Voet D., Voet Ju.G. Biochemistry
Подождите немного. Документ загружается.

that contains heme c
1
and to which cytochrome c docks
(Figure 22-23). The ISP is likewise anchored by a single
transmembrane helix and extends into the intermembrane
space. Interestingly, the two ISPs of the dimeric complex
are domain swapped (intertwined) such that the [2Fe–2S]
cluster-containing domain of one protomer interacts with
the cytochrome b and cytochrome c
1
subunits of the other
protomer. The distances between the various metal cen-
ters are all quite large, ranging from 21 to 34 Å. The por-
tion of the complex that occupies the matrix, which ac-
counts for more than half the mass of cytochrome bc
1
,
consists largely of the structurally homologous Core 1 and
Core 2 proteins.
The route of electrons through the cytochrome bc
1
com-
plex is discussed in Section 22-3B, together with the mech-
anism by which the complex preserves the free energy of
electron transfer from CoQH
2
to cytochrome c for ATP
synthesis.
4. Cytochrome c
Cytochrome c, whose evolution we discussed in Section
7-3B, is a peripheral membrane protein of known crystal
structure (Figs. 8-42 and 9-41c) that is loosely bound to the
outer surface of the inner mitochondrial membrane. It
alternately binds to cytochrome c
1
(of Complex III) and to
cytochrome c oxidase (Complex IV) and thereby functions
to shuttle electrons between them.
The X-ray structure of yeast cytochrome bc
1
in complex
with cytochrome c,determined by Carola Hunte, reveals, as
expected, that cytochrome c binds to the cytochrome c
1
subunit of cytochrome bc
1
(Fig. 22-23). This association ap-
pears to be particularly tenuous because its interfacial area
(880 Å
2
) is significantly less than that exhibited by
protein–protein complexes known to have low stability
(typically ⬍1600 Å
2
). Such a small interface is well suited
for fast binding and release. The closest approach between
the heme groups of the contacting proteins is 4.1 Å
between atoms of their respective thioether-bonded sub-
stituents, and their Fe–Fe distance is 17.4 Å. This accounts
for the 8.3 ⫻ 10
6
s
⫺1
rate of electron transfer between these
two redox centers (see below).
a. Protein Structure Influences the Rate
of Electron Transfer
Reduced hemes are highly reactive entities; they can
transfer electrons over distances of 10 to 20 Å at physiolog-
ically significant rates. Hence cytochromes, in a sense, have
the opposite function of enzymes: Instead of persuading
unreactive substrates to react, they must prevent their
hemes from transferring electrons nonspecifically to other
cellular components. This, no doubt, is why these hemes
are almost entirely enveloped by protein. However,
cytochromes must also provide a path for electron transfer
to an appropriate partner.
In proteins, electron transfers occur between redox-
active cofactors such as hemes and/or Fe–S clusters. How-
ever, a survey of proteins of known structure that function
in electron transfer reveals that electrons travel no more
than 14 Å between protein-embedded redox centers and
that transfers over longer distances always involve chains
of redox-active cofactors (e.g., Figs. 22-19b and 22-22b).
Electron transfers occur far more efficiently through bonds
than through space via a quantum mechanical process
known as electron tunneling. Thus, as Harry Gray has ex-
perimentally demonstrated, electron tunneling within
proteins occurs largely through the polypeptide chains be-
tween bound redox-active groups and the rate of electron
transfer varies with the structure of the intervening
polypeptide. Moreover, tunneling across protein–protein
interfaces is largely mediated by van der Waals interactions
and water-bridged hydrogen bonds. Nevertheless, Leslie
Dutton has shown that the experimentally measured elec-
tron transfer rates within proteins vary only with the dis-
tance between the electron donor and the electron accep-
tor and fall off with an ⬃10-fold decrease in rate for each
1.7 Å increase in this distance.
5. Complex IV (Cytochrome c Oxidase)
Cytochrome c oxidase (COX or CcO), the terminal en-
zyme of the electron-transport chain, catalyzes the one-
electron oxidations of four consecutive reduced cytochrome
c molecules and the concomitant four-electron reduction of
one O
2
molecule to yield 2H
2
O:
Eukaryotic COX is an ⬃200-kD transmembrane pro-
tein composed of 8 to 13 subunits, whose largest and most
hydrophobic chains, Subunits I, II, and III, are encoded by
mitochondrial genes, with the remaining subunits encoded
by nuclear genes. Eukaryotic COX exists in membranes as
a dimer. Subunits I and II of this complex contain all four
of its redox-active centers: two a-type hemes, a and a
3
, and
two Cu-containing centers, Cu
A
and Cu
B
. The Cu
A
center
and heme a are of low potential (0.245 and 0.210 V; Table
22-1), whereas Cu
B
and heme a
3
are of higher potential
(0.340 and 0.385 V). Spectroscopic studies indicate that
electrons are passed from cytochrome c to the Cu
A
center,
then to the heme a, and finally to a binuclear complex of
heme a
3
and Cu
B
.O
2
binds to this binuclear complex and is
reduced to H
2
O in a complex four-electron reaction (see
below).
a. X-Ray Structures of Cytochrome c Oxidase
The X-ray structures of three species of cytochrome c
oxidase have been determined: two relatively simple
forms from the soil bacterium Paracoccus denitrificans
(1106 residues) by Michel and the purple photosynthetic
bacterium Rhodobacter sphaeroides by Iwata, and a
more complex form from bovine heart (1806 residues) by
Shinya Yoshikawa. Each protomer of the 2-fold symmetric
dimeric bovine COX has an ellipsoidal (potatolike)
shape comprised of a 48-Å-thick transmembrane portion
and hydrophilic portions that protrude 32 and 37 Å into
the mitochondrial matrix and the intermembrane space,
4 cytochrome c
3⫹
⫹ 2H
2
O
4 cytochrome c
2⫹
⫹ 4H
⫹
⫹ O
2
¡
Section 22-2. Electron Transport 841
JWCL281_c22_823-870.qxd 3/19/10 11:00 AM Page 841
respectively (Fig. 22-24).These protomers each consist of 13
different subunits that mainly form 28 transmembrane
helices. The protomer surfaces that comprise the dimer in-
terface are concave (Fig. 22-24) and hence their relatively
tenuous contacts enclose a lipid-filled cavity. Moreover,
mitochondrial COX is active as a monomer.Thus a mecha-
nistic role for dimer formation seems unlikely. The P. deni-
trificans and Rb. sphaeroides COXs are monomeric com-
plexes, each of which consists of only 4 subunits that
collectively contain 22 transmembrane helices.
The structures of bovine COX Subunits I (12 transmem-
brane helices) and II (2 transmembrane helices), which
bind all of the complex’s four redox centers, are closely
similar to those of P. denitrificans and Rb. sphaeroides
COXs.These are the subunits that carry out the main func-
tions of the complex: transporting electrons from cy-
tochrome c to O
2
to yield water, while pumping protons
from inside (as we shall refer to the mitochondrial matrix
and the bacterial cytoplasm; also called the N-side due to
its negative charge) to outside (as we shall refer to the
mitochondrial intermembrane space and the bacterial
periplasmic space; also called the P-side due to its positive
charge). Bovine Subunit III (7 transmembrane helices),
whose structure also resembles that of its P. denitrificans
and Rb. sphaeroides counterparts, does not appear to di-
rectly participate in electron transfer or proton transloca-
tion. Indeed, a complex consisting of only P. denitrificans
Subunits I and II can actively transport electrons and pump
protons. Thus the function of Subunit III is unknown,
although there is some evidence that it facilitates the assem-
bly of Subunits I and II to form the active complex. Note,
however, that Subunit III does not contact Subunit II.
