Voet D., Voet Ju.G. Biochemistry
Подождите немного. Документ загружается.

5. Succinyl-CoA synthetase converts succinyl-coenzyme
A to succinate.The free energy of the thioester bond is con-
served in this reaction by the formation of “high-energy”
GTP from GDP ⫹ P
i
.
6. The remaining reactions of the cycle serve to oxidize
succinate back to oxaloacetate in preparation for another
round of the cycle. Succinate dehydrogenase catalyzes the
oxidation of succinate’s central single bond to a trans dou-
ble bond, yielding fumarate with the concomitant reduc-
tion of the redox coenzyme FAD to FADH
2
(the molecular
formulas of FAD and FADH
2
and the reactions through
which they are interconverted are given in Fig. 16-8).
7. Fumarase then catalyzes the hydration of fumarate’s
double bond to yield malate.
8. Finally, malate dehydrogenase reforms oxaloacetate
by oxidizing malate’s secondary alcohol group to the corre-
sponding ketone with concomitant reduction of a third
NAD
⫹
to NADH. Acetyl groups are thereby completely
oxidized to CO
2
with the following stoichiometry:
The citric acid cycle functions catalytically as a consequence
of its regeneration of oxaloacetate: An endless number of
acetyl groups can be oxidized through the agency of a single
oxaloacetate molecule.
NADH and FADH
2
are vital products of the citric acid
cycle.Their reoxidation by O
2
through the mediation of the
electron-transport chain and oxidative phosphorylation
(Chapter 22) completes the breakdown of metabolic fuel
in a manner that drives the synthesis of ATP. Other func-
tions of the cycle are discussed in Section 21-5.
B. Historical Perspective
The citric acid cycle was proposed in 1937 by Hans Krebs, a
contribution that ranks as one of the most important
achievements of metabolic chemistry.We therefore outline
the intellectual history of this cycle’s discovery.
By the early 1930s, significant progress had been made
in elucidating the glycolytic pathway (Section 17-1A). Yet
the mechanism of glucose oxidation and its relationship to
cellular respiration (oxygen uptake) was still a mystery.
Nevertheless, the involvement of several metabolites in
cellular oxidative processes was recognized. It was well
known, for example, that in addition to lactate and acetate,
the dicarboxylates succinate, malate, and ␣-ketoglutarate,
as well as the tricarboxylate citrate, are rapidly oxidized by
muscle tissue during respiration. It had also been shown
that malonate (Section 21-3F), a potent inhibitor of succi-
nate oxidation to fumarate, also inhibits cellular respira-
tion, thereby suggesting that succinate plays a central role
in oxidative metabolism rather than being just another
metabolic fuel.
In 1935, Albert Szent-Györgyi demonstrated that cellu-
lar respiration is dramatically accelerated by catalytic
amounts of succinate, fumarate, malate, and oxaloacetate;
3NADH ⫹ FADH
2
⫹ GTP ⫹ CoA ⫹ 2CO
2
3NAD
⫹
⫹ FAD ⫹ GDP ⫹ P
i
⫹ acetyl-CoA
¡
that is, the addition of any of these substances to minced
muscle tissue stimulates O
2
uptake and CO
2
production far
in excess of that required to oxidize the added dicarboxylic
acid. Szent-Györgyi further showed that these compounds
were interconverted according to the reaction sequence:
Shortly afterward, Carl Martius and Franz Knoop dem-
onstrated that citrate is rearranged, via cis-aconitate, to
isocitrate and then dehydrogenated to ␣-ketoglutarate.
␣-Ketoglutarate was already known to undergo oxidative
decarboxylation to succinate and CO
2
. This extended the
proposed reaction sequence to
What was necessary to close the circle so as to make the
system catalytic was to establish that oxaloacetate is con-
verted to citrate. In 1936, Martius and Knoop demon-
strated that citrate could be formed nonenzymatically from
oxaloacetate and pyruvate by treatment with hydrogen
peroxide under basic conditions. Krebs used this chemical
model as the point of departure for the biochemical exper-
iments that led to his proposal of the citric acid cycle.
Krebs’ hypothesis was based on his investigations, starting
in 1936, on respiration in minced pigeon breast muscle (which
has a particularly high rate of respiration). The idea of a cat-
alytic cycle was not new to him: In 1932, he and Kurt Hense-
leit had elucidated the outlines of the urea cycle, a process in
which ammonia and CO
2
are converted to urea (Section
26-2). The most important observations Krebs made in sup-
port of the existence of the citric acid cycle were as follows:
1. Succinate is formed from fumarate, malate, or oxalo-
acetate in the presence of the metabolic inhibitor malonate.
Since malonate inhibits the direct reduction of fumarate to
succinate, the succinate must be formed by an oxidative cycle.
2. Pyruvate and oxaloacetate can form citrate enzymat-
ically. Krebs therefore suggested that the metabolic cycle is
closed with the reaction:
3. The interconversion rates of the cycle’s individual
steps are sufficiently rapid to account for observed respira-
tion rates, so it must be (at least) the major pathway for
pyruvate oxidation in muscle.
Although Krebs had established the existence of the cit-
ric acid cycle, some major gaps still remained in its com-
plete elucidation. The mechanism of citrate formation did
not become clear until Nathan Kaplan and Fritz Lipmann
discovered coenzyme A in 1945 (Section 21-2), and Severo
Ochoa and Feodor Lynen established, in 1951, that acetyl-
CoA is the intermediate that condenses with oxaloacetate
to form citrate. Oxidative decarboxylation of ␣-ketoglutarate
to succinate was also shown to involve coenzyme A with
succinyl-CoA as an intermediate.
Pyruvate ⫹ oxaloacetate
¡
citrate ⫹ CO
2
¡
malate
¡
oxaloacetate
¡
␣-ketoglutarate
¡
succinate
¡
fumarate
Citrate
¡
cis-aconitate
¡
isocitrate
Succinate
¡
fumarate
¡
malate
¡
oxaloacetate
Section 21-1. Cycle Overview 791
JWCL281_c21_789-822.qxd 3/17/10 11:33 AM Page 791
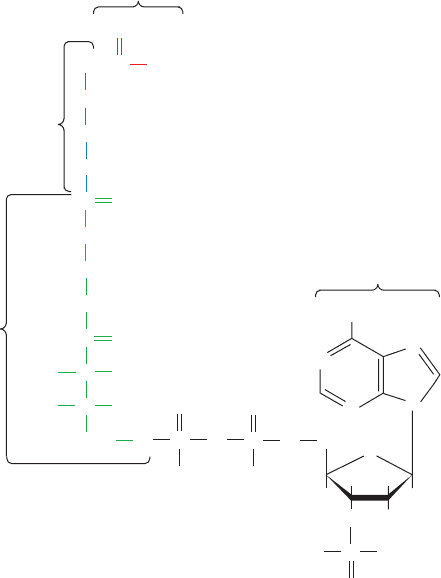
Acetyl-coenzyme A (acetyl-CoA)
⬃
O
C
S
CH
2
O
O
H
H
O OH
N
N
NH
2
N
N
CH
3
CH
2
NH
O
C
CH
2
CH
2
NH
O
C
C
C
CH
2
H
H
3
C
OH
CH
3
O
P O P
O CH
2
O
HH
O
–
O
–
O
–
O
P
O
–
Adenosine-3⬘-
phosphate
Pantothenic
acid residue
Acetyl group
-Mercaptoethylamine
residue
The elucidation of the citric acid cycle was a major
achievement and, like all achievements of this magnitude,
required the efforts of numerous investigators. Indeed, bio-
chemists are still working to understand the cycle on a mo-
lecular and enzymatic level. We shall study the eight en-
zymes that catalyze the cycle after first discussing the cycle’s
major fuel, acetyl-CoA, and its formation from pyruvate.
