Voet D., Voet Ju.G. Biochemistry
Подождите немного. Документ загружается.

B. The Mechanism of Dihydrolipoyl Dehydrogenase
The reaction catalyzed by dihydrolipoyl dehydrogenase
(E3) is more complex than Reactions 4 and 5 in Fig. 21-6
suggest. Vincent Massey demonstrated that oxidized dihy-
drolipoyl dehydrogenase contains a “redox-active” disulfide
bond, which in the enzyme’s reduced form has accepted an
electron pair through bond cleavage to form a dithiol:
He established this through the following observations in-
volving arsenite (which, as we saw in Section 21-2Ai, reacts
with vicinal sulfhydryl groups but not with disulfides).
1. The spectrum of oxidized dihydrolipoyl dehydroge-
nase (E) is unaffected by arsenite.
2. When NADH reacts with the oxidized enzyme in
the presence of arsenite, the resulting reduced enzyme
(EH
2
) binds arsenite to form an enzymatically inactive
species.
3. The oxidation state of the flavin in a flavoprotein
(flavin-containing protein) is readily established from its
characteristic UV–visible spectrum: FAD is an intense yel-
low, whereas FADH
2
is pale yellow. The spectrum of the
arsenite-inactivated EH
2
indicates that its FAD prosthetic
group is fully oxidized.
Oxidized dihydrolipoyl dehydrogenase must therefore
have a second electron acceptor in addition to FAD; ar-
senite’s known specificity suggests that the acceptor is a
disulfide. Dihydrolipoyl dehydrogenase’s amino acid se-
quence indicates that its redox-active disulfide bond
forms between its Cys 43 and Cys 48, which occur on a
highly conserved segment of the enzyme’s polypeptide
chain.
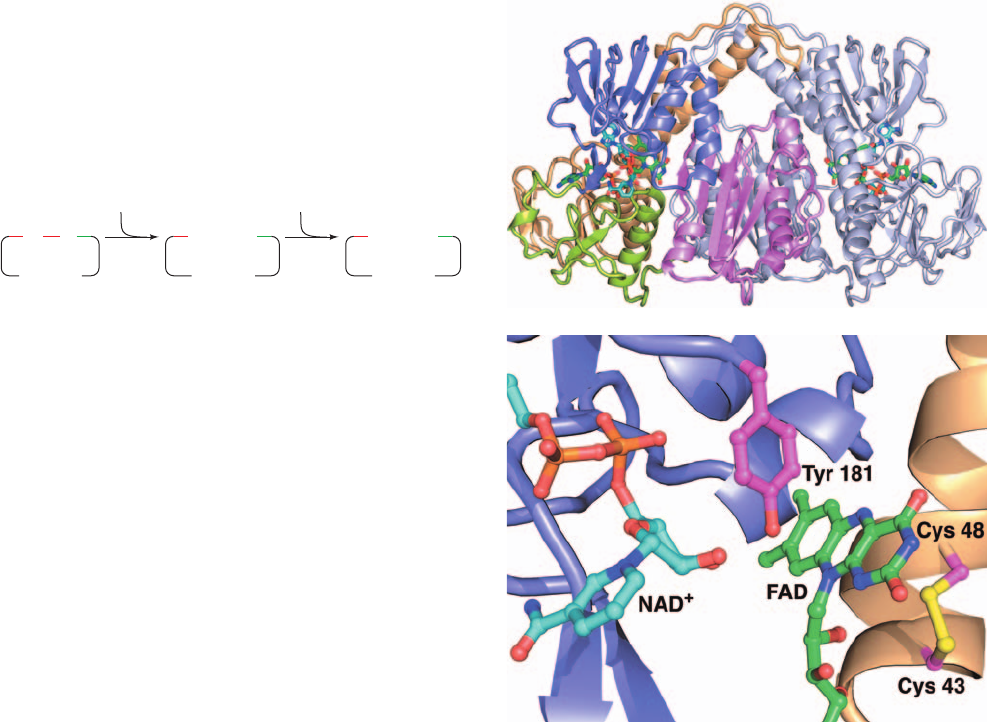
a. The X-Ray Structures of Dihydrolipoyl
Dehydrogenase and Glutathione Reductase
The X-ray structures of dihydrolipoyl dehydrogenase
from several microorganisms, mostly determined by Hol,
reveal that each ⬃470-residue subunit of these homo-
dimeric enzymes is folded into four domains, all of which
participate in forming the subunit’s catalytic center (Fig.
21-13a). An E2 PSBD (not present in Fig. 21-13) binds to
the bottom of the enzyme along its 2-fold axis in much
the same way that it binds to E1 (Fig. 21-12). The flavin is
almost completely buried in the protein, which prevents
the surrounding solution from interfering with the electron-
transfer reaction catalyzed by the enzyme (FADH
2
, but not
NADH or thiol, is rapidly oxidized by O
2
).The redox-active
disulfide, which is located on the opposite side of the
flavin ring from the nicotinamide ring (Fig. 21-13b), links
successive turns in a distorted segment of an ␣ helix (in
an undistorted helix, the C
␣
atoms of Cys 43 and Cys 48
would be too far apart to permit the disulfide bond
to form).
S S
Enzyme
SH HS
Enzyme
S S
Enzyme
_ _
+
2H
_
2e
E3’s catalytic mechanism has been largely determined
in analogy with that of the homologous (⬃33% identical)
but structurally more extensively characterized glutathione
reductase (GR). This nearly ubiquitous enzyme catalyzes
Section 21-2. Metabolic Sources of Acetyl-Coenzyme A 801
Figure 21-13 X-ray structure of dihydrolipoyl dehydrogenase
(E3) from the gram-negative bacterium Pseudomonas putida in
complex with FAD and NAD
ⴙ
. (a) The homodimeric enzyme is
viewed with its 2-fold axis of symmetry vertical and the E2
dodecahedron below. One subunit is gray and the other is colored
according to domain, with its FAD-binding domain (residues
1–142) light orange, its NAD
⫹
-binding domain (residues 143–268)
purple, its central domain (residues 269–337) yellow-green, and its
interface domain (residues 338–458) pink.The NAD
⫹
and FAD
in both subunits are shown in stick form with FAD C green, NAD
⫹
C cyan, N blue, O red, and P orange. (b) The active site region of
the left subunit in Part a in which the central and interface
domains have been deleted for clarity. The view is very roughly in
the same direction as in Part a.The redox-active portions of the
bound NAD
⫹
and FAD cofactors, the side chains of Cys 43 and
Cys 48 forming the redox-active disulfide bond, and the side chain
of Tyr 181 are shown in ball-and-stick form with FAD C green,
NAD
⫹
C cyan, side chain C magenta, N blue, O red, P magenta,
and S yellow. Note that the side chain of Tyr 181 is interposed
between the flavin and the nicotinamide rings. [Based on an X-ray
structure by Wim Hol, University of Washington. PDBid 1LVL.]
(a)
(b)
JWCL281_c21_789-822.qxd 6/5/10 9:53 AM Page 801
802 Chapter 21. Citric Acid Cycle
EH
2
•
NAD
+
6
Stage II:
NAD
+
reduction
EH
2
4
EH
2
•
L
E–S–S–L
•
S
–
NADH
3
2
H
+
1
–
S
C
O
O
–
N
...
H
S
N
H
...
OH
NAD
+
S
C
O
O
–
N
...
H
S
N
HO
–
S
C
O
O
–
N
...
H
S
N
H
...
–
S
C
O
O
–
N
...
H
S
N
H
S
C
O
O
–
N
...
H
S
N
–
S
C
O
O
–
N
...
H
S
N
H
...
