Voet D., Voet Ju.G. Biochemistry
Подождите немного. Документ загружается.

small C-terminal helix. No channel leading from either side
of the membrane to this binding cavity is apparent.
f. Cardiac Glycosides Specifically Inhibit the
(Na
⫹
–K
⫹
)–ATPase
Study of the (Na
⫹
–K
⫹
)–ATPase has been greatly facili-
tated by the use of cardiac glycosides (also called car-
diotonic steroids), natural products that increase the inten-
sity of heart muscle contraction. Indeed, digitalis, an extract
of purple foxglove leaves (Fig. 20-21a), which contains a
mixture of cardiac glycosides including digitalin (Fig. 20-
21b), has been used to treat congestive heart failure for cen-
turies. The cardiac glycoside ouabain (pronounced wabane;
Fig. 20-21b), a product of the East African ouabio tree, has
been long used as an arrow poison. These two steroids,
which are still among the most commonly prescribed car-
diac drugs, inhibit the (Na
⫹
–K
⫹
)–ATPase by binding
strongly to an externally exposed portion of the enzyme
(the drugs are ineffective when injected inside cells) so as to
block Step 5 in Fig. 20-19.The resultant increase in intracel-
lular [Na
⫹
] stimulates the cardiac (Na
⫹
–Ca
2⫹
) antiport sys-
tem, which pumps Na
⫹
out of and Ca
2⫹
into the cell (Sec-
tion 22-1Bb). The increased cytosolic [Ca
2⫹
] boosts the
[Ca
2⫹
] in other cellular organelles, principally the sarcoplas-
mic reticulum (SR). Thus, the release of Ca
2⫹
to trigger
Section 20-3. ATP-Driven Active Transport 761
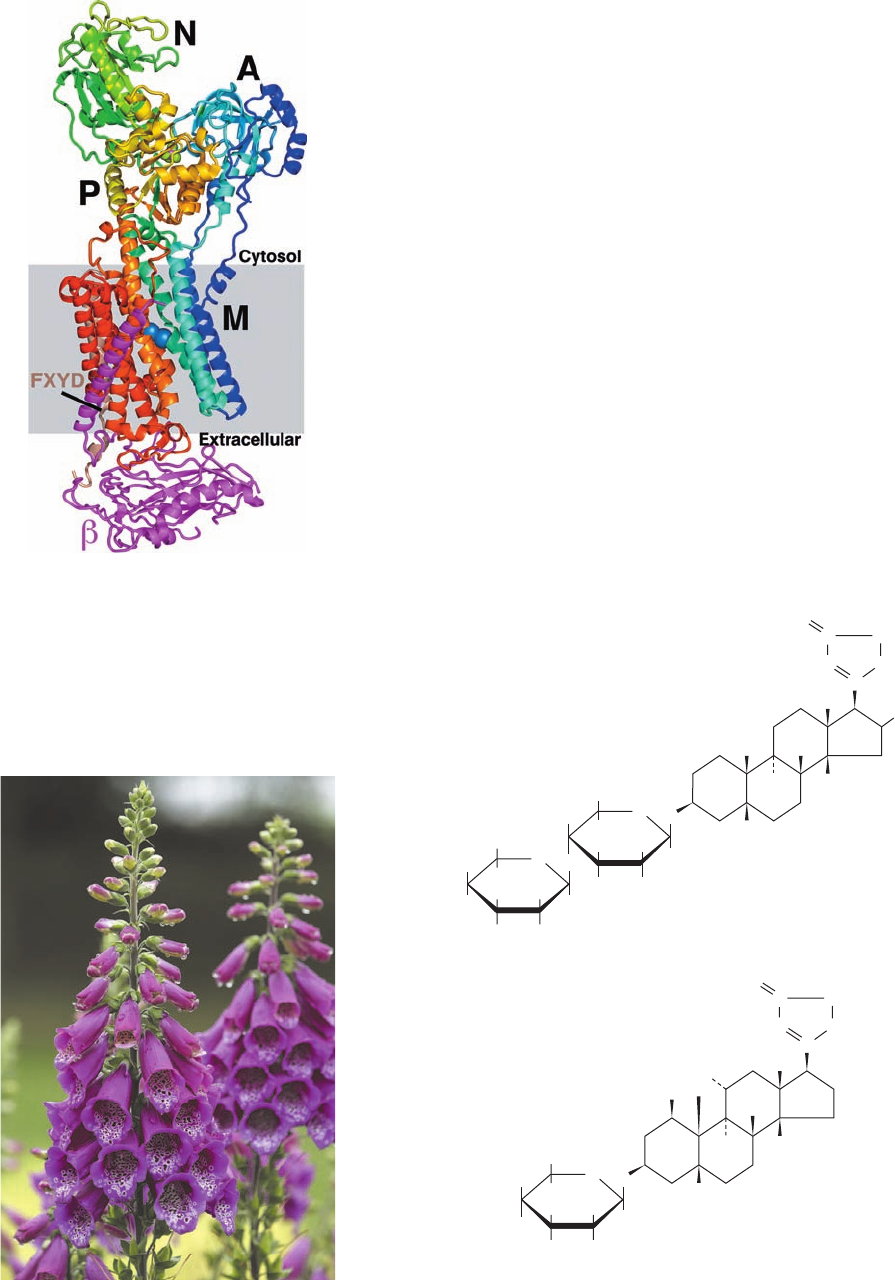
Figure 20-20 X-ray structure of shark (Na
⫹
–K
⫹
)–ATPase in
complex with K
⫹
and MgF
4
2⫺
ions. The protein is drawn in ribbon
form viewed parallel to the plane of the membrane (gray box) with
the cytosol above.The ␣ subunit is colored in rainbow order from
its N-terminus (blue) to its C-terminus (red), the  subunit is
magenta, and the FXYD subunit is brown.The ␣ subunit’s bound
K
⫹
and MgF
4
2⫺
ions are drawn in space-filling form with K light
blue, Mg pink, and F light green. [Based on an X-ray structure by
Chikashi Toyoshima, University of Tokyo, Japan. PDBid 2ZXE.]
Ouabain
CH
3
HOH
2
C
CH
2
O
O
H
OH
OH
HO
HO
HO
HC
C
CO
O
H
H
H
H
Digitalin
H
OCH
3
CH
3
H
3
C
CH
2
O
O
H
OH
H
OH
H
H
OH
H
H
HC
C
CO
O
O
H
H
OH
CH
2
OH
O
H
OH
H
H
HO
H
H
H
H
3
C
CH
3
OH
OH
(b)
Figure 20-21 Cardiac glycosides. (a) The leaves of the purple
foxglove plant are the source of the heart muscle stimulant
digitalis. [iStockphoto.] (b) Digitalin, a major component of
digitalis, and ouabain, a cardiac glycoside isolated from the East
African ouabio tree, are among the most commonly prescribed
cardiac drugs.