2 METABOLIC SOURCES OF
ACETYL-COENZYME A
Acetyl groups enter the citric acid cycle as acetyl-coenzyme
A (acetyl-SCoA or acetyl-CoA; Fig. 21-2), the common
product of carbohydrate, fatty acid, and amino acid break-
down. Coenzyme A (CoASH or CoA) consists of a -
mercaptoethylamine group bonded through an amide linkage
to the vitamin pantothenic acid, which, in turn, is attached to
a 3¿-phosphoadenosine moiety via a pyrophosphate bridge.
The acetyl group of acetyl-CoA is bonded as a thioester to the
sulfhydryl portion of the -mercaptoethylamine group. CoA
thereby functions as a carrier of acetyl and other acyl groups
(the A in CoA stands for “Acetylation”).
Acetyl-CoA is a “high-energy” compound: The ⌬G°¿ for
the hydrolysis of its thioester bond is ⫺31.5 kJ ⴢ mol
⫺1
,
which makes this reaction slightly (1 kJ ⴢ mol
⫺1
) more
exergonic than that of ATP hydrolysis (Section 16-4A).
The formation of this thioester bond in a metabolic inter-
mediate therefore conserves a portion of the free energy of
oxidation of a metabolic fuel.
A. Pyruvate Dehydrogenase Multienzyme
Complex (PDC)
The immediate precursor to acetyl-CoA from carbohydrate
sources is the glycolytic product pyruvate. As we saw in Sec-
tion 17-3, under anaerobic conditions the NADH produced
by glycolysis is reoxidized with concomitant reduction of
pyruvate to lactate (in muscle) or ethanol (in yeast). Under
aerobic conditions, however, NADH is reoxidized by the
mitochondrial electron-transport chain (Section 22-2), so
that pyruvate, which enters the mitochondrion via a spe-
cific pyruvate–H
⫹
symport (membrane transport nomen-
clature is discussed in Section 20-3), can undergo further
oxidation. (The formation of acetyl-CoA from fatty acids
and amino acids is discussed in Sections 25-2 and 26-3.)
Acetyl-CoA is synthesized from pyruvate and CoA
through oxidative decarboxylation by a multienzyme com-
plex named pyruvate dehydrogenase. In general, multien-
zyme complexes are groups of noncovalently associated en-
zymes that catalyze two or more sequential steps in a
metabolic pathway. The pyruvate dehydrogenase multien-
zyme complex (PDC) consists of three enzymes: pyruvate
dehydrogenase (E1), dihydrolipoyl transacetylase (E2), and
dihydrolipoyl dehydrogenase (E3). The E. coli pyruvate de-
hydrogenase complex, whose characterization was pio-
neered by Lester Reed, is an ⬃4600-kD polyhedral particle
that is ⬃300 Å in diameter (Fig. 21-3a). Isolated E. coli E2
forms a particle with 24 identical subunits, which electron
micrographs (Figs. 21-3b and 21-4a), together with an X-ray
structure by Wim Hol (Fig. 21-5a), indicate are arranged
with cubic symmetry.The E1 subunits form dimers that asso-
ciate with the E2 cube at the centers of the cube’s 12 edges
(Fig. 21-4b,c), whereas the E3 subunits form dimers that are
located at the centers of this cube’s 6 faces.We discuss the X-
ray structures of the E1, E2, and E3 subunits below.
a. Some PDCs Have a Dodecahedral Form
Although all PDCs catalyze the same reactions by similar
mechanisms, they may have different quaternary structures.
Whereas the PDCs of E. coli and most other gram-negative
bacteria have the foregoing cubic symmetry, those of
792 Chapter 21. Citric Acid Cycle
Figure 21-2 Chemical structure of acetyl-CoA.
The thioester bond is drawn with a squiggle (
⬃
) to
indicate that it is a “high-energy” bond. In CoA, the
acetyl group is replaced by a hydrogen atom.
JWCL281_c21_789-822.qxd 3/17/10 11:33 AM Page 792
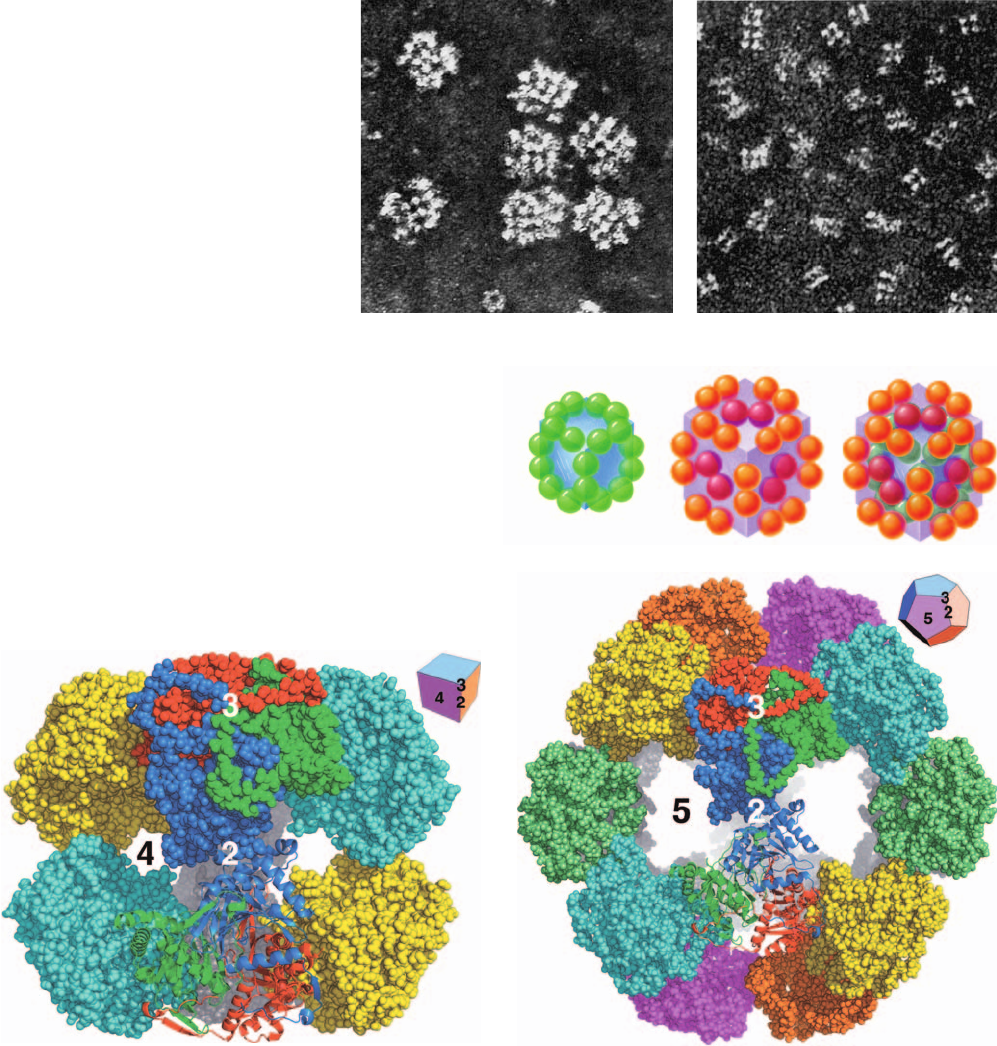
(a) (b) (c)
Section 21-2. Metabolic Sources of Acetyl-Coenzyme A 793
Figure 21-3 Electron micrographs of the
E. coli pyruvate dehydrogenase multienzyme
complex. (a) The intact complex. (b) The
dihydrolipoyl transacetylase (E2) “core”
complex. [Courtesy of Lester Reed,
University of Texas.]