E
His 451'
Glu 456'
HO
HO
HO
HO
Tyr 181
Cys 43
Cys 48
FAD
5
NAD
Stage I:
Dihydrolipoamide
oxidation
+
+
S
E
•
LH
2
H
S
H
S
S
–
S
S
S
S
Lipoamide
(L)
SH
SH
Dihydro-
lipoamide
(LH
2
)
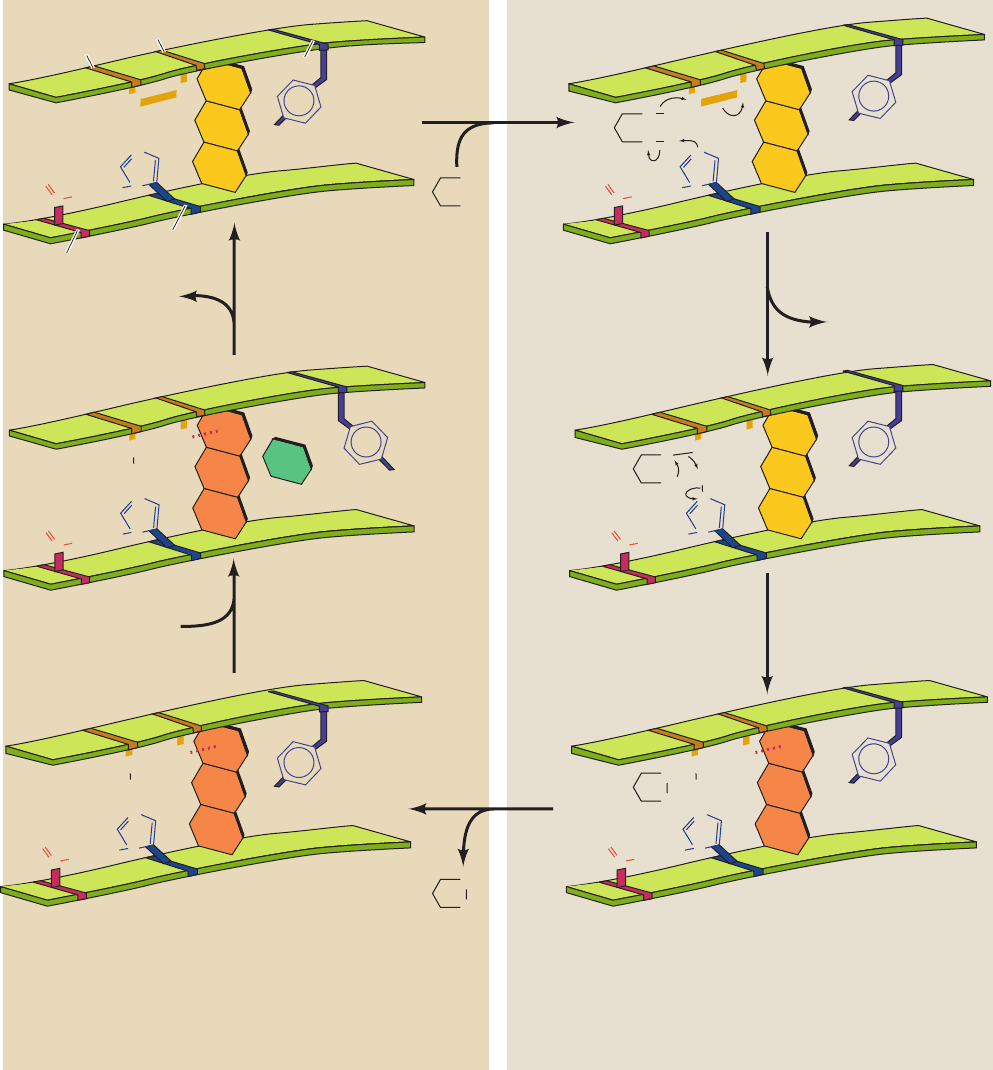
Figure 21-14 Catalytic reaction cycle of dihydrolipoyl
dehydrogenase. The catalytic center is surrounded by protein so
that the NAD
⫹
and dihydrolipoamide binding sites are in deep
pockets.The catalytic cycle consists of six steps: (1) The oxidized
enzyme, E, which contains a redox-active disulfide bond between
Cys 43 and Cys 48, binds dihydrolipoamide, LH
2
, the enzyme’s
first substrate, to form an enzyme–substrate complex, E ⴢ LH
2
.
(2) A substrate S atom nucleophilically attacks S
43
to yield a
disulfide bond and release S
48
as a thiolate ion.The proton on the
substrate’s second thiol group is abstracted by His 451¿ to yield a
second thiolate ion, E–S–S–L ⴢ S
⫺
. (3) The substrate thiolate ion
nucleophilically displaces S
43
aided by general acid catalysis by
His 451¿ to yield the enzyme’s lipoamide product in its complex
with the stable reduced enzyme, EH
2
ⴢ L, in which S
48
forms a
charge-transfer complex with the flavin ring (dotted red line to
the red flavin ring). (4) The lipoamide product is released,
yielding EH
2
.The phenol side chain of Tyr 181 continues to block
access to the flavin ring of FAD, so as to prevent the enzyme’s
oxidation by O
2
. (5) The enzyme’s second substrate, NAD
⫹
,
binds to EH
2
to form EH
2
ⴢ NAD
⫹
.The phenol side chain of Tyr
181 is pushed aside by the nicotinamide ring of NAD
⫹
. (6) The
catalytic cycle is then closed by the reduction of the NAD
⫹
by
EH
2
to reform the oxidized enzyme E and yield the enzyme’s
second product, NADH.
JWCL281_c21_789-822.qxd 3/17/10 11:33 AM Page 802
the NADPH-dependent reduction of glutathione disulfide
(GSSG) to the intracellular reducing agent glutathione
(GSH; its physiological function is discussed in Sections
23-4E and 26-4C):
Note that this reaction is analogous to that catalyzed by di-
hydrolipoyl dehydrogenase, but that these two reactions
normally occur in opposite directions; that is, dihydrolipoyl
dehydrogenase normally uses NAD
⫹
to oxidize two thiol
groups to a disulfide (Fig. 21-6), whereas GR uses NADPH
to reduce a disulfide to two thiol groups. Nevertheless, the
active sites of these two enzymes are closely superim-
posable.
The arrangement of the groups in dihydrolipoyl dehy-
drogenase’s catalytic center and its reaction sequence are
diagrammed in Fig. 21-14. The substrate-binding site is lo-
cated in the interface between the enzyme’s two subunits
in the vicinity of the redox-active disulfide. In the absence
of NAD
⫹
, the phenol side chain of its Tyr 181 covers the
nicotinamide-binding pocket so as to shield the flavin from
contact with the solution. Indeed, Fig. 21-13b shows an
enzyme–product complex of oxidized dihydrolipoyl dehy-
drogenase with NAD
⫹
in which the side chain of Tyr 181 is
interposed between the nicotinamide ring and its binding
site at the enzyme’s catalytic center. However, in the X-ray
structure of GR in complex with NADPH, determined by
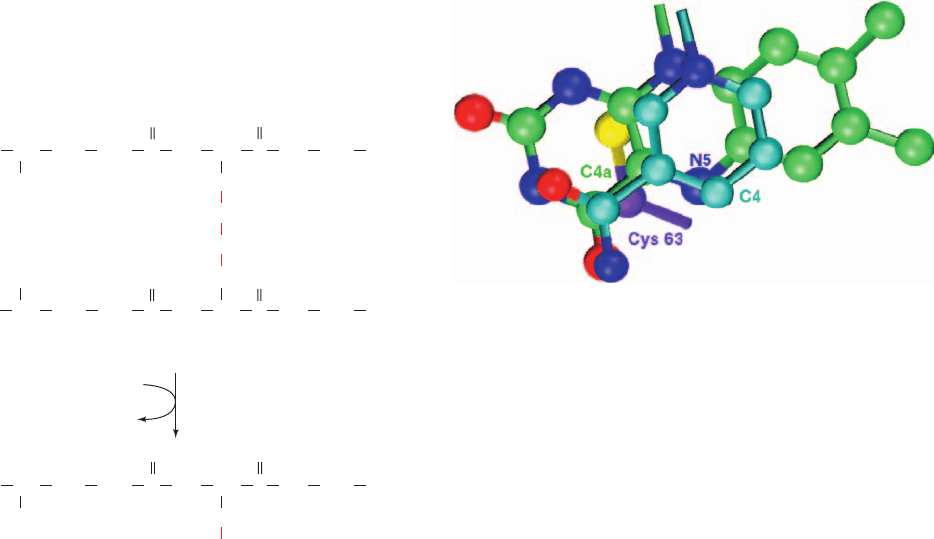
Georg Schulz and Heiner Schirmer, this side chain has
moved aside such that the reduced nicotinamide ring binds
parallel to and in van der Waals contact with the flavin ring
of the fully oxidized enzyme, E (Fig. 21-15). The H
S
sub-
stituent to this reduced nicotinamide’s prochiral C4 atom
(that facing the flavin), the H atom that is known to be lost
in the GR reaction, lies near flavin atom N5, the position
through which electrons often enter a flavin ring on its re-
duction. This positioning is particularly significant in view
of the catalytic mechanism described below.