JWCL281_c20_744-788.qxd 6/12/10 11:07 AM Page 761
muscle contraction (Section 35-3Cb) produces a larger than
normal increase in cytosolic [Ca
2⫹
], thereby intensifying the
force of cardiac muscle contraction. Ouabain, which was
once thought to be produced only by plants, is now known
to also be an animal hormone that is secreted by the adre-
nal cortex and functions to regulate cell [Na
⫹
] and overall
body salt and water balance.
762 Chapter 20. Transport Through Membranes
B. Ca
2⫹
–ATPase
Calcium ion often acts as a second messenger in a manner
similar to cAMP (Section 19-4). Transient increases in cy-
tosolic [Ca
2⫹
] trigger numerous cellular responses, including
muscle contraction (Section 35-3Ca), release of neurotrans-
mitters (Section 20-5Cb), and, as we have seen, glycogen
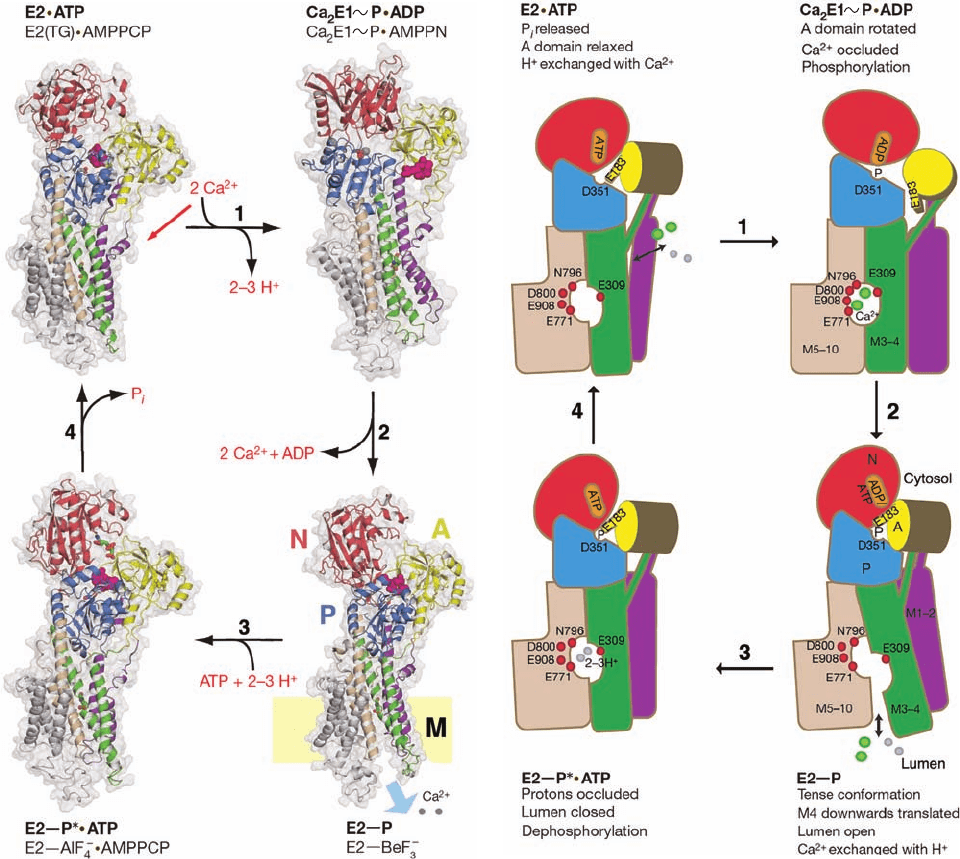
Figure 20-22 Mechanism of SERCA based on four X-ray
structures. (a) The X-ray structures of the SERCA complexes
indicated in lightface are models for the conformational states
indicated in boldface. These states roughly correspond to those in
the corners of Fig. 20-19. The structures are represented by their
ribbon diagrams embedded in their transparent surfaces (gray)
as viewed along the plane of the membrane with the cytosol
above. The A, N, and P domains are colored yellow, red, and blue,
the transmembrane helices M1–2, M3–4, M5–6, and M7–10 are
purple, green, tan, and gray, and bound Ca
2⫹
ions are represented
by gray spheres.ATP and its mimics are drawn in stick form
with C green, N blue, O red, and P orange. A conserved TGES
(a)
(b)
sequence is drawn in space-filling form in magenta. In the lower
right drawing, the position of the lipid bilayer is indicated by the
yellow rectangle. (b) Schematic diagram of the conformational
changes made by SERCA during its catalytic cycle.The protein
components are colored as in Part a except that helices M5–10
are all tan and Ca
2⫹
ions are represented by green spheres. In
addition, protons are represented by gray spheres, and the
residues that ligand Ca
2⫹
ions in the binding cavity are indicated
by red spheres. [Modified from drawings by Poul Nissen,
University of Aarhus, Denmark. PDBids 2C88, 3BA6, 3B9B, and
3B9R.]
JWCL281_c20_744-788.qxd 6/12/10 11:07 AM Page 762
phosphorylated [via a “high energy” bond, which is repre-
sented by a squiggle (
⬃
)] and in complex with the ADP
analog adenosine-5ⴕ-(-amino)diphosphate (AMPPN;
Fig. 22-23c).
2. The A domain rotates toward the phosphorylation
site, thereby contacting the P and N domains. This move-
ment pushes down on the M3–4 segment and pulls up on
the M1–2 segment, thereby separating the lower portions
of these segments from that of the M5–6 segment. This ex-
poses the Ca
2⫹
-binding cavity to the lumen of the SR and
forces apart the residues that had bound the Ca
2⫹
ions in
the cavity, resulting in the release of these ions into the lu-
men.A model for this E2¬P state is provided by the X-ray
structure of SERCA in its E2 state in which BeF
3
⫺
is cova-
lently linked to Asp 351 to form a mimic of a phosphate
group.
3. Two or three protons bind in the lumenally exposed
binding cavity. This, together with the exchange of the ADP
bound to the N domain for ATP results in movements of
transmembrane helices that again occludes the cavity from
the lumen. A model for the resulting E2¬P* ⴢ ATP state
[where the star (*) is indicative of a transition state] is pro-
vided by the X-ray structure of SERCA in its E2 state in
breakdown (Section 18-3Ce). Moreover, Ca
2⫹
is an impor-
tant activator of oxidative metabolism (Section 22-4).
The use of phosphate as a basic energy currency requires
cells to maintain a low internal [Ca
2⫹
] because, for example,
Ca
3
(PO
4
)
2
has a maximum aqueous solubility of 65 M.