Figure 21-4 Structural organization of the E. coli PDC. (a)
The dihydrolipoyl transacetylase (E2) “core.” Its 24 subunits
(green spheres) associate as trimers located at the corners of a
cube to form a particle that has cubic symmetry (O symmetry;
Section 8-5B). (b) The 24 pyruvate dehydrogenase (E1) subunits
(orange spheres) form dimers that associate with the E2 core
(shaded cube) at the centers of each of its 12 edges, whereas the
12 dihydrolipoyl dehydrogenase (E3) subunits (purple spheres)
form dimers that attach to the E2 cube at the centers of each of its
6 faces. (c) Parts a and b combined to form the entire 60-subunit
complex.
Figure 21-5 Comparison of the X-ray structures of the
dihydrolipoyl transacetylase (E2) cores of PDCs. Both complexes
are drawn mainly in space-filling representation and viewed
along their respective 2-fold axes of symmetry. (a) The 125-Å
high cubic (O symmetry; Fig. 8-65c) E2 core from the gram-
negative bacterium Azotobacter vinelandii. It consists of 24
subunits that form 8 trimers, shown here in different colors. The
positions of a 2-fold, a 3-fold, and a 4-fold axis of symmetry are
indicated.The inset (upper right) is a perspective drawing of a
cube. (b) The 237-Å-diameter dodecahedral (I symmetry;
Fig. 8-65c) E2 core from the gram-positive bacterium Bacillus
stearothermophilus. It consists of 60 subunits that form 20 trimers,
shown here in different colors.The positions of a 2-fold, a 3-fold,
and a 5-fold axis of symmetry are indicated.The inset (upper
right) is a perspective drawing of a dodecahedron.The rear
portion of each complex, which is largely eclipsed by the front
portion, is gray. The subunits forming the trimers closest to the
viewer in each drawing are individually colored with the lower
trimer drawn in ribbon form. Note that the subunits in each
trimer are extensively associated and that the trimers in the two
complexes have closely similar structures. In contrast, the
interactions between contacting trimers in both types of
complexes are relatively tenuous.Also note that these contacting
trimers form the 4- and 5-membered rings that comprise the
square and pentagonal faces of the cubic and dodecahedral
complexes, respectively. [Based on X-ray structures by Wim Hol,
University of Washington. PDBids 1EAB and 1B5S.]
(a)
(b)
(a)
(b)
JWCL281_c21_789-822.qxd 6/5/10 9:53 AM Page 793
eukaryotes and some gram-positive bacteria have an analo-
gous dodecahedral form [Fig. 21-5b; a dodecahedron is a
regular polyhedron with I symmetry (Section 8-5B) that has
20 vertices, each lying on a 3-fold axis, and 12 pentagonal
faces having an aggregate of 30 edges]. Thus, the mitochon-
drially located ⬃10,000-kD eukaryotic complex, the largest
known multienzyme complex, consists of a dodecahedral
core of 20 E2 trimers (one centered on every vertex) sur-
rounded by 30 E1 ␣
2

2
heterotetramers (one centered on
every edge) and 12 E3 dimers (one centered in every face).
b. Multienzyme Complexes Are Catalytically Efficient
Multienzyme complexes are a step forward in the evolu-
tion of catalytic efficiency.They offer the following mecha-
nistic advantages:
1. Enzymatic reaction rates are limited by the frequency
at which enzymes collide with their substrates (Section 14-
2Ba). If a series of reactions occurs within a multienzyme
complex,the distance that substrates must diffuse between ac-
tive sites is minimized, thereby achieving a rate enhancement.
2. Complex formation provides the means for channel-
ing (passing) metabolic intermediates between successive
enzymes in a metabolic pathway, thereby minimizing side
reactions.
3. The reactions catalyzed by a multienzyme complex
may be coordinately controlled.
c. Acetyl-CoA Formation Occurs in Five Reactions
The PDC catalyzes five sequential reactions (Fig. 21-6)
with the overall stoichiometry:
The coenzymes and prosthetic groups required in this reac-
tion sequence are thiamine pyrophosphate (TPP;Fig.17-26),
flavin adenine dinucleotide (FAD; Fig. 16-8), nicotinamide
adenine dinucleotide (NAD
⫹
; Fig. 13-2), and lipoamide
acetyl-CoA ⫹ CO
2
⫹ NADH
Pyruvate ⫹ CoA ⫹ NAD
⫹
¡
(Fig. 21-7); their functions are listed in Table 21-1.
Lipoamide consists of lipoic acid joined in amide linkage to
the ε-amino group of a Lys residue. Reduction of its cyclic
disulfide to a dithiol, dihydrolipoamide, and its reoxidation
(Fig. 21-7) are the “business” of this prosthetic group.
The five reactions catalyzed by the PDC are as follows
(Fig. 21-6):
1. Pyruvate dehydrogenase (E1), a TPP-requiring enzyme,
decarboxylates pyruvate, with the intermediate formation of
hydroxyethyl-TPP.This reaction is identical with that catalyzed
by yeast pyruvate decarboxylase (Section 17-3B):
+
+
+
_
_
R⬘
H
3
C
NR
SE1
E1TPP
Pyruvate
Hydroxyethyl-
TPP
C
O
O
CCCH
3
CO
2
H
3
C
HOC CH
3
+
R⬘
NR
SE1
C
E1
+
_
R⬘
H
3
C
NR
SE1
C
_
OCCCH
3
O
H
O OH
794 Chapter 21. Citric Acid Cycle
O
C C
O
–
O
pyruvate
dehydrogenase
(E1)
CO
2
CH
3
OH
C
–
TPPCH
3
TPP
1
Hydroxyethyl-
TPP
2
O
CCH
3
S
S
R
Lipoamide
S
HS
R
Acetyl-dihydrolipoamide
HS
HS
R
dihydrolipoyl
transacetylase
(E2)
4
dihydrolipoyl
dehydrogenase
(E3)
3
CoA
O
CCH
3
S CoA
Acetyl-CoA
FAD
SH
SH
FAD
S
S
5
NADH
+ H
+
NAD
+
Pyruvate
Figure 21-6 The five reactions of the PDC. E1 (pyruvate
dehydrogenase) contains TPP and catalyzes Reactions 1 and 2.