None of the 10 nuclear-encoded subunits of bovine COX
resemble Subunit IV of P.denitrificans and Rb. sphaeroides
COXs (1 transmembrane helix). Seven of these bovine
subunits each have one transmembrane helix, all oriented
with their N-terminal ends on the matrix side of the mem-
brane. These helices are distributed about the periphery of
the dimeric core formed by Subunits I, II, and III. The re-
maining three bovine subunits are globular and associate
entirely with the extramembrane portions of the complex.
The X-ray structure of bovine COX provides little indica-
tion of the function of any of its nuclear-encoded subunits.
Perhaps they have regulatory roles.
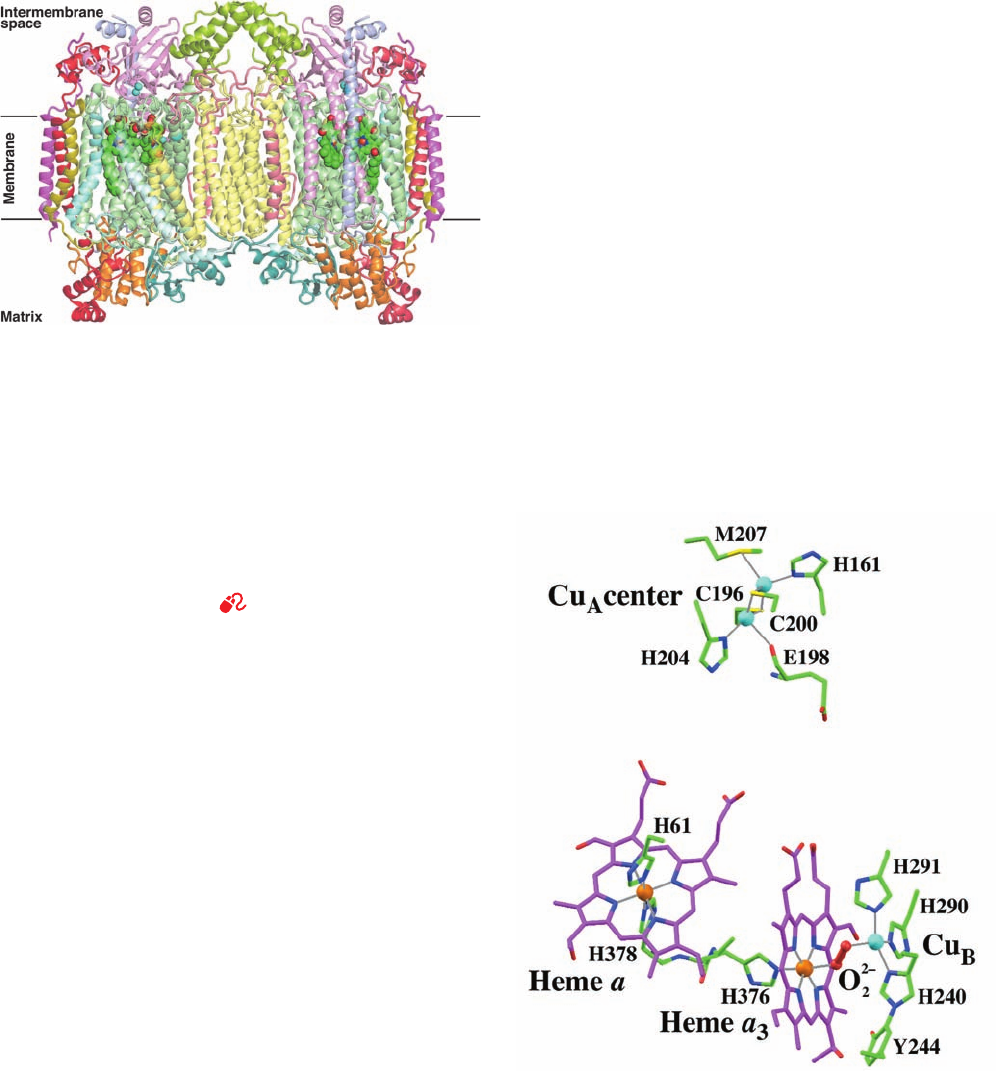
Subunit I binds heme a and the heme a
3
–Cu
B
binu-
clear center (Fig. 22-25), whose metal ions are all located
⬃13 Å below the membrane surface on its intermem-
brane/periplasmic side (Fig. 22-24).The heme a
3
Fe has one
axial His ligand, the heme a Fe has two axial His ligands
842 Chapter 22. Electron Transport and Oxidative Phosphorylation
Figure 22-24 X-ray structure of the bovine heart cytochrome c
oxidase homodimer. The view is perpendicular to its 2-fold axis
and parallel to the membrane with the matrix below. The 13
subunits in each protomer, which collectively have 28
transmembrane helices, are drawn in semitransparent ribbon
form and differently colored according to type with Subunit I
pale green, Subunit II pink, and Subunit III pale yellow. The
protein’s bound heme groups and Cu ions are drawn in
space-filling form with C green, N blue, O red, Fe red-brown, and
Cu cyan.The horizontal black lines delineate the inferred
position of the inner mitochondrial matrix. [Based on an X-ray
structure by Shinya Yoshikawa, Himeji Institute of Technology,
Hyogo, Japan. PDBid 1V54.]
See Interactive Exercise 18.
Figure 22-25 The redox centers in the X-ray structure of
bovine heart cytochrome c oxidase. The view is similar to that of
the left protomer in Fig. 22-24. The Fe and Cu ions are
represented by orange and cyan spheres. Their liganding heme
and protein groups (from subunit II for the Cu
B
center and
subunit I for the others) are drawn in stick form colored
according to atom type with heme C magenta, protein C green,
N blue, O red, and S yellow. The peroxy group that bridges the
Cu
B
and heme a
3
Fe ions is shown in ball-and-stick form in red.
Coordination bonds are drawn as thin gray lines. Note that the
side chains of His 240 and Tyr 244 are joined by a covalent bond
(lower right). [Based on an X-ray structure by Shinya Yoshikawa,
Himeji Institute of Technology, Hyogo, Japan. PDBid 2OCC.]
JWCL281_c22_823-870.qxd 10/19/10 8:07 AM Page 842
(as in Fig. 22-21b, top), and the Cu
B
atom has three His
ligands, whose coordinating N atoms are arranged in an
equilateral triangle that is centered on Cu
B
and is paral-
lel to heme a
3
. The X-ray structure of fully oxidized
bovine COX reveals a peroxide group (O
2⫺
2
; Fig. 22-25)
that bridges the heme a
3
Fe atom and Cu
B
(which are
separated by 4.9 Å), thereby liganding Cu
B
via a dis-
torted square-planar arrangement, a stable coordination
geometry for Cu(II). However, in the X-ray structure of
the fully reduced form of bovine COX (in which the
heme a
3
Fe¬Cu
B
distance is 5.2 Å), this ligand is absent,
so that Cu
B
is trigonally liganded, a stable coordination
geometry for Cu(I). The closest approach of the two
heme groups is 4 Å, and the distance between their Fe
atoms is 13.2 Å.
Subunit II, in addition to its two transmembrane helices,
has a globular domain on the outside surface that binds the
Cu
A
center and largely consists of a 10-stranded  barrel
(Fig. 22-24). The Cu
A
center is located ⬃8 Å above the out-
side membrane surface. Although the Cu
A
center was, for
many years, widely believed to contain only one Cu atom,
the X-ray structures of COX clearly indicate that Cu
A
con-
tains two Cu atoms (Fig. 22-25). These are bridged by two
Cys S atom ligands and have two additional protein ligands
each to form an arrangement similar to that of a [2Fe–2S]
cluster (Fig. 22-15b) in which the two Cu atoms are 2.4 Å
apart. Spectroscopic measurements indicate that in the Cu
A
center’s reduced form, both of its Cu atoms are in their
Cu(I) states, whereas in its fully oxidized form, an electron
appears to be delocalized between the two Cu atoms such
that they assume the [Cu
1.5⫹
...
