Voet D., Voet Ju.G. Biochemistry
Подождите немного. Документ загружается.


Porter, D.J.T. and Bright, H.J., 3-Carbanionic substrate analogues
bind very tightly to fumarase and aspartase, J. Biol. Chem. 255,
4772–4780 (1980).
Remington, S.J., Structure and mechanism of citrate synthase,
Curr. Top. Cell Regul. 33, 202–229 (1992); and Mechanisms of
citrate synthase and related enzymes (triose phosphate iso-
merase and mandelate racemase), Curr. Opin. Struct. Biol. 2,
730–735 (1992).
Wolodk,W.T., Fraser, M.E., James, M.N.G., and Bridger,W.A.,The
crystal structure of succinyl-CoA synthetase from Escherichia
coli at 2.5 Å resolution, J. Biol. Chem. 269, 10883–10890 (1994).
Zheng, L., Kennedy, M.C., Beinert, H., and Zalkin, H. Mutational
analysis of active site residues in pig heart aconitase, J. Biol.
Chem. 267, 7895–7903 (1992).
Metabolic Poisons
Gibble, G.W., Fluoroacetate toxicity, J. Chem. Educ. 50, 460–462
(1973).
Jones, D.E.H. and Ledingham, K.W.D., Arsenic in Napoleon’s
wallpaper, Nature 299, 626–627 (1982).
Lauble, H., Kennedy, M.C., Emptage, M.H., Beinert,H., and Stout,
C.D., The reaction of fluorocitrate with aconitase and the crys-
tal structure of the enzyme-inhibitor complex, Proc. Natl.
Acad. Sci. 93, 13699–13703 (1996).
Winslow, J.H., Darwin’s Victorian Malady, American Philosophi-
cal Society (1971).
Control Mechanisms
Hurley, J.H., Dean, A.M., Sohl, J.L., Koshland, D.E., Jr., and
Stroud, R.M., Regulation of an enzyme by phosphorylation at
the active site, Science 249, 1012–1016 (1990).
Owen, O.E., Kalhan, S.C., and Hanson, R.W., The key role of
anaplerosis and cataplerosis for citric acid cycle function, J.
Biol. Chem. 277, 30409–30412 (2002).
Reed, L.J., Damuni, Z., and Merryfield, M.L., Regulation of mam-
malian pyruvate and branched-chain ␣-keto-acid dehydroge-
nase complexes by phosphorylation and dephosphorylation,
Curr. Top. Cell. Regul. 27, 41–49 (1985).
Srere, P.A., Sherry, A.D., Malloy, C.R., and Sumegi, B., Chan-
nelling in the Krebs tricarboxylic acid cycle, in Agius, L. and
Sherratt, H.S.A. (Eds.), Channelling in Intermediary Metabo-
lism, pp. 201–217, Portland Press (1997).
Stroud, R.M., Mechanisms of biological control by phosphoryla-
tion, Curr. Opin. Struct. Biol. 1, 826–835 (1991). [Reviews,
among other things, the inactivation of isocitrate dehydroge-
nase by phosphorylation.]
Vélot, C., Mixon, M.B., Teige, M., and Srere, P.A., Model of a
quinary structure between Krebs TCA cycle enzymes: A model
for the metabolon, Biochemistry 36, 14271–14276 (1997).
Vélot, C. and Srere,P.A., Reversible transdominant inhibition of a
metabolic pathway. In vivo evidence of interaction between
two sequential tricarboxylic acid cycle enzymes in yeast. J.
Biol. Chem. 275, 12926–12933 (2000).
Problems 821
1. Trace the course of the radioactive label in [2-
14
C]glucose
through glycolysis and the citric acid cycle.At what point(s) in the
cycle will the radioactivity be released as
14
CO
2
? How many turns
of the cycle will be required for complete conversion of the ra-
dioactivity to CO
2
? Repeat this problem for pyruvate that is
14
C-
labeled at its methyl group.
2. The reaction of glutathione reductase with an excess of
NADPH in the presence of arsenite yields a nonphysiological
four-electron reduced form of the enzyme. What is the chemical
nature of this catalytically inactive species?
3. Two-electron reduced dihydrolipoyl dehydrogenase (EH
2
),
but not the oxidized enzyme (E), reacts with iodoacetate
(ICH
2
COO
⫺
) to yield an inactive enzyme. Explain.
4. Given the following information, calculate the physiologi-
cal ⌬G of the isocitrate dehydrogenase reaction at 25°C and pH
7.0: [NAD
⫹
]/[NADH] ⫽ 8; [␣-ketoglutarate] ⫽ 0.1 mM; [isoci-
trate] ⫽ 0.02 mM; assume standard conditions for CO
2
(⌬G°¿ is
given in Table 21-2). Is this reaction a likely site for metabolic con-
trol? Explain.
5. The oxidation of acetyl-CoA to two molecules of CO
2
in-
volves the transfer of four electron pairs to redox coenzymes. In
which of the cycle’s reactions do these electron transfers occur?
Identify the redox coenzyme in each case. For each reaction, draw
the structural formulas of the reactants, intermediates, and
products and show, using curved arrows, how the electrons are
transferred.
6. The citrate synthase reaction has been proposed to proceed
via the formation of the enol(ate) form of acetyl-CoA. How, then,
would you account for the observation that
3
H is not incorporated
into acetyl-CoA when acetyl-CoA is incubated with citrate syn-
thase in
3
H
2
O?
7. Malonate is a competitive inhibitor of succinate in the suc-
cinate dehydrogenase reaction. (a) Sketch the graphs that would
be obtained on plotting 1/v versus 1/[succinate] at three different
malonate concentrations. Label the lines for low, medium, and
high [malonate]. (b) Explain why increasing the oxaloacetate con-
centration in a cell can overcome malonate inhibition.
8. Krebs found that malonate inhibition of the citric acid cycle
could be overcome by raising the oxaloacetate concentration. Ex-
plain the mechanism of this process in light of your findings in
Problem 7.
*9. (2R,3R)-2-Fluorocitrate contains F in the pro-S carboxy-
methyl arm of citrate [note that the rules of organic nomenclature re-
quire that atom C2 in citrate (Fig. 21-20) be renumbered as C4 in
(2R,3R)-2-fluorocitrate]. This compound, but not its diastereomer, is
a potent inhibitor of aconitase. (a) Draw the aconitase-catalyzed re-
action pathway of (2R,3R)-2-fluorocitrate assuming it follows the
same reaction pathway as citrate (Fig. 21-20). (b) Aconitase, in fact,
does not catalyze the foregoing reaction with (2R,3R)-2-fluorocitrate
but, rather, yields the following tight-binding inhibitor:
Draw an alternative aconitase-catalyzed reaction that would gener-
ate this inhibitor. (c) Draw the aconitase-catalyzed reaction of
H
–
OOC
H
OH
COO
–
COO
–
C
C
C
PROBLEMS
JWCL281_c21_789-822.qxd 3/17/10 11:34 AM Page 821
(2S,3R)-3-fluorocitrate,the diastereomer of (2R,3R)-2-fluorocitrate
(fluorocitrate containing F in the pro-R carboxymethyl arm of
citrate; here the atom numbering scheme is the same as that in
Fig. 21-20). Would a tight-binding inhibitor be formed?