E2 (dihydrolipoyl transacetylase) contains lipoamide and
catalyzes Reaction 3. E3 (dihydrolipoyl dehydrogenase) contains
FAD and a redox-active disulfide and catalyzes Reactions 4
and 5.
JWCL281_c21_789-822.qxd 3/17/10 11:33 AM Page 794
E2
CoA
SH
C
C
O
O
CH
3
CoA
S
CH
3
Acetyl-CoA
Acetyl-
dihydrolipoamide–E2
Dihydrolipoamide–E2
S
HS
E2
HS
HS
+
2. Unlike pyruvate decarboxylase, however, pyruvate
dehydrogenase does not convert the hydroxyethyl-TPP in-
termediate into acetaldehyde and TPP. Instead, the hy-
droxyethyl group is transferred to the next enzyme in the
multienzyme sequence, dihydrolipoyl transacetylase (E2).
The reaction occurs by attack of the hydroxyethyl group
carbanion on the lipoamide disulfide, followed by the
elimination of TPP from the intermediate adduct to form
acetyl-dihydrolipoamide and regenerate active E1. The
hydroxyethyl carbanion is thereby oxidized to an acetyl
group by the concomitant reduction of the lipoamide
disulfide bond:
H
+
H
+
H
3
CR⬘
R
CH
O
CH
3
Lipoamide-E2
Reaction 1
S • E1
N
+
C
S
S
E2
H
3
CR⬘
R
CH
O
CH
3
S • E1
N
+
C
S
HS
E2
Acetyl-
dihydrolipoamide-E2
C
O
CH
3
S
HS
E2
H
3
CR⬘
R
S • E1
N
+
C
TPP • E1
+
3. E2 then catalyzes the trans-
fer of the acetyl group to CoA,
yielding acetyl-CoA and dihy-
drolipoamide–E2:
This is a transesterification in which the sulfhydryl group of
CoA attacks the acetyl group of acetyl-dihydrolipoamide–
E2 to form a tetrahedral intermediate (not shown), which
decomposes to acetyl-CoA and dihydrolipoamide–E2.
Section 21-2. Metabolic Sources of Acetyl-Coenzyme A 795
Figure 21-7 Interconversion of lipoamide and
dihydrolipoamide. Lipoamide is lipoic acid covalently joined to
the ε-amino group of a Lys residue via an amide linkage.
S
O
S
CH
2
CH
2
CH
CH
2
CH
2
CH
2
CH
2
C NH
NH
CH
C O
(CH
2
)
4
Lipoamide
Lipoic acid Lys
2H
+
+
2e
–
HS
O
HS
CH
2
CH
2
CH
CH
2
CH
2
CH
2
CH
2
C NH
NH
CH
C O
(CH
2
)
4
Dihydrolipoamide
Table 21-1 The Coenzymes and Prosthetic Groups of Pyruvate Dehydrogenase
Cofactor Location Function
Thiamine pyrophosphate (TPP) Bound to E1 Decarboxylates pyruvate, yielding a
hydroxyethyl-TPP carbanion
Lipoic acid Covalently linked to a Lys on Accepts the hydroxyethyl carbanion from
E2 (lipoamide) TPP as an acetyl group
Coenzyme A (CoA) Substrate for E2 Accepts the acetyl group from acetyl-
dihydrolipoamide
Flavin adenine dinucleotide (FAD) Bound to E3 Reduced by dihydrolipoamide
Nicotinamide adenine dinucleotide (NAD
⫹
) Substrate for E3 Reduced by FADH
2
JWCL281_c21_789-822.qxd 3/17/10 11:33 AM Page 795
H
3
N
+
~35 residues
n
COO
–
~80 residues each
Lipoyl
domains
Outer linker Inner linker
Catalytic
domain
Peripheral
subunit-binding
domain
(
(
~250 residues
S
S
FAD
E3 (oxidized)
SH
SH
FAD
S
S
FADH
2
NAD
+
NADH
+
H
+
Reaction 4
4. Dihydrolipoyl dehydrogenase (E3; also called dihy-
drolipoamide dehydrogenase) reoxidizes dihydrolipo-
amide, thereby completing the catalytic cycle of E2:
Oxidized E3 contains a reactive disulfide group and a
tightly bound FAD.The oxidation of dihydrolipoamide is a
disulfide interchange reaction (Section 9-1A):The lipoamide
disulfide bond forms with concomitant reduction of E3’s
reactive disulfide to two sulfhydryl groups.
5. Reduced E3 is reoxidized by NAD
⫹
:
The enzyme’s active sulfhydryl groups
are reoxidized by the enzyme-bound
FAD, which is thereby reduced to
FADH2.The FADH2 is then reoxidized
to FAD by NAD1, producing NADH.
