Voet D., Voet Ju.G. Biochemistry
Подождите немного. Документ загружается.

resulting depolarization of the postsynaptic membrane ini-
tiates a new action potential. The AChR is also permeable
to K
⫹
and Ca
2⫹
ions. However, since the thermodynamic
driving forces exerted by the resting membrane potential
and the K
⫹
concentration gradient oppose each other and
largely balance, relatively few K
⫹
ions are transferred (for
Na
⫹
ions, these forces reinforce each other).The extracel-
lular concentration of Ca
2⫹
is so much less than that of
Na
⫹
that it makes only a negligible contribution to the in-
ward ionic current. After 1 to 2 ms, the ACh sponta-
neously dissociates from the receptor and the channel
closes.
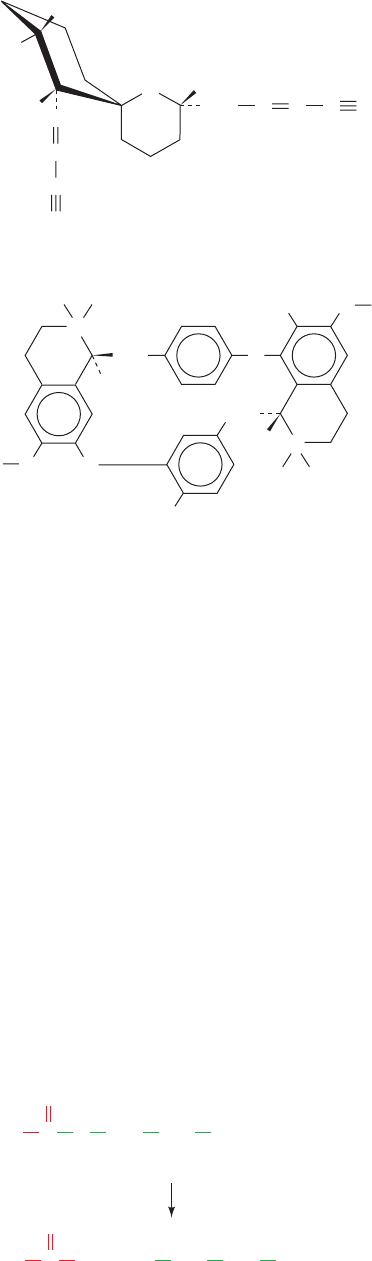
The electron crystallography–based structure (Section
12-3Ab) of the AChR in its closed (unliganded) form, de-
termined by Nigel Unwin, when viewed from the synaptic
cleft, is an 80-Å-diameter 5-fold pseudosymmetric assem-
bly whose subunits are arranged in the clockwise order -␣-
-␦-␣-␥- (Fig. 20-39a).When viewed parallel to the plane of
the postsynaptic membrane, it is a 160-Å-long wedge-
shaped particle (Fig. 20-39b). Each of its subunits consists
of three domains: an N-terminal extracellular assembly
that consists mainly of a 10-stranded  barrel; a transmem-
brane 4-helix down–up–down–up bundle (M1–M4); and an
intracellular component composed mainly of a disordered
segment extending from M3 followed by a curved helix
(MA) that precedes M4.The ACh-binding sites are located
between the ␣–␥ and ␣–␦ interfaces of their extracellular
domains.
The ACh receptor’s most striking structural feature is an
⬃20-Å-diameter and ⬃65-Å-long water-filled central
channel that extends from the receptor’s synaptic entrance
to the level of the lipid bilayer.There it forms a more con-
stricted ⬃30-Å-long pore, constructed from the ring of the
five M2 helices, that is blocked near the middle of the bi-
layer. This blockage, which is presumably the channel’s
gate, consists of rings of conserved Leu 264 and Val/Ile 268
side chains that project inward from the M2 helices. Unwin
has proposed that the binding of ACh to the AChR al-
losterically induces clockwise rotations (viewed from the
synaptic cleft) of its ␣ subunit’s M2 helices that results in
the opening of the gate.
The intracellular end of the AChR contains an ⬃20-Å-
diameter central cavity that is ⬃20 Å long and is connected
to the cytosol via lateral openings between adjacent MA
helices at a level that is ⬃30 Å below the membrane sur-
face.These openings are largely formed by anionic residues
and have widths of ⬃8 Å, which is comparable to the diam-
eters of K
⫹
and Na
⫹
ions surrounded by their first hydra-
tion shells. Thus, they likely serve as filters that prevent the
passage of cytoplasmic anions and large cations. Recall that
Kv channels have similar lateral openings to the cytoplasm
(Section 20-5Ac).
The ACh receptor is the target of some of the most
deadly known neurotoxins (death occurs through respira-
tory arrest), whose use has greatly aided in the elucidation
of receptor function. Histrionicatoxin, an alkaloid secreted
by the skin of the Colombian arrow-poison frog Dendro-
bates histrionicus, and d-tubocurarine, the active ingredient
of the Amazonian arrow-poison curare as well as a med-
ically useful paralytic agent, are both ACh antagonists that
prevent ACh receptor channel opening:
Similarly, a family of homologous 7- to 8-kD venom pro-
teins from some of the world’s most poisonous snakes, in-
cluding ␣-bungarotoxin from snakes of the genus Bungarus,
erabutoxin from sea snakes, and cobratoxin from cobras,
prevent ACh receptor channel opening by binding specifi-
cally and all but irreversibly to its ␣ subunits. Indeed,
detergent-solubilized ACh receptor has been purified by
affinity chromatography on a column containing covalently
attached cobra toxin.Myasthenia gravis (Greek: mys, muscle
⫹ astheneia, weakness and Latin: gravis, serious), an au-
toimmune disease characterized by muscle weakness, is
caused by circulating antibodies that bind to the AChR so
as to lead to its destruction or block its binding of ACh.
d. Acetylcholine Is Rapidly Degraded
by Acetylcholinesterase
An ACh molecule that participates in the transmission of a
given nerve impulse must be degraded in the few milliseconds
before the potential arrival of the next nerve impulse.This es-
sential task is accomplished by acetylcholinesterase (AChE),
a 75-kD fast-acting enzyme that is GPI anchored (Section
12-3B) to the surface of the postsynaptic membrane:
Acetylcholine
Acetate Choline
H
3
C CH
2
++
+
C
O
CH
2
+
HO
H
2
O
N(CH
3
)
3
+
CH
2
CH
2
N(CH
3
)
3
O
H
+
H
3
CC
O
acetylcholinesterase
O
–
H
3
C
H
3
C
CH
3
CH
2
CH
3
H
3
CCH
3
CH
2
CH
2
H
H
CH
OH
CH
HC
H
N
C
Histrionicatoxin
d-Tubocurarine
H
H
C
C
H
CCH
OO
H
O
O
H
OH
OH
N
+
N
+
Section 20-5. Neurotransmission 781
JWCL281_c20_744-788.qxd 7/1/10 7:20 AM Page 781
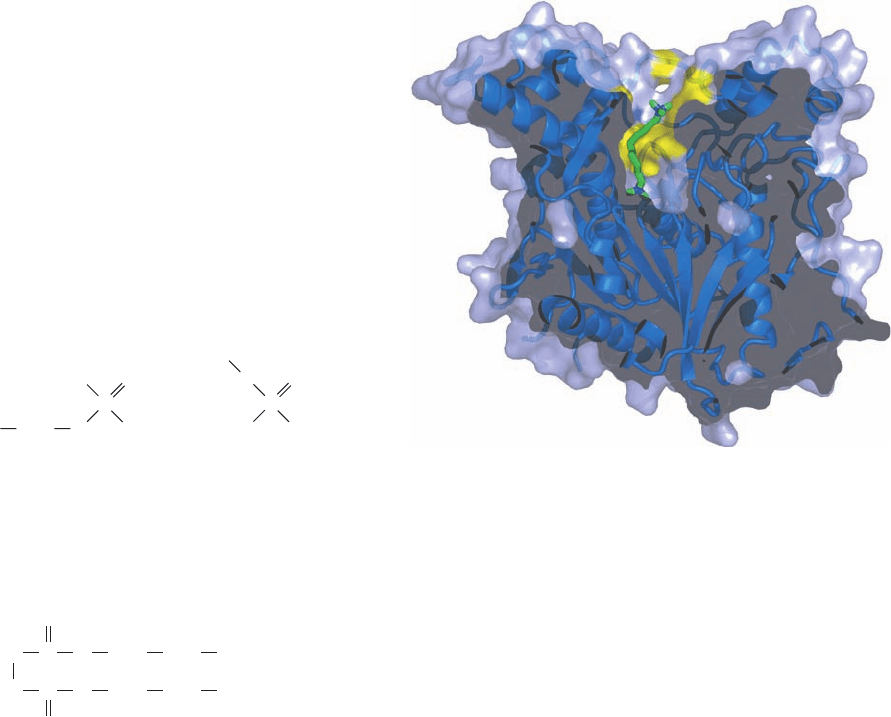
(the turnover number of AChE, k
cat
⫽ 14,000 s
⫺1
; the en-
zyme’s catalytic efficiency,k
cat
/K
M
⫽ 1.5 ⫻ 10
8
M
⫺1
ⴢ s
⫺1
,is
close to the diffusion-controlled limit, so that it is a nearly
perfect catalyst; Section 14-2Bb). The resulting choline is
taken up by the presynaptic cell via an Na
⫹
–choline sym-
port for use in the resynthesis of ACh.The operation of this
transporter is similar to that of the Na
⫹
–glucose symport of
intestinal brush border cells (Section 20-4A).