H
3
N
H
3
N
CH
2
CH
2
CH
2
CH
2
CH
2
CH
2
CH
2
CHCH NHNH CC
S
S
CH
2
COOCCCH CHNH NH
+
+
_
COO
_
COO
_
COO
_
SH
O
O
O
O
H
3
N
NADPH
NADP
H
2 CH
2
CH
2
CH
2
CH
2
COOC
O
CCH CHNH NH
+
+
+
+
_
COO
_
O
Glutathione disulfide (GSSG)
Glutathione (GSH)
(␥-
L-Glutamyl-L-cysteinylglycine)
glutathione
reductase
b. Catalytic Mechanism
Protein X-ray structures neither reveal H atom posi-
tions (except in the most highly resolved structures) nor in-
dicate pathways of electron transfer. The electron-transfer
pathway in the glutathione reductase reaction has never-
theless been inferred from the X-ray structures of a series
of stable enzymatic reaction intermediates as augmented
with a variety of enzymological data. Here we present this
mechanism in terms of the reaction catalyzed by the closely
similar dihydrolipoyl dehydrogenase (Fig. 21-14).
Dihydrolipoyl dehydrogenase has a Ping Pong mecha-
nism (Section 14-5Aa); each of its two substrates, dihy-
drolipoamide and NAD
⫹
, react in the absence of the other
(Fig. 21-6, Reactions 4 and 5). Stage I of the catalytic reac-
tion (Fig. 21-14, Steps 1–4) involves a disulfide interchange
reaction between the first substrate, dihydrolipoamide
(LH
2
), and the redox-active disulfide on the enzyme, a
process in which His 451¿ functions as a general acid–base
(primed residues refer to the opposite subunit). In fact, Glu
456¿, His 451¿, and Cys 43 are arranged much like the cat-
alytic triad of serine proteases (Section 15-3B), with the
Cys SH replacing the Ser OH. The importance of His 451¿
as an acid–base catalyst was established by Charles
Williams through his observation that mutationally chang-
ing it to Gln yields an enzyme that retains only ⬃0.4% of
the wild-type catalytic activity.
The thiolate anion formed from Cys 48 in Step 2 forms a
charge-transfer complex with the flavin in which S
–
48
(the S
atom of Cys 48) contacts the flavin ring near its 4a position
(a charge-transfer complex is a noncovalent interaction in
Section 21-2. Metabolic Sources of Acetyl-Coenzyme A 803
Figure 21-15 The complex of the flavin ring, the nicotinamide
ring, and the side chain of Cys 63 observed in the X-ray structure
of human erythrocyte glutathione reductase. The view is
perpendicular to the flavin ring.Atoms are colored according to
type with nicotinamide C cyan, flavin C green, Cys 63 C purple,
N blue, O red, and S yellow. The two planar heterocycles are
parallel and in van der Waals contact. The Cys 63 S atom
(equivalent to S
48
of dihydrolipoyl dehydrogenase), a member of
the redox-active disulfide, is also in van der Waals contact with
the flavin ring on the opposite side of it from the nicotinamide
ring. [Based on an X-ray structure by Andrew Karplus and
Georg Schulz, Institut für Organische Chemie und Biochemie,
Freiburg, Germany. PDBid 1GRB.]
JWCL281_c21_789-822.qxd 3/17/10 11:33 AM Page 803
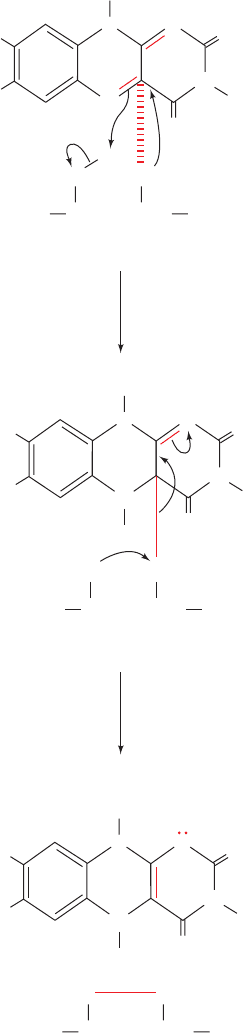
which an electron pair is partially transferred from a donor,
in this case S
–
48
, to an acceptor, in this case the oxidized
flavin ring; the red color of this complex is indicative of the
formation of the charge-transfer complex).
Stage II of this Ping Pong reaction (Fig. 21-14, Steps 5
and 6) involves the binding and reduction of NAD
⫹
fol-
lowed by the release of NADH to regenerate the oxidized
enzyme. The path of the electrons from the reactive disul-
fide in its reduced form through the FAD to NAD
⫹
has
been elucidated by spectroscopic studies and the chemistry
of model compounds.These indicate that an electron pair is
rapidly transferred from S
–
48
to the flavin ring through the
transient formation of a covalent bond from S
48
to flavin
atom 4a (Fig. 21-16, Step 1). His 451¿ then abstracts the pro-
ton from the S
43
thiol to form a thiolate ion, which nucle-
ophilically attacks S
48
to reform the redox-active disulfide
group (Fig. 21-16, Step 2). The resulting reduced flavin an-
ion (FADH
⫺
) has but transient existence.The H atom sub-
stituent to its N5 is immediately transferred (formally as a
hydride ion) to the juxtaposed C4 atom of the nicoti-
namide ring (Fig. 21-15), yielding FAD and the reaction’s
second product, NADH, thereby completing the catalytic
cycle.Thus, the FAD appears to function more like an elec-
tron conduit between the reduced form of the redox-active
disulfide and NAD
⫹
than as a source or sink of electrons.
C. Control of Pyruvate Dehydrogenase
The PDC regulates the entrance of acetyl units derived from
carbohydrate sources into the citric acid cycle. The decar-
boxylation of pyruvate by E1 is irreversible and, since there
are no other pathways in mammals for the synthesis of
acetyl-CoA from pyruvate, it is crucial that the reaction be
carefully controlled.Two regulatory systems are employed:
1. Product inhibition by NADH and acetyl-CoA
(Fig. 21-17a).
2. Covalent modification by phosphorylation/dephos-
phorylation of the pyruvate dehydrogenase (E1) subunit
(Fig.21-17b; enzymatic regulation by covalent modification
is discussed in Section 18-3Ba).
a. Control by Product Inhibition
NADH and acetyl-CoA compete with NAD
⫹
and CoA
for binding sites on their respective enzymes.They also drive
the reversible transacetylase (E2) and dihydrolipoyl dehy-
drogenase (E3) reactions backward (Fig. 21-17a). High ra-
tios of [NADH]/[NAD
⫹
] and [acetyl-CoA]/[CoA] there-
fore maintain E2 in the acetylated form, incapable of
accepting the hydroxyethyl group from the TPP on E1.