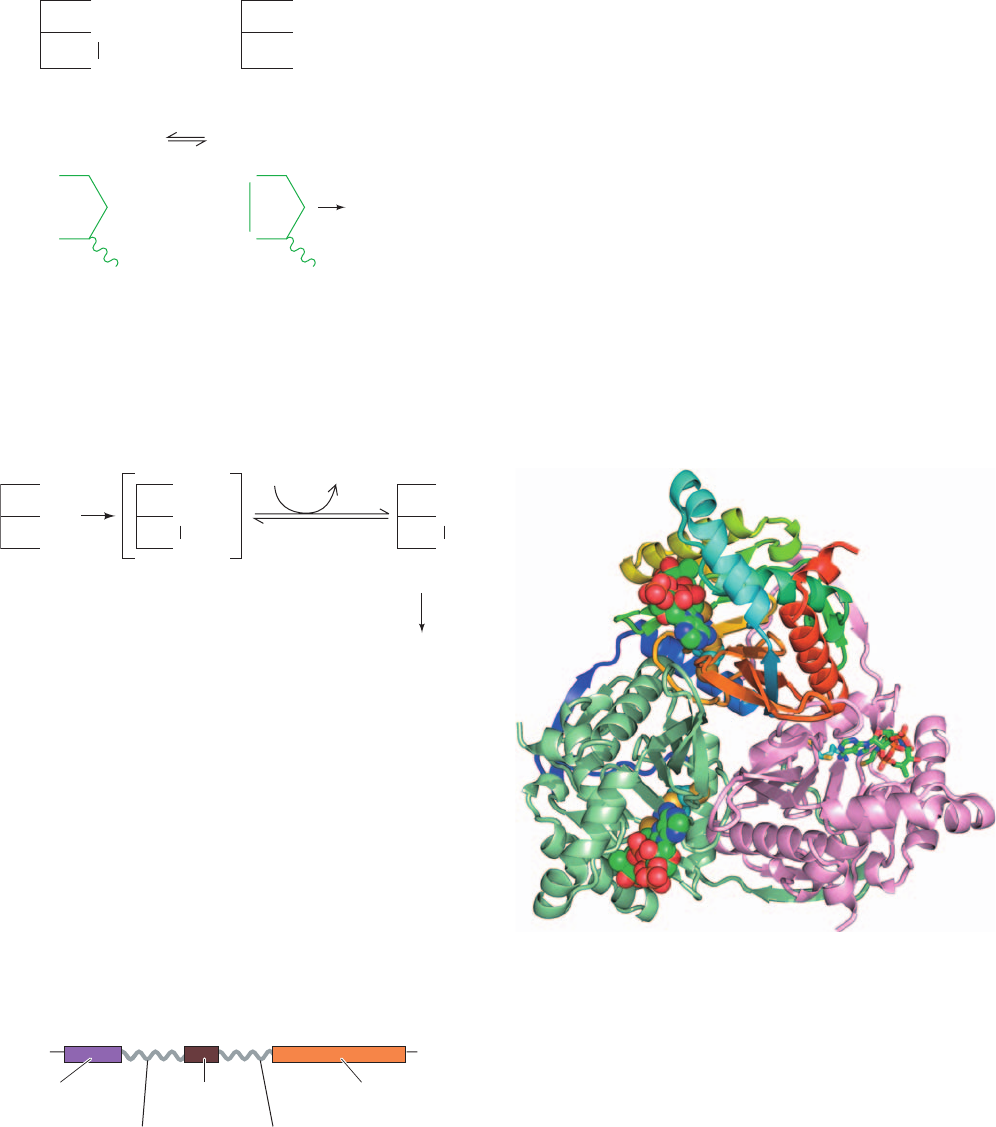
d. The Structure of E2
Dihydrolipoyl transacetylase (E2) consists of several do-
mains (Fig. 21-8): one to three N-terminal lipoyl domains
(⬃80 residues) that each covalently bind a lipoyl group; a
peripheral subunit-binding domain (PSBD; ⬃35 residues)
that binds to both E1 and E3 and hence holds the complex
together; and a C-terminal catalytic domain (⬃250
residues) that contains the enzyme’s catalytic center and its
intersubunit binding sites.These domains are linked by 20- to
S
S
E2
E3
(oxidized)
FAD
S
S
+
H
H
S
S
E2
E3
(reduced)
FAD
S
H
SH
+
Reaction 2
40-residue Pro- and Ala-rich segments that are largely ex-
tended and highly flexible and thereby provide the lipoyl
domains with the mobility they require to interact with E1
and E3 and with neighboring E2 subunits (see below).
The hollow cagelike structures formed by the E2 cat-
alytic domains (Fig. 21-5) contain channels large enough to
allow substrates to diffuse in and out. In fact, coenzyme A
and lipoamide bind in extended conformations at opposite
ends of a 30-Å-long channel that is located at the interface
between each pair of the subunits in a trimer (Fig. 21-9).
This arrangement requires CoA to approach its binding
site from inside the cage.
The NMR structures of the lipoyl domains of E2 from
several sources verify the exposed nature of the site of the
lipoyl group.They consist of a  barrel/sandwich containing
a 4-stranded and a 3-stranded antiparallel  sheet, with the
Lys to which the lipoyl group would be attached extending
from an exposed position on a type I  bend linking two of
the  strands in the 4-stranded sheet (Fig. 21-10a).
The NMR structure of the PSBD from B. stearother-
mophilus, determined by Richard Perham, reveals that its
⬃35-residue ordered region consists of two parallel helices
separated by a loop that form a close-packed hydrophobic
796 Chapter 21. Citric Acid Cycle
Figure 21-8 Domain structure of the dihydrolipoyl
transacetylase (E2) subunit of the PDC. The number of lipoyl
domains, n, is species dependent: n ⫽ 3 for E. coli and A.
vinelandii, n ⫽ 2 for mammals and Streptococcus faecalis, and
n ⫽ 1 for B. stearothermophilus and yeast.
Figure 21-9 X-ray structure of a trimer of A. vinelandii
dihydrolipoyl transacetylase (E2) catalytic domains in complex
with CoA and lipoamide. The trimer, a portion of the cubic
complex (Fig. 21-5a), is drawn in ribbon form and viewed along
its 3-fold axis (the cubic complex’s body diagonal) from inside
the cube. The three identical domains have different colors with
the upper domain colored in rainbow order from its N-terminus
(blue) to its C-terminus (red). The CoA and lipoamide bound to
the upper and lower left subunits are drawn in space-filling form
and those bound to the lower right subunit are drawn in stick form,
with CoA C green, lipoamide C cyan, N blue, O red, P orange, and
S yellow. Note how the N-terminal “elbow” of each subunit
extends behind the counterclockwise adjacent subunit; its deletion
greatly destabilizes the complex. [Based on an X-ray structure by
Wim Hol, University of Washington. PDBid 1EAB.]
