Voet D., Voet Ju.G. Biochemistry
Подождите немного. Документ загружается.

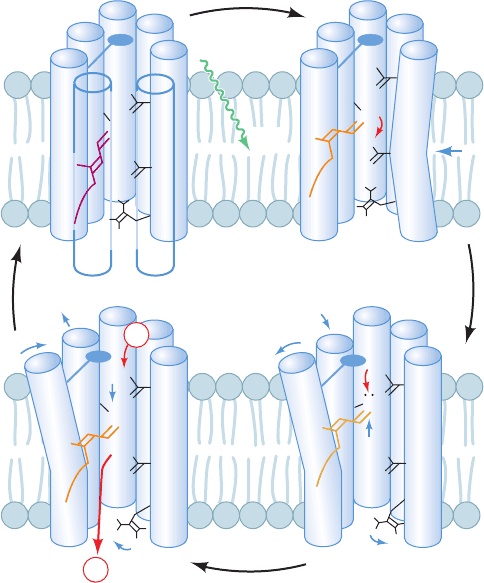
brings Asp 85 closer to the Schiff base N atom. The depro-
tonated retinal straightens and, in doing so, moves upward
(toward the inside) by 0.7 to 1.0 Å. It thereby pushes
against the F helix, causing its inside (cytoplasmic) end to
tilt outward from the channel by ⬃3.5 Å and the G helix to
partially replace it.
3. The movement of the F helix opens up the central
channel on the inside of the membrane, admitting several
water molecules that form a hydrogen bonded chain be-
tween Asp 96 and the Schiff base. One of these water mol-
ecules hydrogen bonds with Asp 96 so as to lower its pK.
This permits Asp 96 to protonate the Schiff base via the in-
termediacy of the chain of hydrogen bonded water mole-
cules, thereby yielding the N state.
4. Asp 96 is reprotonated by the cytoplasmic solution.
The loops forming bacteriorhodopsin’s inside surface bear
numerous charged residues and hence it appears that they
function as “antennas” to capture protons from the alka-
line cytoplasmic medium.Asp 85 transfers its proton to the
extracellular medium via a hydrogen bonded network that
includes several bound water molecules. This process is fa-
cilitated by a preceding 1.6-Å displacement of the Arg 82
side chain toward a complex of residues that includes Glu
194 and Glu 204, which reduces the pK of this complex.The
retinal then relaxes, via the O state, to its original all-trans
form and helices F and G return to their original positions,
thereby re-forming the ground state of the protein and
completing the catalytic cycle.
The retinal, which occupies the center of the protein chan-
nel, thereby acts as a one-way proton valve. The vectorial
nature of this process arises from the unidirectional series
of conformational changes made by the photoexcited reti-
nal as it relaxes to its ground state. The protein’s principal
proton pumping motions are remarkably small, involving
group movements of ⬃1 Å or less in response to the light-
induced flexing of the retinal.These,nevertheless, cause pK
changes in various residues that facilitate proton transfer as
well as making and breaking hydrogen bonded networks of
protein groups and water molecules in the proper sequence
to transport a proton.A similar mechanism is likely to oper-
ate in COX, but with its conformational changes motivated
by redox reactions rather than by photoexcitation.
i. COX Has Two Proton Translocation Channels
Two channels that are candidates for translocating pro-
tons from the inside to the vicinity of the O
2
-reducing cen-
ter have been described in bovine, P. denitrificans, and Rb.
sphaeroides COX (Fig. 22-35). These channels, which are
both contained in Subunit I, are named the K- and
D-channels after their respective key residues (K319 and
D91 in the bovine numbering scheme, which we shall use in
the subsequent discussion). Both putative channels are
similar in character to that present in bacteriorhodopsin in
that they consist of chains of hydrogen bonded and poten-
tially hydrogen bonded protein groups, bound water mole-
cules, and water-filled cavities.
The K-channel leads from K319, which is exposed to the
inside, to Y244, the residue that is the proposed substrate
electron and proton donor in the reaction forming the
P state (Step 4 of Fig. 22-27). The K319M mutant has an
extremely low activity (⬍0.05% of wild type), which is
not increased by supplying additional protons from the out-
side. Hence it appears that the K-channel is not connected
to the putative exit channel (Fig. 22-35) that leads to the
outside. It therefore seems likely that the K-channel only
functions to supply chemical protons to the O
2
-reduction
center.
The entrance to the D-channel is within a region on the
protein surface that appears likely to act as a proton-
gathering antenna. The mutation of D91 to any noncar-
boxylate residue eliminates proton pumping but reduces
the rate of O
2
reduction to only 45% of wild type (in
Section 22-3. Oxidative Phosphorylation 851
Figure 22-34 Proton pump of bacteriorhodopsin. See the text
