Voet D., Voet Ju.G. Biochemistry
Подождите немного. Документ загружается.

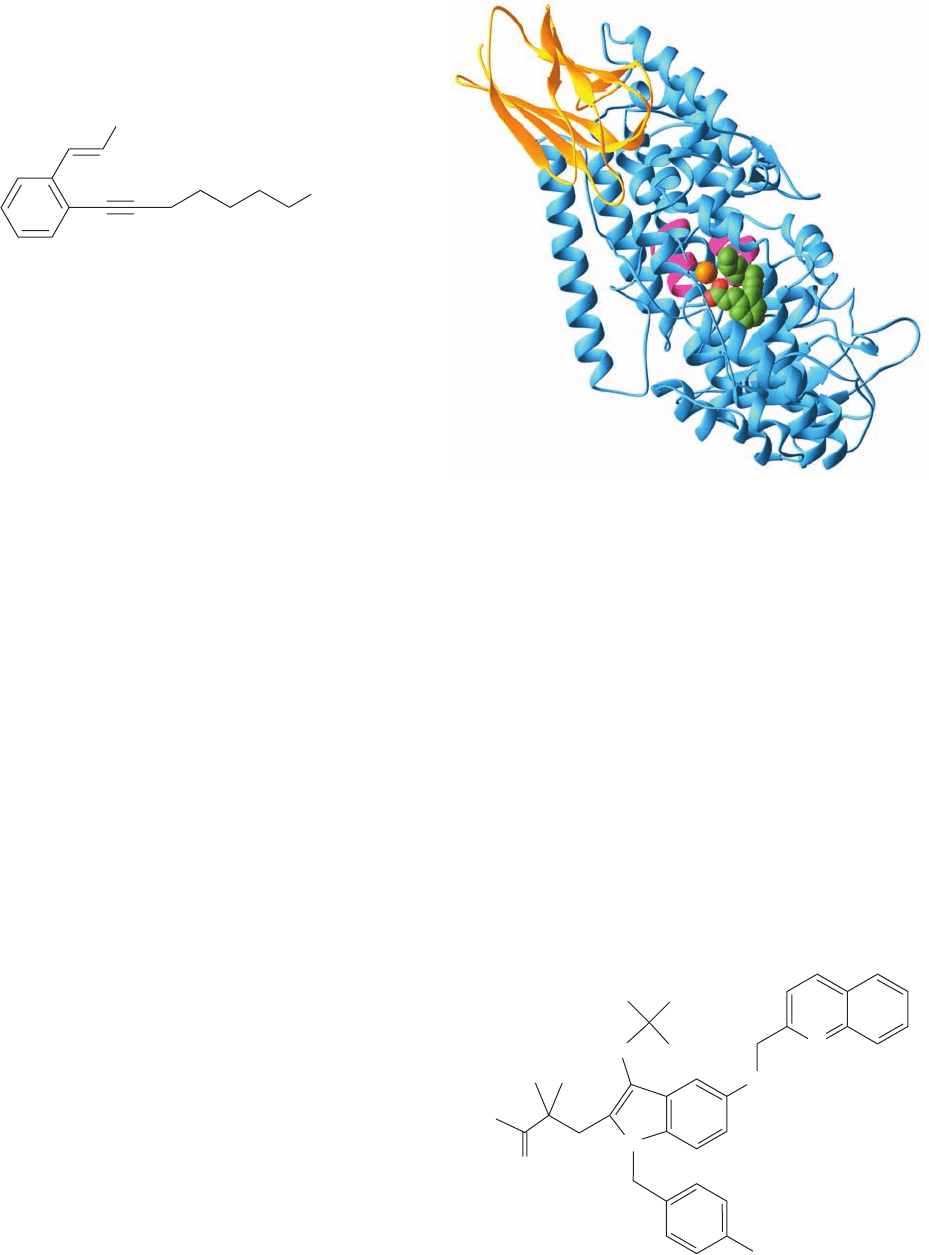
The X-ray structure of the rabbit reticulocyte 15-LO, a
homolog of 5-LO, in complex with the competitive in-
hibitor RS75091
was determined by Michelle Browner. This 663-residue
monomeric protein consists of an N-terminal 8-stranded
barrel domain and a C-terminal catalytic domain (Fig.25-77).
Its active site Fe atom is coordinated by four invariant His
residues and by a C-terminal carboxylate oxygen in a lig-
anding arrangement that is best described as a distorted oc-
tahedron with one of its six vertices unoccupied. The Fe,
which is well below the protein surface, faces an internal
cavity occupied by RS75091. This identifies the substrate
binding cavity, which is lined with mostly hydrophobic
residues and follows an irregular pathway past the Fe atom
to the protein surface. Intriguingly, 15-LO (as does soybean
lipoxygenase-1) contains two rarely observed helices
(Fig. 8-14c), each of which contains two of the Fe-liganding
His residues. Each of these helices is embedded in a
longer helix rather than being at the end of an helix as is
the case for all previously observed helices.
The sizes of the substrate-binding cavities of the 5- and
12-LOs have been predicted through their homology mod-
eling (Section 9-3B) with 15-LO. 5-LO and 12-LO have
smaller amino acids substituted for those in 15-LO, such
that, for example, 5-LO is predicted to have a cavity with
⬃20% greater volume than that of 15-LO. The mutagene-
sis of 5-LO by Harmut Kuhn so as to decrease the size of its
cavity yielded an enzyme with the specificity of 15-LO, thus
supporting the proposal that it is the size of the cavity that
determines lipoxygenase specificity.
b. Peptidoleukotrienes
LTA
4
is converted to peptidoleukotrienes by reaction with
LTC
4
synthase, a glutathione-S-transferase that catalyzes
the addition of the glutathione sulfhydryl group to the
LTA
4
epoxide, forming the first of the peptidoleukotrienes,
leukotriene C
4
(LTC
4
, Fig. 25-78). -Glutamyltransferase
removes glutamic acid, converting LTC
4
to leukotriene
D
4
(LTD
4
). LTD
4
is converted to leukotriene E
4
(LTE
4
)
by a dipeptidase that removes glycine. LTA
4
can also be
hydrolyzed to leukotriene B
4
(LTB
4
), a potent chemotac-
tic agent (a substance that attracts motile cells) involved
in attracting certain types of white blood cells to fight
infection.
Various inflammatory and hypersensitivity disorders
(such as asthma) are associated with elevated levels of
leukotrienes. The development of drugs that inhibit
leukotriene synthesis has therefore been an active field of
RS75091
COOH
CH
3
research. 5-LO activity requires the presence of 5-lipoxyge-
nase-activating protein (FLAP), a homotrimeric integral
membrane protein of 161-residue subunits that is located
in both the nuclear and ER membranes. FLAP, which has
no enzymatic activity, binds the arachidonic acid substrate
of 5-LO and facilitates enzyme–substrate binding as well as
5-LO’s interaction with the membrane. Several inhibitors
of leukotriene synthesis, such as MK-591,
bind to FLAP so as to inhibit both of its functions.
O
N
Cl
O
HO
S
N
MK-591
Section 25-7. Eicosanoid Metabolism: Prostaglandins, Prostacyclins, Thromboxanes, Leukotrienes, and Lipoxins 1001
Figure 25-77 X-ray structure of rabbit reticulocyte
15-lipoxygenase (15-LO) in complex with its competitive
inhibitor RS75091. The N-terminal barrel domain is gold and
the C-terminal catalytic domain is light blue with its two
Fe-liganding helical segments magenta. The Fe is represented
by an orange sphere and the RS75091 is drawn in space-filling
form with C green and O red. [Based on an X-ray structure by
Michelle Browner, Roche Bioscience, Palo Alto, California.
PDBid 1LOX.]
