Voet D., Voet Ju.G. Biochemistry
Подождите немного. Документ загружается.

Exit
3CMP
7
trans Golgi
Polypeptide
3UDP
6
871871
CHAPTER 23
Other Pathways of
Carbohydrate
Metabolism
1 Gluconeogenesis
A. The Gluconeogenesis Pathway
B. Regulation of Gluconeogenesis
C. The Cori Cycle
2 The Glyoxylate Cycle
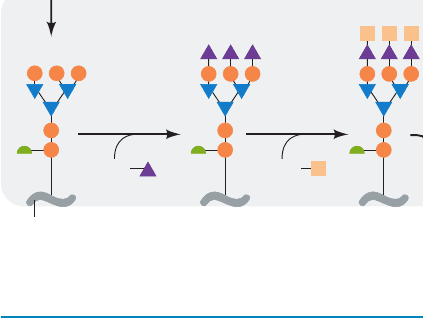
3 Biosynthesis of Oligosaccharides and Glycoproteins
A. Lactose Synthesis
B. Glycoprotein Synthesis
4 The Pentose Phosphate Pathway
A. Oxidative Reactions of NADPH Production
B. Isomerization and Epimerization of Ribulose-5-Phosphate
C. Carbon–Carbon Bond Cleavage and Formation Reactions
D. Control of the Pentose Phosphate Pathway
E. Glucose-6-Phosphate Dehydrogenase Deficiency
Heretofore, we have dealt with many aspects of carbohy-
drate metabolism. We have seen how the free energy of
glucose oxidation is sequestered in ATP through glycolysis,
the citric acid cycle, and oxidative phosphorylation. We
have also studied the mechanism by which glucose is stored
as glycogen for future use and how glycogen metabolism is
controlled in response to the needs of the organism. In this
chapter, we examine several other carbohydrate metabo-
lism pathways of importance:
1. Gluconeogenesis, through which noncarbohydrate
precursors such as lactate, pyruvate, glycerol, and amino
acids are converted to glucose.
2. The glyoxylate cycle, through which plants convert
acetyl-CoA to glucose.
3. Oligosaccharide and glycoprotein biosynthesis,
through which oligosaccharides are synthesized and added
to specific amino acid residues of proteins.
4. The pentose phosphate pathway, an alternate path-
way of glucose degradation, which generates NADPH, the
source of reducing equivalents in reductive biosynthesis,
and ribose-5-phosphate, the sugar precursor of the nucleic
acids.
This chapter completes our study of carbohydrate metabo-
lism in animals; photosynthesis, which occurs only in plants
and certain bacteria, is the subject of Chapter 24.
1 GLUCONEOGENESIS
Glucose occupies a central role in metabolism, both as a
fuel and as a precursor of essential structural carbohy-
drates and other biomolecules. The brain and red blood
cells are almost completely dependent on glucose as an en-
ergy source. Yet the liver’s capacity to store glycogen is
only sufficient to supply the brain with glucose for about
half a day under fasting or starvation conditions. Thus,
when fasting, most of the body’s glucose needs must be met
by gluconeogenesis (literally, new glucose synthesis), the
biosynthesis of glucose from noncarbohydrate precursors.
Indeed, isotopic labeling studies determining the source of
glucose in the blood during a fast showed that gluconeogen-
esis is responsible for 64% of total glucose production over
the first 22 hours of the fast and accounts for almost all the
glucose production by 46 hours.Thus, gluconeogenesis pro-
vides a substantial fraction of the glucose produced in fast-
ing humans, even after a few hours’ fast. Gluconeogenesis
occurs in liver and, to a smaller extent, in kidney.
The noncarbohydrate precursors that can be converted
to glucose include the glycolysis products lactate and pyru-
vate,citric acid cycle intermediates, and the carbon skeletons
of most amino acids. First, however, all these substances
must be converted to oxaloacetate, the starting material for
gluconeogenesis (Fig. 23-1). The only amino acids that can-
not be converted to oxaloacetate in animals are leucine
and lysine because their breakdown yields only acetyl-
CoA (Section 26-3F). There is no pathway in animals for
the net conversion of acetyl-CoA to oxaloacetate. Likewise,
fatty acids cannot serve as glucose precursors in animals
because most fatty acids are degraded completely to
acetyl-CoA (Section 25-2C). Unlike animals, however,
plants do contain a pathway for the conversion of acetyl-
CoA to oxaloacetate, the glyoxylate cycle (Section 23-2), so
that fatty acids can serve as a plant cell’s only carbon
source. Glycerol, a triacylglycerol breakdown product, is
converted to glucose via synthesis of the glycolytic inter-
mediate dihydroxyacetone phosphate, as described in
Section 25-1.
JWCL281_c23_871-900.qxd 6/8/10 9:43 AM Page 871
A. The Gluconeogenesis Pathway
Gluconeogenesis utilizes glycolytic enzymes. Yet three of
these enzymes, hexokinase, phosphofructokinase (PFK),
and pyruvate kinase, catalyze reactions with large negative
free energy changes in the direction of glycolysis. These
reactions must therefore be replaced in gluconeogenesis
by reactions that make glucose synthesis thermodynami-
cally favorable. Here, as in glycogen metabolism (Section
18-1D), we see the recurrent theme that biosynthetic and
degradative pathways differ in at least one reaction.This not
only permits both directions to be thermodynamically fa-
vorable under the same physiological conditions but allows
the pathways to be independently controlled so that one di-
rection can be activated while the other is inhibited.
a. Pyruvate Is Converted to Oxaloacetate before
Conversion to Phosphoenolpyruvate
The formation of phosphoenolpyruvate (PEP) from
pyruvate, the reverse of the pyruvate kinase reaction, is en-
dergonic and therefore requires free energy input. This is
accomplished by first converting the pyruvate to oxaloac-
etate. Oxaloacetate is a “high-energy” intermediate whose
exergonic decarboxylation provides the free energy neces-
sary for PEP synthesis. The process requires the participa-
tion of two enzymes (Fig. 23-2):
1. Pyruvate carboxylase catalyzes the ATP-driven for-
mation of oxaloacetate from pyruvate and .
