate
synthase reaction to completion. ATP hydrolysis is there-
fore required in the ATP-citrate lyase reaction to power
the resynthesis of this thioester bond. Oxaloacetate is re-
duced to malate by malate dehydrogenase. Malate may be
oxidatively decarboxylated to pyruvate by malic enzyme
(Section 25-2Ed) and be returned in this form to the mi-
tochondrion. The malic enzyme reaction resembles that
acetyl-CoA oxaloacetate ADP P
i
Citrate CoA ATP Δ
JWCL281_c25_940-1018.qxd 4/20/10 1:59 PM Page 968
of isocitrate dehydrogenase in which a -hydroxy acid is
oxidized to a -keto acid, whose decarboxylation is
strongly favored (Section 21-3C). Malic enzyme’s coen-
zyme is NADP
⫹
, so when this route is used NADPH is
produced for use in the reductive reactions of fatty acid
biosynthesis.
Citrate transport out of the mitochondrion must be bal-
anced by anion transport into the mitochondrion. Malate,
pyruvate, and P
i
can act in this capacity. Malate may there-
fore also be transported directly back to the mitochon-
drion without generating NADPH.As we have seen in Sec-
tion 25-4C, synthesis of each palmitate ion requires 8
molecules of acetyl-CoA and 14 molecules of NADPH. As
many as 8 of these NADPH molecules may be supplied
with the 8 molecules of acetyl-CoA if all the malate pro-
duced in the cytosol is oxidatively decarboxylated. The re-
maining NADPH is provided through the pentose phos-
phate pathway (Section 23-4).
E. Elongases and Desaturases
Palmitate (16:0), the normal product of the animal fatty acid
synthase pathway, is the precursor of longer chain saturated
and unsaturated fatty acids through the actions of elongases
and desaturases. Elongases are present in both the mito-
chondrion and the ER but the mechanisms of elongation at
the two sites differ.Mitochondrial elongation (a process in-
dependent of the fatty acid synthase pathway) occurs by
successive addition and reduction of acetyl units in a rever-
sal of fatty acid oxidation; the only chemical difference be-
tween these two pathways occurs in the final reduction step
in which NADPH takes the place of FADH
2
as the terminal
Section 25-4. Fatty Acid Biosynthesis 969
Figure 25-38 An example of polyketide biosynthesis: the synthesis of erythromycin A. [After
Pfeifer, B.A., Admiraal, S.J., Gramajo, H., Cane, D.E., and Khosla, C., Science 291, 1790 (2001).]
Loading
Erythromycin A
O
O
O
OH
OH
OH
OH OH
S
O
SSS S SS
OH
N(CH
3
)
2
DEBS: Deoxyerythronolide B synthase
KS: Ketosynthase
A
T: Acyltransferase
A
CP: Acyl carrier protein
KR: Keto reductase
ER: Enoyl reductase
DH: Dehydratase
TE: Thioesterase
Elongation 1
Elongation 2
Elongation 3
Elongation 4
Elongation 5
Elongation 6
glycosylation/
hydroxylation
CH
3
CH
3
CH
3
OCH
3
HO
O
O
O
6-Deoxyerythronolide B
(6dEB)
Propionyl-CoA
(S)-Methylmalonyl-CoA
O
O
O
OH
O
release/cyclization
AT ACP KS AT KR ACP KS AT KR ACP KS AT ACP KS AT DH ER KR
KS AT KR ACP ACPKS AT KR TE
ACP
Module 1 Module 3
DEBS1 (370 kD) DEBS2 (380 kD) DEBS3 (332 kD)
Module 5 Release
Module 2 Module 4 Module 6
O
HO
O
HO
HO
O
HO
HO
SCoA
COOH
O
H
O
SCoA
O
O
HO
HO
O
O
HO
HO
O
HO
O
HO
HO
O
HO
HO
JWCL281_c25_940-1018.qxd 8/9/10 9:44 AM Page 969
970 Chapter 25. Lipid Metabolism
redox coenzyme (Fig. 25-40). Elongation in the ER in-
volves the successive condensations of malonyl-CoA with
acyl-CoA. These reactions are each followed by NADPH-
associated reductions similar to those catalyzed by FAS-I,
the only difference being that the fatty acid is elongated as
its CoA derivative rather than as its ACP derivative.
Unsaturated fatty acids are produced by terminal de-
saturases. Mammalian systems contain four terminal
desaturases of broad chain-length specificities designated
9
-,
6
-,
5
-, and
4
-fatty acyl-CoA desaturases. These
Figure 25-39 Transfer of acetyl-CoA from mitochondrion to
cytosol via the tricarboxylate transport system.
Figure 25-40 Mitochondrial fatty acid elongation. Elongation occurs by the
reversal of fatty acid oxidation with the exception that the final reaction
employs NADPH rather than FADH
2
as its redox coenzyme.
Mitochondrion Cytosol
Inner
mitochondrial
membrane
NAD
+
ATP
ATP-citrate lyase
+
H
ADP
+
P
i
malate dehydrogenase
NADH + H
+
+ CO
malic enzyme
NADP
Acetyl CoA
ADP
+
P
i
citrate synthase
pyruvate carboxylase
ATP
+
HCO
3
–
CH
2
C O
CO
2
–
CO
2
–
CH
2
C
CO
2
–
HHO
CO
2
–
Pyruvate
Malate
Oxaloacetate
Citrate
CH
2
CHO CO
2
–
CH
2
CO
2
–
CO
2
–
SCoA
H
SCoA
+
2
NADPH
SCoACCH
3
O
CH
2
CHO CO
2
–
CH
2
CO
2
–
CO
2
–
Citrate
Pyruvate
CH
3
C O
CO
2
–
Oxaloacetate
CH
2
C O
CO
2
–
CO
2
–
CH
3
C O
CO
2
–
SCoACCH
3
O
+
Tricarboxylate
transport
system
R
CH
2
C
O
SCoA
+
H SCoA
R
CH
2
C
O
Acyl-CoA (C
n
)
Acyl-CoA (C
n+2
)
CH
3
C
O
SCoA
CH
2
C
O
SCoA
R
CH
2
C
H
CH
2
C
O
SCoA
Acetyl-CoA
thiolase
H
2
O
enoyl-CoA hydratase
enoyl-CoA reductase
3-
L-hydroxyacyl-CoA
dehydrogenase
-Ketoacyl-CoA
H
+
+ NADH
NAD
+
H
+
+ NADPH
NADP
+
OH
L--Hydroxyacyl-CoA
R
CH
2
C
H
C
C
O
SCoA
H
,-trans-Enoyl-CoA
RCH
2
CH
2
CH
2
C
O
SCoA
JWCL281_c25_940-1018.qxd 4/20/10 1:59 PM Page 970
