Voet D., Voet Ju.G. Biochemistry
Подождите немного. Документ загружается.

Serine is converted to pyruvate through dehydration by
serine dehydratase. This PLP–enzyme, like the aminotrans-
ferases (Section 26-1), functions by forming a PLP–amino
acid Schiff base, which facilitates the removal of the amino
acid’s ␣-hydrogen atom. In the serine dehydratase reaction,
however, the C
␣
carbanion breaks down with the elimina-
tion of the amino acid’s C

OH, rather than with tautomer-
ization (Fig.26-2,Step 2),so that the substrate undergoes ␣,
elimination of H
2
O rather than deamination (Fig. 26-13).
The product of the dehydration, the enamine aminoacrylate,
tautomerizes nonenzymatically to the corresponding imine,
which spontaneously hydrolyzes to pyruvate and ammonia.
Cysteine may be converted to pyruvate via several
routes in which the sulfhydryl group is released as H
2
S,
or SCN
⫺
.
Glycine is converted to serine by the enzyme serine hy-
droxymethyltransferase, another PLP-containing enzyme
(Fig. 26-12, Reaction 4). This enzyme utilizes N
5
,N
10
-
methylene-tetrahydrofolate (N
5
,N
10
-methylene-THF) as a
cofactor to provide the C
1
unit necessary for this conver-
sion. We shall defer a detailed discussion of THF cofactors
until Section 26-4D.
a. The Glycine Cleavage System Is
a Multienzyme Complex
The methylene group of the N
5
,N
10
-methylene-THF
utilized in the conversion of glycine to serine is obtained
from the methylene group of a second glycine via a reac-
tion in which this glycine’s remaining atoms are released
as CO
2
and (Fig. 26-12, Reaction 3). This reaction is
catalyzed by the glycine cleavage system (also called the
glycine decarboxylase multienzyme system in plants and
glycine synthase when acting in the reverse direction; Sec-
tion 26-5Ae), a complex resembling the pyruvate dehy-
drogenase complex (Section 21-2A) that consists of four
proteins (Fig. 26-14):
NH
⫹
4
SO
2⫺
3
1. A PLP-dependent glycine decarboxylase (P-protein).
2. A lipoamide-containing aminomethyl carrier
(H-protein), which carries the aminomethyl group remain-
ing after glycine decarboxylation.
Section 26-3. Metabolic Breakdown of Individual Amino Acids 1031
C
O
–
N
C
O
H
2
C COO
–
+
H
PLP
+ Serine
2
AminoacrylatePyruvate
1
2–
O
3
PO
H
N
CH
3
H
H
H
...
C
O
–
N
C
O
H
2
C COO
–
+
H
2–
O
3
PO
H
N
CH
3
H
H
+
H
...
5
4
H
+
3
OH
–
6
H
2
ONH
3
–
C
O
–
N
CH
2
C COO
–
+
+++
H
2–
O
3
PO
H
H
2
O
PLP
N
CH
3
H
...
NH
2
CH
2
C COO
–
:
NH
+
2
CH
3
C COO
–
O
CH
3
C COO
–
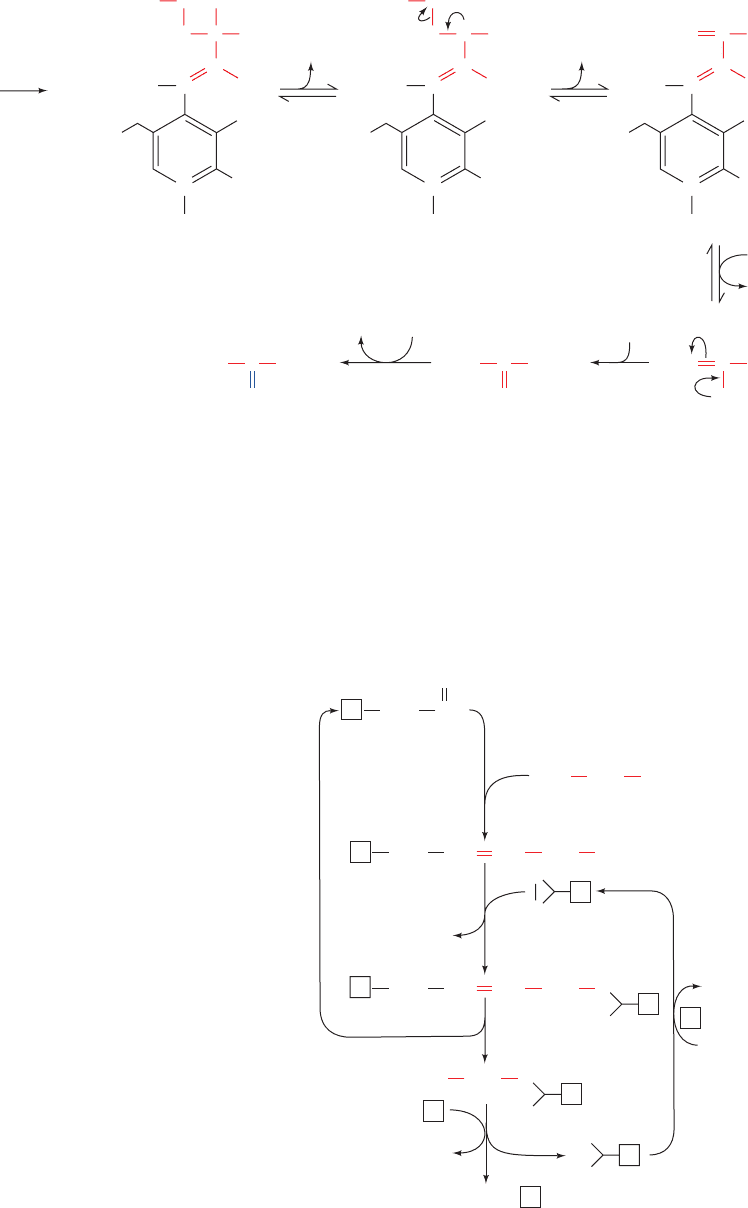
Figure 26-13 The serine dehydratase
reaction. This PLP-dependent enzyme catalyzes
the elimination of water from serine. The steps
in the reaction are (1) formation of a serine–PLP
Schiff base, (2) removal of the ␣-H atom of
serine to form a resonance-stabilized carbanion,
(3)  elimination of OH
⫺
, (4) hydrolysis of the
Schiff base to yield the PLP–enzyme and
aminoacrylate, (5) nonenzymatic tautomerization
to the imine, and (6) nonenzymatic hydrolysis to
form pyruvate and ammonia.
Figure 26-14 The reactions catalyzed by the glycine cleavage
system, a multienzyme complex. The enzymes involved are (1) a
PLP-dependent glycine decarboxylase (P-protein), (2) a
lipoamide-containing protein (H-protein), (3) a THF-requiring
enzyme (T-protein), and (4) an NAD
⫹
-dependent, FAD-requiring
dihydrolipoyl dehydrogenase (L-protein).
CO
2
CH
2
H
3
N
COO
–
CH
O
PLPP
CH
PLPP
CH
PLPP
1
2
2
3
4
+
CH
2
NH
COO
–
+
CH
2
NH
S
S
S
HS
CH
2
NH
3
H
2
N
+
Glycine
H
TTHF •
TN
5
, N
10
-Methylene-THF •
H
H
L
S
HS
H
HS
HS
NADH + H
+
NAD
+
JWCL281_c26_1019-1087.qxd 4/20/10 9:26 AM Page 1031
3. An N
5
,N
10
-methylene-THF synthesizing enzyme
[T-protein; alternatively aminomethyltransferase (AMT)],
which accepts a methylene group from the aminomethyl car-
rier (H-protein; the amino group is released as ammonia).
