Voet D., Voet Ju.G. Biochemistry
Подождите немного. Документ загружается.


where x is at least 5 and where (CH
2
)
x
can contain one or
more double bonds. The (CH
2
)
y
portion of the substrate is
always saturated. Double bonds are inserted between ex-
isting double bonds in the (CH
2
)
x
portion of the substrate
and the CoA group such that the new double bond is three
carbon atoms closer to the CoA group than the next double
bond (not conjugated to an existing double bond) and, in
animals, never at positions beyond C9. Mammalian termi-
nal desaturases are components of mini-electron-transport
systems that contain two other proteins: cytochrome b
5
and
NADH–cytochrome b
5
reductase. The electron-transfer
reactions mediated by these complexes occur at the inner
surface of the ER membrane (Fig. 25-41) and are therefore
not associated with oxidative phosphorylation.
a. Some Unsaturated Fatty Acids Must
Be Obtained in the Diet
A variety of unsaturated fatty acids may be synthesized
by combinations of elongation and desaturation reactions.
However, since palmitic acid is the shortest available fatty
acid in animals, the above rules preclude the formation of
the
12
double bond of linoleic acid [
9,12
-octadecadienoic
acid; 18:2n–6 (this nomenclature is explained in Table
12-1)], a required precursor of prostaglandins. Linoleic
acid must consequently be obtained in the diet (ultimately
from plants that have ⌬
12
- and ⌬
15
-desaturases; it is abun-
dant in most vegetable oils) and is therefore termed an essen-
tial fatty acid. Indeed, animals maintained on a fat-free
diet develop an ultimately fatal condition that is initially
characterized by poor growth, poor wound healing, and
dermatitis. Linoleic acid is also an important constituent of
epidermal sphingolipids that function as the skin’s water-
permeability barrier.
Because of the inability of animal desaturases to add
double bonds to positions beyond C9, another essential
fatty acid is -linolenic acid [ALA;
9,12,15
-octadecatrienoic
acid (18:3n–3, an –3 fatty acid)]. This fatty acid is a pre-
cursor to EPA (
5,8,11,14,17
-eicosapentaenoic acid; 20:5n–3)
and DHA (
4,7,10,13,16,19
-docosahexaenoic acid; 22:6n–3),
polyunsaturated –3 fatty acids recently found to be im-
portant dietary constituents (present in fish oils) that im-
prove cognitive function and vision, and contribute to pro-
tection against inflammation and cardiovascular disease.
DHA is, among other things, the predominant fatty acid in
the phospholipids of retinal rod outer segments. Substitu-
tion of DHA with otherwise identical –6 fatty acids in
phospholipids results in impaired visual acuity. Deficiency
of –3 polyunsaturated fatty acids in brain phospholipids is
associated with memory loss and diminished cognitive
function.
F. Synthesis of Triacylglycerols
Triacylglycerols are synthesized from fatty acyl-CoA esters
and glycerol-3-phosphate or dihydroxyacetone phosphate
(Fig. 25-42). The initial step in this process is catalyzed
either by glycerol-3-phosphate acyltransferase in mito-
chondria and the ER, or by dihydroxyacetone phosphate
acyltransferase in the ER or peroxisomes.In the latter case,
the product acyl-dihydroxyacetone phosphate is reduced
to the corresponding lysophosphatidic acid by an NADPH-
dependent reductase. The lysophosphatidic acid is con-
verted to a triacylglycerol by the successive actions of 1-
acylglycerol-3-phosphate acyltransferase, phosphatidic
acid phosphatase, and diacylglycerol acyltransferase. The
intermediate phosphatidic acid and 1,2-diacylglycerol
(DAG) can also be converted to phospholipids by the path-
ways described in Section 25-8. The acyltransferases are
not completely specific for particular fatty acyl-CoAs,
either in chain length or in degree of unsaturation, but in
triacylglycerols of human adipose tissue, palmitate tends to
be concentrated at position 1 and oleate at position 2.
a. Glyceroneogenesis Is Important for
Triacylglycerol Biosynthesis
The dihydroxyacetone phosphate used to make
glycerol-3-phosphate for triacylglycerol synthesis comes
either from glucose via the glycolytic pathway (Fig. 17-3) or
membrane-bound, nonheme iron–containing enzymes
catalyze the general reaction
CH
3
(CH
2
)
x
(CH
2
)
y
H
C
H
H
C
H
C
O
SCoA NADH
+
++
H
+
O
2
CH
3
(CH
2
)
x
(CH
2
)
y
C
H
C
H
C
O
SCoA
2H
2
O
+
+
NAD
+
Section 25-4. Fatty Acid Biosynthesis 971
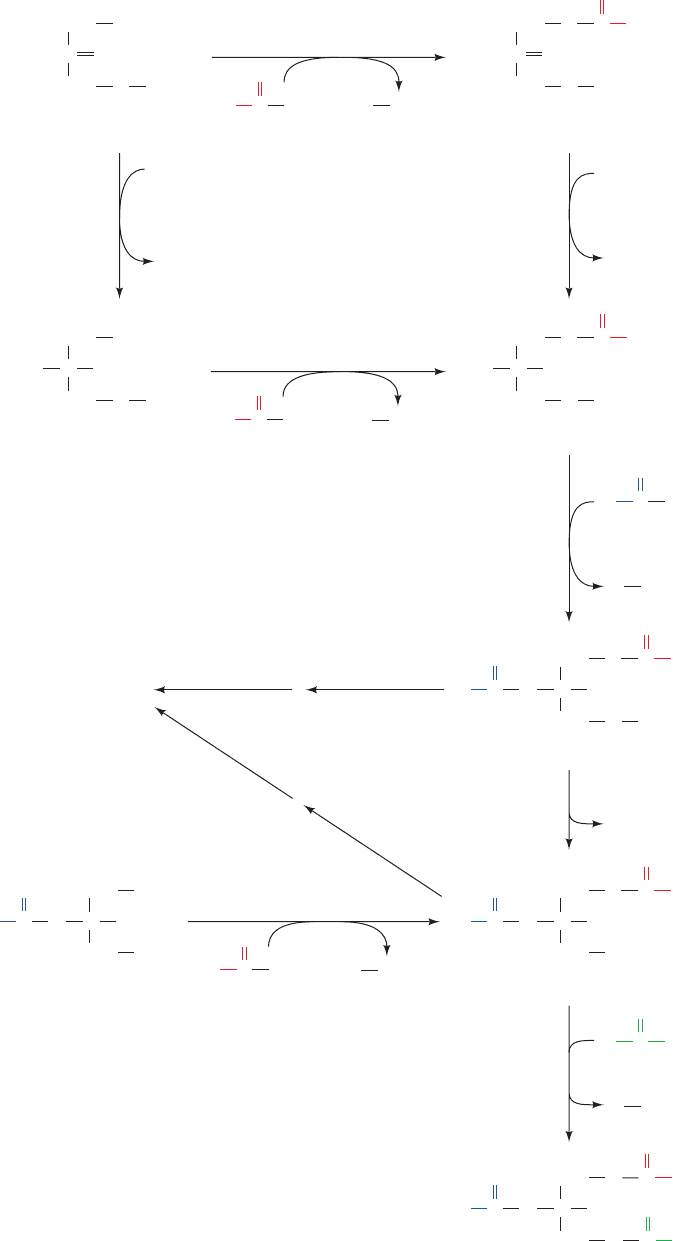
Figure 25-41 The electron-transfer reactions mediated by the
9
-fatty acyl-CoA desaturase complex. Its three proteins, desat-
urase, cytochrome b
5
, and NADH–cytochrome b
5
reductase, are
E
FAD
E
FADH
2
NADH–cytochrome
b
5
reductase
Stearoyl-CoA + 2H
+
+ O
2
desaturase
red
2Fe
2+
NAD
+
NADH + H
+
desaturase
ox
2Fe
3+
(18:0-CoA)
Oleoyl-CoA
+ 2H
2
O
(
9
-18:1-CoA)
2cyt b
5
(ox)
2cyt b
5
(red)
situated in the endoplasmic reticulum membrane. [After Jeffcoat,
R., Essays Biochem. 15, 19 (1979).]