JWCL281_c21_789-822.qxd 6/5/10 9:53 AM Page 796
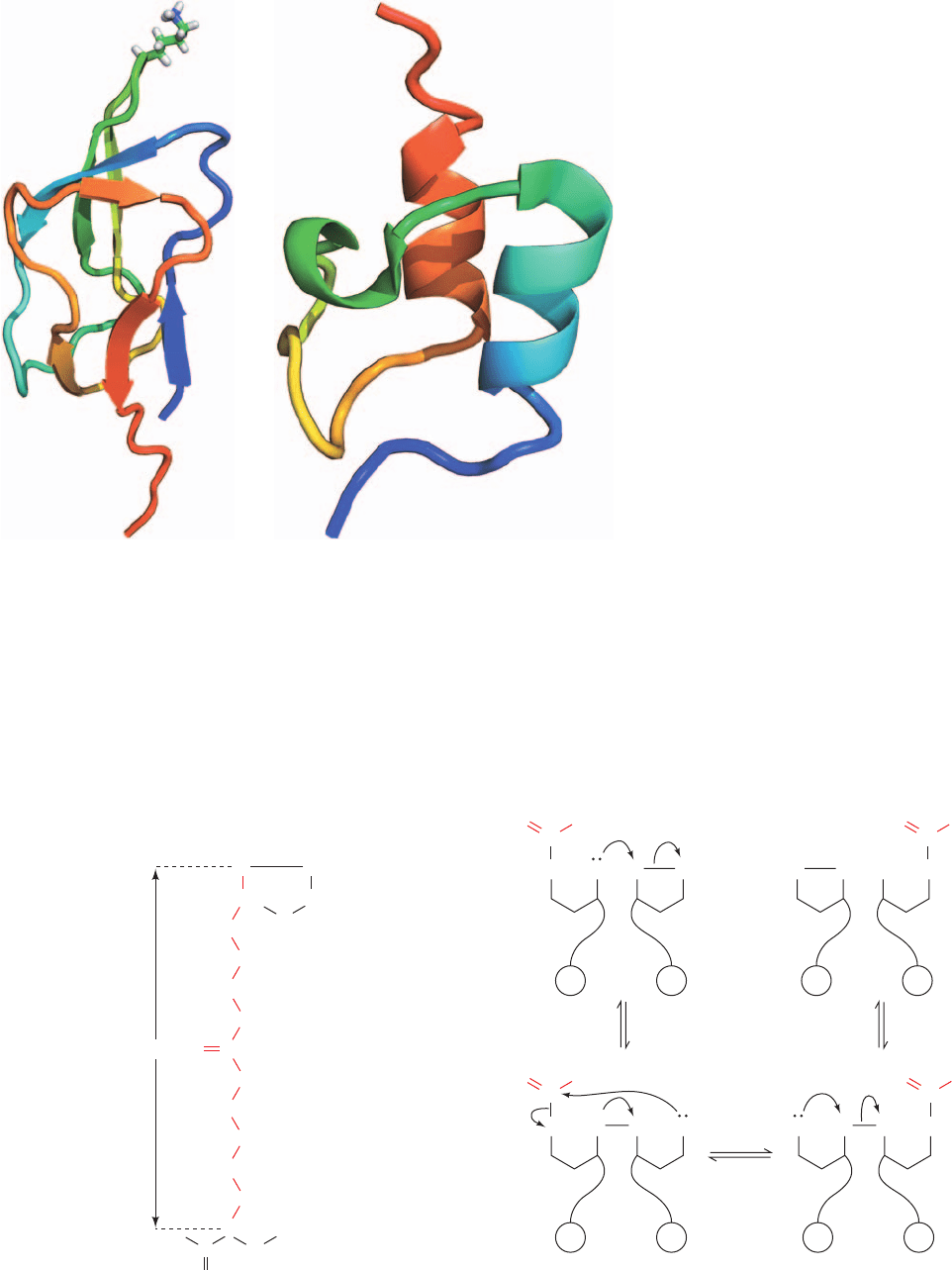
core (Fig. 21-10b). It is one of the smallest known polypep-
tides that have a globular structure but lack disulfide bonds
or prosthetic groups.
e. Intermediates Are Transferred between Enzyme
Subunits by Flexible Tethers
How are reaction intermediates transferred between
the component enzymes of the PDC? The group between
the lipoamide disulfide bond and the E2 polypeptide back-
bone, the so-called lipoyllysyl arm, has a fully extended
length of 14 Å:
CO
C
O
C
H
2
CH
2
CH
S S
CH
2
CH
2
CH
2
CH
2
NH
CH
2
CH
2
CH
2
CH
2
CH
N
H
14 Å
Lipoyllysyl arm
(fully extended)
Evidently, the lipoyllysyl arm, in combination with the
⬎140-Å-long outer linker (Fig. 21-8), acts as a long flexible
tether that swings its attached lipoyl group among the active
sites of E1, E2, and E3. Moreover, there is rapid inter-
change of acetyl groups among the lipoyl groups of the
E2 core (3 ⫻ 24 ⫽ 72 lipoyl groups in an E. coli PDC;
2 ⫻ 60 ⫽ 120 in mammals); the tethered arms also swing
among themselves, exchanging both acetyl groups and
disulfides:
One E1 subunit can therefore acetylate numerous E2 sub-
units and one E3 subunit can reoxidize several dihy-
drolipoamide groups.
O
C
SSSSH
CH
3
E2 E2⬘
O
C
SSSHS
CH
3
E2 E2⬘
O
C
HS S SS
CH
3
E2 E2⬘
O
C
SS SSH
CH
3
E2 E2⬘
Section 21-2. Metabolic Sources of Acetyl-Coenzyme A 797
Figure 21-10 NMR structures of the lipoyl
and peripheral subunit-binding domains of
dihydrolipoyl transacetylase (E2). (a) The
NMR structure of the A. vinelandii
dihydrolipoyl transacetylase (E2) lipoyl
domain.The polypeptide chain is colored in
rainbow order from its N-terminus (blue) to its
C-terminus (red).The Lys side chain to which the
lipoyl group would be bound is shown in stick
form colored according to atom type (C green,
H white, and N blue) and is located in a type I 
turn. [Based on an NMR structure by Aart de
Kok, Wageningen Agricultural University,
Wageningen, Netherlands. PDBid 1IYU.]
(b) The NMR structure of the peripheral
subunit-binding domain (PSBD) from B.
stearothermophilus E2.The polypeptide chain is
colored in rainbow order from its N-terminus
(blue) to its C-terminus (red). [Based on an
NMR structure by Richard Perham, Cambridge
University, U.K. PDBid 2PDD.]
(a)
(b)
JWCL281_c21_789-822.qxd 6/5/10 9:53 AM Page 797
f. Mammalian and Yeast PDCs Contain
Additional Subunits
In mammals and yeast, the dodecahedral PDC’s already
complicated structure has a further level of complexity in
that it contains additional subunits: About 12 copies of E3
binding protein (E3BP) facilitate the binding of E3 to the
E2 core of the eukaryotic dodecahedral complex. E3BP
has a lipoyllysine-containing domain similar to E2 and can
accept an acetyl group, but its C-terminal domain has no
catalytic activity, and the removal of its lipoyllysine domain
does not diminish the catalytic activity of the complex.
E3BP’s main role seems to be to aid in the binding of E3,
since limited proteolysis of E3BP decreases E3’s binding
ability.
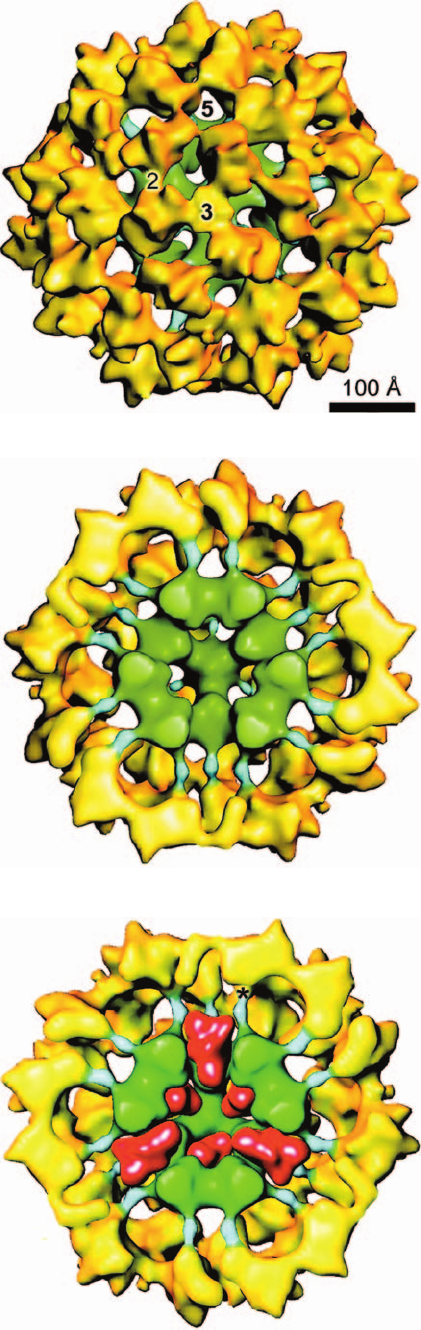
g. Cryoelectron Microscopy Reveals the
Structure of the Dodecahedral PDC
James Stoops and Reed have determined the organiza-
tion of the bovine kidney dodecahedral complex through
cryoelectron microscopy (Fig. 21-11). As expected, the E2
subunits form a dodecahedral core that is surrounded by a
concentric dodecahedron of E1 subunits (Fig. 21-11a,b).