2. PEP carboxykinase (PEPCK) converts oxaloacetate
HCO
3
872 Chapter 23. Other Pathways of Carbohydrate Metabolism
Figure 23-1 Pathways converting lactate, pyruvate, and citric
acid cycle intermediates to oxaloacetate. The carbon skeletons of
OO
CCH
2
–
OO
–
CC
O
C
–
O
O
–
O
O
–
CHCH C
O
C
–
O
CH
2
CH
2
C SCoA
O
O
–
C
O
O
O
O
O
C
–
O
CH
2
CH
2
C
O
Oxaloacetate
Acetyl-CoA
Citrate
Isocitrate
Succinyl-CoA
α-Ketoglutarate
Isoleucine
Methionine
Valine
Succinate
Phenylalanine
Tyrosine
Fumarate
Malate
Arginine
Glutamate
Glutamine
Histidine
Proline
Aspartate
Asparagine
Pyruvate
Alanine
Cysteine
Glycine
Serine
Threonine
Tryptophan
Lactate
Glucose
gluconeogenesis
CITRIC
ACID
CYCLE
H
3
CCC
all amino acids but leucine and lysine may be, at least in part,
converted to oxaloacetate and thus to glucose by these reactions.
JWCL281_c23_871-900.qxd 3/24/10 11:33 AM Page 872
Oxaloacetate
O
C CH
2
O
C
O
C
–
O O
–
Phosphoenol-
pyruvate (PEP)
CH
2
GDP + CO
2
GTP
O
C
O
CO
–
Pyruvate
CH
3
O
C
O
CO
–
PO
2
3
–
2
PEPCK
ADP + P
i
HCO
3
–
+ ATP
1
pyruvate
carboxylase
to PEP in a reaction that uses GTP as a phosphorylating
agent.
b. Pyruvate Carboxylase Has a Biotin
Prosthetic Group
Pyruvate carboxylase, discovered in 1959 by Merton
Utter, is a tetrameric protein of identical ⬃130-kD sub-
units, each of which has a biotin prosthetic group. Biotin
(Fig. 23-3a) functions as a CO
2
carrier by acquiring a car-
boxyl substituent at its ureido group (Fig. 23-3b). Biotin is
covalently bound to the enzyme by an amide linkage be-
tween the carboxyl group of its valerate side chain and the
ε-amino group of an enzyme Lys residue to form a biocytin
(alternatively, biotinyllysine) residue (Fig. 23-3b). The
biotin ring system is therefore at the end of a 16-Å-long
flexible arm, much like that of the lipoic acid prosthetic
group in the pyruvate dehydrogenase multienzyme com-
plex (Section 21-2Ac).
Biotin, which was first identified in 1935 as a growth fac-
tor in yeast, is an essential human nutrient. Its nutritional
deficiency is rare, however, because it occurs in many foods
and is synthesized by intestinal bacteria. Human biotin de-
ficiency almost always results from the consumption of
large quantities of raw eggs. This is because egg whites
contain a protein, avidin, that binds biotin so tightly (disso-
ciation constant, K ⫽ 10
⫺15
M) as to prevent its intestinal
absorption (cooked eggs do not cause this problem be-
cause cooking denatures avidin).The presence of avidin in
eggs is thought to inhibit the growth of microorganisms in
this highly nutritious environment. The avidin homolog
streptavidin, which is secreted by Streptomyces avidinii, is
used as a linking agent in numerous biotechnological appli-
cations (e.g., Section 22-3Ce) because of its particularly
high affinity for biotin.
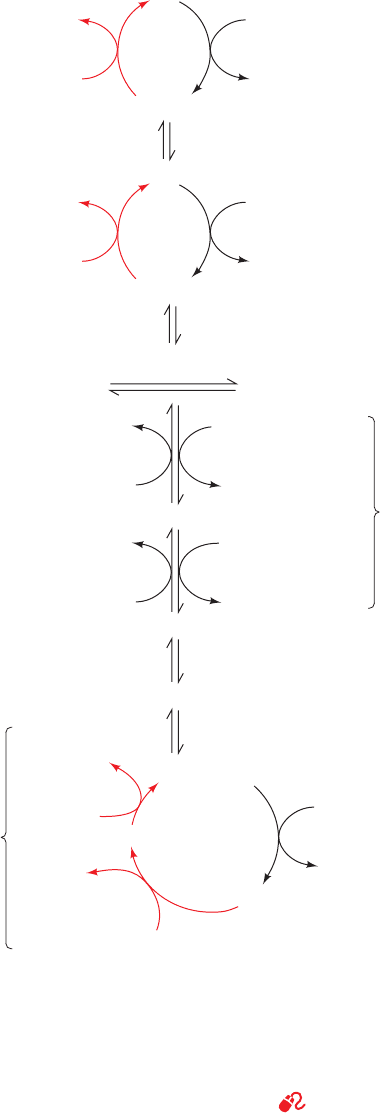
c. The Pyruvate Carboxylase Reaction
The pyruvate carboxylase reaction occurs in two phases
(Fig. 23-4):
Phase I Biotin is carboxylated at its N1 atom by bicar-
bonate ion in a three-step reaction in which the hydrolysis
of ATP to ADP ⫹ P
i
functions, via the intermediate forma-
tion of carboxyphosphate, to dehydrate bicarbonate. This
yields free CO
2
, which has sufficient free energy to carboxy-
late biotin.The resulting carboxyl group is activated relative
to bicarbonate (⌬G°¿ for its cleavage is ⫺19.7 kJ ⴢ mol
⫺1
)
and can therefore be transferred without further free
energy input.
Phase II The activated carboxyl group is transferred
from carboxybiotin to pyruvate in a three-step reaction to
form oxaloacetate.
These two reaction phases occur on different active sites of
the same enzyme.
d. Acetyl-CoA Regulates Pyruvate Carboxylase
Oxaloacetate synthesis is an anaplerotic (filling up) reac-
tion that increases citric acid cycle activity (Section 21-5b).
Accumulation of the citric acid cycle substrate acetyl-CoA
is therefore indicative of the need for more oxaloacetate.
Section 23-1. Gluconeogenesis 873
Figure 23-3 Biotin and carboxybiotinyl–enzyme.
(a) Biotin consists of an imidazoline ring that is cis-fused to a
tetrahydrothiophene ring bearing a valerate side chain.The
chirality at each of its three asymmetric centers is indicated.
Positions 1, 2, and 3 constitute a ureido group. (b) In
carboxybiotinyl–enzyme, N1 of the biotin ureido group is the
carboxylation site. Biotin is covalently attached to carboxylases
by an amide linkage between its valeryl carboxyl group and the
ε-amino group of an enzyme Lys side chain to form biocytin.