JWCL281_c25_940-1018.qxd 4/20/10 1:59 PM Page 971
972 Chapter 25. Lipid Metabolism
O
PO
3
2
–
CH
2
OH
C O
CH
2
O
Dihydroxyacetone
phosphate
PO
3
2
–
CH
2
C
C O
CH
2
O
Acyl-dihydroxyacetone
phosphate
R
O
C SCoAR H SCoA
dihydroxyacetone phosphate
acyltransferase
O
PO
3
2
–
CH
2
OH
C H
CH
2
O
Glycerol-3-
phosphate
PO
3
2
–
CH
2
C
C
CH
2
O
Lysophosphatidic acid
R
O
C SCoAR H SCoA
glycerol-3-phosphate
acyltransferase
HO
O
HHO
O
NADP
+
NADPH
+ H
+
acyl-dihydroxyacetone
phosphate reductase
NAD
+
NADH + H
+
glycerol-3-phosphate
dehydrogenase
1-acylglycerol-3-phosphate
acyltransferase
O
C SCoAR
H
SCoA
O
PO
3
2
–
CH
2
C
C
CH
2
O
Phosphatidic acid
RO
HO
O
CR
phosphatidic acid
phosphatase
P
i
O
CH
2
C
C
CH
2
OH
1,2-Diacylglycerol (DAG)
RO
HO
O
CR
diacylglycerol
acyltransferase
O
C SCoAR
H
SCoA
O
CH
2
C
C
CH
2
O
Triacylglycerol
R
O
HO
O
CR
O
C R
O
C SCoAR H SCoA
2-monoacylglycerol
acyltransferase
CH
2
C
CH
2
OH
2-Monoacylglycerol
(from intestinal digestion)
OH
H
O
O
CR
Phospholipids
Figure 25-42 The reactions of triacylglycerol biosynthesis.
JWCL281_c25_940-1018.qxd 4/20/10 1:59 PM Page 972
from oxaloacetate via an abbreviated version of gluconeo-
genesis (Fig. 23-8) termed glyceroneogenesis. Glyceroneo-
genesis is necessary in times of starvation, since approxi-
mately 30% of the fatty acids that enter the liver during a
fast are reesterified to triacylglycerol and exported as
VLDL (Section 25-1 and 25-6A).Adipocytes also carry out
glyceroneogenesis in times of starvation.They do not carry
out gluconeogenesis but contain the gluconeogenic en-
zyme phosphoenolpyruvate carboxykinase (PEPCK),
which is upregulated when glucose concentration is low,
and participates in the glyceroneogenesis required for tria-
cylglycerol biosynthesis.
5 REGULATION OF FATTY ACID
METABOLISM
Discussions of metabolic control are usually concerned
with the regulation of metabolite flow through a pathway
in response to the differing energy needs and dietary states
of an organism. For example, the difference in the energy
requirement of muscle between rest and vigorous exertion
may be as much as 100-fold. Such varying demands may be
placed on the body when it is in either a fed or a fasted
state. For instance, Eric Newsholme, an authority on the
biochemistry of exercise, enjoys a 2-hour run before break-
fast. Others might wish for no greater exertion than the
motion of hand to mouth. In both individuals, glycogen and
triacylglycerols serve as primary fuels for energy-requiring
processes and are synthesized in times of quiet plenty for
future use.
a. Hormones Regulate Fatty Acid Metabolism
Synthesis and breakdown of glycogen and triacylglyc-
erols, as detailed in Chapter 18 and above, are processes that
concern the whole organism, with its organs and tissues
forming an interdependent network connected by the blood-
stream. The blood carries the metabolites responsible for
energy production: triacylglycerols in the form of chylomi-
crons and VLDL (Section 12-5A), fatty acids as their albu-
min complexes (Section 25-1e), ketone bodies, amino acids,
lactate, and glucose. The pancreatic and cells sense the
organism’s dietary and energetic state mainly through the
glucose concentration in the blood. The cells respond to
the low blood glucose concentration of the fasting and
energy-demanding states by secreting glucagon.The cells
respond to the high blood glucose concentration of the fed
and resting states by secreting insulin. We have previously
discussed (Sections 18-3E and 18-3F) how these hormones
are involved in glycogen metabolism. They also regulate the
rates of the opposing pathways of lipid metabolism and
therefore control whether fatty acids will be oxidized or syn-
thesized. Their targets are the regulatory (flux-generating)
enzymes of fatty acid synthesis and breakdown in specific
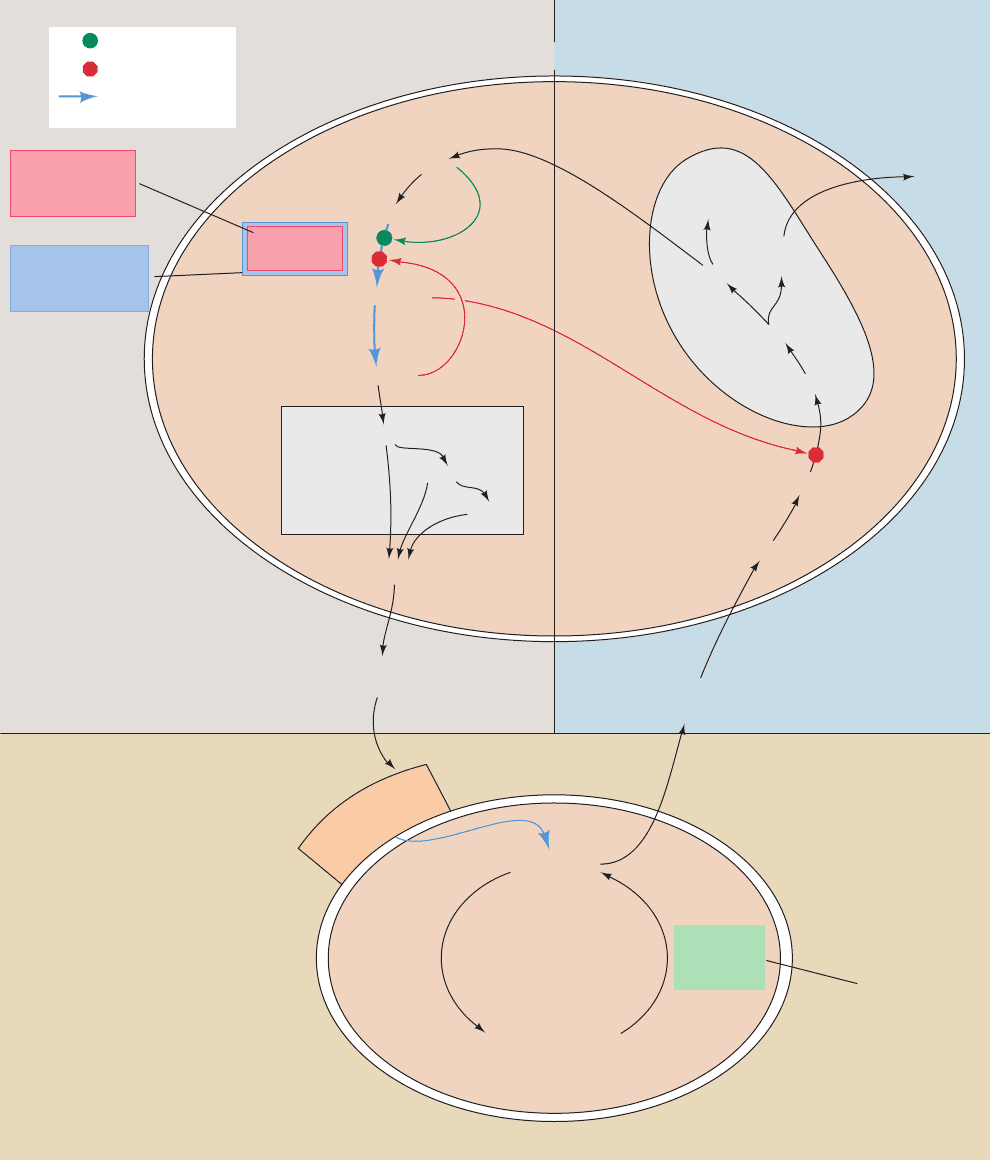
tissues (Fig. 25-43).