10. Which of the following metabolites undergo net oxidation
by the citric acid cycle: (a) ␣-ketoglutarate, (b) succinate, (c) cit-
rate, and (d) acetyl-CoA?
11. Although there is no net synthesis of intermediates by the cit-
ric acid cycle, citric acid cycle intermediates are used in biosynthetic
reactions such as the synthesis of porphyrins from succinyl-CoA.
Write a reaction for the net synthesis of succinyl-CoA from pyruvate.
12. Oxaloacetate and ␣-ketoglutarate are precursors of the
amino acids aspartate and glutamate as well as being catalytic in-
termediates in the citric acid cycle. Describe the net synthesis of
␣-ketoglutarate from pyruvate in which no citric acid cycle inter-
mediates are depleted.
13. Lipoic acid is bound to enzymes that catalyze oxidative
decarboxylation of ␣-keto acids. (a) What is the chemical mode of
attachment of lipoic acid to enzymes? (b) Using chemical struc-
tures, show how lipoic acid participates in the oxidative decar-
boxylation of ␣-keto acids.
14. British anti-lewisite (BAL), which was designed to
counter the effects of the arsenical war gas lewisite, is useful in
treating arsenic poisoning. Explain.
15. The first organisms on Earth may have been chemoau-
totrophs in which the citric acid cycle operated in reverse to “fix”
atmospheric CO
2
in organic compounds. Complete a catalytic cy-
cle that begins with the hypothetical overall reaction succinate ⫹
2 CO
2
→ citrate.
16. Why is it advantageous for citrate, the product of Reaction 1
of the citric acid cycle, to inhibit phosphofructokinase, which
catalyzes the third reaction of glycolysis?
17. Anaplerotic reactions permit the citric acid cycle to supply
intermediates to biosynthetic pathways while maintaining the
proper levels of cycle intermediates.Write the equation for the net
synthesis of citrate from pyruvate.
18. Many amino acids are broken down to intermediates of
the citric acid cycle. (a) Why can’t these amino acid “remnants” be
directly oxidized to CO
2
by the citric acid cycle? (b) Explain why
amino acids that are broken down to pyruvate can be completely
oxidized by the citric acid cycle.
CH
2
SH
CH
2
OH
CH CH CHCl AsCl
2
SH
LewisiteBritish anti-lewisite
(BAL)
822 Chapter 21. Citric Acid Cycle
JWCL281_c21_789-822.qxd 3/17/10 11:34 AM Page 822
823
[H
+
]Low
ATP
ADP
+ P
i
H
2
OO
2
2
1
–
–
–
–
–
––
–
–
–
–
–
–
–
H
+
H
+
H
+
H
+
+
+
+
+
+
+
+
+
+
+
+
[H
+
]High
e
–
I III IV
V
CHAPTER 22
Electron Transport
and Oxidative
Phosphorylation
1 The Mitochondrion
A. Mitochondrial Anatomy
B. Mitochondrial Transport Systems
2 Electron Transport
A. Thermodynamics of Electron Transport
B. The Sequence of Electron Transport
C. Components of the Electron-Transport Chain
3 Oxidative Phosphorylation
A. Energy Coupling Hypotheses
B. Proton Gradient Generation
C. Mechanism of ATP Synthesis
D. Uncoupling of Oxidative Phosphorylation
4 Control of ATP Production
A. Control of Oxidative Phosphorylation
B. Coordinated Control of ATP Production
C. Physiological Implications of Aerobic versus
Anaerobic Metabolism
In 1789,Armand Séguin and Antoine Lavoisier (the father
of modern chemistry) wrote:
. . . in general, respiration is nothing but a slow combustion of
carbon and hydrogen, which is entirely similar to that which
occurs in a lamp or lighted candle, and that, from this point of
view, animals that respire are true combustible bodies that burn
and consume themselves.
Lavoisier had by this time demonstrated that living ani-
mals consume oxygen and generate carbon dioxide. It was
not until the early twentieth century, however,after the rise
of enzymology, that it was established, largely through the
work of Otto Warburg, that biological oxidations are cat-
alyzed by intracellular enzymes.As we have seen,glucose is
completely oxidized to CO
2
through the enzymatic reac-
tions of glycolysis and the citric acid cycle. In this chapter
we shall examine the fate of the electrons that are removed
from glucose by this oxidation process.
The complete oxidation of glucose by molecular oxygen
is described by the following redox equation:
To see more clearly the transfer of electrons, let us break
this equation down into two half-reactions. In the first half-
reaction the glucose carbon atoms are oxidized:
¢G°¿ ⫽⫺2823 kJ ⴢ mol
⫺1
C
6
H
12
O
6
⫹ 6O
2
¡
6CO
2
⫹ 6H
2
O
and in the second, molecular oxygen is reduced:
In living systems, the electron-transfer process connecting
these half-reactions occurs through a multistep pathway that
harnesses the liberated free energy to form ATP.
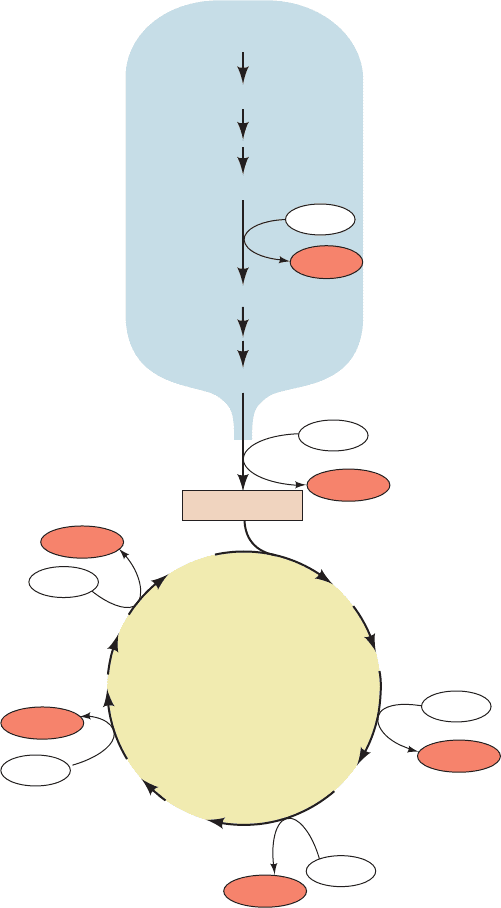
The 12 electron pairs involved in glucose oxidation are
not transferred directly to O
2
. Rather, as we have seen, they
are transferred to the coenzymes NAD
⫹
and FAD to form 10
NADH and 2 FADH
2
(Fig. 22-1) in the reactions catalyzed
by the glycolytic enzyme glyceraldehyde-3-phosphate de-
hydrogenase (Section 17-2F), pyruvate dehydrogenase
(Section 21-2A), and the citric acid cycle enzymes isocitrate
dehydrogenase, ␣-ketoglutarate dehydrogenase, succinate
dehydrogenase (the only FAD reduction), and malate de-
hydrogenase (Section 21-3). The electrons then pass into the
electron-transport chain (alternatively, the respiratory
chain), where, through reoxidation of NADH and FADH
2
,
they participate in the sequential oxidation–reduction of over
10 redox centers before reducing O
2
to H
2
O. In this process,
protons are expelled from the mitochondrion. The free en-
ergy stored in the resulting pH gradient drives the synthesis
of ATP from ADP and P
i
through oxidative phosphoryla-
tion. Reoxidation of each NADH results in the synthesis of
⬃2.5 ATP,and reoxidation of FADH
2
yields ⬃1.5 ATP for a
total of ⬃32 ATP for each glucose completely oxidized to
CO
2
and H
2
O (including the 2 ATP made in glycolysis and
the 2 ATP made in the citric acid cycle).