Cu
1.5⫹
] state.
b. Electron and Proton Acquisition
COX’s cytochrome c binding site is postulated to be in a
corner formed by the globular domain of Subunit II and
the outside surface of Subunit I, since this region is close to
the Cu
A
site and contains 10 acidic side chains that could
interact with a ring of Lys side chains that surrounds cy-
tochrome c’s heme crevice. Indeed, differential labeling of
cytochrome c oxidase’s carboxyl groups in the presence and
absence of cytochrome c demonstrated that cytochrome c
shields the invariant residues Asp 112, Glu 114, and Glu 198
of Subunit II (bovine numbering). Glu 198 is located be-
tween the two Cys residues of Subunit II that ligand Cu
A
(Fig. 22-25). This observation supports the spectroscopic
evidence that places the cytochrome c binding site on Sub-
unit II in close proximity to Cu
A
. Cross-linking studies
have additionally shown that the cytochrome c surface op-
posite the electron-transferring site interacts with Subunit
III, suggesting that Subunit III also participates in binding
cytochrome c.
Time-resolved spectroscopic studies indicate that an
electron obtained from cytochrome c is first acquired by
the Cu
A
center and is then transferred to heme a rather
than heme a
3
, probably because the shortest Cu
A
...
heme a
distance of 11.7 Å is less than the shortest Cu
A
...
heme a
3
distance of 14.7 Å. The electron is then rapidly transferred
to the heme a
3
–Cu
B
binuclear center, where it participates
in reducing the bound O
2
to H
2
O. Note that the fifth ligand
of heme a, His 378, is separated from the fifth ligand of
heme a
3
, His 376, by only one residue, and hence there is a
relatively short through-the-bonds electron transfer path-
way between heme a and a
3
(Fig. 22-25, lower left). In addi-
tion, the closest approach of the two hemes is 4 Å.
COX must acquire four so-called chemical or scalar
protons from the inside for every molecule of O
2
it reduces
to H
2
O.This four-electron process is coupled to the translo-
cation of up to four so-called pumped or vectorial protons
from the inside to the outside, thereby contributing to the
proton gradient that powers ATP synthesis (Section
22-3C). Note that for each turnover of the enzyme,
a total of eight positive charges are transported across the
membrane, thereby contributing to its membrane potential.
c. The Reduction of O
2
by Cytochrome c Oxidase
Occurs in Multiple Steps
The reduction of O
2
to 2H
2
O by cytochrome c oxidase
takes place on the cytochrome a
3
–Cu
B
binuclear complex
(Fig. 22-25). Indeed, a synthetic model of this binuclear
complex (Fig. 22-26), synthesized by James Collman, effi-
ciently catalyzes the reduction of O
2
to H
2
O when attached
to an electrode.
The COX-mediated reduction of O
2
requires, as we
shall see, the nearly simultaneous input of four electrons.
However, the fully reduced – binuclear complex
can readily contribute only three electrons to its bound O
2
in reaching its fully oxidized – state [cytochrome a
3
transiently assumes its Fe(IV) or ferryl oxidation state
during the reduction of O
2
; see below]. What is the source
of the fourth electron?
Cu
2⫹
B
a
4⫹
3
Cu
1⫹
B
a
2⫹
3
4 cyt c
3⫹
⫹ 2H
2
O ⫹ 4H
⫹
outside
8H
⫹
inside
⫹ 4 cyt c
2⫹
⫹ O
2
¡
Section 22-2. Electron Transport 843
HN
HN
HN
N
N
N
N
N
N
NN
N
N
N
NH
O
O
O
O
Fe
2⫹
Cu
Figure 22-26 Synthetic model of the cytochrome a
3
–Cu
B
binuclear complex. This structure efficiently reduces O
2
to H
2
O
when attached to an electrode. The pyridine group that axially
ligands the Fe ion (bottom) can be replaced by an imidazole
group.
JWCL281_c22_823-870.qxd 6/8/10 9:19 AM Page 843
The X-ray structures of both bovine and P. denitrificans
COX clearly indicate that the His 240 ligand of Cu
B
(bovine numbering) is covalently cross-linked to the side
chain of the conserved Tyr 244 (Fig. 22-25, lower right).This
places Tyr 244’s phenolic ¬OH group in close proximity to
the heme a
3
-ligated O
2
such that Tyr 244 can supply the
fourth electron by transiently forming a tyrosyl radical
(TyrOⴢ). In fact, adding peroxide to the resting enzyme
generates a tyrosyl radical, whereas mutating Tyr 244 to
Phe inactivates the enzyme. Moreover, tyrosyl radicals
have been implicated in several enzymatically mediated re-
dox processes, including the generation of O
2
from H
2
O in
photosynthesis (in a sense, the reverse of the COX reac-
tion; Section 24-2Cd), and in the ribonucleotide reductase
reaction (which converts NDP to dNDP; Section 28-3Aa).
Tyr 244’s phenolic¬OH group is within hydrogen bonding
distance of the COX-bound O
2
and hence is a likely H
⫹
donor during O¬O bond cleavage. The formation of the
covalent cross-link is expected to lower both the reduction
potential and the pK of Tyr 244, thereby facilitating both
radical formation and proton donation (the synthetic binu-
clear complex in Fig. 22-26 can function without an associ-
ated tyrosyl radical, presumably because its associated
electrode can supply it with electrons much faster than cy-
tochrome c can supply them to COX).
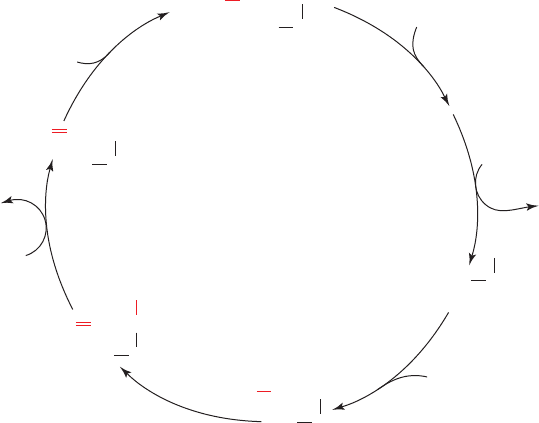
The COX reaction, elucidated in large part by Mårten
Wikström and Gerald Babcock using a variety of spectro-
scopic techniques, involves four consecutive one-electron
transfers from the Cu
A
and cytochrome a sites and occurs
as follows (Fig. 22-27):
1 and 2. The oxidized binuclear complex [Fe(III)
a3
¬OH
⫺
Cu(II)
B
] is reduced to its [Fe(II)
a3
Cu(I)
B
] state by two con-
secutive one-electron transfers from cytochrome c via cy-
tochrome a and Cu
A
.A proton from the matrix is concomi-
tantly acquired and an H
2
O is released in this process. Tyr
244 (Y¬OH) is in its phenolic state.
3. O
2
binds to the reduced binuclear complex so as to
ligand its Fe(II)
a3
atom. It binds to the heme with much the
same configuration it has in oxymyoglobin (Fig. 10-12).
4. Internal electron redistribution rapidly yields the
oxyferryl complex [Fe(IV) O
2⫺
HO
⫺
¬Cu(II)] in which
Tyr 244 has donated an electron and a proton to the com-
plex and thereby assumed its neutral radical state (Y¬Oⴢ).
This is known as compound P because it was originally
thought that this spectroscopically identified state was a
peroxy complex. However, it has since been shown that a
peroxy compound is not on the reaction pathway. The per-
oxy complex displayed in Fig. 22-25 is a two-electron
reduced “mixed valence” state of the enzyme that cannot
reduce O
2
past its peroxy form.
5. A third one-electron transfer from cytochrome c to-
gether with the acquisition of two protons reconverts Tyr
244 to its phenolic state, yielding compound F (for ferryl)
and releasing an H
2
O.
6. A fourth and final electron transfer and proton ac-
quisition yields the oxidized [Fe(III)
a3
¬OH
⫺
Cu(II)
B
]
complex, thereby completing the catalytic cycle.