JWCL281_c25_940-1018.qxd 6/8/10 9:00 AM Page 1001
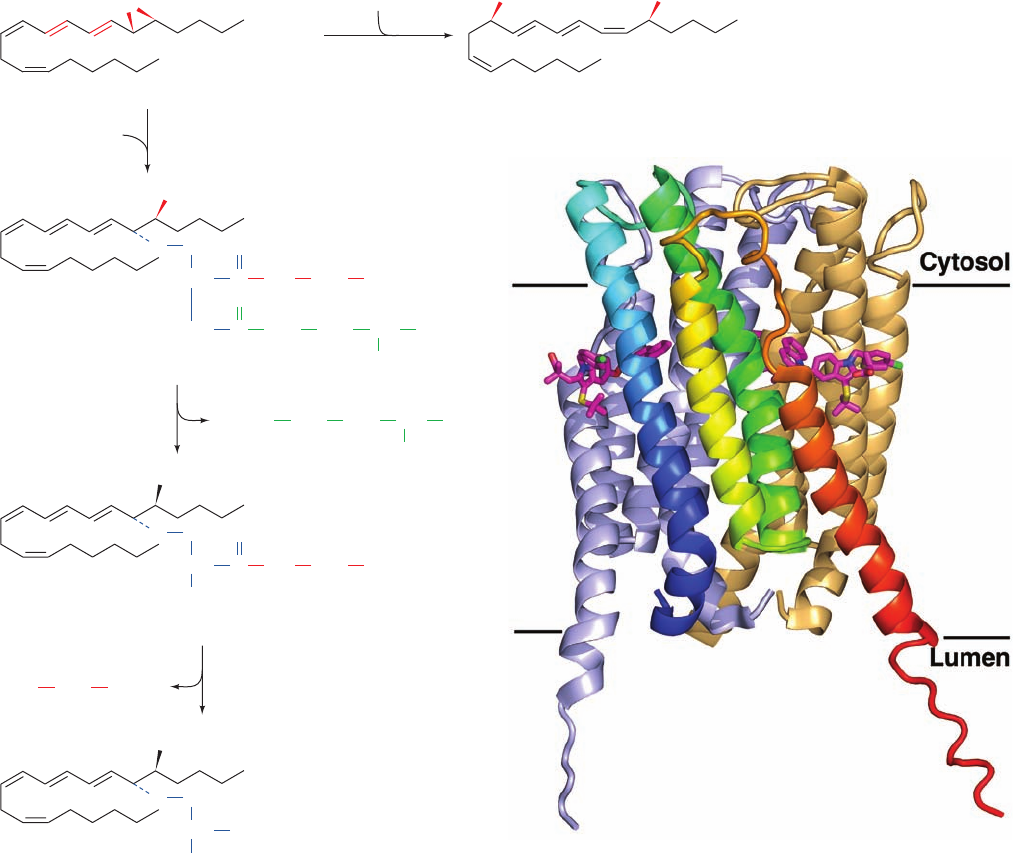
The X-ray structure of human FLAP in complex with
MK-591 (Fig. 25-79), determined by Joseph Becker, reveals
that each subunit forms an up–down–up–down four-helix
bundle whose N- and C-termini are on the luminal side of
the membrane, and that its three identical subunits have
extensive intersubunit contacts, thereby forming a 12-helix
bundle. MK-591 binds in lipid-exposed grooves between
pairs of subunits. Moreover, the trimer forms an elongated
pocket along its 3-fold axis that is open to the lumen. This,
together with biochemical data, suggests that one 5-LO
molecule binds to the cytosolic surface of a FLAP trimer
where, through conformational changes in FLAP, it accepts
arachidonic acid molecules that FLAP has extracted from
the membrane. Inhibitors such as MK-591 presumably
block this extraction process.
c. Diets Rich in Marine Lipids May Decrease
Cholesterol, Prostaglandin, and Leukotriene Levels
Greenland Eskimos have a very low incidence of coro-
nary heart disease and thrombosis despite their high di-
etary intake of cholesterol and fat. Their consumption of
marine animals provides them with a higher proportion of
unsaturated fats than the typical American diet. A major
unsaturated component of marine lipids is 5,8,11,14,17-
1002 Chapter 25. Lipid Metabolism
Figure 25-78 Formation of the leukotrienes from LTA
4
.
Figure 25-79 X-ray structure of human 5-lipoxygenase-
activating protein (FLAP) in complex with MK-591. The protein
is drawn in ribbon form and viewed from within the membrane
with its approximate molecular 3-fold axis vertical.The subunit
closest to the viewer of this homotrimeric protein is colored in
rainbow order from its N-terminus (blue) to its C-terminus (red).
The other subunits are pale blue and pale orange.The bound
MK-591 is drawn in stick form with C magenta, N blue, O red, S
yellow, and C green.The inferred position of the membrane is
indicated by the horizontal black lines. [Based on an X-ray
structure by Joseph Becker, Merck Research Laboratories,
Rahway, New Jersey. PDBid 2Q7M.]
OH
O
Leukotriene A
4
(LTA
4
)
COOH
Leukotriene C
4
(LTC
4
)
Leukotriene D
4
(LTD
4
)
Leukotriene E
4
(LTE
4
)
Glycine
Glutamic acid
COOH
S
Leukotriene B
4
(LTB
4
)
COOH
CH
2
CH
NH
2
NH C
O
CH
2
CH
2
CH
NH CH
2
COOH
COOH
NH
2
CH
2
CH
2
CH COOHHOOC
C
O
leukotriene A
4
hydrolase
LTC
4
synthase
(a glutathione-S-transferase)
Glutathione
-glutamyltransferase
OH
COOH
S
CH
2
CH
NH
2
NH CH
2
COOH
H
2
N
H
2
O
CH
2
COOH
C
O
OH
COOH
S
CH
2
CH
NH
2
COOH
OH
OH
dipeptidase
JWCL281_c25_940-1018.qxd 4/20/10 1:59 PM Page 1002
eicosapentaenoic acid (EPA; Fig. 25-67), an –3 fatty acid,
rather than the arachidonic acid precursor linoleic acid, an
–6 fatty acid. EPA inhibits formation of TxA
2
(Fig. 25-70)
and is a precursor of the series-5 leukotrienes, compounds
with substantially lower physiological activities than their
arachidonate-derived (series-4) counterparts. This suggests
that a diet containing marine lipids should decrease the ex-
tent of prostaglandin- and leukotriene-mediated inflam-
matory responses. Indeed, dietary enrichment with EPA in-
hibits the in vitro chemotactic and aggregating activities of
neutrophils (a type of white blood cell). Moreover, an
EPA-rich diet decreases the cholesterol and triacylglycerol
levels in the plasma of hypertriacylglycerolemic patients.
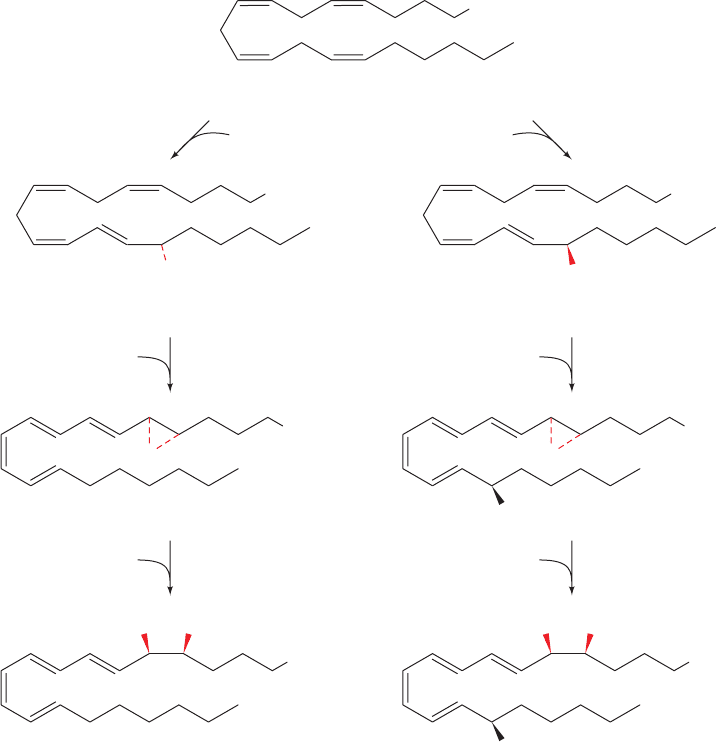
d. Lipoxins and Aspirin-Induced epi-Lipoxins Have
Anti-Inflammatory Properties
Eicosanoids are usually associated with the inflammatory
response.However,some eicosanoids have anti-inflammatory
properties.The lipoxins (LXs), products of the 12- and 15-LO
pathways (sometimes also involving 5-LO), are so named be-
cause they are synthesized through lipoxygenase interactions.