4. An NAD
⫹
-dependent, FAD-requiring dihydrolipoyl
dehydrogenase (L-protein), a protein shared by and
known as E3 in the pyruvate dehydrogenase complex (Sec-
tion 21-2A).
Unlike the pyruvate dehydrogenase complex, the glycine
cleavage system components are only loosely associated
and hence are isolated as individual proteins. Nevertheless,
H-protein has the central role in this multienzyme system: Its
oxidized lipoyllysyl arm (Section 21-2Ad) is reduced as it ac-
cepts an aminomethyl group from P-protein (Fig.26-14, Step
2), it donates the methylene group to THF in complex with
T-protein as ammonia is released (Fig. 26-14, Step 3), and is
then reoxidized by L-protein (Fig. 26-14, Step 4). The X-ray
structure of pea leaf H-protein (Fig. 26-15), determined by
Roland Douce, reveals that it is largely composed of a sand-
wich of 3-stranded and 6-stranded antiparallel  sheets that
structurally resembles the lipoyl domain of E2 in the pyru-
vate dehydrogenase complex (Fig. 21-10a).
The aminomethylthio group is unstable and ordinarily
is rapidly hydrolyzed to formaldehyde and. How-
ever, on its aminomethylation, the previously exposed
lipoyl group (Fig. 26-15a) inserts into a hydrophobic cleft
NH
⫹
4
in the H-protein where its amino group hydrogen-bonds
to Glu 14 (Fig. 26-15b), thereby shielding the aminomethyl
group from hydrolysis. Indeed, the replacement of Glu 14
by Ala results in the rapid hydrolysis of the aminomethyl
group. It therefore appears that the T-protein in the
THF ⴢ T ⴢ H complex functions to release the lipoyl group
from the H-protein cleft and to orient the THF for ap-
proach to the methylene C of the aminomethyl group for
reaction.
Two observations indicate that the above pathway is the
major route of glycine degradation in mammalian tissues:
1. The serine isolated from an animal that has been fed
[2-
14
C]glycine is
14
C labeled at both C2 and C3.This obser-
vation indicates that the methylene group of the N
5
,N
10
-
methylene-THF utilized by serine hydroxymethyltrans-
ferase is obtained from glycine C2.
2. The inherited human disease nonketotic hyper-
glycinemia, which is characterized by mental retardation
and accumulation of large amounts of glycine in body flu-
ids, results from the absence of one of the components of
the glycine cleavage system.
The glycine cleavage system and serine hydroxymethyl-
transferase occupy a vital role in green leaves, catalyzing
the rapid destruction of the huge amounts of glycine pro-
duced by photorespiration (Section 24-3Ca). In fact, these
1032 Chapter 26. Amino Acid Metabolism
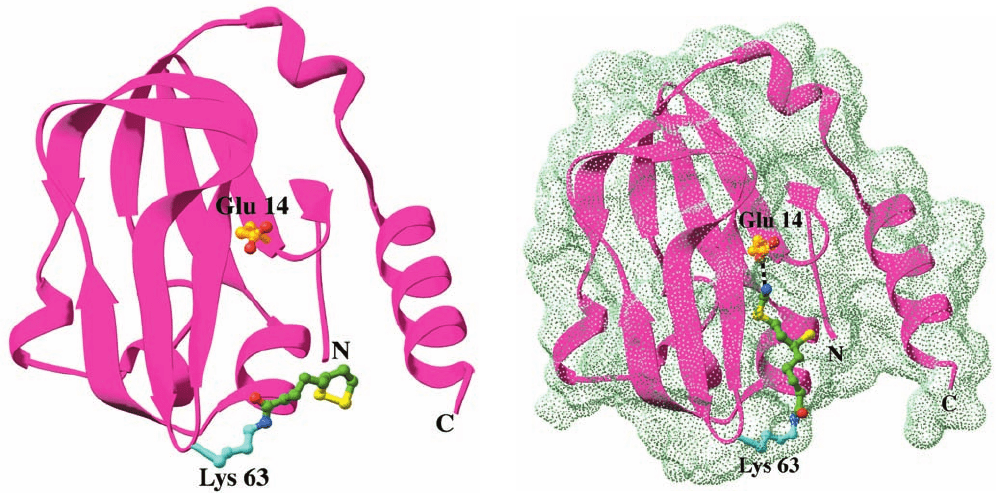
Figure 26-15 X-ray structure of H-protein from the pea leaf
glycine cleavage system. (a) The oxidized lipoamide-containing
form in which the side chain of Glu 14 together with that of Lys
63 with its covalently linked lipoyl group are represented in
ball-and-stick form colored according to atom type (Glu 14 C
gold, Lys 63 C cyan, lipoyl C green, N blue, O red, and S yellow).
(b) The reduced aminomethyl-dihydrolipoamide form of
H-protein viewed and colored as in Part a. The dot surface
represents the protein’s solvent-accessible surface. Note how the
aminomethyl-dihydrolipoamide has changed conformation
relative to the lipoamide in Part a so as to bind in a hydrophobic
cleft in the protein where its amino group is hydrogen bonded to
Glu 14 (dashed black bond).This protects the aminomethyl
group from hydrolysis. [Based on X-ray structures by Roland
Douce, Centre National de la Recherche Scientifique et
Commisariat à l’Energy Atomique, Grenoble, France. PDBids
(a) 1HPC and (b) 1HTP.]
(a)
(b)
JWCL281_c26_1019-1087.qxd 6/8/10 9:38 AM Page 1032
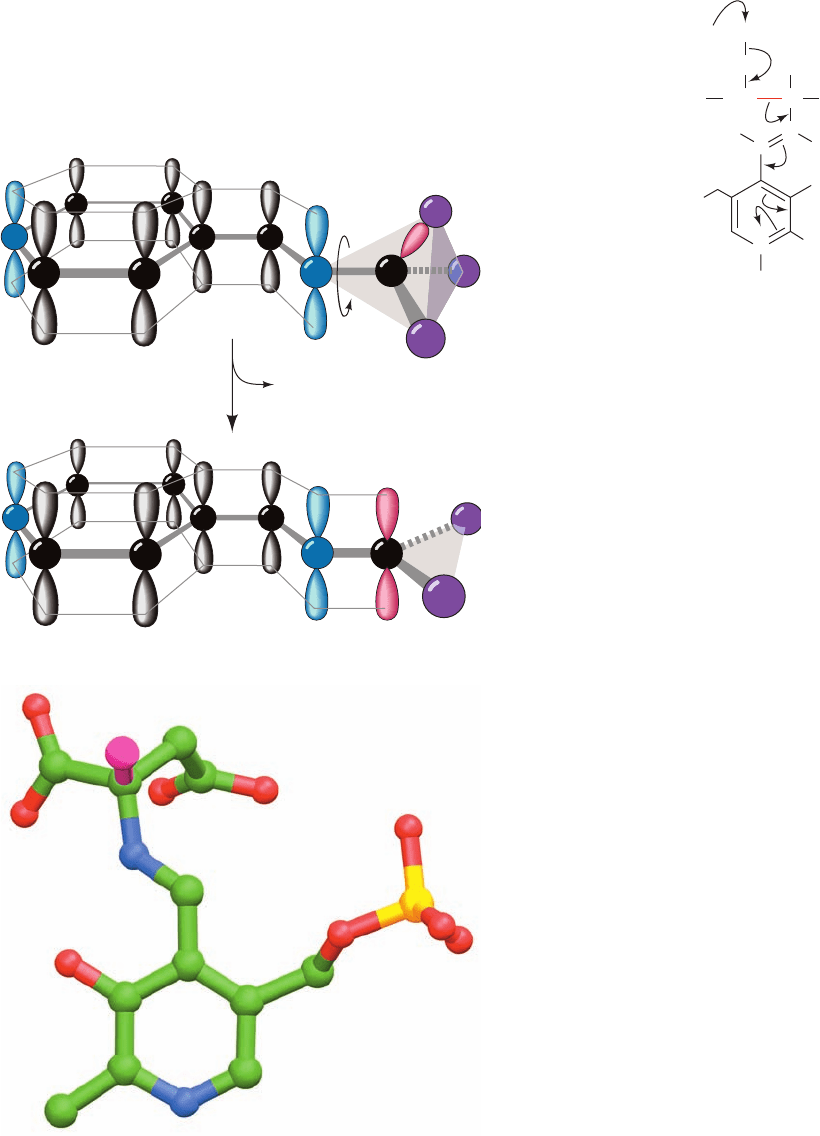
enzymes comprise about half the proteins present in the
mitochondria from pea and spinach leaves.