We are already familiar with most of the mechanisms by
which the catalytic activities of regulatory enzymes may be
controlled: substrate availability, allosteric interactions,
and covalent modification (phosphorylation). These are
examples of short-term regulation, regulation that occurs
with a response time of minutes or less. Fatty acid synthesis
is controlled, in part, by short-term regulation. Acetyl-CoA
carboxylase, which catalyzes the first committed step of
this pathway, is inhibited by palmitoyl-CoA and by the
glucagon-stimulated cAMP-dependent increase in phos-
phorylation, and is activated by citrate and by insulin-
stimulated dephosphorylation (Section 25-4B).
Another mechanism exists for controlling a pathway’s
regulatory enzymes: alteration of the amount of enzyme
present by changes in the rates of protein synthesis and/or
breakdown. This process requires hours or days and is
therefore called long-term regulation (the control of pro-
tein synthesis and breakdown is discussed in Chapters 31
and 32). Lipid biosynthesis is also controlled by long-term
regulation, with insulin stimulating and starvation inhibit-
ing the synthesis of acetyl-CoA carboxylase and fatty acid
synthase. The presence in the diet of polyunsaturated fatty
acids also decreases the concentrations of these enzymes.
The amount of adipose tissue lipoprotein lipase, the en-
zyme that initiates the entry of lipoprotein-packaged fatty
acids into adipose tissue for storage (Section 12-5Ba), is
also increased by insulin and decreased by starvation. In
contrast, the concentration of heart lipoprotein lipase,
which controls the entry of fatty acids from lipoproteins
into heart tissue for oxidation rather than storage, is de-
creased by insulin and increased by starvation. Starvation
and/or regular exercise, by decreasing the glucose concentra-
tion in the blood, change the body’s hormone balance. This
situation results in long-term changes in gene expression that
increase the levels of fatty acid oxidation enzymes and de-
crease those of lipid biosynthesis.
Fatty acid oxidation is regulated largely by the concen-
tration of fatty acids in the blood, which is, in turn, con-
trolled by the hydrolysis rate of triacylglycerols in adipose
tissue by hormone-sensitive triacylglycerol lipase. This
enzyme is so named because it is susceptible to regulation
by phosphorylation and dephosphorylation in response to
hormonally controlled cAMP levels. Epinephrine and
norepinephrine, as does glucagon, act to increase adipose
tissue cAMP concentrations. cAMP allosterically acti-
vates protein kinase A (PKA) which, in turn, increases
the phosphorylation levels of susceptible enzymes. Phos-
phorylation activates hormone-sensitive triacylglycerol li-
pase, thereby stimulating lipolysis in adipose tissue, raising
blood fatty acid levels, and ultimately activating the -
oxidation pathway in other tissues such as liver and muscle.
In liver, this process leads to the production of ketone
bodies that are secreted into the bloodstream for use by
peripheral tissues as an alternative fuel to glucose. PKA,
acting in concert with AMP-dependent protein kinase
(AMPK), also causes the inactivation of acetyl-CoA car-
boxylase (Section 25-4B), one of the rate-determining en-
zymes of fatty acid synthesis, so that cAMP-dependent
phosphorylation simultaneously stimulates fatty acid oxida-
tion and inhibits fatty acid synthesis.
Section 25-5. Regulation of Fatty Acid Metabolism 973
JWCL281_c25_940-1018.qxd 4/20/10 1:59 PM Page 973
Insulin has the opposite effect of glucagon and epineph-
rine: It stimulates the formation of glycogen and triacyl-
glycerols. This protein hormone, which is secreted in re-
sponse to high blood glucose concentrations, triggers a
highly complex signal transduction network (Section 19-4F)
that induces the long-term regulation of numerous enzymes
as well as decreasing cAMP levels. This latter situation
leads to the dephosphorylation and thus the inactivation of
hormone-sensitive triacylglycerol lipase, thereby reducing
the amount of fatty acid available for oxidation. Insulin
974 Chapter 25. Lipid Metabolism
Mitochondrion
Citric acid
cycle
Citrate
Ketone
bodies
Acetyl-CoA
Fatty acyl-CoA
Fatty acyl-CoA
Fatty acid
Citrate
Acetyl-CoA
Malonyl-CoA
Palmitate
Palmitate
Stearate
Oleate
Smooth
ER
fatty acid
synthase
Triacylglycerol
Hepatocyte (liver cell)
Acetyl-CoA
carboxylase
Ketone
bodies
Fatty acid oxidationFatty acid biosynthesis
Triacylglycerol
transport
Fatty acid
transport
Inhibited by
cAMP-dependent
phosphorylation
Activated by
insulin-dependent
dephosphorylation
Free fatty
acids
Triacylglycerols
Activated by
cAMP-dependent
phosphorylation
Fatty acid release
from adipocytes
into bloodstream
Very low density
lipoprotein (VLDL)
Fat storage
Adipocyte
"hormone-
sensitive"
lipase
Under long-term
Inhibition
Activation
regulation
Fatty acid
albumin complex
Lipoprotein
lipase
Figure 25-43 Sites of regulation of fatty acid metabolism.
JWCL281_c25_940-1018.qxd 4/20/10 1:59 PM Page 974
also stimulates the dephosphorylation of acetyl-CoA car-
boxylase, thereby activating this enzyme (Section 25-4Ba).
The glucagon–insulin ratio is therefore of prime impor-
tance in determining the rate and direction of fatty acid
metabolism.
Another control point that inhibits fatty acid oxida-
tion when fatty acid synthesis is stimulated is the inhibi-
tion of carnitine palmitoyltransferase I by malonyl-CoA.