Their activities appear to inhibit those of leukotrienes and
hence they are anti-inflammatory. There are many ways for
LXs to be synthesized by combinations of the actions of 5-,
12-, and 15-LOs. Here we discuss only one such route (Fig.
25-80, left). Lipoxin A
4
(LXA
4
) synthesis from arachidonic
acid begins in endothelial and epithelial cells by the 15-LO-
catalyzed synthesis of (15S)-hydroperoxyeicosatetraenoic
acid [(15S)-HPETE], which is reduced by glutathione
peroxidase to (15S)-hydroxyeicosatetraenoic acid [(15S)-
HETE]. The (15S)-HETE then makes its way to leukocytes
where it is converted to LXA
4
by 5-LO and a hydrolase.
Charles Serhan discovered an additional pathway for
the anti-inflammatory action of aspirin that also involves
lipoxin production.As we have previously seen (Fig. 25-74),
aspirin covalently inhibits the cyclooxygenase activity of
Section 25-7. Eicosanoid Metabolism: Prostaglandins, Prostacyclins, Thromboxanes, Leukotrienes, and Lipoxins 1003
Figure 25-80 Lipoxin biosynthesis. The biosynthesis of the
lipoxin LXA
4
(left) and the aspirin-triggered epi-lipoxin (ATL)
15-epi-LXA
4
(right). In endothelial and epithelial cells,
arachidonic acid is converted by 15-LO and glutathione
15-LXA
4
COOH
OHHO
H
2
O
hydrolase
OH
15-epi-LXA
4
(15S)-HETE
Leukocytes
Endothelial and
epithelial cells
(15R)-HETE
COOH
OH
OHHO
COOH
H
2
O
hydrolase
H
2
OH
2
O
O
2
O
2
5-LO 5-LO
15-LO
+ glutathoine peroxidase
aspirin-acetylated COX-2
COOH
O
OH
COOH
Arachidonic acid
COOH
COOH
≥
OH
OH
≥
OH
O
peroxidase to (15S)-HETE or by aspirin-acetylated COX-2 to
(15R)-HETE.After transfer to leukocytes, 5-LO and a hydrolase
convert these intermediate products to LXA
4
and 15-epi-LXA
4
.
JWCL281_c25_940-1018.qxd 7/21/10 6:21 PM Page 1003
PGHS (COX). However, aspirin-acetylated COX-2 retains
a residual 15-LO activity (Steps 3 and 4 in Fig. 25-71)
through which it initiates a pathway that converts arachi-
donic acid to the anti-inflammatory agents called aspirin-
triggered epi-lipoxins (ATLs; Fig. 25-80, right). This path-
way begins in endothelial and epithelial cells with the
aspirin-acetylated COX-2-catalyzed conversion of arachi-
donic acid to (15R)-hydroxyeicosatetraenoic acid [(15R)-
HETE], the epimer of (15S)-HETE. In leukocytes, 5-LO
and a hydrolase then convert (15R)-HETE to the anti-
inflammatory agent 15-epi-lipoxin A
4
(15-epi-LXA
4
).
These are indeed exciting times in the study of eicosanoid
metabolism and its physiological manifestations. As the
mechanisms of action of the prostaglandins, prostacyclins,
thromboxanes, leukotrienes, and lipoxins are becoming bet-
ter understood, they are providing the insights required for
the development of new and improved therapeutic agents.
8 PHOSPHOLIPID AND
GLYCOLIPID METABOLISM
The “complex lipids” are dual-tailed amphipathic molecules
composed of either 1,2-diacyl-sn-glycerol or N-acylsphingo-
sine (ceramide) linked to a polar head group that is either a
carbohydrate or a phosphate ester (Fig. 25-81; Sections 12-
1C and 12-1D; sn stands for stereospecific numbering, which
assigns the 1 position to the group occupying the pro-S posi-
tion of a prochiral center). Hence, there are two categories
of phospholipids, glycerophospholipids and sphingophos-
pholipids, and two categories of glycolipids, glyceroglyco-
lipids and sphingoglycolipids (also called glycosphingolipids;
GSLs). In this section we describe the biosynthesis of the
complex lipids from their simpler components. We shall see
that the great variety of these substances is matched by the
numerous enzymes required for their specific syntheses.
Note also that these substances are synthesized in mem-
branes, mostly on the cytosolic face of the endoplasmic retic-
ulum, and from there are transported to their final cellular
destinations as indicated in Sections 12-4B–D.
A. Glycerophospholipids
Glycerophospholipids have significant asymmetry in their
C1- and C2-linked fatty acyl groups: C1 substituents are
mostly saturated fatty acids, whereas those at C2 are by and
large unsaturated fatty acids. We shall examine the major
pathways of biosynthesis and metabolism of the glyc-
erophospholipids with an eye toward understanding the
origin of this asymmetry.
a. Biosynthesis of Diacylglycerophospholipids
The triacyglycerol precursors 1,2-diacyl-sn-glycerol and
phosphatidic acid are also the precursors of certain glyc-
erophospholipids (Figs. 25-42 and 25-81). Activated phos-
phate esters of the polar head groups (Table 12-2) react
with the C3 OH group of 1,2-diacyl-sn-glycerol to form the
phospholipid’s phosphodiester bond. In some cases the
phosphoryl group of phosphatidic acid is activated and re-
acts with the unactivated polar head group.
The mechanism of activated phosphate ester formation
is the same for both the polar head groups ethanolamine
and choline (Fig. 25-82):
1. ATP first phosphorylates the OH group of choline or
ethanolamine.
2. The phosphoryl group of the resulting phospho-
ethanolamine or phosphocholine then attacks CTP, dis-
placing PP
i
, to form the corresponding CDP derivatives,
which are activated phosphate esters of the polar head
group.
3. The C3 OH group of 1,2-diacyl-sn-glycerol attacks
the phosphoryl group of the activated CDP–ethanolamine
or CDP–choline, displacing CMP to yield the correspon-
ding glycerophospholipid.
The liver also converts phosphatidylethanolamine to phos-
phatidylcholine by trimethylating its amino group, using S-
adenosylmethionine (Section 26-3Ea) as the methyl donor.
Phosphatidylserine is synthesized from phosphati-
dylethanolamine by a head group exchange reaction cat-
alyzed by phosphatidylethanolamine:serine transferase in
which serine’s OH group attacks the donor’s phosphoryl
group (Fig. 25-83). The original head group is then elimi-
nated, forming phosphatidylserine.
In the synthesis of phosphatidylinositol and phos-
phatidylglycerol, the hydrophobic tail is activated rather
than the polar head group. Phosphatidic acid, the precursor
of 1,2-diacyl-sn-glycerol (Fig. 25-42), attacks the -
phosphoryl group of CTP to form the activated CDP–
1004 Chapter 25. Lipid Metabolism
Figure 25-81 The glycerolipids and sphingolipids. The structures of the common head groups,
X, are presented in Table 12-2.