Threonine is both glucogenic and ketogenic, since one
of its degradation routes produces both pyruvate and
acetyl-CoA (Fig. 26-12, Reactions 6 and 7). Its major route
of breakdown is through threonine dehydrogenase, pro-
ducing ␣-amino--ketobutyrate, which is converted to
acetyl-CoA and glycine by ␣-amino--ketobutyrate lyase.
The glycine may be converted, through serine, to pyruvate.
b. Serine Hydroxymethyltransferase Catalyzes
PLP-Dependent C
␣
¬C

Bond Cleavage
Threonine may also be converted directly to glycine and
acetaldehyde (the latter being subsequently oxidized to
acetyl-CoA), at least in vitro, via Reaction 5 of Fig. 26-12.
Surprisingly, this reaction is catalyzed by serine hydroxy-
methyltransferase. We have heretofore considered PLP-
catalyzed reactions that begin with the cleavage of an amino
acid’s C
␣
¬H bond (Fig. 26-2). Degradation of threonine to
glycine and acetaldehyde by serine hydroxymethyltrans-
ferase demonstrates that PLP also facilitates cleavage of an
amino acid’s C
␣
¬C

bond by delocalizing the electrons of
the resulting carbanion into the conjugated PLP ring:
c. PLP Facilitates the Cleavage of Different
Bonds in Different Enzymes
How can the same amino acid–PLP Schiff base be in-
volved in the cleavage of the different bonds to an amino
acid C
␣
in different enzymes? The answer to this conun-
drum was suggested by Harmon Dunathan. For electrons
to be withdrawn into the conjugated ring system of PLP,
the -orbital system of PLP must overlap with the bond-
ing orbital containing the electron pair being delocalized.
This is possible only if the bond being broken lies in the
plane perpendicular to the plane of the PLP -orbital sys-
tem (Fig. 26-16a). Different bonds to C
␣
can be placed in
this plane by rotation about the C
␣
¬N bond. Indeed, the
X-ray structure of aspartate aminotransferase reveals
that the C
␣
¬H of its aspartate substrate assumes just this
...
H
O
–
CH
3
N
H
2–
O
3
PO
C
β
HC
β
C
α
H
COO
–
C
+
N
+
H
H
3
C
HB:
O
Section 26-3. Metabolic Breakdown of Individual Amino Acids 1033
Figure 26-16 Bond orientation in a PLP–amino acid Schiff
base. (a) The -orbital framework of a PLP–amino acid Schiff base.
The bond to C
␣
in the plane perpendicular to the PLP -orbital
system (from X in the illustration) is labile as a consequence of its
overlap with the system, which permits the broken bond’s
electron pair to be delocalized over the conjugated molecule.
(b) The Schiff base complex of the inhibitor ␣-methylaspartate
with PLP in the X-ray structure of porcine aspartate
aminotransferase as viewed normal to the pyridoxal ring.This
inhibitor is drawn in ball-and-stick form with C green, N blue,
O red, and P gold, with the exception that the methyl C atom and
the bond linking it to the aspartate residue are magenta. Here
the methyl C occupies the position of the H atom that the
enzyme normally excises from aspartate. Note that the bond
linking the methyl C to aspartate is in the plane perpendicular
to the pyridoxal ring and is thus ideally oriented for bond
cleavage. [Part b based on an X-ray structure by David Metzler
and Arthur Arnone, University of Iowa. PDBid 1AJS.]
Delocalized carbanion
X
+
Y
Y
Z
C
α
α
Amino acid–PLP Schiff base
+
+
C
α
–
Z
X
(b)
(a)
JWCL281_c26_1019-1087.qxd 6/8/10 9:38 AM Page 1033
conformation (Fig. 26-16b). Evidently, each enzyme
specifically cleaves its corresponding bond because the
enzyme binds the amino acid–PLP Schiff base adduct with
this bond in the plane perpendicular to that of the PLP
ring. This is an example of stereoelectronic assistance
(Section 15-1Eb): The enzyme binds substrate in a confor-
mation that minimizes the electronic energy of the transi-
tion state.
C. Asparagine and Aspartate Are Degraded
to Oxaloacetate
Transamination of aspartate leads directly to oxaloacetate:
Asparagine is also converted to oxaloacetate in this man-
ner after its hydrolysis to aspartate by
L-asparaginase:
Interestingly,
L-asparaginase is an effective chemothera-
peutic agent in the treatment of cancers that must obtain
asparagine from the blood, particularly acute lymphoblas-
tic leukemia.The cancerous cells express particularly low lev-
els of the enzyme asparagine synthetase (Section 26-5Ab)
and hence die without an external source of asparagine.
However,
L-asparaginase treatment may select for cells
with increased levels of asparagine synthetase expression,
and hence, in these cases, the surviving cancer cells are re-
sistant to this treatment.
H
2
N
O
–
O
C
C
NH
3
+
H
CH
2
C
CC
H
CH
2
C
O
–
O
–
O
O
Asparagine
Aspartate
L-asparaginase
O
NH
4
+
H
2
O
NH
3
+
Aspartate
Oxaloacetate
CO
––
O
O
O
H
C
O
CCH
2
C
–
O
C
O
O
–
O
CCH
2
NH
+
3
aminotransferase
␣-Ketoglutarate
Glutamate
D. Arginine, Glutamate, Glutamine, Histidine, and
Proline Are Degraded to ␣-Ketoglutarate
Arginine, glutamine, histidine, and proline are all degraded
by conversion to glutamate (Fig. 26-17), which in turn is ox-
idized to ␣-ketoglutarate by glutamate dehydrogenase
(Section 26-1). Conversion of glutamine to glutamate in-
volves only one reaction: hydrolysis by glutaminase. Histi-
dine’s conversion to glutamate is more complicated: It is
nonoxidatively deaminated, then it is hydrated, and its imi-
dazole ring is cleaved to form N-formiminoglutamate. The
formimino group is then transferred to tetrahydrofolate
forming glutamate and N
5
-formimino-tetrahydrofolate
(Section 26-4D). Both arginine and proline are converted
to glutamate through the intermediate formation of
glutamate-5-semialdehyde.