AChE is a serine esterase; that is, its catalytic mecha-
nism resembles that of serine proteases such as trypsin.
These enzymes, as we have seen in Section 15-3Ab, are ir-
reversibly inhibited by alkylphosphofluoridates such as di-
isopropylphosphofluoridate (DIPF). Indeed, related com-
pounds such as tabun and sarin
are military nerve gases because their efficient inactivation
of human AChE by reaction with the active site Ser causes
paralysis stemming from cholinergic nerve impulse block-
ade and thus death by suffocation. Succinylcholine,
which is used as a muscle relaxant during surgery, is an ACh
agonist that, although rapidly released by the ACh recep-
tor, is but slowly hydrolyzed by AChE. Succinylcholine
therefore produces persistent depolarization of the postsy-
naptic membrane. Its effects are short-lived, however, be-
cause it is rapidly hydrolyzed by the relatively nonspecific
liver and plasma enzyme butyrylcholinesterase. Certain
snake venoms, such as that of the green mamba snake, in-
activate AChE, although they do so by binding to a site on
AChE distinct from its active site.
e. X-Ray Structure of Acetylcholinesterase
The X-ray structure of the 537-residue AChE from the
electric ray Torpedo californica, determined by Joel Sussman,
Israel Silman, and Michal Harel, confirms that the previ-
ously identified Ser 200 and His 440 are members of
AChE’s catalytic triad. The structure further reveals that
the third member of AChE’s catalytic triad is Glu 327
rather than an Asp residue, only the second instance of a
Glu in this position among the many serine proteases, li-
pases, and esterases of known structure. AChE’s catalytic
triad is arranged in what appears to be the mirror image of
the catalytic triads in trypsin and subtilisin, for example
(Figure 15-20), although, of course, this is not actually the
case since all proteins consist of
L-amino acid residues.
C
O
O
O
O
CCH
2
CH
2
H
2
C
H
2
C
CH
2
CH
2
N(CH
3
)
3
Succinylcholine
N(CH
3
)
3
+
+
(CH
3
)
2
N
(CH
3
)
2
CH
O
O
P
CN
CH
3
CH
2
H
3
CF
O
O
P
Tabun Sarin
AChE’s catalytic site is near the bottom of a narrow and
20-Å-deep gorge that extends halfway through the protein
and widens out near its base (Fig. 20-40). The sides of this
so-called active site gorge are lined with the side chains of
14 aromatic residues that comprise 40% of its surface area.
Since the side chain O atom of the active site Ser is only 4 Å
from the bottom of the gorge, ACh must bind in the gorge
with its positively charged trimethylammonium group sur-
rounded by aromatic side chains.This conclusion came as a
surprise since it had been understandably expected that the
trimethylammonium group would be bound at an anionic
site. Perhaps the weak binding provided by the interactions
of the trimethylammonium group with the electrons of
the aromatic rings facilitates the rapid diffusion of ACh to
the bottom of the gorge, thereby accounting for the en-
zyme’s high turnover number. In fact, model aromatic com-
pounds have been synthesized that also bind quaternary
ammonium compounds.
f. Amino Acids and Their Derivatives Function
as Neurotransmitters
The mammalian nervous system employs well over 30
substances as neurotransmitters. Some of these substances,
such as glycine and glutamate, are amino acids; many others
are amino acid decarboxylation products or their derivatives
(often referred to as biogenic amines). For example, as we
782 Chapter 20. Transport Through Membranes
Figure 20-40 X-ray structure of Torpedo californica
acetylcholinesterase in complex with its inhibitor
decamethonium ion [(CH
3
)
3
N
⫹
(CH
2
)
10
N
⫹
(CH
3
)
3
]. In this
cutaway drawing through the enzyme’s active site gorge, the
protein’s ribbon diagram is embedded in its semitransparent
surface diagram, which is tinted blue with the exception of the
aromatic residues (Phe, Trp, and Tyr) lining the active site gorge,
which are yellow. The decamethonium ion that is bound in the
active site gorge is drawn in stick form with C green and N blue.
[Based on an X-ray structure by Joel Sussman, Israel Silman, and
Michal Harel,The Weizmann Institute of Science, Rehovot,
Israel. PDBid 1ACL.]
JWCL281_c20_744-788.qxd 6/4/10 1:40 PM Page 782
shall see in Section 26-4B, dopamine, norepinephrine, and
epinephrine [which are collectively termed catecholamines
because they are derivatives of catechol (1,2-dihydroxyben-
zene)] are sequentially synthesized from tyrosine, whereas
␥-aminobutyric acid (GABA), histamine, and serotonin are
derived from glutamate, histidine, and tryptophan, respec-
tively (Fig. 20-41). Many of these compounds are hormon-
ally active substances that are present in the bloodstream.
However, since the brain is largely isolated from the general
circulation by a selective filtration system known as the
blood–brain barrier (Section 15-4Ba), the presence of these
substances in the blood has no direct effect on the brain.The
use of the same compounds as hormones and neurotrans-
mitters apparently has no physiological significance but,
rather, is thought to reflect evolutionary opportunism in
adapting already available systems to new roles.
The use of selective staining techniques has established
that each of the different neurotransmitters is used in dis-
crete and often highly localized regions of the nervous sys-
tem. The various neurotransmitters are, nevertheless, not
simply functional equivalents of acetylcholine. Rather,many
of them have distinctive physiological roles. For example,
both GABA and glycine are inhibitory rather than excita-
tory neurotransmitters. The receptors for these substances
are ligand-gated channels that are selectively permeable to
Cl
⫺
. Hence, their opening tends to hyperpolarize the mem-
brane (make its membrane potential more negative) rather
than depolarize it. A neuron inhibited in this manner must
therefore be more intensely depolarized than otherwise to
trigger an action potential (note that these neurons re-
spond to more than one type of neurotransmitter). Thus,
anion channels are inhibitory, whereas cation channels are
excitatory. Ethanol, the oldest and most widely used psy-
choactive drug, is thought to act by inducing GABA re-
ceptors in the brain to open their Cl
⫺
channels.
The subunits of the various neurotransmitter-gated
cation channels have 20 to 40% sequence identity, as do
those of the anion channels. However, the two families of
channel proteins appear to be unrelated. Despite this lack
of homology, the sequences of the two types of channels
suggest that they have considerable structural similarity.