Thus, the [Ca
2⫹
] in the cytosol (⬃0.1 M) is four orders
of magnitude less than it is in the extracellular spaces
(⬃1500 M). This large concentration gradient is maintained
by the active transport of Ca
2⫹
across the plasma mem-
brane, the endoplasmic reticulum (ER; SR in muscle), and
the mitochondrial inner membrane. We discuss the mito-
chondrial system in Section 22-1Bb.The plasma membrane
and sarco(endo)plasmic reticulum each contain a P-type
Ca
2⫹
–ATPase (Ca
2⫹
pump) that actively pumps Ca
2⫹
out
of the cytosol at the expense of ATP hydrolysis. Their ki-
netic mechanisms match that of the (Na
⫹
–K
⫹
)–ATPase
(Fig. 20-19) except that two Ca
2⫹
ions replace the three Na
⫹
ions, two to three H
⫹
ions replace the three K
⫹
ions, and
(out) refers to the outside of the cell for plasma membrane
Ca
2⫹
–ATPase or to the lumen of the sarco(endo)plasmic
reticulum for the Ca
2⫹
–ATPase of that membrane.
a. X-Ray Structures of the Ca
2
⫹
–ATPase
Suggest Its Mechanism
The X-ray structures of the 994-residue Ca
2⫹
–ATPase
from rabbit muscle sarcoplasmic reticulum [also known as
SERCA for sarco(endo)plasmic reticulum Ca
2⫹
-ATPase]
in its complexes with a variety of analogs of ATP and its
components have been determined,the first of which were re-
ported by Toyoshima. SERCA, which constitutes 90% of SR
membrane protein, is a 140-Å-long monomeric protein that
closely resembles the ␣ subunit of the (Na
⫹
–K
⫹
)–ATPase
(Fig. 20-20). It contains a 10-helix transmembrane domain
(M), an actuator domain (A), a nucleotide-binding do-
main (N) to which the ATP binds, and a phosphorylation
domain (P) that contains the phosphorylatable Asp 351.
The two Ca
2⫹
ions are bound in a cavity, similar to that in
the (Na
⫹
–K
⫹
)–ATPase, which is formed, in large part, by
the disruption of helices M4 and M6 in this region.
Four of these structures, all determined by Poul Nissen,
collectively suggest a mechanism of action of P-type ATPases
resulting from a vectorial series of conformational changes
driven by ATP binding, hydrolysis, and release (Figs. 20-22):
1. Let us start with the X-ray structure of SERCA in
its E2 conformation in complex with the inhibitor thapsi-
gargin (TG; Fig. 22-23a), which stabilizes SERCA’s E2
state, and the nonhydrolyzable ATP analog adenosine-5ⴕ-
(,␥-methylene)triphosphate (AMPPCP; Fig. 22-23b).
This structure provides a model for the E2 ⴢ ATP state
(Fig. 20-22, upper left). The exchange of 2 to 3 protons for
2 cytosolic Ca
2⫹
ions causes a rotation of the A domain
that occludes the binding cavity.This activates the ATPase
catalytic site to phosphorylate Asp 351 yielding ADP,
which releases the N domain from the P domain due
to the cleavage of the ATP’s ,␥-phosphodiester bond
that had previously linked these two domains. A model
for the resulting Ca
2
E1⬃P ⴢ ADP state is provided by the
X-ray structure of SERCA in its E1 state with Asp 351
Section 20-3. ATP-Driven Active Transport 763
Figure 20-23 Molecular formulas of several SERCA
inhibitors. (a) Thapsigargin (TG), a product of the plant Thapsia
garganica, (b) AMPPCP, and (c) AMPPN.
Thapsigargin (TG)
(a)
OH
OH
H
O
O
O
O
O
O
O
O
O
O
P
O
O
A
CH
2
CH
2
H
H
H
H
OH OH
O
P
O
–
–
O
Adenosine-5-(β,γ-methylene)triphosphate
(AMPPCP)
O
O
O
–
P
O
O
–
(b)
Adenosine-5-(β-amino)diphosphate
(AMPPN)
PO
A
CH
2
H
H
H
H
OH OH
O
NH
2
O
O
O
–
P
O
O
–
(c)
JWCL281_c20_744-788.qxd 7/1/10 7:18 AM Page 763
C. (H
⫹
–K
⫹
)–ATPase of Gastric Mucosa
Parietal cells of the mammalian gastric mucosa secrete HCl
at a concentration of 0.15M (pH 0.8). Since the cytosolic
pH of these cells is 7.4, this represents a pH difference of
6.6 units, the largest known in eukaryotic cells.The secreted
protons are derived from the intracellular hydration of
CO
2
by carbonic anhydrase:
The secretion of H
⫹
involves the participation of an
(H
⫹
–K
⫹
)–ATPase, an electroneutral antiport with struc-
ture and properties similar to that of Ca
2⫹
–ATPase. Like
other P-type ATPases, it is phosphorylated during the
transport process. In this case, however, the K
⫹
, which en-
ters the cell as H
⫹
is pumped out, is subsequently external-
ized by its electroneutral cotransport with Cl
⫺
. HCl is
therefore the overall transported product.
a. Cimetidine and Omeprazole Prevent Gastric
Ulcers and Heartburn
For many years, effective treatment of peptic ulcers,
which was a frequently fatal condition caused by the attack
of stomach acid on the gastric mucosa, often required sur-
gical removal of the affected portions of the stomach or
sometimes the entire stomach. The discovery, by James
Black, of cimetidine,
which inhibits stomach acid secretion, all but eliminated
the need for this dangerous and debilitating surgery. The
(H
⫹
–K
⫹
)–ATPase of the gastric mucosa is activated by his-
tamine stimulation of the H
2
-receptor in a process medi-
ated by cAMP. Cimetidine (trade name Tagamet), which
became available in 1976, was the first of several widely
used drugs that competitively inhibit the binding of hista-
mine to this receptor. These drugs likewise alleviate the
symptoms of gastroesophageal reflux disease (GERD,
commonly known as heartburn), which is caused by the re-
guritation of stomach acid into the esophagus, a widespread
and painful condition that, when chronic, can damage the
esophagus and cause esophageal cancer. Heartburn can
also be relieved by antacids but the effects of cimetidine
and its analogs are longer lasting (6–10 hours vs 1–2 hours
for antacids, although the former require ⬃30 minutes to
take effect) and can be taken before meals to prevent
heartburn from occurring. A drawback of most of these
substances is that they inhibit several cytochrome P450s
and thereby interfere with the metabolism of numerous
widely used drugs (Section 15-4Bc).
CH
2
S
N
CH
2
Cimetidine
CH
2
N
NH
NH
C
CH
3
C
N
HN
H
3
C
CH
2
CH
2
NH
3
N
HN
+
Histamine
CO
2
⫹ H
2
O Δ HCO
⫺
3
⫹ H
⫹
764 Chapter 20. Transport Through Membranes
complex with AMPPCP and AlF
4
⫺
, which is covalently
linked to Asp 351 to form a trigonal bipyramidal mimic of
the hydrolytic transition state (Fig. 16-6b).
4. The dephosphorylation of Asp 351, as stimulated by
the previous binding of ATP, motivates conformational
changes that opens a channel to the cytosol that permits
the exchange of protons with Ca
2⫹
ions, thereby complet-
ing the catalytic cycle.