(CH
2
)
12
C
O
O
CR
2
CH
2
CH
2
C
O
O
R
1
XO
H
Glycerolipid
C
O
NH
CR
2
CH
CH
2
CC
XO
H
Sphingolipid
OH
H
H
CH
3
X
X
X
= H
= Carbohydrate
= Phosphate ester
1,2-Diacylglycerol
Glyceroglycolipid
Glycerophospholipid
-Acylsphingosine (ceramide)
Sphingoglycolipid (glycosphingolipid)
Sphingophospholipid
N
JWCL281_c25_940-1018.qxd 6/8/10 9:00 AM Page 1004
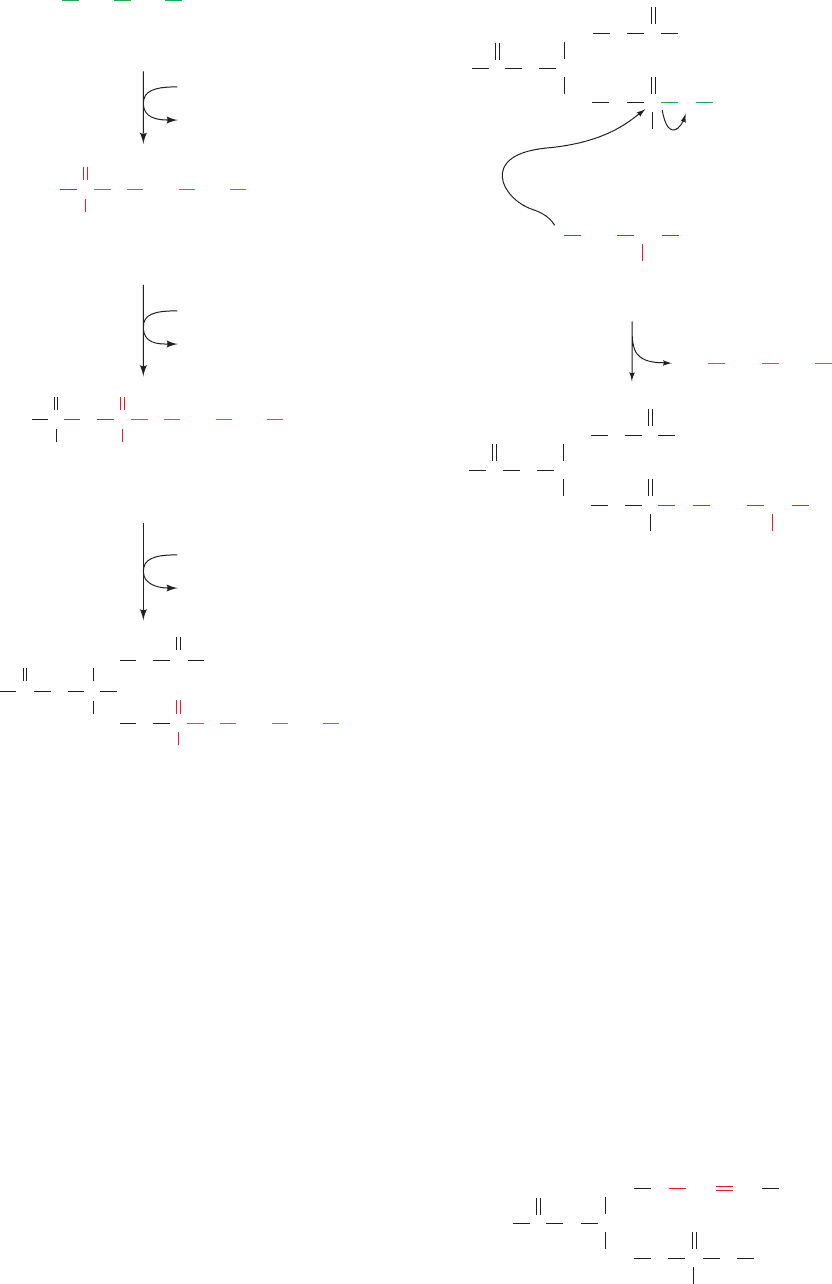
diacylglycerol and PP
i
(Fig. 25-84). Phosphatidylinositol re-
sults from the attack of inositol on CDP–diacylglycerol.
Phosphatidylglycerol is formed in two reactions: (1) attack
of the C1 ¬OH group of sn-glycerol-3-phosphate on
CDP–diacylglycerol, yielding phosphatidylglycerol phos-
phate; and (2) hydrolysis of the phosphoryl group to form
phosphatidylglycerol.
Cardiolipin, an important phospholipid first isolated
from heart tissue, is synthesized from two molecules of
phosphatidylglycerol (Fig. 25-85). The reaction occurs by
the attack of the C1 OH group of one of the phosphatidyl-
glycerol molecules on the phosphoryl group of the other,
displacing a molecule of glycerol.
Enzymes that synthesize phosphatidic acid have a gen-
eral preference for saturated fatty acids at C1 and for un-
saturated fatty acids at C2.Yet, this general preference can-
not account, for example, for the observations that ⬃80%
of brain phosphatidylinositol has a stearoyl group (18:0) at
C1 and an arachidonoyl group (20:4) at C2, and that ⬃40%
of lung phosphatidylcholine has palmitoyl groups (16:0) at
both positions (this latter substance is the major compo-
nent of the surfactant that prevents the lung from collaps-
ing when air is expelled; its deficiency is responsible for res-
piratory distress syndrome in premature infants). William
Lands showed that such side chain specificity results from
“remodeling” reactions in which specific acyl groups of in-
dividual glycerophospholipids are exchanged by specific
phospholipases and acyltransferases.
b. Biosynthesis of Plasmalogens and
Alkylacylglycerophospholipids
Eukaryotic membranes contain significant amounts of
two other types of glycerophospholipids:
1. Plasmalogens, which contain a hydrocarbon chain
linked to glycerol C1 via a vinyl ether linkage:
R
1
C O CH
CH CH
CH
2
CH
2
O
O
O
O
O
–
P OX
A plasmalogen
R
2
Section 25-8. Phospholipid and Glycolipid Metabolism 1005
Figure 25-82 The biosynthesis of phosphatidylethanolamine
and phosphatidylcholine. In mammals, CDP–ethanolamine and
CDP–choline are the precursors of the head groups.
Figure 25-83 Phosphatidylserine synthesis. Serine replaces
ethanolamine in phosphatidylethanolamine by a head group
exchange reaction.