E. Isoleucine, Methionine, and Valine
Are Degraded to Succinyl-CoA
Isoleucine, methionine, and valine have complex degrada-
tive pathways that all yield propionyl-CoA. Propionyl-
CoA, which is also a product of odd-chain fatty acid degra-
dation, is converted, as we have seen, to succinyl-CoA by a
series of reactions involving the participation of biotin and
coenzyme B
12
(Section 25-2E).
a. Methionine Breakdown Involves Synthesis
of S-Adenosylmethionine and Cysteine
Methionine degradation (Fig. 26-18) begins with its re-
action with ATP to form S-adenosylmethionine (SAM; al-
ternatively AdoMet). This sulfonium ion’s highly reactive
methyl group makes it an important biological methylating
agent. For instance, we have already seen that SAM is the
methyl donor in the synthesis of phosphatidylcholine from
phosphatidylethanolamine (Section 25-8Aa). It is also the
methyl donor in the conversion of norepinephrine to epi-
nephrine (Section 26-4B).
Methylation reactions involving SAM yield S-adenosyl-
homocysteine in addition to the methylated acceptor. The
former product is hydrolyzed to adenosine and homo-
cysteine in the next reaction of the methionine degra-
dation pathway. The homocysteine may be methylated
to form methionine via a B
12
-requiring reaction in which
N
5
-methyl-THF is the methyl donor. Alternatively, the ho-
mocysteine may combine with serine to yield cystathionine
in a PLP-requiring reaction, which subsequently forms cys-
teine (cysteine biosynthesis) and ␣-ketobutyrate. The ␣-
ketobutyrate continues along the degradative pathway to
propionyl-CoA and then succinyl-CoA.
b. Hyperhomocysteinemia Is Associated
with Disease
Imbalance between the rate of production of homocys-
teine through methylation reactions utilizing SAM
(Fig. 26-18, Reactions 2 and 3) and its rate of breakdown by
either remethylation to form methionine (Fig. 26-18, Reac-
tion 4) or reaction with serine to form cystathionine in the
1034 Chapter 26. Amino Acid Metabolism
JWCL281_c26_1019-1087.qxd 4/20/10 9:26 AM Page 1034
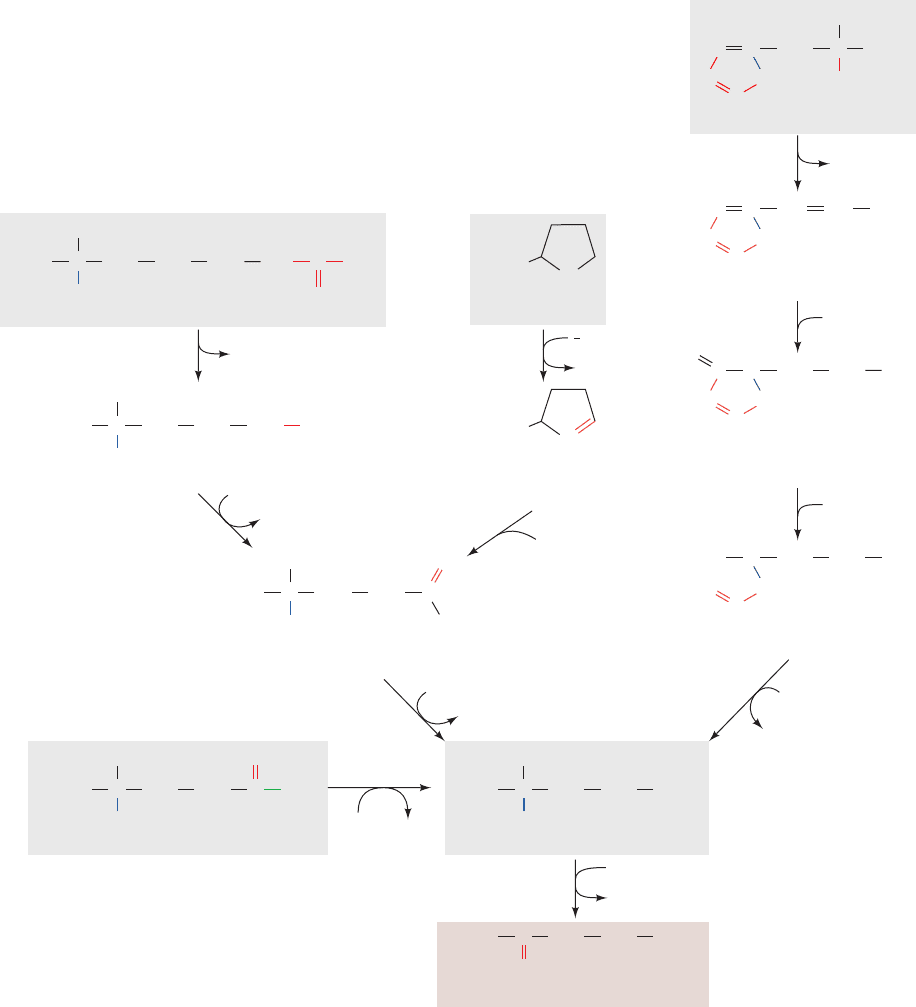
cysteine biosynthesis pathway (Fig. 26-18, Reaction 5) can
result in an increase in the release of homocysteine to the
extracellular medium and ultimately the plasma and urine.
Moderately elevated concentrations of homocysteine in
the plasma, hyperhomocysteinemia, for reasons that are
poorly understood, are closely associated with cardiovas-
cular disease, cognitive impairment, and neural tube de-
fects [the cause of a variety of severe birth defects includ-
ing spina bifida (defects in the spinal column that often
result in paralysis) and anencephaly (the invariably fatal
failure of the brain to develop, which is the leading cause of
infant death due to congenital anomalies)]. Hyperhomo-
cysteinemia is readily controlled by ingesting the vitamin
precursors of the coenzymes that participate in homocys-
teine breakdown, namely, B
6
(pyridoxine, the PLP precur-
sor; Fig. 26-1), B
12
(Fig. 25-21), and folate (Section 26-4D).
Folate, especially, appears to alleviate hyperhomocysteine-
mia; its administration to pregnant women dramatically re-
duces the incidence of neural tube defects in newborns.
This has led to the discovery that 10% of the population is
homozygous for the A222V mutation in N
5
,N
10
-methylene-
tetrahydrofolate reductase (MTHFR; Fig. 26-18, Reaction
12; Section 26-4D), the enzyme that generates N
5
-methyl-
THF for the methionine synthase reaction (Fig. 26-18,
Section 26-3. Metabolic Breakdown of Individual Amino Acids 1035
Figure 26-17 Degradation pathways of arginine, glutamate, glutamine, histidine, and
proline to ␣-ketoglutarate. The enzymes catalyzing the reactions are (1) glutamate
dehydrogenase, (2) glutaminase, (3) arginase, (4) ornithine-␦-aminotransferase,
(5) glutamate-5-semialdehyde dehydrogenase, (6) proline oxidase, (7) spontaneous,
(8) histidine ammonia lyase, (9) urocanate hydratase, (10) imidazolone propionase, and
(11) glutamate formiminotransferase.