The actual nature of a neuron’s response to a neurotrans-
mitter depends more on the characteristics of the correspon-
ding receptor than on the neurotransmitter’s identity. Thus,
as we have seen, nicotinic ACh receptors, which trigger the
rapid contraction of skeletal muscles, respond to ACh within
a few milliseconds by depolarizing the postsynaptic mem-
brane. In contrast, the binding of ACh to muscarinic ACh re-
ceptors in heart muscle inhibits muscle contraction over a
period of several seconds (several heartbeats). This is ac-
complished by hyperpolarizing the postsynaptic membrane
through the closure of otherwise open K
⫹
channels. Slow-
acting neurotransmitters may act by inducing the formation
of a second messenger such as cAMP. In fact, the brain has
the highest concentration of cAMP-dependent kinases in
the body. The binding of catecholamines to their respective
neuronal receptors, through the intermediacy of adenylate
cyclase and cAMP, activates protein kinases to phosphory-
late ion channels so as to alter the neuron’s electrical prop-
erties. The ultimate effect of this process can be either exci-
tatory or inhibitory. Thus catecholamines, whether acting as
hormones (Section 19-1F) or as neurotransmitters, have sim-
ilar mechanisms of receptor activation.
g. Neuropeptides Are Neurotransmitters
A large and growing list of hormonally active polypep-
tides known as neuropeptides also act as neurotransmitters.
Not surprisingly, perhaps, the opioid peptides -endorphin,
met-enkephalin, and leu-enkephalin (Section 19-1K), as
well as the hypothalamic releasing factors TRF, GnRF, and
somatostatin (Section 19-1H), are in this category. What is
less expected is that several gastrointestinal polypeptides,
including the hormones gastrin,secretin,and cholecystokinin
Section 20-5. Neurotransmission 783
Figure 20-41 A selection of neurotransmitters.
CH
2
CH
2
Histamine
γ-Aminobutyric acid (GABA)
Serotonin
(5-Hydroxytryptamine)
Epinephrine
N
H
N
NH
3
CH
2
CH
2
C
H
HO
CH
2
CH
3
+
NH
3
N
+
NH
2
+
H
3
N
+
HO
HO
Glutamate
Glycine
COO
–
COO
–
CH
2
CH
2
CH
H
3
N
+
COO
–
CH
2
Norepinephrine
CH
2
C
H
OH
NH
3
+
HO
HO
OH
Dopamine
CH
2
NH
3
+
HO
HO
CH
2
CH
2
NH
3
+
CH
2
CH
2
–
OOC
JWCL281_c20_744-788.qxd 6/4/10 1:23 PM Page 783
(CCK; Section 19-1C), may also act as neurotransmitters
in discrete regions of the brain, as do the pituitary hor-
mones oxytocin and vasopressin (Section 19-1Ha). Such
neuropeptides differ from the simpler neurotransmitters
in that they seem to elicit complex behavior patterns. For
example, intracranially injecting rats with a nanogram of
vasopressin greatly enhances their ability to learn and re-
member new tasks. Similarly, injecting a male or a female
rat with GnRF evokes the respective postures they re-
quire for copulation. Just how these neuropeptides oper-
ate is but one of the many enigmas of brain function and
organization.
784 Chapter 20. Transport Through Membranes
1 Thermodynamics of Transport Polar molecules and
ions are transported across biological membranes by specific
transmembrane transport proteins. The free energy change of
the species transported depends on the ratio of its concentra-
tions on the two sides of the membrane and, if the species is
charged, on the membrane potential, ⌬⌿.
2 Kinetics and Mechanisms of Transport The rate of
nonmediated diffusion across a membrane is a linear function
of the difference in concentration of the species on the two
sides of the membrane as governed by Fick’s first law of diffu-
sion. Mediated transport is characterized by rapid saturation
kinetics and specificity for the substance transported. It is also
subject to competitive inhibition and chemical inactivation.
Ionophores transport ions through membranes. Carrier
ionophores, such as valinomycin, do so by wrapping a specific
ion in a hydrophobic, membrane-soluble coat that can freely
diffuse through the membrane. Maltoporin is specific for the
passage of maltodextrins because its transport channel
matches their left-handed helical shape and is lined with aro-
matic side chains that form a so-called greasy slide. Glucose
transport across erythrocyte membranes is mediated by
dimeric transmembrane glycoproteins that can assume two
conformations: one with a glucose binding site facing the ex-
ternal cell surface and the other with the glucose site facing
the cytosol. Transport occurs by the binding of glucose to the
protein on one face of the membrane, followed by a conforma-
tional change that closes this site and exposes the other (a
gated pore). GLUT4 is stored in specialized vesicles, which on
insulin stimulation fuse with the plasma membrane.
The KcsA K
⫹
channel, a transmembrane homotetramer,
permits the rapid passage of K
⫹
ions, for which it is highly selec-
tive. It does so, in part, because it forms an aqueous cavity sur-
rounded by the negative ends of helix dipoles that stabilizes K
⫹
ions in the middle of the bilayer. K
⫹
ions, but not the smaller
Na
⫹
ions, are transported because the K
⫹
channel’s selectivity
filter selectively coordinates K
⫹
ions by rings of O atoms in a
way that allows their dehydration, passage, and subsequent hy-
dration without significant activation barriers. ClC Cl
⫺
channels
are TM homodimers, each subunit of which contains an hour-
glass-shaped channel.Aquaporins are TM homotetramers with
an hourglass-shaped channel through each subunit. They facili-
tate the rapid passage of water molecules through membranes
but inhibit the passage of protons via proton jumping by pre-
venting the reorientation of a centrally located water molecule.
3 ATP-Driven Active Transport Active transport of mole-
cules or ions against a concentration gradient requires an input
of free energy. The free energy of ATP hydrolysis is coupled to
the transport of three Na
⫹
ions out of and two K
⫹
ions into the
cell by the (Na
⫹
–K
⫹
)–ATPase. This electrogenic process in-
volves phosphorylation of an Asp residue (by ATP) in the pres-
ence of Na
⫹
and its dephosphorylation (hydrolysis) in the pres-
ence of K
⫹
.Phosphorylation and dephosphorylation are accom-
panied by conformational changes that ensure rapid intercon-
version of all intermediates along the transport pathway. The
X-ray structure of the (Na
⫹
–K
⫹
)–ATPase reveals that it has a
10-helix transmembrane domain that binds 3 Na
⫹
ions or 2 K
⫹
ions near its center and three well-separated cytoplasmic do-
mains. The X-ray structures of four different conformational
states of the sarcoplasmic reticulum Ca
2⫹
–ATPase (SERCA) in-
dicate its transport mechanism and that of P-type ATPases in
general. Plasma membrane Ca
2⫹
–ATPase is activated by
Ca
2⫹
–calmodulin.The (H
⫹
–K
⫹
)–ATPase in the stomach is indi-
rectly inhibited by cimetidine and its analogs, and directly inhib-
ited by omeprazole. Bacteria transport sugars by group translo-
cation, a process in which the transported substance is
chemically modified.The PTS system, which has important reg-
ulatory functions, phosphorylates sugars as they are transported
by utilizing phosphoenolpyruvate as a phosphoryl donor.ABC
transporters use ATP to power conformational changes that
transport a wide variety of substances across membranes.
4 Ion Gradient–Driven Active Transport Active trans-
port may be driven by the free energy stored in ion gradients
(secondary active transport). Glucose is transported into in-
testinal epithelial cells against its concentration gradient by an
Na
⫹
–glucose symport. This process is ultimately powered by
the free energy of ATP hydrolysis since the Na
⫹
gradient is
constantly being replenished via the (Na
⫹
–K
⫹
)–ATPase. The
system conforms to a Random Bi Bi kinetic mechanism,
implying that both Na
⫹
and glucose must be bound for the
transport-producing conformational change to occur. Lactose
is transported into E. coli by lactose permease, an H
⫹
–lactose
symport.This process is driven by the cell’s electrochemical H
⫹
gradient, which is, in turn, maintained by a proton pump cou-
pled with oxidative metabolism.The mitochondrial ATP–ADP
translocator also interacts with the membrane potential in
the asymmetric transport of ATP out of and ADP into the
mitochondrion.