Figure 23-2 Conversion of pyruvate to oxaloacetate and then to phosphoenolpyruvate. The
enzymes involved are (1) pyruvate carboxylase and (2) PEP carboxykinase (PEPCK).
Carboxybiotinyl–enzyme
CH
2
CH
2
CH
2
CH
2
H
2
C
COO
–
(CH
2
)
4
O
CH
C
NH
Lys residue
(CH
2
)
4
O
C NH
C
–
O
N
O
NH
S
O
(b)
Biotin
Valerate side chain
HN
O
NH
S
(a)
C
C
CHH
H
C
6
5
1
4
3
2
JWCL281_c23_871-900.qxd 7/2/10 12:01 PM Page 873
C
OH
O
–
O
–
OOC O
C
CH
2
H
Adenosine
P
i
C
O
OO
+
Biotinyl–enzyme
Biotinyl–enzyme
Carboxybiotinyl–enzyme
Carboxybiotinyl–enzyme
Pyruvate enolate
Oxaloacetate
ATP Carboxyphosphate
OP
O
O
–
OP
O
O
–
P
O
O
–
O
–
HO P
O
O
–
+
ADP
Pyruvate
Biotinyl–enzyme
O
(CH
2
)
4
NH E(CH
2
)
4
C
O
C
O
O
C
O
O
C
O
C
O
O
NH
S
HN
O
(CH
2
)
4
NH E(CH
2
)
4
C
O
NH
S
NC
O
O
NHN
O
HN
C
O
O
–
O
–
O
–
–
OOC O
C
CH
2
H
–
OOC O
C
CH
2
–
OOC O
–
C
CH
2
+
+
+
NHN
O
–
Phase II
Phase I
..
–
..
NH
Indeed, acetyl-CoA is a powerful allosteric activator of
pyruvate carboxylase; the enzyme is all but inactive with-
out bound acetyl-CoA. If, however, the citric acid cycle is in-
hibited (by ATP and NADH, whose presence in high con-
centrations indicates a satisfied demand for oxidative
phosphorylation; Section 21-4), oxaloacetate instead under-
goes gluconeogenesis.
e. X-Ray Structure of Pyruvate Carboxylase
Suggests a Mechanism for Carboxyl Group Transfer
The X-ray structure of pyruvate carboxylase from the
soil bacterium Rhizobium etli in complex with ATPS and
ethyl-CoA (acetyl-CoA with an ethyl group in place of its
acetyl group), determined by Ivan Rayment, reveals that
each subunit of this homotetrameric, 1154-residue protein
consists of four domains (Fig. 23-5a): a biotin carboxylation
(BC) domain (residues 1–465) that carries out Phase I of
the pyruvate carboxylase reaction (Fig. 23-4); an allosteric
domain (residues 466–510 and 1001–1073) that binds
acetyl-CoA; a carboxyltransferase (CT) domain (residues
511–1000) that catalyzes Phase II of the pyruvate carboxy-
lase reaction (Fig. 23-4); and a domain named the biotin
carboxyl carrier protein (BCCP; residues 1074–1154) to
which the enzyme’s biotin prosthetic group is covalently
linked via Lys 1119 (Fig. 23-3).The active site of the BC do-
main is marked by its bound ATPS, the CT domain is
mainly an / barrel (viewed from the side in Fig. 23-5a)
whose active site is located in the mouth of the barrel and
is marked by its bound Zn
2
ion, and the allosteric domain
binds the ethyl-CoA, which like acetyl-CoA, activates the
enzyme.
The biotin prosthetic group on the BCCP domain is dis-
ordered and hence not visible. Nevertheless it is clear that
the ⬃80-Å distance between a subunit’s active sites is too
874 Chapter 23. Other Pathways of Carbohydrate Metabolism
Figure 23-4 Two-phase reaction mechanism of pyruvate
carboxylase. Phase I is a three-step reaction in which
carboxyphosphate is formed from bicarbonate and ATP, followed
by the generation of CO
2
on the enzyme, which then
carboxylates biotin. Phase II is a three-step reaction in which
CO
2
is produced at the active site via the elimination of the
biotinyl enzyme, which accepts a proton from pyruvate to
generate pyruvate enolate. This, in turn, nucleophilically attacks
the CO
2
, yielding oxaloacetate. [After Knowles, J.R., Annu. Rev.
Biochem. 58, 217 (1989).]
JWCL281_c23_871-900.qxd 3/24/10 11:33 AM Page 874
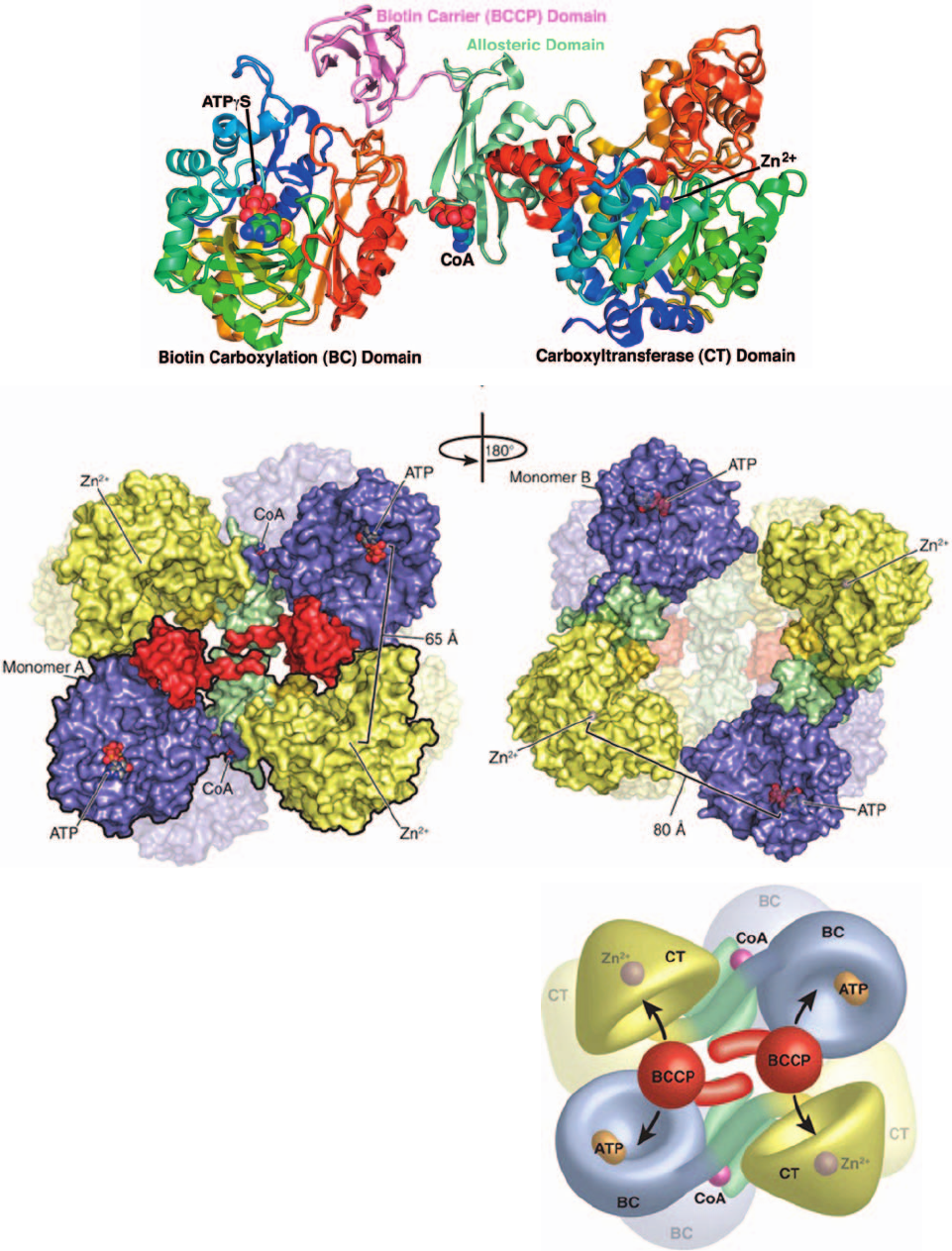
Section 23-1. Gluconeogenesis 875
Figure 23-5 X-ray structure of R. etli pyruvate carboxylase.