This, in turn, ties up the TPP on the E1 subunit in its hy-
droxyethyl form, decreasing the rate of pyruvate decar-
boxylation.
b. Control by Phosphorylation/Dephosphorylation
Control by phosphorylation/dephosphorylation occurs
only in eukaryotic enzyme complexes. These complexes
contain pyruvate dehydrogenase kinase and pyruvate
804 Chapter 21. Citric Acid Cycle
FAD
N
R
N
N
N
O
O
H
H
S
S
–
–
H
3
C
H
3
C
CH
2
CH
2
Cys 48Cys 43
10
10a
4a
5
4
1
Charge-transfer complex
N
R
N
N
N
O
O
H
H
S
S
H
3
C
H
3
C
CH
2
CH
2
Cys 48Cys 43
Covalent adduct
FADH
_
anion
–
N
R
N
N
N
O
O
H
H
S
S
H
3
C
H
3
C
CH
2
CH
2
Cys 48Cys 43
Redox-active disulfide
1
2
Figure 21-16 The reaction transferring an electron pair from
dihydrolipoyl dehydrogenase’s redox-active disulfide in its
reduced form to the enzyme’s bound flavin ring. (1) The collapse
of the charge-transfer complex between the Cys 48 thiolate ion
and the flavin ring (dashed red line) to form a covalent bond
between S
48
and flavin atom C4a. S
48
is located out of the plane
of the flavin, as Fig. 21-15 indicates. Flavin atom N5 acquires a
proton, possibly from S
43
, which becomes a thiolate ion. (2) The
S
43
thiolate nucleophilically attacks S
48
to form the redox-active
disulfide bond, thereby releasing the reduced flavin anion
FADH
⫺
.
JWCL281_c21_789-822.qxd 3/17/10 11:33 AM Page 804
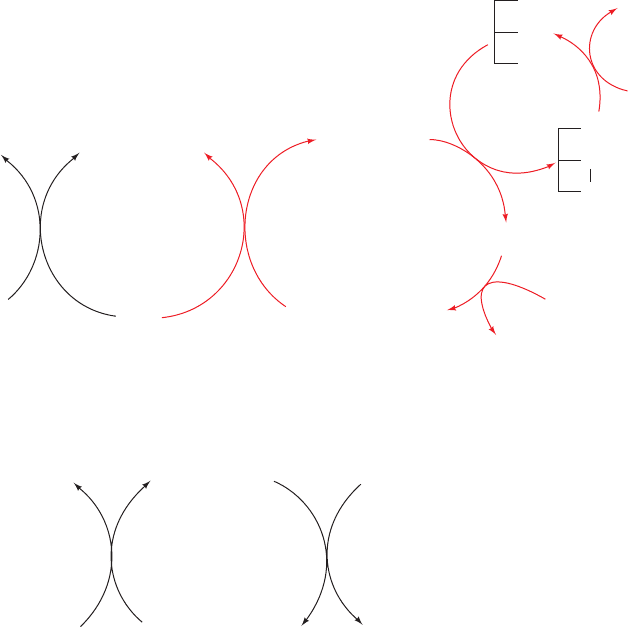
NADH
Acetyl –CoA
NAD
+
pyruvate
dehydrogenase
phosphatase
pyruvate
dehydrogenase
kinase
P
i
H
2
O
E1–OPO
(inactive)
2–
3
E1– OH (active)
ADP
ATP
FAD
S
S
(b)
Activators
Acetyl-CoA
NADH
A
ctivators
Mg
2+
Ca
2+
Inhibitors
Pyruvate
ADP
Ca
2+
(high Mg
2+
)
K
+
Covalent modification
(a)
Product inhibition
FAD
SH
SH
E3
E2
E1
1
2
3
4
5
Dihydrolipoamide
Acetyl-
dihydrolipoamide
CoA
Lipoamide
Hydroxyethyl-
TPP
TPP
Pyruvate
CO
2
dehydrogenase phosphatase bound to the dihydrolipoyl
transacetylase (E2) core. The kinase inactivates the pyru-
vate dehydrogenase (E1) subunit by catalyzing the ATP-
dependent phosphorylation of a Ser residue on the more
C-terminal loop at the entrance of the active site channel
(Fig. 21-17b). Moreover, phosphorylation at only one of
E1’s two active sites inactivates the entire enzyme, thereby
further demonstrating the obligatory nature of the out of
phase coupling between these two active sites (Section 21-
2Al). Hydrolysis of this phosphoSer residue by the phos-
phatase reactivates the complex.
Pyruvate dehydrogenase kinase is activated through its
interaction with the acetylated form of E2. Consequently,
the products of the reaction, NADH and acetyl-CoA, in
addition to their direct effects on the PDC, indirectly acti-
vate pyruvate dehydrogenase kinase. The resultant phos-
phorylation inactivates the complex just as the products
themselves inhibit it.Acetyl-CoA and NADH are products
of fatty acid oxidation (Section 25-2) so that this inhibition
of PDC serves to preserve carbohydrate stores when lipid
fuels are available.
Calcium ion is an important second messenger signaling
the need for increased energy (e.g., for muscle contrac-
tion). Increasing [Ca
2⫹
] enhances pyruvate dehydrogenase
phosphatase activity, thus activating PDC.
Insulin is involved in the control of this system through
its indirect activation of pyruvate dehydrogenase phos-
phatase. Recall that insulin activates glycogen synthesis as
well by activating phosphoprotein phosphatase-1 (Section
18-3Cg). Insulin, in response to increases in blood glucose,
is now seen as promoting the synthesis of acetyl-CoA as
well as glycogen. As we shall see in Section 25-4, acetyl-
CoA is the precursor to fatty acids in addition to being the
fuel for the citric acid cycle. Various other activators and
inhibitors regulate the pyruvate dehydrogenase system
(Fig. 21-17b); in contrast to the glycogen metabolism con-
trol system (Section 18-3), however, it is unaffected by
cAMP.
Section 21-2. Metabolic Sources of Acetyl-Coenzyme A 805
Figure 21-17 Factors controlling the activity of the PDC.
(a) Product inhibition. NADH and acetyl-CoA, respectively,
compete with NAD
⫹
and CoA in Reactions 3 and 5 of the
pyruvate dehydrogenase reaction sequence. When the relative
concentrations of NADH and acetyl-CoA are high, the
reversible reactions catalyzed by E2 and E3 are driven
backward (red arrows), thereby inhibiting further formation of
acetyl-CoA. (b) Covalent modification in the eukaryotic
complex. Pyruvate dehydrogenase (E1) is inactivated by the
specific phosphorylation of one of its Ser residues in a reaction
catalyzed by pyruvate dehydrogenase kinase (right).This
phosphoryl group is hydrolyzed through the action of pyruvate
dehydrogenase phosphatase (left), thereby reactivating E1. The
activators and inhibitors of the kinase are listed on the right and
the activators of the phosphatase are listed on the left.
JWCL281_c21_789-822.qxd 6/5/10 9:53 AM Page 805
3 ENZYMES OF THE CITRIC
ACID CYCLE
In this section we discuss the reaction mechanisms of the
eight citric acid cycle enzymes. Our knowledge of these
mechanisms rests on an enormous amount of experimental
work; as we progress, we shall pause to examine some of
these experimental details. Consideration of how this cycle
is regulated and its relationship to cellular metabolism are
the subjects of the following sections.
A. Citrate Synthase
Citrate synthase (originally named citrate condensing en-
zyme) catalyzes the condensation of acetyl-CoA and ox-
aloacetate (Reaction 1 of Fig. 21-1). This initial reaction of
the citric acid cycle is the point at which carbon atoms are
“fed into the furnace” as acetyl-CoA. The citrate synthase
reaction proceeds via an Ordered sequential kinetic mech-
anism (Section 14-5Aa), with oxaloacetate adding to the
enzyme before acetyl-CoA.