for a description of this mechanism.The protein is represented
by its seven transmembrane helices,A through G (with helices D
and E omitted for clarity in all but the upper left panel), and
several mechanistically important side chains.The retinal is
drawn with the approximate color of the complex in its various
spectroscopically characterized states. Red arrows indicate
proton movements, blue arrows indicate the movements of
groups of atoms, and the “paddle” attached to helix F represents
the bulky side chains that must move aside to open the
cytoplasmic channel. [After Kühlbrandt,W., Nature 406, 569 (2000).]
A
B
C
G
F
E
D
C
G
F
C
G
F
C
G
F
Ground state
all trans retinal protonated
N intermediate
13-cis retinal protonated
Inside
Outside
Membrane
light
L intermediate
13-cis retinal protonated
Late M intermediate
13-cis retinal neutral
H
H
+
H
H
+
O
–
O
–
O
–
O
O
O
O
O
O
O
H
H
H
O
H
O
O
O
O
O
+
+
+
+
+
N
N
N
N
Asp 96
Asp 85
Arg 82
1
3
24
H
+
H
+
JWCL281_c22_823-870.qxd 7/2/10 11:11 AM Page 851
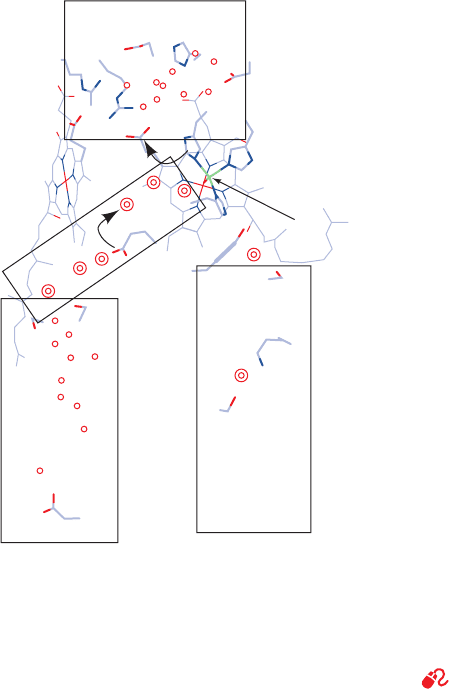
E. coli). Evidently, the D-channel, in series with the exit
channel, is the proton pumping channel. Moreover, the
D-channel, which extends to the vicinity of the heme
a
3
–Cu
B
binuclear center, is also the conduit for the chemi-
cal protons required for the second part of the reaction
cycle (Steps 5 and 6 of Fig. 22-27).
What is the mechanism that couples O
2
reduction to
proton pumping in COX? Unfortunately, X-ray crystallog-
raphy has provided little guidance in answering this ques-
tion because the X-ray structures of only a few different
states of COX have as yet been determined and because
the resolution of several of these structures is too low to re-
liably reveal small structural differences between them.
Consequently, the mechanisms that have been proposed to
explain how COX pumps protons are largely inferences
based on limited structural information, interpretation of
site-directed mutagenesis experiments, spectroscopic data,
theoretical considerations, and chemical intuition. Several
ingenious although largely phenomenological models of
how COX pumps protons have been proposed.These mod-
els agree that at least one proton is pumped during each of
Steps 5 and 6 in the COX reaction cycle (Fig. 22-27) but dis-
agree as to where in the reaction cycle the remaining two
protons are pumped and how the protein actuates this
process. Clearly there is still much to learn about how COX
carries out its function.
j. The Horizontal Helix of Complex I’s Peripheral
Arm Functions Like a Piston.
Complex I pumps four protons out of the matrix for
every electron pair it translocates from NADH to CoQ.
One of these protons appears to be transported at the in-
terface between the peripheral and transmembrane arms
of Complex I (Fig. 2-18) in a way that presumably is driven
by the conformational changes in the peripheral arm as it
translocates electrons from NADH to CoQ. But how are
these conformational changes coupled to the far more dis-
tant antiporter-like subunits, Nqo12, 13, and 14, which pre-
sumably pump one proton each out of the matrix? The low
resolution of the Complex I X-ray structure (Fig. 22-18)
precludes the visualization of the membrane arm’s side
chains and hence a detailed mechanism for proton trans-
port. However, the unusual 110-Å-long horizontal helix
that appears to link the antiporter-like subunits to the con-
formational changes in the peripheral arm has led Sazanov
to postulate that this helix functions like a piston to couple
conformational changes in the peripheral arm to those in
the antiporter-like channels.
C. Mechanism of ATP Synthesis
See Guided Exploration 21: F
1
F
0
–ATP synthase and the binding
change mechanism
The proton-motive force across the mito-
chondrial membrane is harnessed in the synthesis of ATP
by proton-translocating ATP synthase (also known as
F
1
F
0
–ATPase, Complex V, and F-type H
ⴙ
–ATPase). In the
following subsections we discuss the location and structure
of this ATP synthase and the mechanism by which it har-
nesses proton flux to drive ATP synthesis.
a. Proton-Translocating ATP Synthase Is a
Multisubunit Transmembrane Protein