In this chapter we explore the mechanisms of electron
transport and oxidative phosphorylation and their regula-
tion.We begin with a discussion of mitochondrial structure
and transport systems.
1 THE MITOCHONDRION
The mitochondrion (Section 1-2Ac) is the site of eukaryotic
oxidative metabolism. It contains, as Albert Lehninger and
Eugene Kennedy demonstrated in 1948, the enzymes that
mediate this process, including pyruvate dehydrogenase,
the citric acid cycle enzymes, the enzymes catalyzing fatty
acid oxidation (Section 25-2C), and the enzymes and redox
proteins involved in electron transport and oxidative phos-
phorylation. It is therefore with good reason that the mito-
chondrion is described as the cell’s “power plant.”
6O
2
⫹ 24H
⫹
⫹ 24e
⫺
¡
12H
2
O
C
6
H
12
O
6
⫹ 6H
2
O
¡
6CO
2
⫹ 24H
⫹
⫹ 24e
⫺
JWCL281_c22_823-870.qxd 6/8/10 9:18 AM Page 823
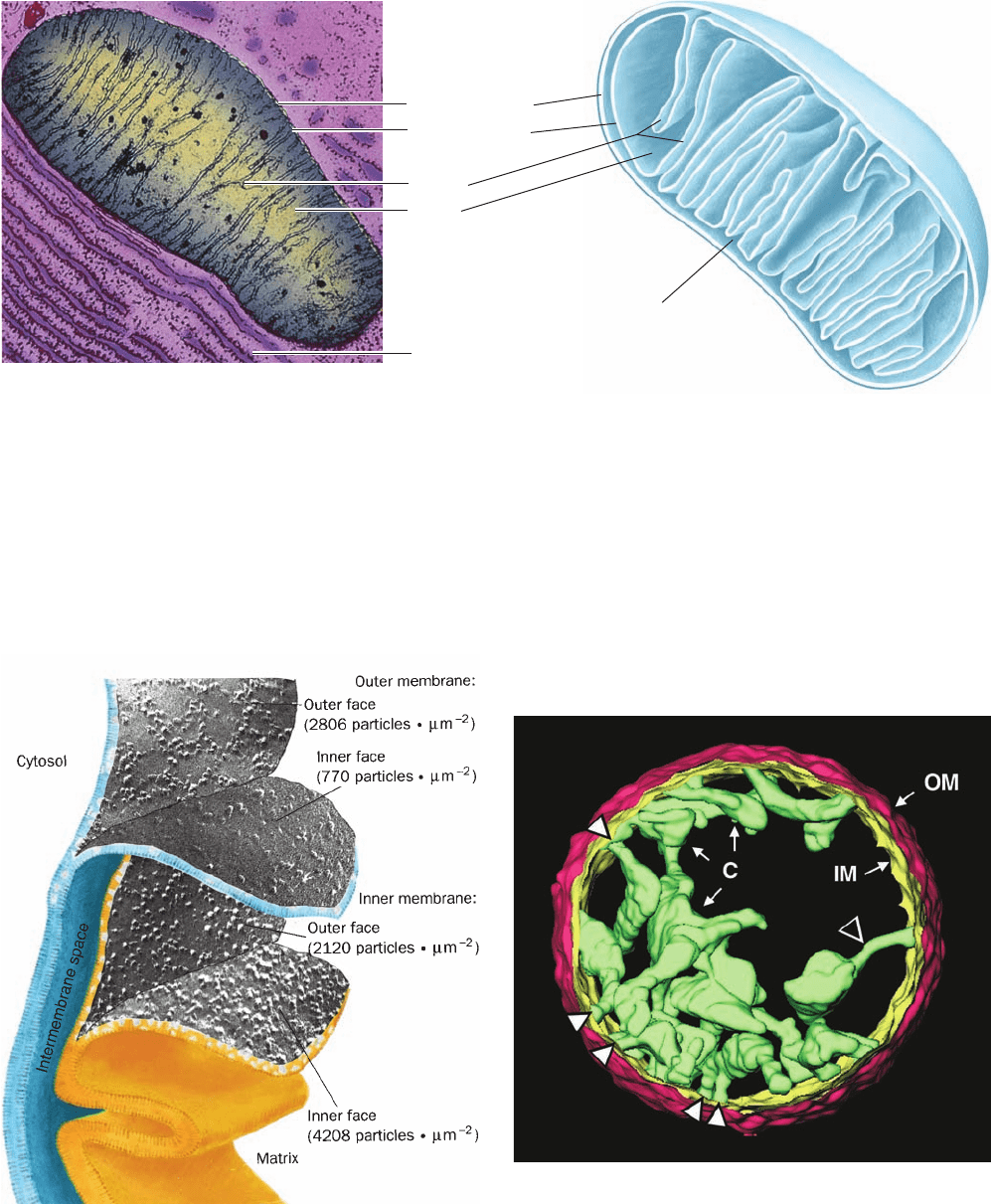
A. Mitochondrial Anatomy
Mitochondria vary considerably in size and shape depend-
ing on their source and metabolic state. They are typically
ellipsoids ⬃0.5 m in diameter and 1 m in length (about
the size of a bacterium; Fig. 22-2). The mitochondrion is
bounded by a smooth outer membrane and contains an ex-
tensively invaginated inner membrane. The number of in-
vaginations, called cristae, varies with the respiratory activ-
ity of the particular type of cell.This is because the proteins
mediating electron transport and oxidative phosphoryla-
tion are bound to the inner mitochondrial membrane, so
that the respiration rate varies with membrane surface
area. Liver, for example, which has a relatively low respira-
tion rate, contains mitochondria with relatively few cristae,
whereas those of heart muscle contain many. Nevertheless,
the aggregate area of the inner mitochondrial membranes
in a liver cell is ⬃15-fold greater than that of its plasma
membrane.
The inner mitochondrial compartment consists of a gel-
like substance of ⬍50% water, named the matrix, which
contains remarkably high concentrations of the soluble en-
zymes of oxidative metabolism (e.g., citric acid cycle en-
zymes), as well as substrates, nucleotide cofactors, and inor-
ganic ions. The matrix also contains the mitochondrial
genetic machinery—DNA, RNA, and ribosomes—that in
mammals expresses only 13 mitochondrial inner mem-
brane proteins together with 22 tRNAs and two ribosomal
RNAs.
a. The Inner Mitochondrial Membrane and Cristae
Compartmentalize Metabolic Functions
The outer mitochondrial membrane contains porin, a
protein that forms nonspecific pores that permit free diffu-
sion of up to 10-kD molecules (the X-ray structures of bac-
terial porins are discussed in Sections 12-3Ad and 20-2D).