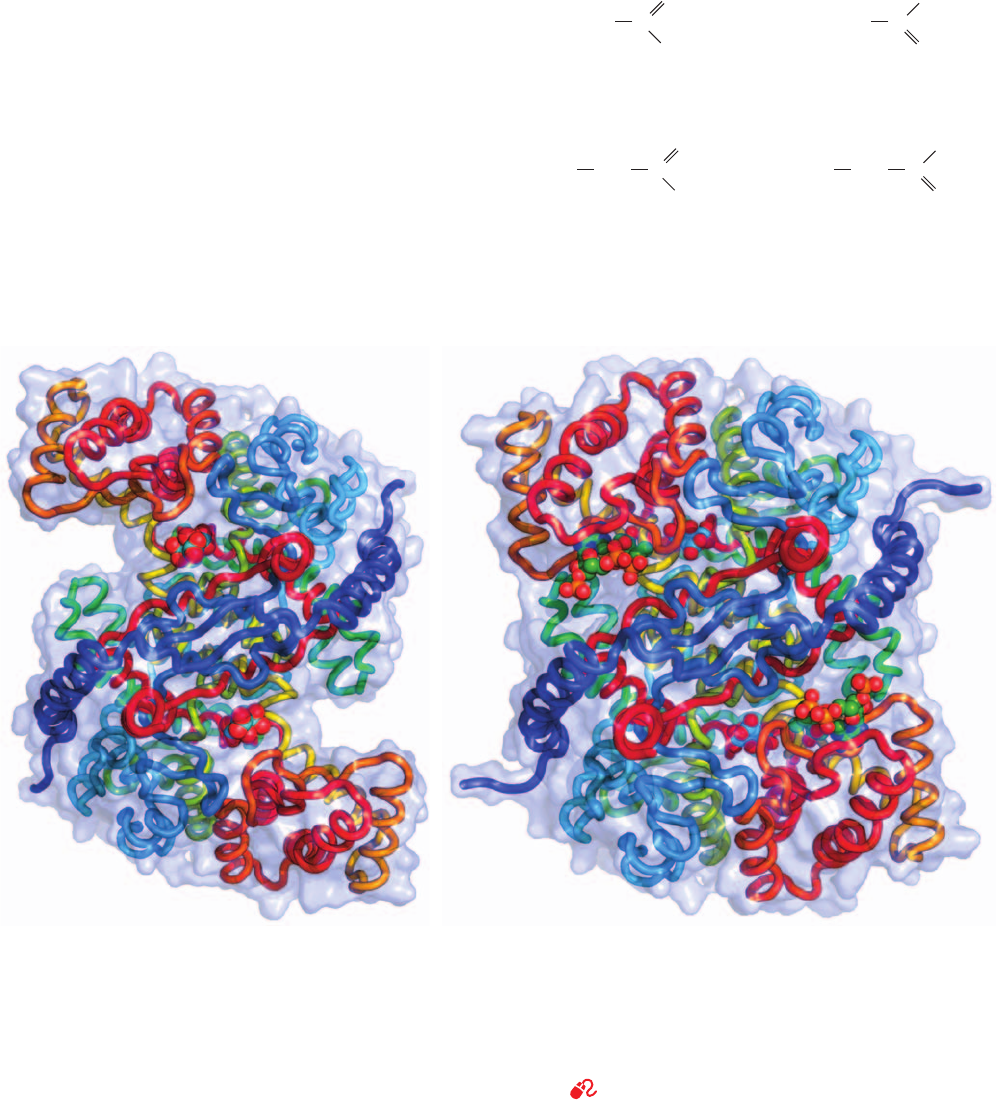
The X-ray structure of the free dimeric enzyme, deter-
mined by James Remington and Robert Huber, shows it to
adopt an “open form” in which the two domains of each sub-
unit form a deep cleft that contains the oxaloacetate binding
site (Fig. 21-18a). On binding oxaloacetate, however, the
smaller domain undergoes a remarkable 18º rotation rela-
tive to the larger domain, which closes the cleft (Fig. 21-18b).
The X-ray structures of two inhibitors of citrate synthase
in ternary complex with the enzyme and oxaloacetate have
also been determined. Acetonyl-CoA, an inhibitory analog
of acetyl-CoA in the ground state, and carboxymethyl-
CoA, a proposed transition state analog (see below),
CoAS C
CH
3
O
Acetyl-CoA
CoAS C
CH
3
CH
2
O
Acetonyl-CoA
(ground-state analog)
CoAS C
O
CH
2
OH
Carboxymethyl-CoA
(transition state analog)
CoAS C
CH
2
OH
Proposed enol
intermediate
806 Chapter 21. Citric Acid Cycle
(a) (b)
Figure 21-18 Conformational changes in citrate synthase.
(a) The open conformation. (b) The closed, substrate-binding
conformation. In both forms, the homodimeric protein is viewed
along its 2-fold axis and is represented by its transparent
molecular surface with its polypeptide chains drawn in worm
form colored in rainbow order from blue at their N-termini to
red at their C-termini.The reaction product citrate, which is
bound to both enzymatic forms, and coenzyme A, which is also
bound to the closed form, are shown in space-filling form with
citrate C cyan, CoA C green, N blue, O red, and P orange.The
large conformational shift between the open and closed forms
entails 18° rotations of the small domains (upper left and lower
right) relative to the large domains resulting in relative
interatomic movements of up to 15 Å. [Based on X-ray
structures by James Remington and Robert Huber, Max-Planck-
Institut für Biochemie, Martinsried, Germany. PDBids 1CTS and
2CTS.]
See Interactive Exercise 15
JWCL281_c21_789-822.qxd 10/19/10 7:42 AM Page 806
bind to the enzyme in its “closed” form, thereby identifying
the acetyl-CoA binding site. The existence of the “open”
and “closed” forms explains the enzyme’s ordered sequen-
tial kinetic behavior: The conformational change induced
by oxaloacetate binding generates the acetyl-CoA binding
site while sealing off the solvent’s access to the bound ox-
aloacetate. This is a classic example of the induced-fit
model of substrate binding (Section 10-4C). Hexokinase
exhibits similar behavior (Section 17-2Aa).
The citrate synthase reaction is a mixed aldol–Claisen es-
ter condensation, subject to general acid–base catalysis and
the intermediate participation of the enol(ate) form of
acetyl-CoA. The X-ray structure of the enzyme in ternary
complex with oxaloacetate and carboxymethyl-CoA reveals
that three of its ionizable side chains are properly oriented
to play catalytic roles: His 274,Asp 375, and His 320.The N1
atoms in both of these His side chains are hydrogen bonded
to two backbone NH groups indicating that these N1 atoms
are not protonated. The participation of His 274, Asp 375,
and His 320 has been confirmed by kinetic studies of mutant
enzymes generated by site-directed mutagenesis. The fol-
lowing three-step mechanism, formulated mainly by Rem-
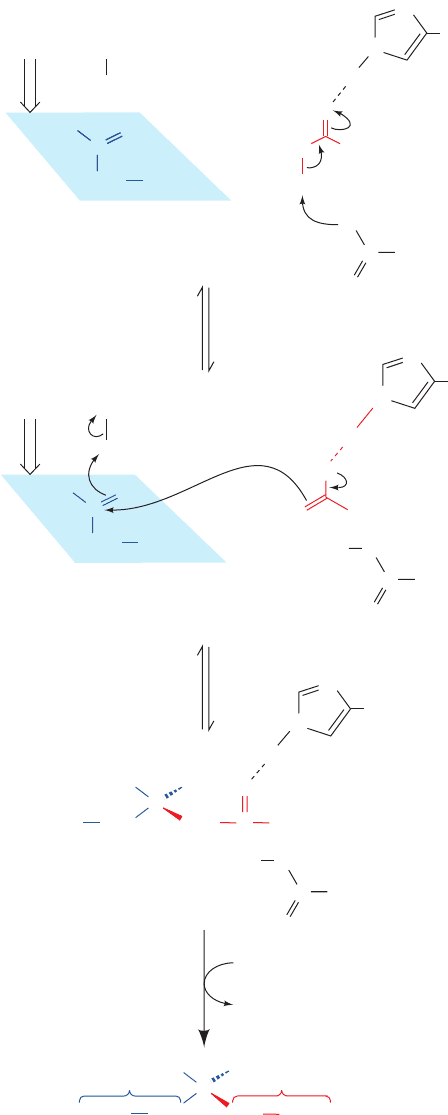
ington, takes into account these observations (Fig. 21-19):
1. The enolate of acetyl-CoA is generated in the rate-
limiting step of the reaction, with the catalytic participation
of Asp 375 acting as a base to remove a proton from the
methyl group and His 274, in its neutral form, H-bonding
the enolate oxygen (whose protonated carbonyl form is
normally far more acidic than a neutral His side chain).