COX typically undergoes 100 to 200 turnovers per second
so that one catalytic cycle takes only a few milliseconds.
Note that the COX reaction proceeds without the release
of the destructive partially reduced reactive oxygen
species (ROS) from its active site. The positions in this
proposed catalytic cycle at which protons appear to be
“
844 Chapter 22. Electron Transport and Oxidative Phosphorylation
Figure 22-27 Proposed reaction sequence for the reduction of
O
2
by the cytochrome a
3
–Cu
B
binuclear complex of cytochrome
c oxidase. The numbered steps are discussed in the text.The
e
–
, H
⫹
e
–
, 2H
⫹
5
4
3
2
1
6
Cu(II)
–
OHFe(III)
HOY
oxidized
Cu(II)
O
2
⫺
Fe(IV)
HO
F
Y
–
OH
Cu(II)
O
2
⫺
Fe(IV)
•
O
P
Y
H
2
O
e
–
, H
⫹
e
–
H
2
O
Cu(I)O
2
Fe(II)
HOY
oxy
Cu(I)Fe(II)
HOY
reduced
O
2
entire reaction is extremely fast; it goes to completion in ⬃1 ms
at room temperature. [Modified from Babcock, G.T., Proc. Natl.
Acad. Sci. 96, 12971 (1999).]
JWCL281_c22_823-870.qxd 3/19/10 11:00 AM Page 844
pumped from the matrix (bacterial cytoplasm) to the in-
termembrane (periplasmic) space are discussed in Section
22-3B. Keep in mind, however,that aspects of this cycle are
uncertain and/or disputed and hence it remains under in-
vestigation.
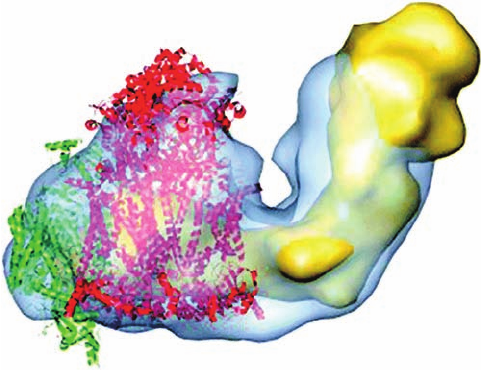
d. Complexes I, III, and IV Form Supercomplexes
For many years, it had been widely assumed that the
complexes of the respiratory chain were laterally mobile
within the inner mitochondrial membrane and hence did
not associate. However, the development of gentle meth-
ods of separating the components of the inner mitochondr-
ial membrane has made it increasingly clear that these al-
ready large protein complexes form supercomplexes. In
fact, a variety of supercomplexes from several organisms
have been characterized, including yeast III
2
IV
2
, bovine
and Arabadopsis thaliana (a plant) I
1
III
2
, and bovine
I
1
III
2
IV
1
(in which, for example, IV
1
represents a protomer
of Complex IV). Electron microscopy–based images such
as Fig. 22-28 indicate that Complex IV monomers associate
with Complex III dimers, which limits the distance that cy-
tochrome c must travel to transport an electron from Com-
plex III to Complex IV to ⬍40 Å. Similarly, the association
of Complex III with Complex I reduces the distance over
which CoQH
2
must diffuse between these two complexes.
Apparently, these supermolecular complexes function to
increase the efficiency of electron transport via the chan-
neling of their intermediates, a conclusion supported by ki-
netic measurements. Note that none of these supercom-
plexes contain Complex II, also in agreement with kinetic
measurements.
3 OXIDATIVE PHOSPHORYLATION
The endergonic synthesis of ATP from ADP and P
i
in mito-
chondria, which, as we shall see, is catalyzed by proton-
translocating ATP synthase (Complex V), is driven by the
electron-transport process. Yet, since Complex V is physi-
cally distinct from the proteins mediating electron trans-
port (Complexes I–IV), the free energy released by electron
transport must be conserved in a form that ATP synthase
can utilize. Such energy conservation is referred to as en-
ergy coupling or energy transduction.
The physical characterization of energy coupling proved
to be surprisingly elusive; many sensible and often ingen-
ious ideas have failed to withstand the test of experimental
scrutiny. In this section we first examine some of the hy-
potheses that have been formulated to explain the cou-
pling of electron transport and ATP synthesis. We shall
then explore the coupling mechanism that has garnered
the most experimental support, analyze the mechanism by
which ATP is synthesized by ATP synthase, and, finally, dis-
cuss how electron transport and ATP synthesis can be
uncoupled.
A. Energy Coupling Hypotheses
In the more than 70 years that electron transport and ox-
idative phosphorylation have been studied, numerous
mechanisms have been proposed to explain how these
processes are coupled. In the following paragraphs, we ex-
amine the mechanisms that have received the greatest ex-
perimental attention.
1. The chemical coupling hypothesis. In 1953, Edward
Slater formulated the chemical coupling hypothesis, in
which he proposed that electron transport yielded reactive
intermediates whose subsequent breakdown drove oxida-
tive phosphorylation. We have seen, for example, that such
a mechanism is responsible for ATP synthesis in glycolysis
(Sections 17-2G and 17-2J). Thus, the exergonic oxidation
of glyceraldehyde-3-phosphate by NAD
⫹
yields 1,3-
bisphosphoglycerate, a reactive (“high-energy”) acyl phos-
phate whose phosphoryl group is then transferred to ADP
to form ATP in the phosphoglycerate kinase reaction. The
difficulty with such a mechanism for oxidative phosphoryla-
tion, which has caused it to be abandoned, is that despite in-
tensive efforts in numerous laboratories over many years,
no appropriate reactive intermediates have been identified.
2. The conformational coupling hypothesis. The con-
formational coupling hypothesis, which Paul Boyer formu-
lated in 1964, proposes that electron transport causes
proteins of the inner mitochondrial membrane to assume
“activated” or “energized” conformational states. These
proteins are somehow associated with ATP synthase such
that their relaxation back to the deactivated conformation
drives ATP synthesis. As with the chemical coupling hy-
pothesis, the conformational coupling hypothesis has
found little experimental support. However, conforma-
tional coupling of a different sort appears to be involved in
ATP synthesis (Section 22-3Cd).
Section 22-3. Oxidative Phosphorylation 845
Figure 22-28 Electron microscopy–based image of the bovine
supercomplex I
1
III
2
IV
1
at 32 Å resolution. The supercomplex is
represented by its semitransparent surface (blue) fitted with the
EM-based structure of Complex I (yellow) and the X-ray
structures of the Complex III dimer (red) and the Complex IV
monomer (green).The view is parallel to the plane of the
membrane with the matrix above. [Courtesy of Eva Schäfer,
Birbeck College, London, U.K.]
JWCL281_c22_823-870.qxd 3/19/10 11:00 AM Page 845
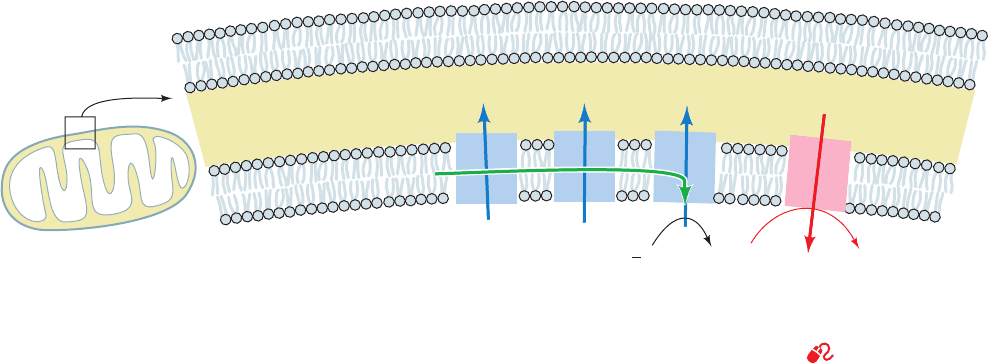
3. The chemiosmotic hypothesis. The chemiosmotic hy-
pothesis, proposed in 1961 by Peter Mitchell, has spurred
considerable controversy, as well as much research, and is
now the model most consistent with the experimental evi-
dence. It postulates that the free energy of electron transport
is conserved by pumping H
⫹
from the mitochondrial matrix
to the intermembrane space so as to create an electrochemi-
cal H
⫹
gradient across the inner mitochondrial membrane.