The inner membrane, which is ⬃75% protein by mass, is
considerably richer in proteins than the outer membrane
(Fig. 22-3). It is freely permeable only to O
2
,CO
2
, and H
2
O
and contains, in addition to respiratory chain proteins, nu-
merous transport proteins that control the passage of
metabolites such as ATP, ADP, pyruvate, Ca
2⫹
, and phos-
phate (see below). This controlled impermeability of the in-
ner mitochondrial membrane to most ions, metabolites, and
low molecular mass compounds permits the generation of
ionic gradients across this barrier and results in the compart-
mentalization of metabolic functions between cytosol and
mitochondria.
Two-dimensional electron micrographs of mitochondria
such as Fig. 22-2a suggest that cristae resemble baffles and
that the intercristal spaces communicate freely with the mi-
tochondrion’s intermembrane space, as Fig. 22-2b implies.
However, electron microscopy–based three-dimensional
image reconstruction methods have revealed that cristae
can vary in shape from simple tubular entities to more
complicated lamellar assemblies that merge with the inner
membrane via narrow tubular structures (Fig. 22-4). Evi-
dently, cristae form microcompartments that restrict the
diffusion of substrates and ions between the intercristal
and intermembrane spaces. This has important functional
implications because it would result in a locally greater pH
gradient across cristal membranes than across inner mem-
branes that are not part of cristae, thereby significantly
influencing the rate of oxidative phosphorylation (Sec-
tion 22-3).
B. Mitochondrial Transport Systems
The inner mitochondrial membrane is impermeable to
most hydrophilic substances. It must therefore contain spe-
cific transport systems to permit the following processes:
824 Chapter 22. Electron Transport and Oxidative Phosphorylation
Figure 22-1 The sites of electron transfer that form NADH
and FADH
2
in glycolysis and the citric acid cycle.
2 NAD
+
2 NADH
2 NADH
2 NAD
+
2 FAD
2 FADH
2
2 NADH
2 NAD
+
2 NADH
2 NAD
+
Glucose-6-phosphate
2 Glyceraldehyde-3-phosphate
2 NAD
+
2 NADH
glyceraldehyde-
3-phosphate
dehydrogenase
2 Pyruvate
2 1,3-Bisphosphoglycerate
pyruvate
dehydrogenase
2 Acetyl-CoA
2 Oxaloacetate
2 Malate
2 Fumarate
2 Succinate
2 Succinyl-CoA
2 α-Ketoglutarate
2 Isocitrate
2 Citrate
malate
dehydrogenase
succinate
dehydrogenase
isocitrate
dehydrogenase
α-ketoglutarate
dehydrogenase
Glucose
Citric acid
cycle
Glycolysis
JWCL281_c22_823-870.qxd 3/19/10 10:59 AM Page 824
Outer membrane
Inner membrane
Cristae
Matrix
Intermembrane space
(b)(a)
Rough endoplasmic reticulum
1. Glycolytically produced cytosolic NADH must gain
access to the electron-transport chain for aerobic oxida-
tion.
2. Mitochondrially produced metabolites such as ox-
aloacetate and acetyl-CoA, the respective precursors for
cytosolic glucose and fatty acid biosynthesis, must reach
their metabolic destinations.
3. Mitochondrially produced ATP must reach the cy-
tosol, where most ATP-utilizing reactions take place,
whereas ADP and P
i
, the substrates for oxidative phospho-
rylation, must enter the mitochondrion.
Section 22-1. The Mitochondrion 825
Figure 22-2 Mitochondria. (a) An electron micrograph of an animal mitochondrion.
[K.R. Porter/Photo Researchers, Inc.] (b) Cutaway diagram of a mitochondrion.
Figure 22-4 Electron microscopy–based three-dimensional
image reconstruction of a rat liver mitochondrion. The outer
membrane (OM) is red, the inner membrane (IM) is yellow, and
the cristae (C) are green.The arrowheads point to tubular
regions of the cristae that connect them to the inner membrane
and to each other. [Courtesy of Carmen Mannella,Wadsworth
Center,Albany, New York.]
Figure 22-3 Freeze-fracture and freeze-etch electron
micrographs of the inner and outer mitochondrial membranes.
The inner membrane contains about twice the density of
embedded particles as does the outer membrane. [Courtesy of
Lester Packer, University of California at Berkeley.]
JWCL281_c22_823-870.qxd 3/19/10 10:59 AM Page 825

We have already studied the ADP–ATP translocator and
its dependence on ⌬⌿, the electric potential difference
across the mitochondrial membrane (Section 20-4C). The
export mechanisms of oxaloacetate and acetyl-CoA from
the mitochondrion are, respectively, discussed in Sections
23-1Ag and 25-4D. In the remainder of this section we ex-
amine the mitochondrial transport systems for P
i
and Ca
2⫹
and the shuttle systems for NADH.
a. P
i
Transport
ATP is generated from ADP ⫹ P
i
in the mitochondrion
but is utilized in the cytosol.The P
i
produced is returned to
the mitochondrion by the phosphate carrier, an electroneu-
tral P
i
–H
⫹
symport that is driven by ⌬pH. The proton that
accompanies the P
i
into the mitochondrion had, in effect,
been previously expelled from the mitochondrion by the
redox-driven pumps of the electron-transport chain (Sec-
tion 22-3B). The electrochemical potential gradient gener-
ated by these proton pumps is therefore responsible for
maintaining high mitochondrial ADP and P
i
concentra-
tions in addition to providing the free energy for ATP
synthesis.
b. Ca
2⫹
Transport
Since Ca
2⫹
, like cAMP, functions as a second messenger
(Section 18-3Ce), its concentrations in the various cellular
compartments must be precisely controlled.The mitochon-
drion, endoplasmic reticulum, and extracellular spaces act
as Ca
2⫹
storage tanks.We studied the Ca
2⫹
–ATPases of the
plasma membrane, endoplasmic reticulum, and sarcoplas-
mic reticulum in Section 20-3B. Here we consider the mito-
chondrial Ca
2⫹
transport systems.
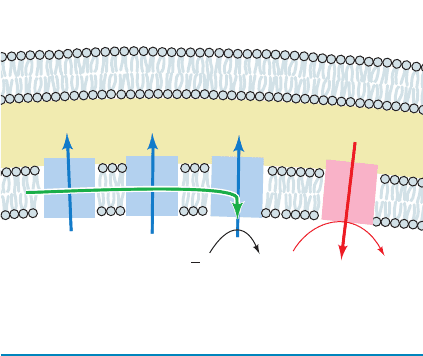
Mitochondrial inner membrane systems separately me-
diate the influx and the efflux of Ca
2⫹
(Fig. 22-5). The Ca
2⫹
influx is driven by the inner mitochondrial membrane’s
membrane potential (⌬°, inside negative), which attracts
positively charged ions. The rate of influx varies with the
external [Ca
2⫹
] because the K
M
for Ca
2⫹
transport by this
system is greater than the cytosolic Ca
2⫹
concentration.
