Voet D., Voet Ju.G. Biochemistry
Подождите немного. Документ загружается.

that are collectively known as statins. Indeed, Lipitor is
presently one of the most widely prescribed drugs in the
United States. The initial decreased cholesterol supply in
the cell caused by the presence of statins is again met by in-
duction of LDL receptors and HMG-CoA reductase so
that, at the new steady state, the HMG-CoA reductase
level is almost that of the predrug state. However, the in-
creased number of LDL receptors causes increased re-
moval of both LDL and IDL (the apoB-containing precur-
sor to LDL), decreasing serum LDL levels appreciably.
Lipitor-treated FH heterozygotes routinely show a serum
cholesterol decrease of 40–50%.
The combined use of these agents, moreover, results in a
clinically dramatic 50 to 60% decrease in serum cholesterol
levels.
e. Overexpression of LDL Receptor Prevents
Diet-Induced Hypercholesterolemia
Experiments are well underway toward the treatment
of hypercholesterolemic individuals by gene therapy
(Section 5-5Hb). A line of transgenic mice has been de-
veloped that overproduce the human LDL receptor.
When fed a diet high in cholesterol, fat, and bile salts,
these transgenic animals did not develop a detectable in-
crease in plasma LDL. In contrast, normal mice fed the
same diet exhibited large increases in plasma LDL levels.
Evidently, the unregulated overexpression of LDL re-
ceptors can prevent diet-induced hypercholesterolemia,
at least in mice.
C. Cholesterol Utilization
Cholesterol is the precursor of steroid hormones and bile
salts. Steroid hormones, which are grouped into five
categories, progestins, glucocorticoids, mineralocorti-
coids, androgens, and estrogens, mediate a wide variety
of vital physiological functions (Section 19-1G). All con-
tain the four-ring structure of the sterol nucleus and are
remarkably similar in structure, considering the enormous
differences in their physiological effects. A simplified
Section 25-6. Cholesterol Metabolism 991
Figure 25-63 Some competitive inhibitors of HMG-CoA
reductase used for the treatment of hypercholesterolemia. The
molecular formulas of lovastatin (Mevacor), pravastatin
(Pravachol), simvastatin (Zocor), and atorvastatin (Lipitor), all
of which are potent competitive inhibitors of HMG-CoA
R
O
O
H
HO
O
CH
3
CH(CH
3
)
2
F
CH
3
H
3
C
X
COO
–
HMG-CoA
H
3
C
X = H
X = H
X = CH
3
R = CH
3
R = OH
R = CH
3
Lovastatin (Mevacor)
Pravastatin (Pravachol)
Simvastatin (Zocor)
H
3
C
HO
HO
HO
N
O
S
O
HN
CoA
COO
–
OH
COO
–
OH
Mevalonate
Atorvastatin (Lipitor)
reductase, are given.The structures of HMG-CoA and
mevalonate are shown for comparison. Note that lovastatin,
pravastatin, and simvastatin are lactones, whereas atorvastatin
and mevalonate are hydroxy acids.The lactones are hydrolyzed
enzymatically in vivo to their active hydroxy-acid forms.
JWCL281_c25_940-1018.qxd 4/20/10 1:59 PM Page 991
biosynthetic scheme (Fig. 25-64) indicates their structural
similarities and differences. We shall not discuss the details
of these pathways.
The quantitatively most important pathway for the excre-
tion of cholesterol in mammals is the formation of bile
acids. The major bile acids, cholate and chenodeoxycholate,
992 Chapter 25. Lipid Metabolism
HO
35
1
4
2
7
8
9
6
10
14
13
15
16
12 17
11
19
18
21 22 24 27
20 23 25
26
Cholesterol
Pregnenolone
HO
CO
CH
3
17-Hydroxypregnenolone
HO
CO
OH
CH
3
Testosterone
OH
OH
Dehydroisoandrosterone
HO
11-Deoxycortisol
O
Estradiol
An estrogen
HO
17-Hydroxyprogesterone
O
CO
OH
CH
3
O
CO
OH
CH
2
OH
Cortisol
A glucocorticoidAn androgen
O
CO
OH
HO
HO
CH
2
OH
O
11-Deoxycorticosterone
O
CO
CH
2
OH
Corticosterone
A mineralo-
corticoid
O
CO
CH
2
OH
HO
OHC
Aldosterone
O
CO
CH
2
OH
Progesterone
A progestin
O
2
1
2
CO
CH
3
3 4
5
6, 78
5
4
Figure 25-64 Simplified scheme of steroid biosynthesis. The
enzymes involved are (1) the cholesterol side chain cleavage
enzyme, (2) steroid C17 hydroxylase, (3) steroid C17, C20 lyase,
(4) steroid C21 hydroxylase, (5) steroid 11-hydroxylase,
(6) steroid C18 hydroxylase, (7) 18-hydroxysteroid oxidase, and
(8) aromatase.
JWCL281_c25_940-1018.qxd 4/20/10 1:59 PM Page 992
are synthesized in the liver and secreted as their glycine or
taurine conjugates (Fig. 25-65), which are known as bile
salts, into the gallbladder. From there, they are secreted
into the small intestine, where they act as emulsifying
agents in the digestion and absorption of fats and fat-
soluble vitamins (Section 25-1). An efficient recycling
system allows the bile salts to reenter the bloodstream and
return to the liver for reuse several times each day. The
1g day
1
of bile salts that normally escape this recycling
system are further metabolized by microorganisms in the
large intestine and excreted. This is the body’s only route
for cholesterol excretion.