The electrochemical potential of this gradient is harnessed to
synthesize ATP (Fig. 22-29).
Several key observations are explained by the chemios-
motic hypothesis:
(a) Oxidative phosphorylation requires an intact inner
mitochondrial membrane.
(b) The inner mitochondrial membrane is imperme-
able to ions such as H
⫹
,OH
⫺
,K
⫹
, and Cl
⫺
, whose
free diffusion would discharge an electrochemical
gradient.
(c) Electron transport results in the transport of H
⫹
out
of intact mitochondria, thereby creating a measura-
ble electrochemical gradient across the inner mito-
chondrial membrane.
(d) Compounds that increase the permeability of the in-
ner mitochondrial membrane to protons, and thereby
dissipate the electrochemical gradient, allow electron
transport (from NADH and succinate oxidation) to
continue but inhibit ATP synthesis; that is, they “un-
couple” electron transport from oxidative phospho-
rylation. Conversely, increasing the acidity outside
the inner mitochondrial membrane stimulates ATP
synthesis.
In the remainder of this section we examine the mecha-
nisms through which electron transport can result in pro-
ton translocation and how an electrochemical gradient can
interact with ATP synthase to drive ATP synthesis.
B. Proton Gradient Generation
Electron transport, as we shall see, causes Complexes I, III,
and IV to transport protons across the inner mitochondrial
membrane from the matrix, a region of low [H
⫹
] and neg-
ative electrical potential, to the intermembrane space
(which is in contact with the cytosol), a region of high [H
⫹
]
and positive electrical potential (Fig. 22-14). The free en-
ergy sequestered by the resulting electrochemical gradi-
ent [which, in analogy to the term electromotive force
(emf), is called proton-motive force (pmf)] powers ATP
synthesis.
a. Proton Pumping Is an Endergonic Process
The free energy change of transporting a proton out of
the mitochondrion against an electrochemical gradient is
expressed by Eq. [20.3], which, in terms of pH, is
[22.1]
where Z is the charge on the proton (including sign), f is
the Faraday constant, and ⌬G is the membrane potential.
The sign convention for ⌬G is that when a positive ion is
transported from negative to positive, ⌬G is positive.
Since pH(out) is less than pH(in), the export of protons
from the mitochondrial matrix (against the proton gradi-
ent) is an endergonic process. In addition, proton trans-
port out of the matrix makes the inner membrane’s internal
surface more negative than its external surface. Outward
transport of a positive ion is consequently associated with
a positive ⌬G and an increase in free energy (endergonic
process), whereas the outward transport of a negative ion
yields the opposite result. Clearly, it is always necessary to
describe membrane polarity when specifying a membrane
potential.
The measured membrane potential across the inner
membrane of a liver mitochondrion, for example,is 0.168 V
¢G ⫽ 2.3 RT[pH(in) ⫺ pH(out)] ⫹ Z f ¢⌿
846 Chapter 22. Electron Transport and Oxidative Phosphorylation
Figure 22-29 Coupling of electron transport (green arrow) and
ATP synthesis. H
⫹
is pumped out of the mitochondrion by
Complexes I, III, and IV of the electron-transport chain (blue
arrows), thereby generating an electrochemical gradient across
the inner mitochondrial membrane. The exergonic return of these
Intermembrane
space
H
+
H
+
H
+
H
+
+
+
+
+
+
+
+
+
+
+
+
+
[H
+
]Low
[H
+
]High
–
–
–
–
–
–
––
–
–
–
–
ATP
ADP
+ P
i
+
–
+
–
+
–
e
–
H
2
OO
2
I
III IV
V
2
1
Outer mitochondrial
membrane
Inner mitochondrial
membrane
protons to the matrix powers the synthesis of ATP (red arrow).
Note that the outer mitochondrial membrane is permeable to
small molecules and ions, including H
⫹
. See the Animated
Figures
JWCL281_c22_823-870.qxd 3/19/10 11:00 AM Page 846
(inside negative; this corresponds to an ⬃210,000 V ⴢ cm
⫺1
electric field across its ⬃80 Å thickness).The pH of the ma-
trix is 0.75 units higher than that of the intermembrane
space. ⌬G for proton transport out of this mitochondrial
matrix is therefore 21.5 kJ ⴢ mol
⫺1
.
b. The Passage of About Three Protons Is
Required to Synthesize One ATP
An ATP molecule’s estimated physiological free en-
ergy of synthesis, around ⫹40 to ⫹50 kJ ⴢ mol
⫺1
, is too
large to be driven by the passage of a single proton back
into the mitochondrial matrix; at least two protons are re-
quired. This number is difficult to measure precisely, in
part because transported protons tend to leak back across
the mitochondrial membrane. However, most estimates
indicate that around three protons are passed per ATP
synthesized.
c. Two Mechanisms of Proton Transport
Have Been Proposed
Three of the four electron-transport complexes, Com-
plexes I, III, and IV, are involved in proton translocation.
Two mechanisms have been entertained that would couple
the free energy of electron transport with the active trans-
port of protons: the redox loop mechanism and the proton
pump mechanism.
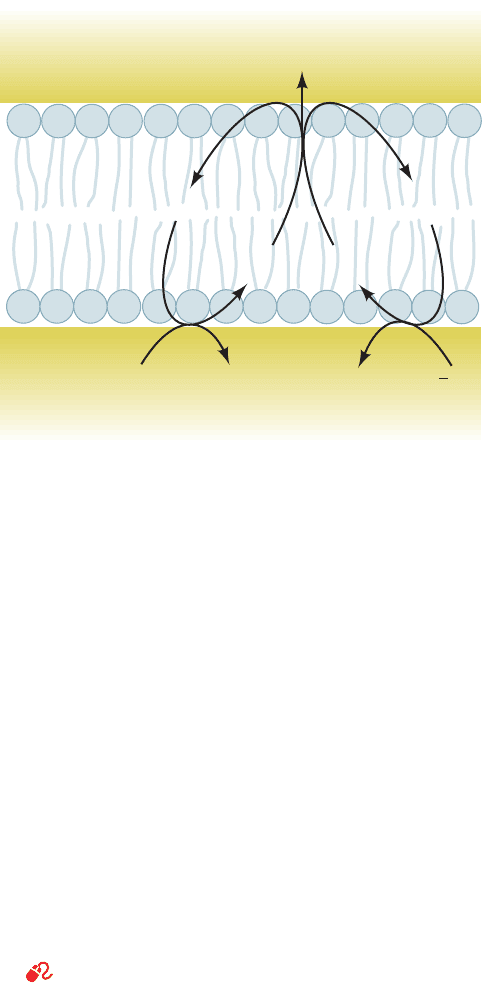
d. The Redox Loop Mechanism
This mechanism, proposed by Mitchell, requires that the
redox centers of the respiratory chain (FMN, CoQ, cy-
tochromes, and iron–sulfur clusters) be so arranged in the
membrane that reduction would involve a redox center si-
multaneously accepting e
⫺
and H
⫹
from the matrix side of
the membrane. Reoxidation of this redox center by the
next center in the chain would involve release of H
⫹
on the
cytosolic side of the membrane together with the transfer
of electrons back to the matrix side (Fig. 22-30). Electron
flow from one center to the next would therefore yield net
translocation of H
⫹
and the creation of an electrochemical
gradient (⌬G and ⌬pH).