In heart, brain, and skeletal muscle mitochondria espe-
cially, Ca
2⫹
efflux is independently driven by the Na
⫹
gra-
dient across the inner mitochondrial membrane. Ca
2⫹
exits
the matrix only in exchange for Na
⫹
, so that this system is an
antiport. This exchange process normally operates at its
maximal velocity. Mitochondria (as well as the endoplasmic
and sarcoplasmic reticulum) therefore can act as a “buffer”
for cytosolic Ca
2⫹
(Fig. 22-6): If cytosolic [Ca
2⫹
] rises, the
rate of mitochondrial Ca
2⫹
influx increases while that of
Ca
2⫹
efflux remains constant, causing the mitochondrial
[Ca
2⫹
] to increase while the cytosolic [Ca
2⫹
] decreases to
its original level (its set point). Conversely, a decrease in cy-
tosolic [Ca
2⫹
] reduces the influx rate, causing net efflux of
[Ca
2⫹
] and an increase of cytosolic [Ca
2⫹
] back to the set
point.
Oxidation carried out by the citric acid cycle in the mito-
chondrial matrix is controlled by the matrix [Ca
2⫹
] (Section
21-4c). It is interesting to note, therefore, that in response
to increases in cytosolic [Ca
2⫹
] caused by increased muscle
activity, the matrix [Ca
2⫹
] increases, thereby activating
the enzymes of the citric acid cycle. This leads to an in-
crease in [NADH], whose reoxidation by oxidative phos-
phorylation (as we study in this chapter) generates the
ATP needed for this increased muscle activity.
826 Chapter 22. Electron Transport and Oxidative Phosphorylation
Figure 22-5 The two mitochondrial Ca
2ⴙ
transport systems.
System 1 mediates Ca
2⫹
influx to the matrix in response to the
membrane potential (negative inside). System 2 mediates Ca
2⫹
efflux in exchange for Na
⫹
.
H
+
Na
+
Ca
2+
(efflux)
(influx)
Ca
2+
Matrix
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
–
–
–
–
–
–
–
–
–
–
–
–
–
–
–
–
–
–
–
–
2 1
Electron transport
Intermembrane
space
10
3
1
0.5 1.0 2.0
Cytosolic concentration of Ca
2+
(μM)
Activity of uptake or efflux pathway
(nmol Ca
2+
•
min
–1
•
mg
–1
)
Set point
Efflux pathway
Uptake pathway
Net uptake
Net efflux
Figure 22-6 The regulation of cytosolic [Ca
2ⴙ
]. The efflux
pathway operates at a constant rate independent of [Ca
2⫹
],
whereas the activity of the influx pathway varies with [Ca
2⫹
].At
the set point, the activities of the two pathways are equal and
there is no net Ca
2⫹
flux.An increase in cytosolic [Ca
2⫹
] results
in net mitochondrial influx, and a decrease in cytosolic [Ca
2⫹
]
results in net mitochondrial efflux. Both effects lead to the
restoration of the cytosolic [Ca
2⫹
]. [After Nicholls, D., Trends
Biochem. Sci. 6, 37 (1981).]
JWCL281_c22_823-870.qxd 3/19/10 10:59 AM Page 826
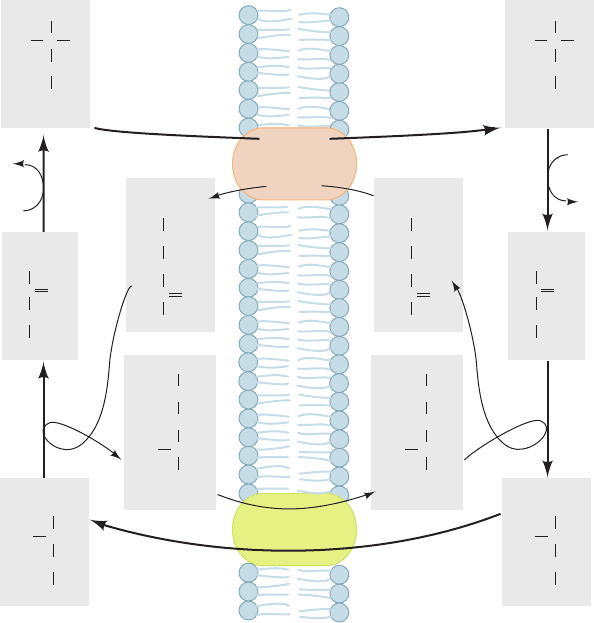
c. Cytoplasmic Shuttle Systems “Transport” NADH
Across the Inner Mitochondrial Membrane
Although most of the NADH generated by glucose oxi-
dation is formed in the mitochondrial matrix via the citric
acid cycle, that generated by glycolysis occurs in the cy-
tosol. Yet the inner mitochondrial membrane lacks an
NADH transport protein. Only the electrons from cytosolic
NADH are transported into the mitochondrion by one of
several ingenious “shuttle” systems. In the malate–aspartate
shuttle (Fig. 22-7), which functions in heart, liver, and kid-
ney, mitochondrial NAD
⫹
is reduced by cytosolic NADH
through the intermediate reduction and subsequent regen-
eration of oxaloacetate. This process occurs in two phases
of three reactions each:
Phase A (transport of electrons into the matrix):
1. In the cytosol, NADH reduces oxaloacetate to yield
NAD
⫹
and malate in a reaction catalyzed by cytosolic
malate dehydrogenase.
2. The malate–␣-ketoglutarate carrier transports
malate from the cytosol to the mitochondrial matrix in ex-
change for ␣-ketoglutarate from the matrix.
3. In the matrix, NAD
⫹
reoxidizes malate to yield
NADH and oxaloacetate in a reaction catalyzed by mito-
chondrial malate dehydrogenase (Section 21-3H).
Phase B (regeneration of cytosolic oxaloacetate):
4. In the matrix, a transaminase (Section 26-1A) con-
verts oxaloacetate to aspartate with the concomitant con-
version of glutamate to ␣-ketoglutarate.
5. The glutamate–aspartate carrier transports aspartate
from the matrix to the cytosol in exchange for cytosolic
glutamate.
6. In the cytosol, a transaminase converts aspartate
to oxaloacetate with the concomitant conversion of
␣-ketoglutarate to glutamate.