Comparison of the structures of cholesterol and the bile
acids (Figs. 25-44 and 25-65) indicates that biosynthesis of
bile acids from cholesterol involves (1) saturation of the 5,6
double bond, (2) epimerization of the 3-OH group, (3) in-
troduction of OH groups into the 7 and 12 positions,
(4) oxidation of C24 to a carboxylic acid,and (5) conjugation
of this side chain carboxylic acid with glycine or taurine.
Cholesterol 7-hydroxylase catalyzes the first and rate-
limiting step in bile acid synthesis and is closely regulated.
7 EICOSANOID METABOLISM:
PROSTAGLANDINS, PROSTACYCLINS,
THROMBOXANES, LEUKOTRIENES,
AND LIPOXINS
Prostaglandins (PGs) were first identified in human semen
by Ulf von Euler in the early 1930s through their ability to
stimulate uterine contractions and lower blood pressure.
von Euler thought that these compounds originated in the
prostate gland (hence their name) but they were later
shown to be synthesized in the seminal vesicles. By the time
the mistake was realized, the name was firmly entrenched.
In the mid-1950s, crystalline materials were isolated from
biological fluids and called PGE (ether-soluble) and PGF
(phosphate buffer–soluble; fosfat in Swedish). This began
an explosion of research on these potent substances.
Almost all mammalian cells except red blood cells pro-
duce prostaglandins and their related compounds, the
prostacyclins, thromboxanes, leukotrienes and lipoxins,
known collectively as eicosanoids since they are all C
20
com-
pounds; Greek: eikosi, twenty). The eicosanoids, like en-
docrine hormones, have profound physiological effects at
extremely low concentrations. For example, they mediate
the following: (1) the inflammatory response, notably as it
involves the joints (rheumatoid arthritis), skin (psoriasis),
and eyes; (2) the production of pain and fever; (3) the reg-
ulation of blood pressure; (4) the induction of blood clot-
ting; (5) the control of several reproductive functions such
as the induction of labor; and (6) the regulation of the
sleep/wake cycle. The enzymes that synthesize these com-
pounds and the receptors to which they bind are therefore
the targets of intensive pharmacological research.
The eicosanoids are also hormonelike in that they bind to
G-protein-coupled receptors (Section 19-2B), and many of
their effects are intracellularly mediated by cAMP. Unlike
endocrine hormones, however, they are not transported in
the bloodstream to their sites of action. Rather, these
chemically and biologically unstable substances (some de-
compose within minutes or less in vitro) are local media-
tors (paracrine hormones; Section 19-1); that is, they act in
the same environment in which they are synthesized.
In this section, we discuss the structures of the
eicosanoids and outline their biosynthetic pathways and
modes of action. As we do so, note the great diversity of
their structures and functions, a phenomenon that makes
the elucidation of the physiological roles of these potent
substances a challenging research area.
A. Background
Prostaglandins are all derivatives of the hypothetical C
20
fatty acid prostanoic acid in which carbon atoms 8 to 12
Section 25-7. Eicosanoid Metabolism: Prostaglandins, Prostacyclins, Thromboxanes, Leukotrienes, and Lipoxins 993
Figure 25-65 Structures of the major bile acids and their glycine and taurine conjugates.
CH
3
R
1
R
1
= OH
R
2
= OH
R
2
= NH
R
2
= NH
Cholic acid
Glycocholic acid
Taurocholic acid
Chenodeoxycholic acid
Glycochenodeoxycholic acid
Taurochenodeoxycholic acid
CH
2
CH
2
COOH
CH
2
SO
3
H
R
1
= H
H
OH
HO
5
1
4
3
2
8
7
9
6
10
13
14
12
16
15
17
11
19
CH
3
C R
2
O
18
21
20
22 24
23
H
H
JWCL281_c25_940-1018.qxd 4/20/10 1:59 PM Page 993
form a cyclopentane ring (Fig. 25-66a). Prostaglandins A
through I differ in the substituents on the cyclopentane ring
(Fig. 25-66b): PGAs are ,-unsaturated ketones, PGEs are
-hydroxy ketones, PGFs are 1,3-diols, etc. In PGF
, the C9
OH group is on the same side of the ring as R
1
; it is on the
opposite side in PGF
. The numerical subscript in the name
refers to the number of double bonds contained on the side
chains of the cyclopentane ring (Fig. 25-66c).
In humans, the most prevalent prostaglandin precursor is
arachidonic acid (5,8,11,14-eicosatetraenoic acid), a C
20
polyunsaturated fatty acid that has four nonconjugated dou-
ble bonds.The double bond at C14 is six carbon atoms from
the terminal carbon atom (the carbon atom), making
arachidonic acid an –6 fatty acid.As Sune Bergström and
Bengt Samuelsson demonstrated, arachidonic acid is syn-
thesized from the essential fatty acid linoleic acid (also an
–6 fatty acid). This occurs via its desaturation with a
6
-
desaturase to yield -linolenic acid (GLA), followed by
elongation and a second desaturation, this time with a
5
-
desaturase (Fig. 25-67; Section 25-4E). Prostaglandins with
the subscript 1 (the “series-1” prostaglandins) are synthe-
sized from dihomo--linolenic acid (DGLA; 8,11,14-
eicosatrienoic acid), whereas “series-2” prostaglandins are
synthesized from arachidonic acid. -Linolenic acid
(ALA), another essential fatty acid since the
15
-desaturase
required for its synthesis occurs only in plants, is a precursor
of 5,8,11,14,17-eicosapentaenoic acid (EPA) and the
“series-3” prostaglandins. Since arachidonate is the primary
prostaglandin precursor in humans, we shall mostly refer to
the series-2 prostaglandins in our examples. Note, however,
that when dietary linoleic acid and -linolenic acid are
equally available, the relative activities of the
5
- and
6
-
desaturases are important in determining the relative
amounts of these prostaglandin precursors.