This mechanism explains how P-type ATPases transport
cations against their steep electrochemical gradients.
b. Calmodulin Regulates the Plasma
Membrane Ca
2⫹
Pump
For a cell to maintain its proper physiological state, it
must regulate the activities of its ion pumps precisely. The
regulation of the Ca
2⫹
pump in the plasma membrane is
controlled by the level of Ca
2⫹
through the mediation of
calmodulin (CaM).This ubiquitous eukaryotic Ca
2⫹
-binding
protein participates in numerous cellular regulatory
processes including, as we have seen, the control of glyco-
gen metabolism (Section 18-3Ce).
Ca
2⫹
–Calmodulin activates the Ca
2⫹
–ATPase of plasma
membranes. The activation, as deduced from the study of the
isolated ATPase, results in a decrease in its K
M
for Ca
2⫹
from
20 to 0.5 M.Ca
2⫹
–CaM activates the Ca
2⫹
pump by binding
to an inhibitory polypeptide segment of the pump in a man-
ner similar to the way in which Ca
2⫹
–CaM activates its tar-
get protein kinases (Section 18-3Cf). Evidence supporting
this mechanism comes from proteolytically excising the
Ca
2⫹
pump’s CaM-binding polypeptide, yielding a truncated
pump that is active even in the absence of CaM. Synthetic
peptides corresponding to this CaM-binding segment not
only bind Ca
2⫹
–CaM but inhibit the truncated pump by in-
creasing its K
M
for Ca
2⫹
and decreasing its V
max
. This sug-
gests that, in the absence of Ca
2⫹
–CaM, the CaM-binding
segment of the pump interacts with the rest of the protein so
as to inhibit its activity. When the Ca
2⫹
concentration in-
creases, Ca
2⫹
–CaM forms and binds to the CaM-binding
segment of the pump in a way that causes it to dissociate
from the rest of the pump, thereby relieving the inhibition.
Now we can see how Ca
2⫹
regulates its own cytoplasmic
concentration: At Ca
2⫹
levels below calmodulin’s ⬃1 M
dissociation constant for Ca
2⫹
, the Ca
2⫹
–ATPase is relatively
inactive due to autoinhibition by its CaM-binding segment. If,
however, the [Ca
2⫹
] rises to this level, Ca
2⫹
binds to calmod-
ulin, which, in turn, binds to the CaM-binding segment so as
to relieve the inhibition, thereby activating the Ca
2⫹
pump:
(CaM* indicates activated calmodulin). This interaction de-
creases the pump’s K
M
for Ca
2⫹
to below the ambient [Ca
2⫹
],
thereby causing Ca
2⫹
to be pumped out of the cytosol.When
the [Ca
2⫹
] decreases sufficiently, Ca
2⫹
dissociates from
calmodulin and this series of events reverses itself,thereby in-
activating the pump.The entire system is therefore analogous
to a basement sump pump that is automatically activated by
a float when the water reaches a preset level.
Ca
2⫹
–CaM* ⴢ pump(active)
Ca
2⫹
⫹ CaM Δ Ca
2⫹
–CaM* ⫹ pump(inactive) Δ
JWCL281_c20_744-788.qxd 7/1/10 7:19 AM Page 764
energy” phosphoryl donor for ATP synthesis in the pyruvate
kinase reaction of glycolysis; Section 17-2J). The PTS simul-
taneously transports and phosphorylates sugars. Since the cell
membrane is impermeable to sugar phosphates, once they
enter the cell, they remain there. Some of the PTS-transported
sugars are listed in Table 20-2.
The PTS system involves two soluble cytoplasmic pro-
teins, Enzyme I (EI) and HPr (for histidine-containing
phosphocarrier protein), which participate in the transport
of all sugars (Fig. 20-24). In addition, for each sugar the sys-
tem transports, there is a specific transmembrane transport
protein EII, which consists of at least three functional com-
ponents: two that are cytoplasmic, EIIA and EIIB, and a
transmembrane channel, EIIC. These three components as-
sociate differently in different EII’s. In E. coli, for example,
EIIA, EIIB, and EIIC are separate subunits in cellobiose-
specific EII; EIIB and EIIC are covalently linked and EIIA
is separate in glucose-specific EII; and all three components
are present on a single peptide in mannitol-specific EII.
Glucose transport, which resembles that of other sugars,
involves the transfer of a phosphoryl group from PEP to
glucose with net inversion of configuration about the phos-
phorus atom.Since each phosphoryl transfer involves inver-
sion (Section 16-2A), an odd number of transfers must be
Omeprazole (trade names Prilosec and Nexium),
directly inhibits the (H
⫹
–K
⫹
)–ATPase by forming an
adduct with the side chain of its Cys 831. Since its introduc-
tion in 1989, omeprazole has greatly supplanted the use of
cimetidine and its analogs due to the irreversible nature of
its inhibition (which reduces acid secretion by up to 99%)
and its lack of drug–drug interactions. Hence,omeprazole is
presently one of the most frequently used drugs worldwide.
D. Group Translocation
Group translocation is a variation of ATP-driven active
transport that most bacteria use to import certain sugars. It
is required for many bacterial processes, both useful and
harmful (to humans), such as those that produce cheese,soy
sauce, and dental cavities. It differs from active transport in
that the molecules transported are simultaneously modified
chemically. The most extensively studied example of group
translocation is the phosphoenolpyruvate-dependent phos-
photransferase system (PTS) of E. coli discovered by Saul
Roseman in 1964. Phosphoenolpyruvate (PEP) is the phos-
phoryl donor for this system (recall that PEP is the “high-
CH
3
CH
3
OCH
3
CH
2
N
H
3
CO
S
..
N
H
N
Omeprazole
O
Section 20-3. ATP-Driven Active Transport 765
Table 20-2 Some of the Sugars Transported by the E.coli
PEP-Dependent Phosphotransferase
System (PTS)
Glucose Galactitol
Fructose Mannitol
Mannose Sorbitol
N-Acetylglucosamine Xylitol
PEP
EI phos-
phorylation
by PEP
1. Phosphoryl
transfer
to HPr
2. Phosphoryl
transfer
to EIIA
3.
EI
HPr
HPr
~ P
Glucose-6-P
ATP
cAMP + PP
i
EIIBC
glc
~ P
EIIBC
glc
Glucose
Glycerol + ATP
Glycerol-3-phosphate
+ ADP
Inside Outside
Phosphoryl
Phosphoryl
transfer
transfer
to EIIBC
to EIIBC
4.
4.
Phosphoryl
transfer
to EIIBC
4.
Phosphoryl transfer
to glucose
5.
EI ~ PPyruvate
E
IIA
glc
E
IIA
glc
~ P
glycerol
kinase
adenylate
cyclase
I
n
h
i
b
i
t
i
o
n
A
c
t
i
v
a
t
i
o
n
lactose
permease
Lactose + H
+
Lactose + H
+
Figure 20-24 Transport of glucose by the PEP-dependent
phosphotransferase system (PTS). HPr and EI are cytoplasmic proteins
common to all sugars transported. EIIA
glc
and EIIBC
glc
are proteins
specific for glucose. EIIA
glc
inhibits non-PTS transport proteins such as
the lactose permease (Section 20-4B) and enzymes such as glycerol
kinase. Adenylate cyclase is activated by the presence of EIIA
glc
⬃P
(or possibly inhibited by the presence of EIIA
glc
).