(a) Ribbon diagram in which the BC and CT domains are each
colored in rainbow order from their N-termini (blue) to their
C-termini (red), the allosteric domain is light green, and the
BCCP domain is pink.The ATP␥S bound at the active site of the
BC domain and the ethyl-CoA bound to the allosteric domain
(with only its nucleotide portion visible) are drawn in space-
filling form with ATP␥S C green, ethyl-CoA C cyan, N blue, O
red, and P orange.The Zn
2⫹
ion bound at the CT domain active
site is represented by a purple sphere. (b) Surface representation
of the tetramer viewed along its 2-fold axis with the two active
subunits closest to the viewer.The BC domain is purple, the
allosteric domain is light green, the CT domain is yellow, and the
BCCP domain is red. For clarity, one of the subunits is outlined
in black.The distance between the ATP␥S in the BC active site
and the Zn
2⫹
ion in the CT active site is 65 Å. (c) View relative to
Part b by a 180° rotation about the vertical axis.The top pair of
subunits have undergone a conformational change relative to
the top pair in Part b such that the ATP␥S–Zn
2⫹
distance
between neighboring subunits is 80 Å. In addition, the BCCP
domains in the top pair here are disordered. (d) Model of the
tetramer indicating how the BCCP domain transfers a carboxyl
group between the BC domain on the same subunit and the CT
domain of its neighboring subunit.The view and domain colors
are the same as in Part b. [Part a based on an X-ray structure by
and Parts b, c, and d courtesy of Ivan Rayment, University of
Wisconsin, Madison,Wisconsin. PDBid 2QF7.]
(a)
(b)
(c)
(d)
JWCL281_c23_871-900.qxd 6/16/10 1:54 PM Page 875
large to be bridged by the 16-Å-long carboxybiotinyl arm
(Fig. 23-3b). However, the BCCP domain is attached to the
enzyme by a flexible polypeptide linker that is 34 Å long,
much like that linking the lipoyl domain(s) to each dihy-
drolipoyl transacetylase (E2) subunit of the pyruvate dehy-
drogenase complex (Section 21-2Ae). Even so, it would re-
quire a dramatic movement of the entire BCCP domain to
transfer substrate between the two active sites of a single
subunit. How, then, does a BCCP domain translocate its
carboxybiotin group between the active sites of a BC do-
main and a CT domain?
Pyruvate carboxylase’s homotetrameric structure is re-
quired for its enzymatic activity; isolated subunits are cat-
alytically inactive. However, the tetramer has only 2-fold
symmetry because the top pair of subunits in Fig. 23-5b
differ in conformation from the top pair in Fig. 23-5c by a
40° rotation and a 40-Å translocation of the BC domain
relative to the CT domain of the same subunit. Indeed,
the BCCP domains in the top pair of subunits in Fig. 23-5c
are disordered, probably because the allosteric domains
on these subunits do not bind ethyl-CoA. Consequently,
the distance between active sites from adjacent subunits is
65 Å for the top pair in Fig. 23-5b, whereas it is 80 Å for
the top pair in Fig. 23-5c. This suggests the model drawn
in Fig. 23-5d in which each BCCP domain at the top of
Fig. 23-5b shuttles CO
2
in the form of carboxybiotin from
the active site of the BC domain on the same subunit to
the CT domain on the adjacent subunit, whereas the
other two subunits are inactive. This is an unusual exam-
ple of allosteric activation coupled with negative cooper-
ativity. It may permit pyruvate carboxylase to carry out
efficient catalysis in association with other metabolic
enzymes.
The foregoing model is supported by experiments
involving two mutant forms of pyruvate carboxylase:
K1119Q, which eliminates the biotinylation of the BC do-
main; and K718Q, which impairs the Phase II reaction.
Tetramers of each of these mutant subunits exhibited 0.1%
and 4% of the wild-type enzymatic activity, respectively.
However, mixed tetramers exhibit 20% activity, thus indi-
cating the formation of neighboring pairs of functional BC
and CT domains.