CH
2
NH
3
+
H
HC
O
C
CH
2
C
H
N NH
C
Histidine
COO
–
HC C
CH
C
H
N NH
Urocanate
COO
–
CH
C
H
C
C
H
N NH
4-Imidazolone-
5-propionate
COO
–
O
CH
2
H
2
O
NH
4
+
CH
2
H
C
C
H
HN NH
-Formiminoglutamate
COO
–
CH
2
H
2
O
–
OOC
N
NH
3
+
H
–
OOC
C
Arginine
NH
Urea
CH
2
CH
2
CH
2
C NH
2
NH
2
+
NH
3
+
H
–
OOC
C
Ornithine
CH
2
CH
2
CH
2
NH
3
+
–
OOC
N
H
2
+
Proline
–
OOC
Pyrroline-
5-carboxylate
NH
3
+
H
–
OOC
C
Glutamate-
5-semialdehyde
CH
2
CH
2
C
H
O
H
2
O
-Ketoglutarateα
Glutamate
NAD(P)
+
NAD(P)H
NH
3
+
H
–
OOC
C
Glutamate
CH
2
CH
2
COO
–
NH
3
+
H
–
OOC
C
Glutamine
CH
2
CH
2
C
NH
2
NH
3
N
5
-Formimino-THF
THF
H
2
O
NADP
NADPH
+
+
NH
3
–
OOC
C
-Ketoglutarate
CH
2
CH
2
COO
–
O
α
3
4
5
2
1
11
10
9
8
2
O
2
1
6
N
H
+
7
H
2
O
JWCL281_c26_1019-1087.qxd 4/20/10 9:26 AM Page 1035
Reaction 4). This mutation does not affect this homo-
tetrameric enzyme’s reaction kinetics but instead increases
the rate at which it dissociates into dimers that readily
lose their essential flavin cofactor. Folate derivatives that
bind to the enzyme decrease its rate of dissociation and
flavin loss, thus increasing the mutant enzyme’s overall
activity and decreasing the homocysteine concentration.
The X-ray structure of E. coli MTHFR (which is 30%
identical with the catalytic domain of human MTHFR),
determined by Rowena Matthews and Martha Ludwig,
1036 Chapter 26. Amino Acid Metabolism
NH
3
+
H
S
Methionine
CH
2
CH
2
C
N
5
-methyl-THF
N
5
,N
10
-
methylene-
THF
ATP
P
i
+
+
PP
i
α
-Ketobutyrate
CH
2
C
COO
–
O
COO
–
CH
3
HH
HH
HO OH
O
NH
3
+
H
S
-Adenosylmethionine (SAM)
CH
2
CH
2
C COO
–
CH
3
CH
2
Adenine
S
HH
HH
HO OH
O
NH
3
+
H
S
-Adenosylhomocysteine
CH
2
CH
2
C COO
–
NAD
+
+ glycine
NADP
+
NADPH + H
+
NADH
+ NH
4
+
+ CO
2
CH
2
Adenine
S
NH
3
+
H
HS
Homocysteine
CH
2
CH
2
C COO
–
NH
3
+
H
S
Cystathionine
CH
2
CH
2
C COO
–
NH
3
+
H
CH
2
C COO
–
NH
3
+
H
CH
2
C COO
–
HS
H
3
C
Propionyl-CoA
CH
2
C
SCoA
O
H
3
C
Succinyl-CoA
CH
2
C
SCoA
O
CH
2
–
OOC
methyl acceptor
methylated acceptor
Adenosine
NADH
CoASH
+
NAD
+
+
H
2
O
+
H
2
O
Serine
H
2
O
THF
Cysteine
NH
3
H
2
O
cysteine
biosynthesis
biosynthetic
methylation
+
CO
2
1
2
3
5
4
6
7
8910
11
12
Figure 26-18 The pathway of methionine degradation,
yielding cysteine and succinyl-CoA as products. The enzymes
involved are (1) methionine adenosyltransferase in a reaction
that yields the biological methylating agent S-adenosylmethionine
(SAM), (2) methyltransferase, (3) adenosylhomocysteinase,
(4) methionine synthase (a coenzyme B
12
–dependent enzyme),
(5) cystathionine -synthase (a PLP-dependent enzyme),
(6) cystathionine ␥-lyase (a PLP-dependent enzyme), (7) ␣-keto
acid dehydrogenase, (8) propionyl-CoA carboxylase,
(9) methylmalonyl-CoA racemase, (10) methylmalonyl-CoA
mutase (a coenzyme B
12
–dependent enzyme; Reactions 8–10 are
discussed in Section 25-2E), (11) glycine cleavage system (Figs.
26-12 and 26-14) or serine hydroxymethyltransferase (Fig. 26-12),
(12) N
5
,N
10
-methylene-tetrahydrofolate reductase (a coenzyme
B
12
– and FAD-dependent enzyme; Figs. 26-19 and 26-49).
JWCL281_c26_1019-1087.qxd 6/8/10 9:38 AM Page 1036
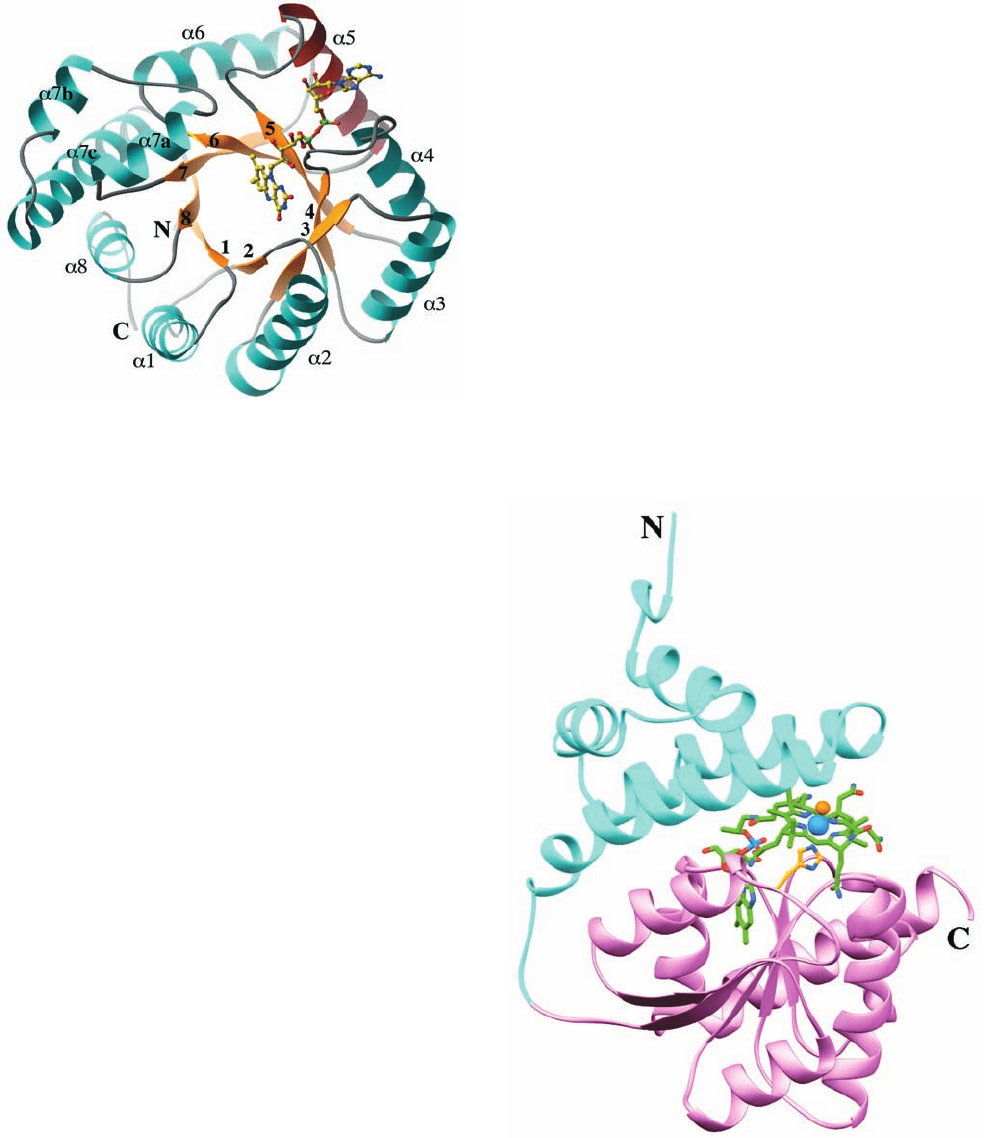
reveals that this 296-residue enzyme forms an ␣/ barrel.The
FAD cofactor binds at the C-terminal ends of barrel strands
3, 4, and 5 and along helix ␣5 (Fig. 26-19).Ala 177, which
corresponds to Ala 222 in the mammalian enzyme, does not
interact directly with the active site FAD. Instead it occupies
a position flush against helix ␣5 (which ends with residue
176). It is postulated that the replacement of Ala 177 by a
bulkier Val residue would force helix ␣5 to reorient. Since
this helix appears to be involved in the subunit interface as
well as in FAD binding, its reorientation is likely to decrease
the strength of subunit and FAD interactions.