R = H
R = CH
3
C
O
O
CR
2
CH
2
CH
2
C
O
O
R
1
O
H
P
O
O
–
O
CH
2
CH
2
Phosphatidylethanolamine
Phosphatidylcholine (lecithin)
R = H
R = CH
3
OP
O
O
–
O
CH
2
CH
2
CDP–ethanolamine
CDP–choline
P
O
O
–
Cytidine
–
OP
O
O
–
O
CH
2
CH
2
Phosphoethanolamine
Phosphocholine
R = H
R = CH
3
CH
2
CH
2
NR
+
Ethanolamine
Choline
HO
ATP
ADP
ethanolamine kinase
choline kinase
or
CTP
PP
i
CTP:phosphoethanolamine
cytidyltransferase
or CTP:phosphocholine
cytidyltransferase
1,2-Diacylglycerol
CMP
CDP–ethanolamine:1,2-diacylglycerol
phosphoethanolamine transferase
CDP–choline:1,2-diacylglycerol
phosphocholine transferase
or
1
2
3
R = H
R = CH
3
3
NR
+
3
NR
+
3
NR
+
3
R
2
R
1
C O CH
CH
2
CH
2
O
O
C
O
O
O
O
–
P OCH
2
CH
2
NH
3
+
Phosphatidylethanolamine
+
HO CH
2
CH COO
–
NH
3
+
Serine
HO CH
2
CH
2
NH
3
+
O
O
OC
CR
1
O
O
–
O
O
P O CH
2
CH
2
CH
2
CHR
2
CH COO
–
NH
3
+
Phosphatidylserine
phosphatidylethanolamine:
serine transferase
JWCL281_c25_940-1018.qxd 4/20/10 1:59 PM Page 1005
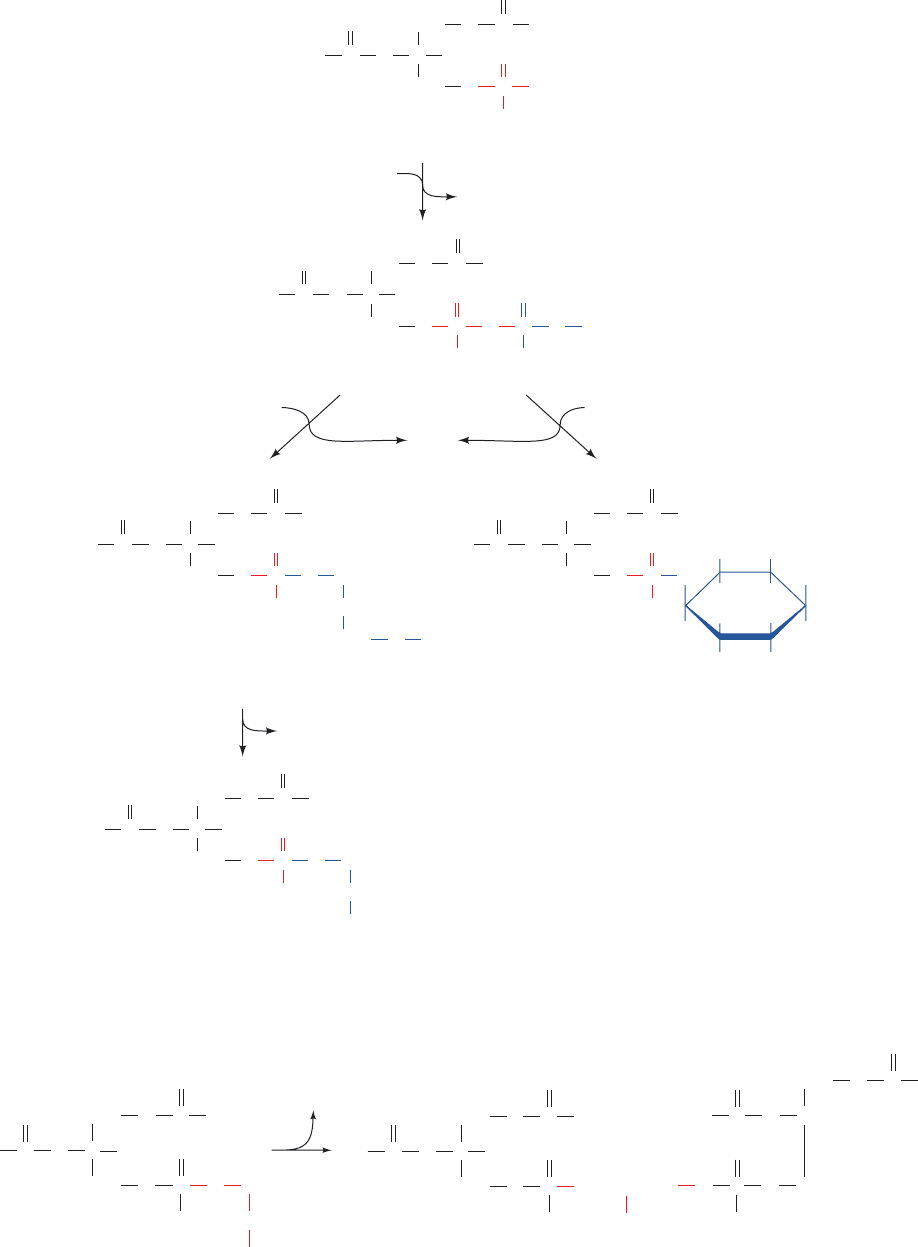
1006 Chapter 25. Lipid Metabolism
Figure 25-84 The biosynthesis of phosphatidylinositol and phosphatidylglycerol. In mammals,
this process involves a CDP–diacylglycerol intermediate.
Figure 25-85 The formation of cardiolipin.
C
O
O
CR
2
CH
2
CH
2
C
O
O
R
1
O
H
P
O
O
–
O
–
Phosphatidic acid
CTP
PP
i
C
O
O
CR
2
CH
2
CH
2
C
O
O
R
1
O
H
P
O
O
–
O
CDP–diacylglycerol
P
O
O
–
O Cytidine
CMP
Glycerol-3-phosphate Inositol
C
O
O
CR
2
CH
2
CH
2
C
O
O
R
1
O
H
P
O
O
–
O
Phosphatidylglycerol phosphate
CH
2
CH
CH
2
O PO
3
2
–
P
i
C
O
O
CR
2
CH
2
CH
2
C
O
O
R
1
O
H
P
O
O
–
O
Phosphatidylglycerol
CH
2
CHOH
CH
2
OH
C
O
O
CR
2
CH
2
CH
2
C
O
O
R
1
O
H
P
O
O
–
O
Phosphatidylinositol
OH HO
OH
OH
OH
H
HH
H
H
OH
H
2 R
2
R
1
C O C
CH
2
CH
2
O
O
C
O
O
O
O
–
P OCH
2
CHOH
CH
2
OH
Phosphatidylglycerol
O
O
OC
CR
1
O
O
–
O
O
P
OCH
2
CHCH
2
CH
2
CH
2
R
2
OH
glycerol
R
2
R
1
C O CH
CH
2
CH
2
O
O
C
O
O
O
O
–
P
O
H
C
H
Cardiolipin
JWCL281_c25_940-1018.qxd 4/20/10 1:59 PM Page 1006
2. Alkylacylglycerophospholipids, in which the alkyl
substituent at glycerol C1 is attached via an ether linkage:
About 20% of mammalian glycerophospholipids are plas-
malogens. The exact percentage varies both from species to
species and from tissue to tissue within a given organism.
While plasmalogens comprise only 0.8% of the phospho-
lipids in human liver,they account for 23% of those in human
nervous tissue. The alkylacylglycerophospholipids are less
abundant than the plasmalogens; for instance, 59% of the
ethanolamine glycerophospholipids of human heart are plas-
malogens, whereas only 3.6% are alkylacylglycerophospho-
lipids. However, in bovine erythrocytes, 75% of the
ethanolamine glycerophospholipids are of the alkylacyl type.
R
1
C O CH
CH
2
CH
2
O
O
O
O
O
–
P O
X
R
2
An alkylacyl-
glycerophospholipid
The pathway forming ethanolamine plasmalogens and
alkylacylglycerophospholipids involves several reactions
(Fig. 25-86):
1. Exchange of the acyl group of 1-acyldihydroxyace-
tone phosphate for an alcohol.
2. Reduction of the ketone to 1-alkyl-sn-glycerol-3-
phosphate.
3. Acylation of the resulting C2 OH group by acyl-CoA.
4. Hydrolysis of the phosphoryl group to yield an
alkylacylglycerol.
5. Attack by the new OH group of alkylacylglycerol on
CDP–ethanolamine to yield 1-alkyl-2-acyl-sn-glycerophos-
phoethanolamine.
6. Introduction of a double bond into the alkyl group to
form the plasmalogen by a desaturase having the same co-
factor requirements as the fatty acid desaturases (Section
25-4E).