The redox loop mechanism requires that the first redox
carrier contain more hydrogen atoms in its reduced state
than in its oxidized state and that the second redox carrier
have no difference in its hydrogen atom content between its
reduced and oxidized states. Are these requirements met in
the electron-transport chain? Some of the redox carriers,
FMN and CoQ, in fact, contain more hydrogen atoms in
their reduced state than in their oxidized state and thus can
qualify as proton carriers as well as electron carriers. If
these centers were spatially alternated with pure electron
carriers (cytochromes and iron–sulfur clusters), such a
mechanism could well be accommodated.
The main difficulty with the redox loop mechanism in-
volves the deficiency of (H
⫹
⫹ e
⫺
) carriers that can alter-
nate with pure e
⫺
carriers. Whereas the electron-transport
chain has as many as 15 pure e
⫺
carriers (up to 8 iron–sulfur
proteins, 5 cytochromes, and 2 Cu centers), it has only 2
(H
⫹
⫹ e
⫺
) carriers. The fact that there are three complexes
with standard reduction potential changes large enough to
provide free energy for ATP synthesis suggests the need
for at least three proton-transport redox carriers. As we
shall see, however, there are, in fact, three proton-transport
sites but only two proton-transport redox carriers: Both the
redox loop mechanism and the proton pump mechanism
(discussed below) are employed.
e. Complex III Pumps Protons via the Q Cycle, a
Type of Redox Loop
See Guided Exploration 20: The Q cycle Mitchell postulated
that Complex III functions in a way that permits one mole-
cule of CoQH
2
, the two-electron carrier, to sequentially re-
duce two molecules of cytochrome c, a one-electron carrier,
while transporting four protons. This occurs via a modified
redox loop mechanism involving a remarkable bifurcation
of the flow of electrons from CoQH
2
to cytochrome c
1
and
to cytochrome b. It is through this so-called Q cycle that
Complex III pumps protons from the matrix to the inter-
membrane space.
The essence of the Q cycle is that CoQH
2
undergoes a
two-cycle reoxidation in which the semiquinone is a sta-
ble intermediate. This involves two independent binding
sites for coenzyme Q: Q
o
, which binds CoQH
2
and is lo-
cated between the ISP and heme b
L
in proximity to the in-
termembrane space (Fig. 22-23); and Q
i
, which binds both
and CoQ and is located near heme b
H
in proximity toQ
⫺
ⴢ
Q
⫺
ⴢ
Section 22-3. Oxidative Phosphorylation 847
Intermembrane
space
Inner
membrane
Matrix
2B
ox
AH
2
SSH
2
2H
+
A
ox
2B
–
red
H
2
OO
2
2H
+
+
2
1
Figure 22-30 The redox loop mechanism for electron transport–
linked H
ⴙ
translocation. AH
2
represents (H
⫹
⫹ e
⫺
) carriers such
as FMNH
2
and CoQH
2
, whereas B represents pure e
⫺
carriers
such as iron–sulfur clusters and the cytochromes. These compo-
nents are so arranged as to require that electron transport be
accompanied by H
⫹
translocation.
JWCL281_c22_823-870.qxd 3/19/10 11:00 AM Page 847
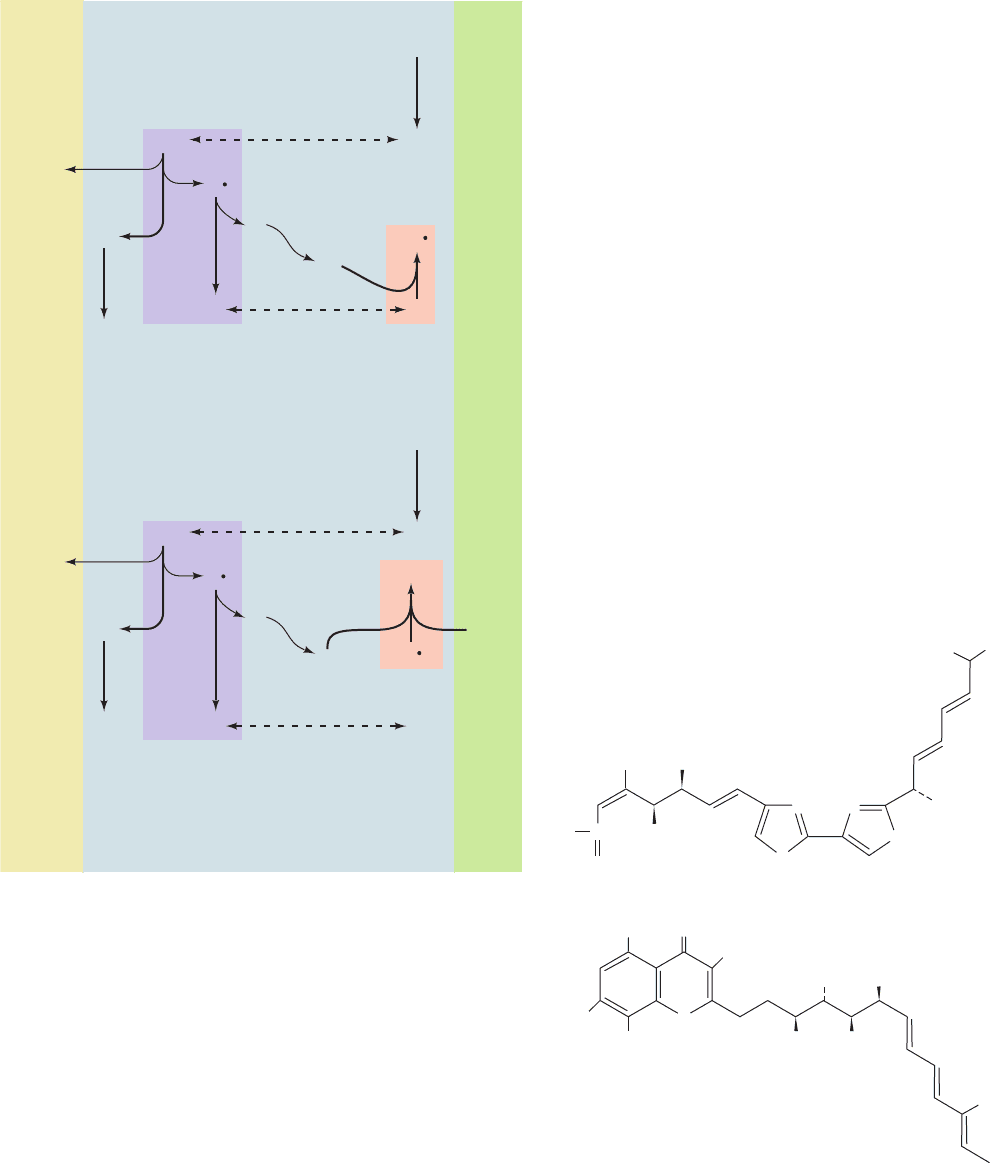
the matrix. In the first cycle (Fig. 22-31a), CoQH
2
, which is
supplied by Complexes I or II on the matrix side of the in-
ner mitochondrial membrane (1), diffuses through the
membrane to its cytoplasmic side, where it binds to the Q
o
site (2).There it transfers one of its electrons to the ISP (3),
releasing its two protons into the intermembrane space
and yielding . The ISP then reduces cytochrome c
1
,
whereas the transfers its remaining electron to heme b
L
(4), yielding fully oxidized CoQ. Heme b
L
then reduces
heme b
H
(6).The CoQ from Step 4 is released from the Q
o
site and diffuses back through the membrane to rebind at
the Q
i
site (5), where it picks up the electron from heme
b
H
(7), reverting to the semiquinone form, .Thus, the re-
action for this first cycle is
In the second cycle (Fig. 22-31b), another CoQH
2
re-
peats Steps 1 through 6: One electron reduces the ISP and
then cytochrome c
1
, and the other electron sequentially re-
duces heme b
L
and then heme b
H
. This second electron
then reduces the at the Q
i
site produced in the first cy-
cle (8), yielding CoQH
2
. The protons taken up in this last
step originate in the mitochondrial matrix.The reaction for