Proton-translocating ATP synthase consists of two ma-
jor substructures comprising 8 to 13 different subunits.
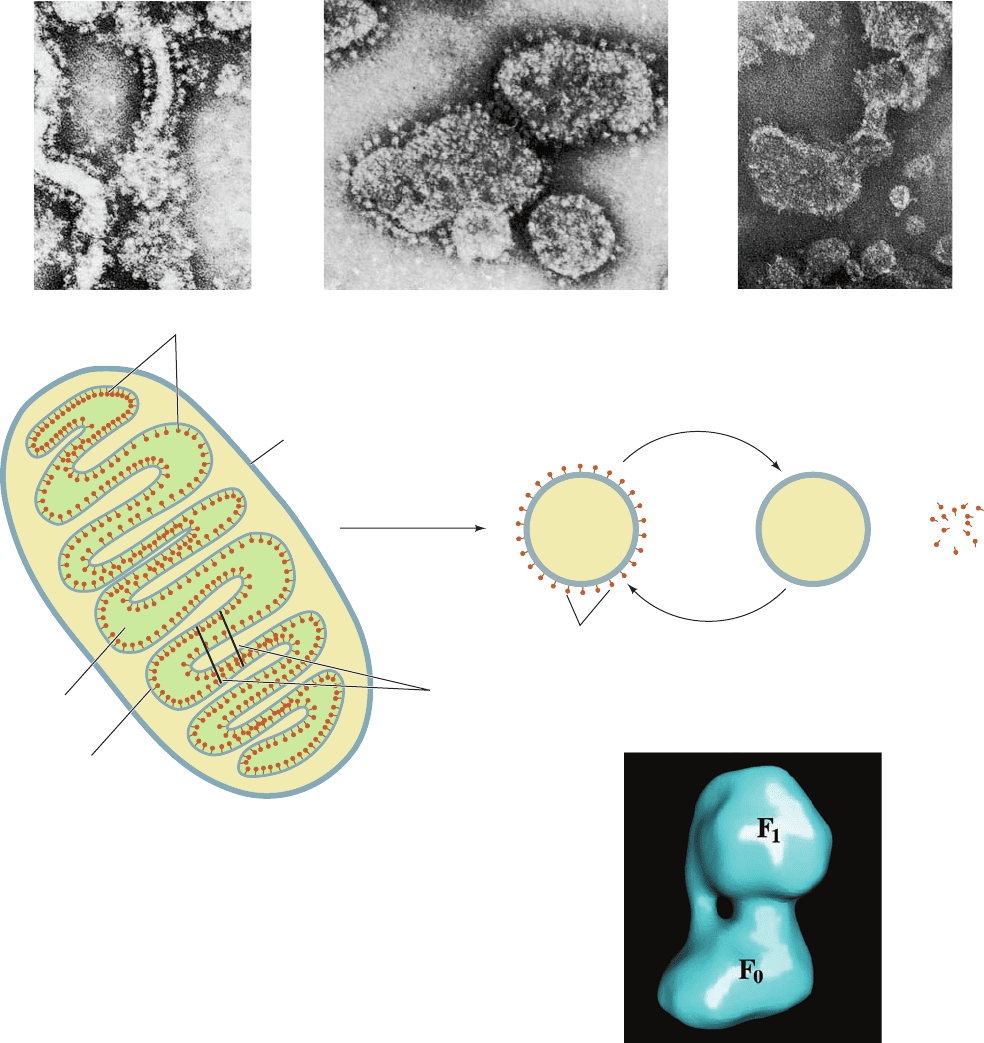
Electron micrographs of mitochondria (Fig. 22-36) show
lollipop-shaped structures studding the matrix surface of
the inner mitochondrial membrane (Fig. 22-36a). Similar
entities have been observed lining the inner surface of the
bacterial plasma membrane and in chloroplasts (Section
24-2Da). Sonication of the inner mitochondrial membrane
yields sealed vesicles, submitochondrial particles, from
which the “lollipops” project (Fig. 22-36b) and which can
carry out ATP synthesis.
Efraim Racker discovered that the proton-translocating
ATP synthase from submitochondrial particles comprises
two functional units, F
0
and F
1
.F
0
is a water-insoluble trans-
membrane protein composed of as many as eight different
types of subunits (although only three in E. coli) that con-
tains a proton translocation channel. F
1
is a water-soluble
852 Chapter 22. Electron Transport and Oxidative Phosphorylation
Outside
Inside
exit channel
heme a
heme a
3
copper B
hydrophobic
cavity
D-channel
K-channel
Asp 369
Asp 364
Arg 439
Arg 438
His 368
His 291
D
Tyr 244
Thr 316
Lys 319
Ser 255
Ser 157
Glu 242
Ser 156
Asp 91
Figure 22-35 The proton-translocating channels in bovine
COX. The enzyme is viewed parallel to the membrane with the
matrix below. The four rectangles delineate the proposed proton
entry and exit conduits. Single and double circles, respectively,
represent water molecules that are observed in X-ray structures
or that theoretical methods suggest are likely to be present.
[After a drawing by Mårten Wikström, University of Helsinki,
Helsinki, Finland.]
JWCL281_c22_823-870.qxd 7/5/10 10:27 AM Page 852
peripheral membrane protein, composed of five types of
subunits, that is easily dissociated from F
0
by treatment
with urea. Solubilized F
1
can hydrolyze ATP but cannot
synthesize it (hence the name ATPase). Submitochondrial
particles from which F
1
has been removed by urea treat-
ment no longer exhibit the lollipops in their electron mi-
crographs (Fig. 22-36c) and lack the ability to synthesize
ATP. If, however, F
1
is added back to these F
0
-containing
submitochondrial particles, their ability to synthesize ATP
is restored and their electron micrographs again exhibit the
lollipops. Thus the lollipops are the F
1
particles. Higher res-
olution cryoelectron micrograph–based images of the ATP
synthase from bovine heart mitochondria reveal that its F
1
and F
0
components are joined by both an ⬃50-Å-long cen-
tral stalk and a less substantial peripheral stalk (Fig. 22-37).
Certain bacterial F
1
F
0
–ATPases translocate Na
⫹
ions
rather than protons.
Section 22-3. Oxidative Phosphorylation 853
Figure 22-37 Cryoelectron microscopy–based image of
F
1
F
0
–ATPase from bovine heart mitochondria. [Courtesy of John
Rubinstein, University of Toronto, Canada, and John Walker and
Richard Henderson, MRC Laboratory of Molecular Biology,
Cambridge, U.K.]
Figure 22-36 Electron micrographs and interpretive drawings
of the mitochondrial membrane at various stages of dissection.
(a) Cristae from intact mitochondria showing their F
1
“lollipops”
projecting into the matrix. [From Parsons, D.F., Science 140, 985
(1963). Copyright © 1963 American Association for the
Advancement of Science. Used by permission.]
(b) Submitochondrial particles, showing their outwardly
projecting F
1
lollipops. Submitochondrial particles are prepared
by the sonication (ultrasonic disruption) of inner mitochondrial
membranes. [Courtesy of Peter Hinkle, Cornell University.]
(c) Submitochondrial particles after treatment with urea.
[Courtesy of Efraim Racker, Cornell University.]
F
1
particles
Outer
membrane
Matrix
Inner
membrane
sonication
urea
reconstitution
F
1
particles F
1
particles
Sonication
cleavage lines
Mitochondrion
Submitochondrial
particle
Membranous
vesicles
+
(a)
(b)
(c)
JWCL281_c22_823-870.qxd 7/2/10 11:11 AM Page 853
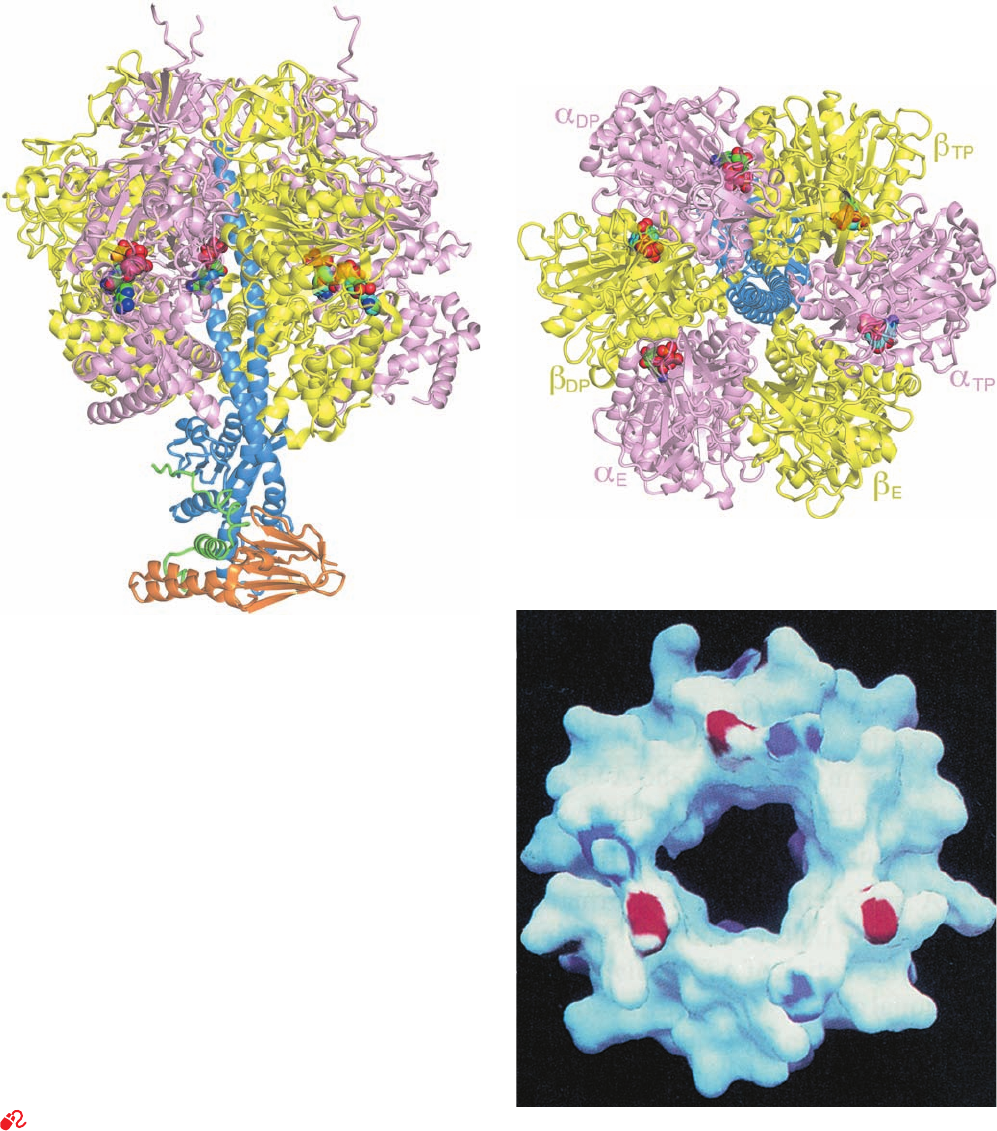
b. The X-Ray Structure of F
1
Reveals the
Basis of Its Lollipoplike Structure
Mitochondrial F
1
is an ␣
3