This inhibition keeps the newly synthesized fatty acids
out of the mitochondrion (Section 25-2B) and thus away
from the -oxidation system. As we have seen (Section
25-4Bc), heart muscle, an oxidative tissue that does not
carry out fatty acid biosynthesis, contains an isoform of
acetyl-CoA carboxylase, ACC2, whose sole function ap-
pears to be the synthesis of malonyl-CoA to regulate
fatty acid oxidation.
AMPK may itself be an important regulator of fatty
acid metabolism.This phosphorylating enzyme is activated
by AMP and inhibited by ATP and thus has been proposed
to serve as a fuel gauge for the cell. When ATP levels are
high, signaling the fed and rested state, this kinase is inhib-
ited, allowing ACC to become dephosphorylated (acti-
vated) so as to stimulate malonyl-CoA production for fatty
acid synthesis in adipose tissue and for inhibition of fatty
acid oxidation in muscle cells.When activity levels increase
causing ATP levels to decrease with a concomitant increase
in AMP levels, AMPK is activated to phosphorylate (inac-
tivate) ACC. The resulting decrease in malonyl-CoA levels
causes fatty acid biosynthesis to decrease in adipose tissue
while fatty acid oxidation increases in muscle to provide
the ATP for continued activity.
6 CHOLESTEROL METABOLISM
Cholesterol is a vital constituent of cell membranes and the
precursor of steroid hormones and bile salts. It is clearly es-
sential to life, yet its deposition in arteries has been associ-
ated with cardiovascular disease and stroke, two leading
causes of death in humans. In a healthy organism, an intri-
cate balance is maintained between the biosynthesis, utiliza-
tion, and transport of cholesterol, keeping its harmful depo-
sition to a minimum. In this section, we study the pathways
of cholesterol biosynthesis and transport and how they are
controlled. We also examine how cholesterol is utilized in
the biosynthesis of steroid hormones and bile salts.
A. Cholesterol Biosynthesis
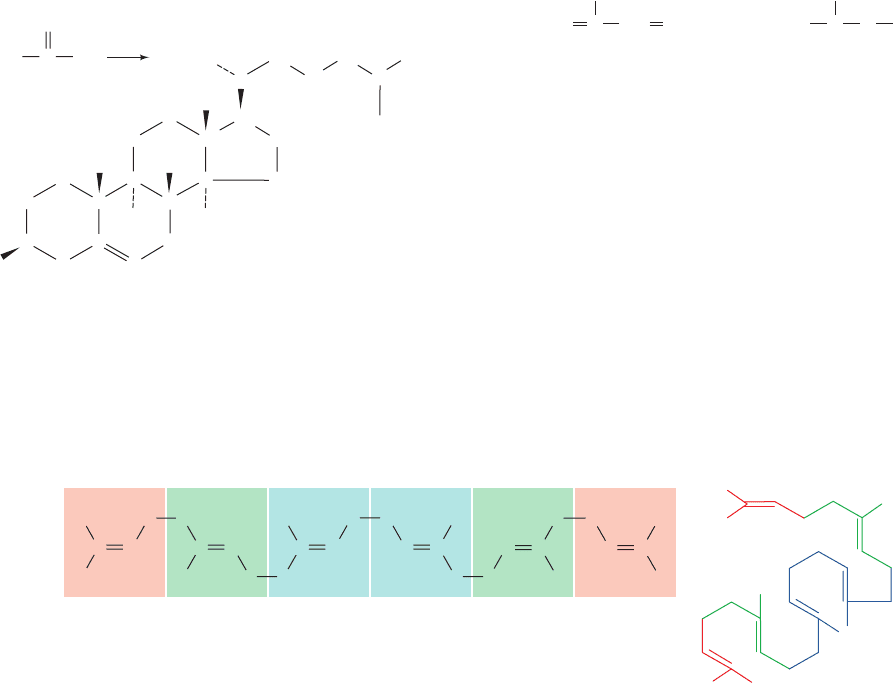
All of the carbon atoms of cholesterol are derived from
acetate (Fig. 25-44). Observation of their pattern of incor-
poration into cholesterol led Konrad Bloch to propose that
acetate was first converted to isoprene units, C
5
units that
have the carbon skeleton of isoprene:
Isoprene units are condensed to form a linear precursor to
cholesterol, and then cyclized.
Squalene, a polyisoprenoid hydrocarbon (Fig. 25-45a),
was demonstrated to be the linear intermediate in choles-
terol biosynthesis by the observation that feeding isotopi-
cally labeled squalene to animals yields labeled choles-
terol. Squalene may be folded in several ways that would
enable it to cyclize to the four-ring sterol nucleus (Section
12-1E).The folding pattern proposed by Bloch and Robert
B.Woodward (Fig. 25-45b) proved to be correct.
Isoprene
(2-Methyl-1,3-butadiene)
An isoprene unit
CH
2
CH
3
CHC
CH
2
C
CCC C
Section 25-6. Cholesterol Metabolism 975
Figure 25-44 All of cholesterol’s carbon atoms are derived
from acetate.
H
2
C
H
3
C
C
C
C
C
C
C
H
2
H
2
HO
H
2
C
C
C
H
2
C
H
C
H
CH
2
H
CH
3
CH
3
H
CH
3
CH
2
CH
2
CH
2
CH
2
C
CH
2
CH
2
CH
3
CH
CH
CH
C
Acetate
O
–
O
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
17
16
19
18
20
21
22
23
24
25
26
27
H
CC
CC
C
CC
C
CCC
C
C
C
C
C
C
C
C
C
C
CC
C
C
C
C
C
C
C
(a)
(b)
Figure 25-45 Squalene. (a) Extended conformation. Each box contains one isoprene
unit. (b) Folded in preparation for cyclization as predicted by Bloch and Woodward.
JWCL281_c25_940-1018.qxd 6/8/10 8:59 AM Page 975
Bloch’s outline for the major stages of cholesterol
biosynthesis was
This pathway has been experimentally verified and its de-
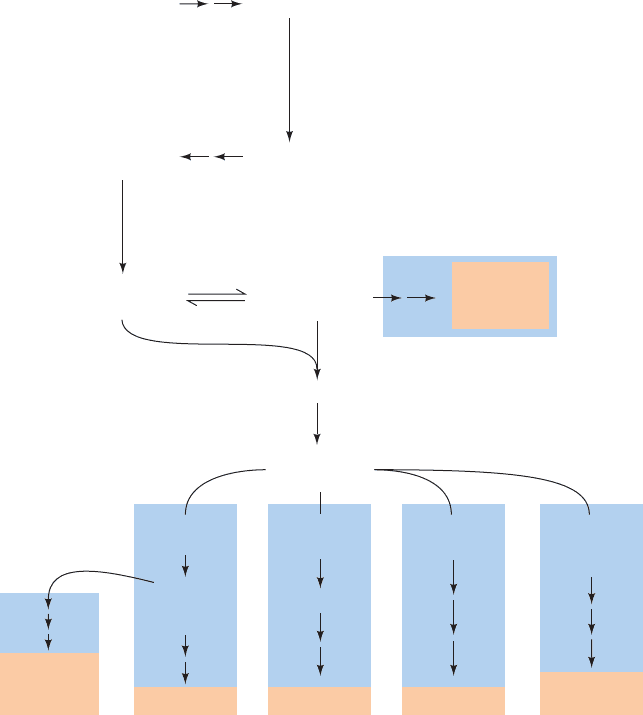
tails elaborated. It is now known to be part of a branched
pathway (Fig. 25-46) that produces several other essential
isoprenoids in addition to cholesterol, namely, ubiquinone
(CoQ; Fig. 22-17b), dolichol (Fig. 23-15), farnesylated and
geranylgeranylated proteins (Fig. 12-29), and isopentenyl-
adenosine (a modified base of tRNA; Fig. 32-10). We shall
examine in detail the portion of this pathway that synthe-
sizes cholesterol. Note, however, that 25,000 isoprenoids
(also known as terpenoids), mostly of plant, fungal, and
bacterial origin, have been characterized. These serve as
membrane constituents (e.g., cholesterol), hormones
squalene
¡
cyclization product
¡
cholesterol
Acetate
¡
isoprenoid intermediate
¡
(steroids), pheromones, defensive agents, photoprotective
agents (e.g., -carotene; Section 24-2Ad), and visual pig-
ments (e.g., retinal; Section 12-3Ab), to name only a few of
their many biological functions.