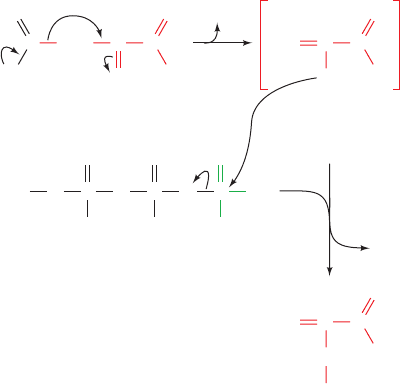
f. PEP Carboxykinase
PEPCK, a monomeric ⬃630-residue enzyme, catalyzes
the GTP-driven decarboxylation of oxaloacetate to form
PEP and GDP (Fig. 23-6). Note that the CO
2
that carboxy-
lates pyruvate to yield oxaloacetate is eliminated in the for-
mation of PEP. Oxaloacetate may therefore be considered
to be “activated” pyruvate, with CO
2
and biotin facilitating
the activation at the expense of ATP hydrolysis. Acetyl-
CoA is similarly activated for fatty acid biosynthesis
through such a carboxylation decarboxylation process
(forming malonyl-CoA; Section 25-4B). In general, -keto
acids may be considered “high-energy” compounds because
of the high free energy of decarboxylation of the -carboxyl
group. The enolates they generate are used to form
carbon–carbon bonds in fatty acid biosynthesis or phospho-
enolpyruvate here in gluconeogenesis.
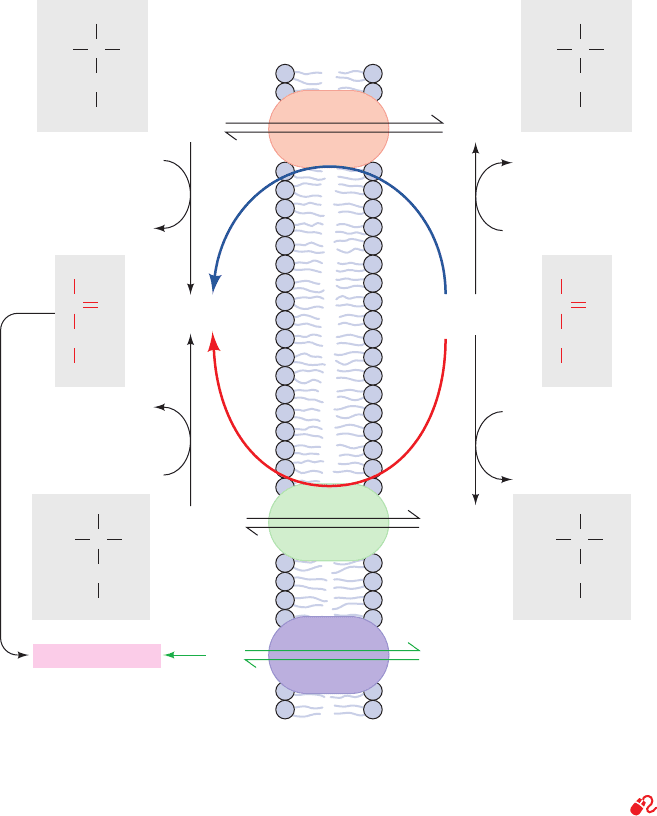
g. Gluconeogenesis Requires Metabolite Transport
between Mitochondria and Cytosol
The generation of oxaloacetate from pyruvate or citric
acid cycle intermediates occurs only in the mitochondrion,
whereas the enzymes that convert PEP to glucose are cy-
tosolic. The cellular location of PEPCK varies with the
species. In mouse and rat liver it is located almost exclu-
sively in the cytosol, in pigeon and rabbit liver it is mito-
chondrial, and in guinea pig and humans it is more or less
equally distributed between both compartments. In order
for gluconeogenesis to occur, either oxaloacetate must
leave the mitochondrion for conversion to PEP or the PEP
formed there must enter the cytosol.
PEP is transported across the mitochondrial membrane
by specific membrane transport proteins. There is, how-
ever, no such transport system for oxaloacetate. It must
first be converted either to aspartate (Fig. 23-7, Route 1) or
to malate (Fig. 23-7, Route 2), for which mitochondrial
transport systems exist (Section 22-1B).The difference be-
tween these two routes involves the transport of NADH
reducing equivalents. The malate dehydrogenase route
(Route 2) results in the transport of reducing equivalents
from the mitochondrion to the cytosol, since it utilizes mi-
tochondrial NADH and produces cytosolic NADH.The as-
partate aminotransferase route (Route 1) does not involve
NADH. Cytosolic NADH is required for gluconeogenesis
so, under most conditions, the route through malate is a ne-
cessity. If the gluconeogenic precursor is lactate, however
(Section 23-1C), its oxidation to pyruvate generates cytoso-
lic NADH, so that either transport route may then be used.
Of course, as we have seen, during oxidative metabolism
876 Chapter 23. Other Pathways of Carbohydrate Metabolism
O
CCC
H
2
O
–
O
–
O
CCC
H
2
O
–
O
O
O
OPGuanosine
O
–
O
OP
O
–
O
O
–
P
O
–
C
O
O
O
CC
CH
2
–
O
O
–
PO
2
3
–
Phosphoenolpyruvate
(PEP)
Pyruvate enolateOxaloacetate
GTP
CO
2
GDP
Figure 23-6 The PEPCK mechanism. Decarboxylation of
oxaloacetate (a -keto acid) forms a resonance-stabilized
enolate anion whose oxygen atom attacks the phosphoryl
group of GTP forming PEP and GDP.
JWCL281_c23_871-900.qxd 6/8/10 9:43 AM Page 876
the two routes may also alternate (with Route 2 reversed)
to form the malate–aspartate shuttle, which transports
NADH reducing equivalents into the mitochondrion (Sec-
tion 22-1Bc).
In the liver, where the urea cycle occurs (Section 26-2),
a third route, a modification of Route 1, may be followed
for transporting oxaloacetate into the cytosol. The aspar-
tate that enters the cytosol by Route 1 may be converted to
fumarate as part of the urea cycle (Fig. 26-8), instead of be-
ing transaminated. Fumarate is then hydrated to malate
and dehydrogenated to oxaloacetate by cytosolic equiva-
lents of citric acid cycle enzymes.This third route generates
cytosolic NADH in the same way as does Route 2.
h. Hydrolytic Reactions Bypass PFK and Hexokinase
The opposing pathways of gluconeogenesis and glycolysis
utilize many of the same enzymes (Fig. 23-8). However, the
free energy change is highly unfavorable in the gluconeogenic
direction at two other points in the pathway in addition to
the pyruvate kinase reaction: the PFK reaction and the
hexokinase reaction. At these points, instead of generat-
ing ATP by reversing the glycolytic reactions, FBP and G6P
are hydrolyzed, releasing P
i
in exergonic processes cat-
alyzed by fructose-1,6-bisphosphatase (FBPase) and
glucose-6-phosphatase, respectively. Glucose-6-phosphatase
is unique to liver and kidney, permitting them to supply
glucose to other tissues.