a. Arachidonate Is Generated by
Phospholipid Hydrolysis
Arachidonate is stored in cell membranes esterified at
glycerol C2 of phosphatidylinositol and other phospho-
lipids. The production of arachidonate metabolites is
controlled by the rate of arachidonate release from these
phospholipids through three alternative pathways (Fig.
25-68):
1. Phospholipase A
2
hydrolyzes acyl groups at C2 of
phospholipids (Fig. 25-68b, left).
2. Phospholipase C (Section 19-4B) specifically hy-
drolyzes the phosphatidylinositol head group to yield a
1,2-diacylglycerol (DAG) and phosphoinositol. DAG is
phosphorylated by diacylglycerol kinase to phosphatidic
acid, a phospholipase A
2
substrate (Fig. 25-68b, center).
(Recall that DAG and the various phosphorylated forms
of phosphoinositol are also important signaling molecules
in that they mediate the phosphoinositide cascade; Sec-
tion 19-4.)
3. The DAG also may be hydrolyzed directly by dia-
cylglycerol lipase (Fig. 25-68b, right). Corticosteroids are
used as anti-inflammatory agents because they inhibit
phospholipase A
2
, reducing the rate of arachidonate pro-
duction.
b. Aspirin Inhibits Prostaglandin Synthesis
The use of aspirin as an analgesic (pain-relieving), an-
tipyretic (fever-reducing), and anti-inflammatory agent has
been widespread since the nineteenth century. Yet, it was
not until 1971 that John Vane discovered its mechanism of
action. Aspirin, as do other nonsteroidal anti-inflammatory
drugs (NSAIDs), inhibits the synthesis of prostaglandins
from eicosanoid precursors (Section 25-7Ba). These in-
hibitors have therefore proved to be valuable tools in the
elucidation of prostaglandin biosynthesis pathways and
have provided a starting point for the rational synthesis of
new anti-inflammatory drugs.
994 Chapter 25. Lipid Metabolism
HO
HO
COOH
(a)
(b)
(c)
11
12
13
14
15
16
17
18
19
20
2
13
4
5
6
7
8
10
9
Prostanoic acid
O
AD
R
2
R
1
R
1
O
HO
HO
HO
R
2
F
R
1
HO
HO
R
2
G+H
R
1
R
1
O
O
O
R
2
I
R
2
E
R
1
R
2
O
B
R
2
R
1
O
O
C
R
2
R
1
OH
OH
O
HO
F
R
1
R
2
COOH
PGE
1
HO
OH
O
COOH
PGE
2
HO
OH
COOH
PGF
2
Figure 25-66 Prostaglandin structures. (a) The carbon
skeleton of prostanoic acid, the prostaglandin parent compound.
(b) Structures of prostaglandins A through I. (c) Structures of
prostaglandins E
1
,E
2
, and F
2
(the first prostaglandins to be
identified).
JWCL281_c25_940-1018.qxd 4/20/10 1:59 PM Page 994
c. Arachidonic Acid Is a Precursor of Leukotrienes,
Thromboxanes, and Prostacyclins
Arachidonic acid also serves as a precursor to com-
pounds whose synthesis is not inhibited by aspirin. In fact,
there are two main pathways of eicosanoid metabolism.
The so-called cyclic pathway, which is inhibited by
NSAIDs, forms prostaglandin’s characteristic cyclopen-
tane ring, whereas the so-called linear pathway, which is
Section 25-7. Eicosanoid Metabolism: Prostaglandins, Prostacyclins, Thromboxanes, Leukotrienes, and Lipoxins 995
Linoleic acid
(9,12- octadeca-
dienoic acid)
-Linolenic acid
(GLA; 6,9,12-
octadecatrienoic
acid)
DihomoGLA
(DGLA; 8,11,14-
Eicosatrienoic
acid)
A
rachidonic acid
(5,8,11,14-eicosa-
tetraenoic acid)
5,8,11,14,17-
Eicosa-
pentaenoic acid (EPA)
COOH
COOH
COOH
COOH
–2H
6
-desaturase
15
-desaturase
(plants only)
–2H
PG
1
+2C elongase
PGH
synthase
PGH
synthase
+2C elongase
–2H
5
-desaturase
–2H
5
-desaturase
20:3
COOH
20:4
20:4
COOH
20:5
18:3
18:2
COOH
–2H
6
-desaturase
18:3
–2H
–2H
PG
2
PG
3
COOH
18:4
-Linolenic acid
(ALA; 9-12-15-octadeca-
trienoic acid)
OCH
CH
2
H
31
C
19
(b)
(a)
C
O
O
O
–
PX
X =
CH
2
OCR
1
Arachidonoyl
group
Phospholipid
(phosphatidylinositol)
Inositol
1,2-Diacylglycerol (DAG)
Phosphoinositol
phospholipase A
2
phospholipase C
phospholipase Cphospholipase A
2
diacylglycerol kinase diacylglycerol lipase
O
O
Phosphatidic acid
Lysophosphatidic acid
+
Arachidonic acid
Monoacylglycerol
+
Arachidonic acid
Lysophospholipid
+
Arachidonic acid
phospholipase A
2
OH H
OHH
HH
OH
OHH
HO
HO
Figure 25-67 Synthesis of prostaglandin precursors. The
linoleic acid derivatives dihomoGLA (DGLA), arachidonic acid,
Figure 25-68 Release of arachidonic acid by phospholipid
hydrolysis. (a) The sites of hydrolytic cleavage mediated by
phospholipases A
2
and C.The polar head group, X, is often
inositol and its various phosphorylated forms (Section 19-4D).