3
␥␦ε nonamer in which the 
subunits contain the catalytic sites for ATP synthesis and
the ␦ subunit is required for binding of F
1
to F
0
. The X-ray
structure of F
1
from bovine heart mitochondria, deter-
mined by John Walker and Andrew Leslie, reveals that this
⬃400-kD protein is a 100-Å-high and 100-Å-wide spheroid
that is mounted on a 50-Å-long stem (Fig. 22-38a,b). F
1
’s ␣
and  subunits (553 and 528 residues), which are 20% iden-
tical in sequence and have nearly identical folds, are
arranged alternately, like the segments of an orange, about
the upper portion of a 114-Å-long, 74-residue ␣ helix
formed by the ␥ subunit (298 residues). The C-terminus of
this helix protrudes into a 15-Å-deep dimple that is cen-
trally located at the top of the spheroid. The lower half of
854 Chapter 22. Electron Transport and Oxidative Phosphorylation
Figure 22-38 X-ray structures of F
1
–ATPase from bovine
heart mitochondria. (a) F
1
–ATPase in complex with ADP and
ATP drawn as a semitransparent ribbon diagram viewed parallel
to the membrane with the matrix above. The ␣, , ␥, ␦, and ε
subunits are pink, yellow, blue, orange, and green, respectively,
and the nucleotides are shown in space-filling form with ATP C
green,ADP C cyan, N blue, O red, and P orange. (b) The
structure in Part a rotated 90° about the horizontal axis such that
the protein is viewed along its pseudo-3-fold axis from the
matrix.The ␦ and ε subunits have been deleted for clarity. Note
that although the ␣
DP
and 
DP
subunits both normally bind ATP,
in this structure they have bound ADP. (c) A surface diagram of
the inner portion of the ␣
3