a. HMG-CoA Is a Key Cholesterol Precursor
Acetyl-CoA is converted to isoprene units by a series of
reactions that begins with formation of hydroxymethylglu-
taryl-CoA (HMG-CoA; Fig. 25-26), a compound we previ-
ously encountered as an intermediate in ketone body
biosynthesis (Section 25-3). HMG-CoA synthesis requires
the participation of two enzymes: thiolase and HMG-CoA
synthase. The enzymes forming the HMG-CoA leading to
ketone bodies occur in the mitochondria, whereas those re-
sponsible for the synthesis of the HMG-CoA that is des-
tined for cholesterol biosynthesis are located in the cytosol.
Their catalytic mechanisms, however, are identical.
976 Chapter 25. Lipid Metabolism
Figure 25-46 The branched pathway of isoprenoid
metabolism in mammalian cells. The pathway produces
ubiquinone, dolichol, farnesylated and geranylgeranylated
cis -Prenyl
transferase
Acetyl-CoA
HMG-CoA
Mevalonate
Mevalonate
pyrophosphate
Isopentenyl
pyrophosphate
Dimethylallyl
pyrophosphate
Isopentenyl
adenosine
(tRNA)
Geranyl pyrophosphate
Farnesyl
pyrophosphate
Squalene
synthase
Squalene
CholesterolUbiquinone Dolichol
HMG-CoA reductase
trans -Prenyl
transferase
Geranyl-
geranyl
phosphate
Geranyl-
geranylated
proteins
Protein
prenyl
transferase
Farnesylated
proteins
proteins, and isopentenyl adenosine, a modified tRNA base, in
addition to cholesterol.
JWCL281_c25_940-1018.qxd 4/20/10 1:59 PM Page 976
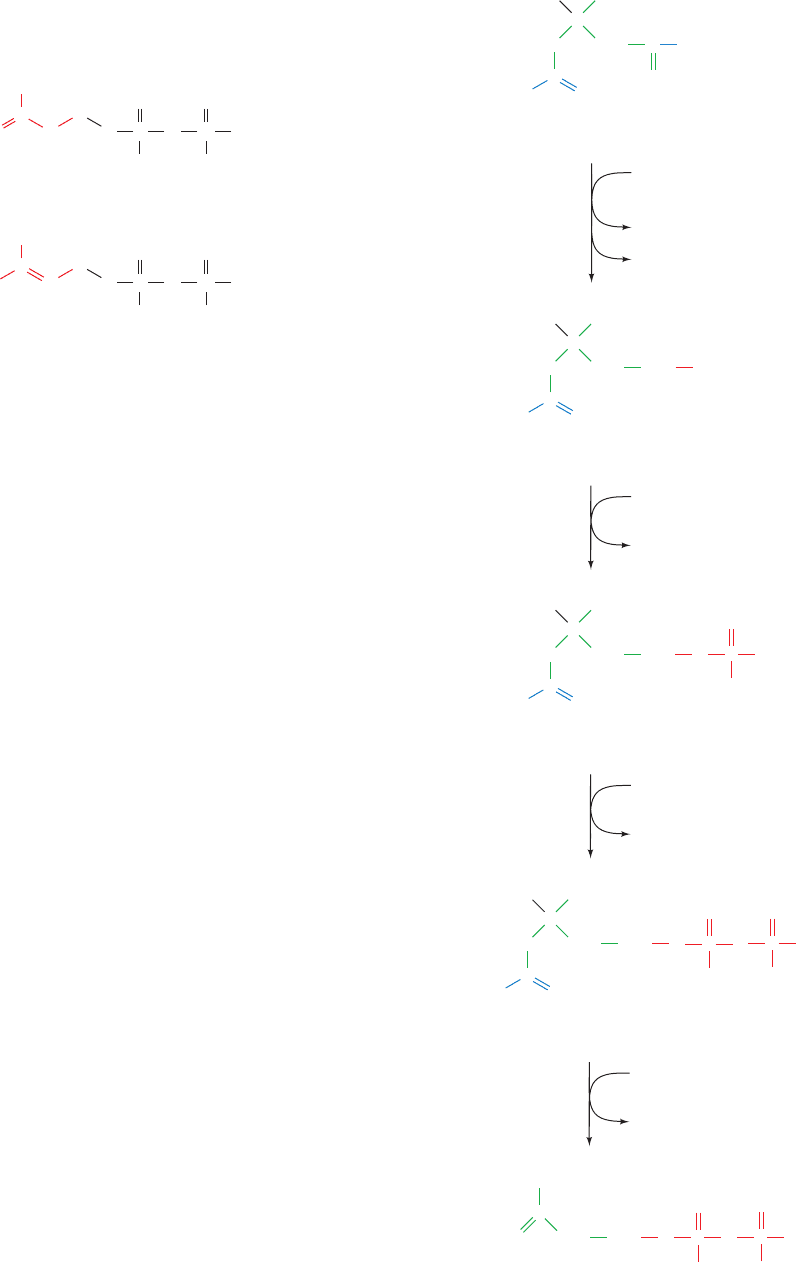
HMG-CoA is the precursor of two isoprenoid interme-
diates, isopentenyl pyrophosphate and dimethylallyl
pyrophosphate:
The formation of isopentenyl pyrophosphate involves four
reactions (Fig. 25-47):
1. The CoA thioester group of HMG-CoA is reduced
to an alcohol in an NADPH-dependent four-electron re-
duction catalyzed by HMG-CoA reductase, yielding
mevalonate.
2. The new OH group is phosphorylated by mevalonate-
5-phosphotransferase.
3. The phosphate group is converted to a pyrophos-
phate by phosphomevalonate kinase.
4. The molecule is decarboxylated and the resulting alco-
hol dehydrated by pyrophosphomevalonate decarboxylase.