(b) Pathways of arachidonic acid liberation from phospholipids.
and 5,8,11,14,17-eicosapentaenoic acid (EPA) are the respective
precursors of the series-1, series-2, and series-3 prostaglandins.
JWCL281_c25_940-1018.qxd 6/8/10 9:00 AM Page 995
996 Chapter 25. Lipid Metabolism
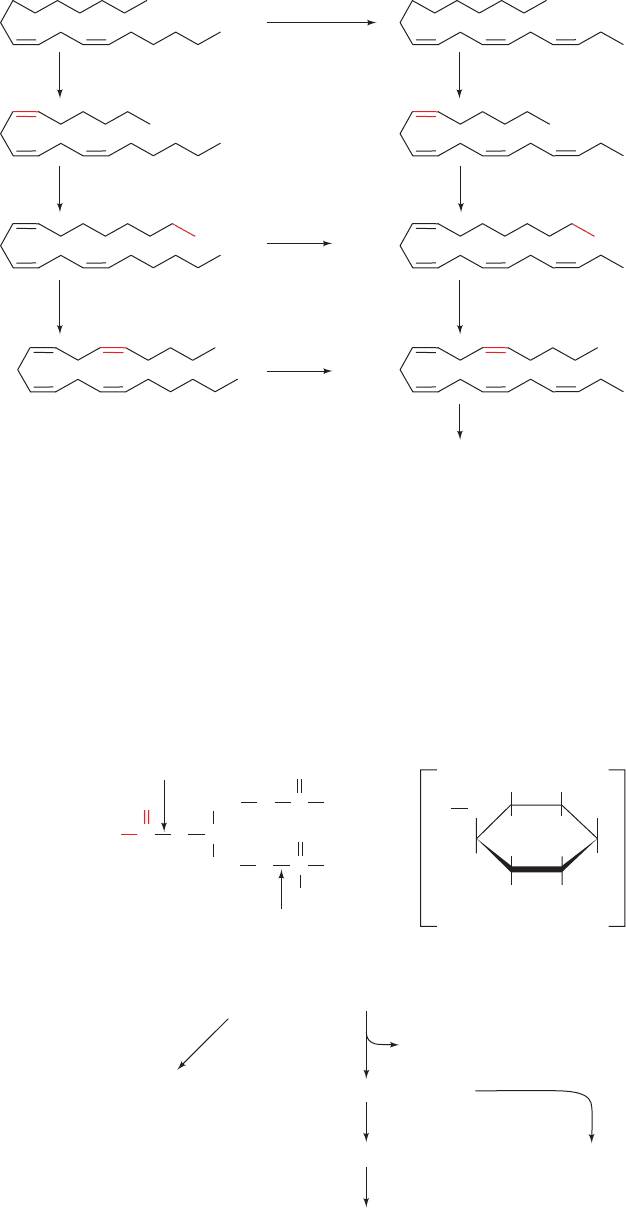
Figure 25-69 The cyclic and linear pathways of arachidonic acid metabolism.
Figure 25-70 The cyclic pathway of arachidonic acid metabolism. This pathway’s branches lead
to prostaglandins, prostacyclins, and thromboxanes.
Prostaglandins
Arachidonic acid
5-Hydroperoxyeicosatetraenoic acid
(5-HPETE)
12-Hydroperoxyeicosatetraenoic acid
(12-HPETE)
COOH
“cyclic pathway”
20:4
“linear pathway”
(lipoxygenases)
or
HOO
or
COOH
COOH
Leukotrienes
Hepoxilins
OOH
COOH
Lipoxins
15-Hydroperoxyeicosatetraenoic acid
(15-HPETE)
HOO
COOH
COOH
Arachidonic acid
PGH synthase
prostacyclin
synthase
thromboxane
synthase
(Aspirin inhibits)
HO
O
COOH
HO
OH
O
PGI
2
(unstable)
6-Oxo-PGF
1
COOH
HO
HO
O
OH
Prostacyclins
PGH
2
TxA
2
(unstable)
TxB
2
Thromboxanes
Prostaglandins
COOH
O
O
OH
COOH
OH
O
O
COOH
OH
PGE
2
COOH
OH
HO
HO
PGF
2
COOH
OH
O
HO
PGD
2
COOH
OH
O
HO
OH
JWCL281_c25_940-1018.qxd 4/20/10 1:59 PM Page 996
not inhibited by these agents, leads to the formation of the
leukotrienes and HPETEs (Fig. 25-69; Section 25-7C).
Studies using NSAIDs helped demonstrate that two
structurally related and highly short-lived classes of
compounds, the prostacyclins and the thromboxanes (Fig.