3
assembly through which the
C-terminal helix of the ␥ subunit penetrates as viewed from the
matrix.The surface is colored according to its electrical potential,
with positive potentials blue, negative potentials red, and neutral
white. Note the absence of charge on the inner surface of this
sleeve. That part of the ␥ subunit’s C-terminal helix which
contacts this sleeve is similarly devoid of charge. [Parts a and b
based on an X-ray structure by Andrew Leslie and John Walker,
MRC Laboratory of Molecular Biology, Cambridge, U.K. PDBid
1E79. Part c from Abrahams, J.P., Leslie,A.G.W., Lutter, R., and
Walker, J.E., Nature 370, 621 (1994). PDBid 1BMF.]
See Interactive Exercise 19
(a)
(b)
(c)
JWCL281_c22_823-870.qxd 10/19/10 8:08 AM Page 854
the helix forms a bent left-handed antiparallel coiled coil
with the N-terminal segment of the ␥ subunit. This coiled
coil forms much of the ⬃50-Å-long central stalk seen in
cryoEM-based images of the F
1
F
0
–ATPase such as Fig.22-37.
The cyclical arrangement and structural similarities of
F
1
’s ␣ and  subunits gives it both pseudo-3-fold and
pseudo-6-fold rotational symmetry. Nevertheless, the pro-
tein is asymmetric.This is in part due to the presence of the
␥, ␦, and ε subunits but, more importantly, because each of
the ␣ and  subunits have a somewhat different conforma-
tion.Thus, one  subunit (designated 
TP
) normally binds a
molecule of ATP, the second (
DP
) binds ADP, and the
third (
E
) has an empty and distorted binding site. The ␣
subunits all normally bind ATP, although they also differ
conformationally from one another (however, ␣
TP
and 
TP
both bind ADP in Fig. 22-38a,b). The ATP and ADP bind-
ing sites each lie at a radius of ⬃20 Å near an interface be-
tween adjacent ␣ and  subunits and, in fact, all incorpo-
rate a few residues from the adjacent subunit. The ␣ and 
subunits both contain two sequence motifs, the Walker A
motif (GXXXXGKT/S, where X is any residue) and the
Walker B motif (R/KXXXGXXL/VhhD, where h is a hy-
drophobic residue), that participate in ATP binding and
which occur widely in nucleotide-binding proteins of all
descriptions.
The ␦ and ε subunits (168 and 51 residues) are wrapped
about the base of the ␥ subunit’s coiled coil. The X-ray
structure of E. coli F
1
resembles its bovine counterpart.
Note, however, that in an unfortunate confusion of nomen-
clature, the E. coli ε subunit is the homolog of the mito-
chondrial ␦ subunit; the E. coli ␦ subunit is the counterpart
of the mitochondrial oligomycin-sensitivity conferral pro-
tein (OSCP; see Problem 11), and the mitochondrial ε sub-
unit has no counterpart in either bacterial or chloroplast
ATP synthases.
c. The c Subunits of F
0
Form a Transmembrane Ring
The F
0
component of the F
1
F
0
–ATPase from E. coli con-
sists of three transmembrane subunits, a, b, and c (271, 161,
and 89 residues) that form an a
1
b
2
c
12
complex. However,
the number c subunits per F
0
assembly varies from 10 to 15,
depending on the species. Mitochondrial F
0
additionally
contains one copy each of three different subunits, d, F
6
,
and OSCP (256, 108, and 213 residues), as well as several
“minor” subunits, e, f, g, and A6L, of unknown function.
Subunits a and A6L are encoded by mitochondrial genes.
A variety of evidence indicates that the hydrophobic c sub-
units associate to form a ring with the ab
2
unit located at its
periphery (see below). The sequence of the a subunit sug-
gests that this highly hydrophobic peptide forms five trans-
membrane helices.
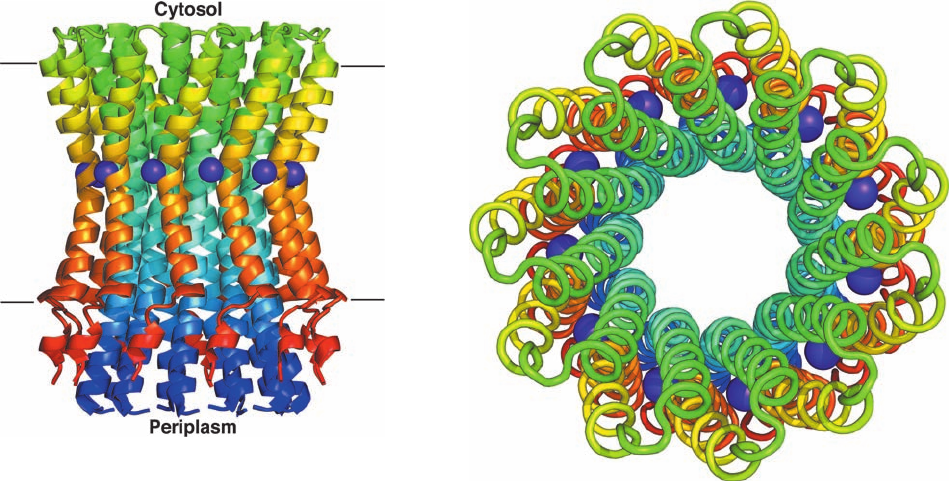
The X-ray structure of the c
11
unit from the Na
⫹
-
translocating F
1
F
0
–ATPase of the gram-negative bac-
terium Ilyobacter tartaricus, determined by Peter Dim-
roth, reveals an 11-fold symmetric cylindrical assembly
that is 70 Å high and ⬃50 Å in diameter (Fig. 22-39). Each
of its identical, largely hydrophobic subunits consists al-
most entirely of an inner helix that defines the length of
the cylinder and an outer helix that is somewhat shorter.
Both helices are bent in the vicinity of the Na
⫹
ion-binding
Section 22-3. Oxidative Phosphorylation 855
Figure 22-39 X-ray structure of the c
11
assembly of the I.
tartaricus Na
⫹
-translocating F
1
F
0
–ATPase. (a) Ribbon diagram
as viewed along the plane of the plasma membrane with the
cytosol above. Each subunit is colored in rainbow order from its
N-terminus (blue) to its C-terminus (red). Bound Na
⫹
ions are
represented by purple spheres.The parallel lines delineate the
inferred position of the plasma membrane. (b) View from the
cytosol parallel to the assembly’s 11-fold axis in which each
subunit is drawn in worm form and colored as in Part a.The
minimally ⬃20-Å-diameter central channel presumably contains
a lipid bilayer. [Based on an X-ray structure by Peter Dimroth,
ETH, Zürich, Switzerland. PDBid 1YCE.]
(a)
(b)
JWCL281_c22_823-870.qxd 7/2/10 11:11 AM Page 855
site, thereby conferring an hourglasslike shape on the c
11
assembly (Fig. 22-39a). Each Na
⫹
ion is liganded by
residues from both helices of a given subunit as well as
those of the clockwise neighboring outer helix as seen in
Fig. 22-29b.
In vertebrates, the peripheral stalk (Fig. 22-37) consists
of the OSCP, b, d, and F
6
subunits, whereas in most
prokaryotes, it consists of the OSCP homolog ␦ and two
copies of b. The X-ray structure of most of the cytosolic
portion of a complex of bovine subunits b, d, and F
6
, con-
stituting 54% of the extramembrane segment of the pe-
ripheral stalk, determined by Leslie and Walker, reveals
that this b fragment forms a 160-Å-long and curved ␣ he-
lix, and that the other subunit fragments are also mainly
helical (Fig. 22-40). The curvature of this assembly closely
matches that of the peripheral stalk in cryoEM-based im-
ages of the F
1
F
0
–ATPase (e.g., Fig. 22-37). Such cryoEM-
based images, together with the foregoing X-ray structures
and the NMR structures of ␦ and the transmembrane seg-
ment of b, both from E. coli, has enabled the construction
of a composite model of the E. coli F
1
F
0
–ATPase (Fig. 22-
41), which, of course, resembles the mitochondrial assem-
bly. Note the extensive contacts between ␥ and ε and the
top of the c cylinder.
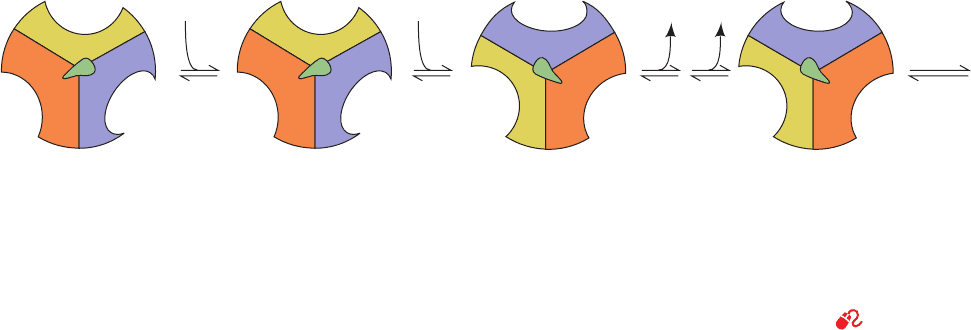
d. The Binding Change Mechanism:
Proton-Translocating ATP Synthase Is
Driven by Conformational Changes
The mechanism of ATP synthesis by proton-translocating
ATP synthase can be conceptually broken down into three
phases:
1. Translocation of protons carried out by F
0
.
2. Catalysis of formation of the phosphoanhydride
bond of ATP carried out by F
1
.
3. Coupling of the dissipation of the proton gradient
with ATP synthesis, which requires interaction of F
1
and F
0
.
The available evidence supports a mechanism for ATP
formation, proposed by Boyer, that resembles the confor-
mational coupling hypothesis of oxidative phosphorylation
(Section 22-3A). However, the conformational changes in
the ATP synthase that power ATP formation are generated
by proton translocation rather than by direct electron
transfer,as proposed in the original formulation of the con-
formational coupling hypothesis.
F
1
is proposed to have three interacting catalytic pro-
tomers, each in a different conformational state: one that
binds substrates and products loosely (L state), one that
binds them tightly (T state), and one that does not bind
them at all (open or O state). The free energy released on
proton translocation is harnessed to interconvert these
three states.The phosphoanhydride bond of ATP is synthe-
sized only in the T state and ATP is released only in the O
state. The reaction involves three steps (Fig. 22-42):
1. Binding of ADP and P
i
to the “loose” (L) binding
site.
2. A free energy–driven conformational change that
converts the L site to a “tight” (T) binding site that cat-
alyzes the formation of ATP.This step also involves confor-
mational changes of the other two subunits that convert
856 Chapter 22. Electron Transport and Oxidative Phosphorylation
Figure 22-40 X-ray structure of a portion of the peripheral
stalk of bovine F
1
F
0
–ATPase. This protein fragment is drawn in
ribbon form embedded in its semitransparent molecular surface,
with b (residues 79–183) magenta, d (residues 3–123) cyan, and
F
6
(residues 5–70) orange. The N- and C-termini of each subunit
are indicated. [Based on an X-ray structure by Andrew Leslie
and John Walker, MRC Laboratory of Molecular Biology,
Cambridge, U.K. PDBid 2CLY.]
Figure 22-41 A composite model of the E. coli F
1
F
0
–ATPase.
This model is based on the X-ray structure of the E. coli F
1
subunit (PDBid 1JNV), which resembles that of bovine F
1
(Fig. 22-38), the structures displayed in Figs. 22-39 and 22-40, and
the NMR structures of ␦ and the transmembrane segment of the
b from E. coli (PDBids 2A7U and 1B9U). The structures of a
and the so-called hinge region of b are unknown. [Courtesy of
Peter Dimroth, ETH, Zürich, Switzerland.]
JWCL281_c22_823-870.qxd 7/2/10 11:11 AM Page 856
the ATP-containing T site to an “open” (O) site and con-
vert the O site to an L site.
3. ATP is synthesized at the T site on one subunit while
ATP dissociates from the O site on another subunit. On the
surface of the active site, the formation of ATP from ADP
and P
i
entails little free energy change, that is, the reaction
is essentially at equilibrium. Consequently, the free energy
supplied by the proton flow primarily facilitates the release
of the newly synthesized ATP from the enzyme; that is, it
drives the T S O transition, thereby disrupting the
enzyme–ATP interactions that had previously promoted
the spontaneous formation of ATP from ADP ⫹ P
i
in the
T site.
How is the free energy of proton transfer coupled to
the synthesis of ATP? Boyer proposed that the binding
changes are driven by the rotation of the catalytic assembly,
␣
3