5 Neurotransmission Voltage-gated cation channels
such as Kv channels open in response to the membrane poten-
tial and close a short time later through the action of a second
gate that functions via a modified “ball-and-chain” mechanism.
Nerve impulses are traveling waves of electrical excitation
along axon plasma membranes known as action potentials that
are generated by the transient opening of voltage-gated Na
⫹
channels to let Na
⫹
ions into the cell followed a short time later
by the transient opening of voltage-gated K
⫹
channels to let K
⫹
ions out of the cell. Nerve impulses are chemically transmitted
across most synapses by the release of neurotransmitters.
Acetylcholine (ACh), the best characterized neurotransmitter,
is packaged in synaptic vesicles that are exocytotically released
CHAPTER SUMMARY
JWCL281_c20_744-788.qxd 7/1/10 7:20 AM Page 784

References 785
into the synaptic cleft. This process is triggered by an increase
in cytosolic [Ca
2⫹
] resulting from the arriving action potential’s
opening of voltage-gated Ca
2⫹
channels. The ACh diffuses
across the synaptic cleft, where it binds to the ACh receptor, a
transmembrane cation channel that opens in response to ACh
binding.The resultant flow of Na
⫹
into and K
⫹
out of the post-
synaptic cell depolarizes the postsynaptic membrane, which, if
sufficient neurotransmitter has been released,triggers a postsy-
naptic action potential. The ACh receptor is the target of nu-
merous deadly neurotoxins, including histrionicatoxin, d-
tubocurarine, and cobra toxin, which all bind to the ACh
receptor so as to prevent its opening. The ACh is rapidly de-
graded, before the possible arrival of the next nerve impulse,
through the action of acetylcholinesterase, a fast-acting serine
esterase that has an unusual aromatic side chain–lined active
site gorge. Nerve gases and succinylcholine inhibit acetyl-
cholinesterase and therefore block nerve impulse transmission
at cholinergic synapses.
Many specific regions of the nervous system employ neuro-
transmitters other than ACh.Most of these neurotransmitters are
amino acids, such as glycine and glutamate, or their decarboxyla-
tion products and their derivatives, including catecholamines,
GABA, histamine, and serotonin. Many of these compounds are
also hormonally active, but they are excluded from the brain by
the blood–brain barrier.Although many neurotransmitters, such
as ACh, are excitatory, others are inhibitory. The latter stimulate
the opening of anion (Cl
⫺
) channels, thereby causing the postsy-
naptic membrane to become hyperpolarized, so that it must be
more highly depolarized than otherwise to trigger an outgoing
action potential.There is also a growing list of polypeptide neuro-
transmitters, many of which are also polypeptide hormones, that
elicit complex behavior patterns.
General
Alberts, B., Johnson, A., Lewis, J., Raff, M., Roberts, K., and
Walter, P., The Molecular Biology of the Cell (5th ed.), Chapter
11, Garland Science (2008).
Ashcroft, F.M., From molecule to malady, Nature 440, 440–447
(2006). [Reviews ion channels and their defects.]
Busch, W. and Saier, M.H., Jr.,The transporter classification (TC)
system, 2002, Crit. Rev. Biochem. Mol. Biol. 37, 287–337 (2002).
[Summarizes the classification of the nearly 400 families of
transport systems and their distribution among the three do-
mains of life.]
Gadsby, D.C., Ion channels versus ion pumps: the principal differ-
ence, in principle, Nature Struct. Mol. Biol. 10, 344–352 (2009).
Gouaux, E. and MacKinnon, R., Principles of selective ion pump
transport in channels and pumps, Science 310, 1461–1465
(2005). [Compares several transport proteins of known struc-
ture and discusses the selectivity of Na
⫹
,K
⫹
,Ca
2⫹
, and
Cl
⫺
transport.]
Lodish, H., Berk, A., Kaiser, C.A., Krieger, M., Scott, M.P.,
Bretscher, A., Ploegh, H., and Matsudaira, P., Molecular Cell
Biology (6th ed.), Chapters 11 and 23, Freeman (2008).
Kinetics and Mechanism of Transport
Clapham, D.E., Unlocking family secrets: K
⫹
channel transmem-
brane domains, Cell 97, 547–550 (1999).
Dutzler, R., Wang, Y.-F., Rizkallah, P.J., Rosenbusch, J.P., and
Schirmer,T., Crystal structures of various maltooligosaccharides
reveal a specific sugar translocation pathway, Structure 4,
127–134 (1996); and Dutzler, R., Schirmer, T., Karplus, M., and
Fischer, S.,Translation mechanism of long sugar chains across the
maltoporin membrane channel, Structure 10, 1273–1284 (2002).
Glucose Transport
Barnett, J.E.G., Holman, G.D., Chalkley, R.A., and Munday, K.A.,
Evidence for two asymmetric conformational states in the hu-
man erythrocyte sugar transport system, Biochem. J. 145,
417–429 (1975).
Huang, S. and Czech, M.P., The GLUT4 glucose transporter, Cell.
Metab. 5, 237–252 (2007).
Watson, R.T. and Pessin, J.E., GLUT4 translocation: The last 200
nanometers, Cell. Signal. 19, 2209–2217 (2007); and Bridging
the GAP between insulin signaling and GLUT4 translocation,
Trends Biochem. Sci. 31, 215–222 (2006).
K
⫹
Channels
Roux, B., Ion conduction and selectivity in K
⫹
channels, Annu.
Rev. Biophys. Biomol. Struct. 34, 153–171 (2005).
Zhou, Y., Morais-Cabral, J.H., Kaufman, A., and MacKinnon, R.,
Chemistry of ion coordination and hydration revealed by a K
⫹
channel–Fab complex at 2.0 Å resolution, Nature 414, 43–48
(2001); and Doyle, D.A., Morais-Cabral, J.M., Pfuetzner, R.A.,
Kuo, A., Gulbis, J.M., Cohen, S.L., Chait, B.T., and MacKinnon,
R., The structure of the potassium channel: Molecular basis of
K
⫹
conduction and selectivity, Science 280, 69–77 (1998). [High
and medium resolution X-ray structures of the KcsA channel.]
Cl
⫺
Channels
Dutzler, R.,The ClC family of chloride channels and transporters,
Curr. Opin. Struct. Biol. 16, 439–446 (2006).
Dutzler,R., Campbell,E.B.,Cadene,M.,Chait, B.T.,and MacKinnon,
R., X-ray structure of a ClC chloride channel at 3.0 Å reveals
the molecular basis of anion selectivity, Nature 415, 287–294
(2002).
Lobet, S. and Dutzler, R., Ion-binding properties of the ClC chlo-
ride selectivity filter, EMBO J. 25, 24–33 (2006).
Aquaporins
Fu, D. and Lu, M., The structural basis of water permeation and
proton exclusion in aquaporins, Mol. Memb. Biol. 24, 366–374
(2007). [A review.]
Gonen, T. and Walz, T., The structure of aquaporins, Q. Rev. Bio-
phys. 39, 361–396 (2006).
King, L.S., Kozono, D., and Agre, P., From structure to disease:
The evolving tale of aquaporin biology, Nature Rev. Mol. Cell
Biol. 5, 687–698 (2004).
Sui, H., Han, B.-G., Lee, J.K., and Jap, B.K., Structural basis of
water-specific transport through the AQP1 water channel,
Nature 414, 872–878 (2001).
(Na
⫹
–K
⫹
)–ATPase
Blaustein, M.P., Physiological effects of endogenous ouabain:
Control of intracellular Ca
2⫹
stores and cell responsiveness,
Am. J. Physiol. 264, C1367–C1378 (1993).