25-70), are also products of the cyclic pathway of eicosanoid
metabolism. The specific products produced by this
branched pathway depend on the tissue involved. For ex-
ample, blood platelets (thrombocytes) produce thrombox-
anes almost exclusively; vascular endothelial cells, which
make up the walls of veins and arteries, predominantly syn-
thesize the prostacyclins; and heart muscle makes PGI
2
,
PGE
2
, and PGF
2
in more or less equal quantities. In the re-
mainder of this section, we study the cyclic and the linear
pathways of eicosanoid metabolism.
B. The Cyclic Pathway of Eicosanoid Metabolism:
Prostaglandins, Prostacyclins, and Thromboxanes
The first step in the cyclic pathway of eicosanoid metabo-
lism is catalyzed by PGH synthase (PGHS; also called
prostaglandin H synthase and prostaglandin endoperoxide
synthase; Fig. 25-71). This heme-containing enzyme con-
tains two catalytic activities: a cyclooxygenase activity and
a peroxidase activity. The former catalyzes the tyrosyl
radical-mediated addition of two molecules of O
2
to
arachidonic acid, forming PGG
2
. The latter converts the
hydroperoxy function of PGG
2
to an OH group, yielding
PGH
2
. PGH
2
is the immediate precursor of all series-2
prostaglandins, prostacyclins, and thromboxanes (Fig.
25-70).The cyclooxygenase activity of the enzyme gives it its
common name, COX [not to be confused with cytochrome c
oxidase, which is also called COX (Section 22-2C5)].
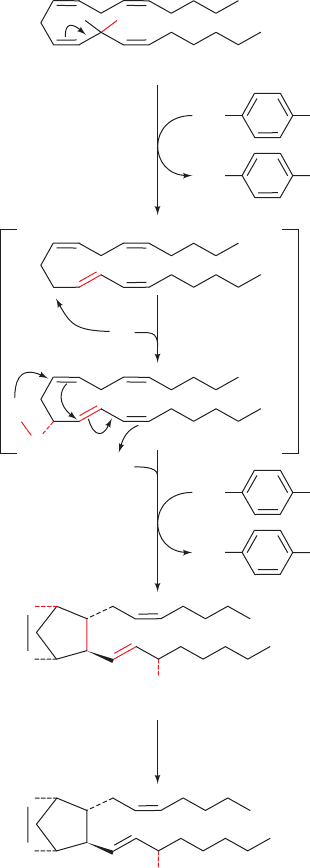
PGHS, a homodimeric glycoprotein of 576-residue sub-
units, is a monotopic membrane protein that extends into
the lumen of the endoplasmic reticulum. Its X-ray struc-
ture, determined by Michael Garavito, reveals that each of
its subunits folds into three domains (Fig. 25-72a): an N-
terminal module that structurally resembles epidermal
growth factor (EGF; a hormonally active polypeptide that
stimulates cell proliferation; Section 12-3Ae); a central
membrane-binding motif; and a C-terminal enzymatic do-
main. The 44-residue membrane-binding motif has a hy-
drophobic surface that faces away from the body of the
protein [as is also true of oxidosqualene cyclase (Fig.
25-58b) and fatty acid amide hydrolase (Fig. 12-28)].
The peroxidase active site of PGHS occurs at the inter-
face between the large and small lobes of the catalytic do-
main, in a shallow cleft that contains the enzyme’s
Fe(III)–heme prosthetic group. The cleft exposes a large
portion of the heme to solvent and is therefore thought to
comprise the substrate binding site.
The cyclooxygenase active site lies on the opposite side
of the heme at the end of a long narrow hydrophobic chan-
nel (⬃8 25 Å) extending from the outer surface of the
membrane-binding motif to the center of each subunit
(Fig. 25-72b).This channel allows access of the membrane-
associated substrate to the active site. Tyr 385, which lies
near the top of the channel, just beneath the heme, has
been shown to form a transient radical during the cy-
clooxygenase reaction as does, for example, Tyr 244 of cy-
tochrome c oxidase (Section 22-2C5c). Indeed, the muta-
genic replacement of PGHS’s Tyr 385 by Phe abolishes its
cyclooxygenase activity. The Tyr 385 radical is generated
via an intramolecular oxidation by the heme cofactor.
Section 25-7. Eicosanoid Metabolism: Prostaglandins, Prostacyclins, Thromboxanes, Leukotrienes, and Lipoxins 997
COOH
cyclooxygenase activity
(inhibited by aspirin)
H
•O
•
•
H
Arachidonic acid
O
2
O
2
COOH
11
13
15
Tyr 385
Tyr 385HO
1
•O
Tyr 385
Tyr 385
HO
3
4
COOH
COOH
2
O
O
O
O
OOH
PGG
2
COOH
O
O
OH
PGH
2
peroxidase
activity
Figure 25-71 Reactions catalyzed by PGH synthase (PGHS).
The enzyme contains two activities: a cyclooxygenase, which cat-
alyzes Steps 1 to 3 and is inhibited by aspirin; and a peroxidase,
which catalyzes Step 4. (1) A radical at Tyr 385 that is generated
by the enzyme’s heme cofactor stereospecifically abstracts a
hydrogen atom from C13 of arachidonic acid, which then
rearranges so that the radical is on C11. (2) The radical reacts with
O
2
to yield a hydroperoxide radical. (3) The radical cyclizes and
reacts with a second O
2
molecule at C15 to yield a peroxide in a
process that regenerates the Tyr radical. (4) The enzyme’s peroxi-
dase activity converts the peroxide at C15 to a hydroxyl group.