3
with respect to other portions of the F
1
F
0
–ATPase.This
hypothesis is supported by the X-ray structure of F
1
.Thus,
the closely fitting nearly circular arrangement of the ␣
and  subunits’ inner surface about the ␥ subunit’s helical
C-terminus is reminiscent of a cylindrical bearing rotating
in a sleeve (Fig. 22-38c). Indeed, the contacting hydropho-
bic surfaces in this assembly are devoid of the hydrogen
bonding and ionic interactions that would interfere with
their free rotation; that is, the bearing and sleeve appear
to be “lubricated.” Moreover, the central cavity in the
␣
3

3
assembly (Fig. 22-38a) would permit the passage of
the ␥ subunit’s N-terminal helix within the core of this
particle during rotation. Finally, the conformational dif-
ferences between F
1
’s three catalytic sites appear to be
correlated with the rotational position of the ␥ subunit.
Apparently the ␥ subunit, which is thought to rotate within
the fixed ␣
3

3
assembly, acts as a molecular cam shaft in
linking the proton-motive force–driven rotational motor to
the conformational changes in the catalytic sites of F
1
.This
concept is also supported by molecular dynamics simula-
tions (Section 9-4) by Leslie, Walker, and Martin Karplus,
which indicate that the conformational changes in the 
subunits arise from both steric and electrostatic interac-
tions with the rotating ␥ subunit.
Rotating assemblies are not unprecedented in biologi-
cal systems. Bacterial flagella, which function as propellers,
had previously been shown to be membrane-mounted ro-
tary engines that are driven by the discharge of a proton
gradient (Section 35-3Ib).
e. The F
1
F
0
–ATPase Is a Rotary Engine
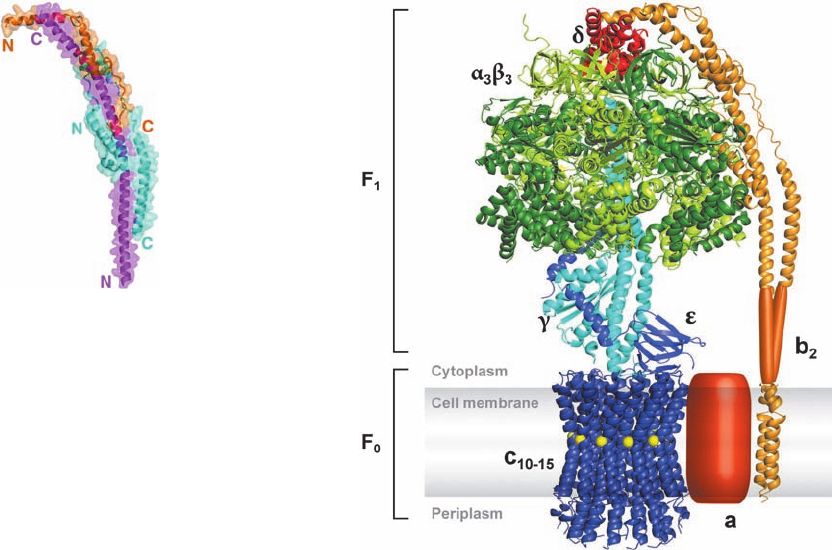
In the F
1
F
0
–ATPase, the rotor is proposed to be an as-
sembly of the c ring and its associated ␥ and (E. coli) ε sub-
units, whereas the ab
2
unit and the (E. coli) ␦ subunit to-
gether with the ␣
3

3
spheroid form the stator (Fig. 22-41).
The rotation of the c ring in the membrane relative to the
stationary a subunit is driven by the migration of protons
from the outside to the inside, as we discuss below. The pe-
ripheral arm (b
2
␦) presumably functions to hold the ␣
3

3
spheroid in place while the ␥ subunit rotates inside it.
The rotation of the E. coli ␥ε–c-ring rotor with respect
to the ab
2
␦–␣
3

3
stator has been ingeniously demonstrated
by Masamitsu Futai using techniques developed by
Kazuhiko Kinosita Jr. and Masasuke Yoshida (Fig. 22-43a).
The ␣
3

3
spheroid of E. coli F
1
F
0
–ATPase was fixed, head
down, to a glass surface as follows: Six consecutive His
residues (a so-called His Tag; Section 6-3Dg) were muta-
genically appended to the N-terminus of the ␣ subunit,which
is located at the top of the ␣
3

3
spheroid as it is drawn in
Fig. 22-38a. The His-tagged assembly was applied to a glass
surface coated with horseradish peroxidase (which, like most
proteins, sticks to glass) conjugated with Ni
2⫹
-nitriloacetic
acid [N(CH
2
COOH)
3
, which tightly binds His tags], thereby
binding the F
1
F
0
–ATPase with its F
0
side facing away from
the surface. The Glu 2 residues of this assembly’s c sub-
units, which are located on the side of the c ring facing away
from F
1
, had been mutagenically replaced by Cys residues,
which were then covalently linked to biotin (a coenzyme
that normally participates in carboxylation reactions; Sec-
tion 23-1Ab). A fluorescently labeled and biotinylated
(at one end) filament of the muscle protein actin (Section
35-3Ac) was then attached to the c subunit through the ad-
dition of a bridging molecule of streptavidin, a protein that
avidly binds biotin to each of four binding sites (Cys 193 of
Section 22-3. Oxidative Phosphorylation 857
Figure 22-42 Energy-dependent binding change mechanism
for ATP synthesis by proton-translocating ATP synthase. F
1
has
three chemically identical but conformationally distinct
interacting ␣ protomers: O, the open conformation, has very
low affinity for ligands and is catalytically inactive; L has loose
binding for ligands and is catalytically inactive;T has tight
binding for ligands and is catalytically active. ATP synthesis
occurs in three steps. (1) Binding of ADP and P
i
to site L.
(2) Energy-dependent conformational change converting
12
L
T
ADP + P
i
Energy
3
ATP H
2
O
ADP • P
i
ADP • P
i
ATP
ATP
ATP
O
L
T O
T
O L
T
ATP
O L
binding site L to T,T to O, and O to L. (3) Synthesis of ATP at
site T and release of ATP from site O. The enzyme returns to its
initial state after two more passes of this reaction sequence. The
energy that drives the conformational change is apparently
transmitted to the catalytic ␣
3

3
assembly via the rotation of the
␥ε assembly (in E. coli; ␥␦ε in mitochondria), here represented
by the centrally located asymmetric pointer (green). [After Cross,
R.L., Annu. Rev. Biochem. 50, 687 (1980).]
See the Animated
Figures
JWCL281_c22_823-870.qxd 7/20/10 6:25 PM Page 857
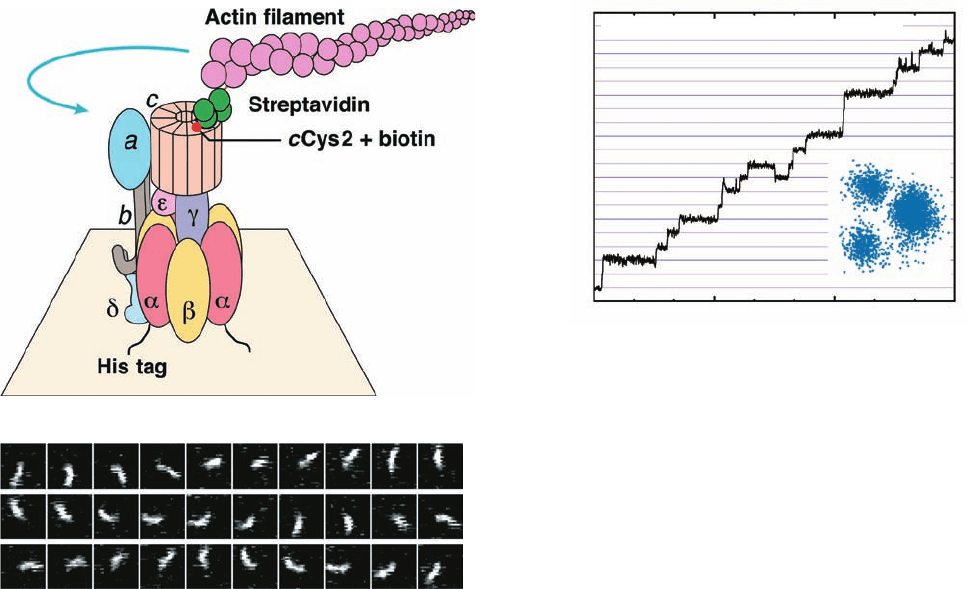
the ␥ subunit, the only other Cys residue in the rotor, was
mutagenically replaced by Ala to prevent it from being
linked to an actin filament).
E. coli F
1
F
0
–ATPase can work in reverse, that is, it can
pump protons from the inside (cytoplasm) to the outside
(periplasm) at the expense of ATP hydrolysis (this enables
the bacterium to maintain its proton gradient under anaer-
obic conditions, which it uses to drive various processes
such as flagellar rotation). Thus, the foregoing preparation
was observed under a fluorescence microscope as a 5 mM
MgATP solution was infused over it. Many of the actin fila-
ments were seen to rotate (Fig. 22-43b), and always in a
counterclockwise direction when viewed looking down on
the glass surface (from the outside). This would permit the ␥
subunit to sequentially interact with the  subunits in the
direction
(Figs. 22-38b and 22-42), the direction expected for ATP
hydrolysis.