Much as we discussed for a similar step in the triose phos-
phate isomerase reaction (Section 17-2Eb), the pK of the
protonated form of the substrate thioester carbonyl oxy-
gen increases to ⬃14 on enolization, which approximates
that of neutral His 274. Hence, it has been proposed that this
step is facilitated through the formation of a low-barrier
hydrogen bond (which, it will be recalled, is a particularly
strong form of hydrogen bonding interaction in which the
hydrogen atom is more or less equally shared between the
“donor” and “acceptor” atoms; Section 15-3Dd). However,
whether or not this low-barrier hydrogen bond actually
forms is a subject of controversy. The presence of the
thioester bond to CoA facilitates the enolization; enoliza-
tion of acetate alone would require the generation of a
much more highly charged and therefore less stable inter-
mediate.
2. Citryl-CoA is formed in a second acid–base-catalyzed
reaction step in which the acetyl-CoA enolate nucleophili-
cally attacks oxaloacetate while His 320, also in its neutral
form, donates a proton to oxaloacetate’s carbonyl group.
The citryl-CoA remains bound to the enzyme.
3. Citryl-CoA is hydrolyzed to citrate and CoA, with
His 320, now in the anionic form, abstracting a proton from
water as it attacks the carbonyl group of the citryl-CoA
and the acidic form of Asp 375 donating a proton to the
CoA as it leaves. This hydrolysis provides the reaction’s
thermodynamic driving force (⌬G°¿ ⫽ –31.5 kJ ⴢ mol
⫺1
).
We shall see presently why the reaction requires such a
large, seemingly wasteful, release of free energy.
Section 21-3. Enzymes of the Citric Acid Cycle 807
O
C
CH
2
–
OOC
COO
–
O
SCoA
H
His
274
Acetyl-CoA
Possible low-barrier
hydrogen bond
H
2
C+
1
C Asp 375
:O
–
O
H
C Asp 375
H
His 320
Oxaloacetate
(S)-Citryl-CoA
–
O
O
C
CH
2
–
OOC
SCoAH
2
C
2
3
C Asp 375
O
O
H
COO
–
H
His 320
C Asp 375C Asp 375
O
O
H
O
C
OH
C
–
OOC
–
OOC
SCoA
CoASH
H
2
C CH
2
H
2
O
His
–
320
Citrate
pro S-arm
COO
–
OH
C
–
OOC
–
OOC H
2
C CH
2
N
N
His
274
N
N
H
His
274
N
N
..
H
si
si
pro R-arm
Figure 21-19 Mechanism and stereochemistry of the citrate
synthase reaction. His 274 and His 320 in their neutral forms and
Asp 375 have been implicated as general acid–base catalysts.The
overall reaction’s rate-limiting step is the formation of the
acetyl-CoA enolate, which may be stabilized by a low-barrier
hydrogen bond to His 274.The acetyl-CoA enolate then
nucleophilically attacks the si face of oxaloacetate’s carbonyl
carbon.The resulting intermediate, (S)-citryl-CoA, is hydrolyzed
to yield citrate and CoA. [Mostly after Remington, S.J., Curr.
Opin. Struct. Biol. 2, 732 (1992)].
JWCL281_c21_789-822.qxd 6/5/10 9:53 AM Page 807
Enzyme-catalyzed reactions, as we have previously
noted, are stereospecific. The aldol–Claisen condensation
that occurs here involves attack of the acetyl-CoA enolate
exclusively at the si face of oxaloacetate’s carbonyl carbon
atom, thereby yielding (S)-citryl-CoA (chirality nomencla-
ture is presented in Section 4-2C).The acetyl group of acetyl-
CoA thereby only forms citrate’s pro-S carboxymethyl group.
B. Aconitase
Aconitase catalyzes the reversible isomerization of citrate
and isocitrate with cis-aconitate as an intermediate (Reac-
tion 2 of Fig. 21-1). Although citrate has a plane of symme-
try and is therefore not optically active, it is nevertheless
prochiral; aconitase can distinguish between citrate’s pro-R
and pro-S carboxymethyl groups.
A combination of X-ray crystallography and site-
directed mutagenesis by Helmut Beinert and David Stout
identified several amino acid residues that participate in
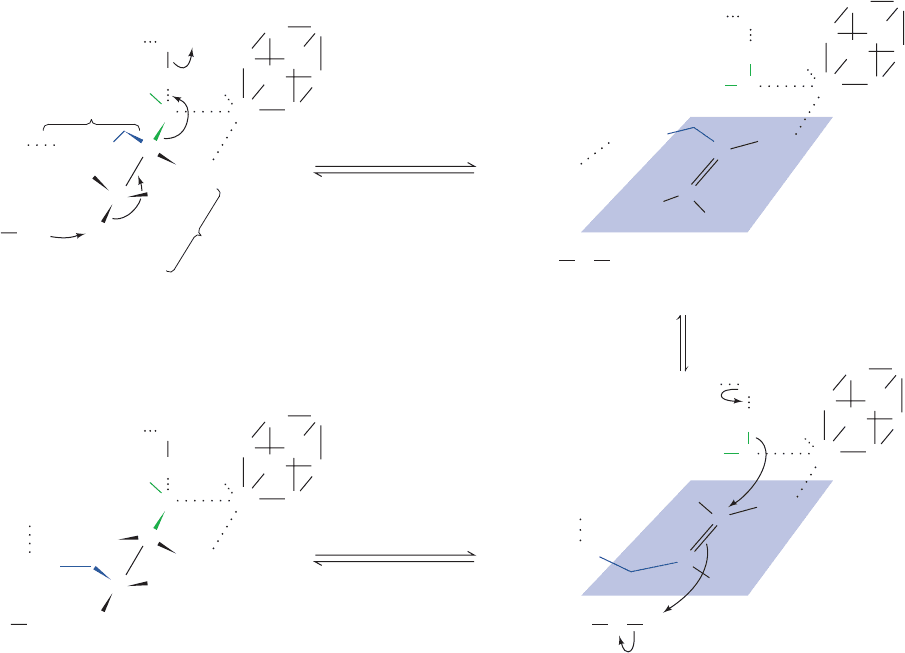
catalysis. Formation of the cis-aconitate intermediate in-
volves a dehydration in which Ser 642 alkoxide, acting as a
general base, abstracts the pro-R proton at C2 of citrate’s
pro-R carboxymethyl group (Fig. 21-20, top). [The O
␥
of
Ser 642 occupies a sort of oxyanion hole (Section 15-3Db)
that apparently stabilizes its otherwise highly basic alkox-
ide form. The carbanion formed in the transition state of
this reaction is not shown.] This is followed by the loss of
the OH group at C3 in a trans elimination of H
2
O to form
the cis-aconitate intermediate. The latter reaction step is
facilitated through general acid catalysis by His 101, whose
imidazolium group is polarized through ion pairing to the
side chain of Asp 100. This model is corroborated by the
observations that the mutagenesis of Asp 100, His 101, or
Ser 642 results in a 10
3
- to 10
5
-fold reduction in aconitase’s
catalytic activity without greatly affecting its substrate
binding affinity.