JWCL281_c25_940-1018.qxd 6/8/10 9:00 AM Page 997
The fate of PGH
2
depends on the relative activities of
the enzymes catalyzing the specific interconversions (Fig.
25-70). Platelets contain thromboxane synthase, which me-
diates the formation of thromboxane A
2
(TxA
2
), a vaso-
constrictor and stimulator of platelet aggregation (an ini-
tial step in blood clotting;Section 35-1).Vascular endothelial
cells contain prostacyclin synthase, which catalyzes the syn-
thesis of prostacyclin I
2
(PGI
2
), a vasodilator and inhibitor
of platelet aggregation. These two substances act in opposi-
tion, maintaining a balance in the cardiovascular system.
a. NSAIDs Inhibit PGH Synthase
Nonsteroidal anti-inflammatory drugs (NSAIDs; Fig.
25-73) inhibit the synthesis of the prostaglandins, prosta-
cyclins, and thromboxanes by inhibiting or inactivating the
cyclooxygenase activity of PGHS. Aspirin (acetylsalicylic
acid), for example, acetylates this enzyme: If [
14
C-acetyl]
acetylsalicylic acid is incubated with the enzyme, radioac-
tivity becomes irreversibly associated with the inactive en-
zyme as Ser 530 becomes acetylated (Fig. 25-74). The X-ray
structure of PGHS reveals that Ser 530, which is not impli-
cated in catalysis, extends into the cyclooxygenase channel
just below Tyr 385 such that its acetylation would block
arachidonic acid’s access to the active site (Fig. 25-72b).The
structure of PGHS, which was crystallized with the NSAID
flurbiprofen (Fig.25-73), indicates that this drug binds in the
cyclooxygenase channel, with its carboxyl group forming an
ion pair with Arg 120 (Fig. 25-72a). Evidently, flurbiprofen,
and by implication other NSAIDs, inhibits the cyclooxyge-
nase activity of PGHS by blocking its active site channel.
Low doses of aspirin, ⬃80 mg (baby aspirin) every day,
significantly reduce the long-term incidence of heart at-
tacks and strokes. Such low doses selectively inhibit
platelet aggregation and thus blood clot formation be-
cause these enucleated cells, which have a lifetime in the
circulation of ⬃10 days, cannot resynthesize their inacti-
vated enzymes.Vascular endothelial cells are not so drasti-
cally affected since, for the most part, they are far from the
site where aspirin is absorbed, are exposed to lesser con-
centrations of aspirin and, in any case, can synthesize addi-
tional PGHS.
b. COX-2 Inhibitors Lack the Side
Effects of Other NSAIDs
PGHS has two isoforms, COX-1 and COX-2, that share
a high degree (60%) of sequence identity and structural
homology. COX-1 is constitutively (without regulation) ex-
pressed in most, if not all, mammalian tissues, thereby sup-
porting levels of prostaglandin synthesis necessary to
maintain organ and tissue homeostasis such as that of the
gastrointestinal mucosa. In contrast, COX-2 is only ex-
pressed in certain tissues in response to inflammatory stim-
998 Chapter 25. Lipid Metabolism
Figure 25-72 X-ray structure of PGH synthase (PGHS) from
sheep seminal vesicles in complex with the NSAID flurbiprofen.
(a) This homodimeric monotopic membrane protein is viewed
parallel to the plane of the ER membrane with its 2-fold axis of
symmetry vertical.The EGF-like module is green, the
membrane-binding motif is orange, and the catalytic domain is
blue. The heme (red); flurbiprofen (yellow); Tyr 385 (magenta),
which forms a transient radical during the cyclooxygenase
reaction; and Arg 120 (green), which forms an ion pair with
flurbiprofen, are drawn in space-filling form. (b) A C
diagram of
a PGHS subunit (green), the left subunit in Part a as viewed from
30° to the left.The peroxidase active site is located above the
heme (pink).The hydrophobic channel, which penetrates the
subunit from the membrane-binding motif at the bottom of the
figure to the cyclooxygenase active site below the heme, is
represented by its van der Waals surface (blue dots). The three
residues in the channel that are shown in stick form in orange
are, from top to bottom:Tyr 385, Ser 530, which is acetylated by
aspirin, and Arg 120. [Courtesy of Michael Garavito, Michigan
State University. PDBid 1CQE.]
(a)
(b)
JWCL281_c25_940-1018.qxd 4/20/10 1:59 PM Page 998
uli such as cytokines, protein growth factors, and endotox-
ins, and hence is responsible for the elevated prostaglandin
levels that cause inflammation. The NSAIDs in Fig. 25-73
are relatively nonspecific and therefore can have adverse
side effects, most notably gastrointestinal ulceration, when
used to treat inflammation or fever.A structure-based drug
design program (Section 15-4Ad) was therefore instituted
to create inhibitors that would target COX-2 but not
COX-1. The three-dimensional structures of COX-1 and
COX-2 are almost identical.However,their amino acid dif-
ferences, specifically I523V, I434V, and H513R (COX-1
amino acid on the left and COX-2 amino acid on the right),
make COX-2’s active site channel ⬃20% larger in volume
than that of COX-1. In addition, the fourth helix of the
membrane-binding domain is oriented slightly differently
so as to provide a larger opening to the channel. Medicinal
chemists therefore synthesized inhibitors, collectively
known as coxibs, that could enter the COX-2 channel but
are excluded from that of COX-1. Two of these inhibitors,
rofecoxib (Vioxx) and celecoxib (Celebrex; Fig. 25-75), be-
came major drugs for the treatment of inflammatory dis-
eases such as arthritis because they lack the major side
Section 25-7. Eicosanoid Metabolism: Prostaglandins, Prostacyclins, Thromboxanes, Leukotrienes, and Lipoxins 999
Figure 25-73 Some nonsteroidal anti-inflammatory drugs (NSAIDs).