Why should this mutation be so prevalent in the hu-
man population? What selective advantage, if any, might
it confer? We have seen that the gene for sickle-cell ane-
mia provides a selective advantage against malaria (Sec-
tion 7-3Ab). However, the selective advantage of the
A222V mutation in human MTHFR is as yet a matter of
speculation.
c. Methionine Synthase Is a Coenzyme
B
12
–Dependent Enzyme
Methionine synthase (alternatively homocysteine methyl-
transferase), the enzyme that catalyzes Reaction 4 in
Fig. 26-18, is the only coenzyme B
12
–associated enzyme in
mammals besides methylmalonyl-CoA mutase (Section
25-2Eb). However, in methionine synthase, the cobalamin
Co ion is axially liganded by a methyl group forming
methylcobalamin rather than by a 5¿-deoxyadenosyl group
as occurs in methylmalonyl-CoA mutase (Fig. 25-21). This
is because the cobalamin functions to accept the methyl
group from N
5
-methyl-THF to yield methylcobalamin (and
THF), which, in turn, donates the methyl group to homo-
cysteine to yield methionine.
The X-ray structure of the 246-residue methylcobal-
amin-binding portion of the 1227-residue monomeric
E. coli methionine synthase, also determined by
Matthews and Ludwig, reveals that it consists of two do-
mains, an N-terminal helical domain and a C-terminal
Rossmann fold-like ␣/ domain, with the corrin ring
sandwiched between them (Fig. 26-20). The ␣/ domain
resembles the corrin-binding ␣/ domain in methyl-
malonyl-CoA mutase (Fig. 25-22) and, in fact, sequence
homologies suggest that this domain is a common binding
motif in B
12
-associated enzymes. The Co ion’s second ax-
ial ligand is a His side chain as is also the case in methyl-
malonyl-CoA mutase; the coenzyme’s 5,6-dimethylbenza-
midazole (DMB) moiety, which ligands the Co ion in free
methylcobalamin, has swung aside to become anchored to
the protein at some distance from the corrin ring.
Section 26-3. Metabolic Breakdown of Individual Amino Acids 1037
Figure 26-19 X-ray structure of E. coli N
5
,N
10
-methylene-
tetrahydrofolate reductase (MTHFR). The structure is viewed
along the axis of its ␣/ barrel looking toward the C-terminal
ends of its  strands.The protein is colored according to its
secondary structure with  strands yellow and ␣ helices cyan
except for helix ␣5, which is red. The enzyme’s bound FAD is
drawn in ball-and-stick form with C yellow, N blue, O red, and P
green. Note that the AMP moiety of the FAD is in contact with
helix ␣5. [Courtesy of Rowena Matthews and Martha Ludwig,
University of Michigan. PDBid 1B5T.]
Figure 26-20 X-ray structure of the B
12
-binding domains of
E. coli methionine synthase. Its N-terminal helical domain
(residues 651–743) is cyan and its C-terminal ␣/ domain
(residues 744–896) is pink.The methylcobalamin cofactor and its
axially liganded His 759 side chain are drawn in stick form with
cobalamin C green, His C gold, N blue, O red, and the Co ion
and its axially liganded methyl group represented by light blue
and orange spheres, respectively. [Based on an X-ray structure by
Rowena Matthews and Martha Ludwig, University of Michigan.
PDBid 1BMT.]