Because of the presence of separate gluconeogenic en-
zymes at the three irreversible steps in the glycolytic conver-
sion of glucose to pyruvate, both glycolysis and gluconeo-
genesis are rendered thermodynamically favorable. This is
accomplished at the expense of the free energy of hydrol-
ysis of two molecules each of ATP and GTP per molecule
of glucose synthesized by gluconeogenesis in addition to
that which would be consumed by the direct reversal of
glycolysis.
Section 23-1. Gluconeogenesis 877
COO
–
COO
–
C
CH
2
HHO
COO
–
COO
–
C
CH
2
HH
3
N
+
COO
–
COO
–
C
CH
2
HH
3
N
+
COO
–
COO
–
C
CH
2
HHO
COO
–
COO
–
C
CH
2
O
COO
–
COO
–
C
CH
2
O
Gluconeogenesis
Cytosol Mitochondrion
NAD
+
MalateMalate
Oxaloacetate
Route
1
Route
2
AspartateAspartate
Oxaloacetate
NADH + H
+
malate
dehydrogenase
Amino acid
α-Keto acid
aspartate
aminotransferase
PEPPEP
Amino acid
α-Keto acid
aspartate
aminotransferase
NAD
+
NADH + H
+
malate
dehydrogenase
Inner
mitochondrial
membrane
Figure 23-7 Transport of PEP and oxaloacetate from the
mitochondrion to the cytosol. PEP is directly transported
between these compartments. Oxaloacetate, however, must first
be converted to either aspartate through the action of aspartate
aminotransferase (Route 1) or to malate by malate
dehydrogenase (Route 2). Route 2 involves the mitochondrial
oxidation of NADH followed by the cytosolic reduction of
NAD
and therefore also transfers NADH reducing equivalents
from the mitochondrion to the cytosol.
See the Animated
Figures
JWCL281_c23_871-900.qxd 3/24/10 11:33 AM Page 877
NADH + H
+
NADH + H
+
NAD
+
+ P
i
Glucose
Glucose-6-phosphate
glucose-6-phos-
phatase
(–5.1)
hexokinase (32.9)
Fructose-6-phosphate
Fructose-1,6-bisphosphate
fructose bisphos-
phatase
(–8.6)
ATP
ADP
phosphofructokinase (24.5)
H
2
O
H
2
O
P
i
P
i
ATP
ADP
triose phosphate
isomerase
Glyceraldehyde-3-
phosphate
Dihydroxyacetone
phosphate
P
i
+ NAD
+
glyceraldehyde-3-phos-
phate dehydrogenase
1,3-Bisphosphoglycerate
ATP
ADP
phosphoglycerate
kinase
phosphoglucose isomerase (1.1)
aldolase (12.1)
3-Phosphoglycerate
phosphoglycerate mutase (1.3)
2-Phosphoglycerate
enolase (1.2)
ATP
ADP
Phosphoenolpyruvate
pyruvate kinase
(26.4)
ATP
ADP
Pyruvate
Oxaloacetate
GTP
CO
2
+ GDP
PEPCK
P
i
+ ADP
ATP
+ CO
2
pyruvate
carboxylase
1
2
3
3
(4.1)
(–22.6)
Glycolysis:
Gluconeogenesis:
Overall:
Such free energy losses in a cyclic process are thermody-
namically inescapable.They are the price that must be paid
to maintain independent regulation of the two pathways.
B. Regulation of Gluconeogenesis
If both glycolysis and gluconeogenesis were to proceed in
an uncontrolled manner, the net effect would be a futile
cycle wastefully hydrolyzing ATP and GTP. This does not
occur. Rather, these pathways are reciprocally regulated so
as to meet the needs of the organism. In the fed state, when
the blood glucose level is high, the liver is geared toward
fuel conservation: Glycogen is synthesized and the gly-
colytic pathway and pyruvate dehydrogenase are acti-
vated, breaking down glucose to acetyl-CoA for fatty acid
biosynthesis and fat storage. In the fasted state, however,
the liver maintains the blood glucose level both by glyco-
gen breakdown and by reversing the flux through glycol-
ysis toward gluconeogenesis [using mainly protein degra-
dation products via the glucose–alanine cycle (Section
26-1Ad) and glycerol from triacylglycerol hydrolysis
(Section 25-1e)].
a. Glycolysis and Gluconeogenesis Are Controlled
by Allosteric Interactions and Covalent Modifications
The rate and direction of glycolysis and gluconeogenesis
are controlled at the points in these pathways where the for-
ward and reverse directions can be independently regulated:
the reactions catalyzed by (1) hexokinase/glucose-6-
phosphatase, (2) PFK/FBPase,and (3) pyruvate kinase/pyru-
vate carboxylase–PEPCK (Fig. 23-8). Table 23-1 lists these
regulatory enzymes and their regulators. The dominant
mechanisms are allosteric interactions and cAMP-dependent
covalent modifications (phosphorylation/dephosphoryla-
tion; Section 18-3). cAMP-dependent covalent modification
renders this system sensitive to control by glucagon and
other hormones that alter cAMP levels.
One of the most important allosteric effectors involved
in the regulation of glycolysis and gluconeogenesis is
fructose-2,6-bisphosphate (F2,6P), which activates PFK
and inhibits FBPase (Section 18-3F). The concentration of
F2,6P is controlled by its rates of synthesis and breakdown
by phosphofructokinase-2 (PFK-2) and fructose bisphos-
phatase-2 (FBPase-2), respectively. Control of the activi-
ties of PFK-2 and FBPase-2 is therefore an important
aspect of gluconeogenic regulation even though these
2ATP ⫹ 2GTP ⫹ 4H
2
O
¡
2ADP ⫹ 2GDP ⫹ 4P
i
¡
glucose ⫹ 2NAD
⫹
⫹ 4ADP ⫹ 2GDP ⫹ 6P
i
2 Pyruvate ⫹ 2NADH ⫹ 4H
⫹
⫹ 4ATP ⫹ 2GTP ⫹ 6H
2
O
2 pyruvate ⫹ 2NADH ⫹ 4H
⫹
⫹ 2ATP ⫹ 2H
2
O
Glucose ⫹ 2NAD
⫹
⫹ 2ADP ⫹ 2P
i
¡
enzymes do not catalyze reactions of the pathway. PFK-2
and FBPase-2 activities, which occur on separate domains
of the same bifunctional enzyme, are subject to allosteric
878 Chapter 23. Other Pathways of Carbohydrate Metabolism
Figure 23-8 Pathways of gluconeogenesis and glycolysis. The
three numbered steps, which are catalyzed by different enzymes
in gluconeogenesis, have red arrows. The ⌬G’s for the reactions
in the direction of gluconeogenesis under physiological
conditions in liver are given in parentheses in kJ ⴢ mol
⫺1
.[⌬G’s
obtained from Newsholme, E.A. and Leech, A.R., Biochemistry
for the Medical Sciences, p. 448, Wiley (1983).]