Kaplan, J.H., Biochemistry of the Na,K-ATPase, Annu. Rev.
Biochem. 71, 511–535 (2002).
Morph, J.P., Pedersen, B.P., Toustrop-Jensen, M.S., Sørensen, T.L.-M.,
Petersen, J., Andersen, J.P., Vilsen, B., and Nissen, P., Crystal
REFERENCES
JWCL281_c20_744-788.qxd 3/17/10 1:49 PM Page 785
786 Chapter 20. Transport Through Membranes
structure of the sodium–potassium pump, Nature 450, 1043–1049
(2007). [The 3.4-Å resolution structure of the porcine enzyme.]
Shinoda, T., Ogawa, H., Cornelius, F., and Toyoshima, C., Crystal
structure of the sodium–potassium pump at 2.4 Å resolution,
Nature 459, 446–450 (2009). [The shark enzyme.]
Ca
2⫹
–ATPase
Enyedi, A., Vorherr, T., James, P., McCormick, D.J., Filoteo, A.G.,
Carafoli, E., and Penniston, J.T., The calmodulin binding do-
main of the plasma membrane Ca
2⫹
pump interacts both with
calmodulin and with another part of the pump, J. Biol. Chem.
264, 12313–12321 (1989).
Jencks, W.P., Coupling of hydrolysis of ATP and the transport of
Ca
2⫹
by the calcium ATPase of sarcoplasmic reticulum,
Biochem. Soc. Trans. 20, 555–559 (1992). [An insightful discus-
sion of the mechanism of coupling chemical energy to the vec-
torial transport of ions against a concentration gradient.]
Olesen, C., Picard, M., Winther, A.-M.L., Gyrup, C., Morth, J.P.,
Oxvig, C., Møller, J.V., and Nissen, P., The structural basis of
calcium transport by the calcium pump, Nature 450, 1036–1042
(2006).
Toyoshima, C. and Inesi, G., Structural basis of ion pumping by
Ca
2⫹
-ATPase of the sarcoplasmic reticulum, Annu. Rev.
Biochem. 73, 269–292 (2004).
Toyoshima, C., Nakasako, M., and Ogawa, H., Crystal structure of
the calcium pump of sacroplasmic reticulum at 2.6 Å resolu-
tion, Nature 405, 647–655 (2000).
(H
⫹
–K
⫹
)–ATPase
Besanèon, M., Shin, J.M., Mercier, F., Munson, K., Rabon, E.,
Hersey, S., and Sachs, G., Chemomechanical coupling in the
gastric H,K ATPase, Acta Physiol. Scand. 146, 77–88 (1992).
Pedersen, B.P., Buch-Pedersen, M.J., Morth, J.P., Palmgren, M.G.,
and Nissen, P., Crystal structure of the plasma membrane
proton pump, Nature 450, 1111–1114 (2007).
PEP-Dependent Phosphotransferase System
Herzberg, O. and Klevit, R., Unraveling a bacterial hexose trans-
port pathway, Curr. Opin. Struct. Biol. 4, 814–822 (1994).
Hurley, J.H., Faber, H.R.,Worthylake,D., Meadow, N.D., Roseman, S.,
Pettigrew, D.W., and Remington, S.J., Structure of the regulatory
complex of Escherichia coli E III
glc
with glycerol kinase, Science
259, 673–677 (1993). [EIIA was previously named EIII.]
Meadow, N.D., Fox, D.K., and Roseman, S.,The bacterial phospho-
enolpyruvate:glucose phosphotransferase system, Annu. Rev.
Biochem. 59, 497–542 (1990); and Feese, M., Pettigrew, D.W.,
Meadow, N.D., Roseman, S., and Remington, S.J., Cation pro-
moted association (CPA) of a regulatory and target protein is
controlled by protein phosphorylation, Proc.Natl.Acad Sci.91,
3544–3548 (1994).
Saier, M.H., Jr., Chauvaux, S., Deutscher, J., Reizer, J., and Ye, J.-J.,
Protein phosphorylation and regulation of carbon metabolism
in gram-negative versus gram-positive bacteria, Trends
Biochem. Sci. 20, 267–271 (1995).
ABC Transporters
Higgins, C.F., Multiple molecular mechanisms for multidrug re-
sistance transporters, Nature 446, 749–757 (2007).
Hollerstein, K., Dawson, R.J.P., and Locher, K.P., Structure and
mechanism of ABC transporter proteins, Curr. Opin. Struct.
Biol. 17, 412–418 (2007).
Jones, P.M., O’Mara, M.L., and George,A.M.,ABC transporters: a
riddle wrapped in a mystery inside an enigma, Trends Biochem.
Sci. 34, 520–531 (2009).
Oldham, M.L., Davidson, A.L., and Chen, J., Structural insights
into ABC transporter mechanism, Curr. Opin. Struct. Biol. 18,
726–733 (2008).
Rees, D.C., Johnson, E., and Lewinson, O., ABC transporters: the
power to change, Nature Rev. Mol.Cell Biol. 10, 218–227 (2009).
Ward, A., Reyes, C.L.,Yu, J., Roth, C.B., and Chang, G., Flexibility
in the ABC transporter MsbA:Alternating access with a twist,
Proc. Natl. Acad Sci. 104, 19005–19010 (2007).
Na
⫹
–Glucose Symport
Faham, S.,Watanabe,A., Besserer, G.M., Cascio, D., Specht,A., Hi-
rayama, B.A., Wright, E.M., and Abramson, J., The crystal
structure of a sodium galactose transporter reveals insights
into Na
⫹
/sugar symport, Science 321, 810–814 (2008).
Wright, E.M., Hirayama, B.A., and Loo, D.F., Active sugar trans-
port in health and disease, J. Intern. Med. 261, 32–43 (2007).
Lactose Permease
Abramson, J., Smirnova, I., Kasho, V., Verner, G., Kaback, H.R.,
and Iwata, S., Structure and mechanism of the lactose perme-
ase of Escherichia coli, Science 301, 610–615 (2003).
Guan, L. and Kaback, H.R., Lessons from lactose permease,
Annu. Rev. Biophys. Biomol. Struct. 35, 67–91 (2006).
ATP–ADP Translocator
Klingenberg, M., Molecular aspects of the adenine nucleotide car-
rier from mitochondria, Arch. Biochem. Biophys. 270, 1–14
(1989).
Nury, H., Dahout-Gonzalez, C., Trézéguet, V., Lauquin, G.J.M.,
Brandolin, G., and Pebay-Peyroula, E., Relations between
structure and function of the mitochondrial ADP/ATP carrier,
Annu. Rev. Biochem. 75, 713–741 (2006).
Neurotransmission
Bezanilla, F., How membrane proteins sense voltage, Nature Rev.
Mol. Cell Biol. 9, 323–332 (2008).
Catterall, W.A., Structure and regulation of voltage-gated Ca
2⫹
channels, Annu. Rev. Cell Dev. Biol. 16, 521–555 (2000).
Geppert, M. and Südhof, T.C., Rab3 and synaptotagmin. The yin
and yang of synaptic transmission, Annu. Rev. Neurosci. 21,
75–95 (1998).
Gulbis, J.M. and Doyle, D.A., Potassium channel structures: do
they conform? Curr. Opin. Struct. Biol. 14, 440–446 (2004).
Hille, B., Ionic Channels of Excitable Membranes (3rd ed.),
Sinauer Associates (2001).
Lin, R.C. and Scheller, R.H., Mechanisms of synaptic vesicle exo-
cytosis, Annu. Rev. Cell Dev. Biol. 16, 19–49 (2000).
Long, S.B., Campbell, E.B., and MacKinnon, R., Crystal structure
of a mammalian voltage-dependent Shaker family K
⫹
channel;
and Voltage sensor of Kv1.2: Structural basis of electromechan-
ical coupling, Science 309, 897–903; and 903–908 (2005).