The E2 subunits bind the E1 subunits via the radially ex-
tending, ⬃50-Å-long inner linkers that precede the PSBD
of E2 (Fig. 21-8; these linkers are not present in Fig. 21-5b
because that X-ray structure only contains E2’s catalytic
domain). Although the bovine kidney PDC used in the
structure determination lacked sufficient E3BP ⴢ E3 to be
visible, its position in the electron microscopy–based struc-
ture of yeast PDC indicates that an E3 dimer occupies and
largely fills each pentagonal opening of the E2 core
(Fig. 21-11c). Hence the E3 subunits are not arranged with
dodecahedral symmetry (similarly, the E3 subunits in the
cubic E. coli PDC are not arranged with cubic symmetry;
798 Chapter 21. Citric Acid Cycle
Figure 21-11 Electron microscopy–based images of the bovine
kidney pyruvate dehydrogenase complex at ⬃35 Å resolution.
(a) The entire particle as viewed along its 3-fold axis of
symmetry. E1 is yellow, the E2 catalytic core is green, and the
inner linkers that connect E2’s catalytic domains to its
E1-binding domains, the PSBDs (Fig. 21-8), are cyan.The particle
is viewed along a 3-fold axis of symmetry with the positions of a
5-fold axis and a 2-fold axis also marked. (b) A cutaway diagram
viewed and colored as in Part a but with the particle’s closest half
removed to reveal the E2 catalytic core and the inner linkers.
Compare the green portion with the X-ray structure of a
dodecahedral E2 catalytic core as viewed along a 2-fold axis
(Fig. 21-5b). (c) A cutaway diagram as in Part b but with E3
dimers (Fig. 21-13a) shown at 20 Å resolution (red) modeled
into the pentagonal openings of the E2 core. The position of a
peripheral subunit-binding site at the end of an E2 inner linker is
marked by an asterisk (*). [From Zhou, Z.H., McCarthy, D.B.,
O’Connor, C.M., Reed, L.J., and Stoops, J.K., Proc. Natl. Acad.
Sci. 98, 14802 (2001).]
(a)
(b)
(c)
JWCL281_c21_789-822.qxd 3/17/10 11:33 AM Page 798
Fig. 21-4b).The PSBD, which is located at the end of the E2
inner linker (* in Fig. 21-11c), is the point from which the
E2 lipoyl domains (Fig. 21-10a) swing. It is ⬃50 Å from the
nearest active sites of E1, E2, and E3.
In addition to E3BP, the mammalian PDC contains one
to three copies each of pyruvate dehydrogenase kinase and
pyruvate dehydrogenase phosphatase. The kinase and
phosphatase function to regulate the catalytic activity of
the complex (Section 21-2Cb).
h. Two Other Multienzyme Complexes Closely
Resemble the PDC
In addition to the PDC, most cells contain two other
closely related multienzyme complexes: the ␣-ketoglu-
tarate dehydrogenase complex [also called 2-oxoglutarate
dehydrogenase (OGDH), which catalyzes Reaction 4 of
the citric acid cycle; Fig. 21-1] and the branched-chain
␣-keto acid dehydrogenase complex (BCKDH; which par-
ticipates in the degradation of isoleucine, leucine, and va-
line; Section 26-3Ee). These multienzyme complexes all
catalyze similar reactions: the NAD
⫹
-linked oxidative de-
carboxylation of an ␣-keto acid with the transfer of the re-
sulting acyl group to CoA.In fact, all three members of this
2-ketoacid dehydrogenase family (alternatively, the 2-oxo
acid dehydrogenase family) of multienzyme complexes
share the same E3 subunit, and their E1 and E2 subunits,
which are specific for their corresponding substrates, are
homologous and use identical cofactors.Thus, to differenti-
ate them, the E1s for PDC,OGDH, and BCKDH are often
referred to as E1p, E1o, and E1b, respectively, and like-
wise, their E3s are called E3p, E3o, and E3b.
i. Arsenic Compounds Are Poisonous because
They Sequester Lipoamide
Arsenic has been known to be a poison since ancient
times.As(III) compounds, such as arsenite (AsO
3
3⫺
) and or-
ganic arsenicals, are toxic because of their ability to cova-
lently bind sulfhydryl compounds. This is particularly true
of vicinal (adjacent) sulfhydryls such as those of lipoamide
because they can form bidentate adducts:
The resultant inactivation of lipoamide-containing enzymes,
especially pyruvate dehydrogenase and ␣-ketoglutarate
dehydrogenase (Section 21-3D), brings respiration to a
halt.
Organic arsenicals are more toxic to microorganisms
than to humans, apparently because of differences in the
sensitivities of their various enzymes to these compounds.
This differential toxicity is the basis for the early twentieth
century use of organic arsenicals in the treatment of
syphilis (now superseded by penicillin) and trypanosomia-
sis (typanosomes are parasitic protozoa that cause several
diseases including African sleeping sickness and Chagas-
Cruz disease). These compounds were really the first
antibiotics, although, not surprisingly, they had severe side
effects.
j. Arsenic Poisoning, Napoleon, and Darwin
Arsenic is often suspected as a poison in untimely
deaths. In fact, it has long been thought that Napoleon
Bonaparte died from arsenic poisoning while in exile on
St. Helena, an island in the Atlantic Ocean. This suspicion,
and the chemical analyses it sparked, makes a fascinating
chemical anecdote. The finding that a lock of Napoleon’s
hair indeed contains a high level of arsenic strongly sup-
ports the notion that arsenic poisoning at least contributed
to his death. But was it murder or environmental pollu-
tion? A sample of the wallpaper from Napoleon’s drawing
room was found to contain the commonly used (at the
time) green pigment copper arsenate (CuHAsO
4
). It was
eventually determined that in a damp climate, as occurs on
St. Helena, fungi growing on the wallpaper eliminate the
arsenic by converting it to the volatile and highly toxic
trimethyl arsine [(CH
3
)
3
As]. Indeed, Napoleon’s regular
visitors also suffered from symptoms of arsenic poisoning
(e.g., gastrointestinal disturbances), which appeared to
moderate when they spent much of their time outdoors.
Thus, Napoleon’s arsenic poisoning may have been unin-
tentional.
Retrospective detective work also suggests that
Charles Darwin was a victim of chronic arsenic poison-
ing. For most of his life after he returned from his epic
voyage, Darwin complained of numerous ailments, in-
cluding eczema, vertigo, headaches, arthritis, gout, palpi-
tations, and nausea, all symptoms of arsenic poisoning.