HMG-CoA reductase mediates the rate-determining step
of cholesterol biosynthesis and is the most elaborately regu-
lated enzyme of this pathway. This 888-residue ER
membrane-bound enzyme is regulated, as we shall see in
Section 25-6Bb, by competitive and allosteric mechanisms,
phosphorylation/dephosphorylation, and long-term regu-
lation.Cholesterol itself is an important feedback regulator
of the enzyme.
b. Pyrophosphomevalonate Decarboxylase
Catalyzes an Apparently Concerted Reaction
5-Pyrophosphomevalonate is converted to isopentenyl
pyrophosphate by an ATP-dependent dehydration–decar-
boxylation reaction catalyzed by pyrophosphomevalonate
decarboxylase (Fig. 25-48). When [3-
18
O]5-pyrophospho-
mevalonate (*O in Fig. 25-48) is used as a substrate, the la-
beled oxygen appears in P
i
. This observation suggests that
3-phospho-5-pyrophosphomevalonate is a reaction inter-
mediate. Since all attempts to isolate this intermediate
have failed, however, it has been proposed that phosphory-
lation, the , elimination of CO
2
, and the elimination of P
i
occur in a concerted reaction.
OOP
O
O
–
O
–
H
2
H
2
H
2
C
C
C
P
O
O
–
CH
3
C
Isopentenyl pyrophosphate
OOP
O
O
–
O
–
H
H
2
H
3
C
C
C
P
O
O
–
CH
3
C
Dimethylallyl pyrophosphate
Section 25-6. Cholesterol Metabolism 977
Figure 25-47 Formation of isopentenyl pyrophosphate from HMG-CoA.
CH
2
CH
3
C
O
C
C
H
2
C SCoA
O
–
O
HO
HMG-CoA
OH
CH
2
CH
3
CH
2
C
C
H
2
C
O
–
O
HO
Mevalonate
mevalonate-5-
phosphotransferase
ATP
ADP
2
Phosphomevalonate
O
O
P
CH
2
CH
3
CH
2
C
C
H
2
C
O
–
O
HO
O
–
O
–
phosphomevalonate
kinase
ATP
ADP
3
5-Pyrophosphomevalonate
O
O
O
P
CH
2
CH
3
CH
2
C
C
H
2
C
O
–
O
HO
O
–
O
P
O
–
O
–
pyrophospho-
mevalonate
decarboxylase
ATP
ADP
+ P
i
+ CO
2
4
Isopentenyl pyrophosphate
O
O
O
–
O
P
CH
2
CH
3
CH
2
C
H
2
C
O
–
O
P
O
–
HMG-CoA
reductase
CoA
2 NADP
+
2 NADPH
1
JWCL281_c25_940-1018.qxd 4/20/10 1:59 PM Page 977
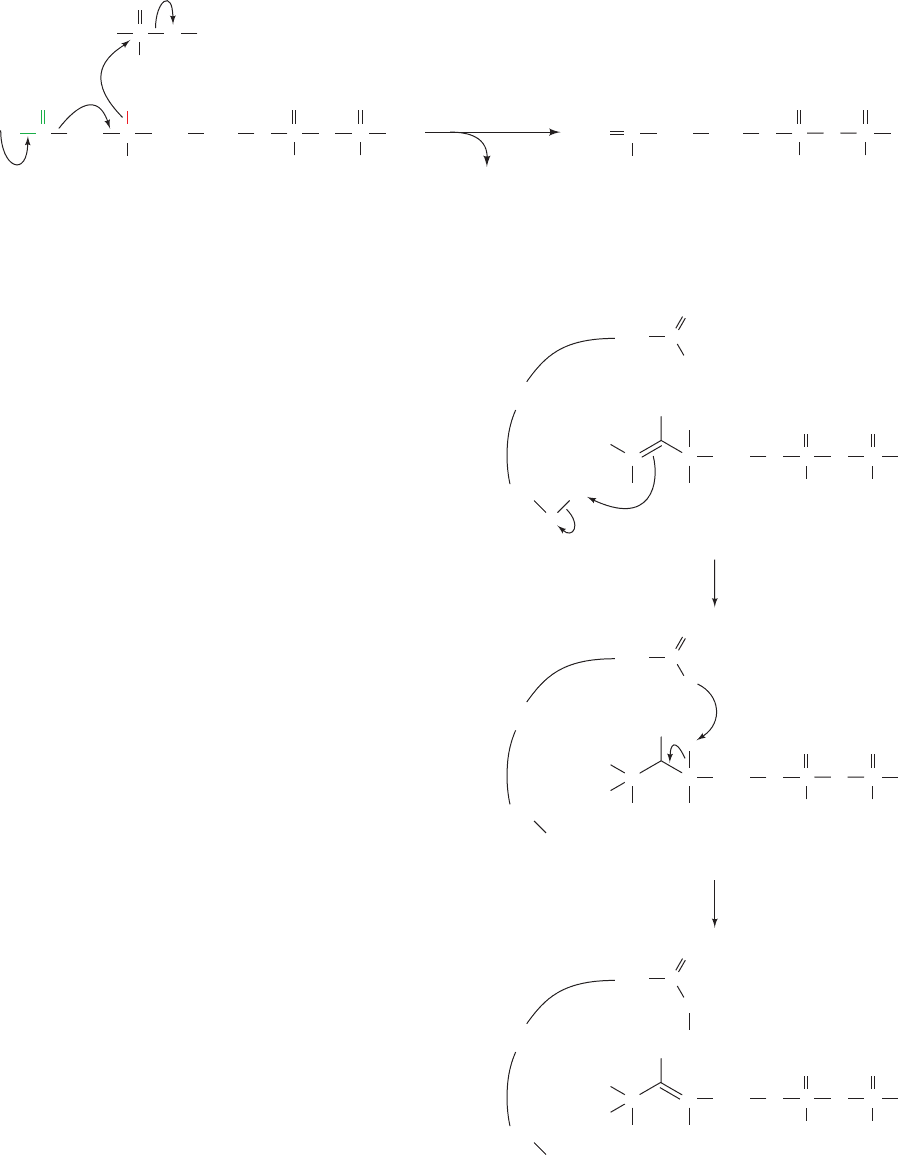
OO
CC
P
O
O
–
O
–
P
O
O
–
H
E
Isopentenyl
pyrophosphate
CH
2
H
3
C
H
H
H
H
S
Cys
OO
C
C
C
P
O
O
–
O
–
P
O
O
–
+
O
–
O
H
H
E
Tertiary
carbocation
CH
2
H
3
C
H
H
H
S
–
Cys
Glu
C
O
–
O
Glu
OO
C
C
C
P
O
O
–
O
–
P
O
O
–
O
O
H
H
E
Dimethylallyl
pyrophosphate
CH
2
H
3
C
H
H
H
S
–
Cys
Glu
The equilibration between isopentenyl pyrophosphate
and dimethylallyl pyrophosphate is catalyzed by isopen-
tenyl pyrophosphate isomerase. The reaction appears to
occur via a protonation/deprotonation reaction involving
the intermediacy of a tertiary carbocation intermediate.
Cys and Glu residues have been implicated as the general
acid and base catalysts, respectively (Fig. 25-49), as sup-
ported by site-directed mutagenesis and the X-ray struc-
ture of the enzyme. The carbocation is thought to be sta-
bilized through interactions with the aromatic cloud of
an adjacent Trp residue. Aromatic residues provide elec-
tron-rich interactions with positively charged groups
without forming covalent bonds that would destroy the
intermediate.