the second cycle is therefore
For every two CoQH
2
that enter the Q cycle, one
CoQH
2
is regenerated. The combination of both cycles, in
which two electrons are transferred from CoQH
2
to cy-
tochrome c
1
, results in the overall reaction
X-ray studies of Complex III provide direct evidence
for the independent existence of the Q
o
and Q
i
sites. The
antifungal agents myxothiazol and stigmatellin,
CoQ ⫹ 2 cytochrome c
1
(Fe
2⫹
) ⫹ 4H
⫹
(outside)
CoQH
2
⫹ 2 cytochrome c
1
(Fe
3⫹
) ⫹ 2H
⫹
(matrix)
¡
CoQ ⫹ CoQH
2
⫹ cytochrome c
1
(Fe
2⫹
) ⫹ 2H
⫹
(outside)
CoQH
2
⫹ Q
⫺
ⴢ ⫹ cytochrome c
1
(Fe
3⫹
)⫹ 2H
⫹
(matrix)
¡
Q
⫺
ⴢ
Q
⫺
ⴢ ⫹ cytochrome c
1
(Fe
2⫹
) ⫹ 2H
⫹
(outside)
CoQH
2
⫹ cytochrome c
1
(Fe
3⫹
)
¡
Q
⫺
ⴢ
Q
⫺
ⴢ
Q
⫺
ⴢ
848 Chapter 22. Electron Transport and Oxidative Phosphorylation
Figure 22-31 The Q cycle. The Q cycle is an electron-
transport cycle in Complex III that accounts for H
⫹
translocation
during the transport of electrons from cytochrome b to
cytochrome c: The overall cycle is actually two cycles, the first
(a) requiring reactions 1 through 7 and the second (b) requiring
reactions 1 through 6 and 8. (1) Coenzyme QH
2
is supplied by
Complex I on the matrix side of the membrane. (2) QH
2
diffuses
to the outside of the membrane. (3) QH
2
reduces the Rieske
iron–sulfur protein (ISP) forming semiquinone and releasing
2H
⫹
.The ISP goes on to reduce cytochrome c
1
. (4) reduces
heme b
L
to form coenzyme Q. (5) Q diffuses to the matrix side.
(6) Heme b
L
reduces heme b
H
.(7, cycle 1 only) Q is reduced to
by heme b
H
.(8, cycle 2 only) is reduced to QH
2
by heme
b
H
. [After Trumpower, B.L., J. Biol. Chem. 265, 11410 (1990).]
Q
⫺
ⴢQ
⫺
ⴢ
Q
⫺
ⴢ
Q
⫺
ⴢ
2H
+
ISP
Inter-
membrane
space
Cycle 1
Matrix
QQ
Q
o
Q
i
c
1
1
2
4
3
5
6
e
–
e
–
e
–
b
L
e
–
From
complex I
ISP
Q
o
Q
i
c
1
e
–
7
e
–
b
H
2H
+
2H
+
QH
2
QH
2
QH
2
QH
2
QH
2
Cycle 2
QQ
1
2
4
3
5
6
e
–
e
–
e
–
e
–
b
L
From
complex I
8
b
H
Q
–
Q
–
Q
–
Q
–
(b)
(a)
H
3
CO
OCH
3
CH
3
C
O
H
2
N
S
S
NN
Myxothiazol
Stigmatellin
OCH
3
O
CH
3
CH
3
OCH
3
OCH
3
CH
3
CH
3
OH CH
3
CH
3
CH
3
H
3
CO
H
3
C
O
JWCL281_c22_823-870.qxd 6/8/10 9:19 AM Page 848
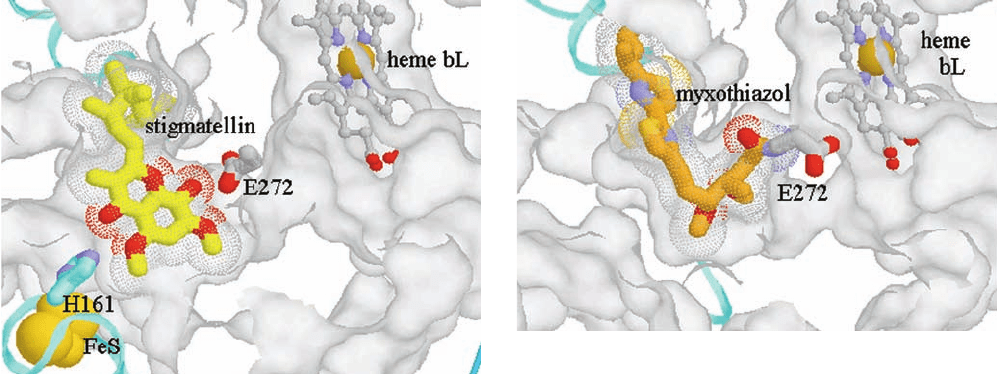
which both block electron flow from CoQH
2
to the ISP and
to heme b
L
(Steps 3 and 4 of both cycles), bind in a pocket
within cytochrome b between the ISP and heme b
L
(Fig.
22-23). Evidently, this binding pocket overlaps the Q
o
site.
Similarly, antimycin (Section 22-2Ba), which blocks elec-
tron flow from heme b
H
to CoQ and (Step 7 of Cycle 1
and Step 8 of Cycle 2), binds in a pocket near heme b
H
,
thereby identifying this pocket as site Q
i
.
The circuitous route of electron transfer in Complex III
is tied to the ability of coenzyme Q to diffuse within the
hydrophobic core of the membrane in order to bind to
both the Q
o
and Q
i
sites.This process is facilitated by an in-
dentation in the surface of cytochrome b’s transmembrane
region that contains Q
o
from one protomer and Q
i
from
the other. When CoQH
2
is oxidized, two reduced cy-
tochrome c molecules and four protons appear on the outer
side of the membrane. Proton transport by the Q cycle thus
follows the redox loop mechanism of proton transport in
which a redox center itself (CoQ) is the proton carrier.As
we shall see below, however, Complexes I and IV follow a
different mechanism of proton transport, the proton pump
mechanism.
f. The Bifurcation of the Q Cycle’s Electron Flow
Occurs via Domain Movement
Why does Q
o
-bound exclusively reduce heme b
L
(Step 4 of the Q cycle) rather than the Rieske [2Fe–2S]
cluster of the ISP, despite the greater reduction potential
difference (⌬e) favoring the latter reaction (Table 22-1)?
Q
⫺
ⴢ
Q
⫺
ⴢ
The remarkable answer to this question provides a fasci-
nating insight into the inner workings of Complex III. Al-
though the binding of stigmatellin and myxothiazol to Q
o
are mutually exclusive, these inhibitors affect this site dif-
ferently: Stigmatellin perturbs the spectrum and redox
properties of the ISP’s Rieske [2Fe–2S] cluster as well as
prevents it from oxidizing cytochrome c
1
, whereas myxoth-
iazol does not interact with the ISP but, instead, shifts the
spectrum of heme b
L
. Evidently, stigmatellin is a CoQH
2
analog and myxothiazol is a mimic.