JWCL281_c20_744-788.qxd 6/4/10 12:16 PM Page 765
involved. Four phosphorylated protein intermediates have
been identified, indicative of five phosphoryl transfers:
The transport process occurs as follows (Fig. 20-24):
1. PEP phosphorylates EI at N3 (N
ε
) of His 189 to form
a reactive phosphohistidine adduct.
2. The phosphoryl group is transferred to N1 (N
␦
) of
His 15 on HPr. His is apparently a favored phosphoryl
group acceptor in phosphoryl-transfer reactions. It also
participates in the phosphoglycerate mutase reaction of
glycolysis (Section 17-2Ha).
3. HPr
⬃
P continues the phosphoryl-transfer chain by
phosphorylating EIIA
glc
at N3 of His 90.
4. The fourth phosphoryl transfer is to Cys 421 of EIIB
glc
.
5. The phosphoryl group is finally transferred from
EIIB
glc
to glucose, which, in the process, is transported
across the membrane by EIIC
glc
. Glucose is released into
the cytoplasm only after it has been phosphorylated to
glucose-6-phosphate (G6P).
Thus the transport of glucose is driven by its indirect, exergonic
phosphorylation by PEP. The PTS is an energy-efficient sys-
tem since only one ATP equivalent is required to both trans-
port and phosphorylate glucose. When the active transport
and phosphorylation steps occur separately, as they do in
many cells, two ATPs are hydrolyzed per glucose processed.
a. Bacterial Sugar Transport Is
Genetically Regulated
The PTS is more complex than the other transport sys-
tems we have encountered, probably because it is part of a
complicated regulatory system governing sugar transport.
When any of the sugars transported by the PTS is abun-
dant, the active transport of sugars, which enter the cell via
other transport systems, is inhibited.This inhibition, called
catabolite repression, is mediated through the cAMP con-
centration (Section 31-3C). cAMP activates the transcrip-
tion of genes that encode various sugar transport proteins,
including lactose permease (Section 20-4B). The presence
of glucose results in a decrease in [cAMP], which, in turn,
represses the synthesis of these other sugar transport pro-
teins. Direct inhibition of the sugar transport proteins
themselves, as well as of certain enzymes, also occurs.
The mechanism for control of [cAMP] is thought to reside
in EIIA
glc
,which is transiently phosphorylated in Step 3 of the
PTS transport process (Fig.20-24).When glucose is plentiful,
this enzyme is present mostly in its dephospho form since
CH
2
NH
Phosphohistidine residue
PO
O
–
O
–
C
C
H
3
5
4
O
N
N
CH
2
1
PEP S EI S HPr S EIIA
glc
S EIIBC
glc
S glucose
EIIA
glc
⬃
P rapidly transfers its phosphoryl group through
EIIBC
glc
to glucose. Under these conditions, adenylate cy-
clase is inactive, although whether dephospho EIIA
glc
in-
hibits this enzyme or EIIA
glc
⬃
P activates it is unclear.
However, dephospho EIIA
glc
binds to and inhibits many
non-PTS transporters and enzymes that participate in the
metabolism of sugars other than glucose (the metabolite of
choice for many bacteria), including lactose permease and
glycerol kinase (Section 17-5A). In the absence of glucose,
EIIA
glc
is converted to EIIA
glc
⬃
P, thereby relieving the inhi-
bition of non-PTS transporters. In addition, adenylate cy-
clase is activated to produce cAMP, which, in turn, induces
the increased production of some of the non-PTS trans-
porters and enzymes that EIIA
glc
inhibits. This is a form of
energy conservation for the cell.Why synthesize the proteins
required for the transport and metabolism of all sugars
when the metabolism of only one sugar at a time will do?
b. The X-Ray Structure of EIIA
glc
in
Complex with Glycerol Kinase
The X-ray structures of EIIA
glc
, both alone and in com-
plex with one of its regulatory targets, glycerol kinase,
which were determined by James Remington and Rose-
man, have revealed how EIIA
glc
inhibits at least some of its
targets and why EIIA
glc
⬃
P does not do so. EIIA
glc
con-
tains two His residues, His 75 and His 90, that are required
for phosphoryl transfer, although only His 90 is necessary
for EIIA
glc
to accept a phosphate from HPr. The X-ray
structure of E. coli EIIA
glc
alone reveals that these two His
residues lie in close proximity (their N3 atoms are 3.3 Å
apart) in a depression on the surface of the protein that is
surrounded by a remarkable ⬃18-Å-diameter hydropho-
bic ring consisting of 11 Phe, Val, and Ile side chains.
The X-ray structure of EIIA
glc
in complex with glycerol
kinase (Fig. 20-25) confirms that this hydrophobic gasket is
indeed the site of interaction between the two proteins and
reveals how the phosphorylation of His 90 disrupts this inter-
action.The two active site His residues, which are completely
buried within the hydrophobic interaction surface, coordi-
nate a previously unanticipated Zn
2⫹
ion, which is addition-
ally coordinated to Glu 478 of glycerol kinase and a water
molecule. The phosphorylation of EIIA
glc
His 90 to yield EI-
IA
glc
⬃
P no doubt disrupts this intermolecular interaction,
thereby releasing glycerol kinase and reversing its inhibition.
E. ABC Transporters
The ABC transporters, which widely occur in all kingdoms of
life, are named for their highly conserved, ⬃100-residue ATP-
binding cassette. They form a large superfamily of transmem-
brane proteins that collectively transport a wide variety of sub-
stances across membranes, including ions, sugars, peptides,
lipids, metabolites, and numerous toxins and drugs. For exam-
ple, the acquired resistance of cancer cells to chemotherapeu-
tic agents is often due to the selection of cells that overexpress
the ABC transporter named multidrug resistance (MDR)
transporter (also called P-glycoprotein), which pumps a wide
assortment of amphiphilic substances—including many
drugs—out of the cell. Similar proteins in bacteria are, in
many cases, responsible for their antibiotic resistance. The
766 Chapter 20. Transport Through Membranes
JWCL281_c20_744-788.qxd 6/4/10 12:16 PM Page 766
(b)
(a)
human genome encodes 48 ABC transporters and their de-
fects are responsible for several inherited diseases includ-
ing cystic fibrosis (Section 12-4Bf),Tangier disease (Section
12-5Cd), and adrenoleukodystrophy (Section 25-2Fa).
ABC transporters consist minimally of four modules: two
highly conserved cytosolic nucleotide-binding domains, and
two transmembrane domains that typically contain six trans-
membrane helices each. In bacteria, the four domains are
contained on two or four separate polypeptides. In eukary-
otes, a single polypeptide includes all four domains. Bacterial
ABC transporters mediate the import as well as the export of
a variety of compounds, whereas their eukaryotic counter-
parts function only as exporters that transport molecules out
of the cell or into intracellular compartments such as the ER.