OH
C
COOH
CH
H
3
CO
C CH
3
OH
NH
CO
CH
3
CH
3
H
3
CO
O
NN
O
O
O
O
Aspirin
(acetylsalicylic acid)
Acetaminophen
Cl
N
CO
Indomethacin
Naproxen
COOH
CH
CH
3
C
4
H
9
F
Flurbiprofen
COOH
CHCH CH
2
H
3
C
CH
3
CH
2
COOH
H
3
C
CH
3
Ibuprofen
Phenylbutazone
O C
O
+
+
COOH
Aspirin
CH
3
OH
COOH
Salicylic
acid
PGH synthase
(inactive)
PGH synthase
(active)
CH
2
Ser 530
E
HO
CH
2
CH
3
Ser 530
E
O
C
O
SO
2
CH
3
Rofecoxib (Vioxx)
O
O
CH
3
SO
2
NH
2
F
3
C
Celecoxib (Celebrex)
N
N
Figure 25-74 Inactivation of PGH synthase by aspirin. Aspirin
acetylates Ser 530 of PGH synthase, thereby blocking the
enzyme’s cyclooxygenase activity.
Figure 25-75 COX-2 inhibitors. Rofecoxib and celecoxib are
specific inhibitors of COX-2 (PGH synthase-2).
JWCL281_c25_940-1018.qxd 4/20/10 1:59 PM Page 999
effects of the nonspecific NSAIDs. However,in 2004,Vioxx
was withdrawn from the pharmaceutical market because of
unanticipated cardiac side effects arising from its attenua-
tion of PGI
2
formation.
c. COX-3 May Be the Target of Acetaminophen
Acetaminophen, which is among the most widely used
analgesic/antipyretic drugs (but possesses little anti-
inflammatory activity, so that it is not really an NSAID),
does not significantly bind to either COX-1 or COX-2.
Thus its mechanism of action remained a mystery until the
discovery by Daniel Simmons of a third COX isozyme,
COX-3, a splice variant of COX-1, that is selectively inhib-
ited by acetaminophen as well as by certain NSAIDs. This
suggests that COX-3 is the primary target of drugs that
decrease pain and fever.
C. The Linear Pathway of Eicosanoid Metabolism:
Leukotrienes and Lipoxins
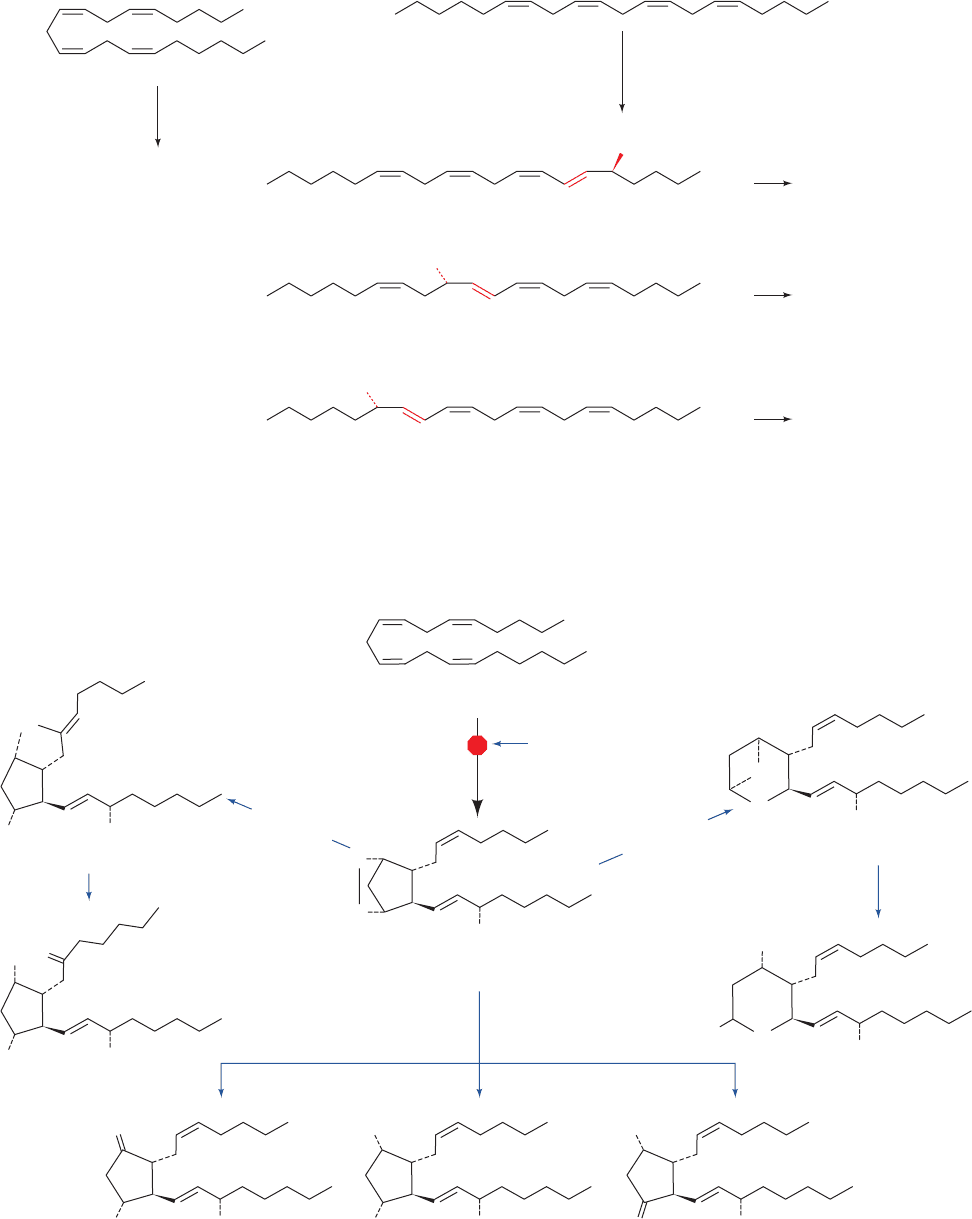
Arachidonic acid can be converted by a linear pathway
to several different hydroperoxyeicosatetraenoic acids
(HPETEs) by the 5-, 12-, and 15-lipoxygenases (5-, 12-,
and 15-LOs; Fig. 25-69). Hepoxilins are hydroxy epoxy de-
rivatives of 12-HPETE whose functions are not as yet well
understood. Lipoxins, the products of a second lipoxyge-
nase acting on 15-HPETE, are anti-inflammatory sub-
stances. Leukotrienes, derived from the 5-LO reaction, are
synthesized by a variety of white blood cells, mast cells
(connective tissue cells derived from the blood-forming
tissues that secrete substances which mediate inflamma-
tory and allergic reactions), as well as lung, spleen, brain,
and heart. Peptidoleukotrienes (LTC
4
,LTD
4