E
(O state)
¡

DP
(L state)
¡

TP
(T state)
In a variation of the above experiment, the ␥ subunit of
the ␣
3

3
␥ complex was directly cross-linked, via its Cys 193,
to a fluorescently labeled actin filament and, in this case,
the  subunits were immobilized by appended His tags. At
very low ATP concentrations (e.g., 0.02 M), video images
(Fig. 22-44) revealed that the fluorescent actin filament ro-
tated counterclockwise in discrete steps of 120°, as the
binding change mechanism predicts. Moreover, the calcu-
lated frictional work done in each rotational step is very
nearly equal to the energy available from the hydrolysis of
one ATP molecule, that is, the F
1
F
0
–ATPase converts chem-
ical to mechanical energy with nearly 100% efficiency.
The foregoing system also works in reverse. An ⬃0.7-
m-diameter magnetic bead that was coated with strepta-
vidin was attached to the biotinylated ␥ subunit of an im-
mobilized ␣
3

3
␥ complex.When the resulting assembly was
placed in a rotating magnetic field in the presence of ADP
and P
i
,ATP was produced when the magnetic field rotated
in the clockwise direction but hydrolyzed when it rotated
in the counterclockwise direction. This further demon-
strates that F
1
is a device that interconverts mechanical and
chemical energy.
f. c-Ring Rotation Is Impelled by H
ⴙ
-Induced
Conformational Changes
The foregoing structural and biochemical information
has led to the model for proton-driven rotation of the F
0
subunit that is diagrammed in Fig. 22-45. Protons from the
outside enter a hydrophilic channel between the a subunit
and the c ring, where they bind to a c subunit. The c ring
858 Chapter 22. Electron Transport and Oxidative Phosphorylation
Figure 22-43 Rotation of the c ring in E. coli F
1
F
0
–ATPase.
(a) The experimental system used to observe the rotation. See
the text for details.The blue arrow indicates the observed
direction of rotation of the fluorescently labeled actin filament
that was linked to the c ring. (b) The rotation of a 3.6-m-long
actin filament in the presence of 5 mM MgATP as seen in
successive video images taken through a fluorescence micro-
scope. [Courtesy of Masamitsu Futai, Osaka University, Osaka,
Japan.]
Figure 22-44 Stepwise rotation of the ␥ subunit of F
1
relative
to an immobilized ␣
3

3
unit at low ATP concentration as
observed by fluorescence microscopy. The graph plots the
cumulative number of rotations made by a fluorescently labeled
actin filament that was linked at one end to the ␥ subunit in a
preparation similar to that diagrammed in Fig. 22-43a (but
lacking F
0
, ␦, and ε). Note that the actin filament rotates in
increments of 120°.This is also evident in the inset, which shows
the superposition of the centers of the actin images (the ␦
3

3
␥
assembly is fixed in the center). [Courtesy of Kazuhiko Kinosita,
Jr., Keio University, Yokohama, Japan.]
Revolutions
Time (s)
a [ATP] = 0.02 μM, Actin length = 1.1 μm
7
6
5
4
3
2
1
0
0306090
(a)
(b)
JWCL281_c22_823-870.qxd 6/8/10 9:19 AM Page 858
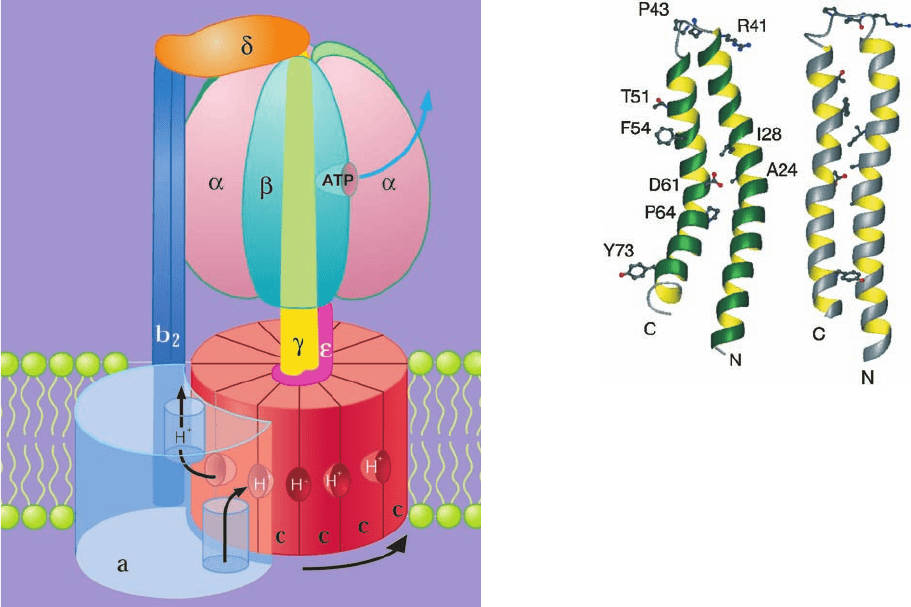
then rotates nearly a full turn (while protons bind to suc-
cessive c subunits as they pass this input channel) until the
subunit reaches a second hydrophilic channel between the
a subunit and the c-ring that opens into the inside. There
the proton is released.Thus, E.coli F
1
F
0
–ATPase, which has
12 c subunits in its F
0
assembly and generates 3ATP per turn,
ideally forms 3/12 ⫽ 0.25 ATP for every proton it passes
from the periplasmic space (outside) to the cytosol (inside).
Organisms with more/less c subunits in their c-rotor tend
to have lesser/greater values of proton-motive force across
their membranes and hence have less/more impetus im-
parted to their c-rotor per proton passed. Hence it takes the
passage of proportionately more/less protons to generate
each ATP, as the first law of themodynamics requires.
How does the passage of protons through this system in-
duce the rotation of the c ring and hence the synthesis of
ATP? The mutation of the c subunit’s conserved Asp 61 to
Asn inactivates E. coli F
1
F
0
–ATPase.The a subunit’s invari-
ant Arg 210 (E. coli numbering) has been similarly impli-
cated in proton translocation. Through the mutagenic con-
version of selected residues on the a and c subunits to Cys,
Robert Fillingame has shown that the outer (C-terminal)
helix of E. coli subunit c (Fig. 22-39), which contains Asp
61, can be disulfide-cross-linked to the putative fourth he-
lix of subunit a, which contains Arg 210. Evidently, these
helices are juxtaposed at some point in the c ring’s rotation
cycle. Thus, it is postulated that the protonation of Asp 61
releases its attraction to Arg 210, thereby permitting the
c ring to rotate.
Comparison of the NMR structures of subunit c at pH 8
and pH 5 (Fig. 22-46), at which Asp 61 is, respectively, de-
protonated and protonated, reveals that its main confor-
mational change on protonation is an ⬃140° clockwise ro-
tation (as viewed from F
1
) of its Asp 61-containing
C-terminal helix with respect to its N-terminal helix. Since
the C-terminal helix is the c ring’s outer helix (Fig. 22-39b),
this suggests that, on protonation, the rotation of the
C-terminal helix mechanically pushes against the juxtaposed
a subunit so as to rotate the c ring in the direction indicated
in Fig. 22-45.
D. Uncoupling of Oxidative Phosphorylation
Electron transport (the oxidation of NADH and FADH
2
by
O
2
) and oxidative phosphorylation (the synthesis of ATP)
are normally tightly coupled due to the impermeability of
the inner mitochondrial membrane to the passage of pro-
tons. Thus the only way for H
⫹
to reenter the matrix is
through the F
0
portion of the proton-translocating ATP
Section 22-3. Oxidative Phosphorylation 859
Figure 22-45 Schematic diagram of the action of the E. coli
F
1
F
0
–ATPase. The ␥ε–c
12
ring complex is the rotor and the
ab
2
–␣
3