Long, S.B., Tao, X., Campbell, E.B., and MacKinnon, R., Atomic
structure of a voltage-dependent K
⫹
channel in a lipid
membrane-like environment, Nature 450, 376–382 (2007).
Orlova, E.V., Rahman, M.A., Gowen, B., Volynski, K.E., Ashton,
A.C., Manser, C., van Heel, M., and Ushkaryov,Y.A., Structure
of ␣-latrotoxin oligomers reveals that divalent cation-dependent
tetramers form membrane pores, Nature Struct. Biol. 7, 48–53
(2000).
Roosild, T.P., Lê, K.-T., and Choe, S., Cytoplasmic gatekeepers of
K
⫹
-channel flux: a structural perspective, Trends Biochem. Sci.
29, 39–45 (2004).
Sussman, J.L., Harel, M., Frolow, F., Oefner, C., Goldman,A.,Toker,
L., and Silman, I.,Atomic structure of acetylcholinesterase from
Torpedo californica: A prototypic acetylcholine-binding pro-
tein, Science 253, 872–879 (1991).
JWCL281_c20_744-788.qxd 7/1/10 7:20 AM Page 786
Swartz, K.J., Sensing voltage across lipid membranes, Nature 456,
891–897 (2008).
Unwin, N., Refined structure of the nicotinic acetylcholine receptor
at 4 Å resolution,J. Mol.Biol. 346, 967–989 (2004);and Miyazawa,
A., Fujiyoshi, Y., and Unwin, N., Structure and gating mechanism
of the acetylcholine receptor pore, Nature 423, 949–955 (2003).
Yellin, G., The voltage-gated potassium channels and their rela-
tives, Nature 419, 35–42 (2002).
Zhou, M., Morais-Cabral, J.H., Mann, S., and MacKinnon, R.,
Potassium channel receptor site for the inactivation gate and
quaternary amine inhibitors, Nature 411, 657–661 (2001).
Problems 787
1. If the glucose concentration outside a cell is 10 mM but that
inside a cell is 0.1 mM, what is glucose’s chemical potential differ-
ence across the membrane at 37°C?
*2. If a solution of an ionic macromolecule is equilibrated with
a salt solution from which it is separated by a membrane through
which the salt ions but not the macromolecule can pass, a mem-
brane potential is generated across the membrane. This so-called
Donnan equilibrium arises because the impermeability of the
membrane to some ions but not others prevents the equalization
of the ionic concentrations on the two sides of the membrane. To
demonstrate this effect, assume that the Cl
⫺
salt of a monocationic
protein, P
⫹
, is dissolved in water to the extent that [Cl
⫺
] ⫽ 0.1M
and is separated by a membrane impermeable to the protein but
not NaCl from an equal volume of 0.1M NaCl solution.Assuming
no volume change in either compartment, what are the concentra-
tions of the various ionic species on either side of the membrane
after the system has equilibrated? What is the membrane poten-
tial across the membrane? (Hint: Mass is conserved and the solu-
tion on each side of the membrane must be electrically neutral.At
equilibrium, )
3. Gramicidin A, a dimer of 15-residue polypeptides that
forms a hollow helix, is a channel-forming ionophore that permits
the passage of ⬃10
7
alkalai metal ions per second through a mem-
brane. How long would it take one molecule of gramicidin A to
transport enough Na
⫹
to change the concentration inside an ery-
throcyte of volume 80 m
3
by 10 mM? Assume the erythrocyte’s
Na
⫹
pumps are inoperative and that gramicidin A does not also
transport ions out of the cell, which it really does.
4. Predict whether the following compounds can cross a mem-
brane without mediation or will require facilitation. Indicate the
criteria you used to make these predictions. (a) Ethanol, (b) glycine,
(c) cholesterol, and (d) ATP.
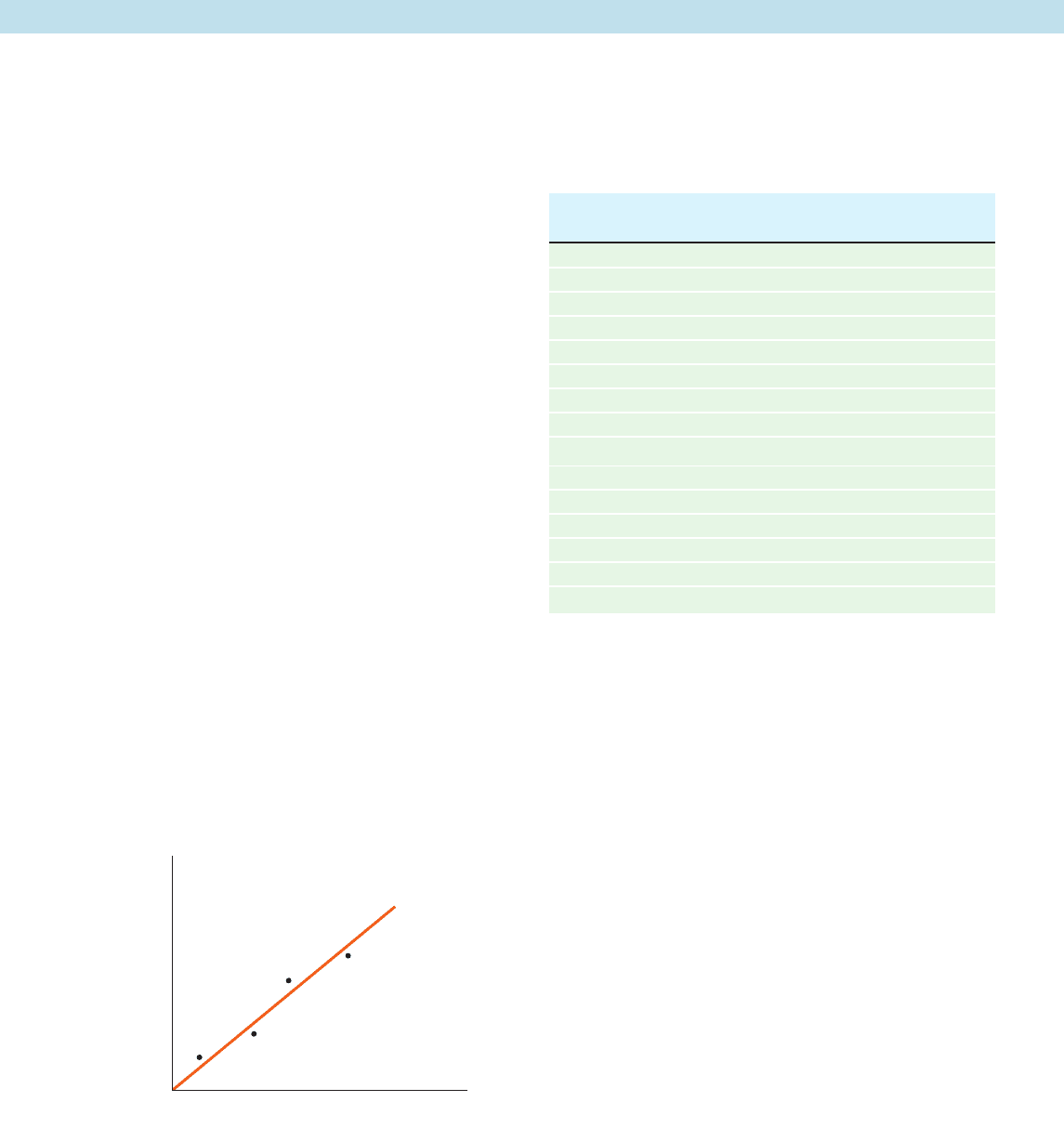
5. The rate of movement (flux) of a substance X into cells was
measured at different concentrations of X to construct the graph
below.
(a) Does this information suggest that the movement of X into
the cells is mediated by a protein transporter? Explain.
(b) What additional experiment could you perform to verify
that a transport protein is or is not involved?
Flux
[X]
¢G
Na
⫹
⫹ ¢G
Cl
⫺
⫽ 0.