, and LTE
4
)
are now recognized to be the components of the slow re-
acting substances of anaphylaxis (SRS-A; anaphalaxis is a
violent and potentially fatal allergic reaction) released
from sensitized lung after immunological challenge. These
substances act at very low concentrations (as little as 10
10
M) to contract vascular, respiratory, and intestinal smooth
muscle. Peptidoleukotrienes, for example, are ⬃10,000-
fold more potent than histamine, a well-known stimulant
of allergic reactions. In the respiratory system, they con-
strict bronchi, especially the smaller airways; increase mu-
cus secretion; and are thought to be the mediators in
asthma.They are also implicated in immediate hypersensi-
tivity (allergic) reactions, inflammatory reactions, and
heart attacks.
a. Leukotriene Synthesis
The first two reactions in the conversion of arachidonic
acid to leukotrienes are both catalyzed by 5-LO, which con-
tains a nonheme, non-[Fe–S] cluster iron atom that must be
in its Fe(III) state to be active.These reactions occur as fol-
lows (Fig. 25-76):
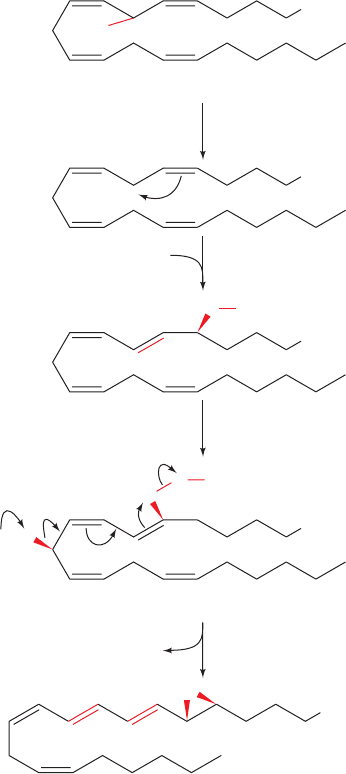
1. The oxidation of arachidonic acid to form 5-HPETE,
a substance that, in itself, is not a physiological mediator.
This reaction occurs in three steps:
(a) The active site iron atom, in its active Fe(III) state,
abstracts an electron from the central methylene
group of the 5,8-pentadiene moiety of arachidonate
and the resulting free radical loses a proton to an
enzymatic base.
(b) The free radical rearranges and adds O
2
to form a
hydroperoxide radical.
(c) The hydroperoxide radical reacts with the active
site iron, now in its Fe(II) form, to yield the hy-
droperoxide in its anionic form, which the enzyme
then protonates to yield the hydroperoxide product,
regenerating the active Fe(III) enzyme.
2. The base-catalyzed elimination of water to form the
unstable epoxide leukotriene A
4
(LTA
4
; the subscript indi-
cates the number of carbon–carbon double bonds in the
molecule, which is also its series number).
1000 Chapter 25. Lipid Metabolism
Figure 25-76 The 5-LO-catalyzed oxidation of arachidonic
acid to LTA
4
via the intermediate 5-HPETE.
OO•
COOH
Fe(III)–E
+
H
5-HPETE
Arachidonic acid
Leukotriene A
4
(LTA
4
)
COOH
Fe(II)–E–
•
Fe(II)–E–•
•
Fe(III)–E+
98 65
7
COOH
O
COOH
O
O
H
H
COOH
O
2
1(b)
1(a)
1(c)
2
BS
H
2
O
JWCL281_c25_940-1018.qxd 4/20/10 1:59 PM Page 1000