808 Chapter 21. Citric Acid Cycle
Asp His100 101
_
_
*
_
_
+
+
H
H
H
O
OSer 642 :
HS
S
S
S
H
2
O
C
C
COO
_
COO
OOC
pro-S
pro-R
Fe
Fe
Fe
Fe
a
3
2
pro-R
Arg 580
_
+
H
C
C
_
COO
OOC
3
2
Arg
Ser 642
580
Citrate cis-Aconitate intermediate
[4Fe-4S] cluster
*H
O
Asp His100 101
_
_
O
H
H
S
S
S
S
H
2
O
COO
Fe
Fe
Fe
Fe
a
Asp His100 101
_
_
*
_
_
+
+
H
H
H
O
OSer 642 :
HS
S
S
S
H
2
O
C
C
COO
_
COO
OOC
Fe
Fe
Fe
Fe
a
3
2
Arg 580
(2R,3S)-Isocitrate
_
+
H
C
C
_
COO
OOC
3
2
Arg
Ser 642
580
*H
O
Asp
His
100
101
_
_
O
H
H
S
S
S
S
H
2
O
COO
Fe
Fe
Fe
Fe
a
180⬚ flip
Figure 21-20 Mechanism and stereochemistry of the aconitase
reaction. Fe
a
of the enzyme’s [4Fe–4S] cluster coordinates the
citrate hydroxyl and central carboxyl groups;Arg 580 forms a
salt bridge with the pro-S carboxyl group; Ser 642, in its alkoxide
form, acts as a general base; and the Asp 100-polarized His 101
acts as a general acid in the elimination of water to form
cis-aconitate. Note the unusual 180° flip that cis-aconitate
apparently undergoes, possibly while remaining bound to the
active site. Thus rehydration takes place on the opposite face of
the substrate from which dehydration occurred, yielding
(2R,3S)-isocitrate.
JWCL281_c21_789-822.qxd 6/5/10 9:53 AM Page 808
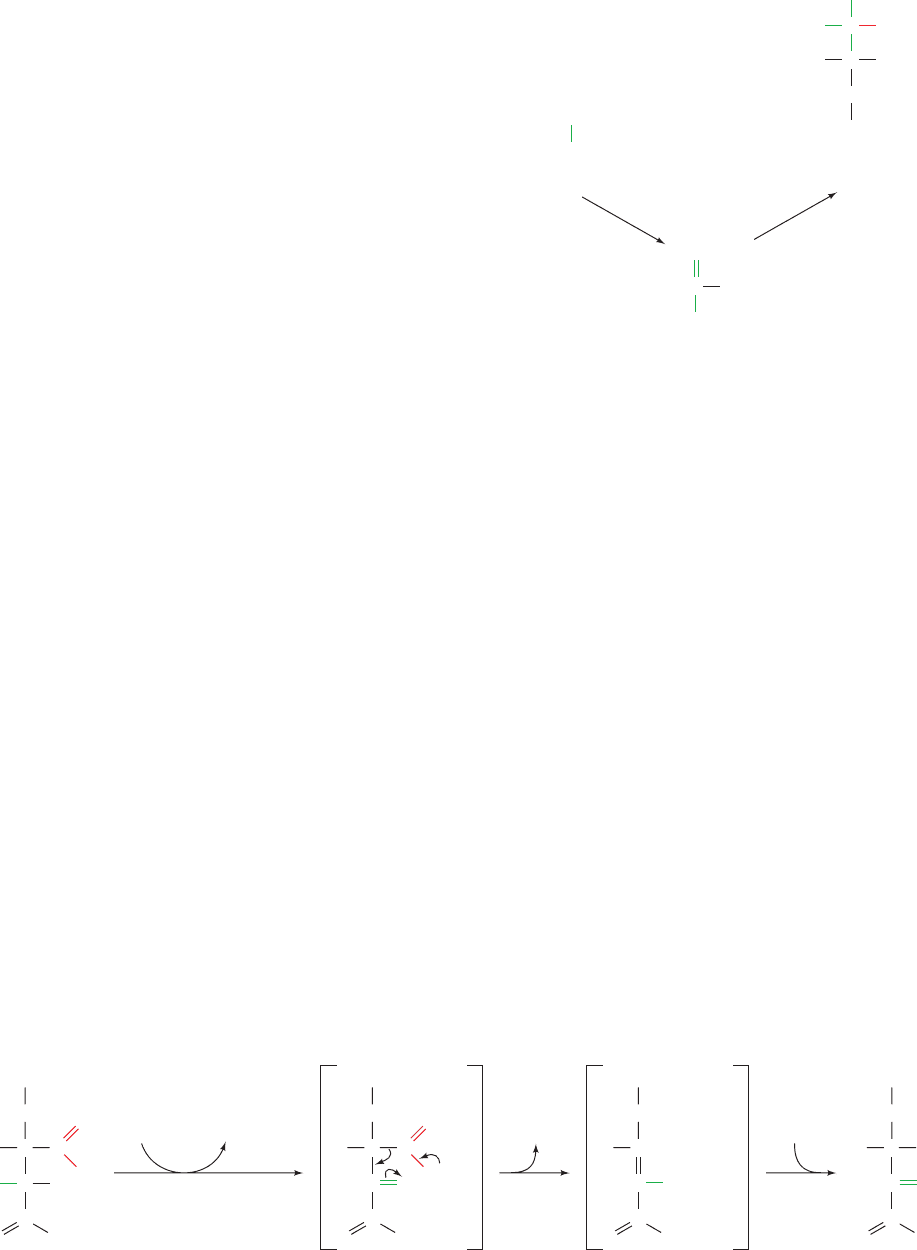
Aconitase contains a covalently bound [4Fe–4S]
iron–sulfur cluster, which is required for catalytic activity
(the properties of iron–sulfur clusters are discussed in Sec-
tion 22-2Ca). A specific Fe(II) atom in this cluster, the so-
called Fe
a
atom, is thought to coordinate the OH group of
the substrate so as to facilitate its elimination. Iron–sulfur
clusters are almost always associated with redox processes
although, intriguingly, not in the case of aconitase. It has
been postulated that the electronic properties of the
[4Fe–4S] cluster permit the Fe
a
atom to expand its coordi-
nation shell from the four ligands observed in the X-ray
structure of the free enzyme (three S
2⫺
ions and a OH
⫺
ion)
to the six octahedrally arranged ligands observed in the
enzyme–substrate complex. A single metal ion, such as
Zn
2⫹
or Cu
2⫹
, is unable to do so, and hence would require
that some of its ligands be displaced on binding substrate.
The second stage of the aconitase reaction is rehydra-
tion of cis-aconitate’s double bond to form isocitrate (Fig.
21-20, bottom). The nonenzymatic addition of H
2
O across
the double bond of cis-aconitate would yield four stereoiso-
mers.Aconitase, however, catalyzes the stereospecific trans
addition of OH
⫺
and H
⫹
across the double bond to form
only (2R,3S)-isocitrate in the forward reaction and citrate
in the reverse reaction.
Although citrate’s OH group is lost to the solvent in the
aconitase reaction, the abstracted H
⫹
is retained by Ser 642.
Remarkably, it adds to the opposite faces of cis-aconitate’s
double bond in forming citrate and isocitrate. The cis-
aconitate must expose a different face to the sequestered
H
⫹
for the formation of isocitrate. This is thought to occur
by a 180º flip of the intermediate on the enzyme’s surface
while maintaining its association with Arg 580 or else by
dissociation from the enzyme and replacement by another
cis-aconitate in the “flipped” orientation.
a. Fluorocitrate Inhibits Aconitase
Fluoroacetate, one of the most toxic small molecules
known (LD
50
⫽ 0.2 mg ⴢ kg
⫺1
of body weight in rats), oc-
curs in the leaves of certain African, Australian, and South
American poisonous plants. Interestingly, fluoroacetate it-
self has little toxic effect on cells; instead, cells enzymati-
cally convert it first to fluoroacetyl-CoA and then to
(2R,3R)-2-fluorocitrate, which specifically inhibits aconi-
tase (see Problem 9):
It is not clear,however,that the inhibition of aconitase fully
accounts for fluorocitrate’s high toxicity. Indeed, fluoro-
citrate also inhibits the transport of citrate across the mito-
chondrial membrane.