6. You have isolated a new strain of bacteria and would like to
know whether leucine and ethylene glycol enter the cells by medi-
ated diffusion or only by a nonmediated route. To do this you
measure the initial rates of uptake of these molecules as a func-
tion of external concentration and obtain the data in the following
table.
Which compound(s) enters by a mediated route? What criteria
did you use for this decision?
7. Aquaporin AQP1 (Fig. 20-15) forms an hourglass-shaped
pore that, in its narrowest region, is 3 Å in diameter. AQP1 is im-
permeable to glycerol [CHOH(CH
2
OH)
2
]. However, a homolo-
gous and structurally similar aquaglyceroporin, which is mini-
mally 3.4 Å wide, permits the passage of glycerol but is only
poorly permeable to water. Discuss the possible differences be-
tween these channels that would account for their different
permeabilities.
8. The (Na
⫹
–K
⫹
)–ATPase is inhibited by nanomolar concen-
trations of vanadate, which forms a pentavalent ion, , with
trigonal bipyramidal symmetry. Explain the mechanism of this in-
hibition. (Hint: See Section 16-2B.)
9. What function might the synthesis of digitalis serve in the
purple foxglove plant?
10. The (H
⫹
–K
⫹
)–ATPase secretes H
⫹
at a concentration of
0.18M from cells that have an internal pH of 7.What is the ⌬G re-
quired for the transport of 1 mol of H
⫹
under these conditions?
Assuming that the ⌬G for ATP hydrolysis is ⫺31.5 kJ ⴢ mol
⫺1
under
these conditions, and that the membrane potential is 0.06 V, inside
negative, how much ATP must be hydrolyzed per mole of H
⫹
transported in order to make this transport exergonic?
VO
5⫺
5
PROBLEMS
Concentration Initial Uptake Rate
Compound (M) (arbitrary units)
Leucine 1 ⫻ 10
⫺6
110
2 ⫻ 10
⫺6
220
5 ⫻ 10
⫺6
480
1 ⫻ 10
⫺5
830
3 ⫻ 10
⫺5
1700
1 ⫻ 10
⫺4
2600
5 ⫻ 10
⫺4
3100
1 ⫻ 10
⫺3
3200
Ethylene glycol 1 ⫻ 10
⫺3
1
5 ⫻ 10
⫺3
5
0.01 10
0.05 50
0.1 100
0.5 500
1.0 1000
JWCL281_c20_744-788.qxd 3/17/10 1:49 PM Page 787
788 Chapter 20. Transport Through Membranes
11. A 100-Å-thick membrane has a membrane potential
of 100 mV. What is the magnitude of this potential difference in
V ⴢ cm
⫺1
? Comment on the magnitude of this potential field in
macroscopic terms.
12. The resting membrane potential (⌬⌿) of a neuron at
37°C is ⫺60 mV (inside negative). If the free energy change asso-
ciated with the transport of one Na
⫹
ion from outside to inside is
⫺11.9 kJ ⴢ mol
⫺1
, and [Na
⫹
] outside the cell is 260 mM, what is
[Na
⫹
] inside the cell?
13. Write a kinetic scheme for the (H
⫹
–K
⫹
)–ATPase that pro-
vides for coupled ATP hydrolysis with H
⫹
transport. Discuss the
order of substrate addition required for coupling. Identify the
steps in which mutual destabilization results in reasonable rates of
transport.
14. If the ATP supply in the cell shown in Fig. 20-27c suddenly
vanished, would the intracellular glucose concentration increase,
decrease, or remain the same?
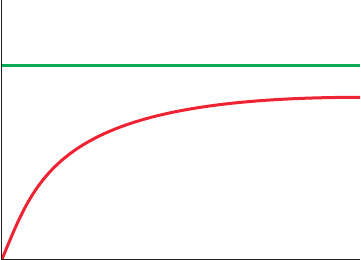
15. Endothelial cells and pericytes in the retina of the eye
have different mechanisms for glucose uptake. The figure below
shows the rate of glucose uptake for each type of cell in the pres-
ence of increasing amounts of sodium. What do these results re-
veal about the glucose transporter in each cell type?
Rate of Glucose Uptake
[Na
+
]
Endothelial cells
Pericytes
16. Why don’t nerve impulses propagate in the reverse
direction?
17. What is the resting membrane potential across an axonic
membrane at 25°C (a) in the presence of tetrodotoxin or (b) with
a high concentration of Cs
⫹
inside the axon (use the data in Table
20-3)? How do these substances affect the axon’s action potential?
18. Decamethonium ion [(CH
3
)
3
N
⫹
(CH
2
)
10
N
⫹
(CH
3
)
3
] is a
synthetic muscle relaxant.What is its mechanism of action?
JWCL281_c20_744-788.qxd 3/17/10 1:49 PM Page 788
789
Fumarate
Succinate
Succinyl-CoA
α-Ketoglutarate
Isocitrate
GTP
ATP
Ca
2+
, A
Ca
2+
NADH
789
CHAPTER 21
Citric Acid Cycle
1 Cycle Overview
A. Reactions of the Cycle
B. Historical Perspective
2 Metabolic Sources of Acetyl-Coenzyme A
A. Pyruvate Dehydrogenase Multienzyme Complex (PDC)
B. The Mechanism of Dihydrolipoyl Dehydrogenase
C. Control of Pyruvate Dehydrogenase
3 Enzymes of the Citric Acid Cycle
A. Citrate Synthase
B. Aconitase
C. NAD
⫹
-Dependent Isocitrate Dehydrogenase
D. ␣-Ketoglutarate Dehydrogenase
E. Succinyl-CoA Synthetase
F. Succinate Dehydrogenase
G. Fumarase
H. Malate Dehydrogenase
I. Integration of the Citric Acid Cycle
J. Evolution of the Citric Acid Cycle
4 Regulation of the Citric Acid Cycle
5 The Amphibolic Nature of the Citric Acid Cycle
In this chapter we continue our metabolic explorations by
examining the citric acid cycle, the common mode of oxida-
tive degradation in eukaryotes and prokaryotes. This cycle,
which is alternatively known as the tricarboxylic acid
(TCA) cycle and the Krebs cycle, marks the “hub” of the
metabolic system: It accounts for the major portion of car-
bohydrate, fatty acid, and amino acid oxidation and gener-
ates numerous biosynthetic precursors. The citric acid cycle
is therefore amphibolic, that is, it operates both cataboli-
cally and anabolically.
We begin our study of the citric acid cycle with an
overview of its component reactions and a historical syn-
opsis of its elucidation. Next, we explore the origin of the
cycle’s starting compound, acetyl-coenzyme A (acetyl-
CoA), the common intermediate formed by the break-
down of most metabolic fuels. Then, after discussing the
reaction mechanisms of the enzymes that catalyze the cycle,
we consider the various means by which it is regulated.
Finally, we deal with the citric acid cycle’s amphibolic
nature by examining its interrelationships with other meta-
bolic pathways.