See the
Animated Figures
JWCL281_c23_871-900.qxd 7/2/10 12:02 PM Page 878

regulation as well as control by covalent modifications
(Table 23-1). Low levels of blood glucose result in hor-
monal activation of gluconeogenesis through regulation of
[F2,6P] (Fig. 23-9).
Activation of gluconeogenesis in liver also involves in-
hibition of glycolysis at the level of pyruvate kinase. Liver
pyruvate kinase is inhibited both allosterically by alanine
(a pyruvate precursor; Section 26-1Ad) and by phosphory-
lation. Glycogen breakdown, in contrast, is stimulated by
phosphorylation (Section 18-3C). Both pathways then flow
toward G6P, which is converted to glucose for export to
muscle and brain. Muscle pyruvate kinase, an isozyme of
the liver enzyme, is not subject to these controls. Indeed,
such controls would be counterproductive in muscle since
this tissue lacks glucose-6-phosphatase and thus the ability
to synthesize glucose via gluconeogenesis.
b. PEPCK Concentration Is Transcriptionally
Controlled
PEPCK is the enzyme that catalyzes the first commit-
ted reaction of gluconeogenesis. It is therefore of interest
(Table 23-1) that PEPCK’s activity is controlled solely
through the transcriptional regulation of the gene encod-
ing it (transcriptional regulation is outlined in Section 5-
4Aa and discussed in detail in Sections 31-3 and 34-3). In
particular, the transcription of the PEPCK gene is stimu-
lated by glucagon, glucocorticoids, and thyroid hormones,
and is inhibited by insulin. For instance, the cAMP that is
produced in response to stimulation of the liver by
glucagon, in addition to its initiation of phosphorylation
cascades (Section 18-3), induces the transcription of the
PEPCK gene. Richard Hanson has shown that this occurs
because the PEPCK gene promoter (a control region that
precedes the transcriptional initiation site of genes en-
coding proteins; Section 5-4Aa) contains a specific DNA
sequence called the cAMP response element (CRE) that
is bound by a transcription factor named CRE binding
protein (CREB), but only when CREB is also binding
cAMP (recall that a transcription factor is a protein that
binds to a specific segment of its target promoter and, in
doing so, activates RNA polymerase to initiate the tran-
scription of the associated gene; Section 5-4Aa). How-
ever, the PEPCK gene promoter contains numerous
other binding sites for specific transcription factors.
Among them are the thyroid hormone response element
(TRE), which is bound by thyroid hormone receptor in
complex with thyroid hormone (Section 19-1D), and the
glucocorticoid hormone response element (GRE), which
is bound by the glucocorticoid receptor in complex with a
glucocorticoid hormone (Sections 19-1G and 34-3Bn). In
contrast, PEPCK gene transcription is strongly repressed
by protein factors phosphorylated by the PI3K signaling
cascade (Section 19-4D) initiated by the binding of in-
sulin to the insulin receptor (these protein factors may re-
press transcription by interfering with the binding of the
above transcription factors; the mechanism of insulin sig-
naling is discussed in Sections 19-3Ac,19-3Cg, and 19-4F).
The rate of PEPCK mRNA production is determined by
the integration of these various interactions and hence of
the signals that caused them.
Section 23-1. Gluconeogenesis 879
Table 23-1 Regulators of Gluconeogenic Enzyme Activity
Allosteric Allosteric Enzyme Protein
Enzyme Inhibitors Activators Phosphorylation Synthesis
PFK ATP, citrate AMP, F2,6P
FBPase AMP, F2,6P
Pyruvate kinase Alanine F1,6P Inactivates
Pyruvate carboxylase Acetyl-CoA
PEPCK Stimulated by glucagon, thyroid hormone,
and glucocorticoids, and inhibited by insulin
PFK-2 Citrate AMP, F6P, P
i
Inactivates
FBPase-2 F6P Glycerol-3-P Activates
Low blood [glucose]
Increased glucagon secretion
Increased [cAMP]
Increased enzyme phosphorylation
Activation of FBPase-2 and inactivation of PFK-2
Decreased [F2,6P]
Inhibition of PFK and activation of FBPase
Increased gluconeogenesis
Figure 23-9 Hormonal regulation of [F2,6P]. This process
activates gluconeogenesis in liver in response to low blood
[glucose].
JWCL281_c23_871-900.qxd 10/19/10 11:35 AM Page 879
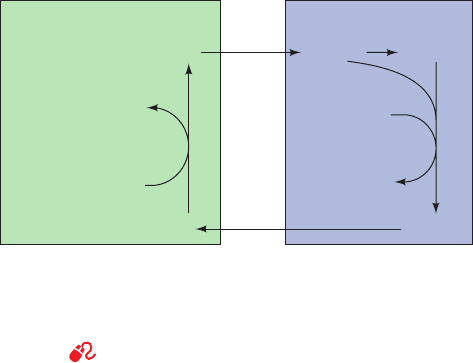
C. The Cori Cycle
Muscle contraction is powered by hydrolysis of ATP,
which is then regenerated through oxidative phosphoryla-
tion in the mitochondria of slow-twitch (red) muscle fibers
and by glycolysis yielding lactate in fast-twitch (white)
muscle fibers. Slow-twitch fibers also produce lactate
when ATP demand exceeds oxidative flux. The lactate is
transferred, via the bloodstream, to the liver, where it is re-
converted to pyruvate by lactate dehydrogenase and then
to glucose by gluconeogenesis. Thus, through the interme-
diacy of the bloodstream, liver and muscle participate in a
metabolic cycle known as the Cori cycle (Fig. 23-10) in
honor of Carl and Gerty Cori, who first described it.This is
the same ATP-consuming glycolysis/gluconeogenesis “fu-
tile cycle” we discussed above. Here, however, instead of
occurring in the same cell, the two pathways occur in dif-
ferent organs. Liver ATP is used to resynthesize glucose
from lactate produced in muscle. The resynthesized glu-
cose is returned to the muscle, where it is stored as glyco-
gen and used, on demand, to generate ATP for muscle
contraction. The ATP utilized by the liver for this process
is regenerated by oxidative phosphorylation. After vigor-
ous exertion, it often takes at least 30 min for all of the lac-
tate so produced to be converted to glycogen and the oxy-
gen consumption rate to return to its resting level, a
phenomenon known as oxygen debt.