The electrons of cytosolic NADH are thereby transferred
to mitochondrial NAD
⫹
to form NADH, which is subject
to reoxidation via the electron-transport chain. The
malate–aspartate shuttle yields ⬃2.5 ATPs for every cytoso-
lic NADH. Note, however, that every NADH that enters
the matrix is accompanied by a proton which, as we shall
Section 22-1. The Mitochondrion 827
H
3
N
Aspartate
COO
–
CH
2
CH
COO
–
+
α -Ketoglutarate
COO
–
CH
2
CH
2
CO
COO
–
Cytosol MatrixInner
mitochondrial
membrane
3
malate–
-ketoglutarate
carrier
α
2
NADH + H
+
NAD
+
malate dehydrogenase
COO
–
CH
2
C
COO
–
HHO
Malate
COO
–
CH
2
CO
COO
–
Oxaloacetate
H
3
N
Aspartate
COO
–
CH
2
CH
COO
–
+
Glutamate
COO
–
CH
2
CH
2
H
3
N CH
+
COO
–
aspartate
aminotransferase
glutamate–
aspartate carrier
5
4
α -Ketoglutarate
COO
–
CH
2
CH
2
CO
COO
–
1
NAD
+
malate dehydrogenase
COO
–
CH
2
C
COO
–
HHO
Malate
COO
–
CH
2
CO
COO
–
Oxaloacetate
Glutamate
COO
–
CH
2
CH
2
H
3
N CH
+
COO
–
6
aspartate
aminotransferase
H
+
+ NADH
Figure 22-7 The malate–aspartate shuttle. The electrons of
cytosolic NADH are transported to mitochondrial NADH
(shown in red as hydride transfers) in Steps 1 to 3. Steps 4 to 6
then serve to regenerate cytosolic oxaloacetate.
JWCL281_c22_823-870.qxd 3/19/10 10:59 AM Page 827
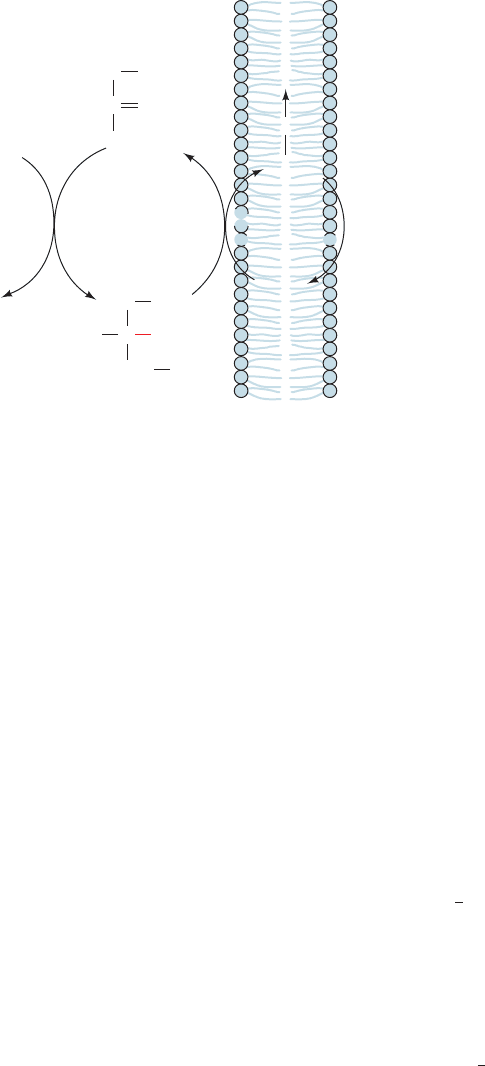
see (Section 22-3C), would otherwise be used to generate
⬃0.3 ATP. Consequently, every cytosolic NADH that is
translocated to the matrix by the malate–aspartate shuttle
yields ⬃2.2 ATPs.
The glycerophosphate shuttle (Fig. 22-8), which is sim-
pler but less energy efficient than the malate–aspartate
shuttle, occurs in brain and skeletal muscle and is particu-
larly prominent in insect flight muscle (the tissue with the
largest known sustained power output—about the same
power-to-weight ratio as a small automobile engine). In it,
glycerol-3-phosphate dehydrogenase catalyzes the oxida-
tion of cytosolic NADH by dihydroxyacetone phosphate to
yield NAD
⫹
, which reenters glycolysis.The electrons of the
resulting glycerol-3-phosphate are transferred to flavopro-
tein dehydrogenase to form FADH
2
.This enzyme, which is
situated on the inner mitochondrial membrane’s outer sur-
face, supplies electrons to the electron-transport chain in a
manner similar to that of succinate dehydrogenase (Sec-
tion 22–2C2). The glycerophosphate shuttle therefore results
in the synthesis of ⬃1.5 ATPs for every cytoplasmic NADH
reoxidized, ⬃0.7 ATP less than the malate–aspartate shuttle.
However, the advantage of the glycerophosphate shuttle is
that, being essentially irreversible, it operates efficiently
even when the cytoplasmic NADH concentration is low
relative to that of NAD
⫹
, as occurs in rapidly metabolizing
tissues. In contrast, the malate–aspartate shuttle is re-
versible and hence is driven by concentration gradients.
2 ELECTRON TRANSPORT
In the electron-transport process, the free energy of electron
transfer from NADH and FADH
2
to O
2
via protein-bound
redox centers is coupled to ATP synthesis. We begin our
study of this process by considering its thermodynamics.
We then examine the path of electrons through the redox
centers of the system and discuss the experiments used to
unravel this pathway. Finally, we study the four complexes
that make up the electron-transport chain. In the next sec-
tion we discuss how the free energy harvested by the
electron-transport process is coupled to ATP synthesis.
A. Thermodynamics of Electron Transport
We can estimate the thermodynamic efficiency of electron
transport through knowledge of standard reduction poten-
tials. As we have seen in our thermodynamic considera-
tions of oxidation–reduction reactions (Section 16-5), an
oxidized substrate’s affinity for electrons increases with its
standard reduction potential, [the voltage generated by
the reaction of the half-cell under standard biochemical
conditions (1M reactants and products with [H
⫹
] defined
as 1 at pH 7) relative to the standard hydrogen electrode;
Table 16-4 lists the standard reduction potentials of several
half-reactions of biochemical interest].The standard reduc-
tion potential difference, for a redox reaction involv-
ing any two half-reactions is therefore expressed:
a. NADH Oxidation Is a Highly Exergonic Reaction
The half-reactions for O
2
oxidation of NADH are
(Table 16-4)
and
Since the O
2
/H
2
O half-reaction has the greater standard
reduction potential and therefore the higher electron affin-
ity, the NADH half-reaction is reversed, so that NADH is
the electron donor in this couple and O
2
the electron ac-
ceptor.The overall reaction is
so that
The standard free energy change for the reaction can then
be calculated from Eq. [16.7]:
where f, the Faraday constant, is 96,485 C ⴢ mol
–1
of elec-
trons and n is the number of electrons transferred per
¢G°¿ ⫽⫺nf ¢e°¿
¢e°¿ ⫽ 0.815 ⫺ (⫺0.315) ⫽ 1.130 V
1
2
O
2
⫹ NADH ⫹ H
⫹
Δ H
2
O ⫹ NAD
⫹
1
2
O
2
⫹ 2H
⫹
⫹ 2e
⫺
Δ H
2
O
e°¿ ⫽ 0.815 V
NAD
⫹
⫹ H
⫹
⫹ 2e
⫺
Δ NADH
e°¿ ⫽⫺0.315 V
¢e°¿ ⫽ e°¿
(e
⫺
acceptor)
⫺ e°¿
(e
⫺
donor)
¢e°¿,
e°¿
828 Chapter 22. Electron Transport and Oxidative Phosphorylation
Figure 22-8 The glycerophosphate shuttle. The electrons of
cytosolic NADH are transported to the mitochondrial electron-
transport chain in three steps (shown in red as hydride transfers):
(1) Cytosolic oxidation of NADH by dihydroxyacetone
phosphate catalyzed by glycerol-3-phosphate dehydrogenase.