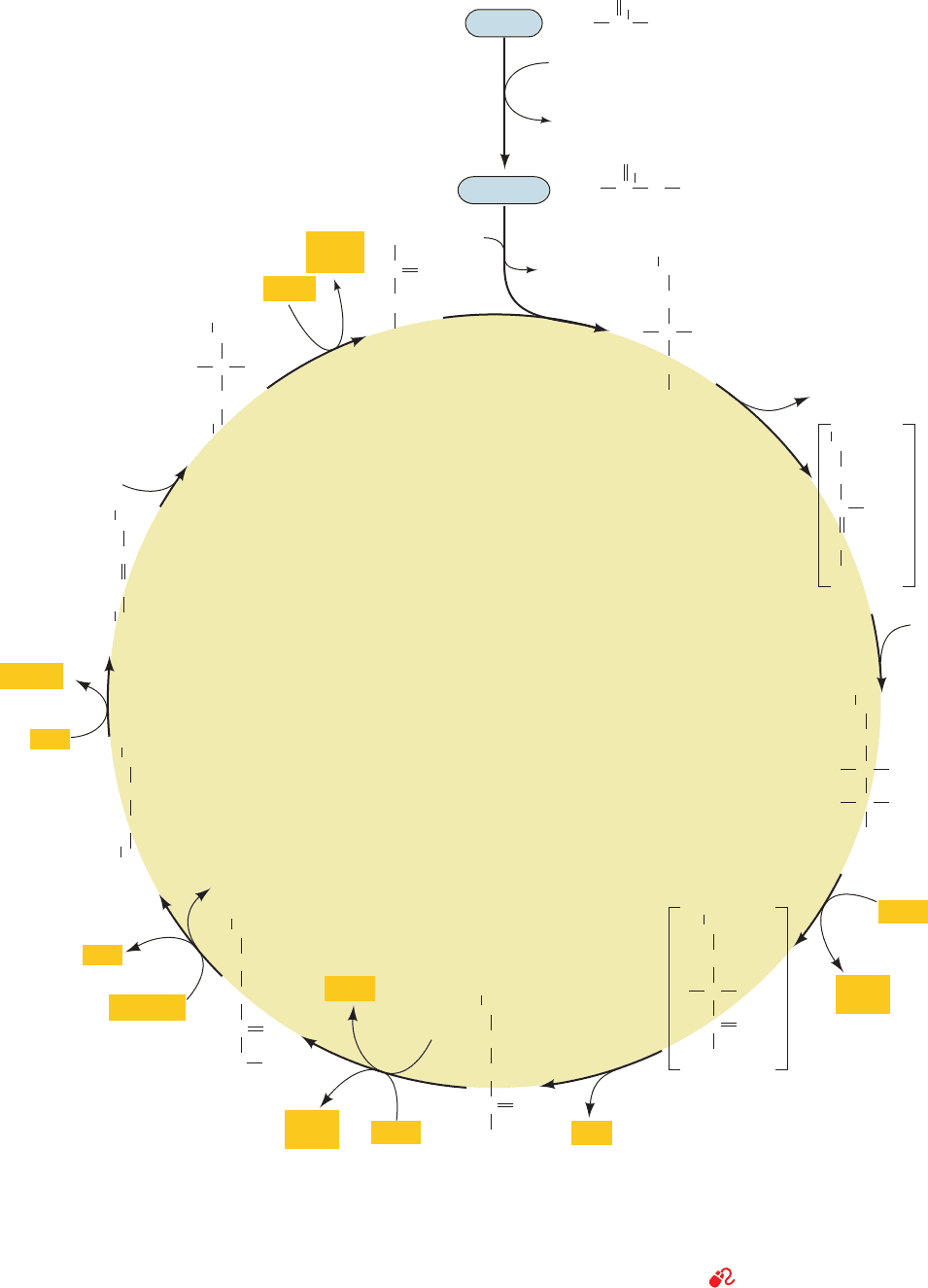
1 CYCLE OVERVIEW
See Guided Exploration 18: Citric acid cycle overview The citric
acid cycle (Fig. 21-1) is an ingenious series of reactions that
oxidizes the acetyl group of acetyl-CoA to two molecules of
CO
2
in a manner that conserves the liberated free energy for
utilization in ATP generation. Before we study these reac-
tions in detail, let us consider the cycle’s chemical strategy
by “walking” through the cycle and noting the fate of the
acetyl group at each step. Following this preview, we shall
consider some of the major discoveries that led to our pres-
ent understanding of the citric acid cycle.
A. Reactions of the Cycle
The eight enzymes of the citric acid cycle (Fig. 21-1) catalyze
a series of well-known organic reactions that cumulatively ox-
idize an acetyl group to two CO
2
molecules with the concomi-
tant generation of three NADHs, one FADH
2
, and one GTP:
1. Citrate synthase catalyzes the condensation of
acetyl-CoA and oxaloacetate to yield citrate, giving the
cycle its name.
2. The strategy of the cycle’s next two steps is to re-
arrange citrate to a more easily oxidized isomer and then
oxidize it. Aconitase isomerizes citrate, a not readily oxi-
dized tertiary alcohol, to the easily oxidized secondary al-
cohol isocitrate. The reaction sequence involves a dehydra-
tion, producing enzyme-bound cis-aconitate, followed by a
hydration, so that citrate’s hydroxyl group is, in effect,
transferred to an adjacent carbon atom.
3. Isocitrate dehydrogenase oxidizes isocitrate to the
-keto acid intermediate oxalosuccinate with the coupled
reduction of NAD
⫹
to NADH; oxalosuccinate is then de-
carboxylated, yielding ␣-ketoglutarate. This is the first step
in which oxidation is coupled to NADH production and
also the first CO
2
-generating step.
4. The multienzyme complex ␣-ketoglutarate dehy-
drogenase oxidatively decarboxylates ␣-ketoglutarate to
succinyl-coenzyme A. The reaction involves the reduction
of a second NAD
⫹
to NADH and the generation of a sec-
ond molecule of CO
2
. At this point in the cycle, two mole-
cules of CO
2
have been produced, so that the net oxidation
of the acetyl group is complete. Note,however, that it is not
the carbon atoms of the entering acetyl-CoA that have
been oxidized.
JWCL281_c21_789-822.qxd 6/5/10 9:53 AM Page 789
790 Chapter 21. Citric Acid Cycle
citrate
synthase
1.
2.
isocitrate
dehydrogenase
3.
α-ketoglutarate
dehydrogenase
4.
succinyl-CoA
synthetase
5.
succinate
dehydrogenase
6.
malate
dehydrogenase
8.
Oxaloacetate
Citrate
Isocitrate
α
-Ketoglutarate
Citric acid
cycle
CoASH
CoASH
Acetyl-CoA
CoASH + NAD
+
CO
2
+ NADH
pyruvate dehydrogenase
H
2
O
H
2
O
H
2
O
H
2
O
aconitase
COO
–
CH
2
FADH
2
FAD
GTP
GDP + P
i
NAD
+
NADH
+ H
+
CO
*COO
–
CCH
3
COO
–
O
CCH
3
S
O
CoA
COO
–
CH
2
CHO COO
–
CH
2
*COO
–
COO
–
CH
2
CH COO
–
*COO
–
CHO H
=
COO
–
CHO H
CH
2
COO
–
=
1
/
2
1
/
2
1
/
2
1
/
2
=
COO
–
CH
HC
COO
–
=
=
COO
–
CH
2
COO
–
=
=
CH
2
COO
–
CH
2
=
CH
2
CO
S CoA
COO
–
CH
2
=
CH
2
CO
*COO
–
COO
–
CH
2
=
C
CH
*COO
–
COO
–
cis-Aconitate
COO
–
CH
2
=
C
CO
*COO
–
H COO
–
OxalosuccinateSuccinyl-CoA
Succinate CoASH
Fumarate
L-Malate
NAD
+
NADH
+ H
+
fumarase7.
CO
2
NAD
+
NADH
+ H
+
isocitrate
dehydrogenase
3.
2. aconitase
=
Pyruvate
=
=
*
CO
2
1
/
2
1
/
2
Figure 21-1 Reactions of the citric acid cycle. The reactants
and products of this catalytic cycle are boxed.The pyruvate S
acetyl-CoA reaction (top) supplies the cycle’s substrate via
carbohydrate metabolism but is not considered to be part of the
cycle.The bracketed compounds are enzyme-bound
intermediates.An isotopic label at C4 of oxaloacetate (*)
becomes C1 of ␣-ketoglutarate and is released as CO
2
in
Reaction 4.An isotopic label at C1 of acetyl-CoA (‡) becomes
C5 of ␣-ketoglutarate and is scrambled in Reaction 5 between
C1 and C4 of succinate (1/2‡).
See the Animated Figures
JWCL281_c21_789-822.qxd 3/17/10 11:33 AM Page 790