JWCL281_c26_1019-1087.qxd 4/20/10 9:26 AM Page 1037

NH
3
+
CH
2
C
SCoA
O
C
H
2
O
CH
COO
–
CH
R
1
R
2
C
COO
–
CH
R
1
R
2
O
C
SCoA
CH
R
1
R
2
O
3
CH
3
Methylacrylyl-CoA
7
CH
2
SCoA
O
C
H
2
O
CH
3
β-Hydroxybutyryl-CoA
8
CH
OH
CH
2
O
–
O
C
CH
3
β-Hydroxyisobutyrate
9
CH
OH
HC COO
–
CH
3
Methylmalonate
semialdehyde
10
CH
O
CoASH
NAD
+
NADH
NADH, CO
2
NAD
+
, CoASH
CH
3
C
SCoA
O
C
H
2
O
CH
3
Tiglyl-CoA
4
CH
SCoA
O
C
CH
3
α-Methyl-β-
hydroxybutyryl-CoA
CH
OH
α-Methylacetoacetyl-CoA
6
CoASH
CH
CH
3
C
SCoA
O
C
CH
3
CH
O
CH
3
5
NAD
+
NADH
C C
SCoA
O
CH
H
2
O
H
3
C
-Methylcrotonyl-CoA
11
CH
2
SCoA
O
C
H
2
O
CH
3
β-Methylglutaconyl-CoA
12
CH
CH
2
SCoA
O
C
β-Hydroxy-β-
methylglutaryl-CoA (HMG-CoA)
13
C
OH
β
C
–
OOC
CH
2
CH
3
–
OOC
CO
2
+ATP
ADP P
i
SCoA
O
C
CH
3
Acetyl-CoA
CH
2
O
C
–
OOC
CH
3
Acetoacetate
SCoA
O
C
CH
3
Acetyl-CoA
SCoA
O
CCH
2
Propionyl-CoA
CH
3
Succinyl-CoA
FADH
2
FAD
NADH, CO
2
NAD
+
, CoASH
Glutamate
␣-Ketoglutarate
1
2
R
1
CH
3
=,
R
2
CH
3
= CH
2
(A) Isoleucine
(B) Valine
(C) Leucine
:
:
:
R
1
CH
3
=,
R
2
CH
3
=
R
1
H=,
R
2
(CH
3
)
2
= CH
(A) α-Keto-β-methylvalerate
(B) α-Ketoisovalerate
(C) α-Ketoisocaproic acid
(A) α-Methylbutyryl-CoA
(B) Isobutyryl-CoA
(C) Isovaleryl-CoA
(A) (B) (C)
H
3
C
+
+
1038 Chapter 26. Amino Acid Metabolism
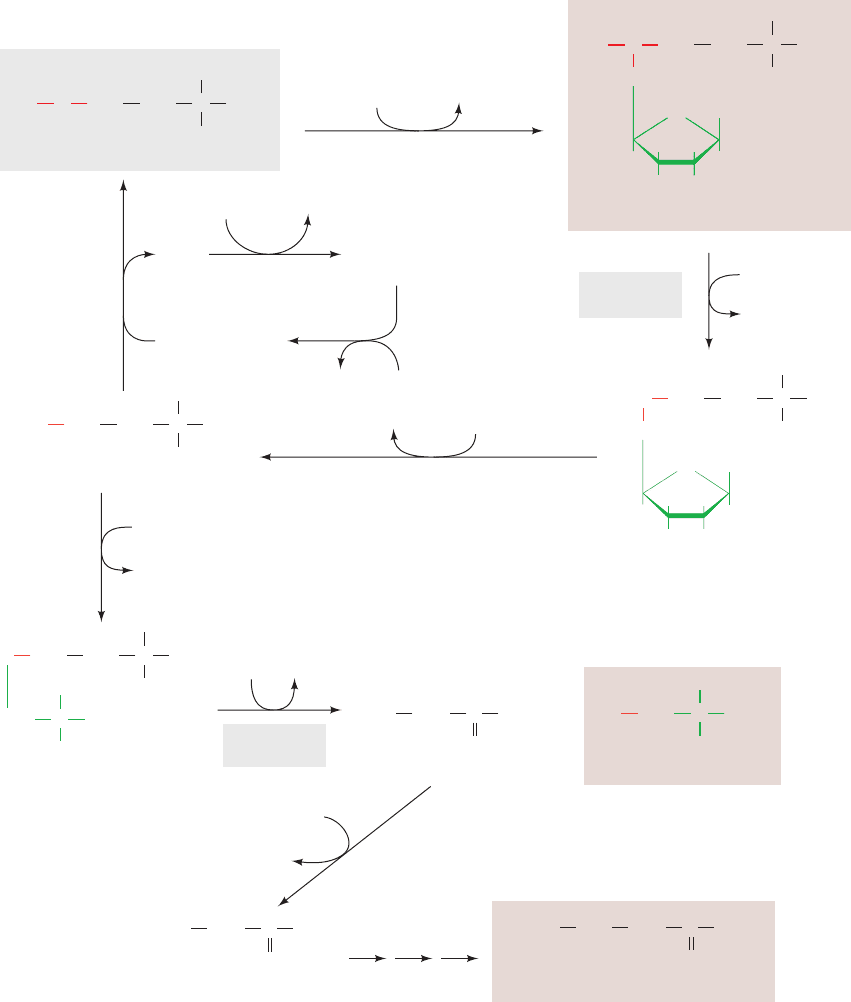
Figure 26-21 The degradation of the branched-chain amino acids (A) isoleucine, (B) valine,
and (C) leucine. The first three reactions of each pathway utilize the common enzymes
(1) branched-chain amino acid aminotransferase, (2) branched-chain ␣-keto acid dehydrogenase
(BCKDH), and (3) acyl-CoA dehydrogenase. Isoleucine degradation then continues (left) with
(4) enoyl-CoA hydratase, (5) -hydroxyacyl-CoA dehydrogenase, and (6) acetyl-CoA
acetyltransferase to yield acetyl-CoA and the succinyl-CoA precursor propionyl-CoA.Valine
degradation (center) continues with (7) enoyl-CoA hydratase, (8) -hydroxyisobutyryl-CoA
hydrolase, (9) -hydroxyisobutyrate dehydrogenase, and (10) methylmalonate semialdehyde
dehydrogenase to also yield propionyl-CoA. Leucine degradation (right) continues with
(11) -methylcrotonyl-CoA carboxylase (a biotin-dependent enzyme), (12) -methylglutaconyl-
CoA hydratase, and (13) HMG-CoA lyase to yield acetyl-CoA and acetoacetate.
JWCL281_c26_1019-1087.qxd 4/20/10 9:26 AM Page 1038
d. Branched-Chain Amino Acid Degradation
Pathways Contain Themes Common to All
Acyl-CoA Oxidations
Degradation of the branched-chain amino acids isoleucine,
leucine, and valine begins with three reactions that employ
common enzymes (Fig. 26-21, top): (1) transamination to the
corresponding ␣-keto acid, (2) oxidative decarboxylation
to the corresponding acyl-CoA, and (3) dehydrogenation
by FAD to form a double bond.
The remainder of the isoleucine degradation pathway (Fig.
26-21, left) is identical to that of fatty acid oxidation (Section
25-2C): (4) double-bond hydration, (5) dehydrogenation by
NAD
⫹
, and (6) thiolytic cleavage yielding acetyl-CoA and
propionyl-CoA, which is subsequently converted to succinyl-
CoA. Valine degradation is a variation on this theme (Fig.
26-21, center): Following (7) double-bond hydration, (8) the
CoA thioester bond is hydrolyzed before (9) the second dehy-
drogenation reaction.The thioester bond is then regenerated
as propionyl-CoA in the sequence’s last reaction (10), an ox-
idative decarboxylation rather than a thiolytic cleavage.
e. Maple Syrup Urine Disease Results from a Defect
in Branched-Chain Amino Acid Degradation
Branched-chain ␣-keto acid dehydrogenase (BCKDH;
also known as ␣-ketoisovalerate dehydrogenase), which
catalyzes Reaction 2 of branched-chain amino acid degra-
dation (Fig. 26-21), is a multienzyme complex containing
three enzymatic components, E1, E2, and E3, together
with BCKDH kinase (phosphorylation inactivates) and
BCKDH phosphatase (dephosphorylation activates),
which impart control by covalent modification. This com-
plex closely resembles the pyruvate dehydrogenase and
␣-ketoglutarate dehydrogenase multienzyme complexes
(Sections 21-2A and 21-3D). Indeed, all three of these mul-
tienzyme complexes share a common protein component,
E3 (dihydrolipoyl dehydrogenase), and employ the coen-
zymes thiamine pyrophosphate (TPP), lipoamide, and
FAD in addition to their terminal oxidizing agent, NAD
⫹
.
A genetic deficiency in BCKDH causes maple syrup
urine disease (MSUD), so named because the consequent
buildup of branched-chain ␣-keto acids imparts the urine
with the characteristic odor of maple syrup. Unless
promptly treated by a diet low in branched-chain amino
acids (but not too low because they are essential amino
acids; Section 26-5), MSUD is rapidly fatal.