(2) Oxidation of glycerol-3-phosphate by flavoprotein
dehydrogenase with the reduction of FAD to FADH
2
.
(3) Reoxidation of FADH
2
with the passage of electrons into
the electron-transport chain. Note that the glycerophosphate
shuttle is not a membrane transport system.
H
CH
2
OPO
3
2
–
CH
2
OPO
3
2–
OH
Inner
mitochondrial
membrane
Electron-
transport
chain
f lavoprotein
dehydrogenase
Matrix
Cytosol
12
FAD
FADH
2
2e
–
CO
H
2
C
H
2
C
Dihydroxyacetone
phosphate
glycerol-3-phosphate
dehydrogenase
NAD
+
NADH
OH
CHO
3
Glycerol-3-phosphate
H
+
+
JWCL281_c22_823-870.qxd 6/8/10 9:18 AM Page 828
mole of reactants. Thus, since 1 V ⫽ 1 J ⴢ C
–1
, for NADH
oxidation:
In other words, the oxidation of 1 mol of NADH by O
2
(the
transfer of 2e
⫺
) under standard biochemical conditions is
associated with the release of 218 kJ of free energy.
b. Electron Transport Is Thermodynamically Efficient
The standard free energy required to synthesize 1 mol
of ATP from ADP ⫹ P
i
is 30.5 kJ. The standard free energy
of oxidation of NADH by O
2
, if coupled to ATP synthesis,
is therefore sufficient to drive the formation of several
moles of ATP. This coupling, as we shall see, is achieved by
an electron-transport chain in which electrons are passed
through three protein complexes containing redox centers
with progressively greater affinity for electrons (increasing
standard reduction potentials) instead of directly to O
2
.
This allows the large overall free energy change to be broken
up into three smaller packets, each of which is coupled with
ATP synthesis in a process called oxidative phosphoryla-
tion. Oxidation of 1 NADH therefore results in the synthe-
sis of ⬃2.5 ATP. (Oxidation of FADH
2
, whose entrance
⫽⫺218 kJ ⴢ mol
⫺1
¢G°¿ ⫽⫺2
mol e
⫺
mol reactant
⫻ 96,485
C
mol e
⫺
⫻ 1.13 J ⴢ C
⫺1
into the electron-transport chain is regulated by a fourth
protein complex, is similarly coupled to the synthesis of
⬃1.5 ATP.) The thermodynamic efficiency of oxidative
phosphorylation is therefore 2.5 ⫻ 30.5 kJ ⴢ mol
⫺1
⫻
100/218 kJ ⴢ mol
⫺1
⫽ 35% under standard biochemical
conditions. However, under physiological conditions in
active mitochondria (where the reactant and product con-
centrations as well as the pH deviate from standard condi-
tions), this thermodynamic efficiency is thought to be
⬃70%. In comparison, the energy efficiency of a typical
automobile engine is ⬍30%.
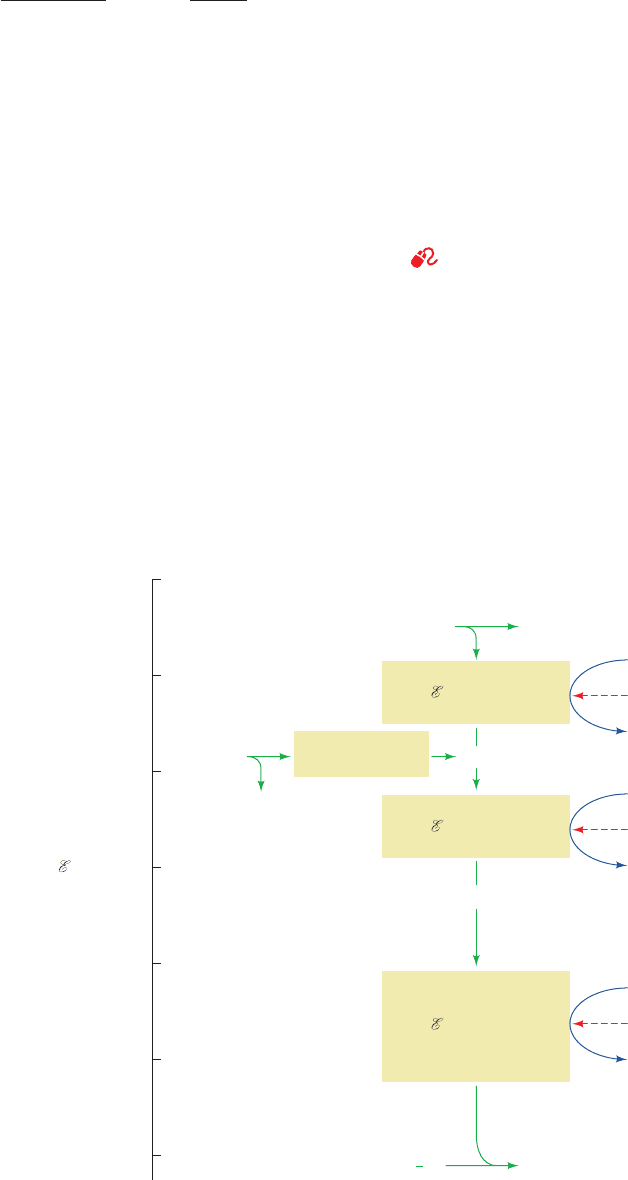
B. The Sequence of Electron Transport
See Guided Exploration 19: Electron transport and oxidative phos-
phorylation overview
The free energy necessary to generate ATP
is extracted from the oxidation of NADH and FADH
2
by the
electron-transport chain, a series of four protein complexes
through which electrons pass from lower to higher standard
reduction potentials (Fig. 22-9). Electrons are carried from
Complexes I and II to Complex III by coenzyme Q (CoQ
or ubiquinone; so named because of its ubiquity in respir-
ing organisms), and from Complex III to Complex IV by
the peripheral membrane protein cytochrome c (Sections
7-3B and 9-6A).
Section 22-2. Electron Transport 829
H
2
O2H
+
+ O
2
NADH NAD
+
(–0.315 V)
2e
–
Succinate
Fumarate
Complex
II
FADH
2
CoQ
(+0.031 V)
(+0.045 V)
Cytochrome c (+0.235 V)
(+0.815 V)
2e
–
2e
–
+0.8
+0.6
+0.4
+0.2
–0.2
–0.4
0
°′(V)
Complex I
Δ °′ = 0.360 V
(ΔG°′ = –69.5 kJ · mol
–1
)
Complex III
Δ °′ = 0.190 V
(ΔG°′ = –36.7 kJ · mol
–1
)
Complex IV
Δ °′ = 0.580 V
(ΔG°′ = –112 kJ · mol
–1
)
ADP + P
i
CN
–
ATP
ADP + P
i
Antimycin
A
ATP
ADP + P
i
Rotenone or
Amytal
ATP
2
1
Figure 22-9 The mitochondrial electron-transport chain. The
standard reduction potentials of its most mobile components
(green) are indicated, as are the points where sufficient free
energy is harvested to synthesize ATP (blue) and the sites of action
of several respiratory inhibitors (red). (Note that Complexes I,
III, and IV do not directly synthesize ATP but, rather, sequester
the free energy necessary to do so by pumping protons outside
the mitochondrion to form a proton gradient; Section 22-3.)