The ABC transporter of gram-negative bacteria named
MsbA, a close homolog of the MDR transporter, functions
to transport lipopolysaccharides (Section 11-3Bc) and their
glycolipid component,lipid A, from the cytoplasmic leaflet of
the plasma membrane to its periplasmic leaflet; that is, MsbA
is a flippase (Section 12-4Aa). X-ray structures of Msb,deter-
mined by Geoffrey Chang, reveal that its two identical
582-residue subunits are extensively intertwined (Fig. 20-26).
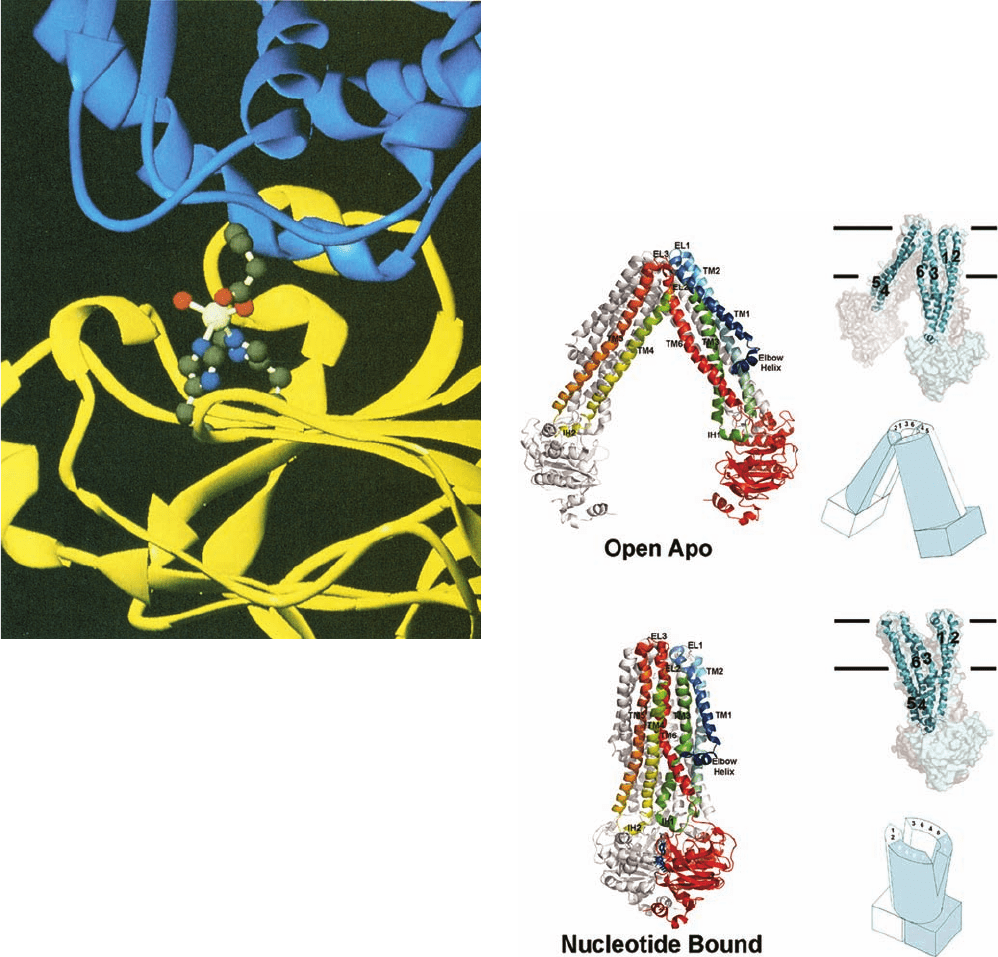
In the nucleotide-free (apo) structure (Fig. 20-26a), each
subunit contributes four of its six transmembrane helices
Section 20-3. ATP-Driven Active Transport 767
Figure 20-25 X-ray structure of E. coli EIIA
glc
(yellow, a
168-residue monomer) in complex with one of its regulatory
targets, glycerol kinase (blue, a tetramer of identical 501-residue
subunits). The two proteins associate, in part, by tetrahedrally
coordinating a Zn
2⫹
ion via the side chains of His 75 and His 90 of
EIIA
glc
, a carboxylate oxygen from Glu 478 of glycerol kinase, and
a water molecule. These groups are shown in ball-and-stick form
with C gray, N blue, O red, and Zn
2⫹
white. The Zn
2⫹
-mediated
interaction between EIIA
glc
and glycerol kinase inactivates
glycerol kinase, presumably through an induced-fit mechanism.
The phosphorylation of EIIA
glc
at His 90 disrupts this interaction,
thereby reversing the inhibition of glycerol kinase. [Courtesy of
James Remington, University of Oregon. PDBid 1GLA.]
Figure 20-26 X-ray structures of the ABC transporter MsbA.
(a) E. coli MsbA in the absence of bound nucleotide (Open
Apo). (b) S. typhimurium MsbA in complex with AMPPNP
(Nucleotide Bound). On the left, the structures are drawn in
ribbon form viewed along the plane of the membrane with the
periplasmic space above. One subunit in each homodimer is
colored in rainbow order from N-terminus (blue) to C-terminus
(red) and the other subunit is gray. The AMPPNP in Part b is
drawn in stick form in blue. Transmembrane helices (TM1–6),
extracellular loops (EL1–3), and intracellular helices (IH1–2) are
labeled. On the upper right, the structures are drawn as
semitransparent surface diagrams with one subunit cyan and the
other gray, and showing the embedded transmembrane helices
(1–6) of the cyan subunit.The horizontal lines delineate the lipid
bilayer. On the lower right, the structures are represented by
schematic diagrams with one subunit cyan and the other white
and with the positions of the transmembrane helices indicated.
[Modified from drawings by Geoffrey Chang,The Scripps
Research Institute, La Jolla, California. PDBids 3B5W and 3B60.]
JWCL281_c20_744-788.qxd 7/1/10 7:19 AM Page 767
(TM1–3 and 6) to one leg of the ⌳-shaped homodimer and
two (TM4–5) to the other leg. The nucleotide-binding do-
mains (NBDs; globular portions at the end of each leg) are
⬃50 Å apart. However, in its complex with AMPPNP (Fig.
20-26b), the two legs of the ⌳ have swung together by
⬃60° to bring the NBDs into contact, with TM3 and 6 hav-
ing swung away from TM1–2 to contact TM4–5.This opens
a space between the subunits on the periplasmic side of
the membrane, which presumably permits a lipopolysac-
charide that had bound to MsbA from the cytosolic leaflet
of the plasma membrane to be released into its periplas-
mic leaflet. The unidirectional transport of substrate may
be facilitated by the different sizes of the openings, and
possibly, by a reduced affinity for substrate by MsbA’s
ATP-bound form.