MSUD is an autosomal recessive disorder that is caused
by defects in any of four of the complex’s six subunits, E1␣,
E1, E2, or E3 (E1 is an ␣
2

2
heterotetramer). The determi-
nation of the X-ray structure of human BCKDH E1 by Wim
Hol (Fig. 26-22) has enabled the interpretation of several of
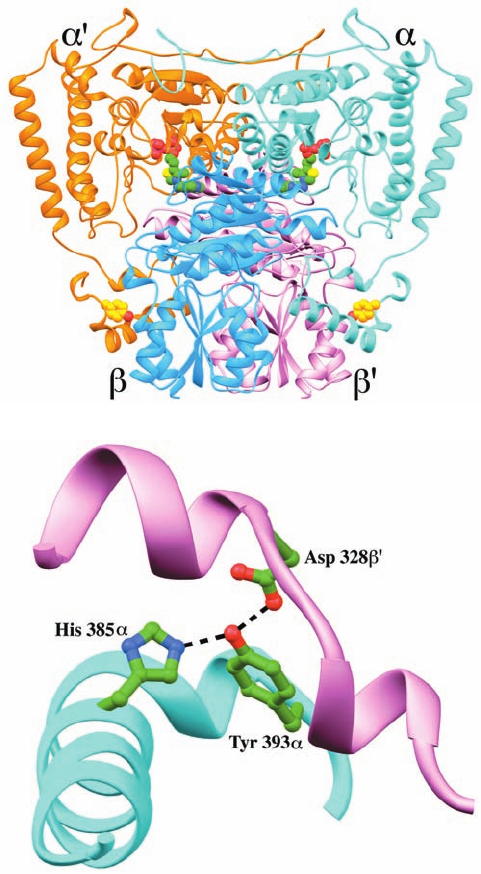
the mutations causing MSUD.The most common mutation is
Y393N-␣, the so-called Mennonite mutation, which occurs
once in every 176 live births in the Old Order Mennonite
population (versus 1 in 185,000 worldwide). This mutation is
so common among Old Order Mennonites that it has been
attributed to a founder effect, that is, a mutation that origi-
nated in one of the handful of founders of this isolated com-
munity. The E1 tetramer can be considered to be a dimer of
␣ heterodimers with a TPP cofactor at the interface be-
tween an ␣ subunit and a  subunit and with each ␣ subunit
contacting both the  and ¿ subunits (Fig. 26-22a). The
amino acid change in the Mennonite mutation occurs at the
␣–¿ interface:Tyr 393␣ is hydrogen bonded to both His 385␣
and Asp 328¿ (Fig. 26-22b). Its mutation to Asn disrupts
these interactions and thereby impedes tetramerization.
Section 26-3. Metabolic Breakdown of Individual Amino Acids 1039
Figure 26-22 X-ray structure of the E1 component of the
human branched-chain ␣-keto acid dehydrogenase multienzyme
complex. (a) The ␣
2

2
heterotetramer.The ␣ subunits are colored
cyan and orange, and the  subunits are blue and pink. The
thiamine pyrophosphate (TPP) cofactor and Tyr 393␣ (which is
mutated to Asn in the Mennonite mutation, causing maple syrup
urine disease) are shown in space-filling form with TPP C green,
Tyr 393␣ C gold, N blue, O red, S yellow, and P magenta. Note
the similarity of this structure to that of the E1 component of the
pyruvate dehydrogenase multienzyme complex (Fig. 21-12a).
(b) The ␣–¿ interface colored as in Part a and showing the
interactions of Tyr 393␣ with His 385␣ and Asp 328¿. The side
chains of these residues are drawn in ball-and-stick form with C
green, N blue, and O red and with the hydrogen bonds between
them represented by dashed lines. [Based on an X-ray structure
by Wim Hol, University of Washington. PDBid 1DTW.]
(b)
(a)
JWCL281_c26_1019-1087.qxd 4/20/10 9:26 AM Page 1039
CH
3
CH
2
HC
O
CH
3
N
+
CH
2
CH
2
CH
2
CH
2
H
NH
3
+
COO
–
Lysine
CNH CH
2
CH
2
CH
2
CH
2
H
NH
3
+
COO
–
Saccharopine
C
COO
–
CH
2
COO
–
H
α-Ketoglutarate
NADPH
+
NADP
+
CH
2
CH
2
CH
2
C
H
NH
3
+
COO
–
α
-Aminoadipate-6-
semialdehyde
–
OOC
CCH
2
CH
2
CH
2
H
NH
3
+
COO
–
α
-Aminoadipate
–
OOC
CH
2
CH
2
CH
2
C
O
COO
–
α
-Ketoadipate
–
OOC
CCH
2
CH
2
CH
2
O
SCoA
Glutaryl-CoA
–
OOC
CH CCH
2
CH
O
SCoA
Glutaconyl-CoA
CH CH
3
C
O
SCoA
Crotonyl-CoA
CH
CH
2
CH
3
C
O
SCoA
β
-Hydroxybutyryl-CoA
CH
OH
CH
2
CH
3
C
O
SCoA
Acetoacetyl-CoA
C
O
CH
2
C
O
SCoA
HMG-CoA
C
OH
CH
2
–
OOC
CH
2
COO
–
Acetoacetate
CCH
3
O
NADH
NAD
+
Glutamate
NAD(P)H
NAD(P)
+
α-Ketoglutarate
Glutamate
NADH
NAD
+
+
+
CoA
CO
2
FADH
FAD
2
CO
2
H
2
O
NADH
NAD
+
CoA
Acetyl-CoA
Acetyl-CoA
1
2
3
4
5
6
7
8
9
10
11
H
2
O +
F. Leucine and Lysine Are Degraded to
Acetoacetate and/or Acetyl-CoA
Leucine is oxidized by a combination of reactions used in 
oxidation and ketone body synthesis (Fig. 26-21, right).The
first dehydrogenation and the hydration reactions are in-
terspersed by (11) a carboxylation reaction catalyzed by
the biotin-containing enzyme -methylcrotonyl-CoA car-
boxylase. The hydration reaction (12) then produces
-hydroxy--methylglutaryl-CoA (HMG-CoA), which is
cleaved by HMG-CoA lyase to form acetyl-CoA and the
ketone body acetoacetate (13) (which, in turn, may be con-
verted to 2 acetyl-CoA; Section 25-3).
Although there are several pathways for lysine degrada-
tion, the one that proceeds via formation of the ␣-ketoglu-
tarate–lysine adduct saccharopine predominates in mam-
malian liver (Fig. 26-23).This pathway is of interest because
we have encountered 7 of its 11 reactions in other pathways.
Reaction 4 is a PLP-dependent transamination. Reaction 5
is the oxidative decarboxylation of an ␣-keto acid by a
multienzyme complex similar to pyruvate dehydrogenase
1040 Chapter 26. Amino Acid Metabolism
Figure 26-23 The pathway
of lysine degradation in
mammalian liver. The
enzymes involved are
(1) saccharopine dehydrogenase
(NADP
⫹
, lysine forming),
(2) saccharopine
dehydrogenase (NAD
⫹
,
glutamate forming),
(3) aminoadipate
semialdehyde dehydrogenase,
(4) aminoadipate
aminotransferase (a PLP
enzyme), (5) ␣-keto acid
dehydrogenase, (6) glutaryl-
CoA dehydrogenase,
(7) decarboxylase, (8) enoyl-CoA
hydratase, (9) -hydroxyacyl-
CoA dehydrogenase,
(10) HMG-CoA synthase,
and (11) HMG-CoA lyase.
Reactions 10 and 11 are
discussed in Section 25-3.
JWCL281_c26_1019-1087.qxd 4/20/10 9:26 AM Page 1040