JWCL281_c22_823-870.qxd 3/19/10 10:59 AM Page 829
Top view
Platinum cathode
++
–
Silver anode
Bubble
escape
slit
Glass
sample
chamber
Sample
KCl
Bubble slit
Magnetic stirrer
O
2
-Permeable Teflon membrane
Lucite holder
Epoxy plug
Side view
Complex I catalyzes oxidation of NADH by CoQ:
Complex III catalyzes oxidation of CoQ (reduced) by cy-
tochrome c:
Complex IV catalyzes oxidation of cytochrome c (reduced)
by O
2
, the terminal electron acceptor of the electron-
transport process:
The changes in standard reduction potential of an electron
pair as it successively traverses Complexes I, III, and IV
correspond,at each stage,to sufficient free energy to power
the synthesis of nearly one ATP molecule.
Complex II catalyzes the oxidation of FADH
2
by CoQ.
This redox reaction does not release sufficient free energy to
synthesize ATP; it functions only to inject the electrons from
FADH
2
into the electron-transport chain.
¢G°¿ ⫽⫺16.4 kJ ⴢ mol
⫺1
¢e°¿ ⫽ 0.085 V
FADH
2
⫹ CoQ (oxidized)
¡
FAD ⫹ CoQ (reduced)
¢G°¿ ⫽⫺112 kJ ⴢ mol
⫺1
¢e°¿ ⫽ 0.580 V
2 cytochrome c (oxidized) ⫹ H
2
O
2 cytochrome c (reduced)
⫹
1
2
O
2
¡
¢G°¿ ⫽⫺36.7 kJ ⴢ mol
⫺1
¢e°¿ ⫽ 0.190 V
CoQ (oxidized) ⫹ 2 cytochrome c (reduced)
CoQ (reduced) ⫹ 2 cytochrome c (oxidized)
¡
¢G°¿ ⫽⫺69.5 kJ ⴢ mol
⫺1
¢e°¿ ⫽ 0.360 V
NAD
⫹
⫹ CoQ (reduced)
NADH ⫹ CoQ (oxidized)
¡
a. The Workings of the Electron-Transport Chain
Have Been Elucidated through the Use of Inhibitors
Our understanding of the sequence of events in elec-
tron transport is largely based on the use of specific in-
hibitors. This sequence has been corroborated by meas-
urements of the standard reduction potentials of the redox
components of each of the complexes as well as by deter-
mining the stoichiometry of electron transport and the
coupled ATP synthesis.
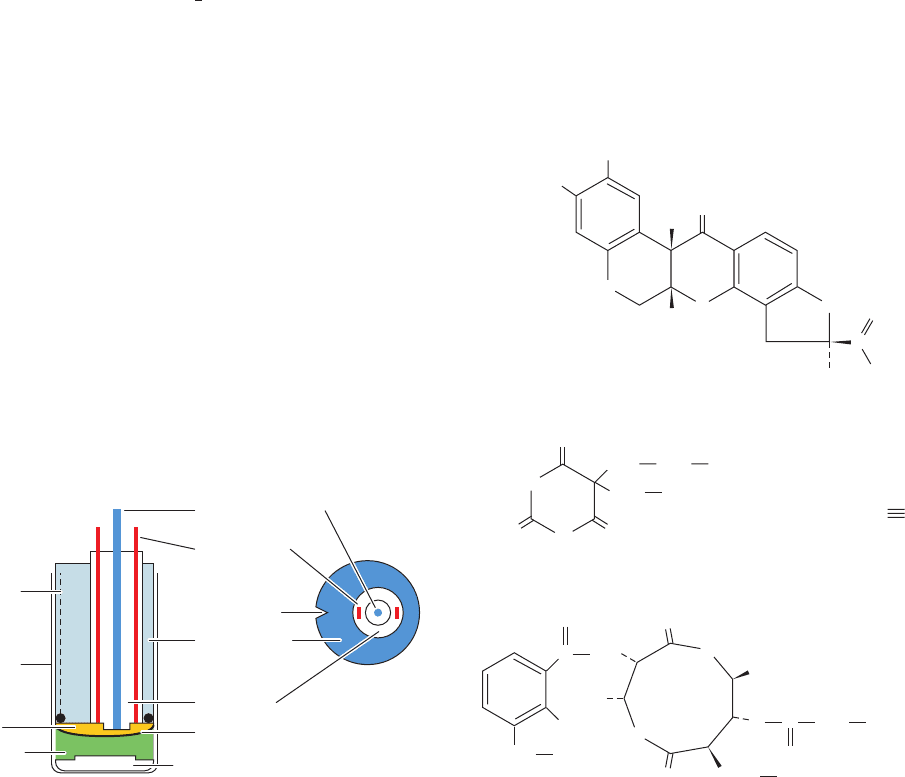
The rate at which O
2
is consumed by a suspension of mi-
tochondria is a sensitive measure of the functioning of the
electron-transport chain. It is conveniently measured with
an oxygen electrode (Fig. 22-10). Compounds that inhibit
electron transport, as judged by their effect on O
2
disap-
pearance in such an experimental system, have been
invaluable experimental probes in tracing the path of
electrons through the electron-transport chain and in
determining the points of entry of electrons from various
substrates. Among the most useful such substances are
rotenone (a plant toxin used by Amazonian Indians to poi-
son fish and which is also used as an insecticide), amytal (a
barbiturate), antimycin (an antibiotic), and cyanide:
The following experiment illustrates the use of these
inhibitors:
A buffered solution containing excess ADP and P
i
is
equilibrated in the reaction vessel of an oxygen electrode.
O
O
H
H
H
O
O
O
O
O
CH
3
O
OCH
3
C
CH
3
CH
2
CH
2
CH
2
(CH
2
)
5
H
3
C
CH
3
CH
3
CH
3
CH
2
CH
2
CH(CH
3
)
2
C
CH(CH
3
)
2
Rotenone
HN
O
O
O
O
O
O
O
N
H
–
CN
Amytal Cyanide
OH
NH
CHO
C
NH
Antimycin
830 Chapter 22. Electron Transport and Oxidative Phosphorylation
Figure 22-10 The oxygen electrode. This electrode consists of
an Ag/AgCl reference electrode and a Pt electrode, both
immersed in a KCl solution and in contact with the sample
chamber through an O
2
-permeable Teflon membrane. O
2
is
reduced to H
2
O at the Pt electrode, thereby generating a voltage
with respect to the Ag/AgCl electrode that is proportional to the
O
2
concentration in the sealed sample chamber. [After Cooper,
T.G., The Tools of Biochemistry, p. 69, Wiley (1977).]
JWCL281_c22_823-870.qxd 6/8/10 9:19 AM Page 830