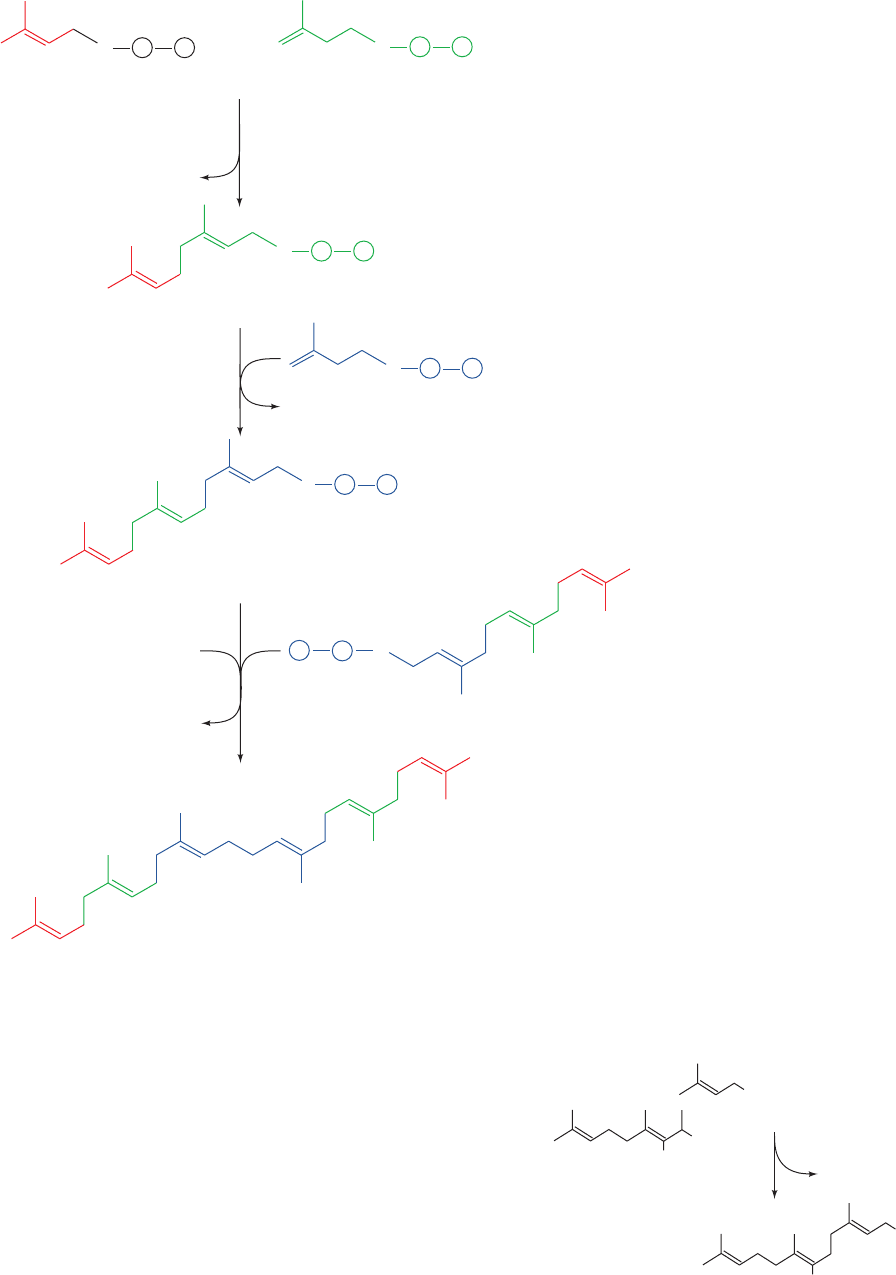
c. Squalene Is Formed by the Condensation
of Six Isoprene Units
Four isopentenyl pyrophosphates and two dimethylallyl
pyrophosphates condense to form the C
30
cholesterol pre-
cursor squalene in three reactions catalyzed by two en-
zymes (Fig. 25-50):
1. Prenyltransferase (farnesyl pyrophosphate synthase)
catalyzes the head-to-tail (1¿–4) condensation of dimethy-
lallyl pyrophosphate and isopentenyl pyrophosphate to
yield geranyl pyrophosphate.
2. Prenyltransferase catalyzes a second head-to-tail
condensation of geranyl pyrophosphate and isopentenyl
pyrophosphate to yield farnesyl pyrophosphate (FPP).
3. Squalene synthase (SQS) then catalyzes the head-to-
head (1–1¿) condensation of two farnesyl pyrophosphate
molecules to form squalene. Farnesyl pyrophosphate is
also a precursor to dolichol, farnesylated and geranylger-
anylated proteins, and ubiquinone (Fig. 25-46).
Prenyltransferase catalyzes the condensation of isopen-
tenyl pyrophosphate with an allylic (conjugated to a C“C
double bond) pyrophosphate. It is specific for isopentenyl
pyrophosphate but can use either the 5-carbon dimethyl-
allyl pyrophosphate or the 10-carbon geranyl pyrophosphate
as its allylic substrate.The prenyltransferase-catalyzed con-
densation mechanism is particularly interesting since it is
one of the few known enzyme-catalyzed reactions that pro-
978 Chapter 25. Lipid Metabolism
Figure 25-48 Action of pyrophosphomevalonate decarboxylase. The enzyme catalyzes an ATP-dependent
concerted dehydration–decarboxylation of pyrophosphomevalonate, yielding isopentenyl pyrophosphate.
Figure 25-49 Mechanism of isopentenyl pyrophosphate
isomerase. The enzyme interconverts isopentenyl pyrophosphate
and dimethylallyl pyrophosphate by a protonation/deprotonation
reaction involving a carbocation intermediate in which a Cys and
a Glu residue act as a proton donor and acceptor.The carbo-
cation intermediate appears to be stabilized by interactions
with a nearby Trp side chain.
OOP
O
O
–
O
–
CH
2
CH
2
CH
2
P
O
O
–
O
–
OP
O
–
O
C
O
O
–
CH
3
C
*
OH
ADP
+
OOP
O
O
–
O
–
CH
2
CH
2
CH
2
P
*
P
i
+ ADP +
O
O
–
CH
3
C
pyrophospho-
mevalonate
decarboxylase
CO
2
5-Pyrophosphomevalonate Isopentenyl pyrophosphate
JWCL281_c25_940-1018.qxd 4/20/10 1:59 PM Page 978
ceed via a carbocation intermediate. Two possible conden-
sation mechanisms can be envisioned (Fig. 25-51):
Scheme I An S
N
1 mechanism in which an allylic carbo-
cation forms by the elimination of PP
i
. Isopentenyl py-
rophosphate then condenses with this carbocation, forming
a new carbocation that eliminates a proton to form product.
Scheme II An S
N
2 reaction in which the allylic PP
i
is
displaced in a concerted manner. In this case, an enzyme
nucleophile, X, assists in the reaction. This group is elimi-
nated in the second step with the loss of a proton to form
product.
Dale Poulter and Hans Rilling used chemical logic to
differentiate between these two mechanisms. Capitalizing
on the observation that S
N
1 reactions are much more sensi-
tive to electron-withdrawing groups than S
N
2 reactions,
they synthesized a geranyl pyrophosphate derivative in
which the H at C2 is replaced by the electron-withdrawing
group F. This allylic substrate for the second (1¿–4) conden-
sation catalyzed by prenyltransferase, not surprisingly, has
the same K
M
as the natural substrate (F and H have similar
atomic radii):
It is, however,the V
max
of this reaction that tells the story. If
the reaction is an S
N
2 displacement, the fluoro derivative
OPP
F
OPP
+
PP
i
, H
+
OPP
F
Section 25-6. Cholesterol Metabolism 979
Figure 25-50 Formation of squalene from isopentenyl
pyrophosphate and dimethylallyl pyrophosphate. The
pathway involves two head-to-tail condensations catalyzed
by prenyltransferase and a head-to-head condensation
catalyzed by squalene synthase.