X-ray structures of Complex III reveal that its Q
o
site is
a bifurcated pocket in which stigmatellin binds close to the
ISP docking interface (see below), whereas myxothiazol
binds in the vicinity of heme b
L
(Fig. 22-32; their binding
to Q
o
is mutually exclusive because their hydrophobic
tails would overlap). Furthermore, the globular Rieske
[2Fe–2S] cluster–containing domain of the ISP (Fig. 22-23,
top) is conformationally mobile and assumes a conforma-
tional state that is controlled by the ligand-binding state of
Q
o
: It binds to cytochrome b near the heme b
L
site when
stigmatellin is bound to the Q
o
site, but has swung around
by ⬃20 Å (via an ⬃57º hinge motion that leaves intact its
tertiary structure) to bind to the cytochrome c
1
near its
heme c when myxothiazol is bound to Q
o
. Apparently the
globular domain of the ISP functions to shuttle an electron
from CoQH
2
, which is bound to Q
o
near the ISP docking
interface, to heme c
1
by mechanically swinging the reduced
Rieske [2Fe–2S] cluster between these sites. The resulting
shifts to the position near heme b
L
(probably via aQ
⫺
ⴢ
Q
⫺
ⴢ
Section 22-3. Oxidative Phosphorylation 849
Figure 22-32 X-ray structures of the Q
o
binding site of the
chicken cytochrome bc
1
complex occupied by inhibitors. The
structures show (a) its complex with stigmatellin and (b) its
complex with myxothiazol.The protein surface (white) has been
cut away to show the Q
o
pocket. Heme b
L
(upper right) is shown
in ball-and-stick form with C gray, N blue, O red, and Fe tan. In
Part a, stigmatellin is drawn as a stick model with C yellow and
its volume represented by the dotted surface. The Rieske [2Fe–2S]
cluster (lower left) is represented by gold spheres, and the ISP
domain to which it is bound is drawn as a cyan ribbon. Note that
His 161, which is a ligand of the Rieske [2Fe–2S] cluster, forms a
hydrogen bond to stigmatellin. In Part b, the myxothiazol is
drawn as a stick model with C orange. Note that the semiquinone-
mimicking portion of myxothiazol binds to Q
o
in proximity to
heme b
L
, whereas its hydrophobic tail occupies the same position
as that of stigmatellin.Also note that the [2Fe–2S] cluster–
containing ISP domain is not visible in this diagram; it has
rotated into proximity with cytochrome c
1
. [Courtesy of Antony
Crofts, University of Illinois at Urbana–Champagne, and Edward
Berry, University of California at Berkeley. PDBid 3BCC.]
(a)
(b)
JWCL281_c22_823-870.qxd 3/19/10 11:00 AM Page 849
rotation about a bond connecting the semiquinone ring to its
nonpolar tail), which it then reduces. Thus, is unable to
reduce the ISP (after it has reduced cytochrome c
1
) be-
cause it is too far away to do so. This novel mechanism is
supported by the observation that mutagenically inserting
a disulfide bond either within the hinge of the ISP or be-
tween it and cytochrome b greatly diminishes the activity
of Complex III but that its activity is restored on exposure
to reducing agents.
g. The Proton Pump Mechanism
Complex IV (COX) transports four protons from the
matrix to the intermembrane space for each O
2
it reduces
(2H
⫹
per electron pair; Fig. 22-14). It contains no (H
⫹
⫹ e
⫺
)
carriers and hence cannot do so via a redox loop (Q cycle-
like) system. Rather, as we shall see, it does so via the pro-
ton pump mechanism (Fig. 22-33), which does not require
that the redox centers themselves be H
⫹
carriers. In this
model, the transfer of electrons results in conformational
changes to the complex. The unidirectional translocation of
protons occurs as a result of the influence of these confor-
mational changes on the pK’s of amino acid side chains and
their alternate exposure to the internal and external side of
the membrane. We have previously seen that conformation
can influence pK. The Bohr effect in hemoglobin, for ex-
ample, results from conformational changes induced by O
2
binding, which causes pK changes in protein acid–base
groups (Section 10-2E). If such a protein were located in a
membrane and if, in addition to pK changes, the conforma-
tional changes altered the side of the membrane to which
the affected amino acid side chains were exposed, the re-
sult would be H
⫹
transport and the system would be a
proton pump.
Keep in mind that protons, being atomic nuclei, must al-
ways be associated with molecules or ions. Consequently, a
proton cannot be transported across a membrane in the
same way that, say, a K
⫹
ion is. Rather, protons are translo-
cated by hopping along chains of hydrogen bonded groups
Q
⫺
ⴢ
in the transport protein in much the same way that hydro-
nium ions migrate through an aqueous solution (Fig. 2-10),
that is, they move along a proton wire (Section 21-2Al).
However, unlike a wire in an electrical circuit, all elements
of a proton wire need not be connected at the same time
and, moreover, internal water molecules, which are not al-
ways apparent in protein X-ray structures, are likely to be
integral parts of the proton wire. Hence, elucidating the
precise pathway of proton transport through a protein is a
difficult and uncertain task. Note that proton pumps, like
other active transport systems, must be gated to prevent
protons from leaking back through the pump and thus
short-circuiting the system.
h. Bacteriorhodopsin Is a Light-Driven Proton Pump
The simplest known and best characterized proton
pump is the intrinsic membrane protein bacteriorhodopsin
of Halobacterium halobium. It consists mainly of seven
transmembrane helices, A through G, that form a central
polar channel (Section 12-3Ab). This channel contains a
retinal prosthetic group that is covalently linked, via a pro-
tonated Schiff base, to Lys 216, which extends from helix G
(Fig. 12-24). The protein obtains the free energy required
for unidirectionally pumping protons from the absorption
of a photon by the retinal. This initiates a sequence of
events in which the protein conformationally adjusts
through successive spectroscopically characterized inter-
mediates, designated J, K, L, M, N, and O, as the system de-
cays back to its ground state over a period of ⬃10 ms. The
result of this cycle is the net translocation of one proton
from the cytoplasm to the extracellular medium, thereby
converting light energy to proton-motive force. The as yet
incompletely understood mechanism of this process, which
was elucidated through detailed structural, mutational, and
time-resolved spectroscopic studies carried out in several
laboratories, is outlined in Fig. 22-34:
1. On absorbing a photon, the ground state all-trans-
retinal photoisomerizes to its 13-cis form. This is a multi-
step process that rapidly passes (in ⬃3 ps) through the J
and K states. The free end of the retinal, which is now
twisted around the newly cis double bond, moves relative
to the protein scaffold such that retinal’s C13 methyl group
and its C14 atom shift toward the inside by 1.3 and 1.7 Å,
respectively. This yields the L state.
2. Further conformational adjustments yield the M
state. Here the N atom of the Schiff base has rotated and
shifted from its ground state position, in which it is hydro-
gen bonded to an internal water molecule, to one in which
it points toward the inside face of the protein in the vicinity
of the hydrophobic side chains of Val 49 and Leu 93. This
reduces the pK of the protonated Schiff base. In contrast,
the pK of Asp 85 increases. This is because, in the ground
state,Asp 85 effectively serves as the counterion of the pro-
tonated Schiff base and participates in a hydrogen bonded
network with three internal water molecules, but in the M
state, it is only associated with a single water molecule.
Consequently, the Schiff base protonates Asp 85. This
process is facilitated by a slight movement of helix C that
850 Chapter 22. Electron Transport and Oxidative Phosphorylation
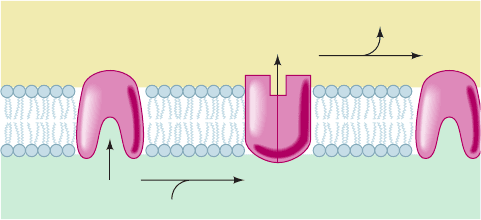
Figure 22-33 Proton pump mechanism of electron
transport–linked proton translocation. At each H
⫹
translocation
site, n protons bind to amino acid side chains on the matrix side
(inside) of the membrane. Reduction causes a conformational
change that decreases the pK’s of these side chains and exposes
them to the cytosolic side (outside) of the membrane, where the
protons dissociate. Reoxidation results in a conformational
change that restores the pump to its original conformation.
nH
+
nH
+
e
–
e
–
Intermembrane
space
Matrix
reduction
reoxidation
JWCL281_c22_823-870.qxd 6/8/10 9:19 AM Page 850