4 ION GRADIENT–DRIVEN
ACTIVE TRANSPORT
Systems such as the (Na
⫹
–K
⫹
)–ATPase discussed above
utilize the free energy of ATP hydrolysis to generate elec-
trochemical potential gradients across membranes. Con-
versely, the free energy stored in an electrochemical poten-
tial gradient may be harnessed to power various endergonic
physiological processes. Indeed, ATP synthesis by mito-
chondria and chloroplasts is powered by the dissipation of
proton gradients generated through electron transport and
photosynthesis (Sections 22-3C and 24-2D). In this section
we discuss active transport processes that are driven by the
dissipation of ion gradients. We consider three examples:
intestinal uptake of glucose by the Na
⫹
–glucose symport,
uptake of lactose by E. coli lactose permease, and the mito-
chondrial ATP–ADP translocator.
A. Na
⫹
–Glucose Symport
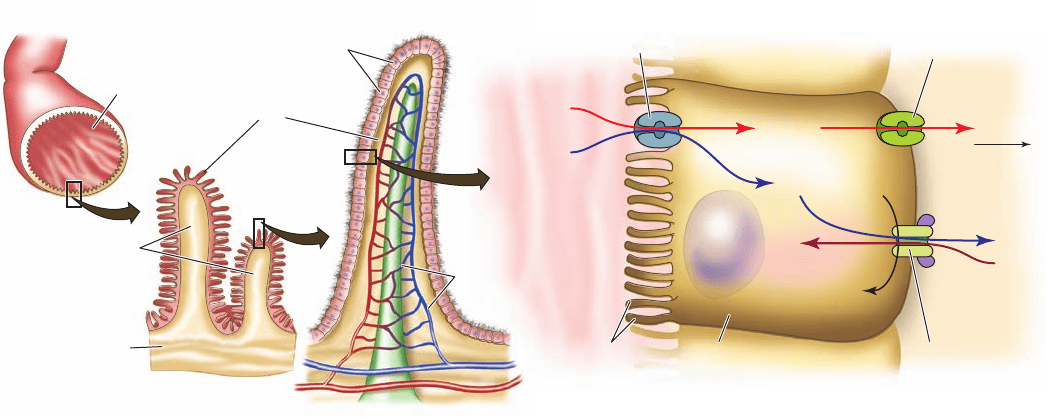
Nutritionally derived glucose is actively concentrated in
brush border cells of the intestinal epithelium by an Na
⫹
-
dependent symport (Fig. 20-27). It is transported from
these cells to the circulatory system via GLUT2 (Section
20-2Eb), a passive-mediated glucose uniport located on the
capillary side of the cell. Note that although the immediate
energy source for glucose transport from the intestine is the
Na
⫹
gradient, it is really the free energy of ATP hydrolysis
that powers this process through the maintenance of the Na
⫹
gradient by the (Na
⫹
–K
⫹
)–ATPase. Nevertheless, since glu-
cose enhances Na
⫹
resorption, which in turn osmotically
enhances water resorption, glucose (possibly as sucrose), in
addition to salt and water, should be fed to individuals suf-
fering from severe salt and water losses resulting from diar-
rhea (drinking only water or a salt solution is ineffective
since they are rapidly excreted from the gastrointestinal
tract). It is estimated that the introduction of this simple
and inexpensive treatment,called oral rehydration therapy,
has decreased the annual number of human deaths from
severe diarrhea, mostly in children in less-developed coun-
tries, from 4.6 to 1.6 million.
a. Active and Passive Glucose Transporters Exhibit
Differential Drug Susceptibilities
The two glucose transport systems are inhibited by dif-
ferent drugs:
1. Phlorizin inhibits Na
⫹
-dependent glucose transport.
768 Chapter 20. Transport Through Membranes
(c) Glucose transport
ATP
Glucose uniport,
GLUT2
Na
+
glucose symport
ADP + P
i
Na
+
Na
+
Na
+
(Na
+
–K
+
)–ATPase
K
+
K
+
Glucose
Glucose
Glucose
Microvilli
Intestinal
lumen
To capillaries
Brush border cell
(a) Small intestine
(b) Villus
Intestinal folds
Intestinal
mucosa
Lumen
Capillaries
Columnar epithelial cells
(brush border)
Villi
Figure 20-27 Glucose transport in the intestinal epithelium.
The brushlike villi lining the small intestine greatly increase its
surface area (a), thereby facilitating the absorption of nutrients.
The brush border cells from which the villi are formed (b)
actively concentrate glucose from the intestinal lumen in
symport with Na
⫹
(c), a process that is driven by the
(Na
⫹
–K
⫹
)–ATPase, which is located on the capillary side of the
cell and functions to maintain a low internal [Na
⫹
].The glucose is
exported to the bloodstream via GLUT2, a separate passive-
mediated uniport system (Section 20-2Eb).
JWCL281_c20_744-788.qxd 7/1/10 7:30 AM Page 768
2. Cytochalasin B inhibits Na
⫹
-independent glucose
transport.
Phlorizin binds only to the external surface of the Na
⫹
-
dependent glucose transporter,whereas cytochalasin B binds
to the cytoplasmic surface of the Na
⫹
-independent glucose
transporter. This further indicates that these proteins are
asymmetrically inserted into membranes. The use of these
inhibitors permits the actions of the two glucose trans-
porters to be studied separately in intact cells.
Kinetic studies indicate that the Na
⫹
–glucose symport
binds its substrates, Na
⫹
and glucose, in random order (Fig.
20-28),although binding of Na
⫹
increases the affinity of the
transporter for glucose to such an extent that the upper
pathway is heavily favored. Only when both substrates are
bound, however, does the protein change its conformation
to expose the binding sites to the inside of the cell.This re-
quirement for concomitant Na
⫹
and glucose transport pre-
vents the wasteful dissipation of the Na
⫹
gradient.
OH
CH
3
HO
OH
O
OH
O
O
OH
O
H
N
O
D-Glucose-β
Phlorizin
Cytochalasin B
CH
2
CH
2
CH
3
B. Lactose Permease
Gram-negative bacteria such as E. coli contain several ac-
tive transport systems for concentrating sugars.We have al-
ready discussed the PTS system.Another extensively stud-
ied system, lactose permease (also known as galactoside
permease), utilizes the proton gradient across the bacterial
cell membrane to cotransport H
⫹
and lactose (Fig. 20-29).
The proton gradient is metabolically generated through
oxidative metabolism in a manner similar to that in mito-
chondria (Section 22-3B). The electrochemical potential
gradient created by both these systems is used mainly to
drive the synthesis of ATP.
Section 20-4. Ion Gradient–Driven Active Transport 769
Figure 20-28 The Na
⫹
–glucose symport system represented
as a Random Bi Bi kinetic mechanism. Binding of Na
⫹
increases
the affinity of the transporter for glucose to such an extent that
the upper pathway is heavily favored.T
o
and T
i
, respectively,
T
o
T
o
T
i
••
Glc
Glc
Glc Glucose
Glc
Na
+
Na
+
Na
+
T
o
•
Na
+
T
i
•
Na
+
Na
+
••
Glc
Glc
Glc
T
i
T
i
•
Glc
T
o
•
Glc
Na
+
Na
+
Recovery
InsideOutside
Transport
Release
H
+
+ Lactose
E-1E-2
Binding E-2
• H
+
Lactose
Oxidative
metabolism
H
+
E-2 • H
+
• Lactose E-1 • H
+
• Lactose
Figure 20-29 Kinetic mechanism of lactose permease in
E. coli. H
⫹
binds first to E-2 outside the cell, followed by lactose.