3
␦ complex is the stator. Rotational motion is imparted to
the rotor by the passage of protons from the outside (periplasm)
to the inside (cytoplasm). Protons entering from the outside bind
to a c subunit where it interacts with the a subunit, and exit to
the inside after the c ring has made a nearly full rotation as
indicated (black arrows), so that the c subunit again contacts the
a subunit.The b
2
␦ complex presumably functions to prevent the
␣
3

3
assembly from rotating with the ␥ subunit. [Courtesy of
Richard Cross, State University of New York, Syracuse, New York.]
Figure 22-46 NMR structures of the c subunit of E. coli
F
1
F
0
–ATPase. The structures, which closely resemble that in Fig.
22-39, were determined in chloroform–methanol–water (4:4:1)
solution at (a) pH 8 (at which D61 is deprotonated) and (b) pH 5
(at which D61 is protonated). Selected side chains are shown to
aid in the comparison of the two structures. Note that the
C-terminal helix in the pH 8 structure has rotated by 140°
clockwise, as viewed from the top of the drawing, relative to that
in the pH 5 structure. [Courtesy of Mark Girvin,Albert Einstein
College of Medicine. PDBids (a) 1C99 and (b) 1C0V.]
JWCL281_c22_823-870.qxd 7/2/10 11:13 AM Page 859
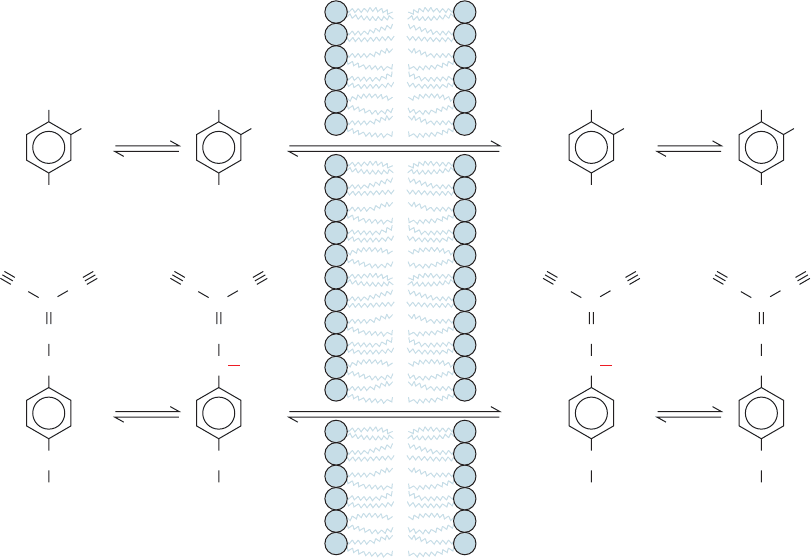
synthase. In the resting state, when oxidative phosphoryla-
tion is minimal, the proton-motive force across the inner
mitochondrial membrane builds up to the extent that the
free energy to pump additional protons is greater than the
electron-transport chain can muster,thereby inhibiting fur-
ther electron transport. However, many compounds, in-
cluding 2,4-dinitrophenol (DNP) and carbonylcyanide-p-
trifluoromethoxyphenylhydrazone (FCCP), have been
found to “uncouple” these processes. The chemiosmotic
hypothesis has provided a rationale for understanding the
mechanism by which these uncouplers act.
The presence in the inner mitochondrial membrane of an
agent that renders it permeable to H
⫹
uncouples oxidative
phosphorylation from electron transport by providing a
route for the dissipation of the proton-motove force that
does not require ATP synthesis. Uncoupling therefore
allows electron transport to proceed unchecked even when
ATP synthesis is inhibited. DNP and FCCP are lipophilic
weak acids that therefore readily pass through membranes.
In a pH gradient, they bind protons on the acidic side of the
membrane, diffuse through, and release them on the alka-
line side, thereby dissipating the gradient (Fig. 22-47).Thus,
such uncouplers are proton-transporting ionophores (Sec-
tion 20-2C).
Even before the mechanism of uncoupling was known,
it was recognized that metabolic rates were increased by
such compounds. Studies at Stanford University in the
early part of the twentieth century documented an increase
in respiration and weight loss caused by DNP. The com-
pound was even used as a “diet pill” for several years. In
the words of Efraim Racker (A New Look at Mechanisms
in Bioenergetics, p. 155):
In spite of warnings from the Stanford scientists, some
enterprising physicians started to administer dinitrophenol to
obese patients without proper precautions. The results were
striking. Unfortunately in some cases the treatment eliminated
not only the fat but also the patients, and several fatalities were
reported in the Journal of the American Medical Association in
1929.This discouraged physicians for a while....
a. Hormonally Controlled Uncoupling in Brown
Adipose Tissue Functions to Generate Heat
The dissipation of an electrochemical H
⫹
gradient, which
is generated by electron transport and uncoupled from ATP
synthesis, produces heat. Heat generation is the physiologi-
cal function of brown adipose tissue (brown fat). This tissue
is unlike typical (white) adipose tissue in that, besides con-
taining large amounts of triacylglycerols, it contains numer-
ous mitochondria whose cytochromes color it brown. New-
born mammals that lack fur, such as humans, as well as
hibernating mammals, contain brown fat in their neck and
upper back that functions in nonshivering thermogenesis,
that is, as a “biological heating pad.” (The ATP hydrolysis
that occurs during the muscle contractions of shivering—or
860 Chapter 22. Electron Transport and Oxidative Phosphorylation
Figure 22-47 Uncoupling of oxidative phosphorylation. The
proton-transporting ionophores DNP and FCCP uncouple
oxidative phosphorylation from electron transport by
H
+
+
NO
2
NO
2
diffusion
Matrix
high pH
Mitochondrial
membrane
Cytosol
low pH
2,4-Dinitrophenol (DNP)
NO
2
NO
2
+ H
+
O
–
O
–
NO
2
NO
2
OHOH
NO
2
NO
2
diffusion
Carbonylcyanide-p-trifluoro-
methoxyphenylhydrazone (FCCP)
+ H
+
H
+
+
O
N
NN
CF
3
N
–
C
CC
O
N
NN
CF
3
N
–
C
CC
H
O
N
NN
CF
3
N
C
CC
H
O
N
NN
CF
3
N
C
CC
discharging the electrochemical proton gradient generated by
electron transport.
JWCL281_c22_823-870.qxd 3/19/10 11:00 AM Page 860