Recall that the precursor–product relationship between
the alkylacylglycerophospholipid and the plasmalogen was
Section 25-8. Phospholipid and Glycolipid Metabolism 1007
Figure 25-86 The biosynthesis of ethanolamine plasmalogen via
a pathway in which 1-alkyl-2-acyl-sn-glycerophosphoethanolamine
is an intermediate. The participating enzymes are (1) alkyl-DHAP
synthase, (2) 1-alkyl-sn-glycerol-3-phosphate dehydrogenase, (3)
acyl-CoA:1-alkyl-sn-glycerol-3-phosphate acyltransferase, (4) 1-alkyl-
2-acyl-sn-glycerol-3-phosphate phosphatase, (5) CDP–ethanolamine:
1-alkyl-2-acyl-sn-glycerophosphoethanolamine transferase, and
(6) 1-alkyl-2-acyl-sn-glycerophosphoethanolamine desaturase.
1-Acyldihydroxyacetone phosphate
CO
CH
2
OPO
3
2–
H
2
C OCR
O
CR SCoA
NADPH
+ H
+
NADP
+
O
OHCR
O
R CH
2
CH
2
OH
1
1-Alkyl-2-acyl-sn-glycerol-3-phosphate
CHO
CH
2
OPO
3
2–
CR
CH
2
O
R
CH
2
CH
2
1-Alkyl-sn-glycerol-3-phosphate
CHHO
CH
2
OPO
3
2–
CH
2
O
R
CH
2
CH
2
O
1-Alkyl-2-acyl-sn-glycerol
5
3
2
CHO
CH
2
OH
CR
CH
2
O
R
CMP
CoASH
CDP–ethanolamine
CH
2
CH
2
O
1-Alkyl-2-acyl-sn-glycerophosphoethanolamine
CHO
CH
2
CH
2
CR
CH
2
O
R
O OP
CH
2
NH
3
+
CH
2
CH
2
O
O
O
–
Ethanolamine plasmalogen
CHO
CH
2
CH
2
CR
CH
2
O
OOP
CH
2
NH
3
+
C
H
CR
H
O
O
O
–
1-Alkyldihydroxyacetone phosphate
CO
CH
2
OPO
3
2–
CH
2
CH
2
CH
2
OR
4
P
i
6 2H
2
O
O
2
+ NADH + H
+
cytochrome b
5
JWCL281_c25_940-1018.qxd 4/20/10 1:59 PM Page 1007
established through studies using [
14
C]ethanolamine (Sec-
tion 16-3Be).
The alkylacylglycerophospholipid with an acetyl group
at R
2
and a choline polar head group (X), 1-O-hexadecyl-
2-acetyl-sn-glycero-3-phosphocholine, is known as
platelet-activating factor (PAF). This molecule has diverse
functions and acts at very low concentrations (10
10
M) to
lower blood pressure and to cause blood platelets to ag-
gregate.
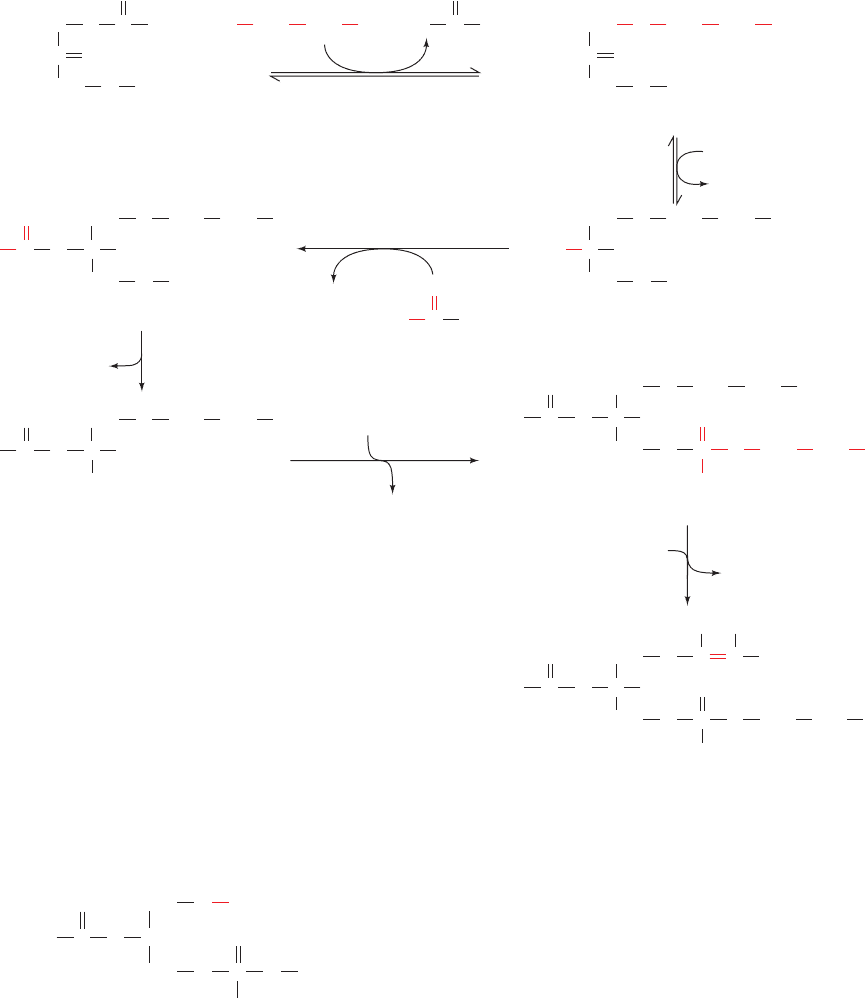
B. Sphingophospholipids
Only one major phospholipid contains ceramide
(N-acylsphingosine) as its hydrophobic tail: sphingomyelin
(N-acylsphingosine phosphocholine; Section 12-1D), an
important structural lipid of nerve cell membranes. The
molecule was once thought to be synthesized from
N-acylsphingosine and CDP–choline. However, it is now
known that the main route of sphingomyelin synthesis oc-
curs through donation of the phosphocholine group of
phosphatidylcholine to N-acylsphingosine (Fig. 25-87).
These pathways were differentiated by establishing the
precursor–product relationships between CDP–choline,
phosphatidylcholine, and sphingomyelin (Section 16-3Be).
Mouse liver microsomes were isolated and incubated for a
short time with [
3
H]choline. Radioactivity appeared in
sphingomyelin only after first appearing in both
CDP–choline and phosphatidylcholine, ruling out the
direct transfer of phosphocholine from CDP–choline to
N-acylsphingosine.
The most prevalent acyl groups of sphingomyelin are
palmitoyl (16:0) and stearoyl (18:0) groups. Longer chain
fatty acids such as nervonic acid (24:1) and behenic acid
(22:0) occur with lesser frequency in sphingomyelins.
C. Sphingoglycolipids
Most sphingolipids are sphingoglycolipids, that is, their polar
head groups consist of carbohydrate units (Section 12-1D).
The principal classes of sphingoglycolipids, as indicated in
Fig. 25-88, are cerebrosides (ceramide monosaccharides),
sulfatides (ceramide monosaccharide sulfates), globosides
(neutral ceramide oligosaccharides), and gangliosides
(acidic, sialic acid–containing ceramide oligosaccharides).
The carbohydrate unit is glycosidically attached to the N-
acylsphingosine at its C1 OH group (Fig. 25-81).
The lipids providing the carbohydrate that covers the
external surfaces of eukaryotic cells are sphingoglycolipids.
Along with glycoproteins (Section 23-3), they are biosyn-
thesized on the luminal surfaces of the endoplasmic reticu-
lum and the Golgi apparatus and reach the plasma mem-
brane through vesicle flow (Sections 12-4C and 12-4D),
where membrane fusion results in their facing the external
surface of the lipid bilayer (Fig. 12-60). Degradation of
sphingoglycolipids occurs in the lysosomes after endocyto-
sis from the plasma membrane.
In the following subsections, we discuss the biosynthesis
and breakdown of N-acylsphingosine and sphingoglyco-
lipids and consider the diseases caused by deficiencies in
their degradative enzymes.