They are released in random order from E-1 inside the cell. E-2
must bind both lactose and H
⫹
in order to change conformation
to E-1, thereby cotransporting these substances into the cell. E-1
changes conformation to E-2 when neither lactose nor H
⫹
is
bound, thus completing the transport cycle.
represent the transport protein with its binding sites exposed to
the outer and inner surfaces of the membrane. [After Crane,
R.K. and Dorando, F.C., in Martonosi, A.N. (Ed.), Membranes
and Transport, Vol. 2, p. 154, Plenum Press (1982).]
JWCL281_c20_744-788.qxd 6/4/10 2:09 PM Page 769
How do we know that lactose transport requires the
presence of a proton gradient? Ronald Kaback has estab-
lished the requirement for this gradient through the fol-
lowing observations:
1. The rate of lactose transport into bacteria is in-
creased enormously by the addition of
D-lactate, an energy
source for transmembrane proton gradient generation.
Conversely, inhibitors of oxidative metabolism, such as
cyanide, block both the formation of the proton gradient
and lactose transport.
2. 2,4-Dinitrophenol, a proton ionophore that dissi-
pates transmembrane proton gradients (Section 22-3D),
inhibits lactose transport into both intact bacteria and
membrane vesicles.
3. The fluorescence of dansylaminoethylthiogalactoside,
a competitive inhibitor of lactose transport, is sensitive to
the polarity of its environment and thus changes when it
binds to lactose permease. Fluorescence measurements in-
dicate that it does not bind to membrane vesicles that con-
tain lactose permease in the absence of a transmembrane
proton gradient.
a. Lactose Permease Has Two Major
Conformational States
Lactose permease is a 417-residue monomer that, like
the mammalian glucose transporters (Section 20-2Eb), to
which it is distantly related, consists mainly of 12 trans-
membrane helices with its N- and C-termini in the cyto-
plasm. Like the (Na
⫹
–K
⫹
)–ATPase, it has two major con-
formational states (Fig. 20-29):
1. E-1, which has a low-affinity lactose binding site fac-
ing the interior of the cell.
2. E-2, which has a high-affinity lactose binding site fac-
ing the exterior of the cell.
E-1 and E-2 can interconvert only when their H
⫹
and lac-
tose binding sites are either both filled or both empty. This
prevents not only dissipation of the H
⫹
gradient without
cotransport of lactose into the cell, but also transport of
CH
2
OH
H
OH H
HOH
HH
O
O
HO
CH
2
OH
H
OH H
HOH
H
O
HOH
CH
2
OH
H
OH H
HOH
HH
O
S
HO
S
O
O
C
H
H
NH
H
H
C
Lactose
Dansylaminoethylthiogalactoside
CH
3
CH
3
N
lactose out of the cell without cotransport of H
⫹
against its
concentration gradient.
The X-ray structure of lactose permease in complex
with a tight-binding lactose analog, determined by Kaback
and So Iwata, reveals that this protein consists of two
structurally similar and 2-fold pseudosymmetrically re-
lated domains containing six transmembrane helices each
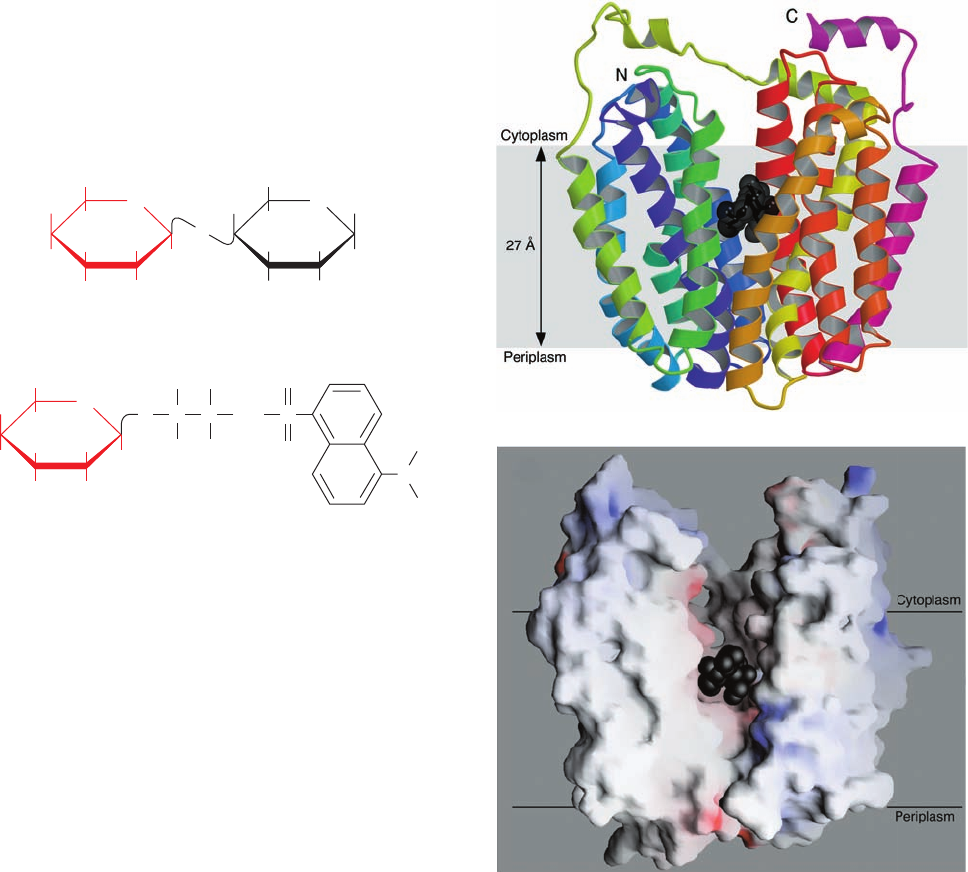
(Fig. 20-30a).A large internal hydrophilic cavity is open to
770 Chapter 20. Transport Through Membranes
Figure 20-30 X-ray structure of lactose permease from E. coli.
(a) Ribbon diagram as viewed from the membrane with the
cytoplasmic side up.The protein’s 12 transmembrane helices are
colored in rainbow order with the N-terminus purple and the
C-terminus pink.The bound lactose analog is represented by
black spheres. (b) Surface model viewed as in Part a but with the
two helices closest to the viewer in Part a removed to reveal the
lactose-binding cavity. The surface is colored according to its
electrostatic potential with positively charged areas blue,
negatively charged areas red, and neutral areas white. [Courtesy
of H. Ronald Kaback, UCLA. PDBid 1PV7.]
(a)
(b)
JWCL281_c20_744-788.qxd 6/4/10 1:37 PM Page 770