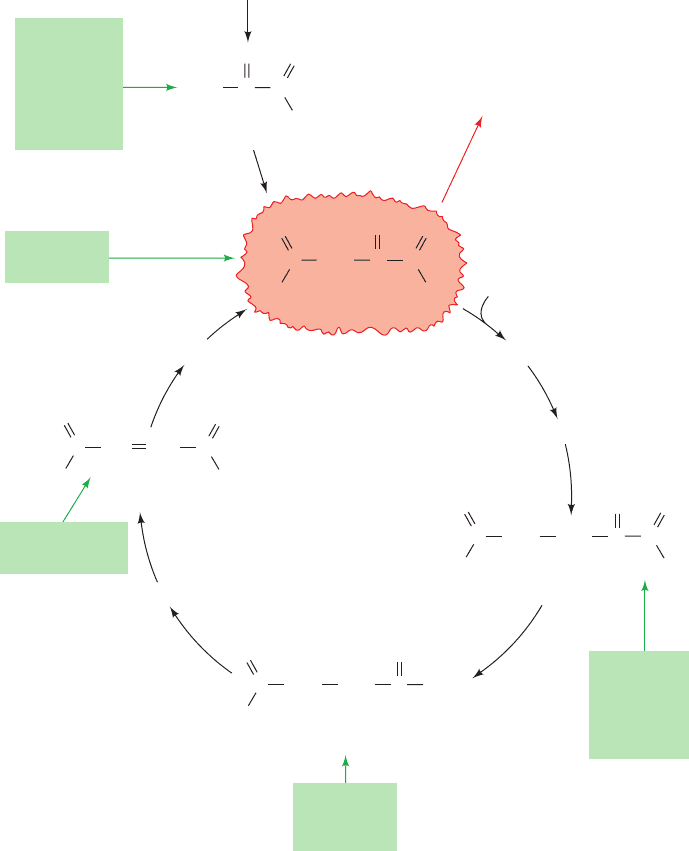
2 THE GLYOXYLATE CYCLE
Plants, but not animals, possess enzymes that mediate the
net conversion of acetyl-CoA to succinate, which is then
converted, via malate,to oxaloacetate.This is accomplished
via the glyoxylate cycle (Fig. 23-11), a pathway involving
enzymes of the glyoxysome (a membranous plant or-
ganelle; Section 1-2Ad). The glyoxylate cycle involves five
enzymes, three of which also participate in the citric acid cy-
cle: citrate synthase, aconitase, and malate dehydrogenase.
The two other enzymes, isocitrate lyase and malate syn-
thase, are unique to the cycle.
The glyoxalate cycle consists of five reactions (Fig. 23-11):
Reactions 1 and 2. Glyoxysomal oxaloacetate is con-
densed with acetyl-CoA to form citrate, which is isomer-
ized to isocitrate as in the citric acid cycle. Since the gly-
oxysome contains no aconitase, Reaction 2 presumably
takes place in the cytosol.
Reaction 3. Glyoxysomal isocitrate lyase cleaves the
isocitrate to succinate and glyoxylate (hence the cycle’s
name).
Reaction 4. Malate synthase, a glyoxysomal enzyme,
condenses glyoxylate with a second molecule of acetyl-
CoA to form malate.
Reaction 5. Glyoxysomal malate dehydrogenase cat-
alyzes the oxidation of malate to oxaloacetate by NAD
,
thereby completing the cycle.
The glyoxylate cycle therefore results in the net conversion
of two acetyl-CoA to succinate instead of to four molecules
of CO
2
, as would occur in the citric acid cycle.The succinate
produced in Reaction 3 is transported to the mitochon-
drion, where it enters the citric acid cycle and is converted
to malate, which has two alternative fates: (1) It can be con-
verted to oxaloacetate in the mitochondrion, continuing
the citric acid cycle and thereby making the glyoxylate
pathway an anaplerotic process (Section 21-5b); or (2) it
can be transported to the cytosol, where it is converted to
oxaloacetate for entry into gluconeogenesis.
The overall reaction of the glyoxylate cycle can be con-
sidered to be the formation of oxaloacetate from two mol-
ecules of acetyl-CoA.
Isocitrate lyase and malate synthase, the only enzymes of
the glyoxylate pathway unique to plants, enable germinat-
ing seeds to convert their stored triacylglycerols, through
acetyl-CoA, to glucose. It had long been assumed that this
was a requirement of germination. However, a mutant of
Arabidopsis thaliana (an oilseed plant) lacking isocitrate
lyase, and hence unable to convert lipids to carbohydrate,
nevertheless germinated. This process was only inhibited
when the mutant plants were subjected to low light condi-
tions. It therefore appears that the glyoxylate cycle’s im-
portance in seedling growth is its anaplerotic function in
providing 4-carbon units to the citric acid cycle, which can
then oxidize the triacylglycerol-derived acetyl-CoA.
3 BIOSYNTHESIS OF
OLIGOSACCHARIDES AND
GLYCOPROTEINS
Oligosaccharides consist of monosaccharide units joined
together by glycosidic bonds (linkages between C1, the
anomeric carbon, of one unit and an OH group of a second
oxaloacetate 2CoA 2NADH FADH
2
2H
2 Acetyl-CoA 2NAD
FAD
¡
880 Chapter 23. Other Pathways of Carbohydrate Metabolism
Figure 23-10 The Cori cycle. Lactate produced by muscle
glycolysis is transported by the bloodstream to the liver, where it
is converted to glucose by gluconeogenesis.The bloodstream
carries the glucose back to the muscles, where it may be stored as
glycogen.
See the Animated Figures
Liver Blood Muscle
Glucose
Lactate Lactate
Glucose Glycogen
ADP + GDP
ATP ATP
ADP
+ GTP
gluconeogenesis
P
i
P
i
+
+
glycogenolysis
and glycolysis
JWCL281_c23_871-900.qxd 3/24/10 11:33 AM Page 880