Squalene
squalene synthase
(head to head)
NADPH
NADP
+
+ 2 PP
i
3
prenyltransferase
(head to tail)
PP
i
2
prenyltransferase
(head to tail)
PP
i
1
P
P
O
P
PO
P PO
P PO
P PO
P PO
Farnesyl pyrophosphate
Farnesyl pyrophosphate
Geranyl pyrophosphate
Dimethylallyl pyrophosphate
Isopentenyl pyrophosphate
+
JWCL281_c25_940-1018.qxd 4/20/10 1:59 PM Page 979
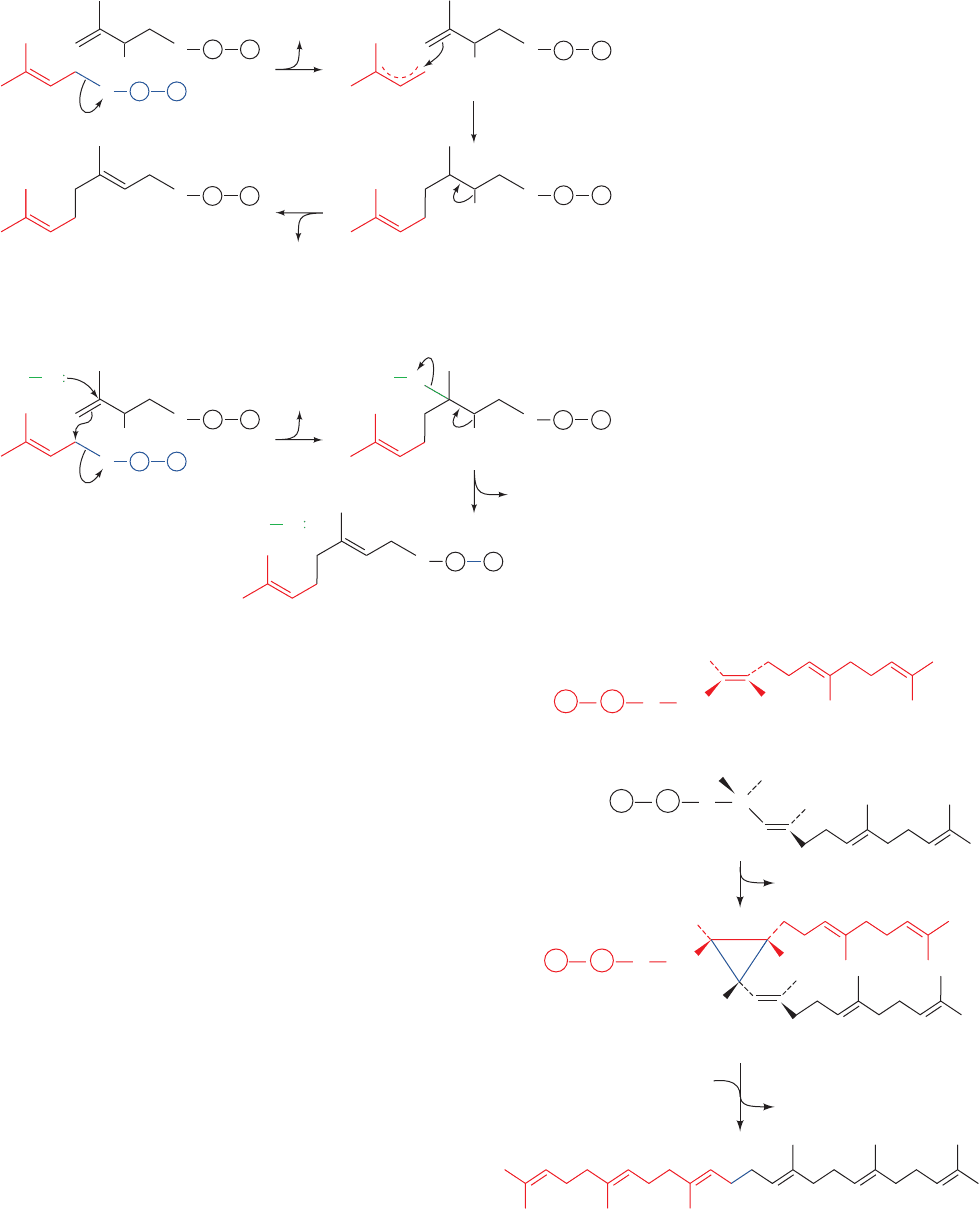
should react at a rate similar to that of the natural sub-
strate. If, instead, the reaction has an S
N
1 mechanism, the
fluoro derivative should react orders of magnitude more
slowly than the natural substrate. In fact, 3-fluorogeranyl
pyrophosphate forms product at 1% of the rate of the
natural substrate, strongly supporting an S
N
1 mechanism
with a carbocation intermediate.
Carbocations are now known to participate in several
reactions of isoprenoid biosynthesis. The enzymes are clas-
sified according to how they generate these carbocations.
Class I enzymes do so via the release of pyrophosphate, as
we have seen for prenyltransferase. Class II enzymes do so
by protonating a double bond, as does isopentenyl py-
rophosphate isomerase (Fig. 25-49), or an epoxide, as we
shall see below for oxidosqualene cyclase.
Squalene, the immediate sterol precursor, is formed by
the head-to-head condensation of two FPP molecules by
SQS.Although the enzyme is a Class I enzyme that is struc-
turally related to prenyltransferase and generates carboca-
tions by the release of pyrophosphate, the reaction is not a
simple head-to-tail condensation, as might be expected,
but, rather, proceeds via a complex two-step mechanism
with each step catalyzed by a different active site on the en-
zyme (Fig. 25-52):
Step I The reaction of two FPP molecules to yield the
stable intermediate presqualene pyrophosphate. This reac-
980 Chapter 25. Lipid Metabolism
R
O
P
P
OP P
H
R
O
O
P
P
H
PP
i
S
N
1
Scheme I
Ionization–condensation–elimination
+
+
R
OP P
R
P P
H
H
+
R
O
P
P
OP P
H
R
PP
i
S
N
2
Scheme II
Condensation–elimination
H
+
O
P
E
X
P
H
R
O
P P
E
X
–
E
X
–
CH
2
PP
i
C
H
H
H
H
CH
3
H
3
C
O
+
1
23
1
1
1
1
1
23
PP
CH
3
NADP
+
+ PP
i
NADPH
H
CH
3
CH
2
OPP
OPP
Farnesyl
pyrophosphate
Farnesyl
pyrophosphate
Presqualene
pyrophosphate
Squalene
I
II
Figure 25-51 Two possible mechanisms for
the prenyltransferase reaction. Scheme I
involves the formation of a carbocation
intermediate, whereas Scheme II involves the
participation of an enzyme nucleophile, X.
Figure 25-52 Action of squalene synthase. The enzyme
catalyzes the head-to-head condensation of two farnesyl
pyrophosphate molecules to form squalene.
JWCL281_c25_940-1018.qxd 4/20/10 1:59 PM Page 980