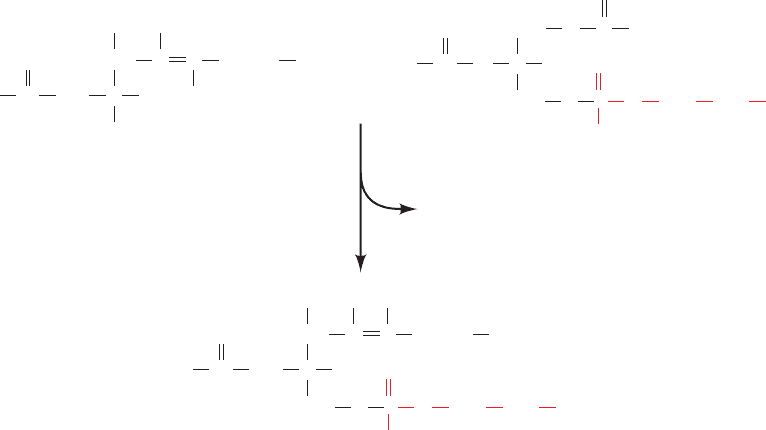
a. Biosynthesis of Ceramide (N-Acylsphingosine)
Biosynthesis of N-acylsphingosine occurs in four reactions
from the precursors palmitoyl-CoA and serine (Fig. 25-89):
1. 3-Ketosphinganine synthase (serine palmitoyltrans-
ferase), a pyridoxal phosphate–dependent enzyme, cat-
alyzes the condensation of palmitoyl-CoA with serine yield-
ing 3-ketosphinganine (pyridoxal phosphate–dependent
reactions are discussed in Section 26-1A).
1008 Chapter 25. Lipid Metabolism
Figure 25-87 The synthesis of sphingomyelin from N-acylsphingosine and phosphatidylcholine.
Ceramide (N-acylsphingosine)
RCNHC
CH
2
OH
H
+
CH
OH
CC
H
(CH
2
)
12
CH
3
H
Phosphatidylcholine
Diacylglycerol
R
2
COC
CH
2
O POCH
2
CH
2
N(CH
3
)
3
+
CH
2
OO
O
O
O
C
R
1
H
Sphingomyelin
R C NH C
CH
2
O POCH
2
CH
2
N(CH
3
)
3
+
O
–
O
–
CH
OH H H
CO
O
C
(CH
2
)
12
CH
3
H
JWCL281_c25_940-1018.qxd 6/8/10 9:00 AM Page 1008
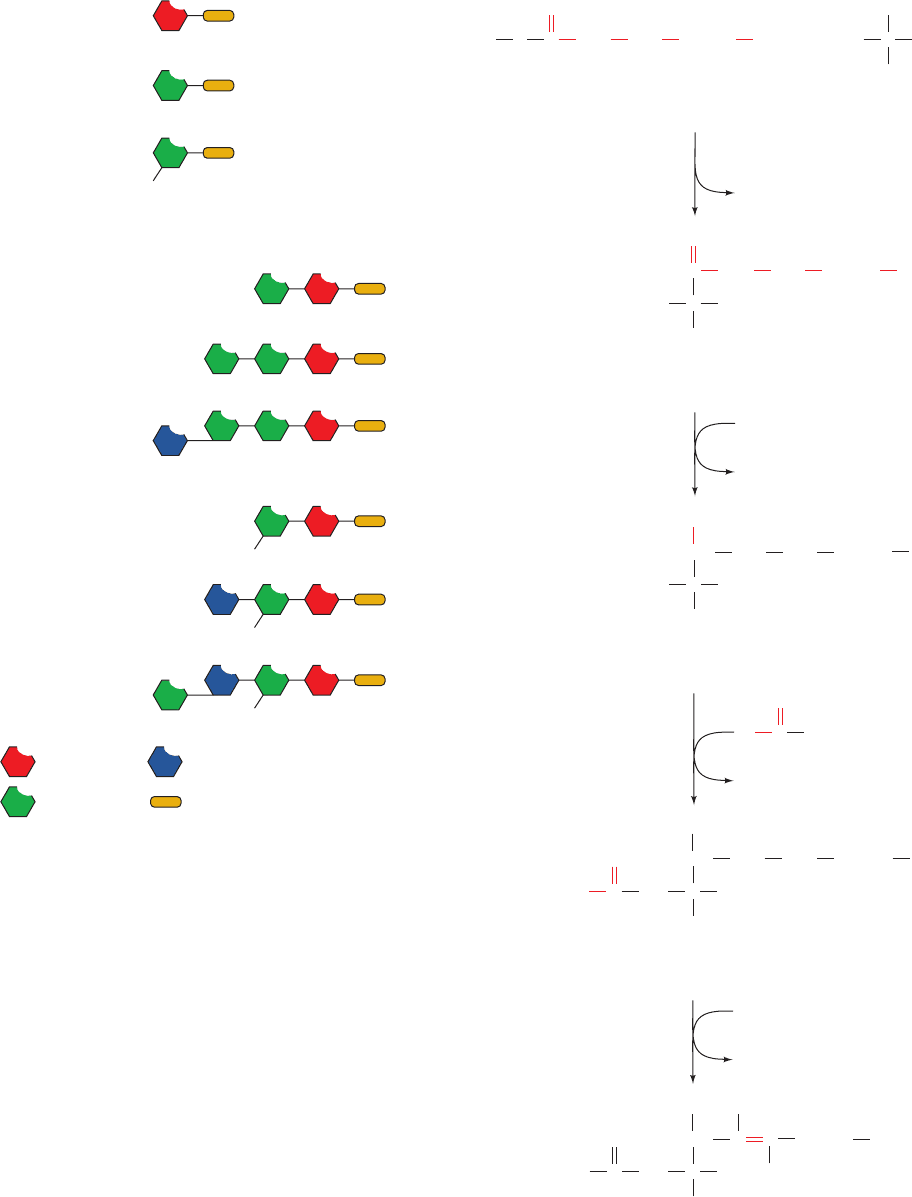
2. 3-Ketosphinganine reductase catalyzes the NADPH-
dependent reduction of 3-ketosphinganine’s keto group to
form sphinganine (dihydrosphingosine).
3. Dihydroceramide is formed by transfer of an acyl
group from an acyl-CoA to the sphinganine’s 2-amino
group, forming an amide bond.
4. Dihydroceramide dehydrogenase converts dihydro-
ceramide to ceramide by an FAD-dependent oxidation
reaction.
b. Biosynthesis of Cerebrosides (Glycosylceramides)
Galactocerebroside (1--galactosylceramide) and glu-
cocerebroside (1--glucosylceramide) are the two most
common cerebrosides. In fact, the term cerebroside is often
Section 25-8. Phospholipid and Glycolipid Metabolism 1009
Figure 25-88 Diagrammatic representation of the principal
classes of sphingoglycolipids. The G
M
ganglioside structures are
presented in greater detail in Fig. 12-7.
Figure 25-89 The biosynthesis of ceramide
(N-acylsphingosine).