Fowler’s solution, a common nineteenth century tonic,
contained 10 mg of arsenite ⴢ mL
⫺1
. Many individuals,
quite possibly Darwin himself, took this “medication” for
years.
k. The Structure of E1
In dodecahedral PDCs, E1 (pyruvate dehydrogenase) is
a 2-fold symmetric ␣
2

2
heterotetramer. However, in cubic
PDCs and in ␣-ketoglutarate dehydrogenase, which is also
cubic, the ␣ and  subunits are genetically fused to form a
single polypeptide and hence a homodimeric enzyme.
The X-ray structure of E1 from the (dodecahedral)
B. stearothermophilus PDC in complex with TPP and a
Section 21-2. Metabolic Sources of Acetyl-Coenzyme A 799
OH
OH
HS
HS
As
–
O
Arsenite
+
R R
–
O As
S
S
+ 2 H
2
O
HS
HS
AsR⬘ +
R R
R⬘ As
S
S
+ H
2
O
Dihydro-
lipoamide
O
Organic
arsenical
JWCL281_c21_789-822.qxd 3/17/10 11:33 AM Page 799
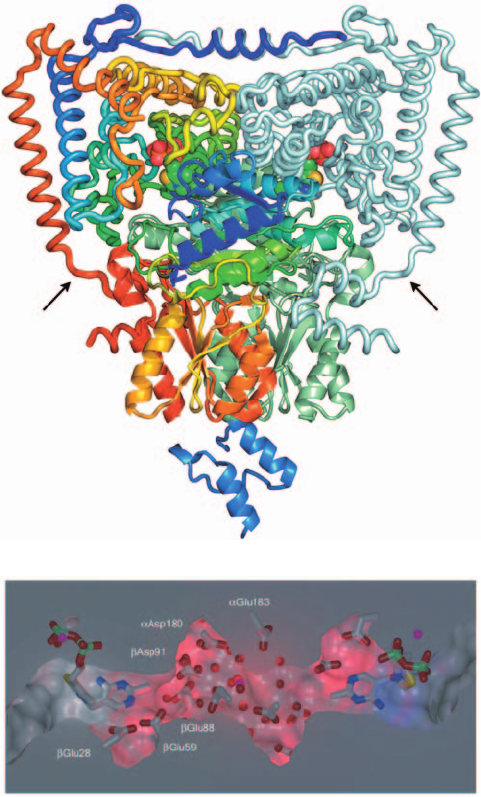
PSBD (Fig. 21-12a), determined by Perham and Ben
Luisi, reveals a tightly associated ␣
2

2
heterotetramer,
whose 368-residue ␣ subunits and 324-residue  sub-
units each consist of two domains. A single PSBD binds
to the C-terminal domains of the  subunits along the 2-
fold axis relating them in a way that sterically prevents
the binding of a second PSBD. Hence the PSBD interacts
asymmetrically with the two chemically identical do-
mains in a manner reminiscent of the way that human
growth hormone interacts with its homodimeric receptor
(Section 19-1J). E1’s conserved ⬃310-residue core occurs
in other TPP-utilizing enzymes of known structure, in-
cluding pyruvate decarboxylase (Section 17-3Ba). Each
TPP is bound between the N-terminal domains of an ␣
and a  subunit at the end of an ⬃21-Å-deep funnel-
shaped channel with its thiazolium ring (its reactive
group) closest to the channel entrance. Apparently, the
lipoyllysyl arm at the end of an E2 lipoyl domain is in-
serted into this channel in an extended conformation for
the transfer of the TPP’s hydroxyacyl substituent to
lipoamide.
l. E1 Active Sites Are Coordinated via a Proton Wire
When apo-E1 is mixed with TPP, the first TPP to bind
to this apparently 2-fold symmetric protein does so or-
ders of magnitude faster than the second TPP. This indi-
cates that these two binding sites, which are ⬃20 Å apart,
somehow communicate. In fact, the enzyme’s two active
sites in the foregoing X-ray structure have different
structures in that two conserved loops (residues 203–212
and 276–287 of the ␣ subunits) at the entrance to the
channel leading to TPP are ordered in one subunit in a
way that blocks the active site but are disordered in the
other subunit.That this is not an artifact of crystallization
was demonstrated by the observation that limited prote-
olysis cleaves the more C-terminal of these loops on one
subunit, but not the other. Similar asymmetries have
been observed in all TPP-dependent enzymes of known
structure.
The X-ray structure of E1 reveals a solvent-filled tunnel
linking its two active sites that is lined with 10 conserved
acidic residues (Fig. 21-12b). Similar acidic tunnels are
present in all TPP-dependent enzymes of known structure,
all of which are either dimeric or tetrameric.This led Luisi
and Perham to propose that as pyruvate proceeds through
the E1-catalyzed Reactions 1 and 2 discussed in Section 21-
2Ac, the proton required by Reaction 1 in one active site is
supplied by the proton released in Reaction 2 in the other
active site. The protons are presumably transferred be-
tween the two active sites via series of proton jumps
(Section 2-1C) through the acidic tunnel, which thereby
functions as a proton wire. Consequently, as the two active
sites progress through their catalytic cycles, they must
remain out of phase with one another; that is, one site re-
quires a general base when the other site requires a general
acid and vice versa so that they reciprocate their catalytic
needs via the proton wire.This explains the observed nega-
tive cooperativity of substrate binding exhibited by E1.
Structurally, this is rationalized by the alternate closing and
opening of the active sites through the out of phase order-
ing and disordering of the loops at the entrance of each
active site channel.
800 Chapter 21. Citric Acid Cycle
Figure 21-12 X-ray structure of B. stearothermophilus E1 in
complex with TPP and the peripheral subunit-binding domain
(PSBD). (a) The E1 ␣
2

2
heterotetramer, viewed with its 2-fold
axis of symmetry vertical and the E2 dodecahedron below, is
drawn with its ␣ subunits in worm form and its  subunits in
ribbon form.The ␣ and  subunits that are closest to the viewer
and predominantly on the left are each colored in rainbow order
from their N-termini (blue) to their C-termini (red); the remaining
␣ and  subunits are pale cyan and pale green. The PSBD (below)
is light blue. The TPP is shown in space-filling form with C green,
N blue, O red, P orange, and S yellow. The entrances to the
enzyme’s active site channels are indicated by black arrows. (b) A
cutaway surface diagram of the solvent-filled tunnel connecting
the enzyme’s two active sites. The hydroxyethyl-TPPs bound at
the enzyme’s active sites and the Asp and Glu side chains lining
the tunnel are drawn in stick form with C gray, N blue, O red, P
green, and S yellow. Water molecules and Mg
2⫹
ions are
represented by red and magenta spheres. [Part a based on an
X-ray structure by and Part b courtesy of Richard Perham and
Ben Luisi, University of Cambridge, U.K. PDBid 1W85.]
(a)
(b)
JWCL281_c21_789-822.qxd 7/2/10 9:05 AM Page 800