C. NAD
ⴙ
-Dependent Isocitrate Dehydrogenase
Isocitrate dehydrogenase catalyzes the oxidative decarboxy-
lation of isocitrate to a-ketoglutarate to produce the citric
acid cycle’s first CO
2
and NADH (Reaction 3 of Fig. 21-1).
Mammalian tissues contain two isoforms of this enzyme.
Although they both catalyze the same reaction, one iso-
form participates in the citric acid cycle, is located entirely
in the mitochondrion, and utilizes NAD
⫹
as a cofactor.The
other isoform occurs in both the mitochondrion and the
cytosol,utilizes NADP
⫹
as a cofactor,and generates NADPH
for use in reductive biosynthesis.
NAD
⫹
-dependent isocitrate dehydrogenase, which re-
quires an Mn
2⫹
or Mg
2⫹
cofactor, catalyzes the oxidation of
a secondary alcohol (isocitrate) to a ketone (oxalosuccinate)
followed by the decarboxylation of the carboxyl group  to
the ketone (Fig. 21-21). In this sequence, the keto group  to
the carboxyl group facilitates the decarboxylation by acting
as an electron sink.The oxidation occurs with the stereospe-
cific reduction of NAD
⫹
at its re face (A-side addition; Sec-
tion 13-2Aa). Mn
2⫹
coordinates the newly formed carbonyl
group so as to polarize its electronic charge.
COO
Fluoroacetate
Fluoroacetyl-CoA
(2R,3R)-2-
Fluorocitrate
_
CH
2
F
CH
2
F
acetate
thiokinase
citrate
synthase
COO
_
COO
_
COO
_
CH
2
CHO
C
SCoA
C
O
H F
Section 21-3. Enzymes of the Citric Acid Cycle 809
...
...
COO
–
Isocitrate
H
+
+ NADHNAD
+
O
–
O
CH
2
C C
O
–
Mn
2+
O
H
HHO
C
C
COO
–
Oxalosuccinate
CO
2
H
+
O
–
O
CH
2
C C
O
–
O
H
O
C
C
...
...
Mn
2+
COO
–
O
–
O
CH
2
CH
O
–
C
C
COO
–
␣-Ketoglutarate
O
–
O
CH
2
CHH
O
C
C
Figure 21-21 Probable reaction mechanism of isocitrate dehydrogenase. Oxalosuccinate is
shown in brackets because it does not dissociate from the enzyme.
JWCL281_c21_789-822.qxd 3/17/10 11:34 AM Page 809

Although the intermediate formation of oxalosuccinate
is a logical chemical prediction, evidence for it has been
difficult to obtain because it has only a transient existence
in reactions catalyzed by the wild-type enzyme. However,
an enzymatic reaction rate can be slowed by the mutation
of particular catalytically important residues, resulting in
the accumulation of specific intermediates.Thus, when Lys
230, which facilitates the decarboxylation of the oxalosuc-
cinate intermediate (Fig. 21-21) is mutated to Met in
NADP
⫹
-dependent isocitrate dehydrogenase, the oxalo-
succinate intermediate accumulates. This accumulated in-
termediate was directly visualized in the X-ray structure
of the mutant enzyme, in the presence of a steady-state
flow of substrate, through the use of fast X-ray intensity
measurements.
D. ␣-Ketoglutarate Dehydrogenase
a-Ketoglutarate dehydrogenase catalyzes the oxidative de-
carboxylation of an a-keto acid (a-ketoglutarate), releasing
the citric acid cycle’s second CO
2
and NADH (Reaction 4
of Fig.21-1).The overall reaction, which chemically resem-
bles that catalyzed by the PDC (Fig. 21-6), is mediated
by a homologous multienzyme complex consisting of
␣-ketoglutarate dehydrogenase (E1o),dihydrolipoyl trans-
succinylase (E2o), and dihydrolipoyl dehydrogenase (E3)
in which the E3 subunits are identical to those in the PDC
(Section 21-2A).
Individual reactions catalyzed by the complex occur by
mechanisms identical to those of the pyruvate dehydroge-
nase reaction (Section 21-2A), the product likewise being a
“high-energy” thioester, in this case succinyl-CoA. There
are no covalent modification enzymes in the ␣-ketoglu-
tarate dehydrogenase complex, however.
E. Succinyl-CoA Synthetase
Succinyl-CoA synthetase (also called succinate thiokinase)
hydrolyzes the “high-energy” compound succinyl-CoA with
the coupled synthesis of a “high-energy” nucleoside triphos-
phate (Reaction 5 of Fig. 21-1). (Note: Enzyme names can
refer to either the forward or the reverse reaction; in this
case, succinyl-CoA synthetase and succinate thiokinase re-
fer to the reverse reaction.) GTP is synthesized from
GDP ⫹ P
i
by the mammalian enzyme; plant and bacterial
enzymes utilize ADP ⫹ P
i
to form ATP. These reactions
are nevertheless equivalent since ATP and GTP are rapidly
interconverted through the action of nucleoside diphos-
phate kinase (Section 16-4C):
a. The Succinyl-CoA Thioester Bond Energy Is
Preserved through the Formation of a Series of
“High-Energy” Phosphates
How does succinyl-CoA synthetase couple the exergonic
hydrolysis of succinyl-CoA (⌬G°¿ ⫽⫺32.6 kJ ⴢ mol
⫺1
)to
the endergonic formation of a nucleoside triphosphate
(⌬G°¿ ⫽ 30.5 kJ ⴢ mol
⫺1
)? This question was answered
GTP ⫹ ADP Δ GDP ⫹ ATP
¢G°¿ ⫽ 0
through the creative use of isotope tracers. In the absence
of succinyl-CoA, the spinach enzyme (which utilizes ade-
nine nucleotides) catalyzes the transfer of ATP’s ␥ phos-
phoryl group to ADP as detected by
14
C-labeling ADP and
observing the label to appear in ATP. Such an isotope ex-
change reaction (Section 14-5D) suggests the participation
of a phosphoryl–enzyme intermediate that mediates the
reaction sequence:
Indeed, this information led to the isolation of a kineti-
cally active phosphoryl–enzyme in which the phosphoryl
group is covalently linked to the N3 position of a His
residue.
When the succinyl-CoA synthetase reaction, which is
freely reversible, is run in the direction of succinyl-CoA
synthesis (opposite to its direction in the citric acid cycle)
using [
18
O]succinate as a substrate,
18
O is transferred from
succinate to phosphate. Evidently, succinyl phosphate, a
“high-energy” mixed anhydride, is transiently formed dur-
ing the reaction.
These observations suggest the following three-step
sequence for the mammalian succinyl-CoA synthetase
reaction (Fig. 21-22):
1. Succinyl-CoA reacts with P
i
to form succinyl phos-
phate and CoA (accounting for the
18
O-exchange reaction).
2. Succinyl phosphate’s phosphoryl group is transferred
to an enzyme His residue, releasing succinate (accounting
for the 3-phosphoHis residue).
3. The phosphoryl group on the enzyme is transferred
to GDP, forming GTP (accounting for the nucleoside
diphosphate exchange reaction).
Note how in each of these steps the “high-energy” succinyl-
CoA’s free energy of hydrolysis is conserved through the
successive formation of “high-energy” compounds: First
succinyl phosphate, then a 3-phosphoHis residue, and
finally GTP. The process is reminiscent of passing a hot
potato.
b. A Pause for Perspective
Up to this point in the cycle, one acetyl equivalent has
been completely oxidized to two CO
2
. Two NADHs and
one GTP (in equilibrium with ATP) have also been gener-
ated. In order to complete the cycle, succinate must be con-
verted back to oxaloacetate.This is accomplished by the cy-
cle’s remaining three reactions.
P
PP+
A +
Step 1
P P P E
ATP
A
P P E
ADP ATP*
ADP*
Phosphoryl-
enzyme
+
A +
Step 2
P P E
A P P E
810 Chapter 21. Citric Acid Cycle
JWCL281_c21_789-822.qxd 7/2/10 9:06 AM Page 810