OSO
–
3
β
O
Cerebrosides
Sulfatide
Globosides
Gangliosides
Glucocerebroside
Galactocerebroside
Lactosyl ceramide
Trihexosyl ceramide
Globoside
G
M3
G
M2
G
M1
β
O
β
O
β
O
β
O
β
O
α
O
β
O
β
O
β
O
β
O
α
α
O
β
O
β
O
NANA
α
β
O
β
O
O
= glucose
O
= N-acetylgalactosamine
= ceramide
O
= galactose
β
O
NANA
α
β
O
β
O
β
O
NANA
NANA
β
O
= N-acetylneuraminic acid (sialic acid)
CH
3
CH
2
C
O
S +CH
2
(CH
2
)
12
CH
3
CH
2
C
O
CH
2
(CH
2
)
12
C
O
Palmitoyl-CoA
H
2
N
CH
2
OH
C
CO
–
H
Serine
3-ketosphinganine reductase
3-ketosphinganine synthase
CO
–
+
H
+
+NADPH
NADP
+
H
2
N
CH
2
OH
CH
2
CoA
2
CoASH
3-Ketosphinganine
(3-ketodihydrosphingosine)
CH
3
CH
2
CH
OH
CH
2
(CH
2
)
12
H
2
N
CH
2
OH
CH
Sphinganine
(dihydrosphingosine)
CH
3
CH
2
CH
OH
(CH
2
)
12
CH
2
NH
CH
2
OH
CH
Dihydroceramide
(N-acylsphinganine)
Ceramide
(N-acylsphingosine)
1
2
acyl-CoA transferase
SCoAR
C
O
R
CH
3
CCCH
OH
(CH
2
)
12
H
NH
CH
2
OH
CH
H
C
O
R
CoASH
3
dihydroceramide dehydrogenase
FAD
FADH
2
4
JWCL281_c25_940-1018.qxd 4/20/10 1:59 PM Page 1009
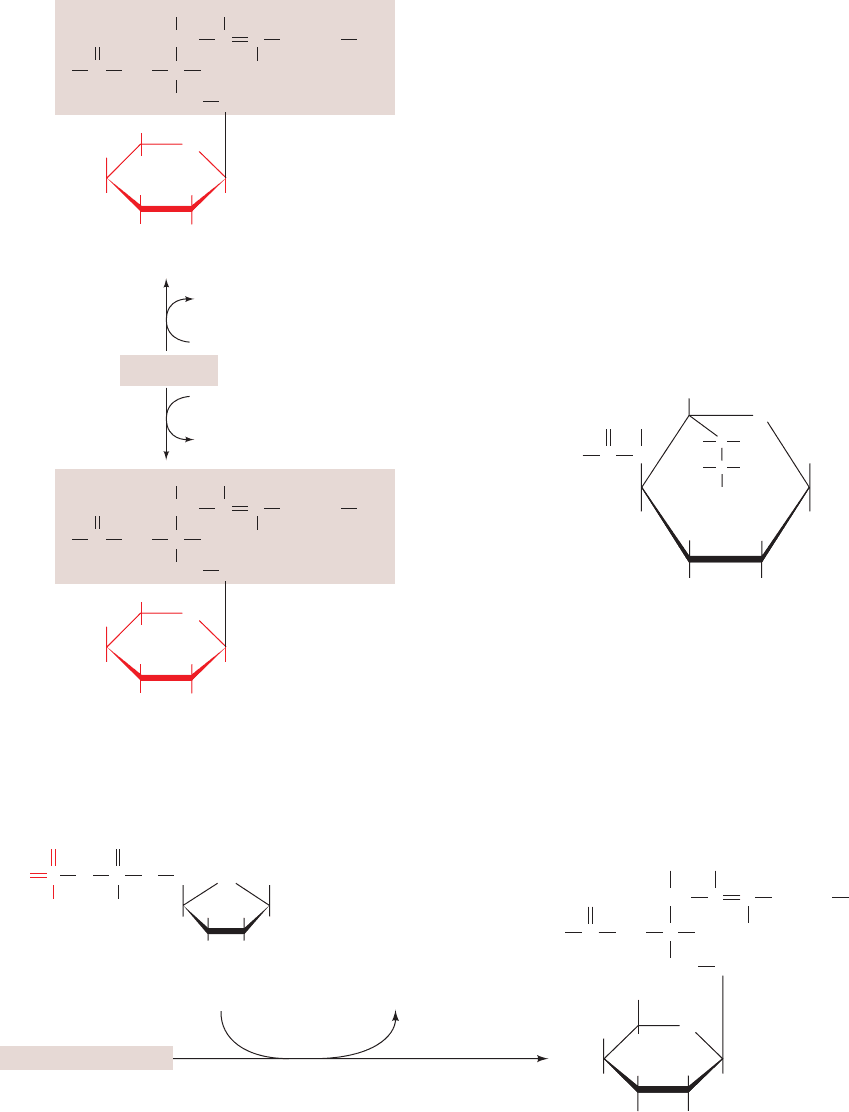
used synonymously with galactocerebroside. Both are syn-
thesized from ceramide by addition of a glycosyl unit from
the corresponding UDP–hexose (Fig. 25-90). Galactocere-
broside is a common component of brain lipids. Glucocere-
broside, although relatively uncommon, is the precursor of
globosides and gangliosides.
c. Biosynthesis of Sulfatides
Sulfatides (galactocerebroside-3-sulfate) account for
15% of the lipids of white matter in the brain. They are
formed by transfer of an “activated” sulfate group from 3-
phosphoadenosine-5-phosphosulfate (PAPS) to the C3
OH group of galactose in galactocerebroside (Fig. 25-91).
d. Biosynthesis of Globosides and Gangliosides
Biosynthesis of both globosides (neutral ceramide
oligosaccharides) and gangliosides (acidic, sialic acid–
containing ceramide oligosaccharides) is catalyzed by a
series of glycosyltransferases. While the reactions are
chemically similar, each is catalyzed by a specific enzyme.
The pathways begin with transfer of a galactosyl unit from
UDP–Gal to glucocerebroside to form a (1 S 4) linkage
(Fig. 25-92). Since this bond is the same as that linking glu-
cose and galactose in lactose, this glycolipid is often re-
ferred to as lactosyl ceramide. Lactosyl ceramide is the pre-
cursor of both globosides and gangliosides. To form a
globoside, one galactosyl and one N-acetylgalactosaminyl
unit are sequentially added to lactosyl ceramide from
UDP–Gal and UDP–GalNAc, respectively. The G
M
gan-
gliosides are formed by addition of N-acetylneuraminic
acid (NANA, sialic acid)
from CMP–NANA to lactosyl ceramide in (2 S 3) linkage
yielding G
M3
. The sequential additions to G
M3
of the
N-acetylgalactosamine and galactose units from
UDP–GalNAc and UDP–Gal yield gangliosides G
M2
and
G
M1
. Other gangliosides are formed by adding a second
COO
–
H
H
HH
HO
OH
H
O
4
3
1
2
6
7
8
9
5
H
HC
H C
CH
2
OH
C N
O
CH
3
OH
OH
N-Acetylneuraminic acid
(NANA, sialic acid)
1010 Chapter 25. Lipid Metabolism
Figure 25-90 The biosynthesis of cerebrosides.
Figure 25-91 The biosynthesis of sulfatides.
H
OH
CH
2
OH
CH
2
(CH
2
)
12
CH
3
CH
CHNH
Glucocerebroside (1-
β
-D-glucosylceramide)
H
H
HO
OH
O
O
HOH
UDP–glucose
UDP
UDP glucose:ceramide
glycosyltransferase
(glucosylceramide synthase)
UDP galactose:ceramide
glycosyltransferase
O
C
R
CC
H
H
OH
HO
OH
CH
2
OH
CH
2
(CH
2
)
12
CH
3
CH
CHNH
Galactocerebroside (1-
β
-D-galactosylceramide)
H
H
H
H
O
O
HOH
O
C
R
CC
H
H
OH
Ceramide
UDP–galactose
UDP
H
H
O
–
CH
2
3-Phosphoadenosine-5-
phosphosulfate (PAPS)
3-Phosphoadenosine-5-
phosphate
Adenine
O
H
H
2
O
3
PO OH
O
PO
O
–
O
O
S
O
Galactocerebroside
OSO
3
H
H
H
HOH
H
HO
O
CH
2
OH
CH
2
(CH
2
)
12
O
Sulfatide (galactocerebroside-3-sulfate)
C
NH
CC CH
3
C
CH
OH H
HH
O
R
JWCL281_c25_940-1018.qxd 4/20/10 1:59 PM Page 1010
